Abstract
The World Health Organisation has set the goal for elimination of Human African Trypanosomiasis (HAT), caused by Trypanosoma brucei gambiense (gHAT), as a public health problem for 2020 and for the total interruption of transmission to humans for 2030. Targeting human carriers and potential animal reservoir infections will be critical to achieving this ambitious goal. However, there is continuing debate regarding the significance of reservoir host animals, wild and domestic, in different epidemiological contexts, whilst the extent and duration of the asymptomatic human carrier state is similarly undefined.
This paper reviews the status of the knowledge of latent infections in wild and domestic animal reservoir hosts towards the goal of better understanding their role in the transmission dynamic of the disease. Focus areas include the transmission cycles in non-human hosts, the infectivity of animal reservoirs to Glossina palpalis s.l., the longevity of infection and the stability of T. b. gambiense biological characteristics in antelopes and domestic animals. There is compelling evidence that T. b. gambiense can establish and persist in experimentally infected antelopes, pigs and dogs for a period of at least two years. In particular, metacyclic transmission of T. b. gambiense has been reported in antelope-G.p.palpalis-antelope and pig-G.p.gambiensis-pig cycles.
Experimental studies demonstrate that the infectiveness of latent animal reservoir infections with T. b. gambiense is retained in animal-Glossina-animal cycles (antelopes and pigs) for periods of three years and human infectivity markers (human serum resistance, zymodeme, DNA) are stable in non-human hosts for the same period. These observations shed light on the epidemiological significance of animal reservoir hosts in specific ecosystems characterized by presently active, as well as known “old” HAT foci whilst challenging the concept of total elimination of all transmission by 2030. This target is also compromised by the existence of human asymptomatic carriers of T. b. gambiense often detected outside Africa after having lived outside tsetse infested areas for many years - sometimes decades.
Non-tsetse modes of transmission may also play a significant but underestimated role in the maintenance of foci and also preclude the total elimination of transmission - these include mother to child transmission and sexual transmission. Both these modes of transmission have been the subject of case reports yet their frequency in African settings remains to be ascertained when the context of residual foci are discussed yet both challenge the concept of the possibility of the total elimination of transmission.
Keywords: Human African Trypanosomiasis (HAT), T. b. gambiense, Elimination, Glossina, Transmission cycles, Wild and domestic animal reservoirs, Infectivity to tsetse, Longevity of parasitaemia, Non-tsetse transmission
1. Introduction
Human African Trypanosomiasis (HAT), known as sleeping sickness, is a fatal protozoan infection caused by sub-species of Trypanosoma brucei and transmitted through the bites of tsetse flies (Glossina spp). Trypanosoma brucei gambiense is found largely in Central and West Africa but also in South Sudan and northern Uganda and causes a chronic infection whereas T. b. rhodesiense found in Eastern and Southern Africa causes the acute form of disease. People living in remote rural areas in sub-Sahara Africa with limited access to health services are under risk of infection although recent maps of distribution show most foci are located in Democratic Republic of Congo (DRC) (Franco et al., 2018).
The disease caused devastating epidemics during the last century (WHO, 2018; Büscher et al., 2017) whilst during the last two decades disease surveillance and control measures implemented by National Sleeping Sickness Control Programmes (NSSCPs), supported by the World Health Organisation (WHO) and numerous stakeholders, have been intensified and better coordinated. These have resulted in reductions in the incidence of HAT (Franco et al., 2018; WHO (World Health Organisation), 2018, WHO (World Health Organisation), 2019). In 2016 only 2164 new cases of HAT were reported to WHO. Of these, 2110 were caused by T. b. gambiense (92% reduction compared to 2000), the remaining by T. b. rhodesiense (2.5% of the total HAT reported cases, 92% reduction compared to 2000). HAT is now targeted for elimination by WHO for the year 2020 (fewer than 2000 cases per year, elimination as public health problem) and the elimination of transmission (zero cases) is targeted for 2030 (WHO (World Health Organisation), 2018, WHO (World Health Organisation), 2019).
The epidemiology of HAT has been reviewed recently (Franco et al., 2014a). Successful intervention and control programmes have been underpinned by knowledge of the transmission dynamics of both forms of the disease. Controlling the zoonotic T. b. rhodesiense disease, with a domestic and wild animal reservoir, requires a multidisciplinary approach that includes veterinary health services and vector control (Franco et al., 2014b; Okello and Welburn, 2014). In the chronic T. b. gambiense form of the disease, humans are generally regarded as the main parasite reservoir (WHO, 2018; Franco et al., 2014a). However, beside the well-known existence of asymptomatic human carriers (Frezil and Carnevale, 1976; Woodruff et al., 1982; Koffi et al., 2006; Jamonneau et al., 2012; Welburn et al., 2016), potential domestic and/or wild animal reservoir hosts have been identified in West and Central Africa during the last decades (Büscher et al., 2018).
The epidemiological significance of these non-human hosts is not fully understood in distinct epidemiological situations and continues to be the subject of contrasting views (Franco et al., 2014b). In this context it should be recalled that despite mass treatment of the human population with pentamidine prophylaxis during the last century and the corresponding decrease in incidence to less than 0.01% in areas of risk (e.g. the then Belgian Congo), a complete elimination of T. b. gambiense has never been achieved (Welburn et al., 2016; Van Hoof, 1937, Van Hoof, 1940; Jannssens, 1971; Jannssens et al., 1984). Persistent “residual foci”, the sporadic occurrence and the resurgence of the disease, especially in rainforest areas, could be explained by the existence of additional latent infections in domestic or wild animal reservoir hosts known to be a food source of G. palpalis s.l., the main vector of T. b. gambiense (Jannssens et al., 1984; Denecke, 1941; Karshima et al., 2016) or asymptomatic human carriers (Jamonneau et al., 2012). The concept of a “cryptic” reservoir is thus hardly a novel or original idea (Büscher et al., 2018).
Given the progress towards elimination of the disease, the Informal Expert Group on Gambiense HAT once again raised the question of the extent to which cryptic animal reservoirs might undermine the permanent elimination of the disease (Büscher et al., 2018). The report of the Expert Group contains a comprehensive catalogue of information on domestic and wild animals that have successfully been infected with T. b. gambiense strains isolated from human patients. It also included information on the infectiveness of animal carriers to tsetse and the duration of infection in non-human hosts. The supplemental information to the article of the Expert Group gave references to publications that consider countries and host species in which T. b. gambiense has been confirmed by isoenzymes, blood incubation infectivity test or hybridization with DNA probes for the period before 1990. For the period after 1990, diagnosis was based on T. b. gambiense-specific PCR and/or immune trypanolysis. The Group concluded that the available information was insufficient to draw final conclusions on the epidemiological impact of animals in gHAT transmission. Consequently, the need for additional studies to clarify the role of reservoir animals was emphasized. This conclusion is precisely what was recommended by the original TDR African Trypanosomiasis Steering Committee in the late 1970s. We document in this paper the research supported by TDR which provided a significant amount of information, some hitherto only available in reports. In keeping with the recommendation of the Expert Group, this article concentrates mostly on previous studies on the transmission dynamic of the disease, typically found in “gray” literature and may have therefore escaped the scientific discussion. The review aims at complementing knowledge on i) the existence of transmission cycles in wild and domestic animals, ii) the longevity of latent infections of T. b. gambiense in animals, iii) the stability of gambiense group 1 in non-human hosts and iv) the infectivity of latent animals reservoir hosts infections to tsetse flies. Conclusions arising from this revisit should improve the understanding of animal reservoirs in the gHAT epidemiology at different endemicity levels. In addition, we also discuss other modes of transmission which should be considered as factors which maintain the existence of circulating parasites within populations both human and animal. Notably emphasizing, in addition the work of Welburn et al. (2016) on the potential role of mother to child transmission, the need to define the extent of the asymptomatic human carriers and possible sexual transmission which has largely been ignored in the debate.
