Abstract
In this work, we studied the structure and function of the adult chicken heart with a focus on the right muscular atrioventricular valve using anatomic and echocardiographic methods. We demonstrated that the free wall thickness of the right and left ventricles changes from the apex to the base of the heart. The right muscular atrioventricular valve (RAVV) is joined directly to both the parietal right ventricle free wall (one attachment) and the interventricular septum (two attachments: ventral and dorsal). This valve does not have chordae tendineae or papillary muscles. The quantitative morphological and functional characterization of the RAVV is given. In color Doppler echo, no regurgitation of blood flow in the RAVV was observed in any of the studied birds. The blood flow velocity in the RAVV is 56.2 ± 9.6 cm s‐1. A contractile function of the RAVV is shown. Based on the findings obtained, we conclude that the RAVV has a sufficient barrier function. In addition, as this valve is an integral part of the right ventricle free wall, it contributes to the right ventricle pump function. An agreed nomenclature of the parts of the RAVV is required.
Keywords: anatomy, atrioventricular valve, echocardiography, Gallus gallus domesticus, heart, intracardiac hemodynamics
Introduction
The vertebrate heart has undergone many adaptations during its evolution, from a two‐chambered heart made up of one atrium and one ventricle in cyclostomes and fish, to a three‐chambered heart made up of two atria and one ventricle in amphibians and reptiles. Subsequently, crocodiles (the only ectothermic vertebrates with a full ventricular septum), birds and mammals evolved a four‐chambered muscular pump (made up of two atria and two ventricles) devoted to loading and unloading a large amount of blood around a closed‐valve circulatory system (Stephenson et al. 2017; Jensen et al. 2018). The most prevalent opinion appears to be that the vertebrate taxa that independently gave rise to mammals and birds, are represented by extant reptiles (Chiappe & Dyke, 2006; Clarke & Middleton, 2008; Jensen et al. 2018). Birds and mammals independently evolved endothermy, and both birds and mammals independently evolved hearts with full ventricular septation and a thick left ventricular wall that allowed for a considerable rise in systemic blood pressure (Jensen et al. 2013b; Burggren et al. 2014). The avian and mammalian hearts circulate blood like rhythmically contracting pumps. These hearts maintain high rates of contraction that in concert with high systemic blood pressures accommodate their high rates of oxygen consumption due to their endothermic state (Jensen et al. 2012). The avian heart enables Arctic terns annually to fly from the Arctic to the Antarctic (the longest animal migration) (Egevang et al. 2010) and bar‐headed geese to fly at high altitude during migration (up to 9 km, where the partial pressure of oxygen is 30% that at sea level (Swan, 1961). Such a heart deserves a comprehensive study.
Literature on the morphometry of the avian heart is limited (Prosheva & Rapota, 1989; Straub et al. 2002; Pees et al. 2006; Kroneman et al. 2019). There is a deficiency in echocardiographic studies of the cardiac structure and function in birds (Martinez‐Lemus et al. 1998; Pees et al. 2004; Gyenai et al. 2012; Prosheva et al. 2015; Masoudifard et al. 2016). There are methodical limitations to the study of avian heart function using echocardiography, e.g. specific features of the heart anatomy and the high heart rate. The right ventricle has a crescent shape and the muscular valve in the atrioventricular (AV) orifice adds complexity to the quantification of its size and function. In general, the avian heart anatomy is very like that of the mamma,l but it has some peculiarities. The most obvious macroscopic difference is the muscular right AV valve (RAVV) in the low pressure circuit to the lungs, the significance of which has never been explained. In the adult avian heart, the RAVV is not a fibrous, cusped valve as in the adult mammalian heart (except in monotremes), but is rather a single, crescent‐shaped muscular flap guarding a long, slit‐like AV orifice (Pettigrew, 1864; Davies, 1930; Prosheva & Rapota, 1989; Pees et al. 2006; Alsafy et al. 2009; Figueiredo et al. 2013; Prosheva et al. 2015; Kroneman et al. 2019). Shaner (1923) made macroscopic observations of the muscular architectonics of the fowl heart ventricles and showed that the fibers of the RAVV partially embrace the base of the left ventricle and merge with the other muscle bundles on the ventral and dorsal surfaces of the heart base. Based on this finding, that author hypothesized that the more vigorous the general cardiac contraction, the more firmly the right AV orifice is shut. Lincoln et al. (2004) proved that, in chicken embryos, the RAVV is almost completely composed of muscle.
In the process of human heart embryogenesis the right and left AV valves are developed in different ways. In the very early stages of the human embryonic heart development, the right AV valve resembles the muscular AV valve of the avian heart. As a result of secondary differentiation, it then acquires the appearance of a membranous valve (Lamers et al. 1995). Persisting muscularity of the right AV valve may lead to the congenital malformation of Ebstein's anomaly (Lamers & Moorman, 2002).
Here, we present anatomical and echocardiographic measurements of parameters of the right ventricle (RV), right muscular AV valve and left ventricle (LV) in the adult chicken heart. We also analyze the connection of the chicken RAVV to the RV free wall and to the interventricular septum. Additionally, we report aspects of intracardiac hemodynamics of Gallus gallus domesticus.
Materials and methods
Animals
Twenty‐nine adult female chickens Gallus gallus domesticus (age 12–16 months; body mass 1.3–2.0 kg) were purchased from a poultry farm. For the anatomical study, the hearts of six chickens were examined. A morphometric investigation of the right muscular AV valve was carried out in the hearts of 15 animals. Eight hens underwent echocardiographic and postmortem examinations. To reduce stress and movement, the chickens were anesthetized by a combination of tiletamine–zolazepam (40 mg kg−1, i.m., Zoletil 100; Virbac, Carros, France) and xylazine (2 mg kg−1, i.m., Xyla; Interchemie, Castenray, The Netherlands). The anesthetized birds breathed spontaneously. The chickens were euthanized by cervical dislocation. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85‐23, revised 1996). The Animal Care and Use Committee of the Institute of Physiology of the Komi Science Center of the Russian Academy of Sciences approved the experimental protocol (approval number: 46).
