Summary
Metastatic cancer cells sense the complex and heterogeneous fibrous extracellular matrix (ECM) by formation of protrusions, and our knowledge of how cells physically recognize these fibers remains in its infancy. Here, using suspended ECM-mimicking isodiameter fibers ranging from 135 to 1,000 nm, we show that metastatic breast cancer cells sense fiber diameters differentially by coiling (wrapping-around) on them in a curvature-dependent manner, whereas non-tumorigenic cells exhibit diminished coiling. We report that coiling occurs at the tip of growing protrusions and the coil width and coiling rate increase in a curvature-dependent manner, but time to maximum coil width occurs biphasically. Interestingly, bundles of 135-nm diameter fibers recover coiling width and rate on 1,000-nm-diameter fibers. Coiling also coincides with curvature-dependent persistent and ballistic transport of endogenous granules inside the protrusions. Altogether, our results lay the groundwork to link biophysical sensing with biological signaling to quantitate pro- and anti-invasive fibrous environments.
Video Abstract
Subject Areas: Cell Biology, Biophysics, Biomechanics, Cancer
Graphical Abstract
Highlights
-
•
Cells sense ECM-mimicking suspended fibers by coiling (wrapping around)
-
•
Coiling occurs at the tip of growing protrusions in a curvature-dependent manner
-
•
Non-tumorigenic cells exhibit diminished coiling compared with metastatic cells
-
•
A bundle of small-diameter fibers recover coiling observed on a large-diameter fiber
Cell Biology; Biophysics; Biomechanics; Cancer
Introduction
Protrusions are extensions from the cell body that are diverse in shape, molecular structure, and location relative to the cell body and play an important role in cell migration and extracellular matrix (ECM) degradation (Blanchoin et al., 2014, Clainche and Carlier, 2008, Koons et al., 2017, Machacek et al., 2009, Pollard and Borisy, 2003). The importance of protrusions as the precursor to migration (Alblazi and Siar, 2015, Friedl and Wolf, 2010, Ponti et al., 2004), in the presence of biophysical (Harland et al., 2011, Lo et al., 2000) and biochemical cues (Rhoads and Guan, 2007, Weber et al., 2013, Wu et al., 2012, Zigmond et al., 2001), is widely documented. In the context of cancer, specialized protrusive structures are known to break down the surrounding extracellular milieu (Buccione et al., 2004, Linder, 2007, Weaver, 2006).
The ECM through which cells navigate is a complex fibrous network that is composed of a wide range of fiber diameters (tens of nanometers to micrometers; Clark et al., 1982, Ushiki, 2002). In vitro assays, by us and others, have shown that the fiber diameter can have a significant impact on cell behavior by inducing changes in the morphology and redistribution of focal adhesion arrangements (Kennedy et al., 2017, Meehan and Nain, 2014). Using 3D gel assays, recent studies have reported that protrusions can remodel collagen fibers in the matrix in a “hand-over-hand” cycle at the leading edge (Meshel et al., 2005, Starke et al., 2013) or lateral to cell body (Wolf and Friedl, 2009), and aligned fibers in the gel limit formation of lateral protrusions, thus promoting cell persistence (Fraley et al., 2015, Riching et al., 2014). It has also been shown that cells seeded in 3D gels form multiple protrusions simultaneously to “probe” the surrounding matrix fibers before extending a single, stable protrusion to define a front-rear axis (Carey et al., 2016). Although these investigations have yielded valuable information on the nature of protrusions, our current understanding of how cells biophysically sense ECM fibers remains in infancy.
Using suspended network of fibers of contrasting curvatures (protrusive assay) that decouples bulk cell body migration from protrusive dynamics, we have previously shown that normal breast epithelial cells MCF 10A form shorter protrusions compared with the highly metastatic breast adenocarcinoma MDA-MB-231 on fibers of varying diameters (Koons et al., 2017). Briefly, our method allows us to constrain the cell body on large-diameter (≥2 μm) base fibers, whereas orthogonally deposited smaller diameter protrusive fibers elicit protrusive events. Using this platform, here, we report that migratory cells coil (wrap-around, Video S1) on fibers of varying curvature differentially. Coiling at the tip of protrusions occurs in bursts during growth phase of protrusions, which synchronizes with endogenous translocation of lipid granules inside protrusions along linear tracks in a persistent superdiffusive manner. Interestingly, depositing a bundle of densely packed small-diameter fibers recovers coiling dynamics of single large-diameter fibers. We also quantitate coiling by migrating cells in spindle shapes on single suspended fibers (migration assay). We report notable differences in coiling behavior between protrusive and migration assays on large-diameter fibers. For the breast cancer model used in this study, we report that non-tumorigenic MCF 10A exhibit diminished coiling in low numbers compared with their metastatic counterparts (MDA-MB-231 and BT-549). Altogether, our results using ECM-mimicking fibers lay the groundwork to link biophysical cell sensing with biological signaling to define pro- and anti-invasive fibrous environments.
Protrusion coiling (wrapping-around) by an MDA-MB-231 (same cell line used in all the subsequent videos unless otherwise specified) cell attached to a suspended fiber. Coiling occurs around the suspended fiber axis. Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
