Abstract
Social jetlag (SJL), the difference in sleep timing between work and free days is a consequence of the discrepancy between the individual’s circadian rhythm and the social clock. SJL is considered a chronic stress factor and has been linked to various health problems. In this field study, we examined for the first time the association between SJL and cardiac regulation during sleep. 33 healthy young men aged 20–26 years participated in the study. The median SJL was used as a cut-off value to assign the participants into two groups with either lower or higher SJL. As a marker of autonomic control we analyzed heart rate variability (HRV) and addressed intra-individual differences between workdays and free days. In subjects with higher SJL, pNN50, an indicator of vagal activity was lower in the first 3 h of sleep on workday as compared to free day (day × sleep block × group, p = 0.015), indicating a more adaptable regulation on free days, when subjects slept according to their own preference. However, in subjects with lower SJL, no HRV differences were found between the two nights. SJL showed correlation with the free day-workday differences of both pNN50 and another vagal index, RMSSD in the first 2 h of sleep (p = 0.023 and 0.047, respectively). In subjects with higher SJL, a different HF power on workdays and free days (p = 0.031) also indicated that a shift in sleep timing is accompanied by an altered parasympathetic activity in the first few hours of sleep. Furthermore, subjective sleep quality on workdays was negatively associated with SJL (p = 0.02), and subjects with higher SJL reported worse sleep quality on workday than on free day (p = 0.027). Taken together, our data call attention on the potential effect of SJL on sleep quality and vagal activity during sleep.
Keywords: autonomic nervous system, heart rate variability, sleep quality, circadian misalignment, social jetlag, cardiovascular risk factor
Introduction
The circadian clock is a fundamental tool enabling organisms to track time internally and thus to adapt their physiology to daily fluctuations in the environment. Circadian time-keeping is organized at the cellular level by the action of molecular oscillators. In mammals, cellular oscillators of peripheral tissues are governed by the central clock located in the suprachiasmatic nucleus of the hypothalamus. A misalignment between the organism’s internal clock and the environmental time is often followed by desynchronization between tissue clocks. Circadian misalignment is considered as a stress factor (Wittmann et al., 2006; Puttonen et al., 2010), and has been linked to the development of various pathological states including cardiovascular diseases (Morris et al., 2017; Strohmaier et al., 2018), metabolic disturbances (Biggi et al., 2008; Vetter et al., 2015), different malignancies (Schernhammer et al., 2006; Papantoniou et al., 2018) and psychological problems (Shields, 2002).
Social jetlag (SJL) is defined as the time difference between the midpoint of sleep on workdays (MSW) and on free days, and it is a consequence of the discrepancy between an individual’s own biological rhythm and the daily timing determined by social constraints (Roenneberg et al., 2003; Wittmann et al., 2006). SJL affects the majority of the adolescent and adult population worldwide. In Europe, more than 30% of the population suffer from an SJL larger than 2 h (Roenneberg et al., 2012). SJL was found to correlate with a higher risk for the development of depression (Levandovski et al., 2011), adverse metabolic changes including obesity and type 2 diabetes (Roenneberg et al., 2012; Rutters et al., 2014; Parsons et al., 2015; Wong et al., 2015; Koopman et al., 2017), and shows association with health-impairing habits such as smoking and excessive caffeine consumption (Wittmann et al., 2006). In addition, our and others’ data indicate that SJL negatively impacts academic performance (Haraszti et al., 2014; Diaz-Morales and Escribano, 2015).
Both epidemiological and laboratory studies indicate that severe forms of circadian misalignment such as shift work or jetlag adversely affect the circulatory system and therefore increase the risk of the development of cardiovascular diseases (Knutsson, 2003; Grimaldi et al., 2016; Morris et al., 2016; Vetter et al., 2016; Hulsegge et al., 2018). SJL is the most frequent type of circadian misalignment, therefore its negative impact on the cardiovascular system would represent a public health concern.
The analysis of heart rate variability (HRV) is a well-established method to assess autonomic cardiac control (Malik, 1996; Laborde et al., 2017). A key advantage of this method is that well-validated programable measuring devices are available for application in field studies without causing any discomfort for the subjects. HRV represents the change in the time interval between successive heartbeats, which is modulated by the sympathetic and parasympathetic nervous system (Malik, 1996). Therefore, HRV is used as a tool to assess autonomic function at the level of the heart. Healthy cardiovascular control is signaled by higher HRV, indicating a stronger parasympathetic activity, whereas low HRV indicates reduced vagal modulation and increased sympathetic activity, which are established risk factors of cardiovascular diseases (Malik, 1996; Thayer et al., 2010). As autonomic control and sleep regulation are interconnected, HRV may be indicative for sleep quality, and efficiency as well. Falling asleep is accompanied by the shift of autonomic balance toward a parasympathetic dominance, measurable in HRV as it increases from wake to slow wave sleep (Elsenbruch et al., 1999; Trinder et al., 2001; Tobaldini et al., 2013; Chouchou and Desseilles, 2014), which is considered to be the most restorative sleep stage (Akerstedt et al., 1997).
In this study, we hypothesized that sleeping out of the endogenous phase on workdays affects cardiovascular regulation and sleep efficiency. To this end we analyzed sleep-related HRV indices and subjective sleep quality in healthy men forming a homogenous sample with respect to age, BMI, and social situation but differing in the extent of SJL, and addressed individual differences between workdays and free days.
Materials and Methods
Participants and Protocol
Participants with regular weekly schedule were recruited via advertisements in social media (Facebook groups) and mailing list of university students. Initially 35 students participated in the study, but two of them were excluded either due to experiencing high emotional stress or for lack of appropriate cooperation. Therefore, data of 33 subjects were involved in the final analysis. The sample was homogenous with respect to age (mean ± SD of 23.2 ± 1.5 years), BMI (mean ± SD of 22.8 ± 2.5), and social situation (university students in Budapest). All subjects were healthy and none of them were taking medication. None of the subjects had a history of sleep disorder or cardiovascular problem and neither one of them experienced jetlag in the last month prior to the study. In a short interview, participants were asked about smoking habits, caffeine consumption, physical activity, blood pressure, and cholesterol levels. Answers did not indicate notable cardiovascular risk factors in any participants. Participants were asked not to undergo extreme physical activity, and to avoid drinking alcohol during the study week. Following the measurements, participants were interviewed about any unexpected events (high psychical and physical stress or any health problems). The study protocol was approved by the Semmelweis University Regional and Institutional Committee of Science and Research Ethics (Ethical approval 170/2016). Participants gave written consent prior to entering the study and were paid for their participation. The study took place in winter (2016/2017) and in the first half of spring (2017) and was paused for 2 weeks after changing to daylight saving time. The number of participants with lower and higher SJL was similar in every month. The study for each subject lasted for a week, during which the participants performed their daily activities according to their usual habit, including waking to an alarm clock on workdays but waking on their own on free days. On a workday (Wednesday) and a free day (Saturday) participants wore an ambulatory heart rate recorder (Actiheart, CamNtech Ltd., United Kingdom) during the night and filled out a sleep quality questionnaire upon waking on the next day. On the days of HRV detection, subjects collected urine samples in the evening before going to bed and in the morning directly after getting up.
