Abstract
The manner of packing together of the cardiomyocytes within the walls of the cardiac ventricles has now been investigated for over half a millennium. In 1669, Lower dissected the ventricular mass, likening the arrangement to skeletal musculature, in the form of a myocardial band extending between the right and left atrioventricular junctions. Pettigrew subsequently showed obvious helical arrangements to be evident within the ventricular walls, but emphasised that the cardiomyocytes were attached to each other, and could not justifiably be compared with skeletal cardiomyocytes. Torrent‐Guasp then reactivated the notion that the ventricular mass was formed of a solitary band. Unlike Lower, he dissected the band as extending between the pulmonary to the aortic roots. Multiple investigations conducted using gross dissection and histology, and more recently diffusion tensor magnetic resonance imaging and computed tomographic analysis, have shown an absence of any anatomical boundaries within the walls that might permit the mass uniformly to be dissected so as to reveal the band. A response to a recent letter to the Journal, nonetheless, claimed that the dissections had been validated by clinicians interpreting the findings so as to provide an explanation for ventricular cardiodynamics, arguing that the findings provided a suitable anatomical model for this purpose. Anatomical models, however, are of no value unless they are anatomically correct. In this review, therefore, we summarise the evidence showing that the cardiomyocytes making up the ventricular walls, rather than forming a ventricular myocardial band, are instead aggregated together to form a three‐dimensional myocardial mesh.
Keywords: aggregated cardiomyocytes, fibrous matrix, helical heart, ventricular myocardial band
Introduction
It is surprising that controversy continues regarding the fashion in which the individual cardiomyocytes are aggregated together within the walls of the ventricles of the heart. The topic has been investigated now for well over 500 years (Keele, 1952). Gross dissection and inspection reveals an obvious ‘grain’ to the ventricular surface (Lower, 1669). Gross dissection, however, is unable to demonstrate the specific components of the walls responsible for producing the visible striations. Lower (1669), nonetheless, was able to unravel the boiled ventricular mass of animal hearts so as to produce a myocardial band extending between the right and left atrioventricular junctions. In this regard, Lower was making comparisons between cardiac and skeletal musculature. Extensive histological examination, including investigations using electron microscopy, has shown that the individual contractile unit within the ventricular walls is the cardiomyocyte (Fox & Hutchins, 1972). The individual cardiomyocytes themselves are bound together within the supporting fibrous matrix (Caulfield & Borg, 1979), but not in such a way as to permit uniform unwrapping of the ventricular cone so as to produce the band as revealed by Lower (1669). Simple analysis of histological sections, however, does not reveal the details of the three‐dimensional patterns produced by virtue of the packing, nor does it demonstrate the extent and alignment of the units thus aggregated together.
Despite these shortcomings, it remains frequent to find, in standard textbooks, descriptions of cardiac ‘fibres’. Definitions regarding the extent and boundaries of such presumed entities are rarely, if ever, forthcoming. Shortly after the turn of the 20th century, it became conventional to describe more extensive sub‐structures within the ventricular walls, such as ‘bulbospiral muscles’. Again, details were not provided as to the extent of such presumed entities, nor the nature of their boundaries (MacCallum, 1900; Mall, 1911). More recent methods have permitted better assessment of the fashion of aggregation of the cardiomyocytes, in particular confocal microscopy (Pope et al. 2008), along with scanning electron microscopy and high‐resolution computed tomography subsequent to pneumatic distension (Lunkenheimer et al. 2017). The findings show that the cardiomyocytes not only form chains joined together in end‐to‐end fashion but, in addition, each cardiomyocyte also has several offspring, which connect the cells in side‐to‐side fashion. It is these junctions between the adjacent cardiomyocytes within the end‐to‐end chains, together with the support provided by the endomysial component of the fibrous matrix, which serve to create the aggregated entities. As yet, nonetheless, we are still not able to define their precise dimensions. Hence, we currently describe them simply as aggregates. The aggregated entities themselves are weakly connected by less abundant cross‐connecting branches, and are separated by the loose perimysial component of the fibrous matrix. The three‐dimensional myocardial mesh thus formed extends throughout the ventricular walls.
The notion that the ventricular walls could be analysed in a fashion comparable to skeletal musculature, as initially perceived by Lower (1669), was revived by the concept promulgated by Torrent‐Guasp (1957). As had been the case with Lower (1669), he proposed that the ventricular cone could be unwrapped to produce a ventricular myocardial band. Unlike Lower, however, the band produced by Torrent‐Guasp (1957) was shown as taking its origin from the pulmonary root, and inserting into the aorta. Torrent‐Guasp made no attempt to supplement his dissections so as to demonstrate anatomic boundaries that might have served to guide his dissection. By neglecting such validation, he ignored the caveat voiced by Lev & Simkins (1956), namely that there are no discrete anatomical boundaries to be found within the ventricular cone permitting its uniform dissection so as to demonstrate pre‐existing band‐like configurations. Despite the lack of supporting histological or anatomical validation, the notion of the myocardial band has been accepted as fact by some of those editing textbooks of anatomy (Moore et al. 2013). The concept was also embraced enthusiastically by clinicians such as Buckberg et al. (2015a,b), although Hoffman (2017) subsequently expressed reservations regarding its anatomical accuracy. Such concerns regarding its anatomical foundation were disregarded by Stephenson et al. (2018) when they responded to a letter recently published in the Journal pointing to its morphological deficiencies (Anderson, 2018). In their response to this letter, they suggested that the concept of the helical band had, indeed, been validated by Buckberg and his colleagues (Stephenson et al. 2018). They further opined that the model ‘does not have to be correct to be useful’. When considered in the context of anatomical accuracy, this is a disturbing premise, more so when we consider the extent of the evidence that reveals the anatomical inaccuracies in a concept initially proposed 350 years ago (Lower, 1669). In this review, we summarise the evidence already extant that demonstrates the anatomic deficiencies relating to the concept of the helical ventricular myocardial band.