Results
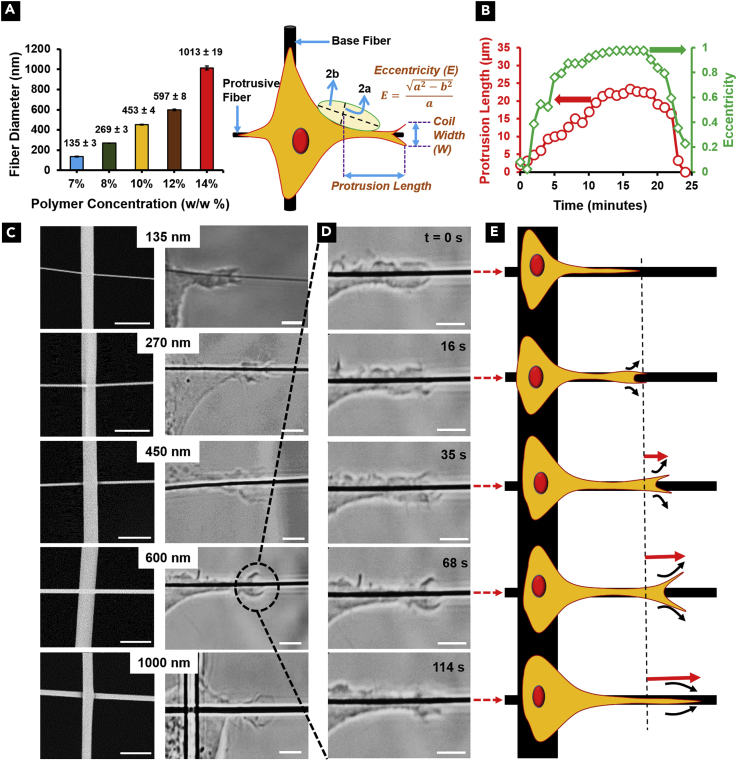
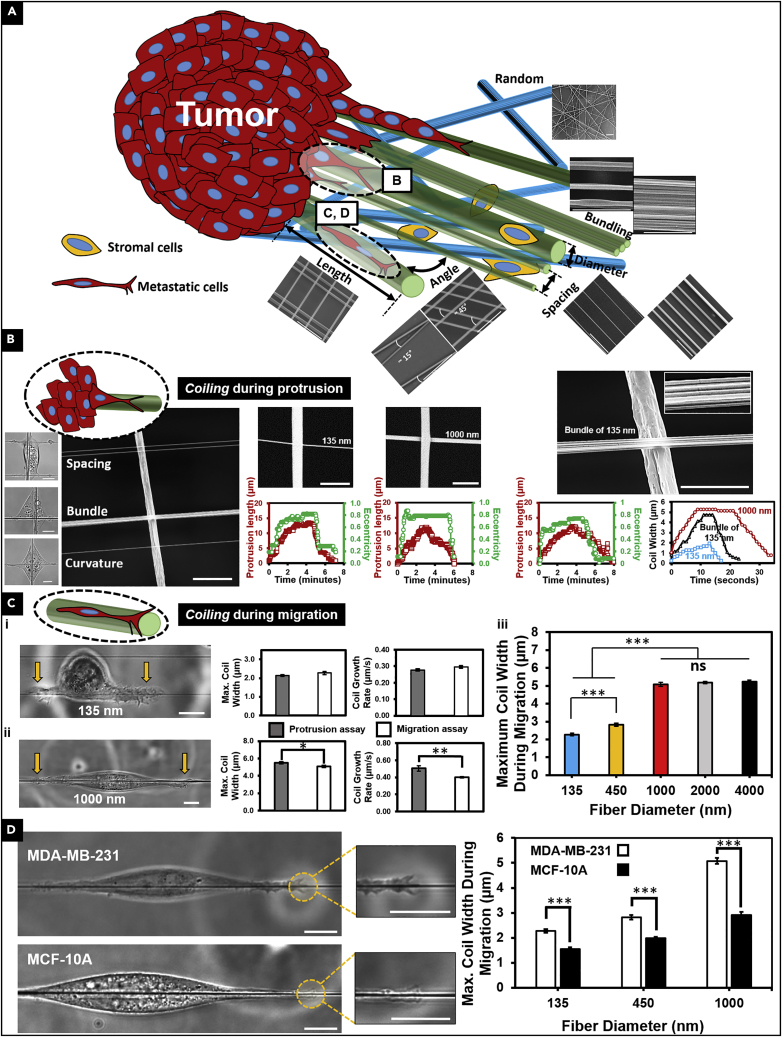
We used the non-electrospinning Spinneret-based Tunable Engineered Parameters (STEP) platform (Nain et al., 2009, Wang and Nain, 2014) to quantitate protrusion coiling behavior and the dynamics of endogenous granule transport into the protrusions as a function of protrusive fiber diameter at high spatiotemporal resolutions (sub-micron and 1 s). To study individual protrusions perpendicular to cell body direction, we used mismatch of fiber diameters with the base fiber at least 2 μm in diameter while five different protrusive fiber isodiameters (Figure 1A) were selected to be 135 ± 3 nm (n = 71), 269 ± 3 nm (n = 51), 453 ± 4 nm (n = 36), 597 ± 8 nm (n = 30), and 1,013 ± 19 nm (n = 40) (Nain and Wang, 2013). We use two morphodynamic metrics, the protrusion length (L) and eccentricity (E), to quantitate protrusion formation, growth, and retraction to the main cell body (protrusive cycle) (Figures 1A and 1B).(Koons et al., 2017) From optical microscopic images, acquired at 63× and 1-s interval (Videos S2, S3, and S4 for 135, 600, and 1,000 nm diameter, respectively), we observed that the protrusion tip coils (wraps around the suspended fiber axis; Figure 1C) such that a coiling cycle is characterized by an increase in the width of the coil till a maximum width is reached followed by a subsequent decrease (Figures 1D and 1E).
Figure 1.
Morphodynamic Metrics used to Quantify Protrusive Behavior and Coiling Dynamics on Protrusive Assay
(A) Schematic showing the STEP protrusion platform (large-diameter base fibers deposited orthogonally onto smaller diameter protrusive fibers) along with metrics used to quantitate protrusive cycle and protrusion coiling. On left are data showing control on protrusive fiber diameter using STEP platform (n = 71, 51, 36, 30, and 40 for 135, 270, 450, 600, and 1,000 nm, respectively).
(B) Representative transient protrusive cycle showing protrusion growth and retraction to cell body.
(C) Representative images of protrusion coiling on the different fiber diameter categories studied. Left panels show scanning electron microscopic images of base-protrusive fiber combinations.
(D) Temporal evolution of protrusion coiling on a 600-nm-diameter fiber.
(E) Schematic showing the growth of the protrusion during a coiling cycle.
Scale bars, 5 μm. Data are represented as mean ± SEM.
Coiling taking place during growth in the protrusion length. Location of coiling is shown by a white arrow. Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
Coiling taking place during growth in the protrusion length. Location of coiling is shown by a white arrow. Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
Coiling taking place during growth in the protrusion length. Location of coiling is shown by a white arrow. Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
Timing of Coiling Is Modulated by Fiber Curvature
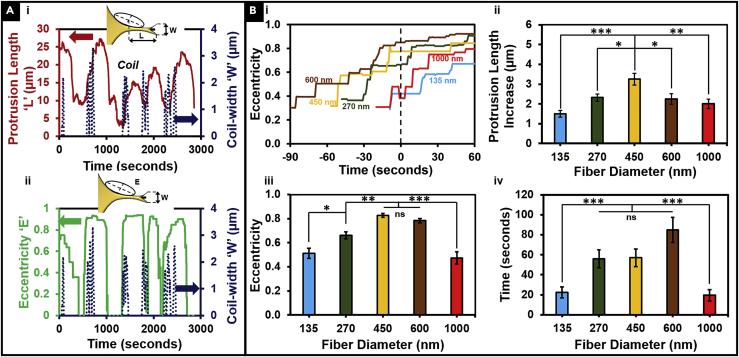
To develop the framework to study the details of the coiling behavior on fibers of varying diameters, we defined additional metrics: maximum coil width occurring during one coiling cycle (Figure 1E), coil growth rate, and the time taken to reach maximum coil width. We observed that coiling occurred in “spurts” at the onset of protrusion growth (Figure 2A) where the protrusion growth phase is quantitatively represented by a concomitant increase in both protrusion length and eccentricity (Figure 2A (i, ii), more representative profiles are included in Figure S1). Next, we wanted to determine the relationship between protrusion eccentricity (broadening of protrusion base) and coiling. Given the dynamic, fluctuating nature of protrusions, we conducted a transient analysis of the evolution of eccentricity before and after coiling initiation (Figure 2B (i), with coiling initiation shown by t = 0). We observed that for the intermediate protrusive fiber categories (∼270–∼600 nm), growth in eccentricity occurred before the initiation of coiling. However, for the fiber diameters on either end of the spectrum (high curvature 135 nm and low curvature 1,000 nm), we observed that coiling started independent of the broadening in the protrusion base. During the coiling process, protrusions were found to elongate (elongation shown schematically in Figure 1E) in a biphasic diameter-dependent fashion (Figure 2B (ii)), and the protrusions continued to increase in length even after the termination of the coiling behavior. To quantify eccentricity-coiling relationship, we looked at the average eccentricity value at the initiation of coiling as a function of fiber diameter and found that for the intermediate-fiber-diameter category (∼270, ∼450, and ∼600 nm), the average eccentricity at the onset of coiling was at least 0.76 ± 0.02, whereas for the ∼135- and ∼1,000-nm cases it averaged 0.51 ± 0.04 and 0.47 ± 0.05, respectively (Figure 2B (iii)). We quantitated the time taken to initiate coiling (total time taken from the initial increase in protrusion eccentricity to the initiation of coiling), and consistent with average eccentricity values, found that on the small- and large-diameter fibers it took less time to initiate coiling (Figure 2B (iv)). Interestingly, we did not observe significance in total length of protrusion at coiling initiation or the number of coiling events occurring in an hour (Figure S2). Overall, we found that the average eccentricity at coiling initiation and its timing exhibited a biphasic response with increase in diameter followed by drop at ∼1,000 nm.
