Abstract
This study aimed to determine the prevalence of three common hemoparasites (Anaplasma marginale, Babesia bovis and Babesia bigemina) in cattle from 16 counties in the Campos de Lages region, Santa Catarina state, Brazil, and the factors affecting disease occurrence. The study population consisted of 257 clinically healthy animals from 21 rural farms. Bovine blood samples were collected by jugular venipuncture. DNA was extracted from whole blood by the phenol/ chloroform method. Genomic DNA extracted from blood samples was subjected to Multiplex PCR for screening of B. bovis, B. bigemina, and A. marginale using specific primers. Prevalences of A. marginale, B. bigemina, and B. bovis were 27%, 16%, and 29%, respectively. Mixed infection was observed in 17.5% of samples. The most frequent was Babesia bovis and Babesia bigemina in 6.62% of samples. A. marginale infection rates were statistically correlated with age groups of cattle. The infections detected in the study population were considered to be subclinical, based on the presence pathogen DNA and absence of clinical symptoms. Seasonality of the pathogens resulted in various degrees of infection, related to the age of the animals and the season. The Campos de Lages region is characterized by enzootic instability for these pathogens because of its climatic and geographic features.
Keywords: Multiplex PCR, Tick-borne disease, Brazil, Enzootic instability
1. Introduction
The unicellular parasites Babesia bovis and Babesia bigemina (order Piroplasmida, family Babesiidae), and the rickettsia Anaplasma marginale (order Rickettsiales, family Anaplasmataceae), are transmitted by Rhipicephalus microplus ticks, among others (Guglielmone, 1995; Kocan, 1995).
These tick-borne disease (TBD) agents are widely distributed, have similar clinical signs and laboratory findings, and are major causes of economic losses in the livestock industry, worldwide [3, 4. Inbred strains of domesticated ungulates, particularly cattle (Bos taurus), are often susceptible to infection by single or mixed pathogenic agents, which may be present at any bovine age, in clinical or subclinical forms (Gonçalves, 2000). Annual expenditures in the Brazilian cattle industry are roughly US$ 500 million for basic control and prophylaxis of TBD agents, and US$ 2 billion for dealing with tick/ TBD complexes (Grisi et al., 2002). Economic losses are due primarily to increased mortality, reduced milk production, and poor feed conversion (Dantas-Torres and Otranto, 2016; Dantas-Torres et al., 2016).
Brazil is considered an enzootic country for TBD (Dantas-Torres and Otranto, 2016; Dantas-Torres et al., 2016). However, climatic conditions in some regions do not favor the development of ticks throughout the year, resulting in the existence of specific TBD “enzootic instability” areas. This situation is changing, as studies show that climate variations is every decade causing an expansion of the area of habitat suitability for R. microplus to regions previously considered colder in South America where the vector was absent. (Estrada-Pena et al., 2005).
Studies conducted in various regions of Brazil has demonstrated, by serological surveys, the prevalence of A. marginale, B. bovis, and B. bigemina in many states with variable epidemiological features (Souza et al., 2013; da Silva et al., 2015; Santos et al., 2017). In Santa Catarina state, the seroprevalence of Babesia sp. was evaluated in a few counties (Dalagnol et al., 1999; Souza et al., 2002).
Polymerase chain reaction (PCR)-based techniques are often utilized in epidemiological surveys of TBD (Martins et al., 2010; Shebish et al., 2012; Zhou et al., 2016) and a modified technique known as multiplex PCR (mPCR) has been particularly successful (Figueroa et al., 1993; Canever et al., 2014; Rodríguez et al., 2015). We used mPCR, to be able in a single reaction to demonstrate the presence of the amplicon related to A. marginale, B. bovis, and B. bigemina infections and the prevalence of these agents in bovine herds in the Campos de Lages region of Santa Catarina state.
2. Materials and methods
2.1. Sample populations
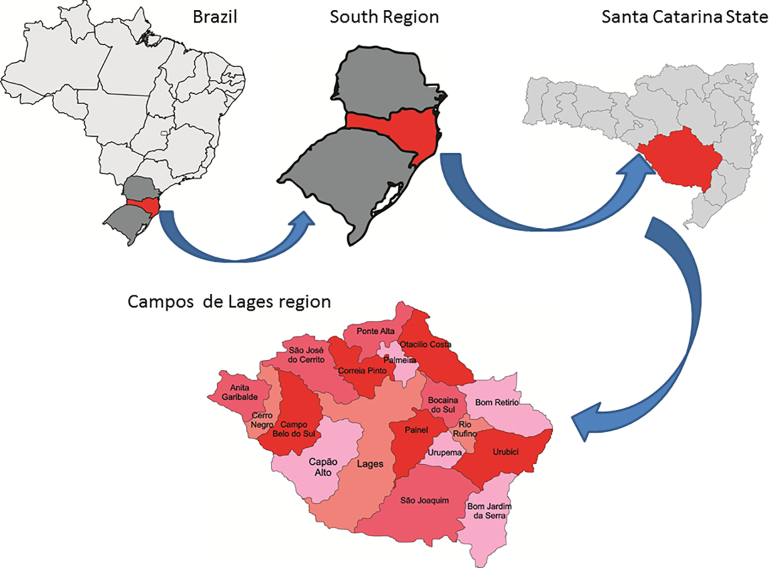
Blood samples were obtained from bovine herds in 16 counties in the Campos de Lages region, Santa Catarina state (Fig. 1), located at altitudes ranging from 850 to 1400 m above sea level. This region is the largest (area 15,726,010 km2) in Santa Catarina state. Its cities are among the coldest in Brazil, and frequently experience intense frost with occasional snowfall during the winter. Its Köppen climate classification is Cfb (Temperate oceanic climate; coldest month averaging above 0 °C (or −3 all months with average temperatures below 22 °C and at least four months averaging above 10 °C. No significant precipitation difference between seasons) (Peel et al., 2007).
