Summary
The presence of nonhuman RNAs in man has been questioned and it is unclear if food-derived miRNAs cross into the circulation. In a large population study, we found nonhuman miRNAs in plasma by RNA sequencing and validated a small number of pine-pollen miRNAs by RT-qPCR in 2,776 people. The presence of these pine-pollen miRNAs associated with hay fever and not with overt cardiovascular or pulmonary disease. Using in vivo and in vitro models, we found that transmission of pollen-miRNAs into the circulation occurs via pulmonary transfer and this transfer was mediated by platelet-pulmonary vascular cell interactions and platelet pollen-DNA uptake. These data demonstrate that pollen-derived plant miRNAs can be horizontally transferred into the circulation via the pulmonary system in humans. Although these data suggest mechanistic plausibility for pulmonary-mediated plant-derived miRNA transfer into the human circulation, our large observational cohort data do not implicate major disease or risk factor association.
Subject Areas: Biological Sciences, Molecular Biology, Omics, Transcriptomics
Graphical Abstract
Highlights
-
•
Pollen-derived miRNAs can be found in the human circulation via pulmonary transfer
-
•
This process is partially mediated by platelets' ability to uptake and digest pollen DNA
-
•
The presence of circulating pollen miRNAs has modest clinical implications
Biological Sciences; Molecular Biology; Omics; Transcriptomics
Introduction
There is a growing appreciation for the importance of non-protein coding genes in development and disease. The discovery of small RNAs, including microRNAs (miRNAs), has dramatically altered our understanding of gene expression regulation (Ambros, 2001) and their modulation of mRNA expression (Carrington and Ambros, 2003, Ambros et al., 2003). Through their regulation of many processes including transitions between pluripotency and differentiation, miRNAs help to orchestrate developmental events and play a role in cellular and tissue homeostasis that are often important for disease (Ambros, 2004, Ambros, 2008, Ambros, 2011). Extracellular miRNAs (exRNAs) are present in a variety of bodily fluids, including plasma, urine, and saliva, and these molecules are notably stable and resist degradation despite the presence of RNase (Mitchell et al., 2008, Argyropoulos et al., 2013). Stability of exRNA in the circulation is due to encapsulation of these small RNAs in vesicles such as exosomes or microparticles that are protected from circulating RNAses. Exosomes serve as a form of cell-to-cell communication by transferring intracellular RNA from one cell to another in close proximity or to cells in distant tissues (Maia et al., 2018). The discovery of stable RNA outside of cells has transformed our understanding of the role RNA may play in cell-to-cell communication and other complex processes. Changes in the signatures of circulating miRNAs have also been associated with the manifestation of a wide array of diseases, including myocardial infarction and chronic lymphocytic leukemia (Laterza et al., 2009, Cheng et al., 2010, Calin et al., 2005).
A very limited number of studies have shown the expression of small noncoding RNAs beyond miRNAs, but the presence of nonhuman RNAs, particularly plant-derived RNAs, in the human circulation has been questioned (Snow et al., 2013). How plant-based exRNAs enter the human circulation or if these exRNAs are genuine is not clear from the available data and the limited published information addressing this issue is contentious. Although in worms there is evidence for horizontal transfer (Timmons et al., 2001), dietary transfer of RNA in humans is not established. Functional studies suggest that rice miRNA168a could inhibit LDLRAP1 expression (Zhang et al., 2012). Several carefully conducted studies, however, cast doubt on these findings and show ineffective delivery of diet-derived miRNAs to recipient animals (Snow et al., 2013, Witwer et al., 2013, Dickinson et al., 2013, Baier et al., 2014).
We recently published the first systematic description of exRNAs, including miRNAs, from an observational cohort, currently, the largest dataset of exRNAs from human samples (Freedman et al., 2016). Characterization of these extracellular transcripts (Freedman et al., 2016) showed an association with thrombotic and inflammatory disease (Mick et al., 2017, Shah et al., 2017). In this same population, we also found sequences consistent with plant-derived miRNAs. As opposed to the currently hypothesized oral horizontal transfer of non-human miRNAs, the presence of sequences consistent with pollen-derived miRNAs led us to speculate about pulmonary transfer, a mode of horizontal miRNA transfer that has not been previously proposed or investigated. It has long been known that pollen inhalation may lead to it penetrating the respiratory tract and altering disease (Michel et al., 1977). It is also known that pollen proteases compromise the airway epithelial barrier through degradation of transmembrane adhesion proteins and lung bioactive peptides (Vinhas et al., 2011). It is not currently known, however, if pollen miRNAs can be transferred into the pulmonary/systemic circulation.