Gross anatomy and morphometry
The body cavity was opened after euthanasia. The hearts were quickly removed, and immersed and washed in normal saline. The heart was then dried with blotting paper and dissected under standardized conditions (Pees et al. 2006). In six chickens the thicknesses of the RV free wall, LV free wall and interventricular septum were measured in three parts of the heart (Fig. 1C). Schematic illustration of the measured sites of the RAVV (n = 15) is shown in Fig. 2B. A vernier caliper and an ocular micrometer of a stereoscopic microscope (MBS‐9, Russia) were used to obtain measurements.
Figure 1.
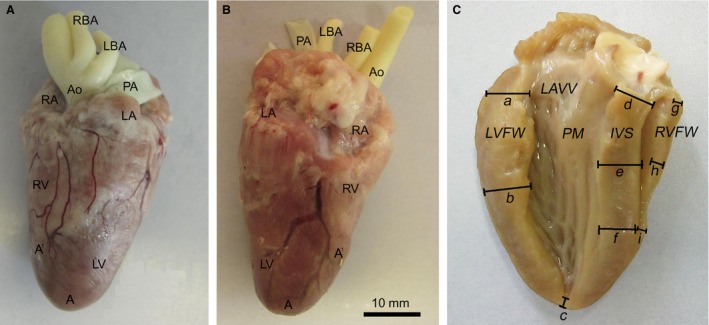
Photographs of an adult chicken heart. The adipose tissue in the coronary sulcus is removed. (A) Ventral surface. (B) Dorsal surface. (C) Illustration of the measuring points for the postmortem evaluation of the myocardial thickness. (a–c) Left ventricular free wall: (a) basal, (b) middle, (c) apical. (d–f) Interventricular septum: (d) basal, (e) middle, (f) apical. (g –i) Right ventricular free wall: (g) basal, (h) middle, (i) apical. A, apex of the heart, A`, apex of the right ventricle; Ao, aorta; IVS, interventricular septum; LA, left atrium; LAVV, left atrioventricular valve; LBA, left brachiocephalic artery; LV, left ventricle; LVFW, left ventricular free wall; PA, pulmonary artery; PM, papillary muscle; RA, right atrium; RBA, right brachiocephalic artery; RV, right ventricle, RVFW, right ventricular free wall.
Figure 2.
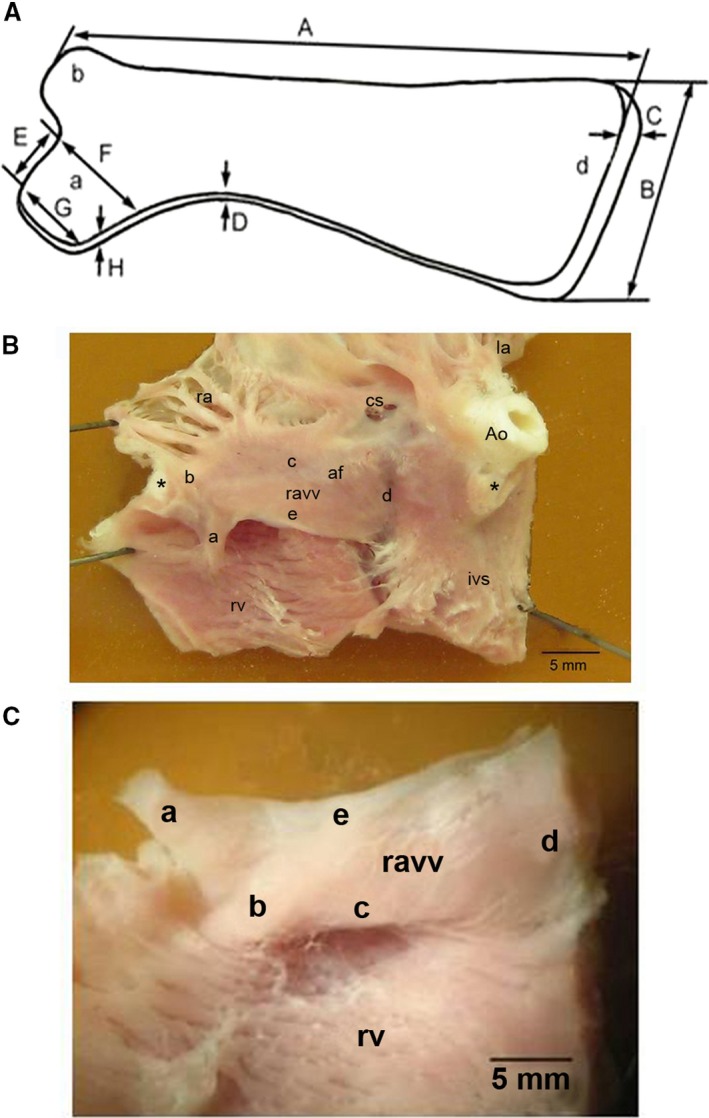
External view of the right muscular atrioventricular valve in the adult chicken heart. (A) Scheme explaining measurement of morphological parameters of the valve: A, valve length; B, valve width in the area d; C, thickness in the area d; D, thickness of the free edge of the valve; E, length of the a area; F, width of the large basis of the a area; G, width of the small basis of the a area; H, thickness of the a area. (B) Photograph of the endocardial surface of an adult chicken heart (a view of the base of the heart after some parts of the atria and the ventricles were removed). The black asterisk shows the place of the ventral septal attachment of the right muscular atrioventricular valve. Adapted and modified from Prosheva & Kaseva (2016). (C) Photograph of the ventricular surface of the right muscular atrioventricular valve; Ao, aorta; af, annulus fibrosus; cs, coronary sinus; ivs, interventricular septum; la, left atrium; ravv, right muscular atrioventricular valve; ra, right atrium; rv, right ventricle; a, part of the free edge of the valve, attached to the right ventricle free wall; b, area of the valve junction with the ventral side of the interventricular septum; c, base of the valve; d, area of the valve junction with the dorsal side of the interventricular septum; e, free edge of the valve.