Fig. 1.
Geographical location of the Campos de Lages region, Santa Catarina state, Brazil.
Twenty-one rural farms, having a total of 257 healthy crossbred cattle were selected for sampling in a non-probabilistic manner from March 2012 to June 2013. Determination of sample quantity was based on the equation:
where n is sample number, Z is a critical value (confidence level 95%), p is population proportion of positive individuals (admitted 85%), q is the population proportion of individuals who are negativeE is a maximum error of estimation.
Blood samples were collected from 1% or more of animals in each bovine herd.
The properties where the samples were collected had on average 113 animals being, 18 properties of beef cattle and 03 of dairy cattle. Only in two properties there were no reports of previous cases of babesiosis or anaplasmosis.
The bovines were classified into five groups according to age: group 1: ≤6 months; group 2: >6 and ≤ 12 months; group 3: >12 and ≤ 24 months; group 4: >24 and ≤ 36 months; group 5: >36 months. Altitudes of farms were classified into three groups: 1200–1400, 901–1199, and 800–900 m.
2.2. Blood collection
Blood samples were collected by jugular venipuncture using vacuum tubes containing ethylenediaminetetraacetic acid (EDTA). The tubes were placed inside isothermal boxes, and shipped to the Laboratório de Bioquímica de Hemoparasitas e Vetores, Universidade do Estado de Santa Catarina, Lages, Brazil for blood DNA extraction. Ethical and institutional approval was given by the Ethics Committee on the Use of Animals, State University of Santa Catarina Lages-SC, Brazil Number 1.29.11.
2.3. DNA extraction
DNA was extracted from whole blood by the phenol/ chloroform method. In brief, 200 μl whole blood was mixed with lysis buffer (10 mM Tris, pH 7.4, 10 mM NaCl, 25 mM EDTA, 1% SDS) containing 100 μg/ml proteinase K (Sigma; St. Louis, MO, USA), incubated at 42 °C for 12 h, washed sequentially with phenol, phenol/ chloroform (1:1), and chloroform, and centrifuged at 14,000 ×g for 10 min. Supernatant was removed, and DNA was precipitated with isopropanol and then washed with 70% ethanol. Tubes were placed in an oven at 37 °C until ethanol was completely evaporated. Resulting DNA was resuspended in 50 μl DNAse-free Milli-Q water, stored at −20 °C, and DNA quantification was measured with a spectrophotometer (NanoDrop 2000H, Thermo Scientific; Waltham, MA, USA).
2.4. Multiplex-PCR
Genomic DNA extracted from blood samples, described as above, was subjected to mPCR amplification for the screening of B. bovis, B. bigemina, and A. marginale.
The set of primers used for A. marginale was described by (Lew et al., 2002), based on the gene encoding Msp1a protein and amplifying 1000 base pairs amplicon. The BoF/BoR and BiIA/BiIB primers were derived from DNA sequences of B. bovis 356-bp (Palmer et al., 1991) and B. bigemina 278 bp (Figueroa et al., 1992), respectively, which were used by (Figueroa et al., 1993) in multiplex PCR.
PCR assay was performed in a total volume of 25 μl containing 80 ng genomic DNA and 8.5 pmol of appropriate primer pair (Table 1), added with 1 U Taq DNA polymerase, GoTaq® Hot Start Polymerase (Promega Corp.; Madison, WI, USA), 0.2 mM dNTPs, 25 mM MgCl2, 5 μl 5× Green GoTaq® Flexi buffer (Promega Corp.; Madison, WI, USA, and ultrapure water. The reaction was performed in a MaxyGene H Thermal Cycler (Axygen; Union City, CA, USA). Amplification steps were: hot start of 5 min at 95 °C, 35 cycles of 1 min at 95 °C, 1 min at 58 °C, 1 min at 72 °C, and final extension step 10 min at 72 °C. The mPCR sensitivity was based in previous study (Figueroa et al., 1993).