Figure 2.
Protrusion Coiling Behavior Intrinsically Linked to Fiber Curvature
(A) (A i) Coiling behavior (blue dashed curve) occurs in bursts and takes place primarily during protrusion length increase (red curve). (A ii) Typically, eccentricity (green curve) increase precedes coiling behavior. Both (A i) and (A ii) are representative profiles for a 450-nm-diameter protrusive fiber case.
(B) (B i) Representative profiles showing the temporal evolution of eccentricity, with t = 0 representing the onset of coiling. (B ii) Increase in protrusion length during coiling. n = 61, 64, 81, 76, and 45 for 135, 270, 450, 600, and 1,000 nm, respectively. (B iii) Average eccentricity at the initiation of protrusion coiling demonstrating that for the intermediate fiber diameters, a high eccentricity value is required before coil initiation. (B iv) Time at which coiling occurs on fibers of different diameters demonstrating that 135- and 1,000-nm-diameter fibers induce faster coiling at lower eccentricities. n = 21, 21, 23, 20, and 20, for the diameters 135, 270, 450, 600, and 1,000 nm, respectively.
See Methods section for a discussion of the statistical significance parameters. Data are represented as mean ± SEM. ∗p < 0.05; ∗∗p < 0.01; and ∗∗∗p < 0.001.
Individual Coiling Dynamics Are Fiber Curvature Dependent
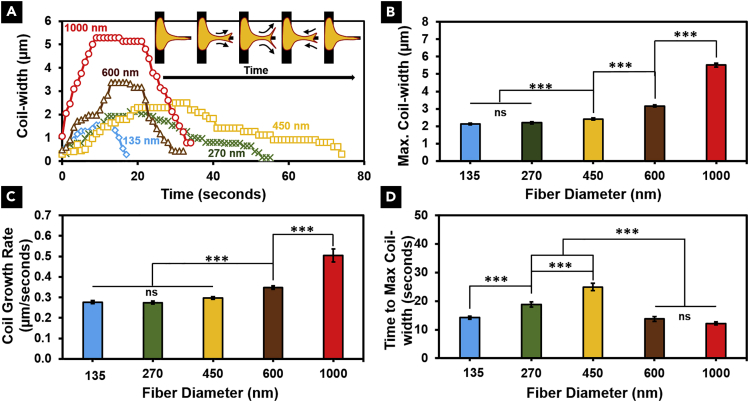
Having quantitated the interplay between increase in protrusion length during coiling, eccentricity, and coiling initiation, we next wanted to analyze the kinetics of coil growth. Observing coiling profiles on different fiber diameters (representative profiles in Figure 3A), we found that the maximum width of the coil, the rate at which the coil grows, and the time taken to reach the maximum coil width are modulated by fiber diameter. For both maximum coil width and the growth rate, we observed no significant differences between the two smallest diameter categories (∼135 and ∼270 nm). However, with further increase in diameter, we found both parameters to increase in a non-linear manner (Figures 3B and 3C), whereas the time taken to reach the maximum coil width displayed a biphasic relationship (Figure 3D). Combined with our findings in Figure 2, we conclude that low curvature ∼1,000-nm diameter fibers support widest coils that initiate independent of protrusion broadening, and do so at the fastest coil growth rate.
Figure 3.
Individual Coiling Dynamics Are Fiber Curvature Dependent
(A) Representative coiling profiles show fiber diameter-dependent dynamics of coiling.
(B and C) (B) Maximum coil width and (C) average coil growth rate increase as a function of protrusive fiber diameter in a non-linear manner.
(D) A biphasic relationship is observed between the time to maximum width and protrusive fiber diameter. In the case of all three parameters (maximum coil width, coil growth rate, and time to maximum width), n values are as follows: 135 nm, 117; 270 nm, 75; 450 nm, 113; 600 nm, 111; 1,000 nm, 77.
See Methods section for a discussion of the statistical significance parameters. Data are represented as mean ± SEM. ∗∗∗p < 0.001.
Granule Translocation Occurs in a Fiber Curvature-Dependent Manner and Coincides with Both the Protrusion Growth and Coiling Cycle
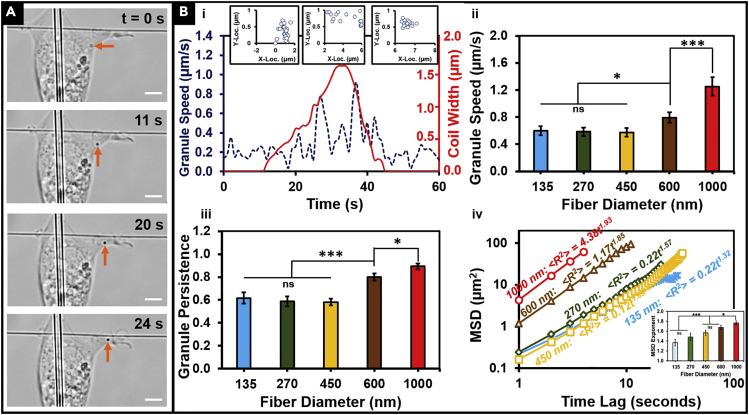
In conjunction with the protrusions coiling, we observed that individual granules entered the protrusions at high speeds persistently (Figure 4A). The granule translocation dynamics showed a near-stationary particle exhibiting fast travel through the protrusion and coming to a near-stationary state again within the protrusion (Video S5). Thus, we inquired if their translocation correlated with the kinetics of coiling.
Figure 4.
Dynamics of Granules Entering Protrusions
(A) Representative time-lapse images of a single granule (shown by orange arrow) entering a protrusion. Scale bar, 5 μm.
(B) (i) Representative transient profile showing that granule speed (dashed blue line) increases with coil width (solid red line). Inset plots of granule position show near-stationary granules translocate persistently at high speeds during coiling cycle, following which they become near-stationary again. (ii, iii) Speed and persistence of the granules increase with increasing protrusive fiber diameter. (iv) Representative MSD versus time profiles and their calculated MSD for granules on different protrusive fiber diameters show that on all tested diameters, the granules enter the protrusions in a superdiffusive manner (exponent of the scaling law fit >1). Inset, MSD exponent as a function of protrusive fiber diameter shows an increase (on average) with increasing diameter. n values are as follows: 135 nm, 19; 270 nm, 17; 450 nm, 12; 600 nm, 21; 1,000 nm, 20.