The concept of the helical ventricular myocardial band
When Torrent‐Guasp (1957) attempted to reduce the biventricular mass of myocardium into a continuum, he likened the arrangement to a wound rope. Unlike Lower (1669), however, who had unwrapped the ventricular cone so as to produce a band extending between the right and left atrioventricular junctions (Fig. 1A), the band produced by Torrent‐Guasp started at the pulmonary trunk, and ended at the aortic root (Fig. 1B). The model included a turn of the long chains of cardiomyocytes contained within it along the long axis of the dissected rope. The rope itself was prepared as coursing around the circumference of the ventricular cone from the endocardium to the epicardium, and then back to the endocardium. Paradoxically, when assessed on this basis, the myocardium forming the rope can also be considered to represent a three‐dimensional continuum. Such a continuum, as we will discuss, was the fashion in which Streeter (1979) viewed the arrangement subsequent to his own collaborations with Torrent‐Guasp. For reasons that remain unknown, Torrent‐Guasp subsequently changed the concept of the wound rope to that of the helical ventricular band, as presented in his collaborations with Buckberg and other colleagues (Kocica et al. 2006). He had deemed from the outset that any transmural components of the initial three‐dimensional mesh from which the band was sculpted were functionally irrelevant. Indeed, when making his initial photographs, Torrent‐Guasp (1957) carefully realigned the so‐called ‘aberrant fibres’ using fine forceps. In this way, he revealed only the alignments seen in the long axis, and hence turned the wound rope into the band‐like structure subsequently modelled by Buckberg et al. (2015a,b). Removal of the components extending in transmural fashion permitted Torrent‐Guasp to create a model in which the components of the dissected band were able freely to glide one against the other. Such freedom of movement of the perceived components remained an essential part of the concept used by Buckberg et al. (2015a,b) to explain cardiodynamics. Prior to dissection, however, the surfaces of the dissected components would have been densely interwoven, and hence fixed to one another. Ignoring this caveat, Torrent‐Guasp (1957), assuming delayed electrical activation, argued that the inner limb of his dissected band would shorten later than the outer limb. Recognising that, subsequent to creation of the band, its inner limb was angulated relative to the outer limb, he postulated a counter‐current action of both limbs. Thus, he envisaged an active systolic ventricular constriction driven by the outer limb, followed by an active early diastolic dilation driven by the inner limb. According to Torrent‐Guasp, both processes would have been accompanied by global ventricular twisting and re‐twisting. This pattern of motion is itself compatible with the frequently cited assumption that ventricular emptying and refilling is comparable to the wringing of a towel. Such a process can be inferred from the illustrations previously provided by Rushmer et al. (1953). It was decades after the initial publication by Torrent‐Guasp (1957) that the model of the ventricular band, as opposed to the rope, was resurrected by Buckberg et al. (2015a,b). Using measurements obtained with ultrasonic crystals, they had demonstrated delayed shortening in the deeper layers of the walls of the left ventricle. As had been the case with Torrent‐Guasp, Buckberg also interpreted the findings on the basis of delayed local electrical activation. In making his measurements, however, Buckberg took no account of local forces. As we will explain, the findings are more readily explained on the basis of auxotonic contraction of ventricular cardiomyocytes (Lunkenheimer et al. 2017). This phenomenon typically prevails in the deeper layers of the ventricular wall. The cardiomyocytes showing such contraction are delayed in their shortening when compared with the larger population of cardiomyocytes responsible for the unloading type of contraction, which prevails in the sub‐epicardial layers of the walls. Such antagonistic actions are part and parcel of the basic behaviour of all contractile structures.
Figure 1.
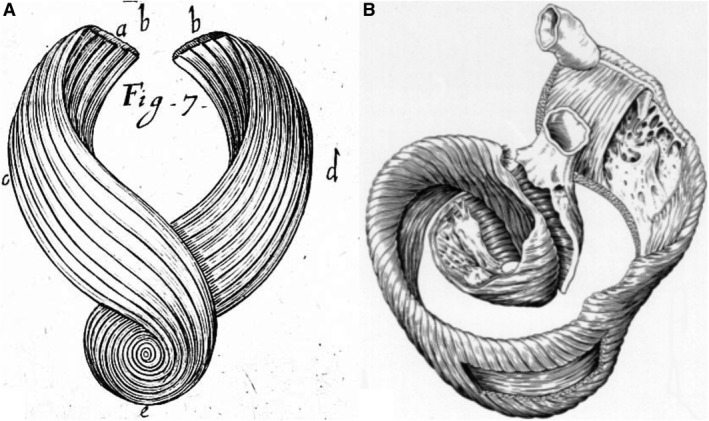
The drawings show the fashion in which the ventricular cone was unwrapped so as to produce a band‐like entity by Richard Lower in 1669 (A), and subsequently by Francisco Torrent‐Guasp in 1957 (B). In neither instance, however, were the prosectors using pre‐existing anatomical planes of cleavage so as to direct their dissections. Panel (B) was reproduced by kind permission of Paul P. Lunkenheimer.