Table 1.
Oligonucleotides primers used in Multiplex-PCR.
| Pathogen | Primer | Sequence (5′ -3′) | Amplicon size | Reference |
|---|---|---|---|---|
| Babesia bigemina | BilA BilB |
CATCTAATTTCTCTCCT ACCCCTCC CCTCGGCTTCAACTCTGATGCCAAAG |
278pb | (Figueroa et al., 1992) |
| Babesia bovis | BoF BoR |
CACGAGGAAGGAACTACCGATGTTGA CCAAGGAGCTTCAACGTACGAGGTCA |
356pb | (Palmer et al., 1991) |
| Anaplasma marginale | 1773F 2957R |
TGTGCTTATGGCAGACATTTCC AAACCTTGTAGCCCCAACTTATCC |
1000pb | (Lew et al., 2002) |
Amplified PCR products were electrophoresed on 0.8% agarose gel, stained with Gel Red dye (Biotium; Fremont, CA, USA), visualized under UV light, and photographed in transilluminator apparatus. Molecular sizes of amplicons were estimated by comparison with a 100-bp ladder standard (Ludwig Biotecnologia; Porto Alegre, Brazil). A. marginale (Strain PR1; Londrina), B. bigemina, and B. bovis strains, kindly provided by Prof. Dr. Odilon Vidotto (State University of Londrina, Brazil), were used as positive controls. Distilled water was used as negative control.
2.5. Sequencing
Purified PCR products were ligated into p-GEM-T-easy® vector (Promega Corp.; Madison, WI, USA) and transformed into calcium-competent Escherichia coli cells (DH10BTM, Life Technologies; Grand Island, NY, USA). Single colonies were grown overnight into LB Medium, and plasmids were purified using a plasmid purification kit (QIAGEN; Hilden, Germany). For confirmation of gene insertion, DNA (1–2 μg) was purified from white positive colonies and sequenced by sequencing service facility ACTGene (Porto Alegre, Brazil). High-quality DNA sequences (Phred P20) were analyzed using the Phred/Phrap/Consed software package. Sequence identities were confirmed with the BLAST tool (www.ncbi.nlm.nih.gov/blast).
2.6. Statistical analysis
Logistic regression analysis of a generalized linear model with binomial distribution was performed using the GENMOD procedure of the SAS/STAT statistical software package (https://support.sas.com/rnd/app/stat/procedures/genmod.html). Values of positive or negative were applied to the variables age, season, and altitude.
3. Results
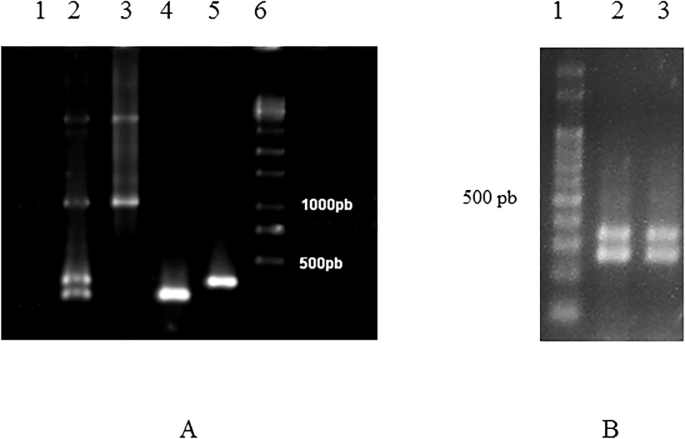
Single PCR and mPCR analysis for positive controls indicated 278-bp, 356-bp, and 1000-bp amplicons for B. bigemina, B. bovis, and A. marginale, respectively (Fig. 2).
Fig. 2.
Multiplex PCR in 0.8% agarose gel. (A) Multiplex PCR and Single PCR for positive controls of Anaplasma marginale, Babesia bovis, and Babesia bigemina, 1: negative control; 2: mPCR; 3: Anaplasma marginale; 4: Babesia bigemina; 5: Babesia bovis; 6: 1000-bp molecular weight marker. (B) Example of samples amplified by Multiplex PCR. 1: 1000-bp molecular weight marker; 2 and 3: B. bigemina and Babesia bovis.
Of the 257 animals tested, 53.8% were positive for at least one of the pathogens tested. Observed prevalences were 27% for A. marginale, 16% for B. bigemina, and 29% for B. bovis. Total percentages of pathogens were 36.3% for single infections and 17.5% for mixed infections (Table 2).
Table 2.
Distribution of individual and concomitant infection in the study population.
| Type of infection | Pathogens | Total (%) | |
|---|---|---|---|
| Individual | Anaplasma marginale | 16.34 | 36.28 |
| Babesia bovis | 14.39 | ||
| Babesia bigemina | 5.55 | ||
| Coinfection | Anaplasma marginale and Babesia bovis | 6.22 | 17.49 |
| Anaplasma marginale and Babesia bigemina | 2.33 | ||
| Anaplasma marginale, Babesia bovis and Babesia bigemina | 2.33 | ||
| Babesia bovis and Babesia bigemina | 6.61 |
Infection rates showed significant differences among the five age groups (Table 3). The rate of A. marginale infection was lowest in the age 24–36 months group. The rates of B. bigemina and B. bovis infection did not differ significantly among the age groups.
