Highlights
-
•
Exercise can be used as an active intervention for the rehabilitation of various diseases.
-
•
Exercise therapy could exert positive effects on alleviating the symptoms and improving the physical performance of patients who suffer from these diseases.
-
•
Exercise prescriptions could provide guidance for patients to engage in suitable physical activities to promote rehabilitation and physical function.
Keywords: Diseases, Exercise prescription, Patients
Abstract
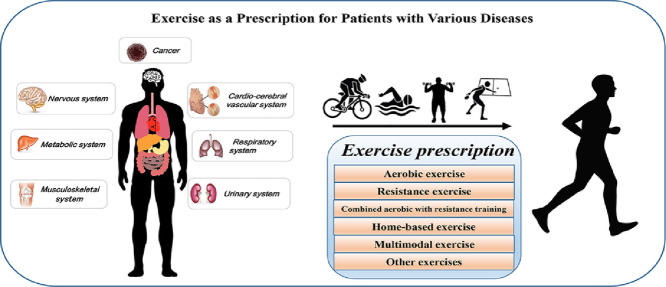
A growing understanding of the benefits of exercise over the past few decades has prompted researchers to take an interest in the possibilities of exercise therapy. Because each sport has its own set of characteristics and physiological complications that tend to occur during exercise training, the effects and underlying mechanisms of exercise remain unclear. Thus, the first step in probing the effects of exercise on different diseases is the selection of an optimal exercise protocol. This review summarizes the latest exercise prescription treatments for 26 different diseases: musculoskeletal system diseases (low back pain, tendon injury, osteoporosis, osteoarthritis, and hip fracture), metabolic system diseases (obesity, type 2 diabetes, type 1 diabetes, and nonalcoholic fatty liver disease), cardio-cerebral vascular system diseases (coronary artery disease, stroke, and chronic heart failure), nervous system diseases (Parkinson's disease, Huntington's disease, Alzheimer's disease, depression, and anxiety disorders), respiratory system diseases (chronic obstructive pulmonary disease, interstitial lung disease, and after lung transplantation), urinary system diseases (chronic kidney disease and after kidney transplantation), and cancers (breast cancer, colon cancer, prostate cancer, and lung cancer). Each exercise prescription is displayed in a corresponding table. The recommended type, intensity, and frequency of exercise prescriptions are summarized, and the effects of exercise therapy on the prevention and rehabilitation of different diseases are discussed.
Graphical Abstract
1. Introduction
Various diseases occurring in the human body are not only detrimental to people's health, but can also reduce quality of life. The lack of physical activity (PA) in modern life is a main reason for the decline of national physical fitness. It affects the growth and development of children and adolescents, as well as the physical wellness and work efficiency of adults. At the same time, a lack of exercise leads to an increase in the incidence of chronic diseases, which increases medical costs and the economic burden on the state and individuals. Guiding people to take part in exercise properly to enhance physical fitness is more urgent and more important than ever.
Lifestyle interventions should be the primary strategy for the prevention and treatment of metabolic diseases owing to their safety and effectiveness. Reasonable diet and weight control have received widespread attention, but the role of an exercise intervention is often overlooked by doctors and patients.1 Research shows that a reasonable exercise intervention can increase energy consumption, strengthen muscle, reduce blood pressure and blood lipids, increase bone density, and regulate psychological processes.2, 3, 4 Therefore, the importance of establishing an individual's level of PA, like the dosage of a drug, prompts investigators to study this problem in different diseases and in different clinical areas related to undesirable living habits. To achieve the desired goal, a specific exercise prescription is important.5
Exercise prescription is generally a specific plan of PA designed for a specific purpose, and it is usually developed by rehabilitation specialists based on the patient's condition. It mainly includes the type, frequency, intensity, and duration of exercise. For resistance exercise (RE) prescription, the total amount of training, interval time, and so on also need to be determined.6 Exercise intensity is critical for patients, and is usually determined by subjects’ heart rate reserve, maximum heart rate (HRmax), maximum oxygen uptake (VO2max), or rating of perceived exertion scales. The overall condition of each patient and the characteristics of the disease should also be considered. Generally, in the early and middle stages of the disease, moderate-intensity or even high-intensity intervals can be adopted. If the disease is in its late stages or if it is shortly after surgery, low-intensity training should be carried out, and the load intensity should be increased gradually. Owing to the special and unique needs of individual patients, the exercise prescription should be personalized and its goal should be evaluated during implementation to achieve the desired effect.
The purpose of this review was to provide effective exercise prescriptions for different diseases as identified in randomized controlled trials (RCTs). After providing background on 26 different diseases in 7 categories, we collated and analyzed the different types of exercise prescriptions and their effects for each disease. We conclude by identifying the best exercise therapy program and its dosage based on evidence, experience, and common sense. The review provides a scientific and methodological basis for choosing an exercise prescription based on to the type of disease being treated and the health needs of the patient, which can aid in preventing and treating diseases and thus promote rehabilitation and improvement in the quality of patients’ lives.
2. Methods
2.1. Data collection
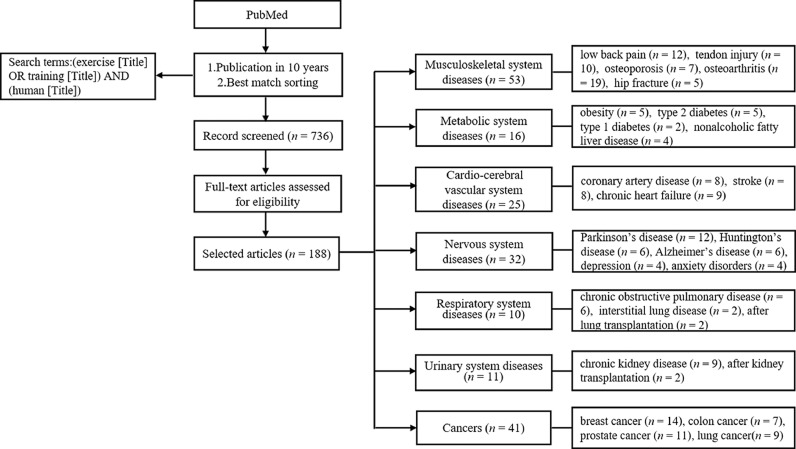
The process used for screening articles is summarized in Fig. 1. A comprehensive literature search was carried out for all forms of exercise in the PubMed of NCBI databases using the following search terms: (exercise [Title] OR training [Title]) AND (human [Title]). To limit the range of search results, we set 2 filters: publication dates within the past 10 years and sorting by best match. We initially found 736 articles corresponding with these limitations. Next, we excluded review articles and gave priority to articles reporting on RCTs with exercise intervention as the main research method, with consideration given to the frequency of the occurrence of the disease and the relative need for exercise therapy. In addition, we focused on the effect of a single exercise intervention and excluded treatments related to diet, drugs, and other measures. Using these filters, we identified 188 articles and used them for data analysis.
Fig. 1.
The flowchart for article searches. The articles included in this review were published before July 2018.
2.2. Data analysis
According to the various targets of exercise intervention, we classified the selected articles into 7 categories: musculoskeletal system diseases, metabolic system diseases, cardio-cerebral vascular system diseases, nervous system diseases, respiratory system diseases, urinary system diseases, and cancers. The selected articles were further subdivided into different disease types associated with each category. The details regarding the exercise protocols that were documented in the articles are listed in the following sections according to the exercise protocol's duration, frequency, and intensity level.
After classifying and analyzing the articles, we identified some commonly used protocols for exercise rehabilitation prescribed for different human diseases. These protocols included aerobic exercise (AE), RE, combined AE with resistance training (CT), home-based exercise (HE), multimodal exercise (ME), and other exercises. The general standards and procedures used for these types of training are described.
2.3. Classification of exercise types
2.3.1. AE
AE (i.e., regular exercise characterized by high repetition and low resistance demands during skeletal muscular contraction)7 is a well-established approach to improving aerobic capacity and health. AE plays a homeostatic role in regulating the rate of energy production, blood flow, and substrate utilization in response to locomotion.8
Typical forms of AE include walking and running on a treadmill, yoga, Tai Chi, Pilates, and cycling. When used for early postoperative rehabilitation or severe disease, each AE regimen can be categorized as being of low, moderate, or high intensity. Endurance training can also be classified as high-intensity AE. Patients who have acceptable cardiorespiratory function are commonly treated, especially at the restorative stage, by cycle, treadmill, or stationary bicycle ergometer using high-intensity interval training (HIIT). In comparison with other types of exercise, HIIT can be considered as a more effective and time-efficient intervention to improve blood pressure and aerobic capacity in obese youths.9
We defined 6 weeks as a tipping point between short-term and long-term exercise; short-term exercise lasts for fewer than 6 weeks while long-term exercise lasts for 6 weeks or more. In the studies we reviewed, the frequency of training ranged from 2 to 6 times per week. Intensity was usually expressed as a percentage of heart rate reserve, a percentage of HRmax, VO2max, or the rating of perceived exertion scales. We followed the intensity classification specified in the original tables from the references articles and, if there was no mention of intensity there, we referred to the classification standard identified in Table 1.10
Table 1.
Classification of exercise intensity.
| Intensity | %HRR or %VO2peak | %HRmax | %VO2max | RPE | %1-RM |
|---|---|---|---|---|---|
| Low intensity | <30 | <57 | <37 | <9 | <30 |
| 30–39 | 57–63 | 37–45 | 9–10 | 30–49 | |
| Moderate intensity | 40–59 | 64–76 | 46–63 | 11–13 | 50–69 |
| High intensity | 60–89 | 77–95 | 64–90 | 14–17 | 70–84 |
| ≥90 | ≥96 | ≥91 | ≥18 | ≥85 |
Note: Table adapted from Garber CE, et al.10
Abbreviations: 1-RM = one-repetition maximum; HRR = heart rate reserve; HRmax = maximum heart rate; RPE = rating of perceived exertion; VO2max = maximum oxygen uptake; VO2peak = peak oxygen uptake.
2.3.2. RE
RE originated in Europe in the 19th century and was quickly adopted in the United States.11 At first, it was believed that RE was detrimental to health and athletic performance. However, with an increased understanding of exercise, RE came to be viewed as helpful to athletes in improving their performance.12 RE is now part of the recommended activities for helping adults maintain their overall health and has been shown to decrease mortality, cardiovascular disease, cholesterol levels, depression, and fatigue, and to improve bone density and insulin sensitivity.13
RE includes strength training and self-managed loaded exercise. Resistance can be achieved by using resistance bands, body weight, or weights such as dumbbells, barbells, machines, and kettle bells. When weights are used, the amount of lift is often defined with respect to a repetition maximum. In this review, RE refers mostly to progressive resistance training (PRT) with weight machines or when different machine-based protocols are used to train major upper and lower muscle groups.
RE protocols involve many factors, including the frequency and intensity of training. Frequency refers to the number of times a muscle group is exercised each week. A repetition refers to 1 full movement of an exercise, and the number of times that movement is done without stopping is the number of repetitions per set. A set refers to the number of times an individual repeats an exercise with a given number of repetitions.14 Intensity is affected by a combination of (1) the amount of resistance used, (2) the number of repetitions, and (3) the number of sets of repetitions performed per muscle group. The intensity of RE varies based on the patient's condition. We followed the intensity classification specified in the original tables from the references articles and, if there was no mention of intensity, we referred to the classification standard identified in Table 1. The training duration for RE is the same as for AE: short term (<6 weeks) and long term (≥6 weeks).
2.3.3. CT
CT greatly benefits body function. Current theoretical studies show that combining AE with resistance training not only enhances cardiorespiratory function,15 but also increases muscle strength.16 Owing to CT including RE, full consideration should be given to the patient's endurance capabilities before CT is prescribed. Depending on the condition of the patient, the most common regimens for CT are walking and riding a bicycle. When the duration of CT is shorter than 6 weeks, we classify it as short-term CT; otherwise it is classified as long-term CT. Intensity of CT is classified in the same way as intensity of AE.
2.3.4. HE
HE (an exercise program that can be undertaken individually in the patient's own home) has been introduced in an attempt to enhance long-term adherence to recommended levels of PA.17 As a type of exercise that is simple and easy, HE is integrated into the daily lives of patients. HE can lead to significant improvements in PA levels, balance, mobility, and muscle strength. Some HE only involves an AE intervention. However, HE patients often first undertake a simple AE regimen, and then do some strength training and other RE. Sometimes HE patients are prescribed specific training, such as high-intensity leg-strengthening exercises or functionally oriented exercises. The duration of HE is classified in the same way that duration of AE is classified. Intensity of HE is classified in the same way as intensity of AE.
2.3.5. ME
ME is a combination of various training methods. Almost all ME trials include AE, RE, strength training, stretching training, balance exercise, and other forms of exercise. ME interventions usually start with AE and use stationary bicycle/cycle ergometers and walking. This is followed by strengthening exercises for the upper limbs and ends with a set of cool-down stretching exercises. Training intensity for ME varies considerably among studies, ranging from 50% to 90% of the HRmax.18 ME is effective in improving muscle strength in the lower extremities, dynamic standing balance, gait speed, and chair stand test scoring. In addition, ME is effective in reducing falls. It is most often prescribed for postoperative treatment and rehabilitation of patients with nervous system diseases. It can also improve cancer-related fatigue symptoms.19 The duration of ME is classified in the same way that duration is classified for AE. The intensity of ME is classified in the same way as the intensity of AE.
2.3.6. Other exercises
There are other special exercise prescriptions, such as balance exercise, aquatic or water-based exercise (distinguished from swimming), whole body vibration (WBV) exercise, concentric and eccentric exercise, and stretching training. The supplementary tables in various sections of this review identify some of the diseases for which these forms of exercise were used in some of the studies we reviewed.
3. Results
3.1. Musculoskeletal system diseases
The musculoskeletal system includes bones, skeletal muscles, tendons, ligaments, cartilage, joints, and other connective tissues, along with vascular and nervous tissues.20 Bones and skeletal muscles are the 2 largest tissues within this system and play fundamental roles in human physiology, enabling locomotion and movement, promoting blood flow to organs, and providing protection for vital organs.20, 21 Sports is a way for people to engage in PA; however, injuries can be a negative consequence of engaging in sports.22 Sports injuries occur during athletic activities or as a result of exercising. Common sports injuries include acute and chronic injuries of muscles, tendons, and ligaments, as well as traumatic fractures and other injuries. The prevention and rehabilitation of sports injuries are addressed through the use of different kinds of training and exercise, or a combination of the 2 modalities.23
3.1.1. Low back pain (LBP)
LBP is one of the main causes of disability and is a major public health issue in many developed countries.24 Chronic nonspecific LBP is usually defined as pain, muscle tension, or stiffness that is localized below the costal margin and above the inferior gluteal folds.25 The vast majority of patients (up to 90%) described having nonspecific LBP, which is defined as symptoms without a clear specific cause.25
Supplementary Table 1 shows that the forms of exercise most commonly used to treat LBP in the studies we reviewed were AE and WBV exercise. At present, exercise therapy is one of the key treatments for the rehabilitation of chronic nonspecific LBP. The modes of AE most commonly used were Tai Chi, yoga, and Pilates. Long-term, low-intensity AE was a safe and effective intervention for patients with nonspecific LBP. AE not only alleviated pain, 26 anxiety, and depression,27 but also improved body function more effectively. For example, AE improved patients’ flexibility, balance,28, 29 and spinal mobility in cases associated with LBP.30 The most frequently used mode of AE was Pilates, which provided position methods that focus on pelvic stability at the waist, including exercises for core muscles and respiratory control, and helped to activate certain muscles. This type of exercise intervention was important to the improvement of pain, disability, and physical and psychological perception of heath and improved stability parameters.31, 32 Furthermore, high-intensity AE was also found to reduce pain, the disability rate, and psychological stress in patients with LBP.33 Short-term daily AE programs that could be used to emphasize postconsciousness and spine self-care were recommended to keep the spine healthy and prevent back pain.34 Long-term WBV exercise was marketed as an intervention for patients with chronic LBP and improved spine strength and endurance, trunk proprioception, and transversus abdominis activation capacity.35 Likewise, long-term WBV exercise was an effective, safe, and suitable intervention for seated working employees with chronic LBP.36 Low-frequency vibrating board therapy was also used to improve postural stability, physical function, health-related quality of life (HRQoL), disability, and foot vibration perception threshold for patients with nonspecific LBP.37
Long-term, low-intensity AE is a common form of exercise for patients with LBP. It is used to decrease pain and enhance quality of life. Pilates, one of the low-intensity AE forms, is the most commonly used among patients with LBP. WBV exercise is also currently being promoted as a treatment for patients with LBP.