Echocardiography
Ultrasound measurements were performed using a SonoAce 8000 Ex (Medison, South Korea) echocardiographic system. The animals were positioned in dorsal recumbency. Water‐soluble, sterile, warmed ultrasound gel was applied to the skin to provide clear visualization of the heart. A 7.5‐MHz transducer placed in a subcostal position was used to generate the images. All measurements were carried out according to the guidelines set out by Pees et al. (2006). Measurements of the LV free wall, interventricular septum, RV free wall and RAVV thicknesses were made at the time of echocardiographic examination. Thicknesses were measured between the AV valves and the papillary muscles for LV (this was accomplished when echocardiographic images included portions of the tips from the left AV leaflets) and at the dorsal septal insertion of RAVV for RV at both end‐systole and end‐diastole. End‐diastole and end‐systole were identified as the largest and smallest distance between the endocardial surfaces of the ventricles during the cardiac cycle that corresponded to the S peak and the end of the T wave on the ECG, respectively. Additional functional parameters measured included velocity of blood flow and pressure gradient in RAVV and aortic valve. These measurements were assessed from a pulsed wave Doppler tracings obtained at the aortic root and at the location of RAVV. The pattern of blood flow in RAVV was evaluated in color Doppler technique. All echocardiographic investigations were performed by the same operator. Following the echocardiographic study, birds underwent postmortem examination. The RV and the RAVV thicknesses were measured in the region where the valve connects with the dorsal side of the interventricular septum, that is, at points similar to the site of measurement in the echocardiographic examination.
Statistical analysis
The results are presented as mean ± SD. The statistic software package SPSS Statistics 17.0 was used for statistical analysis. The t‐test was applied for comparative analysis. A P‐value < 0.05 was considered statistically significant.
Results
Gross anatomy and morphometry of the chicken heart
The anatomical measurements showed that the length and width (measured at the basal region) of the chicken heart are 41.4 ± 3.1 and 22.2 ± 2.6 mm, respectively. The right atrium wall (including the trabecula) is thinner than the left atrium wall (1.2 ± 0.2 vs. 1.7 ± 0.3 mm, P < 0.05). As in various other avian species, the right ventricle of the chicken heart has a crescent‐shaped cavity that does not reach the apex (see Fig. 1). It surrounds the LV. The large LV, with thicker walls, is cone‐shaped and extends to the cardiac apex. The results of measurements of the thickness of the myocardium at three areas of the left and the RV free wall as well as the interventricular septum are presented in Table 1. The LV wall at basal and middle areas is three times thicker than the RV wall (4.4 ± 0.7 vs. 1.3 ± 0.2 mm and 5.2 ± 0.5 vs. 1.7 ± 0.5 mm, respectively, P < 0.05). The thickness of the apical myocardium of the LV is 2.2 ± 0.2 mm. It is significantly thinner (P < 0.05) than the other areas of the LV free wall. The greatest thickness of the LV free wall is observed in the middle level, compared with the basal and apical levels. No significant difference could be demonstrated between the apical and the basal areas of the interventricular septum in terms of the thickness of the myocardium (Table 1). The apical part of the RV free wall is thinner than the middle part (P < 0.05; Table 1). Thus, the wall thickness of the left and right ventricles decreases from the middle area to the apex of the heart.
Table 1.
Thickness of the ventricular myocardium in the chicken heart
| Myocardium | Basal | Middle | Apical |
|---|---|---|---|
| Left ventricle free wall (mm) | 4.4 ± 0.7* | 5.2 ± 0.5* | 2.2 ± 0.2 |
| Interventricular septum (mm) | 5.1 ± 1.1 | 5.4 ± 0.9 | 5.4 ± 1.4 |
| Right ventricle free wall (mm) | 1.3 ± 0.2 | 1.7 ± 0.5† | 1.2 ± 0.04 |
All data values are mean ± SD. P < 0.05 indicates a statistically significant difference.
*Between the thickness of the left ventricle free wall in the base and in the middle as compared with that in the apex.
†Between the thickness of right ventricle free wall in the middle as compared with that in the apex.
Morphology of the right muscular atrioventricular valve
The right AV valve guards the long, slit‐like AV orifice (see Fig. 3). The long and the short diameters of the right AV orifice are 10.2 ± 2.3 and 1.9 ± 0.4 mm, respectively. The long diameter of the left AV orifice is 7.5 ± 1.4 mm and the short diameter 5.4 ± 1.2 mm. Figure 2 shows the right muscular AV valve in the chicken heart. The base of this thick muscular flap (Fig. 2AC,c) is connected with the atrial and ventricular walls. Its sharp free edge (Fig. 2AC,e) projects into the RV cavity. Part of the RAVV free edge is attached to the RV parietal free wall. It represents a trapezoidal muscle (Fig. 2A,a). This valve has neither chordae tendineae nor papillary muscles. There is a straight connection between the right muscular AV valve and the parietal free wall of the RV (Fig. 2AC,a). It should be noted that the connections of the valve to the interventricular septum are also straight (Fig. 2AC,b and AC,d). The RAVV has a ventral and dorsal attachment to the interventricular septum (Fig. 3). The results of the morphological measurements of the RAVV are presented in Table 2. The valve width is different along its whole length, being largest in the region of the valve junction with the dorsal side of the interventricular septum. The thickness of the RAVV in its various parts is also different. The RAVV base and the region of the RAVV junction with the dorsal side of the interventricular septum and parts adjacent to them have the maximal thickness. The sharp RAVV free edge and the region of the RAVV junction with the ventral side of the interventricular septum have a minimal thickness. It should be noted that the width and the thickness of the dorsal attachment to the interventricular septum are more than twice as large as those of the ventral attachment to the interventricular septum (see Table 2). The thickness of the RAVV dorsal attachment to the interventricular septum has the least variability. Therefore, in this ultrasound and postmortem study the RAVV thickness was measured in the region where the valve joins the dorsal side of the interventricular septum. The thickness of the part of the free edge of the valve attached to the RV free wall bears a close resemblance to that of the RAVV dorsal attachment to the interventricular septum (see Table 2).
Figure 3.