Assessment of Sleep Timing
To recruit participants with different SJL and regular weekly schedule, students were asked to fill out the Hungarian version of the Munich Chronotype Questionnaire (MCTQ) (Haraszti et al., 2014). Chronotype was assessed as the midpoint of sleep on free days (MSF) corrected for oversleep on free days (MSFsc), and SJL was calculated by subtracting the midpoint of sleep on workdays (MSW) from MSF, based on Wittmann et al. (2006). During the week of the study each participant kept a sleep diary, based on which the SJL characteristic for the study period was calculated. The mean SJL ± SD was 92.1 ± 52.9 min, which fits well with previously reported SJL values for this age group (Wittmann et al., 2006; Haraszti et al., 2014; Pilz et al., 2018). The median SJL (93 min) was used as a cut-off value to divide the participants into two groups with either lower (SJL ≤ 93 min) or higher SJL (SL > 93 min). Using this cut-off value resulted in a 10-minutes gap between the two groups. There was no significant difference between the two groups regarding age (23.3 ± 1.9 and 23.1 ± 0.9 years mean ± SD for the lower and the higher SJL group, respectively) and BMI (22.1 ± 2.7 and 23.6 ± 2.1 years mean ± SD for the lower and the higher SJL group, respectively). Sleep onset and waking time on the days of HRV detection were estimated by the Actiheart software. These time points were controlled by comparing them with data from the sleep diary. The estimated sleep onset and waking times were used to calculate exact SJL for the days the measurements were performed. This SJL value was used to investigate correlation between SJL and HRV parameters.
Urinary Melatonin
Urine samples collected by the subjects in the evening and morning were stored at −20°C before analysis. Urinary 6-sulfatoxymelatonin, a stable melatonin metabolite, was measured by competitive ELISA according to the instructions of the manufacturer (IBL International). The creatinine concentration of the samples was determined by enzymatic assay (Diagnosticum Zrt., Budapest) to obtain a normalized 6-sulfatoxymelatonin/creatinine ratio (nmol/mmol).
HRV Analysis
The Actiheart monitor was used for the recording of heart rate. Prior to the measurements, participants came into the laboratory where a signal test was performed along with the programing of the device to start recording in the evening. On the next day participants brought the device back for data collection and recharge, before taking it again for the weekend. The Actiheart monitor collects inter-beat-interval (IBI) data, which can be further processed and used for the calculation of HRV parameters. Studies comparing Actiheart and standard ECG recordings did not find any significant difference in IBI detection (Brage et al., 2005; Barreira et al., 2009; Kristiansen et al., 2011). All HRV analyses were performed according to the standards of measurements (Malik, 1996) and the recent recommendations by Laborde et al. (2017). First, reliability of raw data was checked by visual inspection. To ensure a consistent artifact detection and correction, the ARTiiFACT software was used (Kaufmann et al., 2011). According to the international guidelines the data were decomposed into 5 min long segments for analysis of HRV parameters in both the time and frequency domains (Malik, 1996). Segments with more than 10% artifact ratio were excluded. Excluded segments and loss of electrode contact resulted in slightly varying sample sizes in the different analyses. Parameters calculated for the 5 min long segments were averaged for either 1.5- or 2-h blocks, depending on the analysis performed, as indicated in the text and legends.
In the time domain the standard deviation of the normal to normal interval (SDNN), the root mean square of successive differences (RMSSD), and the percentage of successive normal to normal intervals differing by more than 50 ms (pNN50) were calculated using the ARTiiFACT software. For the analysis of HRV in the frequency domain the DADiSP 6.7 software (DSP Development Corp., United States) was used. IBI data were first resampled to 4 Hz using cubic spline interpolation, detrended and tapered with a Hanning window. Power spectral density (PSD) was then calculated with mixed-radix Fast Fourier Transformation, yielding 600 frequency bins in the range of 0–2 Hz. From the average PSD of the successive 5 min segments very low frequency (VLF, 0.003–0.04 Hz), low frequency (LF, 0.04–0.15 Hz), and high frequency (HF, 0.15–0.4 Hz) absolute powers were calculated by numerical integration of the respective frequency ranges. Normalized units of LF (LFnu = LF/LF + HF) were also calculated. Bin-wise and band-wise PSD values were log normalized (natural base logarithm) before the statistical analyses. In our subjects respiratory rate, determined on the basis of the peak in the high frequency region of the FFT plot, was between 0.2 and 0.3 Hz (12–18/min). At this respiratory rate (resting state) HF corresponds to vagal activity, and correction of the HRV parameters for respiration is not required (Larsen et al., 2010; Bertsch et al., 2012; Laborde et al., 2017).
Assessment of Subjective Sleep Quality
For the assessment of subjective sleep quality, we used the Groningen Sleep Quality Scale (GSQS) (Meijman et al., 1988). The Hungarian version of the questionnaire translated from English was validated by Simor et al. (2009). The participants filled out the GSQS questionnaire directly upon waking following the nights with HRV measurements. The questionnaire contains 15 short, true or false questions about the previous night’s sleep. The first question is not evaluated, therefore the GSQS is scored between 0 and 14, a higher score indicating lower quality of sleep.
Statistics
Normality of data (sleep times, HRV measures, questionnaire scores, and 6-sulfatoxymelatonin levels) was assessed by the Kolmogorov-Smirnov test. 6-sulfatoxymelatonin and bin-wise spectral HRV data showed non-normal distribution. For the analysis of the former the non-parametric Mann-Whitney U test was used. Bin-wise spectral HRV data was log normalized to achieve normality. If normal distribution was present, we used parametric statistical procedures as follows. Workday-free day differences were assessed by paired sample t-tests. Group differences were tested by two sample t-tests. For the analysis of HRV data in consecutive time blocks, repeated measures analysis of variance (ANOVA) was performed. Fisher’s LSD test was used as post hoc test. In addition, the association of SJL with sleep quality and HRV was examined by Pearson’s correlation. Log-normalized bin-wise spectral data was expressed as a difference (free day – workday) over the frequency axis (0–0.4 Hz). One-sample t-tests were run in order to test if the differences were significantly deviating from the null hypothesis (difference = 0). Inflation of Type-I error in this case was handled by the procedure of descriptive data analysis (Abt, 1987). Statistical significance threshold was set at p < 0.05. If not otherwise indicated, data are presented as mean ± standard error of the mean (SEM). Statistical analyses were performed using Statistica software version 13.2 (StatSoft, Tulsa, OK, United States).
Results
Basic Rhythm and Sleep Characteristics of the Participants
To control that the participants’ actual sleep schedule during the study week was similar to their usual sleep timing, data from the sleep diary recorded during the study week were compared with data from the MCTQ filled in prior to the study. Reliable correlation for both chronotype (indicated by MSFsc) and SJL were obtained (Supplementary Table S1). Participants were divided into two groups based on the median SJL of the whole sample. Table 1 shows sleep data (timing and duration) for both the workday and free day when subjective sleep quality and HRV were assessed. On workdays the two groups displayed no differences in sleep timing and duration, whereas on free days the group with higher SJL had a significantly later bedtime, sleep onset, and waking time than the other group. Both groups had longer sleep duration on free day compared to workday. As a factor of homeostatic sleep regulation we calculated the time spent awake on the days before the HRV measurements. However, neither a group nor a group x day effect was obtained (Supplementary Table S2).
TABLE 1.