3.1.2. Tendon injury
Common tendon injuries include rotator cuff tendinopathy, tennis elbow, and Achilles tendinopathy (AT). Rotator cuff tendinopathy is the most commonly diagnosed musculoskeletal shoulder condition and is associated with pain, weakness, and loss of function in the shoulder. Tendon swelling may result from acute overload.38 Tendon damage in the shoulder significantly affects function and activities of daily life, including eating, dressing, and working. Tennis elbow, or lateral elbow tendinopathy, is a disorder that affects about 1%–3% of the population.39, 40 A typical symptom is pain at the lateral epicondyle of the humerus, which is aggravated by loading of the common extensor tendinous origin of the forearm extensor muscles.41 AT occurs both in athletic and sedentary people. The incidence among top-level runners has been estimated to be between 7% and 9%.42 About 30% of patients who have AT have a sedentary lifestyle.43
Supplementary Table 2 shows that the exercise interventions used for these conditions included concentric and eccentric training, self-management load training, open chain exercise, and closed chain exercise. Long-term isolated eccentric training was beneficial for shoulder function and helped to alleviate pain in patients.44 When isolated eccentric training was done with a long-term weekly load increase, results showed significant relief of pain in patients with tennis elbow. Muscle contraction was faster and muscles had better elongation.41, 45 Eccentric exercise has become the treatment of choice for AT,46 because it decreases pain and eliminates the need for surgical intervention. It was effective in about 60% of patients.47 In 1 study, Astym therapy, an emerging rehabilitation treatment, was an effective treatment option for patients with lateral elbow tendinopathy, as an initial treatment, and after an eccentric exercise program had failed.48 Alfredson's isolated eccentric and Silbernagel's combined concentric–eccentric exercise programs both showed beneficial results for patients with chronic midportion AT.49 The mechanism of eccentric training might include changes associated with symptomatic tendinopathy that alter fluid movement within the tendon matrix to disrupt remodeling and homeostatic processes.50 Therefore, long-term eccentric training was usually used for tendon injuries in the studies we reviewed. In addition, a self-management program based around a single exercise seemed to be comparable with usual physiotherapy treatment.51 When it was performed with loaded exercise, it also decreased pain as measured by the Shoulder Pain and Disability Index.52 Open chain, closed chain, and range-of-movement exercises all seemed to be effective in bringing about short-term changes in pain and disability in patients with rotator cuff tendinopathy.53
Long-term eccentric exercise has proven to be effective in the management of tendon injuries. It is a fundamental therapeutic resource for the treatment of AT and lateral elbow tendinopathy and may represent a promising and feasible tool for treating tendinopathies.
3.1.3. Osteoporosis (OP)
OP is characterized by low bone mass and bone microarchitecture deterioration, leading to bone fragility and an increased risk of fracture. In recent years, OP has become a metabolic bone disease that affects millions of people around the world.54 Menopause and aging are associated with low PA,55 and a sedentary lifestyle is particularly prevalent in postmenopausal women living in urban areas.56 There is evidence that exercise can prevent some of the complications associated with menopause, such as bone loss, loss of physical fitness, and increased risk of OP.57 Physical exercise effectively decreases risk factors for falling58 and improves balance.59, 60
Supplementary Table 3 shows that commonly used forms of exercise for OP treatment include AE, RE, CT, and balance training. The AE and RE programs stimulated bone synthesis and decreased bone resorption in postmenopausal women with OP, but that exercise while wearing a weighted vest was better for improving balance.61 Long-term Tai Chi exercise decreased the loss of bone mineral density and reduced the risk of fractures in the study population.62 Short-term submaximal AE provided significant improvements in static and dynamic balances in postmenopausal osteoporotic women.63 Long-term, low-intensity balance and strength training significantly improved the strength and balance capability of women with OP.16 Long-term resistance training and balance training had a direct impact on the habitual walking speed of elderly women with a history of OP.64 Supervised long-term, high-intensity CT increased bone mineral density and effectively prevented fractures in senior citizen populations.65, 66
In summary, short-term, moderate-intensity AE and long-term, high-intensity RE can prevent OP and improve balance capacity, aiding in the avoidance of falls and fractures.
3.1.4. Osteoarthritis (OA)
OA is a chronic degenerative musculoskeletal disease that affects many older people around the world.67 In the United States, 33.6% of individuals (12.4 million) age 65 years and older are affected by OA, and this number is projected to increase to 67 million by 2030.68 The main symptoms of OA are joint pain, stiffness, physical limitations, and decreased functional ability in middle-aged and elderly patients.69 The most common joints affected are the knee and hip joints. The strong relationship between obesity and incident knee and hip OA may in part be explained by the increased load on weight-bearing joints experienced by overweight and obese patients. Because OA is a chronic condition that has no effective cure, the clinical management of OA is an enormous challenge. Recent practice guidelines recognize that exercise is a key element of any treatment program for OA.70
Supplementary Table 4 shows the forms of exercise most frequently used for treating OA in the studies we reviewed were aquatic exercise, AE, RE, CT, WBV exercise, and ME. In aquatic exercise protocols, long-term, moderate-intensity treatments significantly decreased body fat proportion,71, 72 and patients showed significant improvements in pain level, disability, and quality of life.71, 73 Aquatic exercise was also an option for obese patients because it minimized joint load.74 Combined with education, it was effective in improving fall risk factors in older adults.75 In addition, an 8-week, dance-based aquatic exercise intervention improved function and cardiorespiratory capacity, and decreased postexercise heart rate and fatigue.76 In AE protocols, long-term, low-intensity yoga improved symptoms and function,77, 78 long-term Tai Ji Quan training improved sleep quality and quality of life,79 and swimming and cycling training improved function and decreased pain.69 Compared with land-based cycling exercise, long-term regular swimming exercise has similar or even better effects on vascular function and inflammatory markers in patients with OA who tend to have a higher risk of cardiovascular disease.80 In RE protocols, long-term strength exercise with gradually increasing intensity improved the motion sense of knee flexion81 and lower extremity function in patients with knee OA.82 For patients with hip OA, muscle power, walking speed, and cadence significantly increased only in the long-term, high-velocity group.83 A WBV exercise program had beneficial effects on the physical performance and neuromuscular control of individuals with knee OA.84 Furthermore, long-term WBV exercise in combination with RE was superior to RE in relation to muscle strength and proprioception.85, 86 ME programs included high-speed resistance training87 and balance training, as well as long-term strengthening, functional, and stretching exercises88 with gradually increasing intensity.
In short, long-term, low- to moderate-intensity exercise focusing on aquatic exercise improves physical function, comorbidities, and quality of life in patients with OA. WBV exercise is also a treatment protocol for OA.
3.1.5. Hip fractures
Over a lifetime, about one-half of women and one-quarter of men will suffer a fragility bone fracture, mostly owing to falling.89, 90 Among the most serious and common fractures are hip fractures, which have significant risks of mortality and disability.90 Increasing evidence shows that exercise rehabilitation interventions have a positive effect on various functional abilities beyond the subacute stage and even later in the nursing stage. Structured exercise improves mobility after a hip fracture.91
Supplementary Table 5 shows that the forms of exercise used for treating hip fractures were AE, HE, and ME. In HE protocols involving PRT, challenging balance training, and neuromuscular functional training of the lower limbs in part overcame some barriers for rehabilitation.7 The HE protocol was sufficient to achieve modest improvement in physical function,92 which might improve measures of balance, strength, and bone density and result in moderate to large improvements in physical performance and quality of life.92 In addition, specific workstation exercise significantly improved balance and strength, which might decrease the risk of falls and fractures in osteopenia women already at risk.93 Among patients who completed standard rehabilitation after a hip fracture, physical function improved modestly 6 months after randomization using a home-based function-oriented exercise program.94 ME programs, including traditional PRT, weight-bearing impact training, and balance training, were recommended to decrease the risk factors of falls and fractures.95
In short, long-term, low- to moderate-intensity exercise focusing on balance training helps in the recovery of hip joint function and improves quality of life.
3.2. Metabolic system diseases
Metabolic disorders have become very concerning because they have negative impacts on people's health and life quality. Lifestyle interventions should be the primary strategy for the prevention and treatment of metabolic diseases. Reasonable diet and weight control have been highly recommended as treatments, but doctors and patients have overlooked the role that exercise interventions can play.96 Such treatments can help to decrease the risk of developing metabolic diseases and can aid in promoting recovery.
3.2.1. Obesity
Obesity is a condition in which excess body fat accumulates. Simple obesity is related to genetics and lifestyle; secondary obesity is associated with multiple endocrine and metabolic diseases. There are a variety of treatments, including exercise therapy, that can be used to intervene in obesity.97
As Supplementary Table 6 shows, exercise prescriptions for obesity included AE and RE combined with HIIT. In AE protocols, the main exercise form was long term and of moderate to high intensity. Specific forms of exercise included treadmill and stationary bicycle aerobic training, which improved body composition and VO2max in obese adults,98 upregulated muscle apelin expression in obese subjects,99 and increased levels serum and platform-based brain derived neurotrophic factor in overweight and obese subjects.100 Additionally, aerobic interval training (AIT) was shown to relieve reactive oxygen species from excessive nicotinamide adenine dinucleotide phosphate oxidase sources, and it improved microvascular endothelial dysfunction in obese patients.9 RE combined with HIIT was used as an intervention among sedentary, overweight, middle-aged individuals, and it was shown that subjects using this method lost weight by improving the function of acute satellite cells. RE combined with HIIT was better than resistance training alone.101
In short, the exercise methods described usually show positive effects, and the most common exercise form for treating or preventing obesity is AE.
3.2.2. Type 2 diabetes (T2D)
T2D, with its high morbidity and mortality, is developing rapidly worldwide and is becoming a significant health problem. Environmental and lifestyle changes, in addition to the ageing of populations, are generally believed to account for the rapid global increase in T2D prevalence and incidence in recent decades.102 Epidemiological investigations demonstrate that there are currently 371 million people with diabetes in the world, and the number is expected to rise to 552 million by 2030.103, 104 Diet therapy, drug therapy, psychotherapy, and self-care monitoring are commonly used intervention methods. Exercise therapy is also a key treatment for patients with T2D and is considered a cornerstone of treatment for T2D, alongside diet and drug treatments.105
As Supplementary Table 7 shows, exercise forms for T2D were CT, RE, and HIIT. AE protocols included treadmill and stationary bicycle. Long-term CT at a moderate intensity alleviated cellular stress in obese adults,106 with no effect on plasma carnosinase content or activity in T2D. This was because the beneficial effects of exercise training on the incidence of diabetic complications were probably not related to a lowering effect on plasma carnosinase content or activity.107 RE was usually performed at moderate and high intensity for a long term. This strategy significantly decrease glucose, insulin, and homeostatic model assessment-insulin resistance levels in elderly T2D patients,108 and altered acute exercise reaction of monocarboxylate transporter 1 in erythrocytes in patients with non-insulin-dependent T2D.109 One session of low-volume HIIT was carried out on a cycle ergometer as an intervention for participants with T2D, and this method had immunomodulatory effects and provided potential anti-inflammatory benefits.110
The most common forms of exercise for treating T2D are AE and RE. Long-term exercise at moderate intensity also shows positive effects in treating T2D patients.
3.2.3. Type 1 diabetes (T1D)
T1D, is the archetypal example of a T-cell-mediated autoimmune disease characterized by selective destruction of pancreatic β cells.111 The pathogenic equation of T1D shows a complex relationship between genetic and environmental factors, most of which have not yet been determined.111 Insulin therapy, psychotherapy, and exercise therapy are intervention methods for treating T1D.
As shown in Supplementary Table 8, HIIT and speed endurance training were the exercise methods used to treat T1D. Cycle sprint training was used as a common speed endurance training method. In the HIIT protocol, cycle sprints with acute interval training carried out on cycle ergometers with high intensity showed a positive effect in that the intervention rapidly decreased patients’ perception of subsequent hypoglycemia and reduced their cognitive dysfunction caused by hypoglycemia.112 However, speed endurance training also showed a negative effect in that it reduced Ca2+-ATPase in skeletal muscles.113
To sum up, long-term speed endurance training at high intensity can improve T1D.
3.2.4. Nonalcoholic fatty liver disease (NAFLD)
NAFLD, the most common chronic liver disease in the world, is predicted to become the most frequent indication for liver transplantation by 2030.114 The disease includes a wide range of liver manifestations, including simple steatosis (also known as nonalcoholic fatty liver), nonalcoholic steatohepatitis, and liver cirrhosis, which may eventually develop into hepatocellular carcinoma. The American Association for the Study of Liver Diseases proposes that exercise can decrease hepatic steatosis in patients with NAFLD.115
As Supplementary Table 9 shows, exercise prescriptions for NAFLD included AE and CT. AE was the main intervention used for NAFLD and was usually performed at moderate to high intensity for both long-term and short-term treatments. Using moderate- and high-intensity AE also decreased the patients’ risk of developing NAFLD116 and yielded small beneficial effects on intrahepatic triglyceride content.117 A 1-year program of AE intervention significantly decreased intrahepatic triglyceride levels and decreased abdominal obesity and blood pressure in obese patients with NAFLD in the moderate exercise group.118 In addition, CT decreased intrahepatic triglyceride in patients with NAFLD.119
To summarize, AE and CT usually showed positive effects, and the most common exercise form used for NAFLD was long-term AE at moderate intensity.
3.3. Cardio-cerebral vascular system diseases
In recent years, the incidence of cardiovascular and cerebrovascular diseases has increased rapidly. Although cardiovascular and cerebrovascular diseases mainly occur in the elderly, they can also occur in young people. Proper exercise can improve the efficiency of heart and lung operation, speed up blood circulation, increase the oxygen supply to the whole body, and improve the elasticity of blood vessels. In addition, exercise can lower blood lipids and prevent arteriosclerosis and thrombosis.120 The effects of exercise help to relieve stress on the heart and effectively prevent cardiovascular diseases.
3.3.1. Coronary artery disease (CAD)
CAD, also known as ischemic heart disease, refers to a group of diseases including stable angina, unstable angina, myocardial infarction, and sudden cardiac death.121 CAD is the most common type of cardiovascular disease.122 Recent research has shown that exercise is an effective way to enhance cardiopulmonary function and improve the overall quality of life for these patients.123
As shown in Supplementary Table 10, exercise interventions for CAD included AE and HE. The most common forms of exercise included walking, cycling, jogging, and Tai Chi. Long-term, moderate-intensity walking exercise decreased the severity of sleep apnea in CAD patients.124 HIIT reduced the morbidity and mortality of CAD and prevent atherosclerosis.125 It had beneficial effects on heart function and quality of life in patients and did not increase the risk of cardiovascular diseases.15, 126 Compared with aerobic continuous training, AIT was more effective for the rehabilitation of patients with CAD in earlier smaller trials, but in another study, the investigators found that similar improvements in exercise capacity and peripheral endothelial function after AIT and aerobic continuous training in a large population of CAD patients.127, 128 Long-term HE was superior to traditional hospital-based cardiac rehabilitation among the hospital group in terms of VO2max and PA, which was more beneficial for postoperative recovery.129 Network-based family cardiac rehabilitation procedures were feasible and effective in improving the PA of patients with CAD.130
In short, exercise interventions usually improve CAD-related risk factors, such as body composition and blood pressure. Compared with moderate continuous training, long-term AE or HE is more effective in improving the physical condition of patients with CAD.
3.3.2. Stroke
Stroke, also known as cerebral vascular accident, is an acute cerebrovascular event, including ischemic and hemorrhagic stroke. The treatment of the acute phase is mainly thrombolysis. After the acute phase, stroke has a very high disability rate. Early exercise intervention after the acute phase is extremely important for the recovery and improvement of exercise capacity.131
As Supplementary Table 11 shows, the types of exercise intervention for stroke were AE, RE, CT, and RE combined with balance training. The forms of exercise included walking, cycling, jogging, power sports, Tai Chi, moving objects in the home, and picking up objects. In AE protocols, moderate-intensity aerobic cycle dynamometer training improved the aerobic capacity and walking ability of stroke patients with hemiplegia132 and had a positive effect on the cognitive ability and exercise control ability of patients with hemiplegia.133, 134 Vigorous AE was also a method used to effectively improve the aerobic capacity of stroke patients with hemiplegia.135 In RE protocols, after long-term PRT combined with balance training, the patient's balance ability was improved in 3 months and the stroke patients’ walking ability improved in 3–6 months.136 Low-intensity RE with balance training was beneficial for improvement in plasma lipids, glucose, and exercise capacity.137 Short-term respiratory muscle training combined with routine physical training improved lung function and exercise capacity.138 Long-term CT improved cognitive ability and reduced mild cognitive impairment in patients with stroke hemiplegia.139
In brief, exercise interventions usually improve cardiopulmonary function, walking ability, balance function, and cognitive ability in stroke patients. Long-term, low- to moderate-intensity AE improves cardiopulmonary function and aerobic capacity and is the most common training method used among stroke patients.
3.3.3. Chronic heart failure (CHF)
CHF refers to the inability of the blood to return to the heart owing to the low relaxation and contraction function of the heart, resulting in venous system deposition and insufficient arterial perfusion, thereby causing cardiac dysfunction.140 The traditional view is that patients with poor heart function should avoid exercise and reduce the stimulation of the heart; however, with continuous developments in sports therapy research, it is now believed that patients with CHF should exercise moderately.
Supplementary Table 12 shows that the types of exercise methods used for CHF included AE, RE, and HIIT. In AE protocols, long-term walking with breathing exercise improved blood oxygen saturation and internal perception, and adjusted mood and quality of life.141 Long-term, moderate-intensity stretching combined with cycling improved and enhanced muscle metabolic reflex control.142 HIIT protocols improved the patients’ quality of life by changing their levels of health.143 HIIT combined with continuous moderate-intensity aerobic training improved the index of submaximal exercise capacity.144 HIIT was easy to implement and was a well-tolerated exercise intervention in cardiac rehabilitation, but it was not more effective compared to continuous AE.145 Long-term HIIT combined with strength training, which is conductive to aortic dilatation ability and increased systolic blood pressure, was more beneficial for vascular response.146, 147 In RE protocols, short-term, low-intensity resistance activities and abdominal exercise improved cardiac function indicators and lung function and had significantly positive benefits for the rehabilitation of elderly and middle-age patients with CHF after acute decompensation.148 Long-term AE and RE were more beneficial for the rehabilitation of patients.149
In short, exercise interventions usually improve heart and lung function in patients with heart failure. Compared with continuous AE training or no exercise, HIIT improved the cardiac contraction function and quality of life. Long-term, HIIT is often used to treat patients with CHF.