2. Methods
A literature search was done in biomedical databases (PubMed, African Index Medicus and Google Scholar) using the following key words: Human African Trypanosomiasis (HAT), West and Central Africa, T. b. gambiense, wild and domestic animal reservoir hosts, parasitaemia, Glossina palpalis s.l., infectivity, xenodiagnosis and host preference. Search was limited to publications after 1980. Further searches were done in: WHO Technical Report Series on Human African Trypanosomiases 1962–2014, the Proceedings of Meetings of the International Scientific Council for Trypanosomiasis Research and Control (ISCTRC) 1977–2017 and Tsetse and Trypanosomiasis Information Quarterly (TTIQ) 1978–2018. Special attention was given to research findings on transmission dynamics of T. b. gambiense in annual and biannual reports of the Liberia Research Unit of the Tropical Institute Hamburg (LRU), Bong Mine, Bong County, Liberia. The focus was on the pre-civil war period (1977 to 1989) reports (available at the Bernhard-Nocht-Institut für Tropenmedizin, Bernhard-Nocht-Strasse 74, D-20359 Hamburg, Germany, and from the authors) and also in Progress and Final Reports of the LRU to the UNDP/WHO/World Bank TDR Special Programme on African Trypanosomiases. During the pre-civil war period in Liberia, extensive studies on the epidemiological significance of animal reservoir hosts and on the prevalence of gHAT were carried out in Liberia. Findings were published in peer reviewed journals, but the majority of the results are only available as “gray” literature (e.g. reports of the LRU, monograph and dissertation theses). The latter are the primary focus of the present review. A total of 63 publications were prioritized for the review, the selection of which depended on whether they addressed aspects of the significance of non-human T. b. gambiense cycles.
3. Current status of knowledge
3.1. Transmission cycles and longevity of infections in non-human hosts
3.1.1. Wild animals
Initial transmission experiments conducted in 1947 in the then Belgian Congo, demonstrated that human derived isolates could be cyclically transmitted through goats, sheep and pigs for over 20 passages without the loss of infectivity to humans (van Hoof, 1947). Thirty years later, additional studies on the occurrence of T. b. gambiense in domestic and wild animals and on transmission cycles of gHAT were conducted in Liberia (Mehlitz, 1977; Gibson et al., 1978; Mehlitz et al., 1982). Intensive studies on the epidemiological significance of non-human-reservoirs followed in the endemic zones of West and Central Africa during the last decades and have been recently up-dated (Büscher et al., 2018).
Transmission cycles in antelopes and pigs are detailed in the reports by the LRU. The detection of T. b. gambiense group 1 in pigs in Liberia and Côte d'Ivoire, as well as the identification of trypanosome strains isolated from kob (Kobus kob) and hartebeest (Alcelaphus buselaphus) in Burkina Faso identical to those isolated from patients in Côte d'Ivoire (Küpper et al., 1981; Mehlitz, 1982) spurred experimental studies on the T. b. gambiense transmission cycles (Sachs and Mehlitz, 1981; Mehlitz et al., 1983).
The antelope-Glossina-antelope cycle study used a T. b. gambiense stock (TH Gamey Dolo/Lib 80, gambiense group 1) (Sachs et al., 1981; Sachs, 1983a, Sachs, 1983b) isolated from a patient in Gbao, Bong County (6°40′N, 9°29′W), (wet film, Giemsa stain, lymph-node aspirate and cerebrospinal fluid positive).
Primary isolation was achieved by inoculation of parasitized blood to a mangabey monkey (Cercocebus torquatus) and a giant rat (Cricetomys emini) followed by ten passages of the stock to Mastomys natalensis. 253 teneral G. p. palpalis which originated from the International Atomic Energy Commission (IAEC), Vienna, Austria, were fed on the monkey (n = 137) and on laboratory rodents (M. natalensis) (n = 116). From 10 days onwards, after hatching, the flies were fed on five black backed duikers (Cephalophus dorsalis). 3.2% of the flies (seven fed on the monkey, one on the rodents) developed salivary gland infections, detected by dissection. In four of five antelopes, a parasitaemia developed after prepatent periods between 18 and 180 days (Mehlitz et al., 1983).
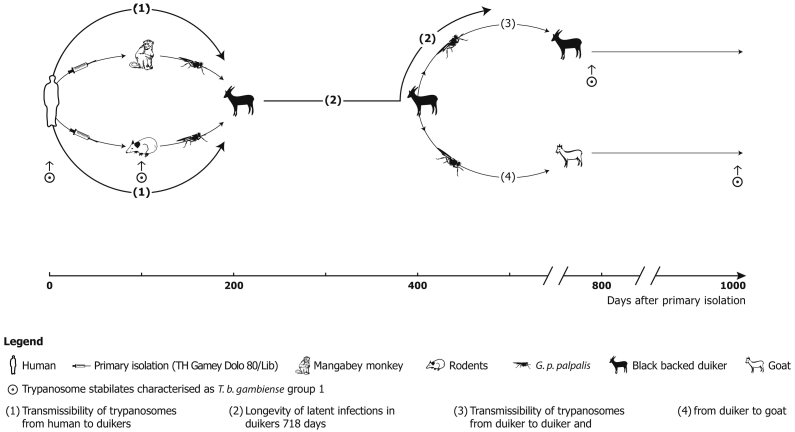
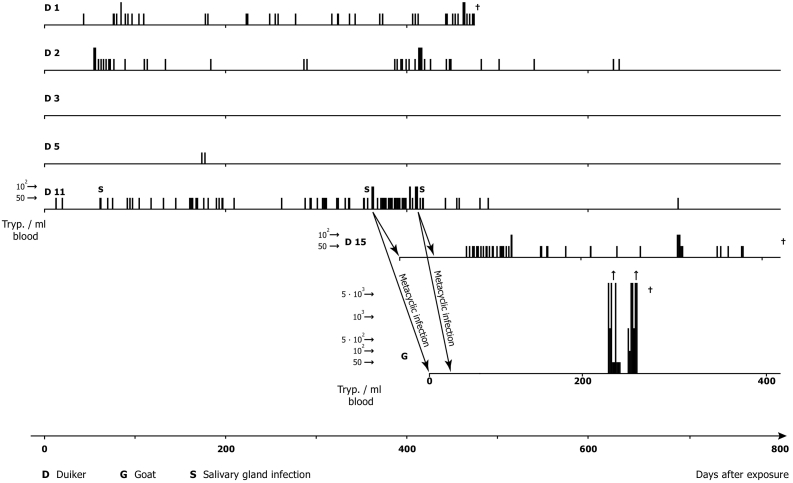
Parasitaemia was checked daily (except public holidays) by the Haematocrit Centrifugation Technique (HCT) (Woo, 1970; Mehlitz, 1978) and the improved miniature-Anion-Exchange Centrifugation Technique (m-AECT) (Sachs, 1983b), allowing the number of trypanosomes/ml blood to be calculated. Parasitaemia was low, often barely exceeding 50 trypanosomes/ml blood, with apparently aparasitaemic periods of more than 100 days. The recorded duration of trypanosome positivity was up to 718 days post exposure in one antelope. Infectivity of trypanosomes for Glossina was demonstrated up to 419 days p.e. (D 11). This was shown by the development of mature salivary gland infections of three of 3017 G. p. gambiensis (0.1%) on days 333, 635 and 678 after primary isolation and of immature infections of 13 of 3017 (0,4%) of teneral flies fed on the antelopes and further by the transmission of the stock to antelope (D 15), which also developed scanty parasitaemia, detectable for a further 363 days. A 3rd cyclical transmission was achieved to a goat (Mehlitz, 1986; Sachs and Mehlitz, 1981). The experimental design of the transmission experiments are shown in Fig.1 and the course, level and duration of parasitaemia in Fig. 1, Fig. 2.