Sleep characteristics of the two groups of participants on the days of the measurements.
| Comparisons | ||||||
| Workday | Free day | Workday | Free day | |||
| Time (hh:mm) | Lower SJL | Higher SJL | Lower SJL | Higher SJL | p | p |
| Bedtime | 23:45 (00:52) | 23:51 (00:49) | 23:56 (00:48) | 01:27 (00:52) | 0.775 | <0.0001 |
| Sleep onset | 23:57 (00:50) | 00:03 (00:51) | 00:09 (00:50) | 01:36 (00:51) | 0.713 | <0.0001 |
| Waking up | 07:09 (00:39) | 07:02 (00:28) | 08:45 (01:09) | 10:00 (01:40) | 0.540 | 0.019 |
| Sleep duration | 07:13 (00:56) | 06:59 (00:43) | 08:35 (01:11) | 08:25 (01:27) | 0.433 | 0.706 |
Comparison of sleep timing and duration between groups with lower and higher SJL on workday and free day. Bedtime was indicated by entries of the sleep diary, whereas sleep onset and waking up were estimated by the Actiheart software and verified by diary data. Mean (SD), two-sample t-test, n = 17 and 16 for the group with lower and higher SJL, respectively.
As a marker of circadian rhythm, urinary levels of 6-sulfatoxymelatonin, the stable metabolite of melatonin, were determined in evening, and morning urinary samples. The marked difference in the levels of 6-sulfatoxymelatonin between the morning and evening samples (mean individual morning/evening ratios ± SEM, 6.75 ± 0.75) confirmed the rhythmic physiology of our participants. However, neither the evening nor the morning excretion of 6-sulfatoxymelatonin was associated with SJL (Supplementary Table S3).
Time-Domain Analysis of HRV
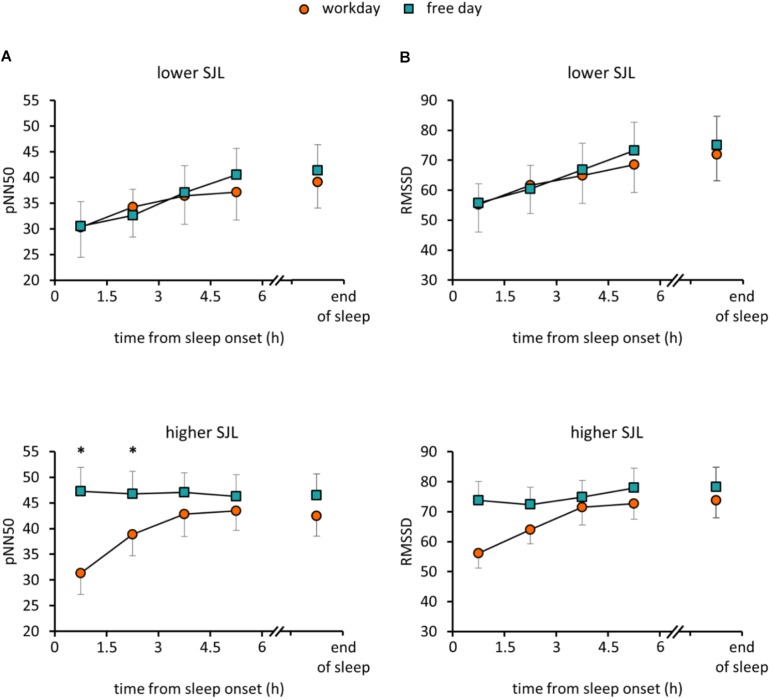
To assess how sleep timing affects autonomic cardiac control during sleep, individual HRV parameters were compared between workdays and free days. Average heart rate (HR) for the whole sleep period did not differ between the two days in either group of participants. However, in the group with higher SJL, SDNN, a basic measure of HRV, was significantly higher on the free day than on the workday (Table 2). To analyze HRV in the course of sleep, we calculated mean HRV parameters for consecutive 1.5-h sleep blocks from the time point of falling asleep. As the minimum sleep length on both days was 6 h, four sleep blocks were analyzed for each night and the data sets of workdays and free days were compared. Figure 1 shows data for RMSSD and pNN50, the time-domain parameters most commonly used for the characterization of vagal control of heart function (Malik, 1996; Laborde et al., 2017). Repeated measures of ANOVA with three factors (group, day, and sleep block) revealed no significant effect of group or day, only sleep block showed significance among the main factors (Supplementary Table S4). Furthermore, a day × sleep block × group interaction was obtained (p = 0.015; F(3,72) = 3.74) for pNN50. While the HRV values nearly overlapped in the group with lower SJL, in the first two time blocks data of subjects with higher SJL showed intra-individual differences as their values were significantly lower on the workday than on the free day (Figure 1A) (higher SJL, workday, first block: 31.3 ± 4.1; second block: 38.9 ± 4.2; free day, first block: 47.3 ± 4.7, second block: 46.8 ± 4.4). Post hoc test did not reveal difference between the two groups in any sleep block. RMSSD showed a similar tendency, as values diverged in the first two blocks in the group with higher SJL, however, the interaction did not reach the level of significance [p = 0.059; F(3,72) = 2.59] (Figure 1B). To exclude that the HRV difference was counteracted by an opposite HRV difference after the sleep period previously analyzed (first 6 h), we performed a further analysis comparing the average HRV in the last 1.5 h of sleep on the workday and in the corresponding sleep segment (same time form sleep onset) on the free day (Figures 1A,B). However, the end-of-sleep HRV data did not display differences between workday and free day.
TABLE 2.
HR and HRV data for the whole sleep period.
| Comparisons | ||||||
| Lower SJL | Higher SJL | Lower SJL | Higher SJL | |||
| Workday | Free day | Workday | Free day | p | p | |
| HR (1/min) | 59.1 (2.1) | 58.1 (1.3) | 57.8 (1.8) | 57.2 (2.1) | 0.520 | 0.739 |
| SDNN | 86.8 (7.3) | 95.1 (6.4) | 91.5 (6.2) | 102.2 (6.9) | 0.088 | 0.018 |
| RMSSD | 61.1 (8.3) | 64.9 (7.4) | 64.1 (4.5) | 72.8 (5.4) | 0.422 | 0.092 |
| pNN50 | 32.8 (5.1) | 35.2 (4.3) | 37.7 (3.6) | 44.2 (3.9) | 0.540 | 0.107 |
Mean HR and HRV data were averaged for the time period from sleep onset to waking up. Data for the workday and the free day were compared within both groups of participants. Mean (SEM), paired t-test, n = 17 and 14 for the group with lower and higher SJL, respectively.
FIGURE 1.
HRV parameters in the course of sleep on workday and free day. PNN50 (A) and RMSSD (B) values were averaged for 1.5-h sleep blocks on workdays and free days for participants with lower (upper panels) and higher SJL (lower panels). The first four consecutive sleep blocks correspond to the first 6 h of sleep. The last sleep block, presented after an axis break, corresponds to the last 1.5 hour of sleep on the workday and the corresponding sleep segment (same time form sleep onset) on the free day. Analyses for the first 6 h of sleep: Repeated measures ANOVA with three levels (day, sleep block, group), post hoc Fisher test, ∗p < 0.05; analyses for the last sleep block (after the axis break): paired t-test; n = 15 for the group with lower SJL and n = 11 for the group with higher SJL.