Historical studies that refute the concept of the myocardial band
Investigations of the arrangement of the cardiomyocytes within the ventricular walls can allegedly be traced back to Galen, who worked in the second century of the modern era. It is said that he identified separate populations of cardiomyocytes aligned in transverse and longitudinal fashion, this arrangement being confirmed by the dissections made by Leonardo (Keele, 1952). The existence of opposing epicardial and endocardial helical striations within the ventricular cone was certainly revealed by the dissections of Lower (1669). As we have emphasised, Lower was perhaps the first dissector to unwrap the cone so as to produce a myocardial band. The change in these helical striations within the depths of the ventricular wall is also well illustrated by one of the dissections made by Sénac (1749). The accuracy of these observations concerning the helical configurations can readily be confirmed by gross dissections made using the porcine heart (Fig. 2). The change in helical angulation of the long axes of the aggregated cardiomyocytes at increasing depths within the ventricular wall was then demonstrated at length by Pettigrew (1860). He claimed to recognise seven layers within the ventricular walls, although he did not demonstrate discrete anatomic boundaries between them. Pettigrew emphasised, nonetheless, the fundamental difference between ventricular cardiomyocytes and skeletal myoctyes. He showed that cardiomyocytes were attached to each other, rather than forming entities with discrete origins and insertions. As already discussed, it was Lev & Simkins (1956) who then pointed to the lack of any discrete anatomical planes of cleavage within the ventricular cone that might permit uniform dissection of its component parts. The findings of Lev & Simkins (1956), in essence, invalidated not only the notion that the cone could be unwrapped to produce an anatomically discrete band, but also the previous suggestions of the existence of sub‐structures within the ventricular cone, as had been proposed by MacCallum (1900) and Mall (1911). Grant (1965) then solidified the notion that the cardiomyocyte was the solitary individual contracting entity within the three‐dimensional myocardial meshwork making up the ventricular walls.
Figure 2.

The image shows the lateral view of the dissected porcine left ventricle positioned on its apex. The dissection has been made so as progressively to remove the thickness of the wall, leaving the wall intact at the base, but removing the myocardium almost to the level of the endocardium at the apex. There is an obvious change in the angulation of the striations, which show reciprocal helical patterns in the epicardial and endocardial components of the wall, with a circumferential arrangement at the middle of the wall. There are, however, no anatomical boundaries within the walls that separate its components. The dissection was made by Paul Lunkenheimer, and is reproduced with his kind permission.
Histological investigations refuting the concept of the myocardial band
Important histological investigations relative to the aggregation of the cardiomyocytes had been performed by German investigators at the end of the 19th century (Krehl, 1891), and again in the middle of the 20th century (Feneis, 1943; Hort, 1957). Krehl emphasised the significance of the cardiomyocytes aggregated within the central parts of the thickness of the left ventricular walls. He considered these parts to provide the driving force, or triebwerkzeug, for ventricular emptying. The investigations of Feneis (1943) revealed the additional complexity to be seen when short axis sections were taken across the ventricular cone. His images show an obvious ‘feathered’ appearance of the aggregated cardiomyocytes, with cleavage planes separating the aggregated units (Fig. 3). These arrangements were replicated in the studies performed later in the 20th century by Greenbaum et al. (1981). The spaces between the aggregated units, as revealed by the histological sectioning, had also been observed subsequent to gross sectioning (Grimm et al. 1976).
Figure 3.
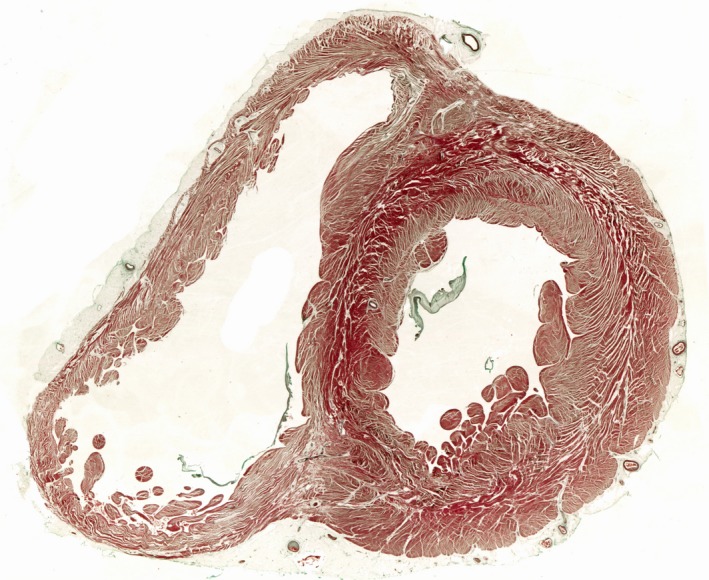
The image shows a short axis section through the ventricular cone taken midway between the ventricular base and the apex. The section is stained with Masson's trichrome technique. There is an obvious ‘feathering’ appearance of the aggregated cardiomyocytic units in the lateral ventricular wall, as was described by Feneis (1943).
It had been generally presumed during the 20th century, however, that all the working cardiomyocytes within the ventricular walls were aligned in tangential fashion (Frank, 1901). This presumption underscored the doctrine of the Frank–Starling mechanism, with this concept in turn providing the basis for the understanding of ventricular cardiodynamics. The process of cutting short axis sections across the ventricular cone, nonetheless, fails to show the alignment of the cardiomyocytes within the aggregated units relative to either the transmural or tangential planes. Serial cross‐sectional planes cut through the full length of the ventricular cone do show the manner of aggregation of the cardiomyocytes. Some of the units extend in circumferential fashion, as had been emphasised by Krehl (1891), while other entities intrude or extrude from the central aggregates (Lunkenheimer et al. 2006). It is the latter aggregates that produce the feathering described by Feneis (Fig. 3). The existence of the circumferential aggregates, denied by those promoting the concept of the ventricular myocardial band, can further be identified by gross dissection (Fig. 4).