Fig. 1.
Diagram of successful experimental cyclical transmissions of patient derived T. b. gambiense (TH Gamey Dolo 80/Lib) to and between antelopes and a goat: Primary isolation by inoculation of patient's blood to a mangabey monkey (C. torquatus) and a giant rat (C. emini) and subpassages to M. natalensis followed by metacyclic infections of black backed duikers (C. dorsalis) with G. p. palpalis fed on the monkey and rodents and another metacyclic infection to a duiker and a goat; T. b. gambiense group 1 characteristics (Paindavoine et al., 1986) are retained until 1017 days after primary isolation.
(Adapted from Mehlitz, 1986).
Fig. 2.
Experimental metacyclic infections (G. p. palpalis) of black backed duikers (C. dorsalis) and a goat with patient derived T. b. gambiense (stock TH Gamey Dolo 80/Lib x): Course, level and duration of parasitaemia and persistence of human infectivity markers [gambiense group 1, (Paindavoine et al., 1986)] after two cyclical transmissions in non-human hosts.
(Adapted from Mehlitz, 1986).
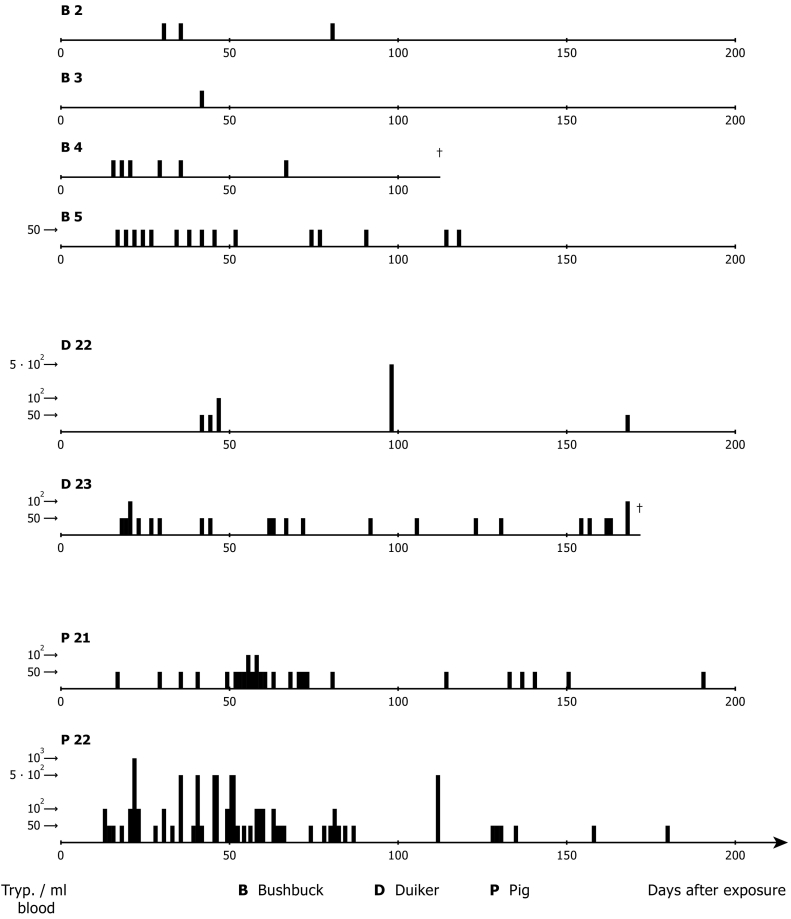
Metacyclic induced infections of additional two black backed duikers (C. dorsalis), four bushbuck (Tragelaphus scriptus) and two pigs as recipient hosts were also demonstrated. This was achieved using teneral G. p. gambiensis [pupae received from Institut d'Elevage et de Médecine Vétérinaire des Pays Tropicaux (IEMVT), Maisons-Alfort, France], fed on the mangabey monkey previously infected with bloodstream forms of T. b. gambiense TH Gamey Dolo/Lib 80 (Mehlitz and Zillmann, 1984). Thirty seven of 147 flies (25.2%) developed metacyclic trypanosomes, which when compared to G. p. palpalis, showed G. p. gambiensis significantly more susceptible to the same human derived T. b. gambiense stock. Bushbuck showed low parasitaemias (below 50 trypanosomes/ml blood), demonstrable until 120 p.e. again with long aparasitaemic periods (Fig. 3). A similar pattern of parasitaemia was observed in the duikers with a period of more than 70 days during which no parasites could be detected, despite the animals daily examination by the m-AECT and HCT. In the pigs, parasites were detected more frequently, but again the parasitaemia rarely exceeded 50 trypanosomes/ml blood. None of the animals showed clinical signs or symptoms of trypanosomiasis. Re-isolation of the parasites by xenodiagnosis was, however, unsuccessful in this experiment.
Fig. 3.
Experimental metacyclic infections (G. p. gambiensis) of antelopes and pigs with patient derived T. b. gambiense (stock TH Gamey Dolo 80/Lib): Course and level of parasitaemia in bushbuck (T. scriptus), duikers (C. dorsalis) and domestic pigs.
(Adapted from Mehlitz and Zillmann, 1984).
Wild ungulates, predominantly Cephalophus spp. and bushbuck, were abundant in the HAT focus (where the prevalence in the human population was 2.0%) in Bong County around the village Gbao and the surrounding biotope during the years 1981/82 (Sachs, 1983a; Mehlitz and Gangpala, 2017). The overall infection rates of wild animals (n = 96) with Trypanosoma spp. was 14%, with T. brucei s.l. 3.6% based on the m-AECT. Despite the use of M. natalensis as a recipient host of blood from animals positive for Trypanozoon parasites, sub-inoculation of parasitized blood into M. natalensis occasionally failed in a proportion of attempts to isolate the organisms for characterisation. This was an experience common when attempting to isolate Trypanozoon parasites of apparently low virulence into a range of hosts (Mehlitz et al., 1984). Studies on the bio-ecology indicated that the risk for humans acquiring infection was low in the villages and adjacent farmland but high at points of human - water contact particularly towards the end of the dry season and along footpaths in farmland throughout the season as well as in the cocoa plantations during the rainy season (Kaminsky, 1987). Host preferences of G. p. gambiensis (125 blood meals identified) showed that 29% were from humans, 29% from antelopes (25% on bushbuck, 4% on duikers), 29% on reptiles, 10% on domestic ruminants and the remaining on dogs and birds. Pigs were not kept in the surveillance area (Mehlitz and Staak, 1984). Mature infections of T. brucei s.l. were not detected in 2882 G. p. gambiensis, either by dissection nor by inoculation of pooled salivary glands to M. natalensis. However, midgut procyclic trypanosome forms (3.9%) could have included also immature T. brucei s.l. development forms (Kaminsky, 1986). These results reflect the observations reviewed by Welburn et al. (2016) who controversially considered that Glossina are rarely infected with T. b. gambiense in the wild proposing other roles of transmission hitherto not considered were important in maintenance of gHAT foci.