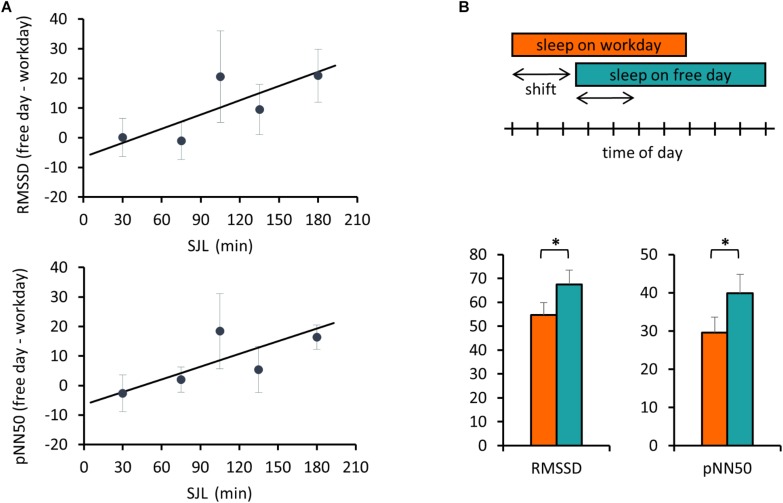
Next, we addressed the relationship between SJL and the HRV difference between the workday and the free day. Analyzing HRV parameters for the first 3 h, corresponding to the first two sleep blocks in Figure 1, a positive correlation between SJL and the free day-workday differences in pNN50 was obtained (Pearson’s r = 0.39, p = 0.033, n = 30). When the analysis was restricted to the first 2 h, both RMSSD and pNN50 differences were positively correlated with SJL (r = 0.365, p = 0.047, n = 30 for RMSSD and r = 0.4135, p = 0.023, n = 30 for pNN50) (Figure 2A).
FIGURE 2.
Free day – workday differences in HRV during sleep are associated with SJL. (A) Correlation of SJL with the difference between free and workday values of RMSSD and pNN50. RMSSD and pNN50 values were averaged for the first 2 h of sleep. Individual free day – workday differences in RMSSD (upper panel) and pNN50 (lower panel) were calculated and correlated with SJL. For better visualization mean values for SJL intervals of either 30 or – in case of low sample number (first and last two intervals) – 60 min are shown. Trend lines for linear regression are shown (upper panel) Pearson’s r = 0.365, p = 0.047, n = 30 and (lower panel) Pearson’s r = 0.4135, p = 0.023, n = 30. (B) Advanced sleep timing on workday is related to a lower HRV. Schematic illustration of the analyzed sleep periods (indicated by the arrows) equal to the shift of sleep onset between work and free day. The boxes represent the time of day from sleep onset until waking (upper panel). RMSSD and pNN50 data were averaged for the shift periods and compared between work and free days (lower panel). Paired t-test, ∗p < 0.05, n = 18.
In the next analysis, we compared the beginning of sleep for a duration equal to the difference of sleep onset between the 2 days, referred as shift of sleep onset (Figure 2B). Data of all participants with more than 25 min shift were involved in this comparison. Both RMSSD and pNN50 parameters were significantly lower on the workday than on the free day (54.7 ± 5.3 and 67.5 ± 6.0, p = 0.016 for RMSSD, and 29.6 ± 4.1 and 39.8 ± 5.0, p = 0.009 for pNN50).
Frequency-Domain Analysis of HRV
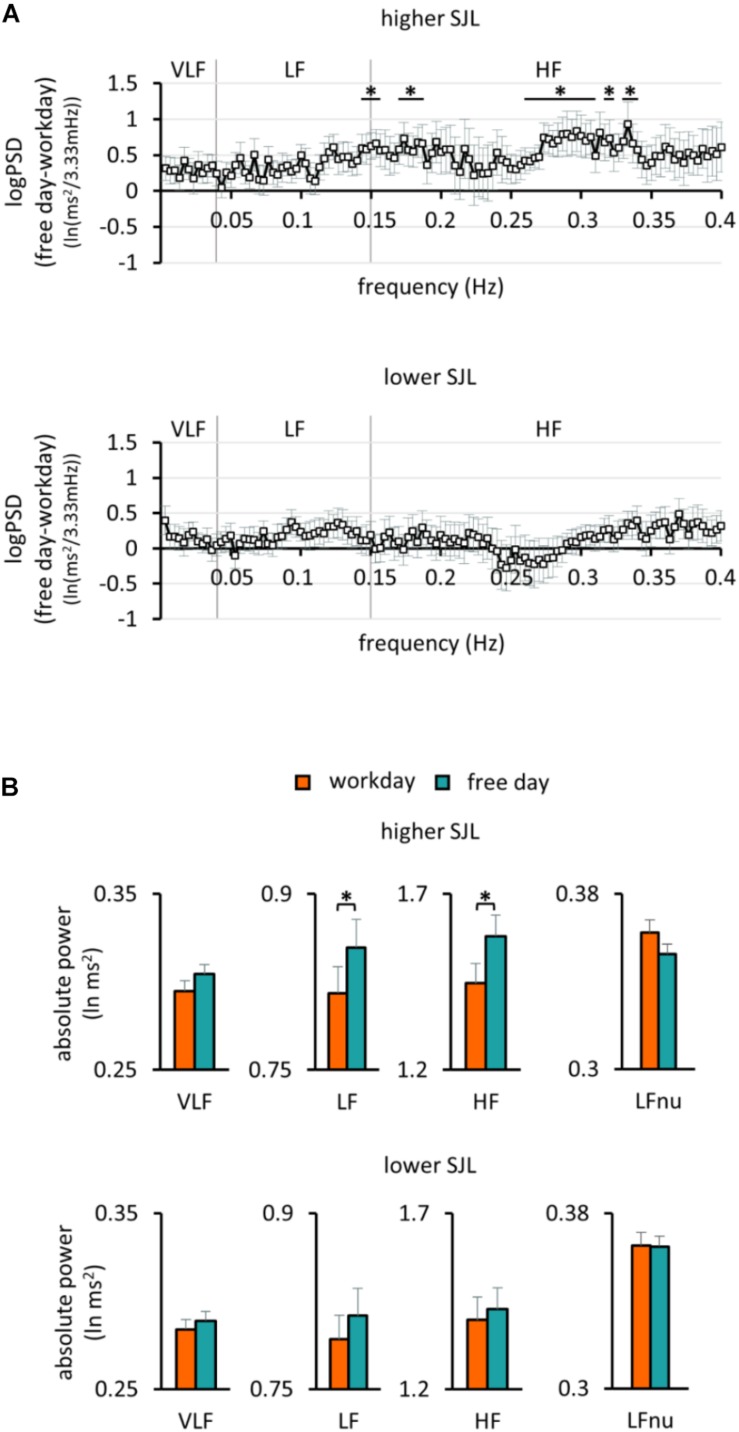
To further characterize the HRV differences in the two groups of participants, frequency domain analyses were also performed. When the PSD of HRV was plotted for the first 2 h of sleep for the work and free day, the curves overlapped in the group with lower SJL, whereas they differed in the group with higher SJL (Supplementary Figure S1). We calculated the individual bin-wise free day – workday differences in the PSD. The difference was significant in several frequency ranges in subjects with higher SJL, but not in those with lower SJL (Figure 3A). The frequency band-based analysis of PSD showed that both LF [0.82 ± 0.02 and 0.85 ± 0.02 (lnms2) on workday and free day, respectively, p = 0.027] and HF [1.45 ± 0.06 and 1.58 ± 0.06 (lnms2) on workday and free day, respectively, p = 0.031], but not VLF [0.29 ± 0.01 and 0.30 ± 0.01 (lnms2) on workday and free day, p = 0.103] bands were involved in the free day elevation of HRV of subjects with higher SJL (Figure 3B). In contrast, no differences between work and free day were found in the group with lower SJL [Figure 3B; VLF, 0.28 ± 0.01 and 0.29 ± 0.01; LF, 0.79 ± 0.02 and 0.81 ± 0.02; HF, 1.40 ± 0.07 and 1.43 ± 0.06 (lnms2) on workday and free day, respectively]. To approach sympathetic modulation, normalized units of LF were also calculated. LFnu powers were relatively low as expected during sleep in healthy young people (Brandenberger et al., 2003; Stein and Pu, 2012). Workday and free day LFnu powers did not differ significantly in either group (Figure 3B).