Results
Pine Pollen Expression in Human Plasma
Using the Framingham Heart Study (FHS) Offspring cohort at their eighth examination visit, we recently analyzed sequencing data from plasma-derived RNA from 40 participants and identified over a thousand human extracellular RNAs and, using a targeted RT-qPCR approach in an additional 2,776 individuals, we characterized the most abundant extracellular transcripts (Freedman et al., 2016, Koupenova et al., 2018b).
The study participants had a mean age of 66.3 ± 9 years and were 54% female; 9% had asthma and 29% had hay fever (Table S1). In addition to the published extracellular RNA data (Freedman et al., 2016) from these RNA sequencing (RNA-seq) and RT-qPCR data, we found three sequences consistent with pine pollen miRNAs, specifically from Pinus taeda (pta) and Pinus densata (pde). One of these pine miRNAs, pta-miR948, was present in 20% of the participants (Table 1). Expression of these miRNAs was not associated with cardiovascular disease or heart failure, chronic obstructive pulmonary disease (COPD), or asthma (Table 1). The presence of pta-miR948 had a modest association with hay fever (Table 1), and the expression of pde-miR946 had a modest association with chronic heart failure, odds ratio = 3.04 95% confidence interval 1.34, 6.90, p-value is 0.01. Covariance adjustment removed the described significance.
Table 1.
Association of Pollen miRNA Expression and Respiratory Diseases
| Offspring 8 (n=2776) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence of exRNA Expression |
exRNA Cq Expression Values |
|||||||||
| Positive Diagnosis |
Negative Diagnosis |
p Value | Positive Diagnosis |
Negative Diagnosis |
p Value | |||||
| N | % | N | % | Mean | SD | Mean | SD | |||
| COPD | ||||||||||
| pde-miR946 | 0 | 0 | 52 | 2 | 0.27 | – | – | 52 | 0.02 | – |
| pta-miR1310 | 4 | 7 | 199 | 7 | 0.79 | 4 | 0.07 | 199 | 0.07 | 0.48 |
| pta-miR948 | 9 | 15 | 528 | 20 | 0.32 | 9 | 0.15 | 528 | 0.20 | 0.92 |
| ASTHMA | ||||||||||
| pde-miR946 | 2 | 1 | 50 | 2 | 0.22 | 2 | 0.01 | 50 | 0.02 | 0.57 |
| pta-miR1310 | 18 | 8 | 189 | 8 | 0.92 | 18 | 0.08 | 189 | 0.08 | 0.67 |
| Pta-miR948 | 50 | 22 | 487 | 20 | 0.46 | 50 | 0.22 | 487 | 0.20 | 0.29 |
| HAY FEVER | ||||||||||
| pde-miR946 | 10 | 1 | 43 | 2 | 0.09 | 10 | 0.01 | 43 | 0.02 | 0.32 |
| pta-miR1310 | 55 | 7 | 152 | 8 | 0.38 | 55 | 0.07 | 152 | 0.08 | 0.79 |
| pta-miR948 | 177 | 22 | 361 | 19 | 0.04 | 177 | 0.22 | 361 | 0.19 | 0.99 |
Expression of pine pollen miRNAs in 2,782 people of Offspring 8 was as follows: pde-miR946 was present in 2% (n = 53), with Cq = 20.7 ± 3; pta-miR1310 was present in 8% (n = 208), with Cq = 8.3 ± 3; pta-miR948 was present in 20% (n = 543), with Cq = 20.8 ± 1.
There was no association with sex in this study.