Whilst the studies reported here demonstrate a proven sylvatic T. b. gambiense cycle can exist, the longevity of parasitaemia in infected antelopes and the tsetse host preferences of G. p. gambiensis point to the possibility of transmission of T. b. gambiense between human and animal hosts in the Liberian rainforest.
Strong support for the involvement of wild fauna in T. b. gambiense transmission also comes from the rainforest area of Southern Cameroon (HAT focus of Bipindi). T. b. gambiense were identified in 8% (n = 164) of wild animals (24 species) examined by PCR using T. b. gambiense group 1 specific primer (Herder et al., 2002). T. b gambiense group 1 was identified in 8% of wild animals sampled and 14% were found infected with non-gambiense Trypanozoon infections. T. b gambiense group 1 parasites were identified in three mammalian orders - ungulates (C. dorsalis 1, C. monticola 1), primates (C. torquatus 1, C. nictitans 2), carnivores (Nandina binotata 2, Genetta servalina 1) and - rodents (Cricetomys gambianus 3, Atherurus africanus 2). Studies in Cameroon were extended to three known HAT foci (Bipindi, Doumé, Campo) and one control zone where HAT has never been reported but where the wild fauna composition was similar to that of the sleeping sickness foci and where livelihoods depended considerably on hunting (Njiokou et al., 2006). Of 1142 wild animals examined using specific PCR primers, 18 (2.2%) were positive for T. b. gambiense group 1 in the endemic zones and none in the control zone. Again, among the positive animal species were primates [(5 of 253 examined, C. torquatus 1, C. nicitans 4); artiodactylis (5 of 234 examined, C. dorsalis 1, C. monticola 4); rodents (5 of 237 examined, C. gambianus 3, A. africanus 2); carnivores (3 of 45 examined, G. servalina 1, N. binotata 2)] (Njiokou et al., 2006).
Evidence of independent transmission cycles in wild animals were found in the Bipindi, HAT focus in Cameroon. This used the Next Generation Matrix, synthesising and evaluating epidemiological and ecological data modelling the potential of transmission of T. b. gambiense in the absence of human cases (Funk et al., 2013). The study stressed that under the assumption of random mixing between vectors and hosts, Gambian HAT could not be maintained in that focus without the contribution of animals. Consequently, animals were considered being crucial for the maintenance of the disease.
Molecular evidence of sylvatic T. b. gambiense cycle has also been reported from studies in Equatorial Guinea by comparing the prevalence of T. b. gambiense in different HAT endemic situations. Together, with a peri-domestic cycle in the Mbindi focus, an additional transmission activity in a sylvatic cycle is predicted with a prevalence rate of 12.1% (T. b. gambiense) in a variety of wild animal hosts in this endemic region (Cordon-Obras et al., 2015).
3.1.2. Domestic animals
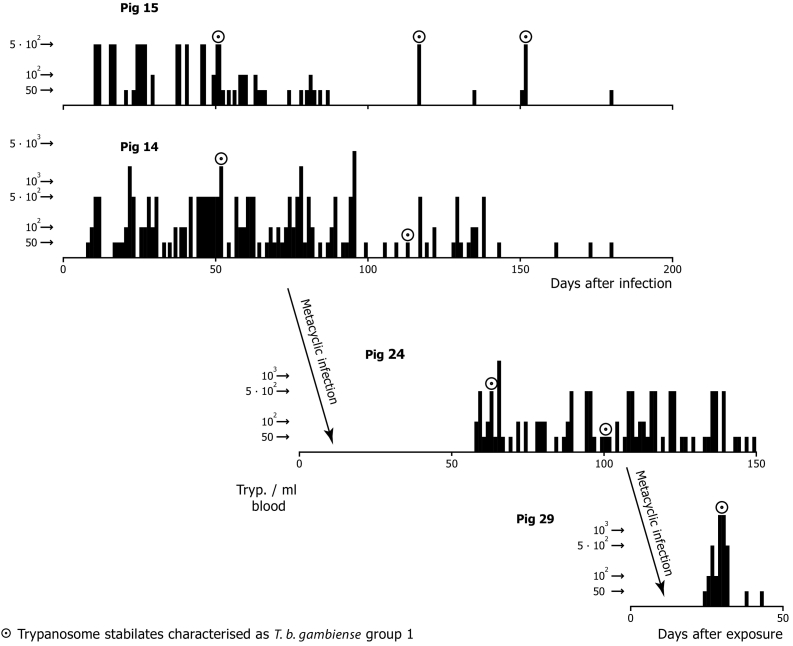
Transmission experiments were undertaken with Liberian domestic pigs infected with metacyclic trypanosomes of T. b. gambiense [TSw 65/82 KP I clone B, pig isolate from Côte d'Ivoire, human serum resistant, DNA hybridization gambiense (Mehlitz et al., 1985; Paindavoine et al., 1986)] and G. p. gambiensis as vector (Fig.4). Following the infection with cloned bloodstream forms of pigs 14 and 15, low and intermittent parasitaemias (below 500 trypanosomes/ml blood) were observed. Again, cyclical transmissibility was shown by metacyclic infection of two more pigs (No 24 and 29). Two of 633 (0.3%) tsetse flies dissected showed salivary gland infections (Table 1), thus, supporting the existence of independent experimental pig-tsetse-pig cycles of T. b. gambiense.
Fig. 4.
Experimentally infected pigs: Level and longevity of parasitaemia after infections with bloodstream forms (pig 15 and 14) and metacyclic infections (G. p. gambiensis) (pig 24 and 29) with T. b. gambiense [TSw 65/82 KP I clone B, pig isolate from Cote D'Ivoire, human serum resistant, DNA hybridization gambiense (Paindavoine et al., 1986) and persistence of these human infectivity markers after two cyclical transmissions.
(Adapted from Mehlitz, 1986).
Table 1.
Localisation of trypanosome development forms in dissected tsetse flies after metacyclic transmission of T. b. gambiense (A) from infected mangabey monkey (Cercocebus torquatus) and from multimammate rats (Mastomys natalensis) to duikers (Cephalophus dorsalis): Infectivity of G. p. palpalis for the Liberian human derived stock TH Gamey Dolo 80/LIB, and (B) from infected pig 14 to pig 24 and from pig 24 to pig 26: Infectivity of G. p. gambiensis for the ivorien pig derived stock TSw 65/82 E KP I (HS) [DNA Gambiense, Paindavoine et al. (1986), adapted from Mehlitz (1986)].
| Gut (n) | Gut salivary gland (n) | Gut hypopharynx salivary gland (n) | Gut hypopharynx labrum salivary gland (n) | Infectious metacyclic trypanosomes (n) | |
|---|---|---|---|---|---|
| (A) G. p. palpalis | |||||
| 137 (fed on Cercocebus) | 22 | 1 | 4 | 2 | 7(5.1%) |
| 116 (fed on Mastomys) | 8 | 1 | 0 | 0 | 1(0.009%) |
| (B) G. p. gambiensis | |||||
| 424 (fed on pig 14) | 16 | 1 | 0 | 0 | 1(0.2%) |
| 209 (fed on pig 24) | 0 | 1 | 0 | 0 | 1(0.5%) |
n denotes the number of flies and the percentage of infectious metacyclic trypanosomes are shown in parenthesis.