FIGURE 3.
Frequency analysis of HRV in the first 2 h of sleep. (A) The bin-wise differences in the power spectral density of HRV between free days and workdays in the group with higher (upper panel) and lower SJL (lower panel). One sample t-test, ∗p < 0.05 indicates above the chance power differences (multiple comparison corrected by the Descriptive Data Analysis protocol), n = 13 and 17 for the group with higher and lower SJL, respectively. (B) Powers of the standard frequency bands on work and free day in the group with higher (upper panel) and lower SJL (lower panel). Paired t-test, ∗p < 0.05, n = 13 and 17 for the group with higher and lower SJL, respectively.
Examination of the Association Between SJL and Subjective Sleep Quality
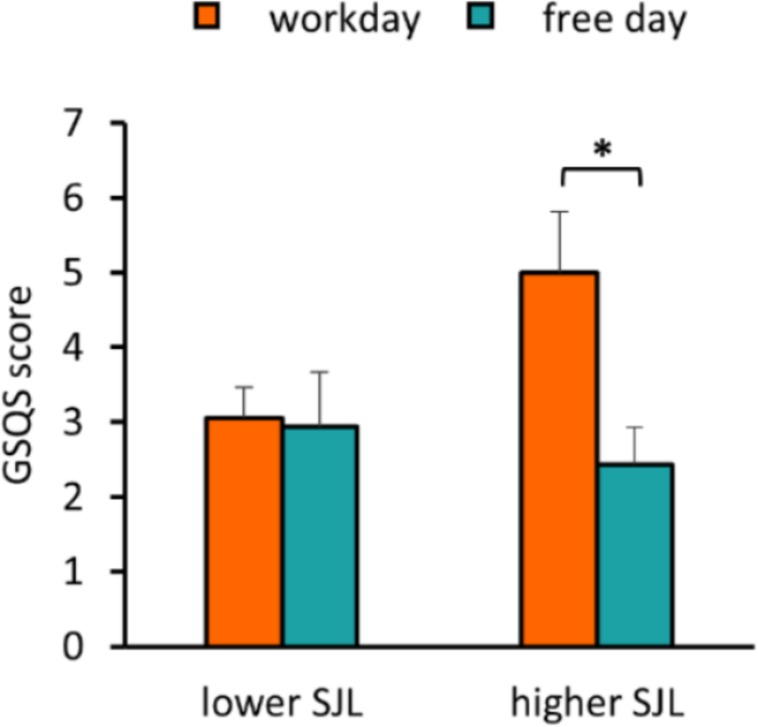
To assess sleep quality, we used the GSQS where a higher score indicates worse quality of sleep. We found that SJL was positively associated with the GSQS score on workday, whereas no association was obtained on the free day (Supplementary Figure S2). While participants in the group with lower SJL reported similar sleep quality for both days, participants with higher SJL had a significantly worse sleep quality on the workday than on the free day (p = 0.027) (Figure 4), and their workday scores were also significantly higher than those of the other group (two-sample t-test, p = 0.043).
FIGURE 4.
Negative association of SJL with sleep quality. Average workday and free day GSQS scores of the participants in the groups with lower and higher SJL are shown. Paired t-test, ∗p < 0.05, n = 17 and 16 for the group with lower and higher SJL, respectively.
Discussion
To our knowledge, this is the first study indicating an interrelationship between SJL and the control of cardiac function. SJL affects most people in our society for shorter or longer periods throughout their life. As a strong and positive correlation between SJL and chronotype was found, and chronotype peaks between ages 20 and 26 years, SJL affects young adults most intensively (Wittmann et al., 2006). Therefore, for this field study we recruited male university students and addressed whether differences in sleep timing between workdays and free days show associations with sleep-related autonomic regulation and subjective sleep quality. Importantly, the measurements were performed in the participants’ home environment and subjects kept their usual sleep schedules both on workdays and on free days. This was confirmed by the correlation of both chronotype (MSFsc) and SJL determined on the basis of the MCTQ (usual sleep timing) and the sleep diary (sleep timing in the study week).
We analyzed HRV, as it is a sensitive marker of the plasticity of the autonomic control of cardiac function. According to literature data, the baseline HRV can show high variations among healthy individuals and the source of these inter-individual differences is largely unclear (Goldberger et al., 2001). Beside the plasticity of autonomic control, other factors such as structural and functional properties of the brain were suggested to influence absolute HRV values (Thayer et al., 2012; Kumral et al., 2019). Therefore, intra-individual HRV changes may be more informative than inter-individual differences in absolute HRV values. Nevertheless, it is interesting to note, that in the group with higher SJL the average HRV was higher in both nights compared to the group with lower SJL. Larger sample and further investigations would be needed to analyze whether a higher HRV during sleep is characteristic for people with higher SJL and/or later chronotype.
When we examined individual changes in HRV between the workday and the free day, we found that in the first 3 h of sleep in the group with higher SJL both RMSSD and pNN50 were lower on workday than on free day. HRV parameters can display circadian variations (Hilton et al., 2000; Vandewalle et al., 2007; Scheer et al., 2009). As we had recordings from only 1/3rd of a day, we cannot assess the daily rhythm of HRV in our subjects. Nevertheless, it might be possible that in the group with higher SJL a decrease in HRV in later sleep segments leads to compensation of the high HRV observed in the first sleep blocks. However, in the end of sleep HRV showed no differences between the 2 days, suggesting that the HRV divergence in the first 3 h of sleep was not counteracted in later sleep segments. A positive correlation between SJL and the free day-workday difference in various HRV parameters suggested that a higher SJL is associated with a larger increase of parasympathetic modulation from the workday to the free day. Results of the frequency-domain analysis were consistent with the time-domain data, as subjects with higher SJL showed different PSD of HRV on free day compared to workday, dominantly in higher frequency regions reflecting the vagal activity (Scholz et al., 1997; Montano et al., 2009; Laborde et al., 2017). Although the sleep duration of most participants was longer on free days than on workdays, this was not dependent on SJL, indicating that there were no differences in sleep deprivation during workdays between the groups of participants. Time spent awake, a main component of homeostatic sleep regulation, was also similar in the two groups. These data suggest that differences in the circadian rather than in the homeostatic sleep regulation are responsible for the workday-free day differences of HRV parameters in people with higher SJL. The apparent deviation of HRV parameters in the first two sleep blocks resulting in a different pattern of HRV in the course of sleep between workday and free day may reflect a difference in sleep structure. In this early period of workday’s night, the biological clock of people with higher SJL (and later chronotype) may not promote sleep. However, the shift of sleep onset from the socially forced earlier time to the preferred later bedtime may cause a deeper sleep with greater vagal tone on free day’s night compared to workday’s night. A recent work showed that timing of sleep onset is associated with changes in the proportion of sleep stages (Akerstedt et al., 2018). In addition, the positive psychical effect of sleep timing without restrictions may also beneficially influence both the autonomic function and the sleep quality of participants with higher SJL.