3.4. Nervous system diseases
Nervous system disorders mainly include central nervous illnesses and diseases, as well as peripheral nervous system diseases, which are often associated with motor control and cognitive dysfunction. Most of these diseases are chronic, with a high disability rate that disrupts the normal life of patients. Exercise is the most common physiological stimulus in humans, because it has the capacity to regulate and reshape tissue function. The patient's motor control abilities can be improved with exercise, and some studies have determined that exercise can also enhance the patient's cognitive function.150
3.4.1. Parkinson's disease (PD)
PD is a common neurodegenerative disorder that brings with it the prominent loss of the dopaminergic neurons in the substantia nigra pars compacta, which is mainly manifested by motor dysfunction, such as rest tremor and muscular rigidity.151 Although medication is currently preferred as a treatment, exercise has drawn more and more attention as a potentially important treatment.
The exercise protocols for patients with PD are summarized in Supplementary Table 13. AE, RE, and ME had some positive effects on patients with PD. AE protocols included walking and the use of bicycles and treadmills. These forms of exercise were usually performed under moderate to high intensity for a long term. AE usually showed positive effects on patients’ walking capacity, exercise function,152, 153 velocity, and step length.154 AE also improved the executive ability of patients with cognitive impairment.155 Additionally, AE was used for early rehabilitation.156 RE involved treadmill training with load or used other machines under moderate to high intensity for short-term and long-term interventions. In regard to cardiovascular function, RE was shown to be safe and improved the patients’ physical performance.157, 158, 159 ME protocols comprised tango, treadmill, cycle ergometer, Tai Chi, Pilates, and boxing. These protocols were performed both as short-term and long-term interventions. They were easy to implement160 and yielded some benefits related to motor function, executive function, and balance.160, 161, 162 A research study reported that aquatic exercise was feasible for individuals in the early stages of PD.163
Long-term AE is frequently prescribed as a treatment for patients with PD and may alleviate PD symptoms.
3.4.2. Huntington's disease (HD)
HD is an inherited disease resulting in neuron apoptosis, which is characterized by jerky and uncoordinated movements of limbs. Unfortunately, there is no effective treatment.164 AE, a combination of AE and RE, and ME can partially alleviate the symptoms of HD, although all these types of exercise are challenging for HD patients, who typically have difficulties in engaging in regular PA.165
As shown in Supplementary Table 14, AE was usually performed long term using moderate-intensity interval training or HIIT. A dosage of 3 times each week for 25–30 min per session yielded improvement in cardiovascular function166 and skeletal muscle mitochondrial function167 and maintained a stabilization of motor function.166, 167 In another study, AE was combined with RE long term at moderate intensity, which benefited cognitive ability and walking.168 In the third study, long-term ME was provided, which included AE, RE, stretching exercise, and task-specific training. Some of these exercise methods proved beneficial for HD patients.169 Surprisingly, the task-specific training did not elicit any effect on HD. The results may have been affected by the insufficiency of the exercise program.170
In short, long-term ME is often prescribed as a novel exercise treatment for HD.
3.4.3. Alzheimer's disease (AD)
AD is a progressive neurodegenerative disease that starts slowly.171 AD patients usually show memory impairment, aphasia, impaired visuospatial skills, executive dysfunction, and personality and behavioral changes, all of which become worse over time and eventually lead to death. Currently, the main treatment of AD is drug therapy, which has not shown satisfactory efficacy because the underlying mechanism of AD is still unclear. However, if patients with AD exercise regularly and systematically, the cognitive, functional, and behavioral symptoms of AD can be reduced.172
As shown in Supplementary Table 15, exercise interventions for AD included AE, ME, and HE. The forms of AE included the use of bicycles and treadmills. As expected, AE improved the patients’ daily activity ability and cognition,173 a change that was independent of the change in Aβ species and total tau protein in cerebrospinal fluid. Long-term balance exercise was also prescribed, and improved the patients’ exercise capacity.174 Long-term ME significantly improved upper and lower body muscle strength and flexibility, agility and dynamic balance, as well as the endurance fitness, gait, and balance abilities of patients with AD.175 Long-term, customized HE decreased the risk of falling in the late stage of the disease176 and improved the executive function of community-dwelling older people with memory disorders.177 It also had beneficial effects on the physical functioning of patients with AD without increasing the total costs of health and social services or causing any significant adverse effects.178
A wide variety of HE is widely used among patients with AD to increase their ability to carry out daily activities and improve their cognitive function.
3.4.4. Depression
Depression is a complex, debilitating disease that affects more than 120 million people worldwide.179 Compared with healthy people, people with depression have a much higher risk of developing complex chronic diseases, such as diabetes, cardiovascular disease, and hypertension, which can significantly decrease their quality of life.180 Clinical studies have shown that traditional depression treatments do not yield satisfactory outcomes. Emerging evidence shows that exercise may be a promising treatment for depression.181
Supplementary Table 16 shows that long-term AE treatments for depression included the use of cycling, treadmills, and other exercise machines at moderate to high intensity. These exercise methods were effective treatments for positive valence symptoms in major depression.182 The combination of AE and sertraline improved the management of late life major depression183 and cognitive abilities,184 decreased the disability rate,184 and improved quality of life.185
In conclusion, long-term, moderate-intensity AE can effectively relieve depressive symptoms and is widely used in the treatment of depression.
3.4.5. Anxiety disorders
The number of patients diagnozed with anxiety disorders is increasing rapidly worldwide.186 Patients with anxiety disorders often not only suffer from severe anxiety disorder, but also have poor metabolic function.187 Epidemiologic investigations have shown that anxiety is one of the world's most common mental illnesses.188 Psychotherapy and medication are usually used for the patients with anxiety disorder.
As Supplementary Table 17 illustrates, the types of exercise interventions used for anxiety were AE, HE, and RE. The forms of AE included cycling, walking, and Tai Chi at moderate to high intensity in long-term doses. Moderate-intensity AE might be a temporary alternative to psychotherapy to decrease anxiety-related psychopathology.189 Long-term HE exercise program had a positive effect on the metabolic index and anxiety level of anxiety patients in Taiwan, China.190 For the patients with an anxiety disorder, RE was performed at low to high intensity acutely or for a short term with a frequency of 2 times per week. So, RE was a low-risk and feasible method to reduce anxiety symptoms in patients with general anxiety disorder.191, 192
In short, moderate-intensity AE and RE can be used to help patients with anxiety disorders.
3.5. Respiratory system diseases
Respiratory system diseases are common diseases that seriously endanger human health.193 Some stretching and chest expansion exercises can enlarge the thorax and increase the strength of the respiratory muscles. It is beneficial to increase lung capacity, lung ventilation, and oxygen utilization capacity to improve the working function of the lungs.194, 195
3.5.1. Chronic obstructive pulmonary disease (COPD)
COPD is a devastating lung disease characterized by incomplete reversible airflow limitation. The main symptoms are chronic cough, cough with sputum, dyspnea, wheezing, and chest tightness. COPD intervention methods mainly include drug therapy, oxygen therapy, and exercise therapy.196
As shown in Supplementary Table 18, exercise prescriptions consisted of individualized AE, RE, and water-based exercise. The forms of exercise included strength training with a pedal exerciser, Tai Chi, and ground-based walking. Patients with COPD individually received long-term, low-intensity physical outpatient training to improve their athletic ability and quality of life.197 Endurance and strength training were performed at high intensity for a long term, which significantly improved endurance.198 Long-term water-based exercise at low intensity constituted a relatively new concept in the management of patients with COPD, and the aquatic environment was well accepted by COPD patients with physical comorbidities.199 Strength training with a pedal exerciser was performed at low intensity for a short term, which improved the muscle strength, balance, and exercise ability of frail elderly patients.200 In addition, long-term Tai Chi exercise enhanced lung function, exercise capacity, and diaphragm strength.201 The results of another study showed that training in ground-based walking was an effective method for improving the quality of life and endurance of patients.202
In brief, patients with COPD can use long-term AE or RE at a low to moderate intensity, combined with training in breathing, to promote disease recovery. Also, water-based exercise is a novel way to treat COPD.
3.5.2. Interstitial lung disease (ILD)
ILD is a group of diseases in which pulmonary interstitial lesions are the main lesions. The chief clinical manifestations are cough and postoperative dyspnea, which often aggravates the condition and eventually leads to respiratory failure. Glucocorticoids combined with immunosuppressive therapy, anti-fibrotic drug therapy, lung transplantation, Chinese herbs, oxygen inhalation, and exercise therapy are intervention methods used to treat ILD.203
As Supplementary Table 19 shows, the exercise method used for ILD was supervised CT. The supervised exercise protocol consisted of long-term, high-intensity AE and resistance training for upper and lower limbs. This method of exercising was effective in patients across the range of ILDs, with clinically meaningful benefits in asbestosis.204 Another study provided certainty regarding the role of in ILD. The results from another study showed that supervised exercise training could be used to inform and optimize the clinical management of people with ILD.205
In short, long-term high-intensity CT can be used to relieve symptoms of ILD patients.
3.5.3. After lung transplantation (LTx)
LTx is a treatment option for patients with end-stage lung disease. Over the past 15 years, with the gradual maturity of lung transplant techniques, donor preservation, and perioperative management, the 1-year survival rate of LTx has increased from 70% to 85%.206 However, there are typically complications after LTx that endanger the patient's health and affect the patient's recovery. Proper exercise can prevent the occurrence of complications and improve the speed of postoperative recovery.
As shown in Supplementary Table 20, exercise methods included WBV exercise and CT. As a feasible and safe exercise modality, short-term WBV exercise was also shown to be a good method for helping patients recover from LTx.207 The forms of CT included cycling, walking, and stair climbing, as well as using leg press equipment. The results of this study showed that supervised endurance strength training immediately after discharge improved functional recovery after LTx in postoperative patients and also improved the body function of patients within 1 year.208
In a word, long-term moderate-intensity CT after LTx improves patients in daily activity, and short-term WBV exercises also have good effects.
3.6. Urinary system diseases
Patients with kidney diseases are characterized by poor physical strength, malnutrition, and a lack of energy. Research shows that exercise can improve the body's function, promote multiple systems, and improve the life quality of patients with kidney disease. Owing to the lack of a comprehensive understanding of the disease, which eventually leads to dysfunction of other systems, most patients with kidney disease need more exercise.209, 210
3.6.1. Chronic kidney disease (CKD)
CKD is a structural or functional abnormality of the kidney. CKD is a compound disease that affects multiple organs and systems in human body.211 Additionally, the lack of exercise and low body function in patients with CKD is an important cause of deterioration.209
Supplementary Table 21 shows that the types of exercise intervention used for CKD were mainly AE, RE, CT, and HE. In AE protocols, short-term, moderate-intensity AIT was used to intervene in patient with CKD Stage 3. The results seemed to show reduced levels of endothelin-1.212 The vasoconstriction function of endothelin-1 is an important factor in cardiovascular disease. In RE protocols, long-term progressive RE was a tolerable intervention for overweight patients with chronic nephropathy. Progressive RE increased muscle the anatomic cross-sectional area, muscle volume, knee extensor strength, and exercise capacity.213 For patients with CKD without deterioration of renal function, modest CT improved the physical performance and avoided muscle wasting.214 In HE protocols, home-based CT was feasible and improved muscle strength and exercise capacity of patients with CKD.215, 216, 217 Additionally, moderate-intensity home-based aerobic training improved the patients’ VO2max and quality of life, although it did not change endothelial function or arterial stiffness.218 Similar studies suggested that AE was effective in decreasing visceral fat in patients and improving cardiopulmonary function.219, 220
Long-term, moderate-intensity HE has a promising effect on CKD, as reflected by improvements in muscle strength, enhanced metabolic capability, regulation of mood, and decrease in other complications.
3.6.2. After kidney transplantation
Kidney transplantation is the most effective way to treat patients with end-stage renal disease and chronic renal failure.221 However, patients sometimes experience organ rejection after surgery, so they need to take immunosuppressive agents to maintain a normal physiological status. These agents sometimes have an ill effect on the patient's physical condition. Recent research shows that regular exercise has a positive effect on kidney transplant patients.222
Supplementary Table 22 shows that the types of exercise intervention for kidney transplantation included AE, RE, and individualized progressive exercise programs. Aerobic and resistance training studies have shown that this type of training is feasible and is an effective treatment that regulates pulse-wave velocity.223 The combination of walking on a treadmill and low-intensity dynamic stretching was beneficial to the physical and psychological rehabilitation of the patients. In addition, this individual intervention method could be appropriately extended to other patients after renal transplantation.224
The exercise regimens described often had a positive effect on patients who have undergone kidney transplantation by improving their movements and limiting the complications they experienced.
3.7. Cancers
Cancer is a major cause of death in the United States and many other parts of the world. In the United States, cancer causes one-quarter of all deaths.225 Increasing numbers of new cancer cases and increasing cancer survival rates have led to a large and rapidly growing population with unique health care needs. PA has emerged as an effective strategy for improving the quality of life, both in physical and emotional terms, among cancer survivors and among those diagnosed with several forms of cancer, including breast, colon, prostate, and lung cancers.
3.7.1. Breast cancer (BC)
BC is the most commonly diagnosed cancer and is the leading cause of death in female patients with cancer around the world. Because anti-cancer treatments and diagnostic technologies have progressed, patients with BC now live longer.226 It is well known that patients with BC suffer from many cancer- and treatment-related side effects, all of which worsen their overall quality of life.227, 228 Exercise is considered to be an effective supportive treatment for patients with BC.229
Supplementary Table 23 shows that the main methods of exercise for patients with BC were AE, RE, and CT. The forms of AE included the use of cycle ergometers, treadmills, elliptical machines, and rowing ergometers. Long-term, high-dose exercise led to loss of fat, improved cardiopulmonary function, and reduced the risk of BC.230, 231, 232 Short-term, low-intensity AE combined with muscle strength enhancement helped to improve shoulder joint mobility and alleviated BC-associated lymphedema and pain.233 Long-term, moderate-intensity AE had significant benefits, but only for women with early stage BC.234 In 1 study, long-term, PRT of moderate intensity using 8 different machines was especially an important part of supportive care.229,235, 236, 237 Moderate-intensity AE combined with RE benefited early adjuvant BC treatment; after 18 weeks of an exercise program, there was a positive effect on physical fatigue, cardiopulmonary function, and muscle strength among participating patients.238 High-intensity AE combined with RE significantly improved patients’ self-confidence, physical fitness, body composition, and chemotherapy completion rate. At the same time, this form of exercise helped to improve sleep quality in patients with BC undergoing chemotherapy.239, 240, 241, 242
In short, for patients with early BC, long-term, low- to moderate-intensity AE and RE are more conducive to patients’ rehabilitation.
3.7.2. Colon cancer
There are 103,000 people diagnosed annually with colon cancer in the United States.238 Among these diagnosed cases, 39% will have localized colon cancer (confined to the primary site, Stages I–II), 36% will have regional colon cancer (the cancer has spread to the regional lymph nodes; Stage III), and 20% will have metastatic disease (the cancer has spread to distant organs, Stage IV).243, 244 The 5-year survival rates for localized and regional colon cancer are 90% and 70%, respectively.243 Observational studies indicate that higher volumes of PA are associated with improved disease outcomes among colon cancer survivors.245
Supplementary Table 24 indicates that the main forms of exercise used for patients with colon cancer were AE and CT. Long-term, moderate-intensity AE reduced the visceral adipose tissue content, increased health-related fitness, and reduced the recurrence risk of colon cancer among patients with Stages I, II, and III disease.246, 247 Long-term, moderate-intensity, high-dose AE improved the favorable prognosis of biomarkers and the multiple HRQoL outcomes of patients with colon cancer.248, 249 Long-term CT had a beneficial effect on reducing patient fatigue.250 Low- to moderate-intensity exercise shortened the length of postoperative hospital stays and improved bowel movement after colectomy.251
In conclusion, for patients with colon cancer, long-term, moderate-intensity, high-dose AE is often used as an adjunct to the treatment of the disease.
3.7.3. Prostate cancer
Prostate cancer is the most prevalent cancer among men in the United States and is the second leading cause of cancer death in men.252 Men over 65 years of age account for 64% of new prostate cancer cases in the United States, and men over 75 years of age account for 23% of patients with prostate cancer.253 Current treatments include radical prostatectomy, radiotherapy, and androgen deprivation therapy (ADT). Patients need active physical rehabilitation after surgery, and one of the ways they can receive it is through exercise.
Supplementary Table 25 reveals that the main forms of exercise used for prostate cancer patients were AE, RE, and CT. AE increased the PA level of patients and had a positive effect on recovery from disease.254 Walking 10,000 paces a day was effective in improving cardiovascular health.255 Long-term RE and impact exercise for patients who were treated with ADT decreased their total body fat.256, 257 The application of ADT in combination with AE and endurance exercise had significant effects on cardiopulmonary capacity,258 resting fat oxidation and glucose,259 body composition,260 and improvement of fatigue.261 Short-term exercise helped to maintain the sexual activity of patients with ADT.262, 263 In addition, long-term, high-intensity exercise significantly improved the peak oxygen uptake and provided more sustainable cardiopulmonary efficacy than low-intensity exercise.264
In summary, for prostate cancer patients, long-term, moderate- and high-intensity CT is often used to assist in the treatment of diseases.