See Methods section for a discussion of the statistical significance parameters. Data are represented as mean ± SEM. ∗p < 0.05 and ∗∗∗p < 0.001.
Endogeneous granule showing rapid, persistent motion through the protrusion. White arrows track the path of the particle. Video was taken at 63x magnification and 1=s imaging interval. Time shown on top left in minutes:seconds.
Near-stationary granules (likely lipid particles, Figure S3) acting as endogenous “tracer particles” were observed to enter the protrusion during the growth phase of the protrusive cycle in ∼80% of the cases, with the remaining particles translocating either in a non-growing protrusion or, rarely, in a retracting protrusion (Figure S4). Of the cases of particle translocation during the protrusion growth phase, we found that 69% of them coincided with the coiling cycle (Figure S5). Thus, we inquired if the granule dynamics were being modulated by the fiber curvature. To quantitate the dynamics of granules entering the protrusion, we manually tracked them using ImageJ software to analyze granule speed and persistence (defined as the ratio of the displacement of the granule to the distance covered by the granule during its journey through the protrusion, Figure 4B (i)). We found that for granules entering a protrusion, the speed and persistence both increased with fiber diameter (Figure 4B (ii, iii)). Granule speeds of up to 1.3 ± 0.2 μm/s at high persistence of 0.89 ± 0.03 were achieved for the low-curvature ∼1,000-nm protrusive fiber diameters representing an ∼2.3 times increase over the speed (0.57 ± 0.06 μm/s) and a ∼1.5 times increase over the persistence (0.58 ± 0.03) recorded for the ∼135- to 450-nm protrusive fiber diameter cases combined. Interestingly, in cases where multiple granules entered the same protrusion at different times, we observed them to follow a narrow spatial set of paths analogous to highways (Figure S6 shows representative cases on different fiber diameters, and Video S6).
Multiple granules (shown by white arrows) follow a narrow set of paths analogous to “highways” when entering the protrusion. Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
As granules were translocating at high speeds in a persistent fashion into the protrusions, we inquired if the process of translocating could be described by mean square displacement (MSD) method used commonly to describe intracellular granule transport (Caspi et al., 2000, Metzler, 2017). Endogenous granule translocation studied using MSD analysis has been previously shown to range from subdiffusion to superdiffusion regimes indicating random to ballistic transport, respectively (Banks and Fradin, 2005, Bressloff and Newby, 2013, Bronstein et al., 2009, Reverey et al., 2015, Tolić-Nørrelykke et al., 2004, Wachsmuth et al., 2000). The MSD for random diffusion of a particle through an unobstructed medium in “d” dimensions is given by < R2> = 2dDt where “R” represents the MSD, “D” is the diffusion coefficient, and “t” is the lag time (Bressloff and Newby, 2013). However, given the densely packed nature of the cell cytoplasm and the presence of molecular motors to assist cargo transport, intracellular transport is better characterized by anomalous diffusion exhibiting a non-linear power-law behavior < R2> = 2dDtα where α = 1 reverts back to the standard diffusion case (Banks and Fradin, 2005, Saxton, 1994). For α < 1, the translocation is termed as subdiffusive, whereas for α > 1 the translocation is termed as superdiffusive, and values of α ∼ 2 represent “ballistic” motion. Our analysis (Figure 4B (iv)) shows that translocation of granules within protrusions occurs in a superdiffusive manner across all the fiber diameter categories studied, and approaches a near-ballistic transport process occurring in protrusions on low-curvature ∼1,000-nm-diameter fibers.
Finally, given the superdiffusive nature of the granule dynamics, we investigated if molecular motors might play a potential role in the transport of granules in the protrusions. Previously, we have shown that the localization of cytoskeletal components inside individual protrusions is dictated by the protrusion morphology (Koons et al., 2017). Specifically, whereas F-actin and microtubules are present in protrusions of all sizes, the intermediate filament vimentin localized only in mature protrusions (eccentricity ∼0.8 and higher). Thus, we investigated the effect of pharmacological inhibitors Monastrol (100 μM) to inhibit kinesin-5 (a microtubule-associated motor protein) and LY249002 (20 μM) to hinder localization of myosin X (an actin filament-associated motor protein) to the cell leading edge by inhibiting phosphatidylinositol (3,4,5)-trisphosphate (PIP3) activity (PIP3 plays a critical role in myosin X recruitment). In both cases (representative cells treated with both drugs shown in Figure S7 and Videos S7, S8, S9, and S10), we observed a change in the protrusive behavior highlighted by an increase in the proportion of relatively short protrusions (<5 μm in length compared with the average protrusion length of 19 μm ± 0.3 μm; Figure S8). In both cases, washing the drug out recovered the original protrusive behavior. Intriguingly, we found that in both drug cases, there was also a decrease in the proportion of protrusions in which a granule translocation was observed. Analysis of the granule dynamics showed that speed and persistence were unaffected in case of the Monastrol addition, whereas a significant decrease in both speed and persistence was observed with addition of LY249002. Washing out the drugs resulted in recovery of original speed and persistence in both cases (Figure S8). Combined, these results suggest that whereas both kinesin-5 and myosin X motor reduce the instances of granule translocation, myosin X significantly impacts the dynamics of granule translocation.
Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
Discussion
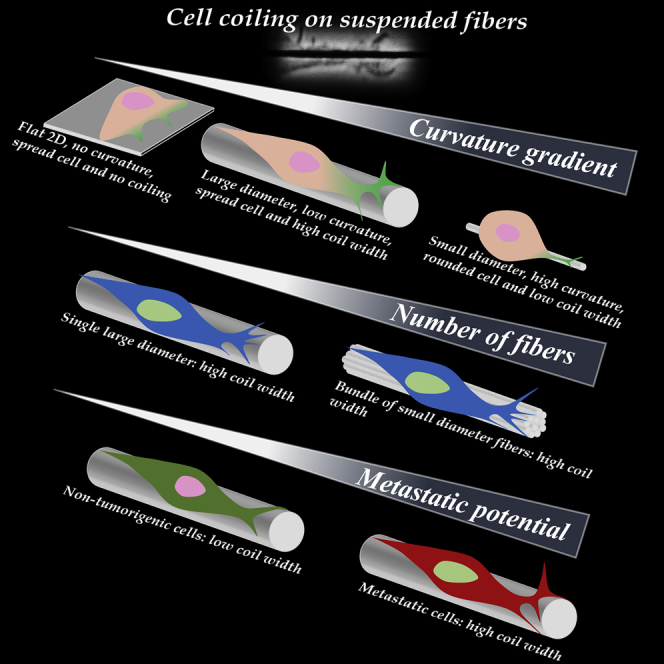
Native fibrous environment surrounding the breast tumor is a complex architecture of fibers with varying diameters, spacing, and orientations (Figure 5A), which collectively make up pro- and anti-invasive biophysical conditions. Invasion along fibers from the edge of the tumor requires cells to first biophysically sense fibers through formation of rod-like protrusions that can mature into broad structures in a fiber curvature-dependent manner (Koons et al., 2017). In our study, we quantitate, at single protrusion resolution, the biophysical probing or recognition of curvature of fibers over a wide range of fiber diameters mimicking the surrounding fibrous environment at the tumor periphery (shown by dashed oval in Figure 5A). We show that fiber curvature modulates the dynamics of the protrusion coiling (size, rate, and time of occurrence). Our findings show that for intermediate-diameter fibers (∼270–600 nm), an increase in eccentricity precedes coiling initiation, whereas for the two ends of the diameter spectrum tested (high curvature, 135 nm, and low curvature, 1,000 nm) coiling occurs independent of protrusion widening (represented by increase in eccentricity, Figure 2). In contrast, the coil width and its rate of increase positively correlate with the fiber diameter (Figure 3). The similarities in time taken to reach maximum coil width observed across fibers on either end of tested diameters can be explained by minimal adhesion-driven contractility on high-curvature 135-nm diameter fibers, and conversely low-curvature 1,000-nm-diameter fibers providing adequate surface area for adhesion sites to mature and increase in number. Indeed, supporting this hypothesis are our previous findings that show that cells attached to (1) small 100-nm-diameter nanoscale fibers are unable to spread and remain rounded while actively probing the fiber curvature (Koons et al., 2017) and (2) large 800-nm-diameter fibers have increased spatial distribution of focal adhesion sites resulting in larger forces (Sheets et al., 2016). However, the adhesion-based contractility on intermediate fiber diameters remains unclear as the time to reach maximum coil width peaks at 450-nm diameter (transient profiles in Figure 3A and data in Figure 3D). Interestingly, substituting single large-diameter fibers with a bundle of smaller diameter fibers recovers coiling dynamics on the large-diameter fibers (Figure 5B and Video S11), thus suggesting the role of both mechanical properties and available surface area in biophysical sensing. To determine if coiling was also exhibited by other breast cancer cell lines, we repeated the study with highly metastatic ductal carcinoma BT-549 (Figure S9). Our data show similarities in coiling dynamics between the two metastatic cancer cell lines.
Figure 5.
Coiling in Recapitulated In Vivo Environments
(A) Schematic showing the heterogeneity observed in environments neighboring breast tumors. Fibers of varied diameters, lengths, orientations, and architectures are interfaced with stromal cells that combined together induce pro- and anti-invasive cell behavior. Inset shows scanning electron microscopic images of fibers deposited in varying configurations (Wang and Nain, 2014) that mimic the native environments including random and aligned configurations. Scale bars, 10 μm.
(B) Cell migration starts with first cells sensing the native environments. Biophysical sensing of fibers can be studied by providing controlled and repeatable fiber architectures (spacing, bundles, and individual fibers of varying curvature). On left are representative phase images of cells on controlled fiber networks (scale bars, 10 μm). Bundling of small-diameter fibers can recover coil width kinetics as that of a single large diameter.
(C) Coiling during cell migration along fibers. Representative images showing “balled-up” cell morphology during migration on (i) high-curvature 135-nm-diameter fiber and elongated, “spindle” morphology during migration on (ii) low-curvature 1,000-nm diameter fiber. Scale bars, 5 μm. Yellow arrows show simultaneous coiling on both sides of the cell. Comparison between coiling dynamics on a protrusion assay and migration assay for ~135-nm-diameter and ~1,000-nm-diameter fiber cases. Sample sizes for the protrusion assay are as follows: 135 nm, 117; 1,000 nm, 77; and for the migration assay are 135 nm, 65; 1,000 nm, 63. (iii) Maximum coil width during migration as a function of the fiber diameter. Sample sizes are as follows: 135 nm, 65; 450 nm, 50; 1,000 nm, 63; 2,000 nm, 61; 4,000 nm, 66.
(D) Representative phase images of coiling dynamics exhibited by non-tumorigenic MCF-10A and metastatic MDA-MB-231 show diminished coiling in non-tumorigenic cell lines. Scale bar, 10 μm. Sample sizes for MDA-MB-231 are as follows: 135 nm, 65; 450 nm, 50; 1,000 nm, 63. Sample sizes for MCF 10A are as follows: 135 nm, 23; 450 nm, 36; 1,000 nm, 20. Data are represented as mean ± SEM. ∗p < 0.05; ∗∗p < 0.01 and ∗∗∗p < 0.001.
Protrusion coiling on a bundle of 135-nm diameter protrusive fibers as opposed to a single 1,000-nm diameter protrusive fiber. Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
After extending protrusions to sense the fibrous environment at the tumor interface, breast cancer cells can detach from the primary tumor and begin migrating along linearized collagen fibers toward the circulatory system for subsequent dissemination to secondary sites (Provenzano et al., 2006). Given that protrusion tip coiling is observed during protrusion extension, we further enquired if coiling also occurred during bulk cell body migration on aligned, single suspended fibers (Figure 5C) of different diameters ranging from ∼135 nm to ∼4,000 nm (hereafter referred to as “migration assay”). On the smallest diameter tested (∼135 nm), cell spreading was hindered as evidenced by frequent “balling-up” of the cell and blebbing (Video S12). In contrast, on relatively larger diameters (∼450 nm and higher, Video S13) tested, cells adopted an elongated “spindle” morphology (Figure 5C (i, ii)). Spindle cells on larger diameter fibers had reduced blebbing, consistent with our previous findings that cell blebbing inversely correlates with cell spread area (Sharma et al., 2013). Interestingly, cells displayed coiling at both ends of the cell body (Figure 5C (i, ii) shown by yellow arrows, and Videos S12 and S13), thus suggesting concurrent sensing at opposite poles of rounded and stretched cells. Next, we quantified the dynamics of coiling at the tip of cells in line with the direction of migration and compared them with coiling quantitated in protrusion assay (perpendicular to the direction of migration) on ∼135-, ∼450-, and ∼1,000-nm diameter fibers. We found that the coiling behavior followed the trends obtained using protrusion assay, whereby the width and rate of coiling increase in a diameter-dependent manner, and the coiling cycle time is the longest for ∼450-nm-diameter fibers (Figure S10). Notably, we found that on ∼1,000-nm-diameter fibers that favor cells to form elongated spindle shapes, coils were significantly smaller in widths and grew at slower rates compared with the protrusion assay (Figure 5C (i,ii)). Furthermore, the maximum coil width in line with migration direction did not change on subsequent larger diameters (∼2,000 and ∼4,000 nm, Figure 5C (iii)). Overall, the similarity in coiling on high-curvature fibers (∼135 and ∼450 nm) and differential response on low-curvature fibers (∼1,000 nm and above) suggests a possible biophysical adaptation from a sensory to a deterministic migratory response with increase in diameter. How these two responses are utilized by cells to establish adhesion-based contractile machinery in single protrusions or at the two poles of a rounded or stretched cell where coiling occurs concurrently remains unclear and is the subject of our future inquiries.
