Abstract
Allergy is a major public health concern, the main treatment for which is symptomatic relief with anti-inflammatory drugs. A key clinical challenge is to induce specific tolerance in order to control allergen-specific memory B and T cells, and specifically block effector cell responses. Our lab recently developed antigen-specific regulatory T-cell (Treg) therapies as a treatment for adverse responses. Recently, we created a chimeric antigen receptor (CAR) approach in which we engineered a target protein antigen, ovalbumin (OVA), linked with the transmembrane and signal transduction domains, CD28-CD3ζ to directly target B cells and sensitized mast cells in an allergy model. We named this receptor “BAR” for B-cell Antibody Receptor. Murine or human Tregs, transduced with a BAR containing OVA or control Tregs expressing an unrelated antigen, were successfully expanded in vitro and tested in the murine OVA-alum allergy model with measurable titers of anti-OVA IgE. Because BAR Tregs express the target antigen and could interact with specific IgE on sensitized mast cells, we first demonstrated that intravenously injected OVA-BAR Tregs did not directly lead to a drop in temperature or release of mediators in plasma indicative of anaphylaxis. Forty-eight hours later, mice were challenged intraperitoneally with 200 μg OVA to induce an anaphylactic reaction, and temperature immediately measured for 30 min. We found that OVA-BAR Tregs protected mice from hypothermia, whereas mice given control BARs (expressing an unrelated antigen) or PBS showed substantial temperature drops indicative of anaphylaxis when systemically challenged with OVA. Importantly, this effect was also demonstrated in a passive anaphylaxis model in which mice that received anti-OVA IgE antibody were protected from hypothermia when treated with OVA-BAR Tregs prior to systemic OVA challenge. These results provide proof of principle that engineered allergen-specific T-regulatory cells can provide clinical protection against severe allergic reactions in individuals already IgE-sensitized to an allergen.
1. Introduction
Allergies to food, venom, or pollen affect up to one in five Americans but treatments primarily involve provision of systemic symptomatic relief and very few offer direct efforts to prevent or reverse the specific responsiveness. While the mechanisms of allergy development are not yet fully elucidated, IgE-mediated allergies typically occur when CD4+ naïve T cells differentiate into T-helper 2 (Th2) cells that produce cytokines such as IL-4, IL-5, and IL-13. IL-4 and IL-13 subsequently drive B cells to class switch into IgE. The specific IgE secreted by plasma cells attach via Fcε receptors on mast cells and basophils. These cells then degranulate and release mediators such as histamine when exposed to the specific allergen recognized by their surface IgE antibodies. Regulatory T-cell (Treg) therapy is potentially promising in re-balancing the immune response, but polyclonal Tregs are not specific. Our lab has developed engineered human and murine regulatory T cells (Tregs), rendered specific by expression of single chain variable fragments (scFv) or T-cell receptors (TCR) as chimeric antigen receptors (CAR), both of which have shown efficacy in vitro and in vivo in models of hemophilia and autoimmunity [1,2]. Recently, we modified this Treg approach to express antigen on Tregs. These cells, which we term BAR (for B-cell Antibody Receptor) Tregs, can interact and suppress specific B cells directly via recognition by the B-cell receptor [3], an approach that has advantages over non-specific immune modulators or other CAR approaches that are MHC restricted, for example. We hypothesize that BAR Tregs have the potential to specifically treat allergy as long as the allergen is known. In this proposal, we focus on BAR Tregs because they target only the relevant specific B cells or IgE-sensitized mast cells.
2. Material and methods
2.1. Mice
Five to six week old female and male BALB/c mice obtained from The Jackson Laboratory (Bar Harbor, ME) were housed at the animal facility at Uniformed Services University of the Health Sciences (USUHS). Animal procedures were approved by the Institutional Animal Care and Use Committee.
2.2. Generation of BAR retroviral vectors
The cDNA sequence for chicken ovalbumin (OVA) and human Factor VIII-A2 (FVIII A2) were derived from GenBank and placed in a construct with a transmembrane region and the intracellular domains of CD28 and CD3zeta. The construct was made as described previously [1,3,4]. The retroviral particles were produced using a pRetroX-IR-ESZsGreen (Clontech, Mountain View, CA) vector using a Phoenix-Ecotropic or Phoenix-Amphotrophic packaging system (Clontech Laboratories) to transduce murine and human cells respectively. Culture supernatants containing the retroviral particles were concentrated with Retro-X Concentrator (Clontech Laboratories), and aliquots were stored at −80 °C until use.