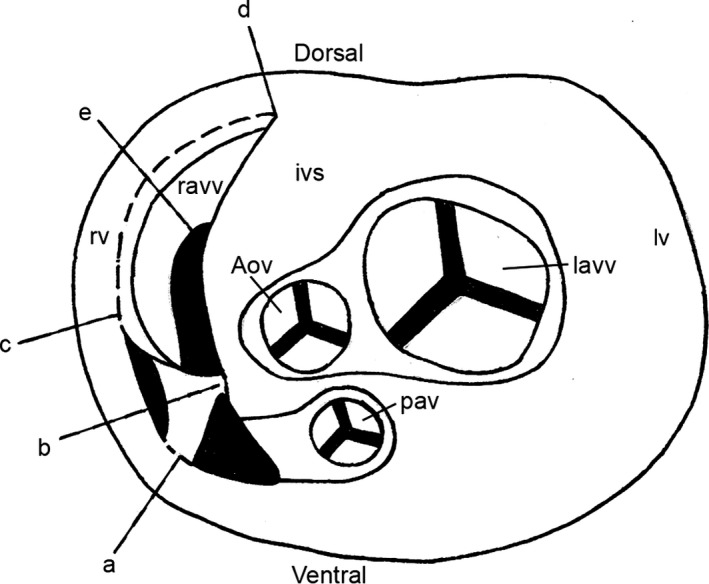
Transverse diagram showing the connections of the chicken right muscular atrioventricular valve to the right ventricle free wall and to the interventricular septum (schematic section at the level of the atrioventricular junction). Adapted and modified from Prosheva & Kaseva (2016). Aov, aortic valve; ivs, interventricular septum; lavv, left atrioventricular valve; lv, left ventricle; ravv, right muscular atrioventricular valve; pav, pulmonary artery valve; rv, right ventricle; a, part of the free edge of the valve, attached to the right ventricle free wall; b, region of the valve junction with the ventral side of the interventricular septum; c, basis of the valve; d, region of the valve junction with the dorsal side of the interventricular septum; e, free edge of the valve.
Table 2.
Morphological parameters of the chicken right muscular atrioventricular valve
| Subject | Parameters | Mean | SD | CV (%) |
|---|---|---|---|---|
| Valve | Length | 19.5 | 1.5 | 8 |
| Width | ||||
| In the region of the valve junction with the dorsal side of the interventricular septum | 7.4 | 1.0 | 14 | |
| In the region of the valve junction with the ventral side of the interventricular septum | 2.9 | 0.5 | 17 | |
| Thickness | ||||
| In the region of the valve junction with the dorsal side of the interventricular septum | 1.7 | 0.1 | 6 | |
| In the region of the valve junction with the ventral side of the interventricular septum | 0.5 | 0.2 | 40 | |
| In the region of the free edge of the valve | 0.6 | 0.1 | 17 | |
| Part of the free edge of the valve, attached to the right ventricle free wall | Length | 3.2 | 0.6 | 19 |
| Width | ||||
| Of the large basis | 3.9 | 0.6 | 15 | |
| Of the small basis | 3.1 | 0.4 | 13 | |
| Thickness | 1.2 | 0.2 | 17 | |
Data are presented in millimeters. SD, standard deviation; CV, coefficient of variation.
Ultrasound observations
The heart rate was 232 ± 12 beats per minute (n = 8). Figure 4 shows the B‐mode image of the chicken heart at the end of the systole. The heart has a wedge‐shaped RV and an ellipsoid‐shaped LV. The results of the ultrasound measurements are presented in Tables 3 and 4. During the systole, the thickness of the RV free wall, LV free wall and interventricular septum increased by 50, 45 and 36%, respectively. Interestingly, at the end of the systole, the thickness of the RV free wall and RAVV were similar (3.0 ± 0.47 vs. 3.0 ± 0.52 mm; see Table 3). During the diastolic filling of the RV, the RAVV shifts towards the ventricular apex under the pressure of blood flow from the right atrium and is finally pressed towards the free wall (Fig. 5A). The boundary of RAVV in this location is very difficult to recognize and its thickness could not be measured. To solve this problem we performed the postmortem measurements to characterize indirectly the RAVV thickness at the end of the diastole. Previously, it was shown that in chickens there was a correlation between diastolic ultrasound measurements and postmortem ones (Martinez‐Lemus et al. 1998). We have found out that the RAVV thickness at the RV end‐systole is higher than that measured in the postmortem examination (3.0 ± 0.5 vs. 1.7 ± 0.5 mm, P < 0.05). This finding implies a contractile function of the RAVV. In two‐dimensional images of the long axis view, motion of the right muscular AV valve is clearly visible. The high mobility of the RAVV should be noted. The RAVV excursion is greater than that of a posterior leaflet of the left membranous AV valve (10.5 ± 0.7 vs. 6.8 ± 0.8 mm, P < 0.05; n = 8). Figure 5 shows a gallery of the RV B‐mode echocardiographic images obtained during a cardiac cycle. While the RV is emptying, RAVV covers the lumen between the right heart chambers (Fig. 5E), performing a barrier function. Figure 6 shows pulsed‐wave Doppler echocardiographic image of a chicken heart. The pulsed Doppler sample volume is placed at the location of the RAVV. In color Doppler echo, no regurgitation of blood flow in the RAVV was observed in any of the eight animals studied. The measured parameter values in pulsed wave echo are presented in Tables 3 and 4. The blood flow velocity and the pressure gradient in the right AV valve are lower than those in the aortic valve.
Figure 4.