3.7.4. Lung cancer (LC)
LC is the leading cause of cancer-related deaths in men and women in the United States, with an estimated 158,040 deaths from LC in 2015.265 The LC mortality rate exceeds that of colon, breast, and prostate cancers combined. Exercise has been shown to improve blood immune function in LC survivors.266
As Supplementary Table 26 shows, the main forms of exercise used for LC patients were HE, AE, CT, and HIIT. Long-term home-based, moderate-intensity walking was a feasible and effective intervention for managing anxiety and depression among patients with LC.267 Long-term Tai Chi helped to improve the proliferation and cell-dissolving activity of peripheral blood mononuclear cells in patients,268 it decrease body fatigue and physical fatigue, and it increased the vitality of patients.269 After receiving supervised CT, the patients’ pain was decreased, but no improvement in HRQoL.270 However, in another study, long-term, moderate-intensity AE or RE had a good effect on HRQoL, physical and psychological symptoms, and tumor treatment effects.271 Home-based, moderate-intensity RE delayed the deterioration of body function and improved muscle strength among patients with advanced LC.272 Patients receiving short-term, high-intensity endurance training and strength training conducted within 5–7 weeks after their operation had good tolerance to the protocol.273 Other studies have shown that preoperative HIIT can significantly improve AE, but cannot reduce early complications after LC resection.274, 275
In brief, for the exercise prescriptions for LC, long-term, moderate- and high-intensity AE or RE is commonly used as adjuvant therapies.
4. Discussion
We have described several types of exercise interventions and their uses in the treatment of various diseases (Table 2). Exercise has a positive effect on many diseases, especially chronic noncommunicable diseases, which is consistent with the results of Pasanen et al's study.276 The clinical significance of exercise intervention is worthy of affirmation. Compared with the huge cost of drugs, exercise intervention is an economic and safe way to prevent and treat diseases, and has few side effects, which reduces the economic burden on families and society. However, not all diseases can be improved by exercise therapy, which sometimes can only be used as an adjuvant treatment for major illness. At present, the types of exercise interventions used as treatments are mainly AE, RE, and CT. Different types of training methods have their own characteristics. For instance, AE primarily enhances cardiopulmonary function, and RE primarily enhances muscle strength and increases basal metabolic rate.
Table 2.
Types of exercise for various diseases.
| Types of exercise | Diseases |
|---|---|
| AE | LBP, OP, OA, hip fractures, obesity, NAFLD, CAD, CHF, PD, AD, depression, anxiety disorders, COPD, CKD, after kidney transplantation, BC, colon cancer, prostate cancer, LC |
| HIIT | Obesity, T2D, T1D, CHF, HD, LC |
| RE | Rotator cuff tendinopathy, tennis elbow, OP, T2D, stroke, CHF, PD, COPD, CKD, after kidney transplantation, BC, prostate cancer, LC |
| CT | OP, T2D, NAFLD, stroke, HD, ILD, after lung transplantation, CKD, after kidney transplantation, BC, colon cancer, prostate cancer, LC |
| HE | Hip fractures, CAD, HD, AD, anxiety disorders, CKD, LC |
| ME | OP, OA, hip fractures, stroke, PD, AD; COPD |
| Aquatic or water-based exercise | OA, PD, COPD |
| Whole body vibration exercise | LBP, OA, after lung transplantation |
| Endurance exercise | T1D |
| Concentric and eccentric exercise | Achilles tendinopathy, tennis elbow |
Abbreviations: AD = Alzheimer's disease; AE = aerobic exercise; BC = breast cancer; CAD = coronary artery disease; CHF = chronic heart failure; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; CT = combined aerobic exercise with resistance training; HD = Huntington's disease; HE = home-based exercise; HIIT = high-intensity interval training; ILD = interstitial lung disease; LBP = low back pain; LC = lung cancer; ME = multimodal exercise; NAFLD = nonalcoholic fatty liver disease; OA = osteoarthritis; OP = osteoporosis; PD = Parkinson's disease; RE = resistance exercise; T1D = Type 1 diabetes; T2D = Type 2 diabetes.
However, avoiding injury is more important than engaging in exercise. Measures for preventing patient from injuries should be taken. When designing an exercise program, we should conduct a comprehensive evaluation of the patient, exclude all possible contraindications, and select the appropriate type and intensity of exercise. During the training, we should keep an eye on the patient's adaptation to the intensity of the exercise and follow the principle of gradual training by starting with a low intensity and gradually increasing the load. The joint efforts of researchers in related fields can help to design the optimal exercise treatment for each individual, yielding a personalized exercise protocol that will help alleviate the symptoms of the illness and improve the patient's quality of life.
There are some limitations to the selection process we used for this review. In screening the articles, we focused on identifying studies that reported on RCTs and ignored comprehensive and systematic reviews or meta-analyses. The reviews and meta-analyses we omitted may have provided additional disease-specific findings. Thus, the search strategy we used may not have identified all relevant studies.
5. Conclusion
Exercise therapy is a safe method for improving patients’ physical performance and alleviating the symptoms of diseases. In this article, we identified the exercise intervention methods used for 26 common diseases and described the main characteristics of each exercise prescription. We then compared the effects of the various exercise intervention methods on the patients’ conditions and selected the recommended forms of exercise to prescribe. For future studies, people should focus on the role of exercise therapy in the prevention of diseases and provide guidance for family doctors and health management consultants that will help them to develop personalized and targeted exercise prescriptions for disease prevention.
Acknowledgments
Acknowledgments
The authors thank all the study participants. This study was supported by Natural Science Foundation of China (No. 31671242) and the National Key R&D Program of China (No. 2018YFC1314701).
Authors’ contributions
Both XL and XT participated in drafting, writing, and revising the manuscript; HZ, RH, and NL made edits and comments to the manuscript; RW and PC helped conceive of the design, provided funding for the study, and helped to draft the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order and presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jshs.2019.04.002.
Contributor Information
Peijie Chen, Email: chenpeijie@sus.edu.cn.
Ru Wang, Email: wangru@sus.edu.cn.
Appendix. Supplementary materials
References
- 1.Humphreys B.R., McLeod L., Ruseski J.E. Physical activity and health outcomes: evidence from Canada. Health Econ. 2014;23:33–54. doi: 10.1002/hec.2900. [DOI] [PubMed] [Google Scholar]
- 2.Lancaster G.I., Febbraio M.A. The immunomodulating role of exercise in metabolic disease. Trends Immunol. 2014;35:262–269. doi: 10.1016/j.it.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Fabbrini E., Sullivan S., Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabol R., Petersen K.F., Dufour S., Flannery C., Shulman G.I. Reversal of muscle insulin resistance with exercise reduces postprandial hepatic de novo lipogenesis in insulin resistant individuals. Proc Natl Acad Sci U S A. 2011;108:13705–13709. doi: 10.1073/pnas.1110105108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stefani L., Galanti G. Physical exercise prescription in metabolic chronic disease. Adv Exp Med Biol. 2017;1005:123–141. doi: 10.1007/978-981-10-5717-5_6. [DOI] [PubMed] [Google Scholar]
- 6.Kraemer W.J., Fleck J.S., Deschenes M.R. Lippincott Williams & Wilkins; Baltimore, MD: 2011. Exercise physiology: integrating theory and application. [Google Scholar]
- 7.Lima C.A., Sherrington C., Guaraldo A., Moraes S.A., Varanda R.D., Melo J.A. Effectiveness of a physical exercise intervention program in improving functional mobility in older adults after hip fracture in later stage rehabilitation: protocol of a randomized clinical trial (REATIVE Study) BMC Geriatr. 2016;16:198. doi: 10.1186/s12877-016-0370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alves C.R., da Cunha T.F., da Paixao N.A., Brum P.C. Aerobic exercise training as therapy for cardiac and cancer cachexia. Life Sci. 2015;125:9–14. doi: 10.1016/j.lfs.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 9.La Favor J.D., Dubis G.S., Yan H., White J.D., Nelson M.A., Anderson E.J. Microvascular endothelial dysfunction in sedentary, obese humans is mediated by NADPH oxidase: influence of exercise training. Arterioscler Thromb Vasc Biol. 2016;36:2412–2420. doi: 10.1161/ATVBAHA.116.308339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garber C.E., Blissmer B., Deschenes M.R., Franklin B.A., Lamonte M.J., Lee I.M. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 11.Todd Jan. Strength is health: George Barker Windship and the first American weight training boom. Iron Game History. 1993;3:3–14. [Google Scholar]
- 12.Dankel S.J., Mattocks K.T., Mouser J.G., Buckner S.L., Jessee M.B., Loenneke J.P. A critical review of the current evidence examining whether resistance training improves time trial performance. J Sports Sci. 2018;36:1485–1491. doi: 10.1080/02640414.2017.1398884. [DOI] [PubMed] [Google Scholar]
- 13.Garber C.E., Blissmer B., Deschenes M.R., Franklin B.A., Lamonte M.J., Lee I.M. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 14.Loveless M.S., Ihm J.M. Resistance exercise: how much is enough? Curr Sports Med Rep. 2015;14:221–226. doi: 10.1249/JSR.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 15.Jaureguizar K.V., Vicente-Campos D., Bautista L.R., de la Pena C.H., Gomez M.J., Rueda M.J. Effect of high-intensity interval versus continuous exercise training on functional capacity and quality of life in patients with coronary artery disease: a randomized clinical trial. J Cardiopulm Rehabil Prev. 2016;36:96–105. doi: 10.1097/HCR.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 16.Otero M., Esain I., Gonzalez-Suarez A.M., Gil S.M. The effectiveness of a basic exercise intervention to improve strength and balance in women with osteoporosis. Clin Interv Aging. 2017;12:505–513. doi: 10.2147/CIA.S127233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Claes J., Buys R., Budts W., Smart N., Cornelissen V.A. Longer-term effects of home-based exercise interventions on exercise capacity and physical activity in coronary artery disease patients: a systematic review and meta-analysis. Eur J Prev Cardiol. 2017;24:244–256. doi: 10.1177/2047487316675823. [DOI] [PubMed] [Google Scholar]
- 18.Liu C.J., Chang W.P., Araujo de Carvalho I., Savage K.E.L., Radford L.W., Amuthavalli Thiyagarajan J. Effects of physical exercise in older adults with reduced physical capacity: meta-analysis of resistance exercise and multimodal exercise. Int J Rehabil Res. 2017;40:303–314. doi: 10.1097/MRR.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 19.Meneses-Echavez J.F., Gonzalez-Jimenez E., Ramirez-Velez R. Effects of supervised multimodal exercise interventions on cancer-related fatigue: systematic review and meta-analysis of randomized controlled trials. Biomed Res Int. 2015;2015 doi: 10.1155/2015/328636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brotto M., Bonewald L. Bone and muscle: interactions beyond mechanical. Bone. 2015;80:109–114. doi: 10.1016/j.bone.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodman C.A., Hornberger T.A., Robling A.G. Bone and skeletal muscle: key players in mechanotransduction and potential overlapping mechanisms. Bone. 2015;80:24–36. doi: 10.1016/j.bone.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Loes M. Medical treatment and costs of sports-related injuries in a total population. Int J Sports Med. 1990;11:66–72. doi: 10.1055/s-2007-1024765. [DOI] [PubMed] [Google Scholar]
- 23.Lauersen J.B., Bertelsen D.M., Andersen L.B. The effectiveness of exercise interventions to prevent sports injuries: a systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. 2014;48:871–877. doi: 10.1136/bjsports-2013-092538. [DOI] [PubMed] [Google Scholar]
- 24.Maher C., Underwood M., Buchbinder R. Non-specific low back pain. The Lancet. 2017;389:736–747. doi: 10.1016/S0140-6736(16)30970-9. [DOI] [PubMed] [Google Scholar]
- 25.Deyo R.A., Rainville J., Kent D.L. What can the history and physical examination tell us about low back pain? JAMA. 1992;268:760–765. [PubMed] [Google Scholar]
- 26.Hall A.M., Maher C.G., Lam P., Ferreira M., Latimer J. Tai chi exercise for treatment of pain and disability in people with persistent low back pain: a randomized controlled trial. Arthritis Care Res (Hoboken) 2011;63:1576–1583. doi: 10.1002/acr.20594. [DOI] [PubMed] [Google Scholar]
- 27.Tekur P., Nagarathna R., Chametcha S., Hankey A., Nagendra H.R. A comprehensive yoga programs improves pain, anxiety and depression in chronic low back pain patients more than exercise: an RCT. Complement Ther Med. 2012;20:107–118. doi: 10.1016/j.ctim.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Lopes S., Correia C., Felix G., Lopes M., Cruz A., Ribeiro F. Immediate effects of Pilates based therapeutic exercise on postural control of young individuals with non-specific low back pain: a randomized controlled trial. Complement Ther Med. 2017;34:104–110. doi: 10.1016/j.ctim.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Valenza M.C., Rodriguez-Torres J., Cabrera-Martos I., Diaz-Pelegrina A., Aguilar-Ferrandiz M.E., Castellote-Caballero Y. Results of a Pilates exercise program in patients with chronic non-specific low back pain: a randomized controlled trial. Clin Rehabil. 2017;31:753–760. doi: 10.1177/0269215516651978. [DOI] [PubMed] [Google Scholar]
- 30.Wajswelner H., Metcalf B., Bennell K. Clinical Pilates versus general exercise for chronic low back pain: randomized trial. Med Sci Sports Exerc. 2012;44:1197–1205. doi: 10.1249/MSS.0b013e318248f665. [DOI] [PubMed] [Google Scholar]
- 31.Notarnicola A., Fischetti F., Maccagnano G., Comes R., Tafuri S., Moretti B. Daily Pilates exercise or inactivity for patients with low back pain: a clinical prospective observational study. Eur J Phys Rehabil Med. 2014;50:59–66. [PubMed] [Google Scholar]
- 32.Patti A., Bianco A., Paoli A., Messina G., Montalto M.A., Bellafiore M. Pain perception and stabilometric parameters in people with chronic low back pain after a Pilates exercise program: a randomized controlled trial. Medicine (Baltimore) 2016;95:e2414. doi: 10.1097/MD.0000000000002414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murtezani A., Hundozi H., Orovcanec N., Sllamniku S., Osmani T. A comparison of high intensity aerobic exercise and passive modalities for the treatment of workers with chronic low back pain: a randomized, controlled trial. Eur J Phys Rehabil Med. 2011;47:359–366. [PubMed] [Google Scholar]
- 34.Hill J.J., Keating J.L. Encouraging healthy spine habits to prevent low back pain in children: an observational study of adherence to exercise. Physiotherapy. 2016;102:229–235. doi: 10.1016/j.physio.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Wang X.Q., Pi Y.L., Chen P.J., Chen B.L., Liang L.C., Li X. Whole body vibration exercise for chronic low back pain: study protocol for a single-blind randomized controlled trial. Trials. 2014;15:104. doi: 10.1186/1745-6215-15-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaeding T.S., Karch A., Schwarz R., Flor T., Wittke T.C., Kuck M. Whole-body vibration training as a workplace-based sports activity for employees with chronic low-back pain. Scand J Med Sci Sports. 2017;27:2027–2039. doi: 10.1111/sms.12852. [DOI] [PubMed] [Google Scholar]
- 37.del Pozo-Cruz B., Hernandez Mocholi M.A., Adsuar J.C., Parraca J.A., Muro I., Gusi N. Effects of whole body vibration therapy on main outcome measures for chronic non-specific low back pain: a single-blind randomized controlled trial. J Rehabil Med. 2011;43:689–694. doi: 10.2340/16501977-0830. [DOI] [PubMed] [Google Scholar]
- 38.Parle P.J., Riddiford-Harland D.L., Howitt C.D., Lewis J.S. Acute rotator cuff tendinopathy: does ice, low load isometric exercise, or a combination of the two produce an analgaesic effect? Br J Sports Med. 2017;51:208–209. doi: 10.1136/bjsports-2016-096107. [DOI] [PubMed] [Google Scholar]