Figure 4.
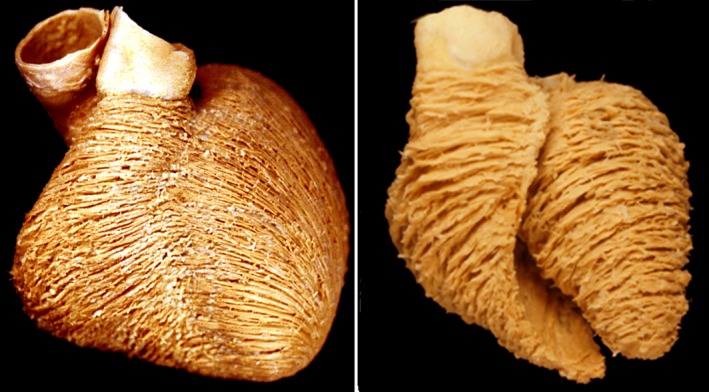
The images show dissections of human hearts, showing the manner of packing of the cardiomyocytes as revealed by gross dissection. The left‐hand panel shows the superficial layers revealed simply by removing the epicardium. Circumferential aggregates on the epicardial surface of the right ventricle become continuous with the epicardial helical layers of the left ventricle. Further dissection of the left ventricle, as shown in the left‐hand panel, reveals the circumferential aggregates found throughout the middle layers of the wall. These aggregates were described by Krehl (1891) as the ‘triebwerkzeug’.
It was histological studies conducted to quantitate the alignment of the cardiomyocytes in the long axis plane, providing the so‐called helical angle, which provided the evidence that came to dominate the discussions of the latter part of the 20th century relative to cardiodynamics (Streeter & Bassett, 1966; Streeter et al. 1969). The overall findings of these investigations were summarised in an extensive chapter published in the Handbook of Physiology of the American Physiological Society (Streeter, 1979). Streeter had confirmed the findings produced by Pettigrew using dissection, namely that the helical angle takes a gradual turn when assessed from the epicardial to the endocardial surfaces of the ventricular walls. His measurements also confirmed that some of the aggregates showed circumferential orientation, hence with zero helical angulation, with these configurations found in the middle component of the walls. Significantly, in addition to providing values of the changing helical angle, the results of the investigations of Streeter showed that, unlike as had been presumed by Frank, not all the cardiomyocytes were aligned in tangential fashion. Thus, Streeter showed that some of the long chains of cardiomyocytes could be traced in transmural fashion, either intruding towards the endocardial surface, or extruding towards the epicardial surface. He described this transmural alignment as representing the angle of imbrication, although the angles he measured were relatively minor. Indeed, those who subsequently used the findings of Streeter to substantiate notions such as contractility continued to presume, following the doctrine of Frank (1901), that all the cardiomyocytes were aligned in tangential fashion.
It is of note that, as already mentioned, Streeter collaborated with Torrent‐Guasp when preparing his chapter for the Handbook of Physiology. Included within his chapter are several illustrations of the rope dissections as initially performed by Torrent‐Guasp (1957). It is of further note that, when discussing his results, Streeter made no mention of the alleged helical ventricular myocardial band. Instead, he concluded that his histological studies endorsed the findings published very much earlier by Krehl (1891). To cite from the chapter ‘In 1891, Krehl synthesised these constructs into a Triebwerk of the left ventricular wall minus the basal valve ring, apex, extreme epicardial fibers, and trabeculae. The Triebwerk was a nested set of fiber paths; each described figures of eight that decreased in amplitude on the inner sets. The innermost set was the ring of circumferential fibers.’ Streeter also considered his findings to be compatible with the investigations previously performed by Feneis and Hort, as discussed above. In keeping with the doctrine established by Grant (1965), he concluded that ‘the heart wall was shown to be a three‐dimensional continuum made up essentially of the one‐dimensional rod element, the cardiac muscle cell.’
In addition to the histological studies spearheaded by Streeter, which themselves replicated the studies conducted by Krehl (1891), Feneis (1943) and Hort (1957), significant histological studies had also been performed by the group working in Auckland, New Zealand (LeGrice et al. 1995). These included an elegant study that used confocal microscopy (Pope et al. 2008). The Auckland group, in their earliest work, had suggested that the cardiomyocytes were aggregated together to form uniform sheets, said to be made up of four–six cardiomyocytes. In a drawing prepared to illustrate these findings, the sheets were shown as separated by additional shelves, with the shelves themselves extending from epicardium to endocardium. The group now recognise that this was an oversimplification of the real arrangement, which is well demonstrated in the confocal reconstruction provided in the study of Pope et al. (2008). When taking account of all the histological studies, it is clear that evidence has yet to be provided to support the notion that anatomical boundaries exist within the ventricular cone that might permit its unwrapping in the form of a ventricular myocardial band, either in the form as initially shown by Lower (1669), or as subsequently alleged to exist by Torrent‐Guasp (1957), this latter version being reproduced decades later by Buckberg et al. (2015a) and, more recently, by Montes (2019).