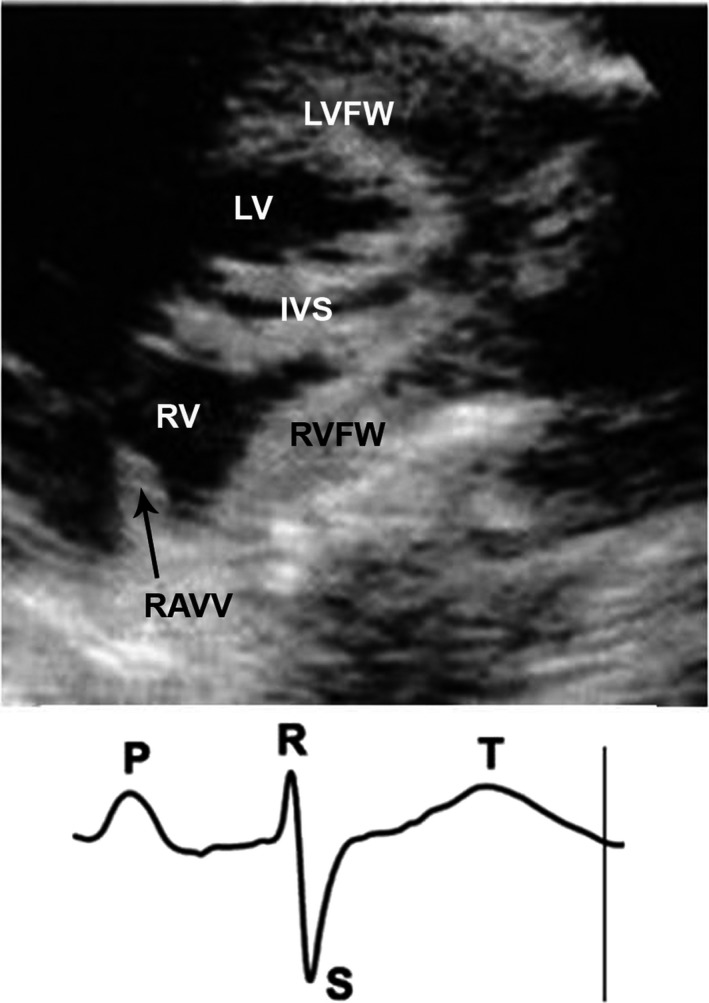
The B‐mode echocardiographic image of a chicken heart obtained at the end of the systole. The marker on the limb lead II ECG shows the moment when the image was obtained. IVS, interventricular septum; LV, left ventricle; LVFW, left ventricular free wall; RAVV, right muscular atrioventricular valve; RV, right ventricle; RVFW, right ventricular free wall.
Table 3.
Measured parameter values of the right ventricle and right muscular atrioventricular valve in eight adult chickens
| Parameters | Mean | SEM | SD | Min | Max |
|---|---|---|---|---|---|
| RVFWTd (mm) | 2.0* | 0.15 | 0.34 | 1.7 | 2.4 |
| RVFWTs (mm) | 3.0* | 0.21 | 0.47 | 2.6 | 3.7 |
| RVFWTpm (mm) | 1.8 | 0.08 | 0.21 | 1.5 | 2.2 |
| RAVVTs (mm) | 3.0 | 0.18 | 0.52 | 2.2 | 3.6 |
| RAVVTpm (mm) | 1.5 | 0.12 | 0.34 | 1.2 | 2.2 |
| V mean in muscular valve (cm s‐1) | 56.2 | 3.40 | 9.61 | 40.1 | 67.9 |
| PG mean in muscular valve (mmHg) | 1.27 | 0.15 | 0.42 | 0.6 | 1.8 |
Max, maximum; Min, minimum; PG mean in muscular valve, the mean pressure gradient in right atrioventricular valve; RAVVTpm, right atrioventricular valve thickness postmortem; RAVVTs, right atrioventricular valve thickness at end‐systole; RVFWTd, right ventricular free wall thickness at end‐diastole; RVFWTpm, right ventricular free wall thickness postmortem; RVFWTs, right ventricular free wall thickness at end‐systole; SD, standard deviation; SEM, the standard error of the mean; V mean in muscular valve, the mean blood flow velocity in right atrioventricular valve.
*Values of RVFWTd and RVFWTs are calculated for five animals.
Table 4.
Echocardiographic measurements of parameters of the left ventricle in eight chicken hearts (longitudinal section)
| Parameters | Mean | SEM | SD | Min | Max |
|---|---|---|---|---|---|
| LVFWTd (mm) | 2.9 | 0.17 | 0.48 | 1.9 | 3.4 |
| LVFWTs (mm) | 4.2 | 0.29 | 0.83 | 2.7 | 5.7 |
| IVSTd (mm) | 3.1 | 0.14 | 0.38 | 2.6 | 3.7 |
| IVSTs (mm) | 4.2 | 0.22 | 0.62 | 3.3 | 4.9 |
| AOD (mm) | 5.2 | 0.22 | 0.63 | 4.7 | 6.7 |
| Vmean in aortic valve (cm/s) | 84.7 | 3.10 | 8.76 | 74.1 | 95.2 |
| PGmean in aortic valve (mmHg) | 2.9 | 0.18 | 0.50 | 2.2 | 3.6 |
AOD, aortic diameter at the root in systole; IVSTd, interventricular septum thickness at end‐diastole; IVSTs, interventricular septum thickness at end‐systole; LVFWTd, left ventricular free wall thickness at end‐diastole; LVFWTs, left ventricular free wall thickness at end‐systole; Max, maximum; Min, minimum; PG mean in aortic valve, the mean pressure gradient at the root of aortic valve; SD, standard deviation; SEM, the standard error of the mean; V mean in aortic valve, the mean blood flow velocity at the root of aortic valve.
Figure 5.
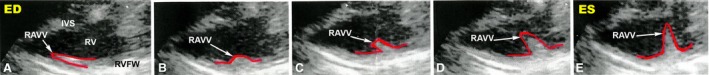
Sequential B‐mode echocardiographic images of the chicken right muscular atrioventricular valve excursion obtained in the course of the cardiac cycle. Arrows indicate the right muscular atrioventricular valve. The right muscular atrioventricular valve contour was outlined by hand and is shown by the red line. ED, end‐diastole; ES, end‐systole; IVS, interventricular septum; RAVV, right muscular atrioventricular valve; RV, right ventricle; RVFW, right ventricle free wall.
Figure 6.