Table 3.
Percentage of infected animals with different pathogens about animals age.
| Age (months) | Animals | Anaplasma marginale | Babesia bovis | Babesia bigemina |
|---|---|---|---|---|
| N | % | % | % | |
| ≤ 6 | 27 | 33.33a | 29.63a | 14.81a |
| > 6 ≤ 12 | 70 | 28.57a | 30.00a | 18.57a |
| > 12 ≤24 |
71 | 26.76a | 35.21a | 19.72a |
| > 24 ≤ 36 | 39 | 7.69b | 20.51a | 12.82a |
| > 36 | 38 | 31.58a | 28.95a | 10.53a |
| P | – | 0.0378 | 0.6082 | 0.6838 |
a,bExistence of statistical difference.
B. bigemina infection rate differed significantly for winter (3.1%) vs. spring, summer, or autumn (>20%) (Table 4). Such seasonal differences for B. bovis and A. marginale infection rates were not stastically significant. However, for all three pathogens, infection rates were highest in spring.
Table 4.
Percentage of infected animals with different pathogens about annual season.
| Season | Animals |
Anaplasma marginale |
Babesia bovis |
Babesia bigemina |
|---|---|---|---|---|
| N | % | % | % | |
| Summer | 90 | 23.33 | 23.33 | 15.56 a |
| Autumm | 107 | 24.30 | 33.64 | 20.56 a |
| Winter | 32 | 28,13 | 28.13 | 3.13 b |
| Spring | 28 | 50.00 | 35.71 | 21.43 a |
| P | – | 0.0793 | 0.0920 | 0.0404 |
a,bExistence of statistical difference.
B. bovis infection rate was significantly higher for the altitude range 901–1199 m (Table 5). A. marginale and B. bigemina infection rates were also higher for this range, but the differences were not stastically significant.
Table 5.
Percentage of infected animals with different pathogens about farm altitude.
| Counties (nt) | Altitude (meters) | Animals | Anaplasma marginale | Babesia bovis | Babesia bigemina |
|---|---|---|---|---|---|
| N | % | % | % | ||
| Bom Jardim da Serra, Urupema, São Joaquim (93) | 1200 a 1400 | 48 | 31.25 | 22.92 b | 14.58 |
| Lages, Capão Alto Painel, Campo Belo do sul (82) | 901 a 1199 | 92 | 31.52 | 45.65 a | 23.91 |
| Ponte Alta, Bom Retiro, Otacílio Costa, Correia Pinto, Bocaina do Sul, Palmeira, São José do Cerrito, Rio Rufino, Anita Garibaldi (82) | 800 a 900 | 117 | 22.22 | 19.66 b | 11.97 |
| P | – | 0.0793 | <0.0001 | 0.1143 |
a,bExistence of statistical difference. nt- number of total animals in the county.
Distributions of the three pathogens varied among the 16 counties sampled (Table 6). In general, prevalences were highest in São Joaquim, Lages, Painel, and Urupema counties, and lowest in Cerro Negro, Anita Garibaldi, and Ponte Alta counties. Palmeira was the only county in which all the 04 samples collected were negative.
Table 6.
Prevalence of Anaplasma marginale, Babesia bovis and Babesia bigemina in the counties.