Findings from diffusion tensor magnetic resonance imaging
When discussing the evidence to support the notion that the helical ventricular myocardial band provided a suitable model with which to interpret ventricular function, Stephenson et al. (2018) pointed to the findings revealed by computational modelling of electromechanical propagation within the ventricular cone (Marce‐Nogue et al. 2013). Similar claims had been made by Buckberg and his associates with regard to the findings of diffusion tensor magnetic resonance imaging. The evidence cited in this regard, however, is based on the obvious helical configuration of the aggregated cardiomyocytes, as revealed by both gross dissections and histological measurements. It is hardly surprising, therefore, that diffusion tensor imaging is able to reveal the presence of such helical configurations, as these tracks represent the spontaneous self‐diffusion of water along the long axes of the adjoined cardiomyocytes. As we have emphasised, this spiralling arrangement of the long chains of cardiomyocytes has been recognised since the earliest works of Lower (1669). Data from diffusion tensor imaging are predominantly assessed in two ways. In the first instance, the orientation of the diffusion vectors can be analysed, thus providing a well‐validated surrogate for the helical alignment of the myocytes (Fig. 5, right hand panel; Agger et al. 2017). Alternatively, the prevailing pathways of the aggregated cardiomyocytes can be assessed using various tracking algorithms so as to produce three‐dimensional maps (Fig. 5, left hand panel; Smerup et al. 2009). With the latter method, the diffusion vectors are connected in an end‐to‐end fashion, without giving consideration to the pronounced side‐to‐side branching of the cardiomyocytes. It is true that tractographies have been proposed to resemble the ventricular myocardial band (Poveda et al. 2013), but the various fibre‐tracking algorithms can be fine‐tuned to connect the vectors virtually at the whim of the investigator, as the technique is open to wide variances that have yet to be determined empirically within the heart. Interpretation of tractography, therefore, constitutes a particular challenge. Results of tractography, therefore, can never stand alone, but should at the very least be supported by numerical analyses of the diffusion raw data. The technique itself does no more than demonstrate the predominant direction of diffusion of water across the three‐dimensional meshwork making up the ventricular walls. Although providing important insights, at present the technique currently lacks sufficient spatial resolution fully to characterise the architectural complexity of the groups of cardiomyocytes that make up the ventricular walls.
Figure 5.
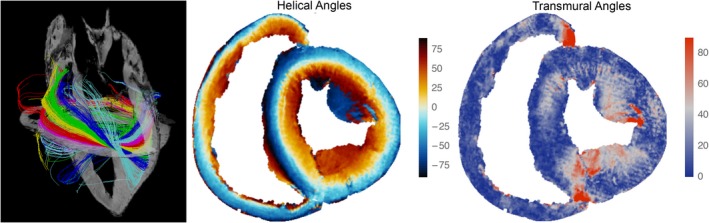
The images show the results of diffusion tensor magnetic resonance imaging. The left panel shows tractography in the left ventricle as viewed from the lateral aspect. Here, the colour coding is arbitrary. It serves only as a visual aid to distinguish the individual tracks. No anatomical or physiological conclusions can be drawn from the colours. The myocardial arrangement correlates with the dissections shown in Fig. 2. The middle panel shows a short axis surface plot of a porcine heart colour‐coded by helical angle. This correlates equally well with the histological image from a human heart as shown in Fig. 3. The right‐hand panel shows the coding that illustrates the transmural angulation in the same short axis section as shown in the middle panel.
The role of the clefts within the three‐dimensional meshwork
One of the conundrums that required explanation in providing the understanding of cardiodynamics was the changes known to occur during systolic ventricular emptying. In this regard, the walls of the left ventricle thicken by between 20 and 40% during systole, depending on the region investigated. The individual cardiomyocytes, in contrast, shorten by no more than 15%, and thicken by only 8% as they contract. So as to account for the additional mural thickening, it is presumed that the aggregations of cardiomyocytes must themselves slide against each other during the change from diastole to systole (Spotnitz et al. 1974). It is the presence of the clefts between the aggregated entities that permits such sliding. The extent of these clefts was revealed by injecting compressed air into the coronary arteries of porcine hearts (Lunkenheimer et al. 1984; Redmann et al. 2011). The pneumatic dissection exaggerated the clefts, with computed tomographic analysis then revealing the spiralling configurations of the opposing epicardial and endocardial helical aggregates (Fig. 6). The analysis failed once more to provide any evidence suggesting that it might be feasible to unwrap the ventricular cone by following pre‐existing anatomical boundaries to produce a ventricular myocardial band. Reconstructions from the computed tomographic datasets, nonetheless, did confirm the presence of aggregated cardiomyocytes aligned in markedly transmural fashion (Fig. 7).
Figure 6.
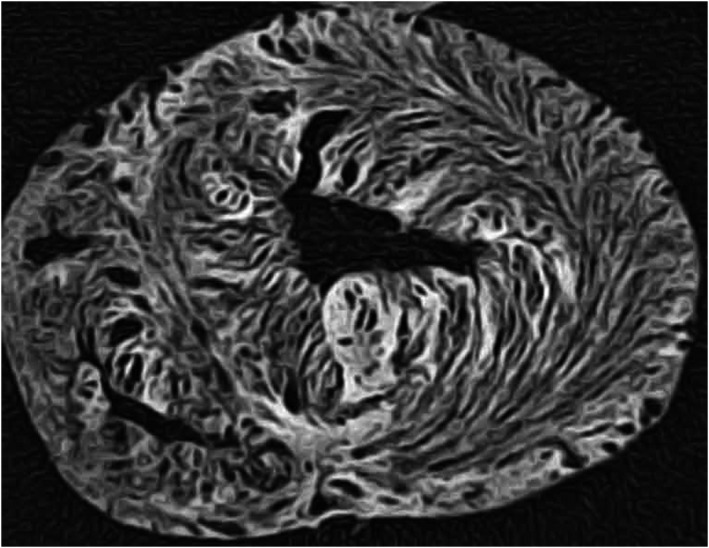
The image shows the short axis of a ventricular cone pneumatically dissected by injection of compressed air into the coronary arteries. The aggregation of the cardiomyocytes is exaggerated by distension of the planes of cleavage. Note the ‘feathering’ in the lateral wall. The pneumatic dissection was made by Paul Lunkenheimer, and the image is reproduced with his kind permission. A similar appearance can be produced if myocardium is progressively dehydrated using alcohol or similar histological solvents.