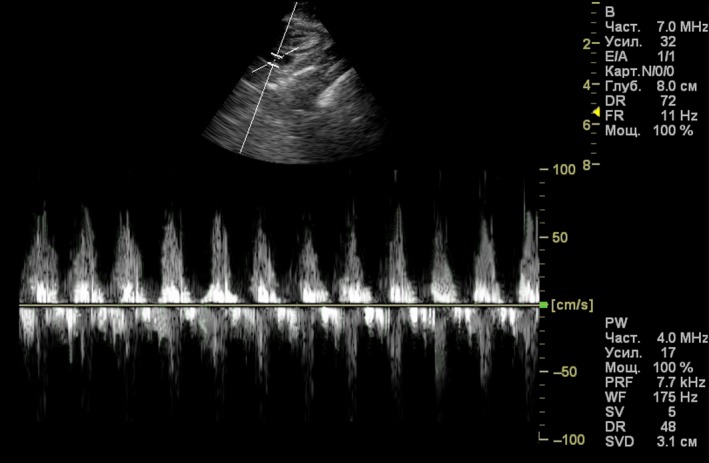
Pulsed‐wave Doppler echocardiographic image of a chicken heart. The pulsed Doppler sample volume is placed at the location of the right muscular atrioventricular valve.
Discussion
The present morphometric study revealed that the wall thickness of the left and right ventricles decreases from the middle region to the apical one in the adult chicken heart. This is consistent with previous findings on the structure of the ventricular myocardium obtained in wild bird species (budgerigars, Alisterus parrots and common buzzards) (Straub et al. 2002). Thus, our anatomical findings support the notion that the existence of areas of different thicknesses in the avian heart ventricles is a physiological norm (Straub et al. 2002). This raises the question: why do these differences in thicknesses exist? We suppose that these differences in the thickness of the ventricles are to a certain extent due to the sequence of the excitation of the ventricles in the bird heart. According to electrophysiological studies, there are no interspecific differences in the sequence of the excitation of the ventricles in the heart of domestic and wild birds (Shmakov et al. 1979; Kharin et al. 2006). Avian hearts have a ventricular conduction system enabling rapid activation of both ventricles in the myocardium from apex to base. The sequence of the excitation of the ventricles in the chicken heart is as follows: apical areas of the right free wall, areas of the apex and the middle of the left free wall, central areas of the free walls, the bases of the free walls (Kharin et al. 2006).
A study of the morphology and functioning of the right muscular AV valve in the avian heart is clearly important to understand the evolution of AV valves in amniotes. It is surprising that a right muscular AV valve is not easily recognized in the reptile heart. Only mature crocodiles have a right muscular AV valve and they are grouped as archosaurs, together with dinosaurs and birds. Birds and monotreme mammals have a muscular valve at the right AV orifice. Adult marsupial and eutherian mammals do not have a right muscular AV valve (Jensen et al. 2013a). Our results have shown that the architecture of the RAVV in the chicken heart is very similar to that described in other avian species (Pees et al. 2006; Alsafy et al. 2009; Jaiswal et al. 2017; Kroneman et al. 2019): a large, thick muscular flap without papillary muscles. Thus, birds have a constant RAVV architecture. Light microscopy shows that the RAVV in the avian heart is a two‐layered structure formed by an outer layer of inverted ventricular muscle and an inner layer of downwardly projecting atrial muscle (Vassall‐Adams, 1982). According to electrophysiological studies, the chicken RAVV, on the atrial side, is made up mainly of working myocardium myocytes as well as conducting and pacemaker cells, and on the ventricular side only of a working myocardium and conducting cells (Prosheva & Kaseva, 2016).
To our knowledge, this is the first study to analyze the connection of the right muscular AV valve to the right ventricle free wall and to the interventricular septum; it is also the first study to perform morphometry of this valve in the avian heart. In the present study we observed one attachment of the RAVV to the RV free wall and two attachments of this valve (ventral and dorsal) to the interventricular septum in the chicken heart. Our results show that the RAVV is connected directly to the parietal free wall of the RV and to the interventricular septum. There are no chordae tendineae in the RV (see Fig. 2AC). Skwarek et al. (2006) studied the different modes of connection between the right AV valve and the papillary muscles in the adult human heart. The following three types were found: type 1 – a straight connection (typical for birds), type 2 – a membranous connection (typical for primates) and type 3 – a connection by means of tendinous chords. The prevalent view appears to be that the types of connection between the right AV valve and the papillary muscles have changed during phylogenesis from straight to membranous connections and then to tendinous chords, as reflected in the ontogenesis. Tendinous chords are the perfected form of the connection (Anderson et al. 2003; Skwarek et al. 2006). Interestingly, in spite of the avian hearts being endowed with a ‘primitive’ muscular AV valve in the right heart, they outperform the hearts of mammals. Birds have higher heart rates and a greater increase in heart rate when transitioning from rest to physical load compared with mammals (Butler & Woakes, 1980; Seymour & Blaylock, 2000). Birds have a larger heart rate range and an extremely effective pump function of the heart in various environmental conditions (e.g. such as temperature, altitude or turbulence during a flight in the upper troposphere). It is an interesting open question whether the avian right heart is a well‐designed pump.
The pattern of RAVV blood flow has been evaluated using the color Doppler technique. In the current study, no color interference representing the regurgitation was observed. Our results are consistent with the data obtained in pigeons (Masoudifard et al. 2016). This suggests a sufficient barrier function of the RAVV in the avian heart. The hemodynamic parameters in the right AV valve and in the aortic valve of the chicken heart has not been previously measured. We discovered that the velocity of blood flow and pressure gradient in the chicken RAVV and aortic valve were both in the same range as those in the pigeon heart (Masoudifard et al. 2016). The blood flow velocity in the chicken RAVV was ≈ 56 cm s‐1 (Table 3), comparable to that in the human right AV valve (≈ 51–64 cm s‐1) (Lang et al. 2015; Wilkenshoff & Kruck, 2017; Dernovoj & Prosheva, 2018).