A long-term study (over four observation periods) in an endemic village (Kouassi-Perita, Daloa-Bouaflé-Vavoua HAT focus, Côte d'Ivoire) was undertaken with the objective of determining the role of animals in the maintenance of T. brucei s.l. infective to man (Mehlitz, 1985). Beside the description of the behaviour and seasonal movement of domestic animals (pigs, goats, dogs, chicken) and human activity, infection rates in pigs of 50,5% to 74% (T. brucei s.l.) were reported. These were diagnosed parasitologically using the m-AECT or M. natalensis subinoculation. On the basis of the quadruple examination of the total pig population (n = 200) over a period of one year, it was shown that all pigs became carriers of T. brucei s.l. The persistent and continuous parasite reservoir in this typical village harboured T. b. gambiense (stock TSw 65/82 E KP I and TSw 125/82 E KP I, Paindavoine et al., 1986), recovered from mixed infection with T. b. brucei by treating trypanosome isolates from pigs with normal human serum (Mehlitz et al., 1984). The role of circulating T. brucei s.l. in pigs and cattle in the neighbouring HAT foci of Bonon and Sinfra has been examined recently. The immune trypanolysis test showed positivity rates in pigs of 27.6% with specific T. b. gambiense variants in the Bonon focus, but these results were not confirmed by PCR using specific T. b. gambiense primers (N'Djetchi et al., 2017). Although these studies in Côte d'Ivoire were geographically close, the results cannot be compared directly because of the long time interval between the investigations (more than 30 years) with potentially resultant in changes in epidemiological determinants. In addition, different diagnostic methods were used. Both studies were carried out in Côte d'Ivoire, and both point to the significant role of pigs as carriers of latent T. b. gambiense infections and supports the findings from Cameroon, although self-cure was seen in experimentally infected pigs with blood from patients in less than six months (Penchenier et al., 2005). These studies in many aspects replicate the studies of van Hoof (1947) in the former Belgian Congo carried out in the early 1940's.
The cyclical transmissibility demonstrated the longevity of experimental animal infections as well as the proof of occurrence of natural T. brucei s.l. mixed infections in pigs, in addition to the known varied host preferences of G. palpalis s.l. for human and pigs in gHAT foci in Côte d'Ivoire (Laveissière et al., 1985), evidence which suggests the transmission of human infective trypanosomes from a pig reservoir to humans a likely phenomenon. However, what proportion of human infections from flies which have acquired infective parasites from an animal source cannot be determined. We know of no way that such a figure could be estimated.
In addition to the pig studies in Liberia, a similar transmission model was successfully applied with autochthonous West African pariah dogs (Hörchner et al., 1985) after they were also identified as reservoir hosts (Zillmann et al., 1984). It was shown that T. b. gambiense (stock TH Gamey Dolo 80/lib, fly 143) could be cyclically transmitted to and between dogs. G. p. gambiensis with mature infections (probing results) fed on pariah dogs and European beagles (Schöning, 1989). Newly, emerged teneral flies obtained their first blood meal on these dogs and flies with mature infections of each group were again used to infect pariah dogs and beagles. The experiment was performed under the same conditions with T. b. brucei (stock TSw 73/77 LRU, Clone A). In the gambiense-group, 1% of the flies developed a mature and 3.7% immature infections. For the brucei-group the numbers were 2.7% and 7.6%, respectively. The animals were monitored for 180 days (parasitaemia, clinical and haematological appearance). Beagles of the T. brucei infected group developed a severe infection, the pariah dogs a milder, chronic clinical presentation. Pariah dogs and beagles in the gambiense infected group showed no clinical symptoms, but parasites were detectable until the end of the observation period rarely exceeding a parasitaemia of 103 trypanosomes/ml blood with intervening aparasitaemic phases between five to ten days (Schöning, 1989).
3.2. Infectiveness of latent animal reservoir hosts to tsetse
Xenodiagnosis is a common method used to increase sensitivity of parasite detection and infectivity of latent infections. The antelope transmission experiment described above (Fig. 1, Fig. 2) demonstrates the diagnostic value of xenodiagnosis for the detection of T. b. gambiense in potential non-human reservoir hosts with low and intermittent parasitaemias and aparasitaemic periods of more than 100 days. Xenodiagnosis again was successful, proven by a third cyclical transmission to a goat, although salivary glands infections were not detected by dissection (Mehlitz et al., 1983). The pig-Glossina-pig cycle also supported the diagnostic value of xenodiagnosis. More recently, the sensitivity of xenodiagnosis for T. b. gambiense was demonstrated in an experimentally infected pig with low parasitaemia estimated at some 10–50 trypanosomes/ml blood. Parasites in recipient G. morsitans submorsitans reared at the Centre International de Recherche-Développement sur L'Élevage en Zone Subhumide (CIRDES) were detectable in the midgut in four out of 37 flies dissected. G. p. gambiensis (n = 308) (CIRDES colony) also used in this experiment remained negative, possibly due to the loss of vector competence (Wombou Toukam et al., 2011; Welburn et al., 2016). The authors emphasized the value of xenodiagnosis of T. b. gambiense using susceptible Glossina (here G. m. submorsitans) to better identify trypanosome carriers and to assess their role in the transmission dynamics.
3.3. Persistence of T. b. gambiense in domestic and wild animals
The persistence and stability of the T. b. gambiense isoenzyme characteristics associated with human serum resistance was reported in experimentally infected mini-pigs (“Göttinger Mini-Pigs”, a special breed developed for laboratory research) as early as 1981. The behavioural and biochemical characteristics of T. brucei clones derived from a domestic pig (stock T. b. brucei, TSw 78E 026) and a human in Côte d'Ivoire (stock T. b. gambiense, TH DAL 0729), did not change in these novel experimental hosts. The characteristics of human serum resistance and isoenzyme patterns remained constant for at least 343 days for T. b. brucei and 154 days for T. b. gambiense p.i. In mixed infections, in which T. b. brucei predominated, a minority of the T. b. gambiense population was recoverable after treatment with human serum by subinoculation into M. natalensis after 140 days p.i. (Schütt and Mehlitz, 1981). Using this method as a screening approach, mixed populations of T. b. brucei and T. b. gambiense were identified among 169 isolates from naturally infected pigs in the Daloa-Bouflé-Vavoua focus (74.0% of which were parasitologically positive with T. brucei s.l.) (Mehlitz et al., 1984). Subsequent evaluation of the T. b. gambiense characteristics confirmed their stability in cyclically and mechanically infected pigs for more than one year based on nuclear DNA analysis of the stocks/clones TSw 65/82 E KP I (b) 2, TSw 125/82 clone B and TSw 125/82 E KP I clone B 2 (Mehlitz et al., 1985; Mehlitz, 1986; Paindavoine et al., 1986). Similarly, the persistence of T. b. gambiense in pigs was also seen in the experimental sylvatic cycle. Behavioural (human serum resistance), biochemical (zymodeme) and molecular (DNA) characteristics were stable for nearly three years (Figures 1and 2). During this period the stock TH Gamey Dolo/Lib 80 did not change its human infectivity markers in non-human hosts throughout the period of the experiments (Mehlitz et al., 1983).
3.4. Alternative modes of transmission of T. b. gambiense
Implications of potential non-tsetse transmission of T. b. gambiense and the potential impact on elimination targets of HAT have been discussed (Welburn et al., 2016) whilst in the paper by the Informal Expert Group on Gambiense HAT Reservoirs (Büscher et al., 2018) made the classical assumption that tsetse transmission is the only factor which drives the epidemiology of gHAT - the only consideration discussed. Alternative transmission can occur through mother to child transmission and by sexual contact in humans and there have been several well documented case reports (Rocha et al., 2004). If these modes of transmission occur on any scale in endemic foci they will have wide reaching implications for elimination; however, as with the role of the animal reservoir the extent to which mother to child and sexual transmission occur and impact on the persistence of T. b. gambiense is unknown and not factored into any modelling.