Cells attached to a 135-nm diameter fiber repeatedly “ball-up” and show extensive blebbing. Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
Cell attached to a 1,000-nm diameter fiber show “spindle” morphology. Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
Breast cancer has a well-known predilection for metastasizing to the bone (Lelekakis et al., 1999, Zhou et al., 2016). Following the attachment of previously circulating breast cancer cells to the vascular endothelia of the bone, their subsequent extravasation into the bone marrow compartment is promoted by bone and marrow-derived chemotactic factors (Mastro et al., 2003). On entering the bone marrow, cancer cells encounter a complex array of stromal cells and growth factors embedded in a highly mineralized ECM, which provide a combination of biophysical and biochemical cues that assist in the colonization of the bone (Gilles et al., 1998, Mastro et al., 2003, Senger and Perruzzi, 1996). The role played by the biochemical cues have been investigated in detail, whereas the influence of the mineralization of the collagen fibers unique to the bone is still unclear. A recent study has shown that MDA-MB-231 breast cancer cells exhibited more rounded morphology on mineralized collagen fibers compared with non-mineralized fibers (Choi et al., 2019). It is unclear if cancer cells could be employing coiling as a physical mechanism to aid invasion (easily visualized as a constant drilling process). Thus, we inquired if non-tumorigenic breast cells also displayed coiling on fibers of various diameters. Interestingly, we found that non-tumorigenic cancer cells (MCF 10A, Video S14) exhibit coiling less frequently (only ∼50% of the cases), and also that the coil width was diminished compared with their metastatic counterpart (Figure 5D). Guided by the coiling behavior observed in the breast model, we inquired if coiling was observed in other cell types. We find that other cancer models (fibrosarcoma HT1080 and thyroid Hras1) as well as migratory NIH 3T3 fibroblasts (Videos S15, S16, and S17) to also exhibit coiling. Collectively, our data suggest that migratory cells advance on fibers by coiling, and the extent of coiling is modulated by the fiber curvature. However, how coiling is utilized in pro- and anti-migratory environments remains unknown.
Protrusion coiling on a 450-nm diameter fiber. Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
In conclusion, using the non-electrospinning STEP platform, we quantitate the biophysical sensing of suspended fibers through coiling by cancer cells. We envision that future studies capable of integrating biophysical quantitation presented in this article with membrane tension, curvature-sensing BAR proteins, family of GTPases, and integrin-driven establishment of contractility will link the biophysical coiling with biological timing and signaling. In addition, by increasing the complexity of the fibrous environment in a repeatable manner (Sheets et al., 2013), cell morphology-driven coiling dynamics could help elucidate the potential role of morphology in cancer cell invasion. Through these future studies, we aim to quantitatively describe the migratory cell decision-making process in pro- and anti-invasive fibrous environments.
Limitations of the Study
We identify key limitations associated with our study along with some of our current and future efforts aimed at addressing them.
At the tumor periphery in vivo, tumor cells interact not only with the ECM fibers but also with the neighboring tumor cells with which they can form cell-cell junctions. However, our reductionist approach investigates the coiling kinetics of single cells interacting with suspended fibers. Thus, we do not replicate the cell-cell interactions that might potentially have an impact on coiling dynamics. In future studies, we aim to include these interactions by interfacing fibers with a monolayer of cells. Using such an approach, we have recently shown protrusion-driven control on invasion modes (single, multichain, and collective) through control of fiber diameter and spacing (Sharma et al., 2017).
The presence of stromal cells provide gradients of soluble biochemical cues in the form of secreted growth factors and chemokines that encourage the invasion of the tumor cells into the surrounding stroma (Oudin and Weaver, 2016). Currently, our studies are conducted in static six-well assays, which do not capture the potential effects of gradients on the coiling dynamics. A recent improvement to our method has been integration of fiber networks in custom microfluidic devices (unpublished data). We envision using this assay to calibrate coiling dynamics in controlled environments of biophysical and biochemical gradients. Similarly, haptotactic gradients (Rhoads and Guan, 2007) can be set up as we have previously shown that different concentrations of fibronectin cause a differential protrusive response (Koons et al., 2017).
Finally, the ECM surrounding the tumor is composed of a mix of mechanical properties (modulus, bending stiffness, and diameter). We have used fibers made of polystyrene, a commonly used material for culturing cells. The modulus of polystyrene (∼1 GPa) matches the modulus of collagen fiber bundles (Silver, 2006, Silver et al., 2003, Wenger et al., 2007), and we have shown the role of bending stiffness (structural stiffness) in modulating cell behavior previously (Meehan and Nain, 2014). Using soft versus stiff polymers (for example, polyurethane with a modulus of ∼ 1–10 MPa) will allow us to understand the role of stiffness in coiling kinetics. Furthermore, although we show that a bundle of small-diameter fibers recover coil widths of larger diameter fibers, future improvements to fiber spinning with precise control on the number of fibers in a bundle will help in dissecting the role of mechanical properties and curvature in coiling kinetics.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work is supported by NSF (1437101 and 1462916) awarded to A.S.N. and NSF CAREER (1454226) to B.B. Authors acknowledge the support from Institute for Critical Technology and Applied Science (ICTAS) and Macromolecules Innovative Institute, Virginia Tech. A.M. and A.S.N. would like to thank members of the STEP Lab, Virginia Tech, for their helpful suggestions and discussions. The authors thank Professor Aime Franco (University of Arkansas) for providing thyroid cancer cells.
Author Contributions
Conceptualization, A.S.N.; Methodology, B.B. and A.S.N.; Investigation, A.M., B.B., and A.S.N.; Writing – Original Draft, A.M. and A.S.N.; Writing – Review & Editing, A.M., B.B., and A.S.N.; Funding Acquisition, B.B. and A.S.N.; Resources, B.B. and A.S.N.; Supervision, B.B. and A.S.N.
Declaration of Interests
Fiber networks used in this study were manufactured by STEP fiber manufacturing platform (US Patents 9029149 and 9902932 to A.S.N., and US Patent 9753023 to A.S.N. and B.B.).
Published: September 27, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.08.023.
A video abstract is available at https://doi.org/10.1016/j.isci.2019.08.023#mmc19.