In the present study, we confirmed our previous finding that the RAVV becomes significantly thicker towards the end of the systole (Prosheva et al. 2015). This implies a contractile function of the RAVV. Generally, contractility is taken as the ability to generate mechanical tension and the ability to shorten. At a given load, the myocyte is shortening but, because cell volume is constant, the shortening is accompanied by myocyte thickening. On the one hand, being a fragment of the RV free wall, the RAVV becomes thicker due to its own contractile function at the RV systole and may contribute to the RV pump‐function. The argument in favor of this opinion is the presence of conducting myocytes (Purkinje fibers) in the RAVV (Prosheva, 1986; Lu et al. 1993; Parto et al. 2013; Prosheva & Kaseva, 2016). As known, conducting myocytes are mainly responsible for the excitation pattern in the heart ventricles of endothermic animals. We suppose that conducting myocytes in the RAVV provide the organization of its excitation as the integral part of the RV free wall to synchronize their contractile performance. In our previous study it was proved that the electrical activation of the RAVV in the chicken heart occurs during excitation of the main bulk of the RV free wall (Prosheva & Shmakov, 1987). Indirect further support for this opinion is the fact that the hypertrophy of the RV wall results in thickening of the RAVV and leads to valvular insufficiency (Pees et al. 2001). On the other hand, contraction of the RAVV results in an increase of its stiffness. One might assume, therefore, that the RAVV takes an active part in separating blood flow between the right heart chambers. In the final part of the systole, a barrier function of the RAVV resembles the function of the sphincter muscles. Thus, the RAVV fulfills two major physiological roles that influence RV filling and performance in the avian heart: (1) acting as the barrier that determines the blood flow separation between the right heart chambers and (2) contracting to support the RV pump function. The contraction assists in the emptying of the RV during the systole.
At present there is no agreed nomenclature for the RAVV parts. The terminology used by authors is extremely confusing. We distinguish the main parts of the RAVV: the base (Fig. 2AC,c), the free edge (Fig. 2AC,e), the dorsal (Fig. 2AC,d) and ventral (Fig. 2AC,b) attachment to the interventricular septum, the part of the valve free edge, attached to the parietal free wall of RV (Fig. 2AC,a). The part of the free edge of the valve, attached to the parietal free wall of the RV, is variously called the ‘anterolateral papillary muscle’ (Cayre et al. 1993), the ‘small papillary muscle’ (Aydinlioğlu et al. 1998), the ‘trabecular muscle’ (Alsafy et al. 2009) and a ‘thick muscular stalk’ (Tadjalli et al. 2009). In our opinion, the above‐mentioned terms are inconsistent because the RAVV does not have the papillary muscles.
In conclusion, it is important to emphasize that when using chicken heart as a biological model for the study of normal and pathological development of the human heart, the differences that exist with respect to the structure and functioning of the right heart in both species should be taken into account.
Conflict of interest
The authors state that there is no conflict of interest.
Author contributions
V.P., N.K. and B.D. acquired and analyzed the data and wrote the paper.
Acknowledgements
We would like to thank Tatyana Shklyar and Felix Blyakhman for discussions.
References
- Alsafy MAM, El‐Gendy SA, Enany S, et al. (2009) Anatomical studies on the atrioventricular valves of the ostrich heart (Struthio camelus). J Vet Anat 2, 67–83. [Google Scholar]
- Anderson RH, Webb S, Brown NA, et al. (2003) Development of the heart: (2) Septation of the atriums and ventricles. Heart 89, 949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydinlioğlu A, Rağbetli MC, Uğraş S, et al. (1998) A morphological study in broiler chick hearts. Folia Morphol 57, 357–362. [PubMed] [Google Scholar]
- Burggren W, Christoffels V, Crossley D, et al. (2014) Comparative cardiovascular physiology: future trends, opportunities and challenges. Acta Physiol 210, 257–276. [DOI] [PubMed] [Google Scholar]
- Butler PJ, Woakes AJ (1980) Heart rate, respiratory frequency and wing beat frequency of free flying barnacle geese Branta leucopsis . J Exp Biol 85, 213–226. [Google Scholar]
- Cayre R, Valencia‐Mayoral P, Coffe‐Ramirez V, et al. (1993) The right atrioventricular valvular apparatus in the chick heart. Acta Anat 148, 27–33. [DOI] [PubMed] [Google Scholar]
- Chiappe LM, Dyke GJ (2006) The early evolutionary history of birds. J Paleont Soc Korea 22, 133–151. [Google Scholar]
- Clarke JA, Middleton KM (2008) Mosaicism, modules, and the evolution of birds: results from a Bayesian approach to the study of morphological evolution using discrete character data. Syst Biol 57, 185–201. [DOI] [PubMed] [Google Scholar]
- Davies F (1930) The conducting system of the bird's heart. J Anat 64, 129–145. [PMC free article] [PubMed] [Google Scholar]
- Dernovoj BF, Prosheva VI (2018) A comprehensive assessment of the cardiovascular system of sportsmen‐skiers in the winter period of preparation for the competition. Hum Ecol 8, 46–51. [Google Scholar]
- Egevang C, Stenhouse IJ, Phillips RA, et al. (2010) Tracking of Arctic terns Sterna paradisaea reveals longest animal migration. PNAS 107, 2078–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo MA, Machado dos Santos C, Pereira‐Sampaio M, et al. (2013) Morphological aspects of atrioventricular valves in the ostrich (Struthio camelus). Biotemas 26, 203–208. [Google Scholar]
- Gyenai K, Kamara D, Geng T, et al. (2012) An assessment of echocardiography as a diagnostic tool for dilated cardiomyopathy in turkey (Meleagris gallopavo). AJAVS 7, 120–125. [Google Scholar]
- Jaiswal S, Singh I, Mahanta D, et al. (2017) Gross and morphometrical studies on the heart of Uttara fowl. J Entomol Zool Stud 5, 2313–2318. [Google Scholar]