Male reproductive organs may act as a refuge for parasites. This could leave patients at risk of a relapse, ultimately allowing them to act as a reservoir for subsequent transmission by tsetse (Biteau et al., 2016) The most recent evidence of a silent parasite carrier and of maternal to child transmission was a case report describing a female patient who had never been in Africa. Her companion had been on a military mission in Angola and following his partners diagnosis he was found to be a symptom-free trypanosome carrier. The female patient's 19 month-old son was diagnosed with late stage HAT which can be attributed to congenital transmission (Rocha et al., 2004). This single well documented case report provides evidence of 1.) sexual transmission 2.) mother to child transmission 3.) the existence of a healthy human carrier. The only question in this situation is did the healthy carrier on a mission to Angola acquire the infection from Glossina or by sexual exposure?
There is evidence from studies in the 1960's and 1970's that trypanosomes have a tropism for testes. T. b. brucei, T. b. rhodesiense and T. b. gambiense can multiply in the interstitial tissue when inoculated into the testicles of rabbits and guinea-pigs whilst T. b. gambiense grows more slowly than the other two parasites (Heisch et al., 1968). Intra testicular inoculation of rabbit‘s testes as an immunologically privileged site was used to isolate T. b. gambiense from cervical lymph gland biopsies and from CSF of gHAT patients (Molyneux, 1973) given the known difficulties of using mice and rats to isolate strains from humans until the susceptibility of M. natalensis was discovered (Mehlitz, 1978). Preferential dissemination into mouse testes of T. b. brucei has recently also been described using bioluminescent imaging (Claes et al., 2009) whilst Carvalho et al. (2018) have shown that T. b. brucei also triggers a marked immune response in male reproductive organs.
However, discussing the potential alternative modes of T. b. gambiense transmission whether mother to child or sexual transmission, there has been no reference to the T. brucei group parasite, T. equiperdum, an exclusively equine parasite which is solely transmitted by coitus. This parasite is extensively distributed largely outside Africa causing dourine. As with T. b. gambiense, T. equiperdum is difficult to establish in laboratory animals and again, as with T. b. gambiense, intratesticular inoculation into rabbits can be successful in establishing strains for laboratory studies. Coital transmission results from trypanosomes present in seminal fluid and in mucus exudate from stallions and the vaginal mucus of mares (Gizaw et al., 2017). We consider that the potential for the sexual mode of transmission as well as mother to child transmission (Welburn et al., 2016) should be recognized as worthy of more detailed consideration. The existence and longevity capacity of healthy carriers of T. b. gambiense has been known for decades (Lapeyssonnie, 1960; Jamonneau et al., 2012). We do not know the prevalence of these carriers, the duration of the asymptomatic carrier state, if they self cure or how they might have acquired infection. We do not know, the frequency of mother to child or sexual transmission versus tsetse transmission.
4. Conclusions
Possible animal reservoirs of gHAT may constrain the goals of its elimination as a public health problem by 2020. It has previously been stated that the current knowledge of T. b. gambiense infections in animals is very limited and fragmented (Büscher et al., 2018). This contribution was intended to highlight existing, but perhaps less accessible information in “gray literature” towards the goal of improving the access to available data. Transmission cycles in non-human hosts, the infectiveness of animal reservoirs to G. palpalis s.l., the duration of infection and the stability of T. b. gambiense characteristics in antelopes and domestic animals have been reviewed. Taken together, it is evident that T. b. gambiense multiplies and can persist in antelopes, pigs and dogs. Metacyclic transmission of T. b. gambiense has been reported in antelope-G.p.palpalis-antelope and pig-G.p.gambiensis-pig-cycles. The infectivity to tsetse of latent animal reservoir infections is retained in the animal-Glossina spp.-animal cycles. Human infectivity markers (human serum resistance, zymodeme, DNA) remain stable in non-human hosts for several years. These facts should be viewed within the wider context of the often controversial discussions and epidemiological hypotheses on the significance of animal reservoir hosts in specific ecosystems of gHAT foci. The NSSCPs, assisted by the WHO Gambiense Network, should include investigations aimed at clarifying the existence of wildlife and domestic latent animal reservoirs in both active, but also known “old” HAT foci role towards targeting elimination (zero transmission) of the disease. Non-tsetse modes of transmission - often regarded as hypothetical (Büscher et al., 2018) - may play a grossly underestimated role in the maintenance of foci and might preclude the total elimination of transmission as envisaged in the latest WHO targets. Why cannot T. b. gambiense behave like and be transmitted as is its cousin T. equiperdum? The results presented here challenge the WHO optimism of the target for complete elimination and zero transmission incidence by 2030 even if the elimination of the public health problem defined as less than 2000 new cases/year is achieved; we postulate that given the “known unknowns” - animal reservoirs, healthy carriers, mother-child and sexual transmission - persistent infections will continue. International health practitioners are encouraged to “learn lessons” and “expect the unexpected”. We urge that in the field of gHAT epidemiology a greater knowledge of historic experiences are factored into the current thinking, that lessons are learnt, the wheel is not reinvented and that the diverse biology of Trypanosoma and its adaptability are recognized.
Author contribution
Both authors contributed to the manuscript.
Declaration of competing interest
The authors declare they have no competing interest.
Acknowledgement
The authors wish to acknowledge the generous support of the Rudolph Geigy Foundation in assisting the publication of this paper.
References
- Biteau N., Asencio C., Izotte J., Rousseau B., Fèvre M., Pillay D. Trypanosoma brucei gambiense infections in mice lead to tropism to the reproductive organs, and horizontal and vertical transmission. PLoS Negl. Trop. Dis. 2016;10(1) doi: 10.1371/journal.pntd.0004350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büscher P., Cecchi G., Jamonneau V., Priotto G. Human African Trypanosomiasis. Lancet. 2017;390(10110):2397–2409. doi: 10.1016/S0140-6736(17)31510-6. [DOI] [PubMed] [Google Scholar]
- Büscher P., Bart J.M., Boelaert M., Bucheton B., Cecchi G., Chitnis N. Do cryptic reservoirs threaten gambiense-sleeping sickness elimination? Trends Parasitol. 2018;34(3):197–207. doi: 10.1016/j.pt.2017.11.008. (Epub 2018 Jan 23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho T, Trindade S, Pimenta S, Santos A.B., Rijo-Ferreira F, Figueiredo L.M., 2018. Trypanosoma brucei triggers a marked immune response in male reproductive organs. PloS Negl. Trop. Dis. 12(8):e0006690. Published 2018 Aug 15. doi: 10.1371/journal.pntd.0006690. [DOI] [PMC free article] [PubMed]
- Claes F., Vodnala S.K., van Reet N, Boucher N, Lunden-Miguel H, Baltz T., et al., 2009. Bioluminescent imaging of Trypanosoma brucei shows preferential testis dissemination which may hamper drug efficacy in sleeping sickness. PLoS Negl. Trop. Dis. 3(7): e486. 10.1371/journal.pntd.0000486. [DOI] [PMC free article] [PubMed]
- Cordon-Obras C., Rodriguez Y.F., Fernandez-Martinez A., Cano J., Ndong-Mabale N., Ncogo-Ada P., et al., 2015. Molecular evidence of a Trypanosoma brucei gambiense sylvatic cycle in the human african trypanosomiasis foci of Equatorial Guinea. Front. Microbiol. 6,765. doi: 10.3389/fmicb.2015.00765. Ecollection2015. [DOI] [PMC free article] [PubMed]
- Denecke K. Menschenpathogene Trypanosomem des Hundes auf FernandoPoo. Ein Beitrag zur Epidemiologie der Schlafkrankheit. Arch. Hyg. (Berl.) 1941;126:38–42. [Google Scholar]
- Franco J.R., Simarro P.P., Diarra A., Jannin J.G. Epidemiology of human African trypanosomiasis. Clin. Epidemiol. A. 2014;6:257–275. doi: 10.2147/CLEP.S39728. (ECollection 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco J.R., Simarro P.P., Diarra A., Ruiz-Postigo J.A., Jannin J.G. 2014b. The journey towards elimination of gambiense human African trypanosomiasis: not far, nor easy. Parasitology 141(6), 748–60. doi: S0031182013002102 [pii] pmid:2470929. [DOI] [PubMed]
- Franco J.R., Cecchi G., Priotto G., Paone M., Diarra A., Grout L. Monitoring the elimination of human African trypanosomiasis: update to 2016. PLoS Negl. Trop. Dis. 2018;12(12) doi: 10.1371/journal.pntd.0006890. Ecollection 2018 Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frezil J.L., Carnevale P., 1976. Le problème du reservoir de virus et du maintien des foyers de trypanosomiase humaine en Afrique Centrale. Cah. O. R. S. T. O. M. Sér. Ent. Méd. Parasitol. 14(4), 307–313.