Figure 7.
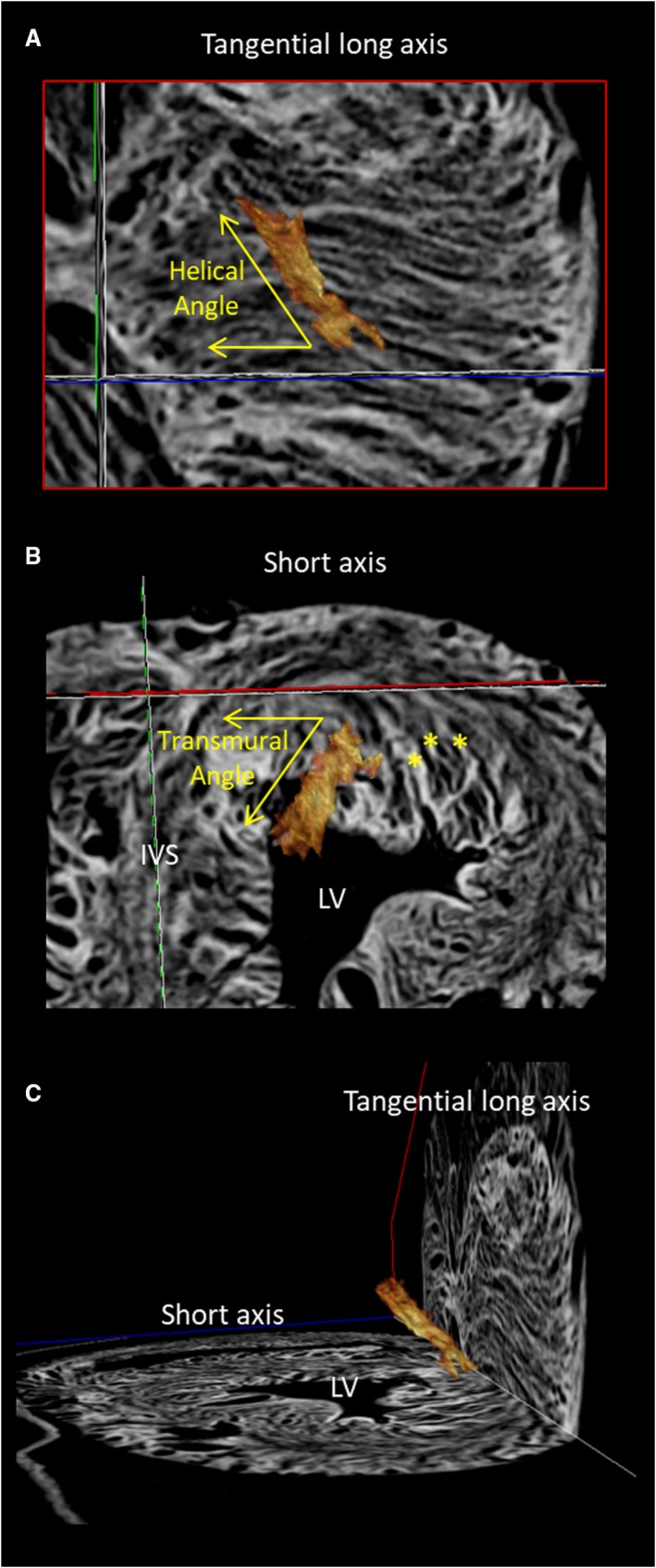
An aggregated unit of cardiomyocytes, shown in brown, has been reconstructed from the computed tomographic dataset produced subsequent to pneumatic distension of the ventricular cone (Fig. 6). The aggregate, and the long chains of cardiomyocytes aggregated within it, can be seen to extend across the ventricular wall in transmural fashion, being obliquely aligned relative to all three orthogonal planes. The stars in (B) show three aggregated units intruding towards the endocardial surface.
The concept of ventricular mural antagonism
It is axiomatic that any anatomical model, if it is to be of value when seeking to understand function, should provide an explanation of all known physiological effects. Models used to explain ventricular cardiodynamics, therefore, must be able to account for the findings regarding the forces generated by the myocardium during ventricular systole. Experiments using force probes carried out by Lunkenheimer et al. (2004) showed that, during ventricular systole, the majority of the identified forces supported ventricular emptying, or unloading. Forces were also identified, nonetheless, that continued to increase during the process of ventricular emptying. These forces, described as being auxotonic, act against systolic mural thickening in the sense that they confine, and hence control, ventricular constriction. The notion of separate populations of cardiomyocytes being responsible, on the one hand, for ventricular emptying, but on the other hand for confining mural thickening, had initially been proposed by Brachet (1813). He had suggested that one set of cardiomyocytes was aligned in long axis fashion, while the alleged second set was radial. Both populations he assumed to be activated separately, the first during systole, the transmural set during diastole. There is no anatomic evidence to support this notion. Lunkenheimer et al. (2004), in contrast, showed that, in areas where they had detected auxotonic forces, there was a predominance of cardiomyocytes aggregated in transmural fashion. Further to explore this finding, Lunkenheimer et al. cut sections across the ventricular walls using circular knives. They hypothesised that, in this fashion, they would better be able to counteract the helical angle, and thus trace the long axes of the transmural entities. The approach is essentially a reinvention of the technique used initially by Streeter (1979). The results were visualised using both histology (Lunkenheimer et al. 2006) and diffusion tensor magnetic resonance imaging (Schmid et al. 2007). The transmural components within the three‐dimensional meshwork were ubiquitous throughout the ventricular cone. The observed arrangement explains well cardiac function on the basis of ventricular antagonism (Lunkenheimer et al. 2017). The presence of the intruding or extruding components of the three‐dimensional mesh, in contrast, is incompatible with the notion that the ventricular cone can be unwrapped to produce an anatomically discrete myocardial band. The three‐dimensional nature of the myocardial architecture is such that the ventricles would be incapable of functioning in the fashion as envisaged at first by Lower (1669), then by Torrent‐Guasp (1957), and subsequently by Buckberg et al. (2015a,b).
