Abstract
The results of calculations of the second interaction (cross) virial coefficient Baw for water vapor and air, based on enhancement data obtained at NBS at 30, 40, and 50 °C, are presented. Comparisons are made with the results of calculations based on the enhancement data of Politzer and Strebel, Webster, and Goff et al. and with the results of the theoretical calculations of Mason and Monchick and of Chaddock. An empirical equation is given for interpolation and extrapolation.
The random (one standard deviation) uncertainty in the mean values of Baw, arising from the scatter of previously obtained NBS values of the enhancement factor, is estimated to range from 0.7 percent at 30 °C to 1.4 percent of 50 °C. The estimated systematic uncertainties range from 4 percent at 30 °C to 6 percent at 50 °C, respectively.
Keywords: Interaction virial coefficients, moist air, second virial coefficient, virial coefficients, water vapor
1. Introduction
The water vapor content of real gases, and of air in particular, saturated under known conditions of pressure and temperature, is not predicted adequately by ideal gas laws [1].1 In air, the deviations from ideality must be accounted for in order to obtain accuracies better than 0.5 percent at pressures as low as 900 millibars [2]. On any isotherm, the saturated water vapor content increases with pressure to some, as yet, undetermined limit, in a manner consistent with that described by Haar and Sengers [3]. For example, at 0 °C and 200 bars, the water vapor concentration or density in air is about twice that of the pure phase.
This increase in water vapor content with pressure is the algebraic sum of the increases in apparent vapor pressure because of the superimposed pressure of air (the Poynting effect), and the Van der Waals type interactions between different molecular species, and the decrease in apparent vapor pressure due to the solution of the air in the liquid water (the Henry’s law effect). Of the three the largest is due to the nonideality of the gas phase (the Van der Waals type interactions).
Given a real gas equation of state of a water vapor-air mixture, say an equation expressed in virial form, it is possible to derive theoretically an expression for the saturation water vapor content of the gas mixture as a function of the mole fraction of the constituents, the parameters of state, and the virial coefficients. In such a formulation, the air-water interactions are in large part characterized by the second cross virial interaction term for the air and water molecules. Unfortunately there are no definitive values for the second cross interaction virial coefficients, although previously obtained values [2] may be sufficient for some applications.
Attempts have been made to derive second cross interaction virial coefficients from statistical mechanics [4, 5]. However, the assumptions (form of potential) on which these derivations are based are far from exact so that the predicted coefficients are of limited value. What are needed are good experimental values of the second (cross) interaction coefficients. Recently, Hyland and Wexler [6] at NBS have reported precise experimental values of enhancement factors for water vapor in CO2-free (CO2 content on the order of 2 ppm) air at temperatures of 30, 40, and 50 °C. It is the purpose of this paper to use these new data, as well as the limited older data in the literature, in order to compute values of the second interaction virial coefficient. The formal derivations and basic experiments strictly apply to CO2-free air. This limitation is unimportant when using the Baw values in real air situations, as any errors introduced by the roughly 300 ppm of CO2 molecules should be well within the limits caused by the uncertainties in Baw.
2. Theory
2.1. General Considerations
We will derive an equation which relates the interaction virial coefficient to the enhancement factor, thermodynamic parameters of state, and several physical constants.2
Initially, it should be stated that the treatment of air as a single-component gas leads to no theoretical inconsistencies, for the following reasons: (1) Over the experimental range of temperatures, we can ignore chemical reactions. (2) The experiments involved a continuous air flow over the liquid surface, so that the molar ratios of the air components are constant, for all experimental conditions of pressure and temperature, once equilibrium has been established. (3) We deal with only the chemical potential of the water in each phase, and since the required chemical potential difference in the liquid phase depends only on the mole fraction of water (see eq (19)) and not on the amount of dissolved species, it doesn’t matter that the gas mixture dissolved in the water is of different composition than that of the gas-phase air. (This difference arises because the degree of absorption in water varies from one air component to the next.) Thus, in this paper, it will be assumed that air acts as a single component substance with a known molecular weight.
Let air be in thermodynamic equilibrium with a surface of the condensed water substance. The chemical potential μi of each component in the gas phase is equal to that in the condensed phase. For our purposes we need only examine the chemical potential of the water, thus
| (1) |
where superscripts g and c designate the gaseous and condensed phases, T is the thermodynamic temperature, P the total system pressure, (where k = g or c) is the number of moles of water, and is the number of moles of air. Water is a vapor in the gas phase, and either liquid or solid (ice) in the condensed phase.
In the equations which follow, the independent variables are always P, T, and , whether stated explicitly or not. It will be understood that the variables which do not explicitly appear are being held constant, so that subscripts are unnecessary for that purpose.
Consider the difference in chemical potential in each phase between pressure states P1 and P2. We may write
| (2) |
We now write
| (3) |
(see e.g., [7]) where Gk is the total Gibbs free energy of either the gas or condensed phase.
To obtain Gk we use the thermodynamic relationship
| (4) |
where Vk is the total volume of the phase. The Gibbs free energy difference between states P1 and P2, obtained by integrating eq (4), is
| (5) |
Differentiating with respect to it follows that
| (6) |
Substituting eq (6) into (2) yields
| (7) |
2.2. Gas Phase
The equation of state of a gas may be expressed in virial form as a power series in reciprocal molar volume
| (8a) |
or as a power series in pressure
| (8b) |
where P is the total pressure, T is the absolute thermodynamic temperature, ν is the molar volume, R is the gas constant, B and B′ are second virial coefficients, and C and C′ are third virial coefficients. In the enhancement measurements considered in this paper, volume is not one of the experimental parameters. For this reason eq (8b) is used in our derivation.
The virial coefficients of the pressure series are related to those of the volume series by
| (8c) |
and
| (8d) |
The virial coefficients are functions only of temperature; those of eq (8a) are derivable from statistical mechanical relationships [8] if the form of the intermolecular potential is known. (A very large “if”, indeed! See, for example, Hanley and Klein [9]). The second virial coefficients may be considered to express the effects of interactions between two molecules, the third virial coefficients may be considered to express the effects of interactions among three molecules, and so forth. The number of terms or coefficients necessary to adequately represent Pv/RT will depend on the gas (or gases) involved and the PvT state.
If the gas under consideration is a mixture, then the coefficients B, C, B′, C′, etc., become mixture virial coefficients Bmix, Cmix, etc., and can be written in terms of the mole fractions of the pure components, the virial coefficients of the pure components, and quantities called interaction (cross) virial coefficients. For a two-component gas mixture, and in particular for water vapor-air mixtures, statistical mechanics shows that [8]
| (9a) |
| (9b) |
where xa and xw are the mole fractions of air and water vapor, Baa and Bww are the second virial coefficients for pure air and pure water vapor, and Caaa and Cwww are the third virial coefficients for pure air and pure water vapor. Baw is the second interaction virial coefficient expressing the effects of interaction between an air molecule and a water molecule. Caaw is the third interaction virial coefficient expressing the effects of interaction between two air molecules and one water molecule whereas Caww is the third interaction virial coefficient expressing the effects of interaction between two water molecules and one air molecule.
The mole fractions of air and water vapor are given by
| (10a) |
and
| (10b) |
The molar volume vmix is related to the total volume Vmix by
| (10c) |
where na and nw are the number of moles of air and water vapor, respectively, in the total volume Vmix.