- Funk S., Nishiura H., Heesterbeek H., Edmunds W.J., Checchi F., 2013. Identifying transmission cycles at the human-animal interface: the role of animal reservoirs in maintaining gambiense human African trypanosomiasis. PLoS Comput. Biol. 9 (1), e1002855. doi: 10.1371/journal.pcbi.1002855. [DOI] [PMC free article] [PubMed]
- Gibson W.C., Mehlitz D., Lanham S., Godfrey D.G. The identification of Trypanosoma brucei gambiense in Liberian pigs and dogs by resistance to human plasma. Trop. Med. Parasitol. 1978;29(3):335–345. [PubMed] [Google Scholar]
- Gizaw Y., Megersa M., Fayera T. Dourine: a neglected disease of equids. Trop. Anim. Health Prod. 2017;49(5):887–897. doi: 10.1007/s11250-017-1280-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisch R.B., Killick-Kendrick R.J., Dorrell J., Mardsen P.D. The behaviour of trypanosomes in testicles. Trans. R. Soc. Trop. Med. Hyg. 1968;62:55. doi: 10.1016/0035-9203(70)90005-2. [DOI] [PubMed] [Google Scholar]
- Herder S., Simo G., Nkinin S., Njiokou F. Identification of trypanosomes in wild animals from Southern Cameroon using the PCR. Parasite. 2002;9:345–349. doi: 10.1051/parasite/2002094345. [DOI] [PubMed] [Google Scholar]
- Hörchner F., Zillmann U., Metzner M., Schönefeld A., Mehlitz D. West African dogs as a model for research on trypanotolerance. Trop. Med. Parasitol. 1985;36(4):257–258. [PubMed] [Google Scholar]
- Jamonneau V., Ilboudo H., Kaboré J., Kaba D., Koffi M., Solano P. Untreated human infections by Trypanosoma brucei gambiense are not 100% fatal. PLoS Negl. Trop. Dis. 2012;6(6) doi: 10.1371/journal.pntd.0001691. accessed June 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannssens P.G. Review of the sleeping sickness situation. Z. Tropenmed. Parasitol. 1971;22:213–224. [PubMed] [Google Scholar]
- Jannssens P.G., Wery M., van Meirvenne N., 1984. Current knowledge and prospects in the field of African trypanosomiasis. In: Medizin in Entwicklungsländer, Verlag Peter Lang, Frankfurt/M, Bd 16, 21–37, ISSN 0721-3247, ISBN 3-8204-5427-6.
- Kaminsky R. Infection rates of tsetse flies (Diptera, Glossinidae) with trypanosomes in the Liberian rain forest during the dry season. Anim. Res. Dev. 1986;23:56–70. [Google Scholar]
- Kaminsky R. Tsetse ecology in a Liberian rain-forest focus of Gambian sleeping sickness. Med. Vet. Entomol. 1987;1(3):257–264. doi: 10.1111/j.1365-2915.1987.tb00352.x. [DOI] [PubMed] [Google Scholar]
- Karshima S.N., Lawal I.A., Bata S.I., Barde I.J., Adamu P.V., Salihu A. Animal reservoirs of Trypanosoma brucei gambiense around the old Gboko sleeping 2010; sickness focus in Nigeria. J. Parasitol. Vector Biol. 2016;8(5):47–54. [Google Scholar]
- Koffi M., Solano P., Denizot M., Courtin D., Garcia A., Lejon V. Aparasitemic serological suspects in Trypanosoma brucei gambiense human African trypanosomiasis: a potential human reservoir of parasites? Acta Trop. 2006;98(2):183–188. doi: 10.1016/j.actatropica.2006.04.001. (Epub 2006 May 24) [DOI] [PubMed] [Google Scholar]
- Küpper W., Dräger N., Mehlitz D., Zillmann U. On the immobilisation of hartebeest and kob in Upper Volta. Trop. Med. Parasitol. 1981;32(1):58–60. [PubMed] [Google Scholar]
- Lapeyssonnie L. Deuxième note concernant un cas exceptionnel de trypanosomiase. Parasitémie observée depuis 21 sans signes clinique apprèciable chez une malade traitée inefficacement pendent les dix premières années. Bull. Soc. Pathol. Exot. 1960;53:28–32. [Google Scholar]
- Laveissière C., Couret D., Staak C., Hervouet J.P., 1985. Glossina palpalis et ses hȏtes en secteur forestier de Cȏte d’Ivoire. Relation avec l’épidémiologie de la trypanosomiase humaine. Cah. O. R. S. T. O. M. Sér. Ent. Méd. Parasitol. 23(4), 297–303.
- Mehlitz D., 1977. The behaviour in the blood inoculation infectivity test of four Trypanozoon strains isolated from pigs in Liberia. Trans. R. Soc. Trop. Med. Hyg. 71(1), 86. [DOI] [PubMed]
- Mehlitz D. Untersuchungen zur Empfänglichkeit von Mastomys natalensis für Trypanosoma (Trypanozoon) brucei gambiense. Trop. Med. Parasitol. 1978;29(1):101–107. [PubMed] [Google Scholar]
- Mehlitz D. Perspectives in Trypanosomiasis Research. Ed. Baker JR, Research Study Press, Chicester; In: 1982. Trypanosomes in African wild animals; pp. 25–35. [Google Scholar]
- Mehlitz D. Final Report to the UNDP/WHO/World Bank Special Programme on African Trypanosmiases. Project No. 820021. 1985. Epidemiological studies on the significance of animal reservoir hosts of Trypanosoma brucei gambiense. 29.01.1985. [Google Scholar]
- Mehlitz D., 1986. Le reservoir animal de la maladie du sommeil à Trypanosoma brucei gambiense. Etudes et Synthèses de l'IEMVT. n.18, Maisons-Alfort: CIRAD-IEMVT, 156 pp. ISBN 2-85985-127-5.
- Mehlitz D., Gangpala L. Sleeping sickness in Liberia-a historical review. Sierra Leone J. Biomed. Res. 2017;9(2):38–46. [Google Scholar]
- Mehlitz D., Staak C., 1984. Host preferences of Glossina spp. in an endemic sleeping sickness area of Bong County. Annual Report 1984 of the Liberia Research Unit (LRU) of the Tropical Institute Hamburg, 6-7.
- Mehlitz D., Zillmann U., 1984. Further experiments on metacyclic infections of antelopes with Typanosoma brucei gambiense. Annual Report 1984 of the Liberia Research Unit (LRU) of the Tropical Institute Hamburg, 15-17.