- 39.Croisier J.L., Foidartdessalle M., Tinant F., Crielaard J.M., Forthomme B. An isokinetic eccentric programme for the management of chronic lateral epicondylar tendinopathy. Br J Sports Med. 2007;41:269–275. doi: 10.1136/bjsm.2006.033324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoogvliet P., Randsdorp M.S., Dingemanse R., Koes B.W., Huisstede B.M. Does effectiveness of exercise therapy and mobilisation techniques offer guidance for the treatment of lateral and medial epicondylitis? A systematic review. Br J Sports Med. 2013;47:1112–1119. doi: 10.1136/bjsports-2012-091990. [DOI] [PubMed] [Google Scholar]
- 41.Peterson M., Butler S., Eriksson M., Svardsudd K. A randomized controlled trial of eccentric vs. concentric graded exercise in chronic tennis elbow (lateral elbow tendinopathy) Clin Rehabil. 2014;28:862–872. doi: 10.1177/0269215514527595. [DOI] [PubMed] [Google Scholar]
- 42.Rompe J.D., Furia J.P., Maffulli N. Mid-portion Achilles tendinopathy–current options for treatment. Disabil Rehabil. 2008;30:1666–1676. doi: 10.1080/09638280701785825. [DOI] [PubMed] [Google Scholar]
- 43.Ames P.R., Longo U.G., Denaro V., Maffulli N. Achilles tendon problems: not just an orthopaedic issue. Disabil Rehabil. 2008;30:1646–1650. doi: 10.1080/09638280701785882. [DOI] [PubMed] [Google Scholar]
- 44.Dejaco B., Habets B., van Loon C., van Grinsven S., van Cingel R. Eccentric versus conventional exercise therapy in patients with rotator cuff tendinopathy: a randomized, single blinded, clinical trial. Knee Surg Sports Traumatol Arthrosc. 2017;25:2051–2059. doi: 10.1007/s00167-016-4223-x. [DOI] [PubMed] [Google Scholar]
- 45.Peterson M., Butler S., Eriksson M., Svärdsudd K. A randomized controlled trial of exercise versus wait-list in chronic tennis elbow (lateral epicondylosis) Ups J Med Sci. 2011;116:269–279. doi: 10.3109/03009734.2011.600476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rees J.D., Maffulli N., Cook J. Management of tendinopathy. Am J Sports Med. 2009;37:1855–1867. doi: 10.1177/0363546508324283. [DOI] [PubMed] [Google Scholar]
- 47.Horstmann T., Jud H.M., Frohlich V., Mundermann A., Grau S. Whole-body vibration versus eccentric training or a wait-and-see approach for chronic Achilles tendinopathy: a randomized clinical trial. J Orthop Sports Phys Ther. 2013;43:794–803. doi: 10.2519/jospt.2013.4762. [DOI] [PubMed] [Google Scholar]
- 48.Sevier T.L., Stegink-Jansen C.W. Astym treatment vs. eccentric exercise for lateral elbow tendinopathy: a randomized controlled clinical trial. PeerJ. 2015;3:e967. doi: 10.7717/peerj.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Habets B., van Cingel R.E.H., Backx F.J.G., Huisstede B.M.A. Alfredson versus Silbernagel exercise therapy in chronic midportion Achilles tendinopathy: study protocol for a randomized controlled trial. BMC Musculoskelet Disord. 2017;18:296. doi: 10.1186/s12891-017-1656-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grigg N.L., Wearing S.C., Smeathers J.E. Achilles tendinopathy has an aberrant strain response to eccentric exercise. Med Sci Sports Exerc. 2012;44:12–17. doi: 10.1249/MSS.0b013e318227fa8c. [DOI] [PubMed] [Google Scholar]
- 51.Littlewood C., Bateman M., Brown K., Bury J., Mawson S., May S. A self-managed single exercise programme versus usual physiotherapy treatment for rotator cuff tendinopathy: a randomised controlled trial (the SELF study) Clin Rehabil. 2016;30:686–696. doi: 10.1177/0269215515593784. [DOI] [PubMed] [Google Scholar]
- 52.Littlewood C., Malliaras P., Mawson S., May S., Walters S.J. Self-managed loaded exercise versus usual physiotherapy treatment for rotator cuff tendinopathy: a pilot randomised controlled trial. Physiotherapy. 2014;100:54–60. doi: 10.1016/j.physio.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 53.Heron S.R., Woby S.R., Thompson D.P. Comparison of three types of exercise in the treatment of rotator cuff tendinopathy/shoulder impingement syndrome: a randomized controlled trial. Physiotherapy. 2017;103:167–173. doi: 10.1016/j.physio.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Montalcini T., Romeo S., Ferro Y., Migliaccio V., Gazzaruso C., Pujia A. Osteoporosis in chronic inflammatory disease: the role of malnutrition. Endocrine. 2013;43:59–64. doi: 10.1007/s12020-012-9813-x. [DOI] [PubMed] [Google Scholar]
- 55.Chien M.Y., Wu Y.T., Hsu A.T., Yang R.S., Lai J.S. Efficacy of a 24-week aerobic exercise program for osteopenic postmenopausal women. Calcif Tissue Int. 2000;67:443–448. doi: 10.1007/s002230001180. [DOI] [PubMed] [Google Scholar]
- 56.Teoman N., Ozcan A., Acar B. The effect of exercise on physical fitness and quality of life in postmenopausal women. Maturitas. 2004;47:71–77. doi: 10.1016/s0378-5122(03)00241-x. [DOI] [PubMed] [Google Scholar]
- 57.Black D.M., Rosen C.J. Clinical practice. Postmenopausal osteoporosis. N Engl J Med. 2016;374:254–262. doi: 10.1056/NEJMcp1513724. [DOI] [PubMed] [Google Scholar]
- 58.Liu-Ambrose T., Eng J.J., Khan K.M., Carter N.D., Mckay H.A. Older women with osteoporosis have increased postural sway and weaker quadriceps strength than counterparts with normal bone mass: overlooked determinants of fracture risk? J Gerontol A Biol Sci Med Soc. 2003;58:M862–M866. doi: 10.1093/gerona/58.9.m862. [DOI] [PubMed] [Google Scholar]
- 59.Halvarsson A, Franzén E, Ståhle A. Balance training with multi-task exercises improves fall-related self-efficacy, gait, balance performance and physical function in older adults with osteoporosis: a randomized controlled trial. Clin Rehabil 29:365–75. [DOI] [PubMed]
- 60.Madureira M.M., Takayama L., Gallinaro A.L., Caparbo V.F., Costa R.A., Pereira R.M. Balance training program is highly effective in improving functional status and reducing the risk of falls in elderly women with osteoporosis: a randomized controlled trial. Osteoporos Int. 2007;18:419–425. doi: 10.1007/s00198-006-0252-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roghani T., Torkaman G., Movasseghe S., Hedayati M., Goosheh B., Bayat N. Effects of short-term aerobic exercise with and without external loading on bone metabolism and balance in postmenopausal women with osteoporosis. Rheumatol Int. 2013;33:291–298. doi: 10.1007/s00296-012-2388-2. [DOI] [PubMed] [Google Scholar]
- 62.Wayne P.M., Kiel D.P., Buring J.E., Connors E.M., Bonato P., Yeh G.Y. Impact of Tai Chi exercise on multiple fracture-related risk factors in post-menopausal osteopenic women: a pilot pragmatic, randomized trial. BMC Complement Altern Med. 2012;12:7. doi: 10.1186/1472-6882-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gunendi Z., Ozyemisci-Taskiran O., Demirsoy N. The effect of 4-week aerobic exercise program on postural balance in postmenopausal women with osteoporosis. Rheumatol Int. 2008;28:1217–1222. doi: 10.1007/s00296-008-0651-3. [DOI] [PubMed] [Google Scholar]
- 64.Stanghelle B., Bentzen H., Giangregorio L., Pripp A.H., Bergland A. Effect of a resistance and balance exercise programme for women with osteoporosis and vertebral fracture: study protocol for a randomized controlled trial. BMC Musculoskelet Disord. 2018;19:100. doi: 10.1186/s12891-018-2021-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kemmler W., von Stengel S., Bebenek M., Engelke K., Hentschke C., Kalender W.A. Exercise and fractures in postmenopausal women: 12-year results of the Erlangen Fitness and Osteoporosis Prevention Study (EFOPS) Osteoporos Int. 2012;23:1267–1276. doi: 10.1007/s00198-011-1663-5. [DOI] [PubMed] [Google Scholar]
- 66.Kemmler W., Bebenek M., Kohl M., von Stengel S. Exercise and fractures in postmenopausal women. Final results of the controlled Erlangen Fitness and Osteoporosis Prevention Study (EFOPS) Osteoporos Int. 2015;26:2491–2499. doi: 10.1007/s00198-015-3165-3. [DOI] [PubMed] [Google Scholar]
- 67.Loeser R.F. Age-related changes in the musculoskeletal system and the development of osteoarthritis. Clin Geriatr Med. 2010;26:371–386. doi: 10.1016/j.cger.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hootman J.M., Helmick C.G. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54:226–229. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- 69.Alkatan M., Baker J.R., Machin D.R., Park W., Akkari A.S., Pasha E.P. Improved function and reduced pain after swimming and cycling training in patients with osteoarthritis. J Rheumatol. 2016;43:666–672. doi: 10.3899/jrheum.151110. [DOI] [PubMed] [Google Scholar]
- 70.Nelson A.E., Allen K.D., Golightly Y.M., Goode A.P., Jordan J.M. A systematic reviewof recommendations and guidelines for the management of osteoarthritis: the chronic osteoarthritis management initiative of the U.S. bone and joint initiative. Semin Arthritis Rheum. 2014;43:701–712. doi: 10.1016/j.semarthrit.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 71.Lim J.Y., Tchai E., Jang S.N. Effectiveness of aquatic exercise for obese patients with knee osteoarthritis: a randomized controlled trial. PM R. 2010;2:723–731. doi: 10.1016/j.pmrj.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 72.Waller B., Munukka M., Rantalainen T., Lammentausta E., Nieminen M.T., Kiviranta I. Effects of high intensity resistance aquatic training on body composition and walking speed in women with mild knee osteoarthritis: a 4-month RCT with 12-month follow-up. Osteoarthritis Cartilage. 2017;25:1238–1246. doi: 10.1016/j.joca.2017.02.800. [DOI] [PubMed] [Google Scholar]
- 73.Cadmus L., Patrick M.B., Maciejewski M.L., Topolski T., Belza B., Patrick D.L. Community-based aquatic exercise and quality of life in persons with osteoarthritis. Med Sci Sports Exerc. 2010;42:8–15. doi: 10.1249/MSS.0b013e3181ae96a9. [DOI] [PubMed] [Google Scholar]
- 74.Yazigi F., Espanha M., Vieira F., Messier S.P., Monteiro C., Veloso A.P. The PICO project: aquatic exercise for knee osteoarthritis in overweight and obese individuals. BMC Musculoskelet Disord. 2013;14:320. doi: 10.1186/1471-2474-14-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arnold C.M., Faulkner R.A. The effect of aquatic exercise and education on lowering fall risk in older adults with hip osteoarthritis. J Aging Phys Act. 2010;18:245–260. doi: 10.1123/japa.18.3.245. [DOI] [PubMed] [Google Scholar]
- 76.Casilda-Lopez J., Valenza M.C., Cabrera-Martos I., Diaz-Pelegrina A., Moreno-Ramirez M.P., Valenza-Demet G. Effects of a dance-based aquatic exercise program in obese postmenopausal women with knee osteoarthritis: a randomized controlled trial. Menopause. 2017;24:768–773. doi: 10.1097/GME.0000000000000841. [DOI] [PubMed] [Google Scholar]
- 77.Cheung C., Wyman J.F., Bronas U., McCarthy T., Rudser K., Mathiason M.A. Managing knee osteoarthritis with yoga or aerobic/strengthening exercise programs in older adults: a pilot randomized controlled trial. Rheumatol Int. 2017;37:389–398. doi: 10.1007/s00296-016-3620-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuntz A.B., Chopp-Hurley J.N., Brenneman E.C., Karampatos S., Wiebenga E.G., Adachi J.D. Efficacy of a biomechanically-based yoga exercise program in knee osteoarthritis: a randomized controlled trial. PLoS One. 2018;13 doi: 10.1371/journal.pone.0195653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu J., Huang L., Wu X., Fu W., Liu Y. Effect of Tai Ji Quan training on self-reported sleep quality in elderly Chinese women with knee osteoarthritis: a randomized controlled trail. Sleep Med. 2017;33:70–75. doi: 10.1016/j.sleep.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 80.Alkatan M., Machin D.R., Baker J.R., Akkari A.S., Park W., Tanaka H. Effects of swimming and cycling exercise intervention on vascular function in patients with osteoarthritis. Am J Cardiol. 2016;117:141–145. doi: 10.1016/j.amjcard.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 81.Chang T.F., Liou T.H., Chen C.H., Huang Y.C., Chang K.H. Effects of elastic-band exercise on lower-extremity function among female patients with osteoarthritis of the knee. Disabil Rehabil. 2012;34:1727–1735. doi: 10.3109/09638288.2012.660598. [DOI] [PubMed] [Google Scholar]
- 82.Lai Z., Zhang Y., Lee S., Wang L. Effects of strength exercise on the knee and ankle proprioception of individuals with knee osteoarthritis. Res Sports Med. 2018;26:138–146. doi: 10.1080/15438627.2018.1431541. [DOI] [PubMed] [Google Scholar]
- 83.Fukumoto Y., Tateuchi H., Tsukagoshi R., Okita Y., Akiyama H., So K. Effects of high- and low-velocity resistance training on gait kinematics and kinetics in individuals with hip osteoarthritis: a randomized controlled trial. Am J Phys Med Rehabil. 2017;96:417–423. doi: 10.1097/PHM.0000000000000640. [DOI] [PubMed] [Google Scholar]
- 84.Lai Z., Wang X., Lee S., Hou X., Wang L. Effects of whole body vibration exercise on neuromuscular function for individuals with knee osteoarthritis: study protocol for a randomized controlled trial. Trials. 2017;18:437. doi: 10.1186/s13063-017-2170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang P., Yang L., Liu C., Wei X., Yang X., Zhou Y. Effects of whole body vibration exercise associated with quadriceps resistance exercise on functioning and quality of life in patients with knee osteoarthritis: a randomized controlled trial. Clin Rehabil. 2016;30:1074–1087. doi: 10.1177/0269215515607970. [DOI] [PubMed] [Google Scholar]
- 86.Trans T., Aaboe J., Henriksen M., Christensen R., Bliddal H., Lund H. Effect of whole body vibration exercise on muscle strength and proprioception in females with knee osteoarthritis. Knee. 2009;16:256–261. doi: 10.1016/j.knee.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 87.Levinger P., Dunn J., Bifera N., Butson M., Elias G., Hill K.D. High-speed resistance training and balance training for people with knee osteoarthritis to reduce falls risk: study protocol for a pilot randomized controlled trial. Trials. 2017;18:384. doi: 10.1186/s13063-017-2129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Svege I., Fernandes L., Nordsletten L., Holm I., Risberg M.A. Long-term effect of exercise therapy and patient education on impairments and activity limitations in people with hip osteoarthritis: secondary outcome analysis of a randomized clinical trial. Phys Ther. 2016;96:818–827. doi: 10.2522/ptj.20140520. [DOI] [PubMed] [Google Scholar]
- 89.Åkesson K., Marsh D., Mitchell P.J., McLellan A.R., Stenmark J., Pierroz D.D. Capture the Fracture: a Best Practice Framework and global campaign to break the fragility fracture cycle. Osteoporos Int. 2013;24:2135–2152. doi: 10.1007/s00198-013-2348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Johnell O., Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16(Suppl. 2):S3–S7. doi: 10.1007/s00198-004-1702-6. [DOI] [PubMed] [Google Scholar]
- 91.Diong J., Allen N., Sherrington C. Structured exercise improves mobility after hip fracture: a meta-analysis with meta-regression. Br J Sports Med. 2016;50:346–355. doi: 10.1136/bjsports-2014-094465. [DOI] [PubMed] [Google Scholar]
- 92.Mangione K.K., Craik R.L., Palombaro K.M., Tomlinson S.S., Hofmann M.T. Home-based leg-strengthening exercise improves function 1 year after hip fracture: a randomized controlled study. J Am Geriatr Soc. 2010;58:1911–1917. doi: 10.1111/j.1532-5415.2010.03076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hourigan S.R., Nitz J.C., Brauer S.G., O'Neill S., Wong J., Richardson C.A. Positive effects of exercise on falls and fracture risk in osteopenic women. Osteoporos Int. 2008;19:1077–1086. doi: 10.1007/s00198-007-0541-7. [DOI] [PubMed] [Google Scholar]
- 94.Latham N.K., Harris B.A., Bean J.F., Heeren T., Goodyear C., Zawacki S. Effect of a home-based exercise program on functional recovery following rehabilitation after hip fracture: a randomized clinical trial. JAMA. 2014;311:700–708. doi: 10.1001/jama.2014.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gianoudis J., Bailey C.A., Ebeling P.R., Nowson C.A., Sanders K.M., Hill K. Effects of a targeted multimodal exercise program incorporating high-speed power training on falls and fracture risk factors in older adults: a community-based randomized controlled trial. J Bone Miner Res. 2014;29:182–191. doi: 10.1002/jbmr.2014. [DOI] [PubMed] [Google Scholar]
- 96.Montesi L., Moscatiello S., Malavolti M., Marzocchi R., Marchesini G. Physical activity for the prevention and treatment of metabolic disorders. Intern Emerg Med. 2013;8:655–666. doi: 10.1007/s11739-013-0953-7. [DOI] [PubMed] [Google Scholar]
- 97.Villareal D.T., Aguirre L., Gurney A.B., Waters D.L., Sinacore D.R., Colombo E. Aerobic or resistance exercise, or both, in dieting obese older adults. N Engl J Med. 2017;376:1943–1955. doi: 10.1056/NEJMoa1616338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Allen J.M., Mailing L.J., Niemiro G.M., Moore R., Cook M.D., White B.A. Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc. 2018;50:747–757. doi: 10.1249/MSS.0000000000001495. [DOI] [PubMed] [Google Scholar]