Substituting eqs (10), (9), (8c), and (8d) into (8b) we obtain
| (11) |
Now Vmix = Vg; therefore eq (11) may be substituted into the left-hand side of eq (7). We let P1 be the pure phase saturation vapor pressure es(T) of the water substance and P2 be any other greater total pressure P. When the total pressure P reduces to es, then xa = 0 and xw = 1. For the sake of simplicity, the superscript g will be deleted. After performing the integration and then the differentiation the left-hand side of eq (7) becomes
| (12) |
The ratio which appears in eq (12) will be called the “enhancement factor” and be designated by the symbol f. It has been variously called “the coefficient f,” “the function f,” and “the correction factor f” [2, 10, 11]. It is closely related to the “vapor concentration enhancement” of Haar and Sengers [3]. Thus
| (13) |
The quantity xwP may be thought of as an “effective” vapor pressure of the water substance in a real gas mixture analogous to a partial pressure in an ideal gas mixture. As P approaches es, xw approaches unity, and at P = es and xw= 1, the enhancement factor f becomes unity.
2.3. Condensed Phase
We will confine our consideration of the condensed phase to the liquid state. Consider the right-hand side of eq (7) which expresses the difference in chemical potential for the water substance in the condensed phase between two pressure states P1 and P2. As before, we let P1 be the pure phase saturation vapor pressure es(T) of the water substance and P2 be any other greater pressure P. Performing the differentiation yields
| (14) |
where Vc is the total volume of the condensed phase (i.e., liquid water containing dissolved air), and where we have emphasized that at P1 = es, . Thus
| (15) |
where is the chemical potential of the pure phase liquid water substance at pressure es.
Consider now the pure phase (single component) liquid water substance at the same pressure states P and es. The difference in chemical potential is
| (16) |
where is the molar volume of pure phase liquid water.
Combining eqs (14), (15), and (16) yields
| (17a) |
or
| (17b) |
The laws of dilute solutions [7] predict that for a solution of dissolved air in water the chemical potential of the solvent, in this case liquid water, at pressure P differs from that of the pure phase of the same substance under the same pressure by an amount given by
| (18) |
where is the mole fraction of water in the solution, and C is a corrective term expressing the excess chemical potential of a real solution over that predicted by the laws of ideal dilute solutions.
Substituting eqs (18) and (17b) into (14) one obtains
| (19) |
Because we may write
| (20) |
For very dilute solutions3 Henry’s law may be used to calculate , i.e.,
| (21) |
where k(T, P) is the Henry’s law “constant” which is a function of T and P, and is the mole fraction of air in the gas phase.
The term C in eq (19) is given by [7]
| (22) |
where W is a function of the interaction energies of the molecular species in the solution. The term C can be shown [see appendix 1] to be negligible in its contribution to Baw and so is dropped from further consideration.
Equation (19) therefore becomes
| (23) |
Kell and Whalley [12] have shown that the specific volume of the pure liquid phase of the water substance can be represented with high accuracy by the following equation of state:
| (24) |
where is the specific volume at temperature t (Celiuis) and pressure P, is the specific volume at temperature t and standard atmospheric pressure (1 atm), and PA is standard atmospheric pressure. Kell [13] has shown also that
| (25) |
It therefore follows that
| (26) |
Let
| (27) |
Inserting eq (27) into (23) we obtain
| (28) |
2.4. Second Interaction Virial Coefficient
By equating eq (12) and (28) and rearranging terms we obtain
| (29a) |
For the sake of simplicity in nomenclature, all superscripts have been omitted, it being understood, however, that mole fractions xa and xw refer only to the gas phase. Let
| (29b) |
| (29c) |
| (29d) |
| (29e) |
| (29f) |
| (29g) |
| (29h) |
then
| (29i) |
so that
| (30) |
The quadratic term appearing in eq (29i) introduces a small correction into the calculated value of Baw. Therefore the value of Baw obtained from eq (29i) without that term must be nearly the same as that calculated from eq (30). It may be shown that this condition is satisfied only if the minus sign is used in front of the radical in eq (30).
3. Sources of Data
3.1. Virial Coefficients
There are several sources of data for the second and third virial coefficients of air [14, 15, 16]. Probably the best and most up to date values of Baa are those of Sengers et al. [16] which cover the temperature range from 100 to 1400 K. Sengers et al. assign a standard deviation of 0.4 cm3/mol to Baa. We have chosen three sigmas as our best estimate of the maximum systematic error, i.e., 1.2 cm3/mol. The Hilsenrath et al. [14] values of Caaa are used here. These range from 90 to 1500 K. We have compared these values with those of Hall and Ibele [15] over temperatures from 0 to 100 °C, our range of interest. The two sets disagree by about 10 percent. This disparity was arbitrarily increased to 15 percent and assigned as the estimated systematic uncertainty in Caaa.
The values given by Goff [2] are used for the second and third virial coefficients of water vapor. Wexler and Greenspan [17] recently have shown that from 0 to 100 °C the Goff values lead to highly precise correlations of theoretical and experimental values of saturation vapor pressure. Other second and third virial coefficients for water vapor are available but only those of Keyes [18] are both experimentally based and cover our experimental range of interest. Goff assigned tolerances to his values, which he stated were equal to twice his estimated probable error. These were converted to one-sigma errors, and compared to the differences between corresponding values of Keyes and Goff. For Bww, the agreement between Keyes and Goff, below 70 °C, is better than 2 percent, while the Goff one-sigma uncertainties range from 8 to 2 percent between 30 and 60 °C. It was felt that the more conservative Goff uncertainties were appropriate here, and as estimates of the maximum systematic uncertainties in Bww we used three-sigma errors. In the case of Cwww below 70 °C, the Keyes and Goff values agree to about 45 percent, whereas the one-sigma errors computed from Goff range from 200 percent at 30 °C to 52 percent at 70 °C. These are felt to be overly conservative. Thus the difference between the Keyes and Goff values was taken as an estimate of the systematic uncertainty in Cwww.
Mason and Monchick [5] and Hyland and Mason [19] give the only known values of Caaw and Caww, respectively.4 These are theoretical calculations based on the Lennard-Jones (12–6) potential, the first covering the temperature range − 80 to + 300 °C, the second from 0 to 100 °C. For purposes of this analysis we ascribe an uncertainty of 50 percent in these values although there is no genuinely sound basis for this choice, and the errors may be larger.
Interpolation equations for the various virial coefficients are tabulated in appendix 2.
3.2. Saturation Vapor Pressure
The following equation, formulated by Wexler and Greenspan [17], is used to calculate es, the saturation vapor pressure of water:
| (31) |
where T48 is the absolute temperature on the International Practical Temperature Scale of 1948 [20, 21] and es is expressed in pascals.5 The coefficients Ei and B are given in table 1.
Table 1.
| E0 | −7.51152 × 103 |
| E1 | 9.65389644 × 101 |
| E2 | 2.3998970 × 10−2 |
| E3 | −1.1654551 × 10−5 |
| E4 | −1.2810336 × 10−8 |
| E5 | 2.0998405 × 10−11 |
| B | −1.2150799 × 101 |
Units: es, pascals; T, kelvins.