- Jensen B, Boukens B, Postma A, et al. (2012) Identifying the evolutionary building blocks of the cardiac conduction system. PLoS ONE 7, e4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen B, van den Berg G, van den Doel R, et al. (2013a) Development of the hearts of lizards and snakes and perspectives to cardiac evolution. PLoS ONE 8, e63651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen B, Wang T, Christoffels V, et al. (2013b) Evolution and development of the building plan of the vertebrate heart. Biochim Biophys Acta 1833, 783–794. [DOI] [PubMed] [Google Scholar]
- Jensen B, Boukens B, Crossley D, et al. (2018) Specialized impulse conduction pathway in the alligator heart. eLife 7, e32120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharin S, Antonova N, Shmakov D (2006) Left ventricular myocardal activation under ventricular paced beats in chickens Gallus gallus domesticus . Comp Biochem Physiol A Mol Integr Physiol 145, 540–545. [DOI] [PubMed] [Google Scholar]
- Kroneman JGH, Faber JW, Schouten JCM, et al. (2019) Comparative analysis of avian hearts provides little evidence for variation among species with acquired endothermy. J Morphol 280, 395–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers WH, Moorman AFM (2002) Cardiac septation. A late contribution of the embryonic primary myocardium to heart morphogenesis. Circ Res 91, 93–103. [DOI] [PubMed] [Google Scholar]
- Lamers WH, Virágh S, Wessels A, et al. (1995) Formation of the tricuspid valve in the human heart. Circulation 91, 111–121. [DOI] [PubMed] [Google Scholar]
- Lang RM, Badano LP, Mor‐Avi V, et al. (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28, 1–39. [DOI] [PubMed] [Google Scholar]
- Lincoln J, Alfieri CM, Yutzey KE (2004) Development of heart valve leaflets and supporting apparatus in chicken and mouse embryos. Dev Dyn 230, 239–250. [DOI] [PubMed] [Google Scholar]
- Lu Y, James TN, Bootsma M, et al. (1993) Histological organization of the right and left atrioventricular valves of the chicken heart and their relationship to the atrioventricular Purkinje ring and the middle bundle branch. Anat Rec 235, 74–86. [DOI] [PubMed] [Google Scholar]
- Martinez‐Lemus LA, Miller MW, Jeffrey JS, et al. (1998) Echocardiographic evaluation of cardiac structure and function in broiler and Leghorn chickens. Poult Sci 77, 1045–1050. [DOI] [PubMed] [Google Scholar]
- Masoudifard M, Bidgoli VR, Madani SA, et al. (2016) Normal echocardiographic findings in healthy pigeons. IJVS 11, 7–13. [Google Scholar]
- Parto P, Tadjalli M, Ghazi SR, et al. (2013) Distribution and structure of Purkinje fibers in the heart of ostrich (Struthio camelus) with the special references on the ultrastructure. Int J Zool 2013, 1–6. [Google Scholar]
- Pees M, Straub J, Krautwald‐Junghanns M (2001) Insufficiency of the muscular atrioventricular valve in the heart of a blue‐fronted amazon (Amazona aestiva aestiva) . Vet Rec 148, 540–543. [DOI] [PubMed] [Google Scholar]
- Pees M, Straub J, Krautwald‐Junghanns M (2004) Echocardiographic examinations of 60 African grey parrots and 30 other psittacine birds. Vet Rec 155, 73–76. [DOI] [PubMed] [Google Scholar]
- Pees M, Krautwald‐Junghanns M, Straub J (2006) Evaluating and treating the cardiovascular system In: Clinical Avian Medicine, vol. 1 (eds Harrison GJ, Lightfoot TL.), pp. 379–394. Palm Beach: Spix Publishing. [Google Scholar]
- Pettigrew JB (1864) On the arrangement of the muscular fibres in the ventricles of the vertebrate heart, with physiological remarks. Philos Trans R Soc Lond B Biol Sci 154, 445–500. [Google Scholar]
- Prosheva V (1986) Electrophysiologic characteristics of cells of the conducting system in the right atrioventricular valve of the pigeon heart. Fiziol Zh Im I M Sechenova 72, 940–946. [PubMed] [Google Scholar]
- Prosheva V, Kaseva N (2016) Location and functional characterization of the right atrioventricular pacemaker ring in the adult avian heart. J Morphol 277, 363–369. [DOI] [PubMed] [Google Scholar]
- Prosheva VI, Rapota IV (1989) Structure of the right muscle atrioventricular valve in the avian heart. Arch Anat Histol Embryol 96, 50–55. [PubMed] [Google Scholar]
- Prosheva VI, Shmakov DN (1987) Activation of the right atrioventricular valve in the avian heart. J Evol Biochem Physiol 23, 269–272. [PubMed] [Google Scholar]
- Prosheva V, Dernovoj B, Kharin S, et al. (2015) Does the right muscular atrioventricular valve in the avian heart perform two functions? Comp Biochem Physiol 184, 41–45. [DOI] [PubMed] [Google Scholar]
- Seymour RS, Blaylock AJ (2000) The principle of Laplace and scaling of ventricular wall stress and blood pressure in mammals and birds. Physiol Biochem Zool 73, 389–405. [DOI] [PubMed] [Google Scholar]
- Shaner RF (1923) On the muscular architecture of the vertebrate ventricle. J Anat 58, 59–70. [PMC free article] [PubMed] [Google Scholar]
- Shmakov DN, Kliushina IV, Roshchevskii MP (1979) Sequence of the spread of excitation in bird heart ventricles. Fiziol Zh SSSR Im I M Sechenova 65, 872–880. [PubMed] [Google Scholar]
- Skwarek M, Dudziak M, Hreczecha J, et al. (2006) The connection between the papillary muscles and leaflets of the tricuspid valve. Folia Morphol 65, 322–328. [PubMed] [Google Scholar]
- Stephenson A, Adams JW, Vaccarezza M (2017) The vertebrate heart: an evolutionary perspective. J Anat 231, 787–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub J, Valerius P, Pees M, et al. (2002) Morphometry of the heart of budgerigars (Melopsittacus undulatus), Alisterus parrots (Alisterus s scapularis) and common buzzards (Buteo buteo). Res Vet Sci 72, 147–151. [DOI] [PubMed] [Google Scholar]
- Swan LW (1961) The ecology of the high Himalayas. Sci Am 205, 68–79. [Google Scholar]
- Tadjalli M, Ghazi SR, Parto P (2009) Gross anatomy of the heart in ostrich (Struthio camelus). Iran J Vet Res 10, 21–27. [Google Scholar]
- Vassall‐Adams PR (1982) The development of the atrioventricular bundle and its branches in the avian heart. J Anat 134, 169–183. [PMC free article] [PubMed] [Google Scholar]
- Wilkenshoff U, Kruck I (2017) Handbuch der Echokardiografie. Stuttgart: Georg Thieme Verlag. [Google Scholar]