- Mehlitz D., Zillmann U., Scott M., Godfrey D.G., 1982. Epidemiological studies on the animal reservoir of gambiense sleeping sickness. Part III. Characterization of Trypanozoon stocks by isoenzymes and sensitivity to human serum. Trop. Med. Parasitol. 33(2), 113–118. [PubMed]
- Mehlitz D., Sachs R., Zillmann U., 1983. Further results on the cyclical transmission of human derived Trypanosoma brucei gambiense to antelopes and a goat. Annual Report of the Liberia Research Unit (LRU) of the Tropical Institute Hamburg for the years 1982 and 1983, 2-3.
- Mehlitz D., Zillmann U., Sachs R., 1984. Studies on the animal reservoir of Gambiense sleeping sickness: the domestic pig as reservoir host of Trypanosoma brucei gambiense. Annual Report 1984 of the Liberia Research Unit (LRU) of the Tropical Institute Hamburg, 10-13.
- Mehlitz D., Zillmann U., Sachs R., 1985. Further evidence of animal reservoir hosts of gambiense sleeping sickness: mixed populations of Trypanosoma brucei gambiense and T. b. brucei in naturally infected pigs in the Ivory Coast. International Scientific Council for Trypanosomiasis Research and Control (ISCTRC), 18th Meeting, Publication No 113, 128-129, Harare, Zimbabwe.
- Molyneux D.H. Isolation of Trypanosoma (Trypanozoon) brucei gambiense in rabbits by the intratesticular inoculation technique. Ann. Trop. Med. Parasitol. 1973;67:391–397. doi: 10.1080/00034983.1973.11686905. [DOI] [PubMed] [Google Scholar]
- N'Djetchi M.K., Ilboudo H., Koffi M., Kaboré, J., Kaboré J.W., Kaba, D., et al., 2017. The study of trypanosome species circulating in domestic animals in two human African trypanosomiasis foci in Côte d‘Ivoire d'Ivoire identifies pigs and cattle as potential reservoirs of Trypanosoma brucei gambiense. PLoS Negl. Trop. Dis. 11(10):e0005993. [DOI] [PMC free article] [PubMed]
- Njiokou F., Laveissiere C., Simo G., Nkinin S., Grebaut P., Cuny G., Herder S. Wild fauna as a probable animal reservoir forTrypanosoma brucei gambiense in Cameroon. Infect. Genet. Evol. 2006;6:147–153. doi: 10.1016/j.meegid.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Okello A.L., Welburn S.C., 2014. The importance of veterinary policy in preventing the emergence and re-emergence of zoonotic disease: examining the case of human African trypanosomiasis in Uganda. Front Public Health; doi.org/10.3389/fpubh.2014.00218, accessed 3 November 2014. [DOI] [PMC free article] [PubMed]
- Paindavoine P., Pays E., Laurent M., Geltemeyer Y., Le Ray D., Mehlitz D., Steinert M. The use of DNA hybridization and numerical taxonomy in determining relationship between Trypanosoma brucei stocks and subspecies. Parasitology. 1986;92(1):31–50. doi: 10.1017/s0031182000063435. [DOI] [PubMed] [Google Scholar]
- Penchenier L., Alhadji D., Bahébégué S., Simo G., Laveissière C., Cuny G. Spontaneous cure of domestic pigs experimentally infected by Trypanosoma brucei gambiense. Implications for the control of sleeping sickness. Vet. Parasitol. 2005;133(1):7–11. doi: 10.1016/j.vetpar.2005.04.034. [DOI] [PubMed] [Google Scholar]
- Rocha G., Martins A., Gama G., Brandão F., Atouguia J. Possible cases of sexual and congenital transmission of sleeping sickness. Lancet. 2004;363(9404):247. doi: 10.1016/S0140-6736(03)15345-7. [DOI] [PubMed] [Google Scholar]
- Sachs R. Sleeping sickness in the Gbarnga area. J. Libr. Med. Dent. Assoc. 1983;12:23–24. [Google Scholar]
- Sachs R., 1983b. Comparison between two concentration methods for the detection of low grade trypanosome parasitaemias in antelopes. Annual Report of the Liberia Research Unit (LRU) of the Tropical Institute Hamburg for the years 1982 and 1983. 5-6.
- Sachs R., Mehlitz D., 1981. Transmission of T. b. gambiense from human patients to antelopes. Annual Report of the Liberia Research Unit (LRU) of the Tropical Institute Hamburg for the years 1981 and 1982, 30.
- Sachs R., Mehlitz D., Koenig J., 1981. Sleeping sickness in the Gbarnga area, Bong County. Annual Report of the Liberia Research Unit (LRU) of the Tropical Institute Hamburg for the years 1981 and 1982. 25-26.
- Schöning B., 1989. Der Hund als Reservoir für Trypanosoma (Trypanozoon) brucei gambiense: Vergleichende Untersuchungen zur Empfänglichkeit für T. b. gambiense Dutton, 1902 und T. b. brucei Plimmer & Bradford, 1899 und zum Infektionsverlauf bei einer europäischen und einer westafrikanischen Hunderasse. Dissertation Thesis (English summary), Faculty of Veterinary Medicine, Free University Berlin, Germany. Journal No. 1481.
- Schütt I.D., Mehlitz D., 1981. On the persistence of human serum resistance and isoenzyme patterns of Trypanozoon in experimentally infected pigs. Acta Trop. 38(4), 367–373. [PubMed]
- Van Hoof L.M., 1937. Sur le rôle du porc comme réservoir deTrypanosoma gambiense. C. R. Soc. Biol. 126, 72–75.
- Van Hoof L.M. Recherche sur le comportement deTrypanosoma gambiense chez le porc. Ann. Soc. Belg. Med. Trop. 1940;20:203–228. [Google Scholar]
- Van Hoof L.M. Observations on trypanosomiasis in the Belgian Congo. Trans. R. Soc. Trop. Med. Hyg. 1947;40:728–761. [PubMed] [Google Scholar]
- Welburn S.C., Molyneux D.H., Maudlin I. Beyond tsetse – implications for research and control of human African Trypanosomiasis epidemics. Trends Parasitol. 2016;32(3):230–241. doi: 10.1016/j.pt.2015.11.008. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organisation), 2018. Trypanosomiasis, human African (sleeping sickness), Fact Sheet, updated February 2018. https://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-african-(sleeping-sickness), accessed 16 February 2018.
- WHO (World Health Organisation), 2019. Human AfricanTrypanosomiasis. Partnership and WHO network for elimination 2019; http://www.who.int/trypanosomiasis.african/surveillance/collaborating_network/en/, accessed 29 March 2019.
- Wombou Toukam C.M., Solano P., Bengaly Z., Jamonneau V., Bucheton B. Experimental evaluation of xenodiagnosis to detect trypanosomes at low parasitaemia levels in infected hosts. Parasite. 2011;18(4):295–302. doi: 10.1051/parasite/2011184295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.T.K. The haematocrit centrifuge technique for the diagnosis of African trypanosomiasis. Acta Trop. 1970;27(4):384–386. [PubMed] [Google Scholar]
- Woodruff A.W., Evans D.A., Owino N.O. A ‘healthy’ carrier of African trypanosomiasis. J. Inf. Secur. 1982;5:89–92. [Google Scholar]
- Zillmann U., Mehlitz D., Sachs R. Identity of Trypanozoon stocks isolated from man and a domestic dog in Liberia. Trop. Med. Parasitol. 1984;35(2):105–108. [PubMed] [Google Scholar]