3.3. Function g(T, P)
The coefficients αij, a and b, given by Kell and Whalley [12] and Kell [13] are tabulated in table 2. These are needed in order to compute using eq (26) which, in turn, permits the computation of g(T, P) using eq (27). Kell and Whalley estimated that the standard deviation of the differences between observed values of and eq (24) does not exceed 10 ppm. Kell estimated that , given by eq (25) has a standard error of 10 ppm or less for temperatures up to 100 °C.
Table 2.
| Coefficients αij | |||
|---|---|---|---|
| i╲j | 1 | 2 | 3 |
| 0 | −50.9769 × 10−6 | 8.2627 × 10−9 | −9.109 × 10−13 |
| 1 | 3.71999 × 10−7 | −1.3794 × 10−10 | 2.626 × 10−14 |
| 2 | −7.01760 × 10−9 | 3.4032 × 10−12 | −8.913 × 10−16 |
| 3 | 6.00227 × 10−11 | −3.6432 × 10−14 | 11.467 × 10−18 |
| 4 | −3.09041 × 10−13 | 2.0836 × 10−16 | −7.102 × 10−20 |
| 5 | 5.93416 × 10−16 | −4.1744 × 10−19 | 14.841 × 10−23 |
| n | Coefficients an | ||
| 0 | 0.9998396 | ||
| 1 | 1.8224944 × 10−2 | ||
| 2 | −7.922210 × 10−6 | ||
| 3 | −5.544846 × 10−8 | ||
| 4 | 1.497562 × 10−10 | ||
| 5 | −3.932952 × 10−13 | ||
| Coefficient b | |||
| 1.8159725 × 10−2 | |||
Units: t, °C; P, bar; PA, 1.01325 bar; , cm3/mol.
3.4. Constants
The solubility data of Winkler [22, 23] for air in water, as reported by Dorsey [24], was used to calculate the Henry’s law “constant” k at standard atmospheric pressure. In the absence of any known air data on the pressure dependence of this “constant,” it was assumed that the percentage changes in k between the same pressure levels at given temperatures were the same for air as for nitrogen, for which there are solubility data both at atmospheric pressure [25, 26, 27, 28], and at high pressure for temperatures above 20 °C [24, 29, 30]. Using solubility data for nitrogen in water and converting these to k, values for air were extropolated accordingly. Below 20 °C, solubilities of nitrogen in water were calculated using the method of Krichevsky and Kasarnovsky [31] and the fugacities reported by Demming and Shupe [32]. The polynomial
| (32) |
was fitted by the method of least squares to the Henry’s law “constant” for air in water at 1, 50, and 100 bars, where k is in units of mole fraction per bar, Ci are coefficients, and t is the temperature in degrees Celsius. Linear interpolations were employed between isobars. Values of k for nitrogen and air are given in table 3 and for Ci in table 4. The values of k for air are considered uncertain, at most, by 10 percent.
Table 3.
Henry’s law “constants” k for nitrogen and air dissolved in waterf
| Temp. °C |
Nitrogena | Air | Aire | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pressure, bars | Pressure, bars | Pressure, bars | |||||||
| 1 | 50 | 100 | lb | 50c | 100d | 1 | 50 | 100 | |
| 0 | 18.65 | 17.00 | 15.62 | 23.25 | 21.19 | 19.48 | 23.20 | 21.20 | 19.49 |
| 5 | 16.42 | 15.11 | 13.96 | 20.45 | 18.82 | 17.39 | 20.53 | 18.81 | 17.37 |
| 10 | 14.89 | 13.69 | 12.70 | 18.26 | 16.80 | 15.58 | 18.30 | 16.79 | 15.58 |
| 15 | 13.51 | 12.41 | 11.60 | 16.49 | 15.15 | 14.15 | 16.46 | 15.12 | 14.10 |
| 20 | 12.52 | 11.42 | 10.72 | 14.93 | 13.62 | 12.78 | 14.96 | 13.75 | 12.88 |
| 25 | 11.61 | 10.68 | 10.01 | 13.84 | 12.73 | 11.92 | 13.76 | 12.64 | 11.90 |
| 30 | 10.88 | 10.00 | 9.46 | 12.83 | 11.79 | 11.16 | 12.79 | 11.77 | 11.13 |
| 35 | 10.23 | 9.44 | 8.97 | 12.00 | 10.08 | 10.53 | 12.02 | 11.10 | 10.52 |
| 40 | 9.67 | 9.02 | 8.57 | 11.34 | 10.58 | 10.05 | 11.40 | 10.58 | 10.05 |
| 45 | 9.27 | 8.70 | 8.25 | 10.84 | 10.17 | 9.64 | 10.87 | 10.19 | 9.68 |
| 50 | 8.90 | 8.44 | 8.00 | 10.44 | 9.90 | 9.39 | 10.39 | 9.89 | 9.38 |
Calculated by assuming that the percent difference for air is the same as for nitrogen between 50 and 1 bars.
Calculated by assuming that the percent difference for air is the same as for nitrogen between 100 and 1 bars.
Calculated from eq (32).
Units: k, 10−6 mole fraction per bar; P, bars.
Table 4.
| i | Pressure, bars | ||
|---|---|---|---|
| 1 | 50 | 100 | |
| 0 | 23.195 | 21.197 | 19.493 |
| 1 | −0.58037 | −0.51768 | −0.46024 |
| 2 | 9.7392 × 10−3 | 8.2103 × 10−3 | 7.3781 × 10−3 |
| 3 | −6.5058 × 10−5 | −4.7585 × 10−5 | −4.4401 × 10−5 |
Units: t, degrees Celsius; k, mole fraction per bar.
On the unified carbon-12 scale the molecular weight of water Mw is 18.0154 g/mol with a maximum total uncertainty of 0.0009 g/mol [33]. The gas constant R is equal to 83.1434 bar cm3/mol K with a standard deviation of 0.0035 bar cm3/mol K [34].
3.5. Enhancement Factor
The NBS enhancement factor data [6] are given in table 5. It is estimated that the systematic uncertainty in f is 0.07 percent and the random uncertainty is 0.2 percent. Two 30°C runs, reported as being suspicious in [6], have not been considered for the calculation of Baw.
Table 5.