Altogether, we suggest that SJL and the chronic changes in sleep timing cause a difference in vagal activity between work and free days’ sleep and may affect the plasticity of cardiac regulation in the first few hours of sleep.
HRV parameters were found to reflect the functional properties of the cardiovascular system in both healthy populations and under pathological conditions. In young male subjects a positive correlation was obtained between vagal indices of HRV and endothelial functions (Pinter et al., 2012). Results of a recent meta-analysis based on eight studies with a total number of almost 22 000 subjects showed that low (but still normal) HRV in healthy populations, i.e., without known cardiovascular diseases, is associated with increased risk of a later cardiovascular event (Hillebrand et al., 2013). Moreover, autonomic dysregulation of the heart may contribute to hypertension (Schroeder et al., 2003), coronary artery calcification (Colhoun et al., 2001), arrhythmias, and congestive heart failure (Chen et al., 2014; Florea and Cohn, 2014; Fukuda et al., 2015).
As autonomic control and sleep regulation are interconnected, HRV can serve as an indicator of sleep quality as well. On the other hand, good subjective sleep quality per se is considered a marker of both healthy cardiovascular control and emotional wellbeing (Massar et al., 2017; Cespedes Feliciano et al., 2018). Our results based on the GSQS showed that SJL negatively impacts sleep quality on workdays. In a recent study using the Pittsburgh Sleep Quality Index (PSQI), Pilz et al. (2018) found that SJL mediates the effect of chronotype on the differences in sleep quality between workdays and free days. As GSQS and PSQI differ in both the items to be answered and the referred period (while the GSQS refers to the sleep quality of the previous night, the PSQI is an instrument assessing sleep quality over the last month), results of Pilz et al. (2018), and our data are complementary and together strongly indicate that SJL negatively impacts sleep quality on workdays.
Data from this study should be interpreted by considering some limitations. The sample size was relatively low. To exclude a possible effect of cycle-dependent changes in sexual hormones, only men were involved in this study. Therefore, it is possible that the interactions examined are characteristic only for men but not for women. Further studies may examine possible sex differences. In order to have a relative homogenous sample, only young adults were involved in the study. Impact of age on the associations found in our study could be the subject of future investigations.
In summary, we suggest that the chronic changes in sleep-wake patterns due to social constraints are associated with lessening of the restorative capacity of sleep on workdays. This is reflected by the differences of autonomic cardiac control between workdays and free days and the lower sleep quality during workday nights. In addition, our findings provide further support for the recent claim indicating that the cardiovascular system is particularly sensitive to circadian variation (Scheer et al., 2009; Janszky et al., 2012; Grimaldi et al., 2016).
Considering the very high prevalence of SJL in both the adolescent and the adult population, our findings together with literature data about the adverse health effects of SJL stress the requirement to develop social strategies for the reduction of SJL. Even small changes or more flexibility in school and work schedules may lessen the harmful effect of SJL. Due to shifting the phase of the circadian clock, daylight saving time (DST) also aggravates SJL and negatively affects health (Kantermann et al., 2007). Therefore, initiatives to abolish DST are currently under consideration. Individual strategies may also be important tools for the reduction of SJL. For example, increasing morning and decreasing evening light exposure can shift the biological clock to an earlier phase and thus lessen SJL. As meal acts as an effective Zeitgeber, appropriate meal timing could also help to adjust the body clock.
Data Availability
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Semmelweis University Regional and Institutional Committee of Science and Research Ethics (Ethical approval 170/2016). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
KE and KK designed the study. ÁS and KE collected the data. ÁS, KE, and RB analyzed the data. KK, ÁS, KE, and RB wrote the manuscript and approved its final version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Till Roenneberg, Norbert Gyöngyösi, and Anita Szőke for helpful comments on the manuscript. We also thank Barna Vásárhelyi for advising in 6-sulfatoxymelatonin measurements and reading of the manuscript. We are especially grateful to Bence Erdős for helping in data processing and critical reading of the manuscript and to Éva Szabó for statistical advice. We also thank Adrienn Kovács and Péter Soós for helping in subjects’ recruitment.
Footnotes
Funding. This work was supported by the National Research, Development and Innovation Office – NKFIH (K115953) and the Higher Education Institutional Excellence Programme of the Ministry of Human Capacities in Hungary, within the framework of the FIKP programme of the Semmelweis University. KK, KE, and RB are Merit Scholars of the Semmelweis University.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2019.00950/full#supplementary-material
References
- Abt K. (1987). Descriptive data analysis: a concept between confirmatory and exploratory data analysis. Methods Inf. Med. 26 77–88. 10.1055/s-0038-1635488 [DOI] [PubMed] [Google Scholar]
- Akerstedt T., Hume K., Minors D., Waterhouse J. (1997). Good sleep–its timing and physiological sleep characteristics. J. Sleep Res. 6 221–229. 10.1111/j.1365-2869.1997.00221.x [DOI] [PubMed] [Google Scholar]
- Akerstedt T., Lekander M., Nilsonne G., Tamm S., D’Onofrio P., Kecklund G., et al. (2018). Effects of late-night short-sleep on in-home polysomnography: relation to adult age and sex. J. Sleep Res. 27:e12626. 10.1111/jsr.12626 [DOI] [PubMed] [Google Scholar]
- Barreira T. K., Kang M., Caputo J. L., Farley R. S., Renfrow M. S. (2009). Validation of the actiheart monitor for the measurement of physical activity. Int. J. Exerc. Sci. 2 60–71. [Google Scholar]
- Bertsch K., Hagemann D., Naumann E., Schachinger H., Schulz A. (2012). Stability of heart rate variability indices reflecting parasympathetic activity. Psychophysiology 49 672–682. 10.1111/j.1469-8986.2011.01341.x [DOI] [PubMed] [Google Scholar]
- Biggi N., Consonni D., Galluzzo V., Sogliani M., Costa G. (2008). Metabolic syndrome in permanent night workers. Chronobiol. Int. 25 443–454. 10.1080/07420520802114193 [DOI] [PubMed] [Google Scholar]
- Brage S., Brage N., Franks P. W., Ekelund U., Wareham N. J. (2005). Reliability and validity of the combined heart rate and movement sensor Actiheart. Eur. J. Clin. Nutr. 59 561–570. 10.1038/sj.ejcn.1602118 [DOI] [PubMed] [Google Scholar]