| Counties (nt) |
Ama |
Bbo |
Bbi |
||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| São Joaquim (47) | Latitude: 28°29′33″S, Longitude: 49° 56′ 1″ W | 15 | 31.9 | 30 | 63.8 | 15 | 32.9 |
| Painel (23) | Latitude: 27° 55′ 30″ S, Longitude: 50° 6′ 12″ W. | 4 | 17.4 | 3 | 13 | 6 | 26 |
| Lages(37) | Latitude: −27°.81′ 67″ S, Longitude: 50° 32′ 64″ W | 16 | 43.2 | 12 | 32.4 | 7 | 18.9 |
| Urupema (30) | Latitude: 28° 17′ 38″ S, Longitude: 49° 55′ 54″ W. | 14 | 46.6 | 12 | 40 | 8 | 26.6 |
| São José do Cerrito (15) | Latitude: 27° 39′ 45″ S, Longitude: 50° 34′ 48″ W. | 5 | 33.3 | 2 | 13.3 | 0 | 0 |
| Rio Rufino(10) | Latitude: 27° 51′ 44″ S, Longitude: 49° 46′ 47″ W. | 3 | 30 | 4 | 40 | 1 | 10 |
| Anita Garibaldi(10) | Latitude: 27° 41′ 9″ S, Longitude: 51° 7′ 50″ W. | 1 | 10 | 1 | 1 | 0 | 0 |
| Ponte Alta (10) | Latitude: 27.48′ 43″ S, Longitude: 50° 22′ 41″ W | 2 | 20 | 2 | 20 | 3 | 30 |
| Bocaina do Sul (4) | Latitude: 27° 44′ 40″ S Longitude: 49° 56′ 40″ W |
1 | 25 | 0 | 0 | 2 | 50 |
| Bom Jardim da Serra (16) | Latitude: 28° 20′ 25″ S, Longitude: 49° 37′ 29″ W. | 0 | 0 | 0 | 0 | 1 | 6.25 |
| Palmeira (4) | Latitude: 27° 57′ 94″,S, Longitude: 50° 9′ 37″ W | 0 | 0 | 0 | 0 | 0 | 0 |
| Otacílio Costa (6) | Latitude: 27° 28′ 59″ S Longitude: 50° 07′ 19″ W |
0 | 0 | 1 | 16.6 | 0 | 0 |
| Correia Pinto (13) | Latitude: 27° 58′ 62″S Longitude: 50° 21′ 55″ W | 1 | 7.7 | 1 | 7.7 | 0 | 0 |
| Campo Belo (11) | Latitude: 27° 89′ 85 S Longitude: 50° 45′ 26″ Oeste | 2 | 18 | 2 | 18 | 0 | 0 |
| Capão Alto (11) | Latitude: 27°93′68 S Longitude: 50° 30′ 51″ W |
3 | 27.3 | 7 | 63.3 | 1 | 9.1 |
| Bom Retiro (10) | Latitude: 27°80′8″ S Longitude: 49° 32′ 1″ W | 4 | 40 | 1 | 10 | 0 | 0 |
n- number of positive animals.
nt- number of total animals in the county.
4. Discussion
Molecular tools have become essential during recent decades for epidemiological investigations of parasites. Multiplex-PCR (mPCR), in particular, is useful for simultaneous identification of two or more pathogenic microorganisms and facilitates analysis of large numbers of samples.
The first study in which mPCR was used for simultaneous detection and sensitive analysis of the hemoparasites B. bigemina, B. bovis, and A. marginale in cattle was described by (Figueroa et al., 1993). One pitfall in mPCR is the possibility of false positive identifications resulting from contamination or nonspecific reactions (Bilgiç et al., 2013). Our experimental approach, based on the original method of (Figueroa et al., 1993), optimized identification techniques for the three pathogenic agents through amplification of A. marginale using the primers described by (Canever et al., 2014) and that of B. bovis using the primers described by (Rodríguez et al., 2015).
In the present work, mPCR results indicated a higher prevalence of B. bovis (29.6%) than of B. bigemina (16.7%) in a single infection. However, this finding should not necessarily be interpreted as a universal characteristic of the study region. In a study of an outbreak in Ponte Alta County, (Canever et al., 2014) observed high seroprevalences of B. bigemina (63.6%) and A. marginale (60.6%) infection. In a study of dairy cows in the Northern Plateau of Santa Catarina state, (Souza et al., 2002) observed a higher seroprevalence of B. bigemina (84.5%) than of B. bovis (76.8%). Dalagnol et al. (Dalagnol et al., 1999), in studies in Lages, Bom Jardim da Serra, Mafra, and Água Doce counties, reported prevalences ranging from 84 to 100% for B. bovis and from 95 to 100% for B. bigemina. However, (Dalagnol et al., 1999; Souza et al., 2002) reports are based solely on serological studies in Santa Catarina State, and none of the above three studies examined A. marginale seroprevalence. Reported infection levels for all three agents are <75%, indicating an area of enzootic instability (Gonçalves, 2000).
Other studies of the variation of pathogen seroprevalence in Brazil (Barros et al., 2005; Guedes Junior et al., 2008; Amorim et al., 2014) indicate the seasonal variation of the microorganisms involved in TBD complexes and the presence of infectious agents in multiple states. Seroprevalence also appears to be closely associated with individual management practices (e.g., intensive tick control) in rural properties, and annual climatic variations, which directly interfere with the development of effective vectors.
In Brazil only few research groups used mPCR for molecular detection of pathogens (Zhou et al., 2016). Studies by other groups have often used PCR or nested PCR (Oliveira-Sequeira et al., 2005). (Brito et al., 2010; Brito et al., 2013) reported high prevalences for B. bovis (98.6%), B. bigemina (96.4%), and A. marginale (95.1%), indicating enzootic stability in Amazonian cattle. In contrast, much lower prevalences were observed for water buffalo (Bubalus bubalis) in the same region (A. marginale 5.4%, B. bovis 15%, B. bigemina 16 (Da Silva et al., 2013a; Da Silva et al., 2013b; Da et al., 2014), suggesting that buffalo are more resistant to the vectors (Da Silva et al., 2014).