- 99.Besse-Patin A., Montastier E., Vinel C., Castan-Laurell I., Louche K., Dray C. Effect of endurance training on skeletal muscle myokine expression in obese men: identification of apelin as a novel myokine. Int J Obes (Lond) 2014;38:707–713. doi: 10.1038/ijo.2013.158. [DOI] [PubMed] [Google Scholar]
- 100.Araya A.V., Orellana X., Godoy D., Soto L., Fiedler J. Effect of exercise on circulating levels of brain-derived neurotrophic factor (BDNF) in overweight and obese subjects. Horm Metab Res. 2013;45:541–544. doi: 10.1055/s-0032-1333237. [DOI] [PubMed] [Google Scholar]
- 101.Pugh J.K., Faulkner S.H., Turner M.C., Nimmo M.A. Satellite cell response to concurrent resistance exercise and high-intensity interval training in sedentary, overweight/obese, middle-aged individuals. Eur J Appl Physiol. 2018;118:225–238. doi: 10.1007/s00421-017-3721-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kolb H., Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017;15:131. doi: 10.1186/s12916-017-0901-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guariguata L. By the numbers: new estimates from the IDF Diabetes Atlas Update for 2012. Diabetes Res Clin Pract. 2012;98:524–525. doi: 10.1016/j.diabres.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 104.Whiting D.R., Guariguata L., Weil C., Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 105.Yang Z., Scott C.A., Mao C., Tang J., Farmer A.J. Resistance exercise versus aerobic exercise for type 2 diabetes: a systematic review and meta-analysis. Sports Med. 2014;44:487–499. doi: 10.1007/s40279-013-0128-8. [DOI] [PubMed] [Google Scholar]
- 106.Khadir A., Kavalakatt S., Cherian P., Warsame S., Abubaker J.A., Dehbi M. Physical exercise enhanced heat shock protein 60 expression and attenuated inflammation in the adipose tissue of human diabetic obese. Front Endocrinol (Lausanne) 2018;9:16. doi: 10.3389/fendo.2018.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stegen S., Sigal R.J., Kenny G.P., Khandwala F., Yard B., De Heer E. Aerobic and resistance training do not influence plasma carnosinase content or activity in type 2 diabetes. Am J Physiol Endocrinol Metab. 2015;309:E663–E669. doi: 10.1152/ajpendo.00142.2015. [DOI] [PubMed] [Google Scholar]
- 108.Amouzad Mahdirejei H., Fadaei Reyhan Abadei S., Abbaspour Seidi A., Eshaghei Gorji N., Rahmani Kafshgari H., Ebrahim Pour M. Effects of an eight-week resistance training on plasma vaspin concentrations, metabolic parameters levels and physical fitness in patients with type 2 diabetes. Cell J. 2014;16:367–374. [PMC free article] [PubMed] [Google Scholar]
- 109.Opitz D., Kreutz T., Lenzen E., Dillkofer B., Wahl P., Montiel-Garcia G. Strength training alters MCT1-protein expression and exercise-induced translocation in erythrocytes of men with non-insulin-dependent type-2 diabetes. Can J Physiol Pharmacol. 2014;92:259–262. doi: 10.1139/cjpp-2012-0405. [DOI] [PubMed] [Google Scholar]
- 110.Durrer C., Francois M., Neudorf H., Little J.P. Acute high-intensity interval exercise reduces human monocyte Toll-like receptor 2 expression in type 2 diabetes. Am J Physiol Regul Integr Comp Physiol. 2017;312:R529–R538. doi: 10.1152/ajpregu.00348.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Michels A., Zhang L., Khadra A., Kushner J.A., Redondo M.J., Pietropaolo M. Prediction and prevention of type 1 diabetes: update on success of prediction and struggles at prevention. Pediatr Diabetes. 2015;16:465–484. doi: 10.1111/pedi.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rooijackers H.M., Wiegers E.C., van der Graaf M., Thijssen D.H., Kessels R.P.C., Tack C.J. A single bout of high-intensity interval training reduces awareness of subsequent hypoglycemia in patients with type 1 diabetes. Diabetes. 2017;66:1990–1998. doi: 10.2337/db16-1535. [DOI] [PubMed] [Google Scholar]
- 113.Harmer A.R., Ruell P.A., Hunter S.K., McKenna M.J., Thom J.M., Chisholm D.J. Effects of type 1 diabetes, sprint training and sex on skeletal muscle sarcoplasmic reticulum Ca2+ uptake and Ca2+-ATPase activity. J Physiol. 2014;592:523–535. doi: 10.1113/jphysiol.2013.261172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Byrne C.D., Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62(Suppl. 1):S47–S64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 115.Chalasani N., Younossi Z., Lavine J.E., Diehl A.M., Brunt E.M., Cusi K. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Am J Gastroenterol. 2012;107:811–826. doi: 10.1038/ajg.2012.128. [DOI] [PubMed] [Google Scholar]
- 116.Winn N.C., Liu Y., Rector R.S., Parks E.J., Ibdah J.A., Kanaley J.A. Energy-matched moderate and high intensity exercise training improves nonalcoholic fatty liver disease risk independent of changes in body mass or abdominal adiposity—a randomized trial. Metabolism. 2018;78:128–140. doi: 10.1016/j.metabol.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 117.Sullivan S., Kirk E.P., Mittendorfer B., Patterson B.W., Klein S. Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology. 2012;55:1738–1745. doi: 10.1002/hep.25548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang H.J., Pan L.L., Ma Z.M., Chen Z., Huang Z.F., Sun Q. Long-term effect of exercise on improving fatty liver and cardiovascular risk factors in obese adults: a 1-year follow-up study. Diabetes Obes Metab. 2017;19:284–289. doi: 10.1111/dom.12809. [DOI] [PubMed] [Google Scholar]
- 119.Brouwers B., Schrauwen-Hinderling V.B., Jelenik T., Gemmink A., Sparks L.M., Havekes B. Exercise training reduces intrahepatic lipid content in people with and people without nonalcoholic fatty liver. Am J Physiol Endocrinol Metab. 2018;314:E165–E173. doi: 10.1152/ajpendo.00266.2017. [DOI] [PubMed] [Google Scholar]
- 120.Chow C.K., Jolly S., Rao-Melacini P., Fox K.A., Anand S.S., Yusuf S. Association of diet, exercise, and smoking modification with risk of early cardiovascular events after acute coronary syndromes. Circulation. 2010;121:750–758. doi: 10.1161/CIRCULATIONAHA.109.891523. [DOI] [PubMed] [Google Scholar]
- 121.Wong N.D. Epidemiological studies of CHD and the evolution of preventive cardiology. Nat Rev Cardiol. 2014;11:276–289. doi: 10.1038/nrcardio.2014.26. [DOI] [PubMed] [Google Scholar]
- 122.Naghavi M., Wang H., Lozano R., Davis A., Liang X., Zhou M. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Heran B.S., Chen J.M., Ebrahim S., Moxham T., Oldridge N., Rees K. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011;7 doi: 10.1002/14651858.CD001800.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mendelson M., Lyons O.D., Yadollahi A., Inami T., Oh P., Bradley T.D. Effects of exercise training on sleep apnoea in patients with coronary artery disease: a randomised trial. Eur Respir J. 2016;48:142–150. doi: 10.1183/13993003.01897-2015. [DOI] [PubMed] [Google Scholar]
- 125.Lim S.T., Min S.K., Kwon Y.C., Park S.K., Park H. Effects of intermittent exercise on biomarkers of cardiovascular risk in night shift workers. Atherosclerosis. 2015;242:186–190. doi: 10.1016/j.atherosclerosis.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 126.Villelabeitia-Jaureguizar K., Vicente-Campos D., Senen A.B., Jimenez V.H., Garrido-Lestache M.E.B., Chicharro J.L. Effects of high-intensity interval versus continuous exercise training on post-exercise heart rate recovery in coronary heart-disease patients. Int J Cardiol. 2017;244:17–23. doi: 10.1016/j.ijcard.2017.06.067. [DOI] [PubMed] [Google Scholar]
- 127.Pattyn N., Vanhees L., Cornelissen V.A., Coeckelberghs E., De Maeyer C., Goetschalckx K. The long-term effects of a randomized trial comparing aerobic interval versus continuous training in coronary artery disease patients: 1-year data from the SAINTEX-CAD study. Eur J Prev Cardiol. 2016;23:1154–1164. doi: 10.1177/2047487316631200. [DOI] [PubMed] [Google Scholar]
- 128.Conraads V.M., Pattyn N., De Maeyer C., Beckers P.J., Coeckelberghs E., Cornelissen V.A. Aerobic interval training and continuous training equally improve aerobic exercise capacity in patients with coronary artery disease: the SAINTEX-CAD study. Int J Cardiol. 2015;179:203–210. doi: 10.1016/j.ijcard.2014.10.155. [DOI] [PubMed] [Google Scholar]
- 129.Smith K.M., McKelvie R.S., Thorpe K.E., Arthur H.M. Six-year follow-up of a randomised controlled trial examining hospital versus home-based exercise training after coronary artery bypass graft surgery. Heart. 2011;97:1169–1174. doi: 10.1136/hrt.2010.202036. [DOI] [PubMed] [Google Scholar]
- 130.Torri A., Panzarino C., Scaglione A., Modica M., Bordoni B., Redaelli R. Promotion of home-based exercise training as secondary prevention of coronary heart disease: a pilot web-based intervention. J Cardiopulm Rehabil Prev. 2018;38:253–258. doi: 10.1097/HCR.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 131.Brazzelli M., Saunders D.H., Greig C.A., Mead G.E. Physical fitness training for patients with stroke: updated review. Stroke. 2012;43:e39–e40. doi: 10.1161/strokeaha.111.647008. [DOI] [PubMed] [Google Scholar]
- 132.Tang A., Sibley K.M., Thomas S.G., Bayley M.T., Richardson D., McIlroy W.E. Effects of an aerobic exercise program on aerobic capacity, spatiotemporal gait parameters, and functional capacity in subacute stroke. Neurorehabil Neural Repair. 2009;23:398–406. doi: 10.1177/1545968308326426. [DOI] [PubMed] [Google Scholar]
- 133.Yeh T.T., Wu C.Y., Hsieh Y.W., Chang K.C., Lee L.C., Hung J.W. Synergistic effects of aerobic exercise and cognitive training on cognition, physiological markers, daily function, and quality of life in stroke survivors with cognitive decline: study protocol for a randomized controlled trial. Trials. 2017;18:405. doi: 10.1186/s13063-017-2153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Quaney B.M., Boyd L.A., McDowd J.M., Zahner L.H., He J., Mayo M.S. Aerobic exercise improves cognition and motor function poststroke. Neurorehabil Neural Repair. 2009;23:879–885. doi: 10.1177/1545968309338193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sandberg K., Kleist M., Falk L., Enthoven P. Effects of twice-weekly intense aerobic exercise in early subacute stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2016;97:1244–1253. doi: 10.1016/j.apmr.2016.01.030. [DOI] [PubMed] [Google Scholar]
- 136.Vahlberg B., Cederholm T., Lindmark B., Zetterberg L., Hellstrom K. Short-term and long-term effects of a progressive resistance and balance exercise program in individuals with chronic stroke: a randomized controlled trial. Disabil Rehabil. 2017;39:1615–1622. doi: 10.1080/09638288.2016.1206631. [DOI] [PubMed] [Google Scholar]
- 137.Tang A., Eng J.J., Krassioukov A.V., Madden K.M., Mohammadi A., Tsang M.Y. Exercise-induced changes in cardiovascular function after stroke: a randomized controlled trial. Int J Stroke. 2014;9:883–889. doi: 10.1111/ijs.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kim J., Park J.H., Yim J. Effects of respiratory muscle and endurance training using an individualized training device on the pulmonary function and exercise capacity in stroke patients. Med Sci Monit. 2014;20:2543–2549. doi: 10.12659/MSM.891112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Marzolini S., Oh P., McIlroy W., Brooks D. The effects of an aerobic and resistance exercise training program on cognition following stroke. Neurorehabil Neural Repair. 2013;27:392–402. doi: 10.1177/1545968312465192. [DOI] [PubMed] [Google Scholar]
- 140.Hunt S.A., Baker D.W., Chin M.H., Cinquegrani M.P., Feldman A.M., Francis G.S. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1995 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2001;38:2101–2113. doi: 10.1016/s0735-1097(01)01683-7. [DOI] [PubMed] [Google Scholar]
- 141.Teng H.C., Yeh M.L., Wang M.H. Walking with controlled breathing improves exercise tolerance, anxiety, and quality of life in heart failure patients: a randomized controlled trial. Eur J Cardiovasc Nurs. 2018;17:717–727. doi: 10.1177/1474515118778453. [DOI] [PubMed] [Google Scholar]
- 142.Antunes-Correa L.M., Nobre T.S., Groehs R.V., Alves M.J., Fernandes T., Couto G.K. Molecular basis for the improvement in muscle metaboreflex and mechanoreflex control in exercise-trained humans with chronic heart failure. Am J Physiol Heart Circ Physiol. 2014;307:H1655–H1666. doi: 10.1152/ajpheart.00136.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chrysohoou C., Tsitsinakis G., Vogiatzis I., Cherouveim E., Antoniou C., Tsiantilas A. High intensity, interval exercise improves quality of life of patients with chronic heart failure: a randomized controlled trial. QJM. 2014;107:25–32. doi: 10.1093/qjmed/hct194. [DOI] [PubMed] [Google Scholar]
- 144.Freyssin C., Verkindt C., Prieur F., Benaich P., Maunier S., Blanc P. Cardiac rehabilitation in chronic heart failure: effect of an 8-week, high-intensity interval training versus continuous training. Arch Phys Med Rehabil. 2012;93:1359–1364. doi: 10.1016/j.apmr.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 145.Koufaki P., Mercer T.H., George K.P., Nolan J. Low-volume high-intensity interval training vs continuous aerobic cycling in patients with chronic heart failure: a pragmatic randomised clinical trial of feasibility and effectiveness. J Rehabil Med. 2014;46:348–356. doi: 10.2340/16501977-1278. [DOI] [PubMed] [Google Scholar]
- 146.Chrysohoou C., Angelis A., Tsitsinakis G., Spetsioti S., Nasis I., Tsiachris D. Cardiovascular effects of high-intensity interval aerobic training combined with strength exercise in patients with chronic heart failure. A randomized phase III clinical trial. Int J Cardiol. 2015;179:269–274. doi: 10.1016/j.ijcard.2014.11.067. [DOI] [PubMed] [Google Scholar]
- 147.Anagnostakou V., Chatzimichail K., Dimopoulos S., Karatzanos E., Papazachou O., Tasoulis A. Effects of interval cycle training with or without strength training on vascular reactivity in heart failure patients. J Card Fail. 2011;17:585–591. doi: 10.1016/j.cardfail.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 148.Acanfora D., Scicchitano P., Casucci G., Lanzillo B., Capuano N., Furgi G. Exercise training effects on elderly and middle-age patients with chronic heart failure after acute decompensation: a randomized, controlled trial. Int J Cardiol. 2016;225:313–323. doi: 10.1016/j.ijcard.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 149.Maiorana A.J., Naylor L.H., Exterkate A., Swart A., Thijssen D.H., Lam K. The impact of exercise training on conduit artery wall thickness and remodeling in chronic heart failure patients. Hypertension. 2011;57:56–62. doi: 10.1161/HYPERTENSIONAHA.110.163022. [DOI] [PubMed] [Google Scholar]
- 150.McDonnell M.N., Smith A.E., Mackintosh S.F. Aerobic exercise to improve cognitive function in adults with neurological disorders: a systematic review. Arch Phys Med Rehabil. 2011;92:1044–1052. doi: 10.1016/j.apmr.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 151.Kalia L.V., Lang A.E. Parkinson's disease. The Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 152.Ferraz D.D., Trippo K.V., Duarte G.P., Neto M.G., Bernardes Santos K.O., Filho J.O. The effects of functional training, bicycle exercise, and exergaming on walking capacity of elderly patients with Parkinson disease: a pilot randomized controlled single-blinded trial. Arch Phys Med Rehabil. 2018;99:826–833. doi: 10.1016/j.apmr.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 153.Uc E.Y., Doerschug K.C., Magnotta V., Dawson J.D., Thomsen T.R., Kline J.N. Phase I/II randomized trial of aerobic exercise in Parkinson disease in a community setting. Neurology. 2014;83:413–425. doi: 10.1212/WNL.0000000000000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cheng S.P., Yang C.Y., Tang F.I., Chen I.J. Training effects of a 12-week walking program on Parkinson disease patients and community-dwelling older adults. NeuroRehabilitation. 2013;32:967–976. doi: 10.3233/NRE-130920. [DOI] [PubMed] [Google Scholar]
- 155.Tabak R., Aquije G., Fisher B.E. Aerobic exercise to improve executive function in Parkinson disease: a case series. J Neurol Phys Ther. 2013;37:58–64. doi: 10.1097/NPT.0b013e31829219bc. [DOI] [PubMed] [Google Scholar]