2.3. Murine T-cell isolation and retroviral transduction and expansion of murine iTregs
Splenocytes were isolated from naïve BALB/c murine spleens, and CD4+ T cells were enriched by positive selection using murine CD4 T-cell isolation kit (Miltenyi Biotech, Somerville, MA) according to the manufacturer’s instructions. These CD4 T cells were activated for 24 to 48 h with anti-CD3 (Clone 1452C11; 4 μg/mL) and anti-CD28 (Clone 37.51; 2 μg/mL) soluble antibody in complete media supplemented with recombinant IL-2 (200 U/mL) in presence of 25 ng/mL of human TGFβ at 37 °C with 5% CO2 to create induced Tregs.
Activated murine CD4 T cells were transduced by spinfection, as described previously [3]. Briefly, transduction was performed by first plating the retroviral particle supernatant onto a 24-well culture plate pretreated with 10 μg/mL RetroNectin, blocking with PBS-BSA 2% for 30 min, and spinning at 2000 ×g at 32 °C for 2 h. Activated T cells were then centrifuged onto the viral particle–coated plate at 500 ×g at 32 °C for 15 min. Transduced cells were transferred to tissue culture plate after 48 h and expanded for 7–10 days. BAR-transduced GFP + CD4 + CD25+ T cells were sorted using FACS Aria Fusion (BD Biosciences, Franklin Lakes, NJ). Sorted murine T cells were rested for 2–3 h without IL-2 and then injected into animals.
2.4. Human T-cell isolation, retroviral transduction and expansion of transduced human Tregs
Buffy-coat blood samples from healthy donors between 26 and 72 years of age (n = 3) were obtained from the Department of Transfusion Medicine, Clinical Center, National Institutes of Health. The procedures were approved by the USUHS Institutional Review Board. The isolation of Tregs (CD4 + CD25hiCD127low) and CD4+ conventional T cells (Teff) (CD4 + CD252CD127hiCD45RA+) from human peripheral blood was as previously described [1–3].
Transduction and expansion of FACS-purified Tregs were as previously described [1–3]. Briefly, thawed Treg cells were pre-stimulated with soluble 5 μg/mL anti-human CD3ε (clone 64.1) and 2 μg/mL anti-human CD28 (clone CD28.2; eBioscience) in complete culture media in the presence of 100 U/mL IL-2 and 2 mM of ODN for 48 to 72 h, as described by Kim et al. [2]. Ten days after the pre-stimulation, the successfully BAR-transduced hTregs were FACS sorted based on GFP expression and restimulated once with 0.5 μg/mL soluble anti-human CD3ε in the presence of 6000 rad γ-irradiated autologous PBMC (PBMC/T cell ratio = 2:1) in the presence of ODN (2 mM) and IL-2 (100 U/mL) and expanded for ten days. Expanded T cells were frozen in FBS containing 10% DMSO and stored at −80 °C until use.
Expression of OVA domain on BAR Tregs on the transduced expanded murine and human Tregs was analyzed as described previously [3]. In addition, intracellular Foxp3 confirmed that these BAR Tregs were > 70–90% positive as previously reported by our lab [1,3] (Supplementary Fig. 1).
2.5. Experimental design and sample collections
Mice were randomly divided into groups of 5–10 animals. All animals were bled from the retro-orbital plexus per approved protocol before immunization and sera were collected and stored at −20 °C. Mice were intraperitoneally immunized weekly with 50 μg of OVA (Grade V; Sigma) adsorbed to Alum (Imject® Alum, Thermo-Fisher) for 3 weeks. Followed by two weeks of rest, mice were bled to establish anti-OVA IgE titers; IgE anti-OVA was detectable in all mice (see below and Supplementary Fig. 2). Mice received an intravenous injection (100 μL by tail vein) of PBS or 0.5 × 106 OVA or irrelevant FVIII domain BAR Tregs (induced murine Tregs or human nTregs). Two days later, mice were intraperitoneally challenged with 200 μg of OVA in PBS and rectal temperature was measured before challenge and every 5 minutes post-challenge for 30 min. Mice were re-challenged with 200 μg of OVA in PBS one month or ten days after receiving murine or human BAR T cells, respectively.