Second interaction virial coefficients based on NBS enhancement data
| Run No. |
Saturation temp. |
Saturation vap. press. | Total pressure |
Mole fraction water vapor | Enhancement factor |
Interaction virial coeff. | Normalized inter. vir. coeff. |
|---|---|---|---|---|---|---|---|
| °C | mbar | bar | cm3/mol | cm3/mol | |||
| t | es | P | xw | f | Baw | Baw | |
| Isotherm temperature, 30 °C | |||||||
| 39 | 29.9994 | 42.4278 | 10.7312 | 0.0040827 | 1.0326 | −29.962 | −29.962 |
| 47 | 30.0065 | 42.4451 | 15.1088 | .0029354 | 1.0449 | −29.389 | −29.392 |
| 42 | 29.9999 | 42.4290 | 19.9344 | .0022534 | 1.0587 | −29.115 | −29.115 |
| 20 | 30.0112 | 42.4566 | 30.1929 | .0015317 | 1.0893 | −28.988 | −28.944 |
| 40 | 29.9999 | 42.4290 | 35.5133 | .0013201 | 1.1049 | −28.763 | −28.763 |
| Isotherm temperature, 40 °C | |||||||
| 34 | 40.0104 | 73.8143 | 14.6417 | 0.0052456 | 1.0405 | −26.286 | −26.289 |
| 19 | 39.9969 | 73.7612 | 30.1957 | .0026497 | 1.0847 | −26.978 | −26.977 |
| 27 | 39.9648 | 73.6350 | 40.4557 | .0020231 | 1.1115 | −26.124 | −26.114 |
| 22 | 40.1505 | 74.3673 | 50.3962 | .0016810 | 1.1392 | −25.831 | −25.876 |
| 21 | 40.1366 | 74.3122 | 60.9945 | .0014285 | 1.1725 | −26.158 | −26.199 |
| Isotherm temperature, 50 °C | |||||||
| 32 | 49.9898 | 123.3233 | 10.5512 | 0.0120190 | 1.0283 | −23.443 | −23.440 |
| 37 | 49.9942 | 123.3503 | 10.6310 | .0119361 | 1.0287 | −23.713 | −23.711 |
| 36 | 50.0042 | 123.4115 | 10.7441 | .0118214 | 1.0292 | −23.915 | −23.916 |
| 43 | 50.0047 | 123.4146 | 10.7546 | .0117992 | 1.0282 | −22.714 | −22.715 |
| 46 | 49.9971 | 123.3680 | 10.7953 | .0117446 | 1.0277 | −21.977 | −21.976 |
| 35 | 50.0011 | 123.3925 | 10.7989 | .0117614 | 1.0293 | −23.946 | −23.946 |
| 31 | 49.9891 | 123.3191 | 14.6231 | 0.0087902 | 1.0423 | −27.135 | −27.132 |
| 33 | 50.0064 | 123.4250 | 14.8620 | .0086263 | 1.0387 | −23.399 | −23.401 |
| 30 | 49.9967 | 123.3656 | 25.3585 | .0051773 | 1.0642 | −23.186 | −23.185 |
| 17 | 50.0101 | 123.4477 | 30.1977 | .0044266 | 1.0828 | −25.846 | −25.849 |
| 16 | 50.0050 | 123.4164 | 30.2324 | .0044135 | 1.0811 | −25.117 | −25.118 |
| 29 | 50.0009 | 123.3913 | 36.5953 | .0036782 | 1.0909 | −22.605 | −22.605 |
| 26 | 49.9766 | 123.2426 | 40.8462 | .0033228 | 1.1013 | −22.502 | −22.495 |
| 23 | 50.0049 | 123.4158 | 50.3229 | .0027681 | 1.1287 | −23.227 | −23.228 |
| 24 | 49.9784 | 123.2536 | 76.3139 | .0019376 | 1.1997 | −23.088 | −23.082 |
| 28 | 49.9815 | 123.2726 | 96.9356 | .0016749 | 1.2355 | −22.298 | −22.292 |
| 25 | 49.9788 | 123.2561 | 102.704 | .0015264 | 1.2719 | −22.483 | −22.477 |
4. Results
Values of Baw calculated from these data using eq (30) are given in table 5. The values were normalized from the experimental temperature to the nominal isotherm temperature. The changes, where they occur, are small.
Our best estimates of the magnitudes of Baw and associated random uncertainties are represented respectively by the means of the normalized isotherm values and the standard deviations of the mean normalized values. These are reported in table 6, along with the standard deviations of the individual determinations.
Table 6.
Mean normalized second interaction virial coefficients based on NBS data
| Isotherm Temp. |
Mean normalized |
Standard deviation of single determination | Standard deviation of mean | ||
|---|---|---|---|---|---|
| °C | cm3/mol | cm3/mol | Percent | cm3/mol | Percent |
| 30 | −29.245 | 0.46 | 1.6 | .21 | 0.7 |
| 40 | −26.291 | .41 | 1.6 | .18 | .7 |
| 50 | −23.595 | 1.35 | 5.7 | .33 | 1.4 |
5. Effect of Third Virial Terms
Statistical mechanics predicts that the virial coefficients are functions solely of temperature [8]. An apparent dependence of Baw on pressure can be introduced via the calculations if a sufficient number of terms are not included in the truncated infinite series of the equation of state. If this equation were terminated at the second virial term rather than the third, the terms associated with the parameters ϵ, δ, and β would reduce to zero and from eq (29i) we would have
| (33) |
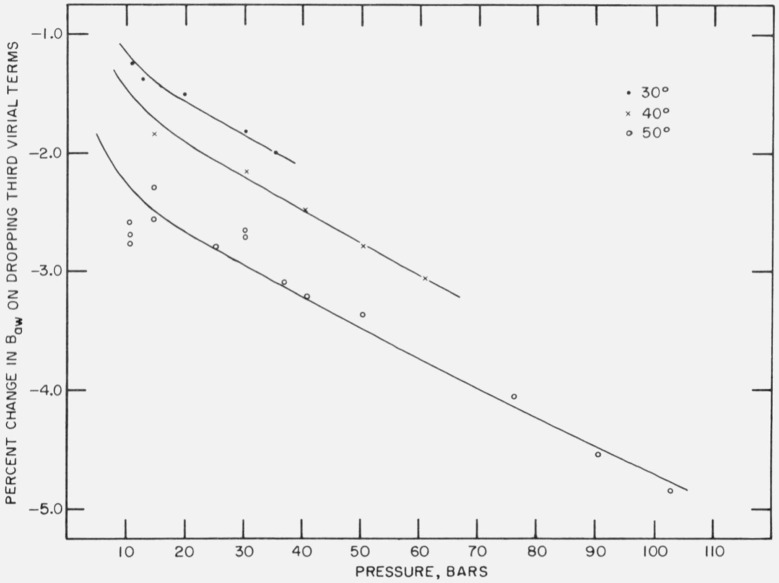
The change introduced by using eq (33) instead of (30) is significant as shown graphically in figure 1 using the NBS enhancement data. Because of this the third virial coefficients were included in the calculation of Baw, even though there are large uncertainties associated with them. It will be shown that the accuracy in Baw is relatively insensitive to the accuracies of the third virial coefficients.
Figure 1.
The percentage changes in calculated values of Baw, when third virial terms are dropped from eq (30).
6. Error Analysis
An analysis was made of the effect of suspected systematic and random errors on the accuracy of the values of Baw given in the results.
6.1. Enhancement Factor
It can be shown that the uncertainties in the experimental parameters P, es, and xw contribute to the error in Baw primarily from their appearance in the enhancement factor term and negligibility because of their presence in the other terms of eq (30).6 Therefore, for purposes of error analysis, the higher order virial coefficients may be neglected and eq (33) used with adequate accuracy for examining the effect of an error in f on Baw. Differentiating Baw with respect to f, replacing the differential with finite increments, and considering only magnitudes, we obtain
| (34) |
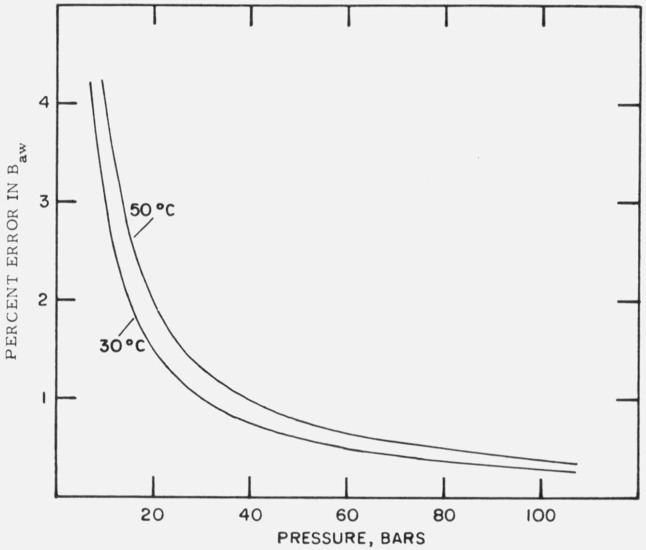
where xa is assumed roughly equal to unity. The systematic uncertainty in the NBS values of enhancement factor is 0.07 percent [6]. By substituting this into eq (34), and using the mean values of Baw given in table 6 to convert ΔBaw to percent, the curves shown in figure 2 were generated. It may be noted that for a fixed relative uncertainty in f the corresponding uncertainty in Baw decreases with increasing pressure. This relationship suggests that for interaction virial coefficient determinations it would be advantageous to perform enhancement measurements at high pressures. However, as we will show below, this gain in accuracy is offset by the increasing uncertainties contributed by the terms in eq (30) containing the third virial coefficients.