- Brandenberger G., Viola A. U., Ehrhart J., Charloux A., Geny B., Piquard F., et al. (2003). Age-related changes in cardiac autonomic control during sleep. J. Sleep Res. 12 173–180. 10.1046/j.1365-2869.2003.00353.x [DOI] [PubMed] [Google Scholar]
- Cespedes Feliciano E. M., Quante M., Rifas-Shiman S. L., Redline S., Oken E., Taveras E. M. (2018). Objective sleep characteristics and cardiometabolic health in young adolescents. Pediatrics 142:e20174085. 10.1542/peds.2017-4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. S., Chen L. S., Fishbein M. C., Lin S. F., Nattel S. (2014). Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ. Res. 114 1500–1515. 10.1161/CIRCRESAHA.114.303772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouchou F., Desseilles M. (2014). Heart rate variability: a tool to explore the sleeping brain? Front. Neurosci. 8:402. 10.3389/fnins.2014.00402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colhoun H. M., Francis D. P., Rubens M. B., Underwood S. R., Fuller J. H. (2001). The association of heart-rate variability with cardiovascular risk factors and coronary artery calcification: a study in type 1 diabetic patients and the general population. Diabetes Care 24 1108–1114. 10.2337/diacare.24.6.1108 [DOI] [PubMed] [Google Scholar]
- Diaz-Morales J. F., Escribano C. (2015). Social jetlag, academic achievement and cognitive performance: understanding gender/sex differences. Chronobiol. Int. 32 822–831. 10.3109/07420528.2015.1041599 [DOI] [PubMed] [Google Scholar]
- Elsenbruch S., Harnish M. J., Orr W. C. (1999). Heart rate variability during waking and sleep in healthy males and females. Sleep 22 1067–1071. 10.1093/sleep/22.8.1067 [DOI] [PubMed] [Google Scholar]
- Florea V. G., Cohn J. N. (2014). The autonomic nervous system and heart failure. Circ. Res. 114 1815–1826. 10.1161/CIRCRESAHA.114.302589 [DOI] [PubMed] [Google Scholar]
- Fukuda K., Kanazawa H., Aizawa Y., Ardell J. L., Shivkumar K. (2015). Cardiac innervation and sudden cardiac death. Circ. Res. 116 2005–2019. 10.1161/CIRCRESAHA.116.304679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberger J. J., Challapalli S., Tung R., Parker M. A., Kadish A. H. (2001). Relationship of heart rate variability to parasympathetic effect. Circulation 103 1977–1983. 10.1161/01.cir.103.15.1977 [DOI] [PubMed] [Google Scholar]
- Grimaldi D., Carter J. R., Van Cauter E., Leproult R. (2016). Adverse Impact of sleep restriction and circadian misalignment on autonomic function in healthy young adults. Hypertension 68 243–250. 10.1161/HYPERTENSIONAHA.115.06847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraszti R. A., Ella K., Gyongyosi N., Roenneberg T., Kaldi K. (2014). Social jetlag negatively correlates with academic performance in undergraduates. Chronobiol. Int. 31 603–612. 10.3109/07420528.2013.879164 [DOI] [PubMed] [Google Scholar]
- Hillebrand S., Gast K. B., de Mutsert R., Swenne C. A., Jukema J. W., Middeldorp S., et al. (2013). Heart rate variability and first cardiovascular event in populations without known cardiovascular disease: meta-analysis and dose-response meta-regression. Europace 15 742–749. 10.1093/europace/eus341 [DOI] [PubMed] [Google Scholar]
- Hilton M. F., Umali M. U., Czeisler C. A., Wyatt J. K., Shea S. A. (2000). Endogenous circadian control of the human autonomic nervous system. Comput. Cardiol. 27 197–200. [PubMed] [Google Scholar]
- Hulsegge G., Gupta N., Proper K. I., van Lobenstein N., IJzelenberg W., Hallman D. M., et al. (2018). Shift work is associated with reduced heart rate variability among men but not women. Int. J. Cardiol. 258 109–114. 10.1016/j.ijcard.2018.01.089 [DOI] [PubMed] [Google Scholar]
- Janszky I., Ahnve S., Ljung R., Mukamal K. J., Gautam S., Wallentin L., et al. (2012). Daylight saving time shifts and incidence of acute myocardial infarction–swedish register of information and knowledge about swedish heart intensive care admissions (RIKS-HIA). Sleep Med. 13 237–242. 10.1016/j.sleep.2011.07.019 [DOI] [PubMed] [Google Scholar]
- Kantermann T., Juda M., Merrow M., Roenneberg T. (2007). The human circadian clock’s seasonal adjustment is disrupted by daylight saving time. Curr. Biol. 17 1996–2000. 10.1016/j.cub.2007.10.025 [DOI] [PubMed] [Google Scholar]
- Kaufmann T., Sutterlin S., Schulz S. M., Vogele C. (2011). ARTiiFACT: a tool for heart rate artifact processing and heart rate variability analysis. Behav. Res. Methods 43 1161–1170. 10.3758/s13428-011-0107-7 [DOI] [PubMed] [Google Scholar]
- Knutsson A. (2003). Health disorders of shift workers. Occup. Med. 53 103–108. 10.1093/occmed/kqg048 [DOI] [PubMed] [Google Scholar]
- Koopman A. D. M., Rauh S. P., van ’t Riet E., Groeneveld L., van der Heijden A. A., Elders P. J., et al. (2017). The association between social jetlag, the metabolic syndrome, and type 2 diabetes mellitus in the general population: the new hoorn study. J. Biol. Rhythms 32 359–368. 10.1177/0748730417713572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen J., Korshoj M., Skotte J. H., Jespersen T., Sogaard K., Mortensen O. S., et al. (2011). Comparison of two systems for long-term heart rate variability monitoring in free-living conditions–a pilot study. Biomed. Eng. Online 10:27. 10.1186/1475-925X-10-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumral D., Schaare H. L., Beyer F., Reinelt J., Uhlig M., Liem F., et al. (2019). The age-dependent relationship between resting heart rate variability and functional brain connectivity. Neuroimage 185 521–533. 10.1016/j.neuroimage.2018.10.027 [DOI] [PubMed] [Google Scholar]
- Laborde S., Mosley E., Thayer J. F. (2017). Heart rate variability and cardiac vagal tone in psychophysiological research - recommendations for experiment planning, data analysis, and data reporting. Front. Psychol. 8:213. 10.3389/fpsyg.2017.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. D., Tzeng Y. C., Sin P. Y., Galletly D. C. (2010). Respiratory sinus arrhythmia in conscious humans during spontaneous respiration. Respir. Physiol. Neurobiol. 174 111–118. 10.1016/j.resp.2010.04.021 [DOI] [PubMed] [Google Scholar]
- Levandovski R., Dantas G., Fernandes L. C., Caumo W., Torres I., Roenneberg T., et al. (2011). Depression scores associate with chronotype and social jetlag in a rural population. Chronobiol. Int. 28 771–778. 10.3109/07420528.2011.602445 [DOI] [PubMed] [Google Scholar]
- Malik M. (1996). Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task force of the european society of cardiology and the north american society of pacing and electrophysiology. Circulation 93 1043–1065. 10.1161/01.cir.93.5.1043 [DOI] [PubMed] [Google Scholar]
- Massar S. A. A., Liu J. C. J., Mohammad N. B., Chee M. W. L. (2017). Poor habitual sleep efficiency is associated with increased cardiovascular and cortisol stress reactivity in men. Psychoneuroendocrinology 81 151–156. 10.1016/j.psyneuen.2017.04.013 [DOI] [PubMed] [Google Scholar]