Prevalences of B. bovis and B. bigemina infection in the northeastern region of Brazil (Köppen climate classification Bsh) were similar to those reported in the southern region, indicating enzootic instability related to dry climate (Amorim et al., 2014). On the other hand, climate in Maranhão state (also in the northeastern region) is more similar to that of the Amazon region, with observed infection prevalences suggesting enzootic stability (Costa et al., 2015). The vectors are not present throughout the year in the region, resulting in later appearance of infection in cattle (Souza et al., 1988). Such seasonality of vectors leads to varying degrees of age-related and season-related infection, as observed. These vectors are able to survive at altitudes >800 m (Baker et al., 1989), explaining the high prevalences of infection observed in cattle at the high-altitude farms we studied.
The cattle in our study population were clinically healthy, with no signs of apathy, jaundice, anemia, or hemoglobinuria, even though 53.3% of them had one or more of the pathogenic agents. Thus, the infections were considered to be subclinical. Some of the cattle were descended from crosses with zebu or European breeds, which are relatively resistant to these vectors, and consequently to clinical manifestation of TBD (Furlong et al., 2004).
We observed mixed infections (two or three agents) in 17.5% of the study animals and single infections in 35.8%. Co-infection of Theileria annulata with A. marginale and/or B. bovis was observed in 22% of cattle in Turkey (Lew et al., 2002), and co-infection of B. bovis and B. bigemina was observed in 12% of ticks of the species Rhipicephalus (formerly Boophilus) annulatus in Egypt (Adham et al., 2009).
At the time of the research cattle rearing was focused in a subsistence way, prioritizing animals that had both meat and milk aptitude, which leads to a very high racial miscegenation scenario in rural properties, in this region. For this reason, it was not possible to estimate the number of purebred animals for each farm and municipality collected. However, this brings new information since it brings the results of a research conducted with crossbred races in the region.
5. Conclusions
The Campos de Lages region, Santa Catarina, Brazil is characterized by the enzootic instability of the pathogens studied, probably due to climatic changes and the absence of R .microplus throughout the year. mPCR was an effective technique for simultaneous analysis of DNA from multiple pathogen species.
Acknowledgments
Acknowledgments
This work was supported by grant from Universidade do Estado de Santa Catarina (2013TR3435). Luisa Lemos Vieira is the recipient of a fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil. The authors are grateful to Dr. S. Anderson for English editing of the manuscript.
Declaration of competing interest
The authors declare that they have no conflict of interest.
References
- Souza A.P., Bellato V., Sartor A.A., Farias L.M. Prevalência de anticorpos anti-Babesia em bovinos no planalto norte de Santa Catarina. Rev Ciências Agrovet. 2002;1:6–10. [Google Scholar]
- Adham F.K., Abd-El-Samie E.M., Gabre R.M., Hussein H.E. Detection of tick blood parasites in Egypt using PCR assay I -- Babesia bovis and Babesia bigemina. Parasitol. Res. 2009;105:721–730. doi: 10.1007/s00436-009-1443-8. [DOI] [PubMed] [Google Scholar]
- Amorim L.S., Wenceslau A.A., Carvalho F.S., Souza-Carneiro P.L., Albuquerque G.R. Bovine babesiosis and anaplasmosis complex: diagnosis and evaluation of the risk factors from Bahia Brazil. Rev. Bras. Parasitol. Vet. 2014;23:328–336. doi: 10.1590/s1984-29612014064. [DOI] [PubMed] [Google Scholar]
- Baker M.K., Ducasse F.B.W., Suthers R.W., Maywald F. The seasonal tick populations on traditional and commercial cattle grazed at four altitudes in Natal. J S Afr Vet Ass. 1989;60:95–101. [PubMed] [Google Scholar]
- Barros S.L., Madruga C.R., Araújo F.R., Menk C.F., Almeida M.A.O., Melo E.P.S., Kessler R.H. Serological survey of Babesia bovis Babesia bigemina and Anaplasma marginale antibodies in cattle from semi-arid region of the state of Bahia Brazil by enzyme-linked immunosorbent assays. Mem. Inst. Oswaldo Cruz. 2005;100:513–517. doi: 10.1590/s0074-02762005000600003. [DOI] [PubMed] [Google Scholar]