Figure 2.
Percentage errors in Baw as a function of temperature and pressure, arising from the estimated systematic error of 7 parts in 104 in the enhancement factor.
6.2. Virial Coefficients
The effects of the estimated systematic uncertainties in the virial coefficients (given in sec. 3.1) on Baw were calculated using eq (30). The results are given in table 7 at the experimental pressures and temperatures.
Table 7.
Estimated systematic uncertainties in Baw
| Temp. | Press. | Source of error | Quadraturee | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baa | BWW | Caaa | CWWW | Caaw | Caww | f | Other | Virial coefficient errors only |
All errors |
||
| Estimated error in parameter, percent | |||||||||||
| (a) | (b) | 15 | (c) | 50 | 50 | .07 | (d) | ||||
| °C | bar | Estimated error in Baw, percent | Estimated error in Baw, percent | ||||||||
| 30 | 10.7 | 2.0 | 1.9 | 0.14 | 0.05 | 0.42 | 0.42 | 2.8 | 0.14 | 2.8 | 4.0 |
| 19.9 | 2.0 | 1.1 | .26 | .03 | .81 | .45 | 1.5 | .14 | 2.5 | 2.9 | |
| 35.5 | 2.0 | .68 | .47 | .02 | 1.4 | .47 | 0.85 | .14 | 2.6 | 2.7 | |
| 40 | 14.6 | 2.2 | 1.6 | .20 | .08 | .62 | .66 | 2.4 | .14 | 2.9 | 3.7 |
| 40.5 | 2.1 | .66 | .56 | .04 | 1.7 | .71 | .85 | .14 | 2.9 | 3.0 | |
| 61.0 | 2.1 | .48 | .84 | .03 | 2.6 | .75 | .57 | .14 | 3.6 | 3.6 | |
| 50 | 10.6 | 2.6 | 2.5 | .16 | .21 | 0.46 | .98 | 3.8 | .14 | 3.8 | 5.4 |
| 25.4 | 2.5 | 1.1 | .38 | .10 | 1.2 | 1.0 | 1.6 | .14 | 3.2 | 3.6 | |
| 50.3 | 2.4 | 0.64 | .76 | .06 | 2.3 | 1.1 | 0.79 | .14 | 3.6 | 3.7 | |
| 76.3 | 2.3 | .48 | 1.2 | .04 | 3.5 | 1.2 | .52 | .14 | 4.5 | 4.6 | |
| 103 | 2.3 | .40 | 1.6 | .04 | 4.8 | 1.2 | .39 | .14 | 5.7 | 5.7 | |
Estimated 3σ error is 1.2 cm3/mol; error is 17, 23, and 34 percent at 30, 40, and 50 °C, respectively.
Estimated error is 24, 16, and 11 percent at 30, 40, and 50 °C, respectively.
Estimated error is nominally 43 percent at 30, 40, and 50 °C.
Quadrature of errors contributed by Henry’s law, gas constant, and ignoring the correction to the law of ideal solutions.
Computed by the square root of the sum of the squares of the individual terms.
6.3. Henry’s Law
It is shown in appendix 1 that dropping the correction term C to Henry’s law, (eq (22)), may lead to an uncertainty in Baw of about 0.13 percent. An uncertainty is contributed also by the “constant” k. The second interaction virial coefficient may be written as follows:
| (35) |
Hence, neglecting the sign,
| (36) |
The estimated uncertainty in k is 10 percent. Over the pressure and temperature range of the NBS data, the variation in k is small. With sufficient accuracy for this calculation, Δk = 1.1 × 10−6 and therefore ΔBaw = 0.014 cm3/mol. Assuming a nominal magnitude of 26 cm3/mol for Baw, k induces an uncertainty in Baw of about 0.05 percent.
6.4. Function g(P, 7); Gas Constant
The largest contributions from the term g(P, T) to the uncertainties in Baw arise from uncertainties in the specific volume of water and the molecular weight of water, while the largest contribution from the gas constant R arises through the enhancement factor term. The uncertainties in P and t in the NBS data are insignificant in their effect on. The systematic errors in , Mw, and R (secs. 3.3 and 3.4) likewise produce negligible uncertainties in the calculated values of Baw.
6.5. Estimated Systematic Uncertainty in Baw
The estimated systematic uncertainties in the individual parameters at the experimental values of P and T are summarized in table 7. These were combined by quadrature to give the estimated overall systematic uncertainty in Baw. Two quadrature columns are shown. The left column is the estimated uncertainty in the calculated value of Baw contributed solely by the virial coefficient terms whereas the right column is the estimated uncertainty in Baw due to all suspected sources of systematic error. Along each isotherm, as the effect on Baw from the fixed percentage uncertainty in enhancement factor decreases with pressure, the effect from the uncertainties in the virial coefficients increases in such a way as to keep the overall systematic uncertainty in Baw more or less constant over the pressure range considered. The systematic uncertainty increases from 4 percent at 30 °C to 6 percent at 50 °C, and applies to the mean value of Baw as well as to the individual values.
6.6. Estimated Random Uncertainty in Baw
The experimental standard deviations of the single determinations and of the mean value of Baw have been given (table 6) as our best estimates of the random uncertainties in Baw. The random uncertainty in Baw arises mainly from the random error in the enhancement factor. The latter, based on the residual standard deviations of fits of f to a pressure function, is 0.02 percent at 30 °C, 0.13 percent at 40, and 0.26 percent at 50 °C [6]. The corresponding calculated uncertainty in a single Baw determination, as a function of pressure along each isotherm, is indicated in table 8. Also given for each isotherm is a mean single-determination uncertainty, based on the three tabulated values. This calculated mean value should be comparable to the experimentally determined precision of a single Baw determination. The latter values are repeated in table 8, and it is seen that the calculated and experimental random uncertainties are in reasonably good agreement.
Table 8.