2.6. Passive anaphylaxis
Naïve mice received an intraperitoneal injection of 7 μg of murine anti-OVA IgE polyclonal antibody. Six to eight hours later, 0.5 × 106 OVA-BAR Tregs or control Tregs were injected intravenously. After two days, mice were intraperitoneally challenged with 200 μg of OVA in PBS and temperature was measured before challenge and every 5 minutes post-challenge as in the active protocol.
2.7. ELISPOT and ELISA assays for antibody formation
To examine the effect of BAR Tregs directly on B cells, spleen cells from naïve mice were stimulated with 1 μg/mL E. coli lipopolysaccharide (LPS) for polyclonal activation for 48 h as described [4]. The cells were washed twice in culture medium and transferred to OVA- or FVIII-coated 96-well ELISPOT plates (EMD Millipore, Billerica, MA) and cultured overnight. The number of spots by specific antibody-secreting B cells (ASCs) was visualized by incubation with HRP-conjugated rabbit anti-mouse IgM/IgG (H + L) (Thermo Fisher) followed by AEC substrate (BD Biosciences). To measure anti-OVA total IgG and IgE levels (Cayman Chemical), an ELISA method was modified from previously reported protocols using antibodies to the specific isotype heavy chains and OVA as the target [5]. Briefly, ELISA plates were coated with 10 μg/mL of OVA (grade V, Sigma) and then blocked for 2 h with PBS-BSA 2% at room temperature. Diluted sera were added and incubated for 1–2 h. After washing, the secondary anti-murine IgE antibody was added for 1 h. The plate was developed using a TMB substrate, 2 N sulfuric acid solution was used to stop the reaction and absorbance was determined using an ELISA plate reader at 450 nm absorbance.
2.8. Histamine and mMCP-1 ELISA
Murine mast cell protease 1 (mMCP-1) (Thermo Fisher Scientific) and histamine ELISAs (Beckman Coulter) were performed by manufacturers’ instructions. Immediately after blood collection serum were extracted and acylated to conserve the histamine prior to performing the ELISA. Absorbance measurements were taken using a Synergy HTX Multi-Mode Microplate reader. Samples were diluted with standard buffer to fit within the linear range of the standard curve.
2.9. Statistical analysis
Statistical analysis was performed using Prism software (v6.0; GraphPad Software, La Jolla, CA). A p-value ≤ .05 was considered statistically significant.
3. Results
3.1. Murine OVA-BAR induced Tregs are safe and do not induce anaphylaxis
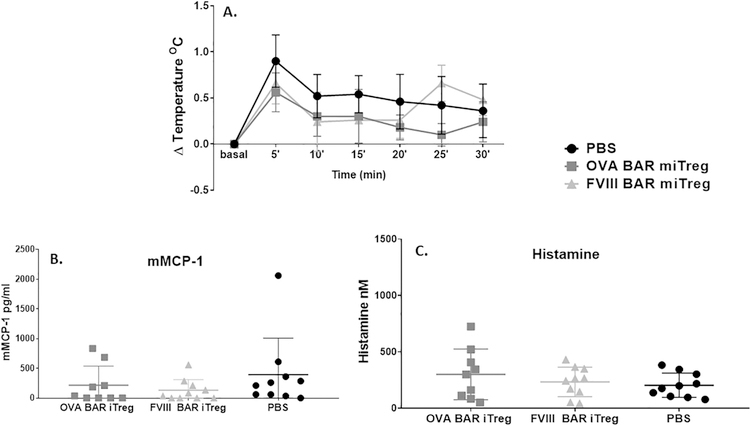
Our hypothesis was that expression of a target antigen on the surface of Tregs would lead to interactions with specific B-cell surface immunoglobulin receptors (BCR) to target and suppress adverse B-cell responses [3]. However, the target antigen of Tregs might also bind to specific IgE bound to IgE receptors on mast cells, thereby signaling for degranulation and mediator release. Therefore, we first evaluated whether injection of OVA BAR-Tregs caused anaphylaxis in OVA-immunized mice. After immunization with OVA/alum and verification of OVA-specific IgE generation, mice received 5 × 105 OVA-expressing BAR Tregs intravenously. Body temperature was measured before and for 30 min after challenge. Serum obtained at 30 min was assessed for levels of mMCP-1 and histamine release, markers of mast cell degranulation. As shown in Fig. 1, we found that injection of OVA BAR Tregs into OVA-sensitized mice caused neither anaphylaxis nor release of histamine or mMCP1.