Conclusions
It is self‐evident that, if any anatomic model can be shown to be wrong, then any interpretations based on the model must themselves similarly be wrong. This is the case with the notion of the so‐called helical ventricular myocardial band. The model has never been validated by histological studies to show boundaries that might permit dissection of its alleged entities in uniform fashion. Anatomists are well aware that it is only by following such boundaries that it proves possible to demonstrate the arrangement of the skeletal muscles of the limbs and trunk. It is the absence of such boundaries that permitted Lower (1669) to dissect the ventricular cone to produce a continuous band starting and finishing at the atrioventricular junctions, yet for Torrent‐Guasp (1957), and now Montes (2019), to produce a band with origin and insertion at the ventriculo‐arterial junctions. In this regard, Stephenson et al. (2018) had argued that the concept of the helical band was, indeed, ‘validated’ by Buckberg et al. (2015a,b) and Buckberg (2016). The works of Buckberg et al. (2015a,b) and Buckberg (2016), however, offered no such anatomical validation. In contrast, there is a large corpus of material, available from multiple sources, which shows that the notion of a helical ventricular band, first proposed 350 years ago, lacks any anatomical foundation. There is no doubt that the cardiomyocytes making up the walls of the ventricular cone are aggregated together in helical fashion. This fact has also been known for centuries. The helical configurations, however, are part and parcel of the three‐dimensional myocardial mesh. It is this model that provides a more appropriate anatomic foundation for the interpretation of ventricular cardiodynamics (Lunkenheimer et al. 2017).
Conflicts of interest
None of the authors has any conflicts of interest to declare.
Acknowledgements
The authors are indebted to Paul Lunkenheimer, University of Munster, for guiding all of them in their analysis of ventricular mural architecture. The authors also thank him for permitting them to produce several of his own images so as to illustrate their review.
References
- Agger P, Ilkjær C, Laustsen C, et al. (2017) Changes in overall ventricular myocardial architecture in the setting of a porcine animal model of right ventricular dilation. J Cardiovasc Magn Reson 19, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RH (2018) Correspondence. Evolution of the vertebrate heart. J Anat 232, 886–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachet J (1813) Sur la cause du mouvement de dilatiation du coeur. PhD thesis, Faculté de Médecine de Paris, Paris.
- Buckberg GD (2016) Echogenic zone in mid‐septum: its structure/function relationship. Echocardiography 33, 1450–1456. [DOI] [PubMed] [Google Scholar]
- Buckberg GD, Hoffman JI, Coghlan HC, et al. (2015a) Ventricular structure‐function relations in health and disease: part I. The normal heart. Eur J Cardiothorac Surg 47, 587–601. [DOI] [PubMed] [Google Scholar]
- Buckberg GD, Hoffman JI, Coghlan HC, et al. (2015b) Ventricular structure‐function relations in health and disease: part II. Clinical considerations. Eur J Cardiothorac Surg 47, 778–787. [DOI] [PubMed] [Google Scholar]
- Caulfield JB, Borg TK (1979) The collagen network of the heart. Lab Invest 40, 364–372. [PubMed] [Google Scholar]
- Feneis H (1943) Das gefüge des herzmuskels bei systole und diastole. Morphologisches Jahrbuch 89, 371–406. [Google Scholar]
- Fox CC, Hutchins GM (1972) The architecture of the human ventricular myocardium. Johns Hopkins Med J 130, 289–299. [PubMed] [Google Scholar]
- Frank O (1901) Isometrie und isotonie des herzmuskels. Z Biol 41, 14–34. [Google Scholar]
- Grant RP (1965) Notes on the muscular architecture of the left ventricle. Circulation 32, 301–308. [DOI] [PubMed] [Google Scholar]
- Greenbaum RA, Ho SY, Gibson DG, et al. (1981) Left ventricular fibre architecture in man. Heart 45, 248–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm AF, Katele KV, Lin HL (1976) Fiber bundle direction in the mammalian heart. An extension of the “nested shells” model. Basic Res Cardiol 71, 381–388. [DOI] [PubMed] [Google Scholar]
- Hoffman JI (2017) Will the real ventricular architecture please stand up? Physiol Rep 5, e13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hort W (1957) Untersuchungen über die muskelfaserdehnung und das gefüge des myokards in der rechten herzkammerwand des meerschweinchens. Virch Archiv Path Anat Physiol 329, 694–731. [DOI] [PubMed] [Google Scholar]
- Keele KD (1952) Leonardo da Vinci, and the Movement of the Heart and Blood. London: Harvey and Blythe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocica MJ, Corno AF, Carreras‐Costa F, et al. (2006) The helical ventricular myocardial band: Global, three‐dimensional, functional architecture of the ventricular myocardium. Eur J Cardiothorac Surg 29S, S21–S40. [DOI] [PubMed] [Google Scholar]