Comparison of estimated random uncertainties in a single determination of Baw
| Temp. | P | Random uncertainty in Baw | |
|---|---|---|---|
| Based on random error in fa | Based on preci-cision of Baw | ||
| °C | Bar | Percent | Percent |
| 30 | 10.7 | 0.80 | |
| 19.9 | .43 | ||
| 35.5 | .24 | ||
| Mean 0.49 | 1.6 | ||
| 40 | 14.6 | 4.4 | |
| 40.5 | 1.6 | ||
| 61.0 | 1.1 | ||
| Mean 2.4 | 1.6 | ||
| 50 | 10.6 | 14.0 | |
| 25.4 | 5.8 | ||
| 50.3 | 2.9 | ||
| 76.3 | 1.9 | ||
| 103 | 1.4 | ||
| Mean 5.2 | 5.7 | ||
The standard deviation of a single determination of f is 0.02 percent at 30 °C, .13 percent at 40 °C, and 0.26 percent at 50 °C. [6].
7. Comparisons
There are three known experimental determinations of the enhancement of water vapor in air from which the interaction virial coefficient may be calculated. Politzer and Strebel [35] performed single saturation isotherm experiments at 50 and 70 °C at total pressure up to 200 bars. Webster [36] obtained values at −35, −20, 0 and 15 °C at total pressures up to 200 bars, also using the single isotherm saturation method. Goff et al. [37, 38, 39] measured a quantity closely related to the interaction virial coefficient, from 5 to 25 °C at total pressures near one bar.
Values of Baw were computed from the Politzer and Strebel data using the same procedures, constants and virial coefficients that had been used with the NBS data. The 50 °C data of Politzer and Strebel yielded values of Baw that appear to scatter independently of pressure around an average value, except for four points at 12 bars and below. These four points are suspect and so were discarded. The mean and the standard deviation of the mean are given in table 9. The 70 °C data of Politzer and Strebel show a strong monotonic pressure dependence, contrary to the predictions of theory. It is probable that there is a significant systematic error in the measurements although the source of this error is not obvious. Because of this, the 70 °C data were excluded from further consideration.
Table 9.
Interaction virial coefficients from literature data
| Temperature °C |
Source | |||||||
|---|---|---|---|---|---|---|---|---|
| Webster | Politzer and Strebel | Goff | Mason and Monchick | Chaddockb | ||||
|
cm3/mola |
cm3/mol |
cm3/mola |
cm3/mol |
−Baw cm3/mol | σ Baw cm3/mol | −Baw cm3/mol | −Baw cm3/mol | |
| −35 | 64.91 | 7.45 | ||||||
| −20 | 50.15 | 3.96 | 48.2 | 3.2 | ||||
| 0 | 38.01 | 1.92 | 42.0 | 3.0 | 50.4 | 62.0 | ||
| 10 | 39.3 | 3.0 | 47.3 | 58.1 | ||||
| 15 | 34.91 | 3.44 | 37.6 | 2.9 | 45.4 | 56.1 | ||
| 20 | 36.8 | 2.9 | 43.9 | 54.4 | ||||
| 30 | 34.5 | 2.8 | 41.0 | 51.1 | ||||
| 40 | 32.3 | 2.7 | 38.2 | 48.1 | ||||
| 50 | 23.16 | 0.39 | 30.4 | 2.7 | 35.8 | 45.2 | ||
| 60 | 28.5 | 2.6 | 33.5 | 42.5 | ||||
= mean value along an isotherm.
Neither Mason and Monchick nor Chaddock assigns an uncertainty to his theoretical values of Baw.
Baw was computed similarly from Webster’s data for each of his isotherms. At −35 and −20 °C a minor change was introduced into the calculations to allow for the solid state ot the condensed phase. This involved the use for the Henry’s law “constant” the value for water at 0 °C and 1 bar and for es the appropriate values of saturation vapor pressure with respect to ice [40]. The resultant error from the choice of k is negligible; in fact, within the uncertainty of the Webster measurements the Henry’s law “constant” could be ignored. The mean value of Baw for each temperature, and the standard deviation of the mean are given in table 9.
A set of Baw values were calculated by an empirical equation given by Goff [2], and converted to units consistent with those employed in this paper. The standard deviation ascribed to the Goff values were derived from the tolerances he assigned, which were stated to be two times the estimated probable error. The values are given in table 9.
No attempt was made to estimate the systematic errors in the above values of Baw. The standard deviations indicate only the precision of the measurements.
Mason and Monchick [5] and Chaddock [4] have made statistical mechanical calculations of Baw by assuming that the forces between air and water vapor molecules can be represented by the Lennard-Jones (12–6) potential and that the separate species potential parameters ϵ0 (the depth of the potential “well”) and σ0 (molecular “diameter”) can be combined by simple combination rules. Their calculations have been extended to encompass our temperature range of interest and the values so obtained are given in table 9.
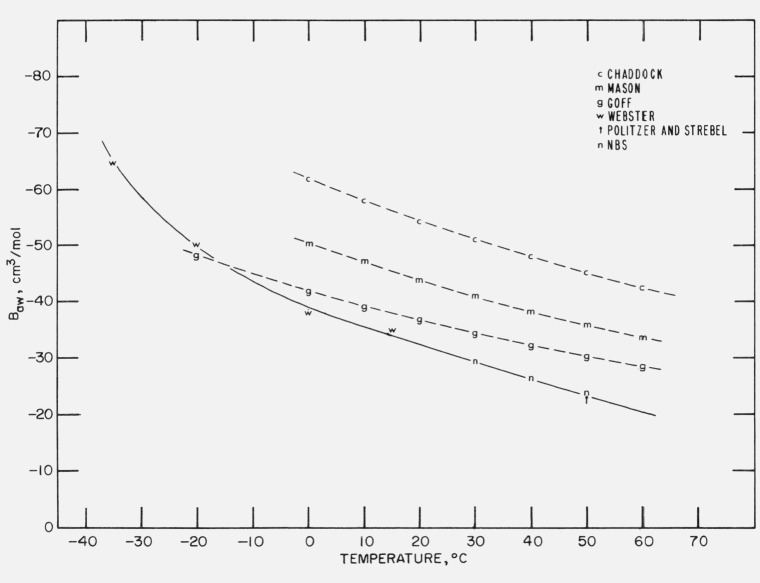
These several sets of values of Baw are compared graphically in figure 3. The curves representing the Goff, Mason and Monchick, and Chaddock values are similar in shape but displaced from each other. It is not surprising that the curves are alike for they are based on similar theoretical formulations, but with somewhat different potential constants. Even Goff’s empirical equation is based on values calculated from a potential function similar to the Lennard-Jones that had been adjusted to yield results consistent with his experimental data [39]. The NBS values of Baw are smaller in absolute value than these other three sets of values. Although the NBS, Webster and the 50 °C Politzer and Strebel values appear to fall on a smooth curve, this may be fortuitous. Until there is additional experimental corroboration, this apparent consistency should be viewed with reservation.
Figure 3.
Baw versus temperature, from various investigators.
8. Smoothing Function
Given experimental values of Baw over a reasonable temperature range, it is feasible to determine the form of the interaction potential between the water vapor and air species and therefore, to derive a theoretically based expression for interpolation and extrapolation of Baw. Unfortunately the NBS data is too limited in temperature range to warrant this approach.
A polynomial equation was fitted, therefore, to the NBS, Politzer and Strebel, and Webster data. The resultant expression is as follows:
| (37) |
where Di are empirical coefficients, t is the temperature in deg Celsius and Baw is in units of cm3/mol. Di are listed in table 10, along with the residual standard deviation of the fit. The experimental and predicted values are compared in table 11.
Table 10.