Fig. 1. Injection of OVA-BAR iTregs did not induce anaphylaxis.
OVA-BAR induced murine induced Tregs (miTreg) injection did not induce hypothermia (A), mMCP-1 release (B) or histamine release (C) in OVA-sensitized mice. BALB/c mice (n = 9–10) received 3 injections of OVA/alum. After two weeks of rest mice received 0.5 × 106 cells and temperature was measured immediately after cell transfer; blood was drawn at 30 minutes post injection and processed immediately for mMCP-1 or histamine release. Similar results were obtained after injection of human nTregs (Supplementary Fig. 3).
3.2. Murine OVA-BAR Tregs protect mice from hypothermia
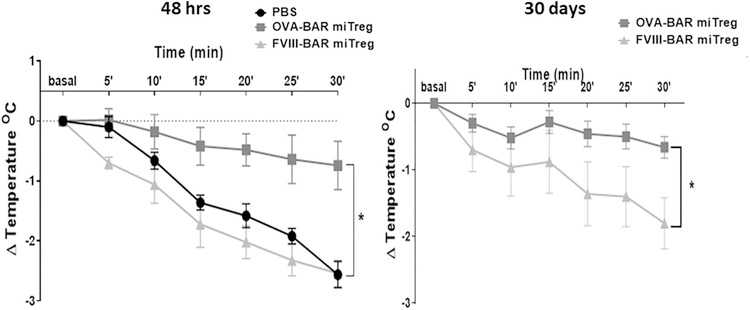
While treating the OVA-sensitized mice with OVA BAR-Tregs did not lead to clinical symptoms of anaphylaxis, mice challenged with 200 μg OVA showed a significant decrease in rectal temperature (Fig. 1, and data not shown). In the experiment shown in Fig. 2A, mice received either 5 × 105 OVA BAR-Tregs, Tregs expressing an unrelated antigen (FVIII A2 domain which is approximately the same molecular weight as OVA), or PBS, and were challenged with 200 μg OVA systemically. The temperature drop was significantly reduced in the group of mice that received OVA BAR-Tregs 48 h before challenge, but not in the groups that received irrelevant Tregs or PBS. Interestingly, mice treated with OVA BAR Tregs were protected from a second challenge with OVA even 30 days after they received the Tregs (Fig. 2B). While IgE was detectable after three immunizations, no drop in titer was observed 9 days after treatment with BAR-Tregs (Supplementary Fig. 2).
Fig. 2. Mouse OVA-BAR iTregs protect mice from anaphylaxis up to one month after injection.
(A) OVA-s mice were challenged as in Materials and Methods and temperature measured immediately after challenge every 5 min for 30 min. (B) Mice were re-challenged after one month and temperature measured as in A. Data was analyzed per paired t-test, *p < .05 between OVA-BARmiTreg and PBS or FVIII-BARmiTreg; N = 5 BALB/c female mice per group.
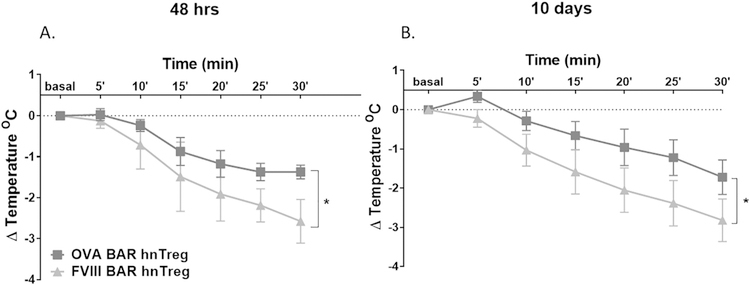
We have also generated BAR-Tregs from expanded human Tregs derived from PBMC, as described previously by our lab [1–3]. These human Tregs did not cause a temperature drop per se (Supplementary Fig. 3). However, mice injected with of 1 × 106 OVA-BAR human Tregs (but not FVIII A2-BAR Tregs) were also protected from anaphylaxis as measured by temperature drop (Fig. 3). This validates the ability of xenogeneic Tregs to suppress adverse responses in a second model, anaphylaxis, as we previously reported in hemophilia [1–3]. This protection was observed up to 10 days after treatment, but then waned due to the rejection of the human T cells by the murine immune system (Fig. 3, and data not shown).
Fig. 3. Human OVA-BAR nTregs protect mice from anaphylaxis up to 10 days after injection.