- Krehl L (1891) Beiträge zur kenntniss der füllung und entleerung des herzens. Abh Math Phys Kl Saechs Akad Wiss 17, 341–362. [Google Scholar]
- LeGrice IJ, Smaill BH, Chai LZ, et al. (1995) Laminar structure of the heart: ventricular myocyte arrangement and connective tissue architecture in the dog. Am J Physiol Heart Circ Physiol 269, H571–H582. [DOI] [PubMed] [Google Scholar]
- Lev M, Simkins CS (1956) Architecture of the human ventricular myocardium; technic for study using a modification of the mall‐maccallum method. Lab Invest 5, 396–409. [PubMed] [Google Scholar]
- Lower R (1669) Tractatus de Corde. London, UK: Early Science in Oxford. [Google Scholar]
- Lunkenheimer PP, Müller RP, Konermann C, et al. (1984) Architecture of the myocardium in computer‐tomography. Invest Radiol 19, 271–278. [DOI] [PubMed] [Google Scholar]
- Lunkenheimer PP, Redmann K, Florek J, et al. (2004) The forces generated within the musculature of the left ventricular wall. Heart 90, 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunkenheimer PP, Redmann K, Kling N, et al. (2006) Three‐dimensional architecture of the left ventricular myocardium. Anat Rec Part A 288A, 565–578. [DOI] [PubMed] [Google Scholar]
- Lunkenheimer PP, Niederer P, Stephenson R, et al. (2017) What is the clinical significance of ventricular mural antagonism. Eur J Cardiothorac Surg 53, 714–723. [DOI] [PubMed] [Google Scholar]
- MacCallum JB (1900) On the muscular architecture and growth of the ventricles of the heart. Johns Hopkins Hosp Rep 9, 307–335. [Google Scholar]
- Mall FP (1911) On the muscular architecture of the ventricles of the human heart. Am J Anat 11, 211–266. [Google Scholar]
- Marce‐Nogue J, Fortuny G, Ballester‐Rodes M, et al. (2013) Computational modeling of electromechanical propagation in the helical ventricular anatomy of the heart. Comput Biol Med 43, 1698–1703. [DOI] [PubMed] [Google Scholar]
- Montes O (2019) Anatomical correlation of the helical structure of the ventricular myocardium through echocardiography. Rev Esp Cardiol. 10.1016/j.rec.2018.10.016 [DOI] [PubMed] [Google Scholar]
- Moore KD, Dalley AF, Agur AMR (2013) Clinically Oriented Anatomy, 7th edn Philadephia, PA: Lippincott Williams & Wilkins. [Google Scholar]
- Pettigrew JB (1860) The Ccroonian lecture: on the arrangement of the muscular fibres of the ventricular portion of the heart of the mammal. Proc R Soc Lond 10, 433–440. [Google Scholar]
- Pope AJ, Sands GB, Smaill BH, et al. (2008) Three‐dimensional transmural organization of perimysial collagen in the heart. Am J Physiol Heart Circ Physiol 295, H1243–H1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poveda F, Gil D, Martí E, et al. (2013) Helical structure of the cardiac ventricular anatomy assessed by diffusion tensor magnetic resonance imaging with multiresolution tractography. Rev Esp Cardiol (English Edition) 66, 782–790. [DOI] [PubMed] [Google Scholar]
- Redmann K, Lunkenheimer PP, Stöppeler S, et al. (2011) Pneumographic imaging of potential cleavage planes within the ventricular myocardium in histology and computed tomography. Bull Georg Natl Acad Sci 5, 115–120. [Google Scholar]
- Rushmer RF, Crystal DK, Wagner C (1953) The functional anatomy of ventricular contraction. Circulation 1, 162–170. [DOI] [PubMed] [Google Scholar]
- Schmid P, Lunkenheimer PP, Redmann K, et al. (2007) Statistical analysis of the angle of intrusion of porcine ventricular myocytes from epicardium to endocardium using diffusion tensor magnetic resonance imaging. Anat Rec 290, 1413–1423. [DOI] [PubMed] [Google Scholar]
- Sénac JB (1749) Traité de la Structure du Coeur, de son Action, et de ses Maladies, Volume 2. Paris: Briasson. [Google Scholar]
- Smerup M, Nielsen E, Agger P, et al. (2009) The three‐dimensional arrangement of the myocytes aggregated together within the mammalian ventricular myocardium. Anat Rec (Hoboken) 292, 1–11. [DOI] [PubMed] [Google Scholar]
- Spotnitz HM, Spotnitz WD, Cottrell TS, et al. (1974) Cellular basis for volume related wall thickness changes in the rat left ventricle. J Mol Cell Cardiol 6, 317–331. [DOI] [PubMed] [Google Scholar]
- Stephenson A, Adams AW, Vaccarezza M (2018) Correspondence: rebuttal letter in response to Professor R.H. Anderson's letter ‘Evolution of the vertebrate heart’. J Anat 232, 888–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter DD (1979) Gross morphology and fiber geometry of the heart In: Handbook of Physiology, Section 2: The Cardiovascular System, pp. 61–112. Bethesda, MD: American Physiological Society. [Google Scholar]
- Streeter DD, Bassett DL (1966) An engineering analysis of myocardial fiber orientation in pig's left ventricle in systole. Anat Rec (Hoboken) 155, 503–511. [Google Scholar]
- Streeter DD, Spotnitz HM, Patel DP, et al. (1969) Fiber orientation in the canine left ventricle during diastole and systole. Circ Res 24, 339–347. [DOI] [PubMed] [Google Scholar]
- Torrent‐Guasp F (1957) Anatomia Funcional del Corazón: La Actividad Ventricular Diastólica y Sistólica. Madrid: Paz Montalvo. [Google Scholar]