Coefficients to eq (37)
| D0 | 38.9221 |
| D1 | −.384587 |
| D2 | +.512266 × 10−2 |
| D3 | −.117467 × 10−3 |
| D4 | +.878093 × 10−6 |
| σres | .842 cm3/mol |
Table 11.
Comparison of experimental and predicted values of Baw
| Temperature | Interaction virial coefficient | Difference | Standard deviation of mean | Source | |||
|---|---|---|---|---|---|---|---|
| °C | Experimental | Predicteda | Pred.—Exp. | Experimental | |||
| cm3/mol | cm3/mol | cm3/mol | Percent | cm3/mol | Percent | ||
| −35 | −64.91 | −65.01 | −0.11 | −0.17 | 7.45 | 11.5 | Webster [36]. |
| −20 | −50.15 | −49.74 | +0.41 | +0.82 | 3.96 | 7.9 | Webster ,36]. |
| 0 | −38.01 | −38.92 | −0.91 | −2.34 | 1.92 | 5.1 | Webster [36]. |
| 15 | −34.91 | −33.95 | +0.96 | +2.75 | 3.41 | 9.8 | Webster [36]. |
| 20 | −32.48 | ||||||
| 30 | −29.24 | −29.53 | −0.29 | −0.99 | 0.21 | 0.7 | NBS. |
| 40 | −26.29 | −26.47 | −0.17 | −0.65 | 0.18 | 0.7 | NBS. |
| 50 | −23.56 | −23.30 | +0.26 | +1.10 | 0.33 | 1.4 | NBS. |
| 50 | −23.16 | −23.30 | −0.14 | −0.60 | 0.39 | 1.7 | P & S [35]. |
| 60 | −20.30 | ||||||
Calculated using eq (37).
The differences between the experimental and predicted values are well within the standard deviation of the mean of the experimental values. The maximum deviation of this equation from NBS values of Baw is 1.1 percent.
Equation (37) is offered as a suitable smoothing function for Baw that is valid from 30 to 50 °C, the temperature range of the NBS experiments. We feel confident in using it from 10 to 60 °C. Because it fits the Webster data well, and within the uncertainty of that data, the equation may be used to −35 °C. However, it should be noted that the systematic uncertainty in the Webster data is unknown so that the reliability of the predicted Baw in the temperature range below, say, 10 °C is unresolved.
9. Appendix 1
For a two component solution of equal-size molecules, such as air dissolved in water, it can be shown [7] that
| (38) |
where is the excess Gibb’s function7, that is, the departure from that predicted through Raoult’s law; na and nw are the total number of moles of air and water; and xa and xw are mole fractions of air and water. The parameter W is given by [7]
| (39) |
where Z is a lattice coordination number (assuming a local crystal structure), N0 is Avogadro’s number, ϵij are interaction energies between the designated molecular species.
Let the mole fractions xi in eq (38) be converted to moles ni. Differentiating with respect to nw we obtain.
| (40) |
This equation gives the excess chemical potential of the water with dissolved air over that predicted from Raoult’s law and is therefore equivalent to the term C in eqs (18), (19), and (22).
Mason and Monchick [5] and Chaddock [4] both give ϵaa/k = 99.2 K and ϵww/k = 380 K (where k is Boltzmann’s constant). Mason and Monchick give values of 194 and 222 K for ϵaw/k, as determined from second virial coefficient data and viscosity data respectively whereas Chaddock gives 220.5 K. For the present purpose, assume ϵaw/k = 210 K. If a coordination number of 15 (which should lead to a conservatively large estimate of C) is assumed, then W is −3.69 × 104 bar cm3/mol. Suppose that this is in error by a factor of 5, then W might be of the magnitude −1.84 × 105 bar cm3/mol.
The mole fraction of air dissolved in water xa may be estimated from Henry’s law using the constants in table 3. The worst case (i.e., the largest value of xa which, in turn maximizes C) occurs at 0 °C and 100 bars. Using this value for xa and W = −1.84 × 105 atm cm3/mol, the magnitude of C is then 0.7 bar cm3/mol.
Equation (30), with the C term included, can be approximated by
| (41) |
The lowest pressure present in the NBS enhancement data is 10 bars. The maximum contribution of C to Baw, therefore, is about 0.035 cm3/mol. For Baw of the order of 26 cm3/mol, the relative contribution of C to Baw is about 1.3 parts in 1000, which is about one order of magnitude smaller than the estimated uncertainty in Baw.
10. Appendix 2
The equations for the virial coefficients for water are essentially those of Goff [2]. Bww is given here with opposite sign to conform to eq (8a). Cwww is a modification of Goff’s equation to conform to eq (8a). Goff suggests the use of his equation for Cwww only at temperatures 60 to 100 °C whereas we extropolate it to − 20 °C. Although this is a potentially dangerous procedure, no other alternative is available.
The equations for the virial coefficients Baa and Caaa were obtained by fitting the Sengers et al. data [16] and the Hilsenrath et al. data [14] respectively, over the temperature range 260 to 380 K, to polynomials.
The equations for the interaction virial coefficients Caaw and Caww were similarly obtained by fitting the Mason and Monchick [5] values from − 20 to + 120 °C and the Hyland and Mason [19] values from 0 to 100 °C, respectively, to polynomials.
The standard deviations of the fits for the latter four equations are given. These equations are; convenient empirical relationships and it is not intended that they be used beyond the fitted temperature ranges.
Footnotes
Figures in brackets indicate the literature references at the end of this paper.
The theoretical basis for our work, which is reviewed below, is discussed in most good thermodynamics texts. It has been used in various derivations similar to ours, for example, by Haar and Sengers [3], and Goff and Bates [37].
At pressures up to at least 200 bars and at temperatures from 0 to 100 °C, the mole fraction of water in an equilibrium solution of dissolved air in water is very near unity. At 0° and 200 bars, .
Incorrect values of Cwww are listed in table 1 of the paper by Hyland and Mason. A term was omitted in the calculation. Although the error propagates through the paper, the preferred Caww values (calculated from viscosities) remain unaltered; the effect on the conclusions of the paper is negligible.
Pascal = 1 N/m2 = 10−5 bar = 10−2 mb = 7.50062 × 10−2 mm Hg.
This was checked by making arbitrary changes in these parameters and noting the corresponding changes in the individual terms contributing to Baw, as well as in Baw itself.
Lewis and Randall [7] call this the excess free energy of mixing and designate it by the symbol .