Supplemental Information
References
- Alblazi K., Siar C. Cellular protrusions - lamellipodia, filopodia, invadopodia and podosomes - and their roles in progression of orofacial tumours: current understanding. Asian Pac. J. Cancer Prev. 2015;16:2187–2191. doi: 10.7314/apjcp.2015.16.6.2187. [DOI] [PubMed] [Google Scholar]
- Banks D.S., Fradin C. Anomalous diffusion of proteins due to molecular crowding. Biophys. J. 2005;89:2960–2971. doi: 10.1529/biophysj.104.051078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchoin L., Boujemaa-Paterski R., Sykes C., Plastino J. Actin dynamics, architecture, and mechanics in cell motility. Physiol. Rev. 2014;94:235–263. doi: 10.1152/physrev.00018.2013. [DOI] [PubMed] [Google Scholar]
- Bressloff P., Newby J. Stochastic models of intracellular transport. Rev. Mod. Phys. 2013;85:136–191. [Google Scholar]
- Bronstein I., Israel Y., Kepten E., Mai S., Shav-Tal Y., Barkai E., Garini Y. Transient anomalous diffusion of telomeres in the nucleus of mammalian cells. Phys. Rev. Lett. 2009;103:018102. doi: 10.1103/PhysRevLett.103.018102. [DOI] [PubMed] [Google Scholar]
- Buccione R., Orth J.D., McNiven M.A. Foot and mouth: podosomes, invadopodia and circular Dorsal ruffles. Nat. Rev. 2004;5:647–657. doi: 10.1038/nrm1436. [DOI] [PubMed] [Google Scholar]
- Carey S.P., Goldblatt Z.E., Martin K.E., Romero B., Williams R.M., Reinhart-King C.A. Local extracellular matrix alignment directs cellular protrusion dynamics and migration through Rac1 and FAK local extracellular matrix alignment directs cellular protrusion dynamics and migration through Rac1 and FAK. Integr. Biol. Integr. Biol. 2016;8:821–835. doi: 10.1039/c6ib00030d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A., Granek R., Elbaum M. Enhanced diffusion in active intracellular transport. Phys. Rev. Lett. 2000;85:5655–5658. doi: 10.1103/PhysRevLett.85.5655. [DOI] [PubMed] [Google Scholar]
- Choi S., Friedrichs J., Song Y.H., Werner C., Estroff L.A., Fischbach C. Intrafibrillar, bone-mimetic collagen mineralization regulates breast cancer cell adhesion and migration. Biomaterials. 2019;198:95–106. doi: 10.1016/j.biomaterials.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clainche C.L.E., Carlier M. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol. Rev. 2008;88:489–513. doi: 10.1152/physrev.00021.2007. [DOI] [PubMed] [Google Scholar]
- Clark R.A.F., Lanigan J.M., DellaPelle P., Manseau E., Dvorak H.F., Colvin R.B. Fibronectin and fibrin provide a provisional matrix for epidermal cell migration during wound reepithelialization. J. Invest. Dermatol. 1982;79:264–269. doi: 10.1111/1523-1747.ep12500075. [DOI] [PubMed] [Google Scholar]
- Fraley S.I., Wu P., He L., Feng Y., Krisnamurthy R., Longmore G.D., Wirtz D. Three-dimensional matrix fiber alignment modulates cell migration and MT1-MMP utility by spatially and temporally directing protrusions. Sci. Rep. 2015;5:14580. doi: 10.1038/srep14580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P., Wolf K. Plasticity of cell migration: a multiscale tuning model. J. Cell Biol. 2010;188:11–19. doi: 10.1083/jcb.200909003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles C., Bassuk J.A., Pulyaeva H., Sage E.H., Foidart J.M., Thompson E.W. SPARC/osteonectin induces matrix metalloproteinase 2 activation in human breast cancer cell lines. Cancer Res. 1998;58:5529–5536. [PubMed] [Google Scholar]
- Harland B., Walcott S., Sun S.X. Adhesion dynamics and durotaxis in migrating cells. Phys. Biol. 2011;8:015011. doi: 10.1088/1478-3975/8/1/015011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy K.M., Bhaw-Luximon A., Jhurry D. Cell-matrix mechanical interaction in electrospun polymeric scaffolds for tissue engineering: implications for scaffold design and performance. Acta Biomater. 2017;50:41–55. doi: 10.1016/j.actbio.2016.12.034. [DOI] [PubMed] [Google Scholar]
- Koons B., Sharma P., Ye Z., Mukherjee A., Lee M.H., Wirtz D., Behkam B., Nain A.S. Cancer protrusions on a tightrope: nanofiber curvature contrast quantitates single protrusion dynamics. ACS Nano. 2017;11:12037–12048. doi: 10.1021/acsnano.7b04567. [DOI] [PubMed] [Google Scholar]
- Lelekakis M., Moseley J.M., Martin T.J., Hards D., Williams E., Ho P., Lowen D., Javni J., Miller F.R., Slavin J., Anderson R.L. A novel orthotopic model of breast cancer metastasis to bone. Clin. Exp. Metastasis. 1999;17:163–170. doi: 10.1023/a:1006689719505. [DOI] [PubMed] [Google Scholar]
- Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17:107–117. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Lo C.M., Wang H.B., Dembo M., Wang Y.L. Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machacek M., Hodgson L., Welch C., Elliott H., Pertz O., Nalbant P., Abell A., Johnson G.L., Hahn K.M., Danuser G. Coordination of Rho GTPase activities during cell protrusion. Nature. 2009;461:99–103. doi: 10.1038/nature08242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastro A.M., Gay C.V., Welch D.R. The skeleton as a unique environment for breast cancer cells. Clin. Exp. Metastasis. 2003;20:275–284. doi: 10.1023/a:1022995403081. [DOI] [PubMed] [Google Scholar]
- Meehan S., Nain A.S. Role of suspended fiber structural stiffness and curvature on single-cell migration, nucleus shape, and focal-adhesion-cluster length. Biophys. J. 2014;107:2604–2611. doi: 10.1016/j.bpj.2014.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshel A.S., Wei Q., Adelstein R.S., Sheetz M.P. Basic mechanism of three-dimensional collagen fibre transport by fibroblasts. Nat. Cell Biol. 2005;7:157–164. doi: 10.1038/ncb1216. [DOI] [PubMed] [Google Scholar]
- Metzler R. Anomalous diffusion in membranes and the cytoplasm of biological cells. Biophys. J. 2017;112:476a. [Google Scholar]
- Nain A.S., Sitti M., Jacobson A., Kowalewski T., Amon C. Dry spinning based Spinneret based tunable engineered parameters (STEP) technique for controlled and aligned deposition of polymeric nanofibers. Macromol. Rapid Commun. 2009;30:1406–1412. doi: 10.1002/marc.200900204. [DOI] [PubMed] [Google Scholar]
- Nain A.S., Wang J. Polymeric nanofibers: isodiametric design space and methodology for depositing aligned nanofiber arrays in single and multiple layers. Polym. J. 2013;45 doi: 10.1038/pj.2013.1. [DOI] [Google Scholar]
- Oudin M.J., Weaver V.M. Physical and chemical gradients in the tumor microenvironment regulate tumor cell invasion, migration, and metastasis. Cold Spring Harb. Symp. Quant. Biol. 2016;81:189–205. doi: 10.1101/sqb.2016.81.030817. [DOI] [PubMed] [Google Scholar]
- Pollard T.D., Borisy G.G. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Ponti A., Machacek M., Gupton S.L., Waterman-Storer C.M., Danuser G. Two distinct actin networks drive the protrusion of migrating cells. Science. 2004;305:1782–1786. doi: 10.1126/science.1100533. [DOI] [PubMed] [Google Scholar]