- Meijman T. F., de Vries-Griever A. H., de Vries G. (1988). The evaluation of the Groningen Sleep Quality Scale. Groningen: Heymans Bulletin (HB 88-13-EX). [Google Scholar]
- Montano N., Porta A., Cogliati C., Costantino G., Tobaldini E., Casali K. R., et al. (2009). Heart rate variability explored in the frequency domain: a tool to investigate the link between heart and behavior. Neurosci. Biobehav. Rev. 33 71–80. 10.1016/j.neubiorev.2008.07.006 [DOI] [PubMed] [Google Scholar]
- Morris C. J., Purvis T. E., Hu K., Scheer F. A. (2016). Circadian misalignment increases cardiovascular disease risk factors in humans. Proc. Natl. Acad. Sci. U.S.A. 113 E1402–E1411. 10.1073/pnas.1516953113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C. J., Purvis T. E., Mistretta J., Hu K., Scheer F. (2017). Circadian misalignment increases C-Reactive protein and blood pressure in chronic shift workers. J. Biol. Rhythms 32 154–164. 10.1177/0748730417697537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papantoniou K., Devore E. E., Massa J., Strohmaier S., Vetter C., Yang L., et al. (2018). Rotating night shift work and colorectal cancer risk in the nurses’ health studies. Int. J. Cancer 143 2709–2717. 10.1002/ijc.31655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons M. J., Moffitt T. E., Gregory A. M., Goldman-Mellor S., Nolan P. M., Poulton R., et al. (2015). Social jetlag, obesity and metabolic disorder: investigation in a cohort study. Int. J. Obes. 39 842–848. 10.1038/ijo.2014.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz L. K., Keller L. K., Lenssen D., Roenneberg T. (2018). Time to rethink sleep quality: PSQI scores reflect sleep quality on workdays. Sleep 41:zsy029. 10.1093/sleep/zsy029 [DOI] [PubMed] [Google Scholar]
- Pinter A., Horvath T., Sarkozi A., Kollai M. (2012). Relationship between heart rate variability and endothelial function in healthy subjects. Auton. Neurosci. 169 107–112. 10.1016/j.autneu.2012.05.005 [DOI] [PubMed] [Google Scholar]
- Puttonen S., Harma M., Hublin C. (2010). Shift work and cardiovascular disease - pathways from circadian stress to morbidity. Scand. J. Work Environ. Health 36 96–108. 10.5271/sjweh.2894 [DOI] [PubMed] [Google Scholar]
- Roenneberg T., Allebrandt K. V., Merrow M., Vetter C. (2012). Social jetlag and obesity. Curr. Biol. 22 939–943. 10.1016/j.cub.2012.03.038 [DOI] [PubMed] [Google Scholar]
- Roenneberg T., Wirz-Justice A., Merrow M. (2003). Life between clocks: daily temporal patterns of human chronotypes. J. Biol. Rhythms 18 80–90. 10.1177/0748730402239679 [DOI] [PubMed] [Google Scholar]
- Rutters F., Lemmens S. G., Adam T. C., Bremmer M. A., Elders P. J., Nijpels G., et al. (2014). Is social jetlag associated with an adverse endocrine, behavioral, and cardiovascular risk profile? J. Biol. Rhythms 29 377–383. 10.1177/0748730414550199 [DOI] [PubMed] [Google Scholar]
- Scheer F. A., Hilton M. F., Mantzoros C. S., Shea S. A. (2009). Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. U.S.A. 106 4453–4458. 10.1073/pnas.0808180106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schernhammer E. S., Kroenke C. H., Laden F., Hankinson S. E. (2006). Night work and risk of breast cancer. Epidemiology 17 108–111. [DOI] [PubMed] [Google Scholar]
- Scholz U. J., Bianchi A. M., Cerutti S., Kubicki S. (1997). Vegetative background of sleep: spectral analysis of the heart rate variability. Physiol. Behav. 62 1037–1043. 10.1016/s0031-9384(97)00234-5 [DOI] [PubMed] [Google Scholar]
- Schroeder E. B., Liao D., Chambless L. E., Prineas R. J., Evans G. W., Heiss G. (2003). Hypertension, blood pressure, and heart rate variability: the atherosclerosis risk in communities (ARIC) study. Hypertension 42 1106–1111. 10.1161/01.HYP.0000100444.71069.73 [DOI] [PubMed] [Google Scholar]
- Shields M. (2002). Shift work and health. Health Rep. 13 11–33. [PubMed] [Google Scholar]
- Simor P., Koteles F., Bodizs R., Bardos G. (2009). A questionnaire based study of subjective sleep quality: the psychometric evaluation of the Hungarian version of the groningen sleep quality scale. Mentálhigiéné és Pszichoszomatika 10 249–261. 10.1556/mental.10.2009.3.5 [DOI] [Google Scholar]
- Stein P. K., Pu Y. (2012). Heart rate variability, sleep and sleep disorders. Sleep Med. Rev. 16 47–66. 10.1016/j.smrv.2011.02.005 [DOI] [PubMed] [Google Scholar]
- Strohmaier S., Devore E. E., Zhang Y., Schernhammer E. S. (2018). A review of data of findings on night shift work and the development of DM and CVD events: a synthesis of the proposed molecular mechanisms. Curr. Diab. Rep. 18:132. 10.1007/s11892-018-1102-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer J. F., Ahs F., Fredrikson M., Sollers J. J., III, Wager T. D. (2012). A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 36 747–756. 10.1016/j.neubiorev.2011.11.009 [DOI] [PubMed] [Google Scholar]
- Thayer J. F., Yamamoto S. S., Brosschot J. F. (2010). The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol. 141 122–131. 10.1016/j.ijcard.2009.09.543 [DOI] [PubMed] [Google Scholar]
- Tobaldini E., Nobili L., Strada S., Casali K. R., Braghiroli A., Montano N. (2013). Heart rate variability in normal and pathological sleep. Front. Physiol. 4:294. 10.3389/fphys.2013.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinder J., Kleiman J., Carrington M., Smith S., Breen S., Tan N., et al. (2001). Autonomic activity during human sleep as a function of time and sleep stage. J. Sleep Res. 10 253–264. 10.1046/j.1365-2869.2001.00263.x [DOI] [PubMed] [Google Scholar]
- Vandewalle G., Middleton B., Rajaratnam S. M., Stone B. M., Thorleifsdottir B., Arendt J., et al. (2007). Robust circadian rhythm in heart rate and its variability: influence of exogenous melatonin and photoperiod. J. Sleep Res. 16 148–155. 10.1111/j.1365-2869.2007.00581.x [DOI] [PubMed] [Google Scholar]
- Vetter C., Devore E. E., Ramin C. A., Speizer F. E., Willett W. C., Schernhammer E. S. (2015). Mismatch of sleep and work timing and risk of Type 2 diabetes. Diabetes Care 38 1707–1713. 10.2337/dc15-0302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter C., Devore E. E., Wegrzyn L. R., Massa J., Speizer F. E., Kawachi I., et al. (2016). Association between rotating night shift work and risk of coronary heart disease among women. JAMA 315 1726–1734. 10.1001/jama.2016.4454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M., Dinich J., Merrow M., Roenneberg T. (2006). Social jetlag: misalignment of biological and social time. Chronobiol. Int. 23 497–509. 10.1080/07420520500545979 [DOI] [PubMed] [Google Scholar]
- Wong P. M., Hasler B. P., Kamarck T. W., Muldoon M. F., Manuck S. B. (2015). Social jetlag, chronotype, and cardiometabolic risk. J. Clin. Endocrinol. Metab. 100 4612–4620. 10.1210/jc.2015-2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.