11. References
- [1].Keyes F. G., and Smith L. B., The present state of psychrometric data, Refrig. Eng. 27, 127 (1934). [Google Scholar]
- [2].Goff J. A., Standardization of thermodynamic properties of moist air, Heating, Piping, and Air Cond. 21, 118 (1949). [Google Scholar]
- [3].Haar L., and Sengers J. M. H. L., The solubility of condensed substances in dense gases and the effect on PVT properties, J. Chem. Phys. 52, 5069 (1970). [Google Scholar]
- [4].Chaddock J. B., Moist air properties from tabulated virial coefficients, Humidity and Moisture, Vol. III, Wexler A. and Wildhack W. A., Eds. (Reinhold Publishing Corp., New York, 1965), p. 273. [Google Scholar]
- [5].Mason E. A., and Monchick L., Survey of the equation of state and transport properties of moist gases, Humidity and Moisture, Vol. III, Wexler A. and Wildhack W. A., Eds. (Reinhold Publishing Corp., New York, 1965), p. 257. [Google Scholar]
- [6].Hyland R. W., and Wexler A., The enhancement of water vapor in air, J. Res. Nat. Bur. Stand. (U.S.), 77A (Phys. and Chem.), No. 1, (Jan-Feb 1973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lewis G. N., and Randall M., Thermodynamics, 2nd Edition, as revised by Pitzer K. S., and Brewer L. (McGraw-Hill Book Co., New York, 1961), Ch. 21. [Google Scholar]
- [8].Hirshfelder J. O., Curtiss C. F. and Bird R. B., The Molecular Theory of Gases and Liquids (John Wiley & Sons, Inc., New York, 1954). [Google Scholar]
- [9].Hanley H. J. M., and Klein M., On the selection of the intermolecular potential function: application of statistical mechanical theory to experiment, Nat. Bur. Stand. (U.S.), Tech. Note 360, 85 pages (November 1967). [Google Scholar]
- [10].Harrison L. P., Fundamental concepts and definitions, Humidity and Moisture, Vol. III, Wexler A. and Wildhack W. A., Eds. (Reinhold Publishing Corp., New York, 1964), p. 8. [Google Scholar]
- [11].Hasegawa S., Hyland R. W., and Rhodes S. W., A comparison between the National Bureau of Standards two-pressure humidity generator and the National Bureau of Standards standard hygrometer, Humidity and Moisture, Vol. III, Wexler A. and Wildhack W. A., Eds. (Reinhold Publishing Corp., New York, 1964), p. 455. [Google Scholar]
- [12].Kell G. S., and Whalley E., The PVT properties of water I. Liquid water in the temperature range 0 to 150 °C and at pressures up to 1 kb, Phil. Trans. Roy. Soc. London, Series A, 258, 565 (1965). [Google Scholar]
- [13].Kell G. S., Precise representation of volume properties of water at one atmosphere, J. Chem. Eng. Data 12, 66 (1967). [Google Scholar]
- [14].Hilsenrath J., et al. , Tables of Thermal Properties of Gases, Nat. Bur. Stand. (U.S.), Circ. 564 (1955). [Google Scholar]
- [15].Hall N. A., and Ibele W. E., The tabulation of imperfect gas properties for air, nitrogen, and oxygen, Trans. ASME 76, 1039 (1957). [Google Scholar]
- [16].Sengers J. M. H. L., Klein M., and Gallagher J. S., Pressure-volume-temperature relationships of gases: Virial coefficients, Report No. AEDC TR–71–39, Arnold Engineering Development Center, AFSC, Arnold Air Force Station Tennessee (1971). [Google Scholar]
- [17].Wexler A., and Greenspan L., Vapor pressure equation for water in the range 0 to 100 °C, J. Res. Nat. Bur. Stand. (U.S.), 75A (Phys. and Chem.), No. 3, 213–230 (May-Jun 1971). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Keyes F. G., The thermodynamic properties of water substance 0° to 150 °C, J. Chem. Phys. 15, 602 (1947). [Google Scholar]
- [19].Hyland R. W., and Mason E. A., Third virial coefficient for air-water vapor mixtures, J. Res. Nat. Bur. Stand. (U.S.) 71A (Phys. and Chem.), No. 3219–224 (May-Jun 1967). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stimson H. F., International temperature scale of 1948, J. Res. Nat. Bur. Stand. (U.S.), 42, 209 (1949) RP1962. [DOI] [PubMed] [Google Scholar]
- [21].Stimson H. F., International temperature scale of 1948. Text revision of 1960, J. Res. Nat. Bur. Stand. (U.S.) 65A, (Phys. and Chem.), No. 3, 139–145 (May-Jun 1961). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Winkler L. W., Die Loslichkeit der Gase in Wasser, Ber. deuts. chem. Ges. 34, 1408 (1901). [Google Scholar]
- [23].Winkler L. W., Chem.-Techn. Untersuchungsmethoden, by Lunge G., 5 ed., 1, 768 (1904). Also 1, 573 (1921 ed.). [Google Scholar]
- [24].Dorsey N. E., Properties of Ordinary Water Substance (Rheinhold Publishing Corp., New York, N.Y., 1940). [Google Scholar]
- [25].Loomis A. G., Solubilities of gases in water, International Critical Tables, Vol. 3, Washburn E. W., Ed. (McGraw-Hill Book Co., New York, 1928), p. 255. [Google Scholar]
- [26].Jones H. B., Solubilities of various gases in water, Handbook of Chemistry and Physics, 33rd. edition, Hodgman C. D., Ed. (Chemical Rubber Publishing Co., Cleveland, Ohio, 1951),p. 1482. [Google Scholar]
- [27].Coste J. H., Review of Nitrogen Solubilities, J. Phys. Chem. 31, 81 (1927). [Google Scholar]
- [28].Van Slyke D. D., Dillion R. T., and Margaria R., Studies of gas and electrolyte equilibria in blood XVIII, Solubility and physical state of atmospheric nitrogen in blood cells and plasma, J. Biol. Chem. 105, 571 (1934). [Google Scholar]
- [29].Wiebe R., Gaddy V. L., and Heins C. Jr., The solubility of nitrogen in water at 50, 75, and 100 °C from 25 to 1000 atmospheres, J. Am. Chem. Soc. 55, 947 (1933). [Google Scholar]
- [30].Saddington A. W., and Krase N. W., Vapor-liquid equilibria in the system nitrogen-water, J. A., Chem. Soc. 56, 353 (1934). [Google Scholar]
- [31].Krichevsky I. R., and Kasarnovsky J. S., Thermodynamical calculations of solubilities of nitrogen and hydrogen in water at high pressures, J. Am. Chem. Soc. 57, 2168 (1935). [Google Scholar]
- [32].Deming W., and Shupe L. E., Some physical properties of compressed gases I. Nitrogen, Phys. Rev. 37, 638 (1931). [Google Scholar]
- [33].Division of Inorganic Chemistry, Commission on Atomic Weights, Atomic weight of the elements, Pure and Applied Chem. 21, 93 (1970). [Google Scholar]
- [34].General Physical Constants Nat. Bur. Stand. (U.S.), Spec. Publ. 344 (1971). [Google Scholar]
- [35].Pollitzer F., and Strebel E., Über der Einfluss indifferenter Gase auf die Sättigungsdampfkonzentration von Flussigkeiten, Zeit. fur phys. Chemie 110, 768 (1924). [Google Scholar]
- [36].Webster T. J., The effect on water vapor pressure of superimposed air pressure, J. S. C. I. 69, 343 (1950). [Google Scholar]
- [37].Goff J. A., and Bates A. C., The interaction constant for moist air, Trans. ASHVE 47, 373 (1941). [Google Scholar]
- [38].Goff J. A., Anderson J. R., and Gratch S., Final values of the interaction constant of moist air, Trans. ASHVE 49, 269 (1943). [Google Scholar]
- [39].Goff J. A., and Gratch S., Thermodynamic properties of moist air, Heating, Piping, and Air Cond. (ASHVE Journal Sect.) 17, 334 (1945). [Google Scholar]
- [40].Goff J. A., Saturation pressure of water on the new Kelvin scale, Humidity and Moisture, Vol. III, Wexler A. and Wildhack W. A., Eds. (Reinhold Publishing Corp., New York, 1965), p. 289. [Google Scholar]