BALB/c female mice (n = 8) were challenged 48 h after receiving human BAR Tregs (hnTreg) from a single donor in each experiment. (A) Temperature measured immediately after challenge every 5 min for 30 min. (B) Mice were re-challenged after 10 days and temperature measured as the first time. Data was analyzed per paired t-test, *p < .05 between OVA-BARhnTreg and PBS or FVIII-BARhnTreg.
3.3. Effect of BAR-Tregs on naïve B cells and antibody formation
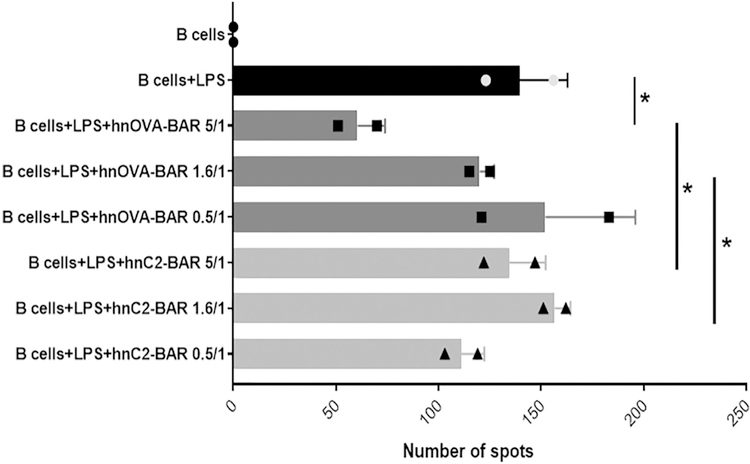
Our hypothesis was that OVA BAR expressing T cells would interact directly with B cells expressing OVA-specific BCR to suppress responsiveness of those B cells. We previously reported that antigen-domain expressing BAR Tregs can suppress specific B cell responses, for example, to FVIII in a prophylactic protocol [3]. However, we did not observe a drop in OVA-specific IgE or IgG in OVA-immunized mice within 9–30 days (Supplementary Fig. 2). To test whether OVA-BAR Tregs could directly target and suppress naïve B cells, we used a short-term assay where B cells were stimulated with E. coli lipopolysaccharide as previously reported by Parvathaneni et al. [4]. As shown in Fig. 4, OVA-BAR human Tregs specifically blocked the development of anti-OVA ELIspots in a dose dependent manner, whereas Tregs expressing FVIII C2 did not.
Fig. 4.
Effect of BAR Tregs on naïve B cells in the presence of human OVA-BAR or FVIII C2 domain BARs + LPS. Tregs and LPS were added at day 0 to naïve spleen cells. ELIspot assay on LPS activated B cells (day 3). *p < .05 between B cells + LPS and hOVA-BAR at 5/1 ratio; hOVA-BAR and hC2BAR at 5/1 ratio; hOVA-BAR at 1.6/1 ratio and hC2BAR at 1.6/1 ratio.
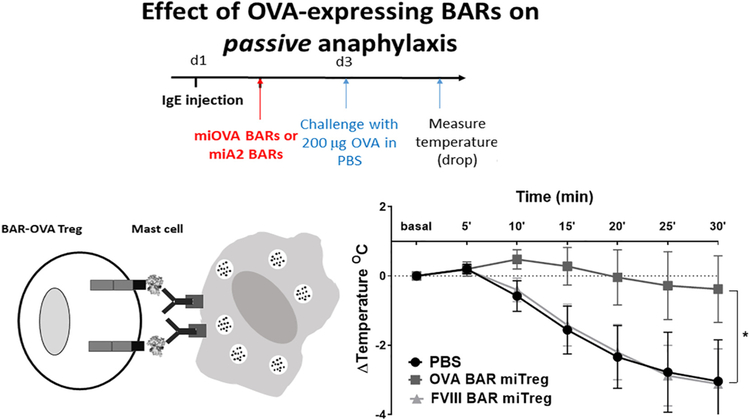
3.4. Effect of BAR-Tregs on passive anaphylaxis and mast cells
We next determined whether OVA-BAR Tregs could suppress passive anaphylaxis. Groups of mice received 20 μL (~7 μg of polyclonal anti-OVA IgE) of murine serum from OVA-immunized mice and 6 to 8 h later received OVA- or FVIII BAR Tregs, or PBS. After 48 h, these mice were challenged with an anaphylaxis-inducing dose of OVA. As shown in Fig. 5, OVA BAR Tregs protected against anaphylaxis after systemic challenge, compared to the control groups. Given that there were no B cells producing OVA-specific IgE antibodies in this experiment, these results suggest that OVA BAR Tregs may have a direct suppressive effect on sensitized mast cells.