- 156.Tseng I.J., Yuan R.Y., Jeng C. Treadmill training improves forward and backward gait in early Parkinson disease. Am J Phys Med Rehabil. 2015;94:811–819. doi: 10.1097/PHM.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 157.Miyasato R., Silva-Batista C., Pecanha T., Low D.A., de Mello M.T., Piemonte M.E. Cardiovascular responses during resistance exercise in patients with Parkinson disease. PM R. 2018;10:1145–1152. doi: 10.1016/j.pmrj.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 158.Rafferty M.R., Prodoehl J., Robichaud J.A., David F.J., Poon C., Goelz L.C. Effects of 2 years of exercise on gait impairment in people with Parkinson disease: the PRET-PD randomized trial. J Neurol Phys Ther. 2017;41:21–30. doi: 10.1097/NPT.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Trigueiro L.C., Gama G.L., Simao C.R., Sousa A.V., Godeiro Junior Cde O., Lindquist A.R. Effects of treadmill training with load on gait in Parkinson disease: a randomized controlled clinical trial. Am J Phys Med Rehabil. 2015;94:830–837. doi: 10.1097/PHM.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 160.Zhang T.Y., Hu Y., Nie Z.Y., Jin R.X., Chen F., Guan Q. Effects of tai chi and multimodal exercise training on movement and balance function in mild to moderate idiopathic Parkinson disease. Am J Phys Med Rehabil. 2015;94:921–929. doi: 10.1097/PHM.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 161.de Oliveira R.T., Felippe L.A., Bucken Gobbi L.T., Barbieri F.A., Christofoletti G. Benefits of exercise on the executive functions in people with Parkinson disease: a controlled clinical trial. Am J Phys Med Rehabil. 2017;96:301–306. doi: 10.1097/PHM.0000000000000612. [DOI] [PubMed] [Google Scholar]
- 162.King L.A., Wilhelm J., Chen Y., Blehm R., Nutt J., Chen Z. Effects of group, individual, and home exercise in persons with Parkinson disease: a randomized clinical trial. J Neurol Phys Ther. 2015;39:204–212. doi: 10.1097/NPT.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Carroll L.M., Volpe D., Morris M.E., Saunders J., Clifford A.M. Aquatic exercise therapy for people with Parkinson disease: a randomized controlled trial. Arch Phys Med Rehabil. 2017;98:631–638. doi: 10.1016/j.apmr.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 164.Dayalu P., Albin R.L. Huntington disease: pathogenesis and treatment. Neurol Clin. 2015;33:101–114. doi: 10.1016/j.ncl.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 165.Busse M., Quinn L., Dawes H., Jones C., Kelson M., Poile V. Supporting physical activity engagement in people with Huntington's disease (ENGAGE-HD): study protocol for a randomized controlled feasibility trial. Trials. 2014;15:487. doi: 10.1186/1745-6215-15-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Frese S., Petersen J.A., Ligon-Auer M., Mueller S.M., Mihaylova V., Gehrig S.M. Exercise effects in Huntington disease. J Neurol. 2017;264:32–39. doi: 10.1007/s00415-016-8310-1. [DOI] [PubMed] [Google Scholar]
- 167.Mueller S.M., Gehrig S.M., Petersen J.A., Frese S., Mihaylova V., Ligon-Auer M. Effects of endurance training on skeletal muscle mitochondrial function in Huntington disease patients. Orphanet J Rare Dis. 2017;12:184. doi: 10.1186/s13023-017-0740-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Busse M., Quinn L., Debono K., Jones K., Collett J., Playle R. A randomized feasibility study of a 12-week community-based exercise program for people with Huntington's disease. J Neurol Phys Ther. 2013;37:149–158. doi: 10.1097/NPT.0000000000000016. [DOI] [PubMed] [Google Scholar]
- 169.Quinn L., Hamana K., Kelson M., Dawes H., Collett J., Townson J. A randomized, controlled trial of a multi-modal exercise intervention in Huntington's disease. Parkinsonism Relat Disord. 2016;31:46–52. doi: 10.1016/j.parkreldis.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 170.Quinn L., Debono K., Dawes H., Rosser A.E., Nemeth A.H., Rickards H. Task-specific training in Huntington disease: a randomized controlled feasibility trial. Physical Therapy. 2014;94:1555–1568. doi: 10.2522/ptj.20140123. [DOI] [PubMed] [Google Scholar]
- 171.Querfurth H.L.F. Mechanisms of disease Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 172.Hernandez S.S., Sandreschi P.F., da Silva F.C., Arancibia B.A., da Silva R., Gutierres P.J. What are the benefits of exercise for Alzheimer's disease? A systematic review of the past 10 years. J Aging Phys Act. 2015;23:659–668. doi: 10.1123/japa.2014-0180. [DOI] [PubMed] [Google Scholar]
- 173.Vidoni E.D., Perales J., Alshehri M., Giles A.M., Siengsukon C.F., Burns J.M. Aerobic exercise sustains performance of instrumental activities of daily living in early-stage Alzheimer's disease. J Geriatr Phys Ther. 2019;42:E129–34. doi: 10.1519/JPT.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ries J.D., Hutson J., Maralit L.A., Brown M.B. Group balance training specifically designed for individuals with Alzheimer disease: impact on Berg Balance Scale, Timed Up and Go, Gait Speed, and Mini-Mental Status Examination. J Geriatr Phys Ther. 2015;38:183–193. doi: 10.1519/JPT.0000000000000030. [DOI] [PubMed] [Google Scholar]
- 175.Santana-Sosa E., Barriopedro M.I., Lopez-Mojares L.M., Perez M., Lucia A. Exercise training is beneficial for Alzheimer's patients. Int J Sports Med. 2008;29:845–850. doi: 10.1055/s-2008-1038432. [DOI] [PubMed] [Google Scholar]
- 176.Hill K.D., LoGiudice D., Lautenschlager N.T., Said C.M., Dodd K.J., Suttanon P. Effectiveness of balance training exercise in people with mild to moderate severity Alzheimer's disease: protocol for a randomised trial. BMC Geriatr. 2009;9:29. doi: 10.1186/1471-2318-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ohman H., Savikko N., Strandberg T.E., Kautiainen H., Raivio M.M., Laakkonen M.L. Effects of exercise on cognition: the Finnish Alzheimer Disease Exercise Trial: a randomized, controlled trial. J Am Geriatr Soc. 2016;64:731–738. doi: 10.1111/jgs.14059. [DOI] [PubMed] [Google Scholar]
- 178.Pitkala K.H., Poysti M.M., Laakkonen M.L., Tilvis R.S., Savikko N., Kautiainen H. Effects of the Finnish Alzheimer disease exercise trial (FINALEX): a randomized controlled trial. JAMA Intern Med. 2013;173:894–901. doi: 10.1001/jamainternmed.2013.359. [DOI] [PubMed] [Google Scholar]
- 179.Lepine J.P., Briley M. The increasing burden of depression. Neuropsychiatr Dis Treat. 2011;7(Suppl. 1):S3–7. doi: 10.2147/NDT.S19617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gaynes B.N., Burns B.J., Tweed D.L., Erickson P. Depression and health-related quality of life. J Nerv Ment Dis. 2002;190:799–806. doi: 10.1097/00005053-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 181.Schuch F.B., Deslandes A.C., Stubbs B., Gosmann N.P., Silva C.T., Fleck M.P. Neurobiological effects of exercise on major depressive disorder: a systematic review. Neurosci Biobehav Rev. 2016;61:1–11. doi: 10.1016/j.neubiorev.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 182.Toups M., Carmody T., Greer T., Rethorst C., Grannemann B., Trivedi M.H. Exercise is an effective treatment for positive valence symptoms in major depression. J Affect Disord. 2017;209:188–194. doi: 10.1016/j.jad.2016.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zanetidou S., Belvederi Murri M., Menchetti M., Toni G., Asioli F., Bagnoli L. Physical exercise for late-life depression: customizing an intervention for primary care. J Am Geriatr Soc. 2017;65:348–355. doi: 10.1111/jgs.14525. [DOI] [PubMed] [Google Scholar]
- 184.Neviani F., Belvederi Murri M., Mussi C., Triolo F., Toni G., Simoncini E. Physical exercise for late life depression: effects on cognition and disability. Int Psychogeriatr. 2017;29:1105–1112. doi: 10.1017/S1041610217000576. [DOI] [PubMed] [Google Scholar]
- 185.Schuch F.B., Vasconcelos-Moreno M.P., Borowsky C., Zimmermann A.B., Rocha N.S., Fleck M.P. Exercise and severe major depression: effect on symptom severity and quality of life at discharge in an inpatient cohort. J Psychiatr Res. 2015;61:25–32. doi: 10.1016/j.jpsychires.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 186.Campbell Burton C.A., Murray J., Holmes J., Astin F., Greenwood D., Knapp P. Frequency of anxiety after stroke: a systematic review and meta-analysis of observational studies. Int J Stroke. 2013;8:545–559. doi: 10.1111/j.1747-4949.2012.00906.x. [DOI] [PubMed] [Google Scholar]
- 187.Huang K.L., Su T.P., Chen T.J., Chou Y.H., Bai Y.M. Comorbidity of cardiovascular diseases with mood and anxiety disorder: a population based 4-year study. Psychiatry Clin Neurosci. 2009;63:401–409. doi: 10.1111/j.1440-1819.2009.01974.x. [DOI] [PubMed] [Google Scholar]
- 188.Stein D.J., Scott K.M., de Jonge P., Kessler R.C. Epidemiology of anxiety disorders: from surveys to nosology and back. Dialogues Clin Neurosci. 2017;19:127–136. doi: 10.31887/DCNS.2017.19.2/dstein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.LeBouthillier D.M., Asmundson G.J. A single bout of aerobic exercise reduces anxiety sensitivity but not intolerance of uncertainty or distress tolerance: a randomized controlled trial. Cogn Behav Ther. 2015;44:252–263. doi: 10.1080/16506073.2015.1028094. [DOI] [PubMed] [Google Scholar]
- 190.Ma W.F., Wu P.L., Su C.H., Yang T.C. The effects of an exercise program on anxiety levels and metabolic functions in patients with anxiety disorders. Biol Res Nurs. 2016;19:258–268. doi: 10.1177/1099800416672581. [DOI] [PubMed] [Google Scholar]
- 191.Broman-Fulks J.J., Kelso K., Zawilinski L. Effects of a single bout of aerobic exercise versus resistance training on cognitive vulnerabilities for anxiety disorders. Cogn Behav Ther. 2015;44:240–251. doi: 10.1080/16506073.2015.1020448. [DOI] [PubMed] [Google Scholar]
- 192.Herring M.P., Jacob M.L., Suveg C., Dishman R.K., O'Connor P.J. Feasibility of exercise training for the short-term treatment of generalized anxiety disorder: a randomized controlled trial. Psychother Psychosom. 2012;81:21–28. doi: 10.1159/000327898. [DOI] [PubMed] [Google Scholar]
- 193.Zhu X.D., Lei X.P., Dong W.B. Resveratrol as a potential therapeutic drug for respiratory system diseases. Drug Des Devel Ther. 2017;11:3591–3598. doi: 10.2147/DDDT.S148868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Miyamoto N., Senjyu H., Tanaka T., Asai M., Yanagita Y., Yano Y. Pulmonary rehabilitation improves exercise capacity and dyspnea in air pollution-related respiratory disease. Tohoku J Exp Med. 2014;232:1–8. doi: 10.1620/tjem.232.1. [DOI] [PubMed] [Google Scholar]
- 195.Williams P.T. Dose-response relationship between exercise and respiratory disease mortality. Med Sci Sports Exerc. 2014;46:711–717. doi: 10.1249/MSS.0000000000000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.O'Donnell D.E., Gebke K.B. Examining the role of activity, exercise, and pharmacology in mild COPD. Postgrad Med. 2014;126:135–145. doi: 10.3810/pgm.2014.09.2808. [DOI] [PubMed] [Google Scholar]
- 197.Boeselt T., Nell C., Lutteken L., Kehr K., Koepke J., Apelt S. Benefits of high-intensity exercise training to patients with chronic obstructive pulmonary disease: a controlled study. Respiration. 2017;93:301–310. doi: 10.1159/000464139. [DOI] [PubMed] [Google Scholar]
- 198.Neunhauserer D., Steidle-Kloc E., Weiss G., Kaiser B., Niederseer D., Hartl S. Supplemental oxygen during high-intensity exercise training in nonhypoxemic chronic obstructive pulmonary disease. Am J Med. 2016;129:1185–1193. doi: 10.1016/j.amjmed.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 199.McNamara R.J., McKeough Z.J., McKenzie D.K., Alison J.A. Acceptability of the aquatic environment for exercise training by people with chronic obstructive pulmonary disease with physical comorbidities: additional results from a randomised controlled trial. Physiotherapy. 2015;101:187–192. doi: 10.1016/j.physio.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 200.Torres-Sanchez I., Valenza M.C., Cabrera-Martos I., Lopez-Torres I., Benitez-Feliponi A., Conde-Valero A. Effects of an exercise intervention in frail older patients with chronic obstructive pulmonary disease hospitalized due to an exacerbation: a randomized controlled trial. COPD. 2017;14:37–42. doi: 10.1080/15412555.2016.1209476. [DOI] [PubMed] [Google Scholar]
- 201.Niu R., He R., Luo B.L., Hu C. The effect of Tai Chi on chronic obstructive pulmonary disease: a pilot randomised study of lung function, exercise capacity and diaphragm strength. Heart Lung Circ. 2014;23:347–352. doi: 10.1016/j.hlc.2013.10.057. [DOI] [PubMed] [Google Scholar]
- 202.Wootton S.L., Ng L.W., McKeough Z.J., Jenkins S., Hill K., Eastwood P.R. Ground-based walking training improves quality of life and exercise capacity in COPD. Eur Respir J. 2014;44:885–894. doi: 10.1183/09031936.00078014. [DOI] [PubMed] [Google Scholar]
- 203.Dowman L., Hill C.J., Holland A.E. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst Rev. 2014;(10) doi: 10.1002/14651858.CD006322.pub3. [DOI] [PubMed] [Google Scholar]
- 204.Dowman L.M., McDonald C.F., Hill C.J., Lee A.L., Barker K., Boote C. The evidence of benefits of exercise training in interstitial lung disease: a randomised controlled trial. Thorax. 2017;72:610–619. doi: 10.1136/thoraxjnl-2016-208638. [DOI] [PubMed] [Google Scholar]
- 205.Dowman L., McDonald C.F., Hill C., Lee A., Barker K., Boote C. The benefits of exercise training in interstitial lung disease: protocol for a multicentre randomised controlled trial. BMC Pulm Med. 2013;13:8. doi: 10.1186/1471-2466-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Langer D. Rehabilitation in patients before and after lung transplantation. Respiration. 2015;89:353–362. doi: 10.1159/000430451. [DOI] [PubMed] [Google Scholar]
- 207.Gloeckl R., Heinzelmann I., Seeberg S., Damisch T., Hitzl W., Kenn K. Effects of complementary whole-body vibration training in patients after lung transplantation: a randomized, controlled trial. J Heart Lung Transplant. 2015;34:1455–1461. doi: 10.1016/j.healun.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 208.Langer D., Burtin C., Schepers L., Ivanova A., Verleden G., Decramer M. Exercise training after lung transplantation improves participation in daily activity: a randomized controlled trial. Am J Transplant. 2012;12:1584–1592. doi: 10.1111/j.1600-6143.2012.04000.x. [DOI] [PubMed] [Google Scholar]
- 209.Howden E.J., Leano R., Petchey W., Coombes J.S., Isbel N.M., Marwick T.H. Effects of exercise and lifestyle intervention on cardiovascular function in CKD. Clin J Am Soc Nephrol. 2013;8:1494–1501. doi: 10.2215/CJN.10141012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Roshanravan B., Robinson-Cohen C., Patel K.V., Ayers E., Littman A.J., de Boer I.H. Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol. 2013;24:822–830. doi: 10.1681/ASN.2012070702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Rhee E.P., Clish C.B., Wenger J., Roy J., Elmariah S., Pierce K.A. Metabolomics of chronic kidney disease progression: a case-control analysis in the chronic renal insufficiency cohort study. Am J Nephrol. 2016;43:366–374. doi: 10.1159/000446484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Headley S., Germain M., Wood R., Joubert J., Milch C., Evans E. Short-term aerobic exercise and vascular function in CKD stage 3: a randomized controlled trial. Am J Kidney Dis. 2014;64:222–229. doi: 10.1053/j.ajkd.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Watson E.L., Greening N.J., Viana J.L., Aulakh J., Bodicoat D.H., Barratt J. Progressive resistance exercise training in CKD: a feasibility study. Am J Kidney Dis. 2015;66:249–257. doi: 10.1053/j.ajkd.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 214.Hamada M., Yasuda Y., Kato S., Arafuka H., Goto M., Hayashi M. The effectiveness and safety of modest exercise in Japanese patients with chronic kidney disease: a single-armed interventional study. Clin Exp Nephrol. 2016;20:204–211. doi: 10.1007/s10157-015-1147-6. [DOI] [PubMed] [Google Scholar]
- 215.Hiraki K., Shibagaki Y., Izawa K.P., Hotta C., Wakamiya A., Sakurada T. Effects of home-based exercise on pre-dialysis chronic kidney disease patients: a randomized pilot and feasibility trial. BMC Nephrol. 2017;18:198. doi: 10.1186/s12882-017-0613-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Howden E.J., Coombes J.S., Strand H., Douglas B., Campbell K.L., Isbel N.M. Exercise training in CKD: efficacy, adherence, and safety. Am J Kidney Dis. 2015;65:583–591. doi: 10.1053/j.ajkd.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 217.Leehey D.J., Collins E., Kramer H.J., Cooper C., Butler J., McBurney C. Structured exercise in obese diabetic patients with chronic kidney disease: a randomized controlled trial. Am J Nephrol. 2016;44:54–62. doi: 10.1159/000447703. [DOI] [PubMed] [Google Scholar]