- Bilgiç H.B., Karagenç T., Simuunza M., Shiels B., Tait A., Eren H., Weir W. Development of a multiplex PCR assay for simultaneous detection of Theileria annulata Babesia bovis and Anaplasma marginale in cattle. Exp. Parasitol. 2013;133:222–229. doi: 10.1016/j.exppara.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito L.G., de Sena M.C.O., Rocha R.B., Netto F.G.S., Marim A.D., Souza G.C.R., Vendrami F.B., Moura M.M.F. Anaplasma marginale infection in cattle from south-western Amazonia. Pesq Vet Bras. 2010;30:249–254. [Google Scholar]
- Brito L.G., Rocha R.B., Barbieri F.S., Ribeiro E.S., Vendrami F.B., Souza G.C.R., Giglioti R., Regitano L.C.A., Falcoski T.O.R.S., Tizioto P.C., Oliveira M.C.S. Babesia bovis infection in cattle in the southwestern Brazilian Amazon. Ticks Tick Borne Dis. 2013;4:78–82. doi: 10.1016/j.ttbdis.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Canever M.F., Vieira L.L., Reck C., Richter L., Miletti L.C. First evaluation of an outbreak of bovine babesiosis and anaplasmosis in Southern Brazil using multiplex. PCR. Kor J Parasitol. 2014;52:507–511. doi: 10.3347/kjp.2014.52.5.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa F.B., Melo S.A., Araujo F., Ramos C.A.N., Carvalho-Neta A.V., Nogueira R.M.S.C.G. Serological parasitological and molecular assessment of Babesia bovis and Babesia bigemina in cattle from state of Maranhão. Rev Caatinga. 2015;28:217–224. [Google Scholar]
- Da Silva J.B., Vinhote W.M.S., Oliveira C.M.C., Andre M.R., Machado R.Z., da Fonseca A.H., Barbosa J.D. Molecular and serological prevalence of Anaplasma marginale in water buffaloes in northern Brazil. Ticks Tick Borne Dis. 2013;5:100–104. doi: 10.1016/j.ttbdis.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Da Silva J.B., Lopes C.T.A., Pinheiro C.P., Lima D.H.S., Silva R.S.L., Fonseca A.H., Araujo F.R., Barbosa-Neto J.D. Molecular and serological prevalence of Babesia bovis and Babesia bigemina in water buffaloes (Bubalus bubalis) on Marajo Island State of Para Brazil. Pesq Vet Bras. 2013;33:847–850. [Google Scholar]
- Da Silva J.B., Dias M.C., Castro G.N.S., Santos P.N., Fonseca A., Reis A.B., S N., Silva J.D. Barbosa. Serological occurrence of Babesia bovis Babesia bigemina and Anaplasma marginale in cattle and water buffaloes of Pará Brazil. Semin Cien Agrar. 2014;35:2495–2500. [Google Scholar]
- Da J.B., Silva C.T.A., Lopes M.G.S., Souza A.F.B., Gibson W.M.S., Vinhote A.H., Fonseca F.R., Araujo J.D. Barbosa-Neto serological and molecular detection of Anaplasma marginale in water buffaloes on Marajo Island state of Para. Brazil Pesq Vet Bras. 2014;34:11–14. [Google Scholar]
- Dalagnol A.A., Martins E., Madruga C.R. Prevalência de agentes da tristeza parasitária bovina em bovinos de corte na região de clima cfb SC. Agrop Cat. 1999;12:46–47. [Google Scholar]
- F. Dantas-Torres, D. Otranto, Anaplasmosis In: Marcondes C B editor. Arthropod Borne Disease Switzerland. Springer 2016 p. 215–222.
- F. Dantas-Torres, L. C. Alves, G. Uilenberg, Babesiois in: Marcondes C B editor. Arthropod Borne Disease. Switzerland. Springer 2016 347–354.
- Estrada-Pena A., Acedo C.S., Quilez J., Del Cacho E.D. A retrospective study of climatic suitability for the tick Rhipicephalus (Boophilus) microplus in the Americas. Glob. Ecol. Biogeogr. 2005;14:565–573. [Google Scholar]
- Figueroa J.V., Chieves L.P., Johnson G.S., Buening G.M. Detection of Babesia bigemina-infected carriers by polymerase chain reaction amplification. J. Clin. Microbiol. 1992;30:2576–2582. doi: 10.1128/jcm.30.10.2576-2582.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa J.V., Chieves L.P., Johnson G.S., Buening G.M. Multiplex polymerase chain reaction-based assay for the detection of Babesia bigemina Babesia bovis and Anaplasma marginale DNA in bovine blood. Vet. Parasitol. 1993;50:69–81. doi: 10.1016/0304-4017(93)90008-b. [DOI] [PubMed] [Google Scholar]
- Gonçalves P.M. Epidemiologia e controle da tristeza parasitária bovina na região sudeste do Brasil. Ciência Rural. 2000;30:187–194. [Google Scholar]