- Provenzano P.P., Eliceiri K.W., Campbell J.M., Inman D.R., White J.G., Keely P.J. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reverey J.F., Jeon J.-H., Bao H., Leippe M., Metzler R., Selhuber-Unkel C. Superdiffusion dominates intracellular particle motion in the supercrowded cytoplasm of pathogenic Acanthamoeba castellanii. Sci. Rep. 2015;5:11690. doi: 10.1038/srep11690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads D.S., Guan J.-L. Analysis of directional cell migration on defined FN gradients: role of intracellular signaling molecules. Exp. Cell Res. 2007;313:3859–3867. doi: 10.1016/j.yexcr.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riching K.M., Cox B.L., Salick M.R., Pehlke C., Riching A.S., Ponik S.M., Bass B.R., Crone W.C., Jiang Y., Weaver A.M. 3D collagen alignment limits protrusions to enhance breast cancer cell persistence. Biophys. J. 2014;107:2546–2558. doi: 10.1016/j.bpj.2014.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton M.J. Anomalous diffusion due to obstacles: a Monte Carlo study. Biophys. J. 1994;66:394–401. doi: 10.1016/s0006-3495(94)80789-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger D.R., Perruzzi C.A. Cell migration promoted by a potent GRGDS-containing thrombin-cleavage fragment of osteopontin. Biochim. Biophys. Acta. 1996;1314:13–24. doi: 10.1016/s0167-4889(96)00067-5. [DOI] [PubMed] [Google Scholar]
- Sharma P., Ng C., Jana A., Padhi A., Szymanski P., Lee J.S.H., Behkam B., Nain A.S. Aligned fibers direct collective cell migration to engineer closing and nonclosing wound gaps. Mol. Biol. Cell. 2017;28:2579–2588. doi: 10.1091/mbc.E17-05-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P., Sheets K., Elankumaran S., Singh Nain A. The mechanistic influence of aligned nanofibers on cell shape, migration and blebbing dynamics of glioma cells. Integr. Biol. Integr. Biol. 2013;5:1036–1044. doi: 10.1039/c3ib40073e. [DOI] [PubMed] [Google Scholar]
- Sheets K., Wang J., Zhao W., Kapania R., Nain A.S. Nanonet force microscopy for measuring cell forces. Biophys. J. 2016;111:197–207. doi: 10.1016/j.bpj.2016.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets K., Wunsch S., Ng C., Nain A.S. Shape-dependent cell migration and focal adhesion organization on suspended and aligned nanofiber scaffolds. Acta Biomater. 2013;9:7169–7177. doi: 10.1016/j.actbio.2013.03.042. [DOI] [PubMed] [Google Scholar]
- Silver F.H. Springer Science & Business Media; 2006. Mechanosensing and Mechanochemical Transduction in Extracellular Matrix: Biological, Chemical, Engineering, and Physiological Aspects. [Google Scholar]
- Silver F.H., Freeman J.W., Seehra G.P. Collagen self-assembly and the development of tendon mechanical properties. J. Biomech. 2003;36:1529–1553. doi: 10.1016/s0021-9290(03)00135-0. [DOI] [PubMed] [Google Scholar]
- Starke J., Maaser K., Wehrle-Haller B., Friedl P. Mechanotransduction of mesenchymal melanoma cell invasion into 3D collagen lattices: filopod-mediated extension–relaxation cycles and force anisotropy. Exp. Cell Res. 2013;319:2424–2433. doi: 10.1016/j.yexcr.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Tolić-Nørrelykke I.M., Munteanu E.-L., Thon G., Oddershede L., Berg-Sørensen K. Anomalous diffusion in living yeast cells. Phys. Rev. Lett. 2004;93:078102. doi: 10.1103/PhysRevLett.93.078102. [DOI] [PubMed] [Google Scholar]
- Ushiki T. Collagen fibers, reticular fibers and elastic fibers. A comprehensive understanding from a morphological viewpoint. Arch. Histol. Cytol. 2002;65:109–126. doi: 10.1679/aohc.65.109. [DOI] [PubMed] [Google Scholar]
- Wachsmuth M., Waldeck W., Langowski J. Anomalous diffusion of fluorescent probes inside living cell nuclei investigated by spatially-resolved fluorescence correlation spectroscopy. J. Mol. Biol. 2000;298:677–689. doi: 10.1006/jmbi.2000.3692. [DOI] [PubMed] [Google Scholar]
- Wang J., Nain A.S. Suspended micro/nanofiber hierarchical biological scaffolds fabricated using non-electrospinning STEP technique. Langmuir. 2014;30:13641–13649. doi: 10.1021/la503011u. [DOI] [PubMed] [Google Scholar]
- Weaver A.M. Invadopodia: specialized cell structures for cancer invasion. Clin. Exp. Metastasis. 2006;23:97–105. doi: 10.1007/s10585-006-9014-1. [DOI] [PubMed] [Google Scholar]
- Weber M., Hauschild R., Schwarz J., Moussion C., de Vries I., Legler D.F., Luther S.A., Bollenbach T., Sixt M. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science. 2013;339:328–332. doi: 10.1126/science.1228456. [DOI] [PubMed] [Google Scholar]
- Wenger M.P.E., Bozec L., Horton M.A., Mesquida P. Mechanical properties of collagen fibrils. Biophys. J. 2007;93:1255–1263. doi: 10.1529/biophysj.106.103192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K., Friedl P. Mapping proteolytic cancer cell-extracellular matrix interfaces. Clin. Exp. Metastasis. 2009;26:289–298. doi: 10.1007/s10585-008-9190-2. [DOI] [PubMed] [Google Scholar]
- Wu C., Asokan S.B., Berginski M.E., Haynes E.M., Sharpless N.E., Griffith J.D., Gomez S.M., Bear J.E. Arp2/3 is critical for lamellipodia and response to extracellular matrix cues but is dispensable for chemotaxis. Cell. 2012;148:973–987. doi: 10.1016/j.cell.2011.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Zhu W., Nowicki M., Miao S., Cui H., Holmes B., Glazer R.I., Zhang L.G. 3D bioprinting a cell-laden bone matrix for breast cancer metastasis study. ACS Appl. Mater. Interfaces. 2016;8:30017–30026. doi: 10.1021/acsami.6b10673. [DOI] [PubMed] [Google Scholar]
- Zigmond S.H., Foxman E.F., Segall J.E. Chemotaxis assays for eukaryotic cells. Curr. Protoc. Cell Biol. 2001 doi: 10.1002/0471143030.cb1201s00. Chapter 12, Unit 12.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protrusion coiling (wrapping-around) by an MDA-MB-231 (same cell line used in all the subsequent videos unless otherwise specified) cell attached to a suspended fiber. Coiling occurs around the suspended fiber axis. Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
Coiling taking place during growth in the protrusion length. Location of coiling is shown by a white arrow. Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
Coiling taking place during growth in the protrusion length. Location of coiling is shown by a white arrow. Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
Coiling taking place during growth in the protrusion length. Location of coiling is shown by a white arrow. Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
Endogeneous granule showing rapid, persistent motion through the protrusion. White arrows track the path of the particle. Video was taken at 63x magnification and 1=s imaging interval. Time shown on top left in minutes:seconds.
Multiple granules (shown by white arrows) follow a narrow set of paths analogous to “highways” when entering the protrusion. Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
Protrusion coiling on a bundle of 135-nm diameter protrusive fibers as opposed to a single 1,000-nm diameter protrusive fiber. Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
Cells attached to a 135-nm diameter fiber repeatedly “ball-up” and show extensive blebbing. Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
Cell attached to a 1,000-nm diameter fiber show “spindle” morphology. Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
Protrusion coiling on a 450-nm diameter fiber. Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.
Video was taken at 63x magnification and 1-s imaging interval. Time shown on top left in minutes:seconds.