Fig. 5. Mouse OVA-BAR iTregs protect from passive anaphylaxis.
Temperature measured after challenge every 5 min for 30 min. Data was analyzed per paired t-test, *p < .05 between OVA-BARmiTreg and PBS or FVIII-BARmiTreg (n = 5 female mice per group).
4. Discussion
The importance of regulatory T cells (Tregs) in moderating the immune system is well established Tregs are important in suppressing adverse immune responses in autoimmunity, transplantation, hemophilia and allergic diseases [6,7]. Indeed, clinical trials with expanded Tregs are ongoing [8–12]. Moreover, polyclonal Tregs have been shown to suppress mast cell degranulation [13,14]. We know that Tregs can inhibit the activation of allergen-specific Th2 cells during allergic reactions [15] and has been demonstrated that Treg promote the production of allergen specific IgG4 antibodies while inhibiting IgE by directly acting on B cells [16].
Thus, using engineered specific Tregs to treat allergy is a promising approach. By utilizing Tregs expressing a specific allergen, this approach targets allergen specific B-cell populations and mast cells without compromising the functioning of normal immune cells. In this paper we used the OVA-induced allergy model. We were able to protect OVA-sensitized mice from anaphylaxis by injecting OVA-specific BAR Tregs prior to systemic allergen challenge and the effect lasted at least 30 days.
These BAR OVA Tregs were also able to protect from anaphylaxis in a passive model where the mice were injected with the anti-OVA antibodies. This result confirms the effect of the Tregs not only on B cells through their BCR, but also on mast cells. Even though we demonstrated in our in vitro experiment that BAR Tregs can prevent OVA-specific B cells from producing antibodies, in this study the predominant protective effect BAR Tregs exerted against anaphylaxis appears to have been independent of actions on B cells in the acute experiments.
In the OVA immunization model, we did not observe reductions in circulating OVA-specific IgE levels, and in the passive model OVA-specific IgE antibodies were not being made by host B cells. Thus, we hypothesize that the protection was most likely occurring by BAR Treg suppression of mast cell function. The mechanism by which these BAR specific Tregs interact with mast cells is unknown and will be investigated in future studies. We hypothesize that this interaction is made through the bound anti-OVA IgE. However, it is also possible that this is due to an interaction through OX40-OX40L, as reported by Gri et al. [13].
BAR T cells, in which Tregs or CD8 cells express antigens recognized by BCRs, have been used in different disease models. BAR-Tregs and BAR-CD8s have been reported by our lab as a prophylactic treatment in a murine model of hemophilia [3,4]. A similar approach has also been reported by Ellebrecht et al. using cytotoxic CD8 T cells in an autoimmune disease model (pemphigus), wherein CD8 T cells expressing the desmoglein 3 (Dsg3) protein were used to target Dsg3-specific B cells, major players in auto-antibodies production leading to pemphigus [17].
This BAR approach was designed to target the B cells but not the long-lived plasma cells. These bone marrow and spleen residing cells lose their BCR expression and may not be recognized by BAR-Tregs. Therefore, additional therapies are needed to reduce the number of IgE producing long lived plasma cells, although long-term effects on memory B cells may result in a decrease in IgE titers. Thus, further testing to determine whether BAR Tregs can exert a long-term effect on memory B cells needs to be done.
Finally, it is not known whether a single BAR-Treg will protect against anaphylaxis in mice sensitized to multiple antigens, but we predict that bystander effects are likely based on the results of Killoran et al. [18]. Since mast cells could bind IgE antibodies of different specificities, this can easily be tested in a future study. Thus, BAR-Tregs therapy has potential to alleviate allergy to multiple antigens.
Supplementary Material
Acknowledgments
MA, EM and DWS designed the experiments, MA, AZ, AL, LK, SV performed the work, and MA and DWS wrote the paper. We thank Kateryna Lund, Kheem Bisht for help with flow cytometry, Claire Monahan for technical assistance, and Yong Chan Kim and John Ryan for critical advice. This work was supported in part by NIH RO1 HL126727 to DWS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Defense.