- 218.Van Craenenbroeck A.H., Van Craenenbroeck E.M., Van Ackeren K., Vrints C.J., Conraads V.M., Verpooten G.A. Effect of moderate aerobic exercise training on endothelial function and arterial stiffness in CKD stages 3-4: a randomized controlled trial. Am J Kidney Dis. 2015;66:285–296. doi: 10.1053/j.ajkd.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 219.Aoike D.T., Baria F., Kamimura M.A., Ammirati A., de Mello M.T., Cuppari L. Impact of home-based aerobic exercise on the physical capacity of overweight patients with chronic kidney disease. Int Urol Nephrol. 2015;47:359–367. doi: 10.1007/s11255-014-0894-8. [DOI] [PubMed] [Google Scholar]
- 220.Baria F., Kamimura M.A., Aoike D.T., Ammirati A., Rocha M.L., de Mello M.T. Randomized controlled trial to evaluate the impact of aerobic exercise on visceral fat in overweight chronic kidney disease patients. Nephrol Dial Transplant. 2014;29:857–864. doi: 10.1093/ndt/gft529. [DOI] [PubMed] [Google Scholar]
- 221.O'Connor E.M., Koufaki P., Mercer T.H., Lindup H., Nugent E., Goldsmith D. Long-term pulse wave velocity outcomes with aerobic and resistance training in kidney transplant recipients - a pilot randomised controlled trial. PLoS One. 2017;12 doi: 10.1371/journal.pone.0171063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Macdonald J.H., Kirkman D., Jibani M. Kidney transplantation: a systematic review of interventional and observational studies of physical activity on intermediate outcomes. Adv Chronic Kidney Dis. 2009;16:482–500. doi: 10.1053/j.ackd.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 223.Greenwood S.A., Koufaki P., Mercer T.H., Rush R., O'Connor E., Tuffnell R. Aerobic or resistance training and pulse wave velocity in kidney transplant recipients: a 12-week pilot randomized controlled trial (the Exercise in Renal Transplant (ExeRT) trial) Am J Kidney Dis. 2015;66:689–698. doi: 10.1053/j.ajkd.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 224.Kastelz A., Tzvetanov I.G., Fernhall B., Shetty A., Gallon L., West-Thielke P. Experimental protocol of a randomized controlled clinical trial investigating the effects of personalized exercise rehabilitation on kidney transplant recipients' outcomes. Contemp Clin Trials. 2015;45:170–176. doi: 10.1016/j.cct.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 225.Siegel R., Ma J., Zou Z., Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 226.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 227.Jones L.W., Eves N.D., Haykowsky M., Freedland S.J., Mackey J.R. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol. 2009;10:598–605. doi: 10.1016/S1470-2045(09)70031-2. [DOI] [PubMed] [Google Scholar]
- 228.Cheema B.S., Kilbreath S.L., Fahey P.P., Delaney G.P., Atlantis E. Safety and efficacy of progressive resistance training in breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2014;148:249–268. doi: 10.1007/s10549-014-3162-9. [DOI] [PubMed] [Google Scholar]
- 229.Wiskemann J., Schmidt M.E., Klassen O., Debus J., Ulrich C.M., Potthoff K. Effects of 12-week resistance training during radiotherapy in breast cancer patients. Scand J Med Sci Sports. 2017;27:1500–1510. doi: 10.1111/sms.12777. [DOI] [PubMed] [Google Scholar]
- 230.Schmitz K.H., Williams N.I., Kontos D., Domchek S., Morales K.H., Hwang W.T. Dose-response effects of aerobic exercise on estrogen among women at high risk for breast cancer: a randomized controlled trial. Breast Cancer Res Treat. 2015;154:309–318. doi: 10.1007/s10549-015-3604-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Courneya K.S., McNeil J., O'Reilly R., Morielli A.R., Friedenreich C.M. Dose-response effects of aerobic exercise on quality of life in postmenopausal women: results from the breast cancer and exercise trial in Alberta (BETA) Ann Behav Med. 2017;51:356–364. doi: 10.1007/s12160-016-9859-8. [DOI] [PubMed] [Google Scholar]
- 232.Brown J.C., Kontos D., Schnall M.D., Wu S., Schmitz K.H. The Dose-response effects of aerobic exercise on body composition and breast tissue among women at high risk for breast cancer: a randomized trial. Cancer Prev Res (Phila) 2016;9:581–588. doi: 10.1158/1940-6207.CAPR-15-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Park J.H. The effects of complex exercise on shoulder range of motion and pain for women with breast cancer-related lymphedema: a single-blind, randomized controlled trial. Breast Cancer. 2017;24:608–614. doi: 10.1007/s12282-016-0747-7. [DOI] [PubMed] [Google Scholar]
- 234.Ligibel J.A., Giobbie-Hurder A., Shockro L., Campbell N., Partridge A.H., Tolaney S.M. Randomized trial of a physical activity intervention in women with metastatic breast cancer. Cancer. 2016;122:1169–1177. doi: 10.1002/cncr.29899. [DOI] [PubMed] [Google Scholar]
- 235.Schmidt M.E., Wiskemann J., Armbrust P., Schneeweiss A., Ulrich C.M., Steindorf K. Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: a randomized controlled trial. Int J Cancer. 2015;137:471–480. doi: 10.1002/ijc.29383. [DOI] [PubMed] [Google Scholar]
- 236.Steindorf K., Schmidt M.E., Klassen O., Ulrich C.M., Oelmann J., Habermann N. Randomized, controlled trial of resistance training in breast cancer patients receiving adjuvant radiotherapy: results on cancer-related fatigue and quality of life. Ann Oncol. 2014;25:2237–2243. doi: 10.1093/annonc/mdu374. [DOI] [PubMed] [Google Scholar]
- 237.Schmidt M.E., Meynkohn A., Habermann N., Wiskemann J., Oelmann J., Hof H. Resistance exercise and inflammation in breast cancer patients undergoing adjuvant radiation therapy: mediation analysis from a randomized, controlled intervention trial. Int J Radiat Oncol Biol Phys. 2016;94:329–337. doi: 10.1016/j.ijrobp.2015.10.058. [DOI] [PubMed] [Google Scholar]
- 238.Travier N., Velthuis M.J., Steins Bisschop C.N., van den Buijs B., Monninkhof E.M., Backx F. Effects of an 18-week exercise programme started early during breast cancer treatment: a randomised controlled trial. BMC Med. 2015;13:121. doi: 10.1186/s12916-015-0362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Courneya K.S., McKenzie D.C., Mackey J.R., Gelmon K., Friedenreich C.M., Yasui Y. Subgroup effects in a randomised trial of different types and doses of exercise during breast cancer chemotherapy. Br J Cancer. 2014;111:1718–1725. doi: 10.1038/bjc.2014.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Courneya K.S., Segal R.J., Mackey J.R., Gelmon K., Friedenreich C.M., Yasui Y. Effects of exercise dose and type on sleep quality in breast cancer patients receiving chemotherapy: a multicenter randomized trial. Breast Cancer Res Treat. 2014;144:361–369. doi: 10.1007/s10549-014-2883-0. [DOI] [PubMed] [Google Scholar]
- 241.Courneya K.S., McKenzie D.C., Mackey J.R., Gelmon K., Friedenreich C.M., Yasui Y. Effects of exercise dose and type during breast cancer chemotherapy: multicenter randomized trial. J Natl Cancer Inst. 2013;105:1821–1832. doi: 10.1093/jnci/djt297. [DOI] [PubMed] [Google Scholar]
- 242.Adams S.C., Segal R.J., McKenzie D.C., Vallerand J.R., Morielli A.R., Mackey J.R. Impact of resistance and aerobic exercise on sarcopenia and dynapenia in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. Breast Cancer Res Treat. 2016;158:497–507. doi: 10.1007/s10549-016-3900-2. [DOI] [PubMed] [Google Scholar]
- 243.Siegel R., Desantis C., Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 244.Siegel R., Desantis C., Virgo K., Stein K., Mariotto A., Smith T. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;64:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 245.Brown J.C., Troxel A.B., Ky B., Damjanov N., Zemel B.S., Rickels M.R. A randomized phase II dose-response exercise trial among colon cancer survivors: purpose, study design, methods, and recruitment results. Contemp Clin Trials. 2016;47:366–375. doi: 10.1016/j.cct.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Cantarero-Villanueva I., Sanchez-Jimenez A., Galiano-Castillo N., Diaz-Rodriguez L., Martin-Martin L., Arroyo-Morales M. Effectiveness of lumbopelvic exercise in colon cancer survivors: a randomized controlled clinical trial. Med Sci Sports Exerc. 2016;48:1438–1446. doi: 10.1249/MSS.0000000000000917. [DOI] [PubMed] [Google Scholar]
- 247.Brown J.C., Zemel B.S., Troxel A.B., Rickels M.R., Damjanov N., Ky B. Dose-response effects of aerobic exercise on body composition among colon cancer survivors: a randomised controlled trial. Br J Cancer. 2017;117:1614–1620. doi: 10.1038/bjc.2017.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Brown J.C., Troxel A.B., Ky B., Damjanov N., Zemel B.S., Rickels M.R. Dose-response effects of aerobic exercise among colon cancer survivors: a randomized phase II trial. Clin Colorectal Cancer. 2018;17:32–40. doi: 10.1016/j.clcc.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Brown J.C., Damjanov N., Courneya K.S., Troxel A.B., Zemel B.S., Rickels M.R. A randomized dose-response trial of aerobic exercise and health-related quality of life in colon cancer survivors. Psychooncology. 2018;27:1221–1228. doi: 10.1002/pon.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Van Vulpen J.K., Velthuis M.J., Steins Bisschop C.N., Travier N., Van Den Buijs B.J., Backx F.J. Effects of an exercise program in colon cancer patients undergoing chemotherapy. Med Sci Sports Exerc. 2016;48:767–775. doi: 10.1249/MSS.0000000000000855. [DOI] [PubMed] [Google Scholar]
- 251.Ahn K.Y., Hur H., Kim D.H., Min J., Jeong D.H., Chu S.H. The effects of inpatient exercise therapy on the length of hospital stay in stages I-III colon cancer patients: randomized controlled trial. Int J Colorectal Dis. 2013;28:643–651. doi: 10.1007/s00384-013-1665-1. [DOI] [PubMed] [Google Scholar]
- 252.Hanchette C.L., Schwartz G.G. Geographic patterns of prostate cancer mortality. Evidence for a protective effect of ultraviolet radiation. Cancer. 1992;70:2861–2869. doi: 10.1002/1097-0142(19921215)70:12<2861::aid-cncr2820701224>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 253.Konstantinos H. Prostate Cancer in the Elderly. Int Urol Nephrol. 2005;37:797–806. doi: 10.1007/s11255-005-0402-2. [DOI] [PubMed] [Google Scholar]
- 254.Gaskin C.J., Craike M., Mohebbi M., Courneya K.S., Livingston P.M. A clinician referral and 12-week exercise training program for men with prostate cancer: outcomes to 12 months of the ENGAGE cluster randomized controlled trial. J Phys Act Health. 2017;14:353–359. doi: 10.1123/jpah.2016-0431. [DOI] [PubMed] [Google Scholar]
- 255.Pernar C.H., Fall K., Rider J.R., Markt S.C., Adami H.O., Andersson S.O. A walking intervention among men with prostate cancer: a pilot study. Clin Genitourin Cancer. 2017;15:e1021–e1028. doi: 10.1016/j.clgc.2017.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Winters-Stone K.M., Dieckmann N., Maddalozzo G.F., Bennett J.A., Ryan C.W., Beer T.M. Resistance exercise reduces body fat and insulin during androgen-deprivation therapy for prostate cancer. Oncol Nurs Forum. 2015;42:348–356. doi: 10.1188/15.ONF.348-356. [DOI] [PubMed] [Google Scholar]
- 257.Winters-Stone K.M., Dobek J.C., Bennett J.A., Dieckmann N.F., Maddalozzo G.F., Ryan C.W. Resistance training reduces disability in prostate cancer survivors on androgen deprivation therapy: evidence from a randomized controlled trial. Arch Phys Med Rehabil. 2015;96:7–14. doi: 10.1016/j.apmr.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Cormie P., Galvao D.A., Spry N., Joseph D., Chee R., Taaffe D.R. Can supervised exercise prevent treatment toxicity in patients with prostate cancer initiating androgen-deprivation therapy: a randomised controlled trial. BJU Int. 2015;115:256–266. doi: 10.1111/bju.12646. [DOI] [PubMed] [Google Scholar]
- 259.Wall B.A., GALVãO D.A., Fatehee N., Taaffe D.R., Spry N., Joseph D. Exercise improves VO2max and body composition in androgen deprivation therapy-treated prostate cancer patients. Med Sci Sports Exerc. 2017;49:1503–1510. doi: 10.1249/MSS.0000000000001277. [DOI] [PubMed] [Google Scholar]
- 260.Buffart L.M., Newton R.U., Chinapaw M.J., Taaffe D.R., Spry N.A., Denham J.W. The effect, moderators, and mediators of resistance and aerobic exercise on health-related quality of life in older long-term survivors of prostate cancer. Cancer. 2015;121:2821–2830. doi: 10.1002/cncr.29406. [DOI] [PubMed] [Google Scholar]
- 261.Cormie P., Newton R.U., Taaffe D.R., Spry N., Joseph D., Akhlil Hamid M. Exercise maintains sexual activity in men undergoing androgen suppression for prostate cancer: a randomized controlled trial. Prostate Cancer Prostatic Dis. 2013;16:170–175. doi: 10.1038/pcan.2012.52. [DOI] [PubMed] [Google Scholar]
- 262.Buffart L.M., Galvao D.A., Chinapaw M.J., Brug J., Taaffe D.R., Spry N. Mediators of the resistance and aerobic exercise intervention effect on physical and general health in men undergoing androgen deprivation therapy for prostate cancer. Cancer. 2014;120:294–301. doi: 10.1002/cncr.28396. [DOI] [PubMed] [Google Scholar]
- 263.Newton R.U., Taaffe D.R., Spry N., Gardiner R.A., Levin G., Wall B. A phase III clinical trial of exercise modalities on treatment side-effects in men receiving therapy for prostate cancer. BMC Cancer. 2009;9:210. doi: 10.1186/1471-2407-9-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Martin E.A., Battaglini C.L., Hands B., Naumann F. Higher-intensity exercise results in more sustainable improvements for VO2peak for breast and prostate cancer survivors. Oncol Nurs Forum. 2015;42:241–249. doi: 10.1188/15.ONF.42-03AP. [DOI] [PubMed] [Google Scholar]
- 265.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 266.Fairey A.S., Courneya K.S., Field C.J., Mackey J.R. Physical exercise and immune system function in cancer survivors: a comprehensive review and future directions. Cancer. 2002;94:539–551. doi: 10.1002/cncr.10244. [DOI] [PubMed] [Google Scholar]
- 267.Chen H.M., Tsai C.M., Wu Y.C., Lin K.C., Lin C.C. Randomised controlled trial on the effectiveness of home-based walking exercise on anxiety, depression and cancer-related symptoms in patients with lung cancer. Br J Cancer. 2015;112:438–445. doi: 10.1038/bjc.2014.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Liu J., Chen P., Wang R., Yuan Y., Wang X., Li C. Effect of Tai Chi on mononuclear cell functions in patients with non-small cell lung cancer. BMC Complement Altern Med. 2015;15:3. doi: 10.1186/s12906-015-0517-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Zhang L.L., Wang S.Z., Chen H.L., Yuan A.Z. Tai Chi exercise for cancer-related fatigue in patients with lung cancer undergoing chemotherapy: a randomized controlled trial. J Pain Symptom Manage. 2016;51:504–511. doi: 10.1016/j.jpainsymman.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 270.Brocki B.C., Andreasen J., Nielsen L.R., Nekrasas V., Gorst-Rasmussen A., Westerdahl E. Short and long-term effects of supervised versus unsupervised exercise training on health-related quality of life and functional outcomes following lung cancer surgery - a randomized controlled trial. Lung Cancer. 2014;83:102–108. doi: 10.1016/j.lungcan.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 271.Jensen W., Oechsle K., Baumann H.J., Mehnert A., Klose H., Bloch W. Effects of exercise training programs on physical performance and quality of life in patients with metastatic lung cancer undergoing palliative chemotherapy–a study protocol. Contemp Clin Trials. 2014;37:120–128. doi: 10.1016/j.cct.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 272.Edbrooke L., Aranda S., Granger C.L., McDonald C.F., Krishnasamy M., Mileshkin L. Benefits of home-based multidisciplinary exercise and supportive care in inoperable non-small cell lung cancer - protocol for a phase II randomised controlled trial. BMC Cancer. 2017;17:663. doi: 10.1186/s12885-017-3651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Edvardsen E., Skjonsberg O.H., Holme I., Nordsletten L., Borchsenius F., Anderssen S.A. High-intensity training following lung cancer surgery: a randomised controlled trial. Thorax. 2015;70:244–250. doi: 10.1136/thoraxjnl-2014-205944. [DOI] [PubMed] [Google Scholar]
- 274.Licker M., Karenovics W., Diaper J., Fresard I., Triponez F., Ellenberger C. Short-term preoperative high-intensity interval training in patients awaiting lung cancer surgery: a randomized controlled trial. J Thorac Oncol. 2017;12:323–333. doi: 10.1016/j.jtho.2016.09.125. [DOI] [PubMed] [Google Scholar]
- 275.Karenovics W., Licker M., Ellenberger C., Christodoulou M., Diaper J., Bhatia C. Short-term preoperative exercise therapy does not improve long-term outcome after lung cancer surgery: a randomized controlled study. Eur J Cardiothorac Surg. 2017;52:47–54. doi: 10.1093/ejcts/ezx030. [DOI] [PubMed] [Google Scholar]
- 276.Pasanen T., Tolvanen S., Heinonen A., Kujala U.M. Exercise therapy for functional capacity in chronic diseases: an overview of meta-analyses of randomised controlled trials. Br J Sports Med. 2017;51:1459–1465. doi: 10.1136/bjsports-2016-097132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.