- Grisi L., Massard C.L., Borja G.E.M., Pereira J.B. Impacto econômico das principais ectoparasitoses em bovinos no Brasil. Hora Veterinária. 2002;125:8–10. [Google Scholar]
- Guedes Junior D.S., Araujo F.R., Silva F.J.M., Rangel C.P., Neto J.D.B., Fonseca A.H. Frequency of antibodies to Babesia bigemina B bovis Anaplasma marginale Trypanosoma vivax and Borrelia burdgorferi cattle from the northeastern region of the state of Para Brazil. Rev. Bras. Parasitol. Vet. 2008;17:105–109. doi: 10.1590/s1984-29612008000200008. [DOI] [PubMed] [Google Scholar]
- Guglielmone A.A. Epidemiology of babesiosis and anaplasmosis in south and Central America. Vet. Parasitol. 1995;57:109–119. doi: 10.1016/0304-4017(94)03115-d. [DOI] [PubMed] [Google Scholar]
- Furlong J., Martins J.R.S., Prata M.C.A. Controle estratégico do carrapato bovino. A Hora Veterinária. 2004;23:53–54. [Google Scholar]
- Kocan K.M. Targeting ticks for control of selected hemoparasitic diseases of cattle. Vet. Parasitol. 1995;57:121–151. doi: 10.1016/0304-4017(94)03116-e. [DOI] [PubMed] [Google Scholar]
- Lew A.E., Bock R.E., Minchin C.M., Masaka S.A. Msp1a polymerase chain reaction assay for specific detection and differentiation of Anaplasma marginale isolates. Vet. Microbiol. 2002;86:325–335. doi: 10.1016/s0378-1135(02)00017-2. [DOI] [PubMed] [Google Scholar]
- Martins T.M., Neves L., Pedro O.C., Fafetine J.M., do Rosário V.E., Domingos A. Molecular detection of Babesia spp and other haemoparasitic infections of cattle in Maputo Province Mozambique. Parasitol. 2010;137:939–946. doi: 10.1017/S003118200999196X. [DOI] [PubMed] [Google Scholar]
- Oliveira-Sequeira T.C.G., Oliveira M.C.S., Araujo J.P., Amarante A.F.T. PCR-based detection of Babesia bovis and Babesia bigemina in their natural host Boophilus microplus and cattle. Int. J. Parasitol. 2005;35:105–111. doi: 10.1016/j.ijpara.2004.09.002. [DOI] [PubMed] [Google Scholar]
- C. E .Suarez, G. H. Palmer, D. P. Jasmer, S. A. Hines, L. E. Perryman, T. F. McElwain, Characterization of the gene encoding a 60-kilodalton Babesia bovis merozoite protein with conserved and surface exposed epitopes. Mol. Biochem. Parasitol. 46 (1991) 45–52. [DOI] [PubMed]
- Peel M.C., Finlayson B.L., McMahon T.A. Updated world map of the Köppen–Geiger climate classification. Hydrol. Earth Syst. Sci. 2007;11:1633–1644. [Google Scholar]
- Rodríguez I., Burri C., Noda A.A., Douet V., Gern L. Multiplex PCR for molecular screening of Borrelia burgdorferi sensu lato Anaplasma spp and Babesia spp. Ann Agric Environ Med. 2015;22:642–646. doi: 10.5604/12321966.1185767. [DOI] [PubMed] [Google Scholar]
- Santos G.B., Gomes I.M.M., Silveira J.A.G., Pires L.C.S.R., Azevedo S., Antonelli S.A.C., Ribeiro M.F.B., Horta M.C. Cattle tick fever in semi-arid of Pernambuco. Pesq Vet Bras. 2017;37:1–7. [Google Scholar]
- Shebish E., Vemulapalli R., Oseto R. Prevalence and molecular detection of Anaplasma marginale Babesia bovis and Babesia bigemina in cattle from Puntarenas Province Costa Rica. Vet. Parasitol. 2012;188:164–167. doi: 10.1016/j.vetpar.2012.03.009. [DOI] [PubMed] [Google Scholar]
- da Silva J.B., Dias C., Manier B.S.M.L., Valim J.R.A., Bom Jardim H.A., Fonseca A.H., Barbosa J.D. Serological detection of Anaplasma marginale Babesia bovis and Babesia bigemina in beef cattle of the northern and central-western regions of Brazil. Semin Cien Agrar. 2015;36:1431–1435. [Google Scholar]
- Souza A.P., Gonzales J.C., Ramos C.I., Paloschi C.G., Moraes A.N. Variação sazonal de Boophilus microplus no planalto catarinense. Pesq Agropec Bras. 1988;23:627–630. [Google Scholar]
- F. A. L. Souza, J. F. V. Braga, L. V. Fires, C. J. S. de Carvalho, E. A. Costa, M. F. B. Ribeiro, R. L. Santos, S. M. M. Silva S Babesiosis and anaplasmosis in dairy cattle in Northeastern Brazil. Pesq Vet Bras. 33 (2013) 1057–1061.
- Zhou M., Cao S., Sevinc F., Sevinc M., Ceylan O., Moumouni P.F., Jirapattharasate C., Liu M., Wang G., Iguchi A., Vudriko P., Suzuki H., Xuan X. Molecular detection and genetic identification of Babesia bigemina Theileria annulata Theileria orientalis and Anaplasma marginale in Turkey. Ticks Tick Borne Dis. 2016;7:126–134. doi: 10.1016/j.ttbdis.2015.09.008. [DOI] [PubMed] [Google Scholar]