Footnotes
Declaration of Competing Interests
The authors declare no conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clim.2019.07.009.
References
- [1].Yoon J, Schmidt A, Zhang AH, Königs C, Kim YC, Scott DW, FVIII-specific human chimeric antigen receptor (CAR) T-regulatory cells suppress T-and B-cell responses to FVIII, Blood 129 (2) (2017) 238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kim YC, Zhang AH, Su Y, Rieder SA, Rossi RJ, Ettinger RA, Pratt KP, Shevach EM, Scott DW, Engineered antigen-specific human regulatory T cells: immunosuppression of FVIII-specific T- and B-cell responses, Blood 125 (7) (2015) 1107–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhang AH, Yoon J, Kim YC, Scott DW, Targeting antigen-specific B cells using antigen-expressing transduced regulatory T cells, J. Immunol 201 (5) (2018) 1434–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Parvathaneni K, Scott DW, Engineered FVIII-expressing cytotoxic T cells target and kill FVIII-specific B cells in vitro and in vivo, Blood Adv 2 (18) (2018) 2332–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang AH, Skupsky J, Scott DW, Effect of B-cell depletion using anti-CD20 therapy on inhibitory antibody formation to human FVIII in hemophilia A mice, Blood 117 (7) (2011) 2223–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Adair PR, Kim YC, Zhang AH, Yoon J, Scott DW, Human Tregs made antigen specific by gene modification: the power to treat autoimmunity and antidrug antibodies with precision, Front. Immunol 8 (2017) 1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sicard A, Levings MK, Scott DW, Engineering therapeutic T cells to suppress alloimmune responses using TCRs, CARs, or BARs, Am. J. Transplant 18 (6) (2018) 1305–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Esensten JH, Muller YD, Bluestone JA, Tang Q, Regulatory T-cell therapy for autoimmune and autoinflammatory diseases: the next frontier, J. Allergy Clin. Immunol 142 (6) (2018) 1710–1718. [DOI] [PubMed] [Google Scholar]
- [9].Gitelman SE, Bluestone JA, Regulatory T cell therapy for type 1 diabetes: may the force be with you, J. Autoimmun 71 (2016) 78–87. [DOI] [PubMed] [Google Scholar]
- [10].Blazar BR, MacDonald KPA, Hill GR, Immune regulatory cell infusion for graft-versus-host disease prevention and therapy, Blood 131 (24) (2018) 2651–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Brunstein CG, Blazar BR, Miller JS, Cao Q, Hippen KL, McKenna DH, Curtsinger J, McGlave PB, Wagner JE, Adoptive transfer of umbilical cord blood-derived regulatory T cells and early viral reactivation, Biol Blood Marrow Transplant 19 (8) (2013) 1271–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Riley JL, June CH, Blazar BR, Human T regulatory cell therapy: take a billion or so and call me in the morning, Immunity 30 (5) (2009) 656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S, Tripodo C, Viola A, Odom S, Rivera J, Colombo MP, et al. , CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction, Immunity 29 (5) (2008) 771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pellerin L, Jenks JA, Begin P, Bacchetta R, Nadeau KC, Regulatory T cells and their roles in immune dysregulation and allergy, Immunol. Res 58 (2–3) (2014) 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Palomares O, Yaman G, Azkur AK, Akkoc T, Akdis M, Akdis CA, Role of Treg in immune regulation of allergic diseases, Eur. J. Immunol 40 (5) (2010) 1232–1240. [DOI] [PubMed] [Google Scholar]
- [16].Meiler F, Klunker S, Zimmermann M, Akdis CA, Akdis M, Distinct regulation of IgE, IgG4 and IgA by T regulatory cells and toll-like receptors, Allergy 63 (11) (2008) 1455–1463. [DOI] [PubMed] [Google Scholar]
- [17].Ellebrecht CT, Bhoj VG, Nace A, Choi EJ, Mao X, Cho MJ, Di Zenzo G, Lanzavecchia A, Seykora JT, Cotsarelis G, et al. , Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease, Science 353 (6295) (2016) 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Killoran KE, Kropp LE, Lindrose AR, Curtis HE, Cook D, Mitre E, Rush desensitization with a single antigen induces subclinical activation of mast cells and protects against bystander challenge in dually sensitized mice, Clin. Exp. Allergy 49 (4) (2019) 484–494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.