Confirmation of Plant Pollen-miRNA Presence in the Circulation
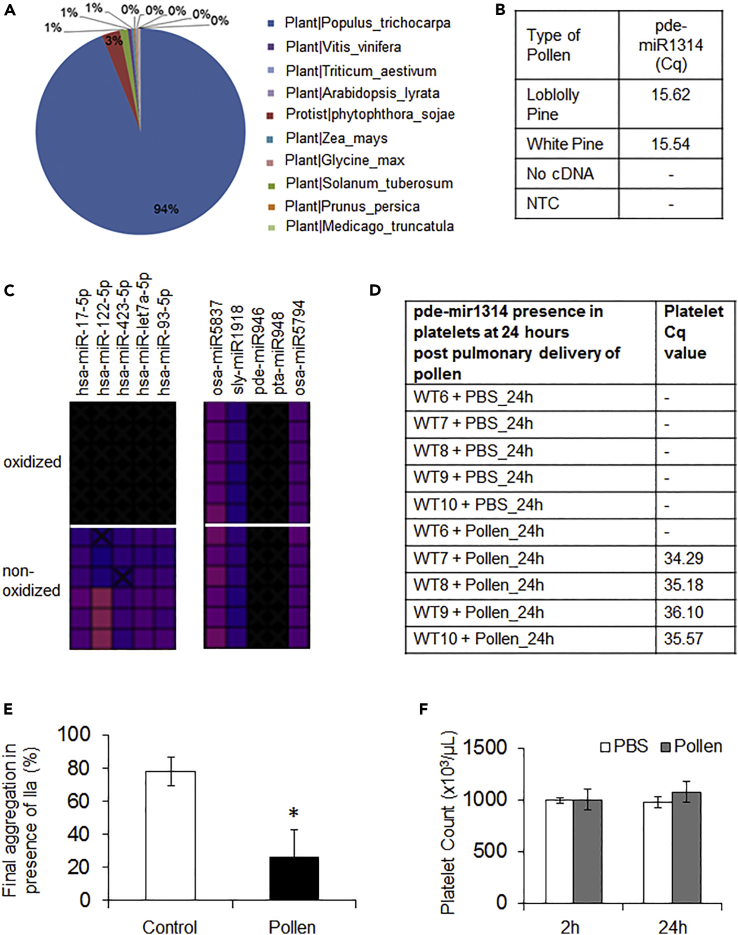
To confirm the validity of our pipeline and verify the species to which the sequences were mapped, we performed additional RNA sequencing studies of plant pollens (corn, pine, and black cottonwood pollen) and analyzed the findings on the Extracellular Consortium (ERCC) pipeline (Rozowsky et al., 2019) (Figure 1A). Importantly, 94% of the black cottonwood (Populus trichocarpa) pollen reads align to the respective genome demonstrating the pipeline's ability to accurately map exogenous miRNA from miRBase and the genomes of sequenced species in Ensembl/NCBI. Of note, the remaining 6% of the genome of black cottonwood maps to other species, these species are only of plant origin, and do not include any mammalian genomes. Sequencing of pine-pollen (Pinus taeda and Pinus strobus) and alignment of the genome using the same pipeline demonstrated the presence of miRNAs for the two types of pine-pollen miRNA identified in the Offspring 8, with an additional pde-miR1314 miRNA that showed high expression. The presence of pde-miR1314 was confirmed by TaqMan RT-qPCR (Figure 1B). For our mechanistic studies, we selected this miRNA owing to its high expression in pollen, as well as pta-miR948, owing to its expression in the FHS participants. We omitted pde-miR946 present only in 2% of the population and pta-miR1310 because of homology with human exRNA. Of note, even though certain reports have proposed that column contamination may skew results toward detection of foreign miRNA (Heintz-Buschart et al., 2018), in our study, pollen miRNAs were not uniformly detected throughout the human population suggesting that pollen miRNA presence does not result from column contamination.
Figure 1.
Verification of Plant miRNA Origin
(A) Cottonwood pollen reads after RNA-seq and analysis on the ERCC bioinformatics pipeline maps only to plant genomes.
(B) RT-qPCR (TaqMan) for one of the most abundant miRNAs in pine pollen identified by RNA-seq confirming that the human plasma-pollen miRNA reads are correctly derived from two types of pine pollen.
(C) Heat plots of miScript RT-qPCR after oxidation of plasma-derived RNA with NaIO4 confirming that the pollen miRNA reads are of plant origin. Plant miRNAs have a methyl group at the 3′ end that cannot be oxidized and the miRNA can be transcribed and detected by miScript chemistry. (D–F) Pollen was delivered intranasally in mice over 3 consecutive days.
(D) Pulmonary transfer of pine pollen-derived miRNA in vivo. Platelets from 12-week-old male C57BL/6J mice were isolated 24 h post the third dose and tested for pollen miRNA presence by RT-qPCR. Mice were challenged intranasally with pollen for 3 consecutive days.
(E) Platelet aggregation was measured in isolated (washed) murine platelets 24 h after the last challenge in the presence of low concentration of thrombin (factor IIa, 0.5 U/mL) (n = 3/group, platelets of two mice were pooled to form one n, p = 0.008).
(F) Platelet number measured in blood at the indicated time points post last delivery of pollen by blood counter (n = 5 mice/group, p = n.s.).
Data in graphs are represented as average ± SEM of n indicated for each panel; significance was assessed by unpaired t test (two-tailed value) and is indicated as a star symbol (*).
To evaluate the methylation status of plant miRNAs in plasma and to confirm that they are of plant origin, we oxidized plasma-derived RNA from healthy donors and screened them by RT-qPCR with miScript PCR chemistry (Zhang et al., 2012). As illustrated in Figure 1C, miRNAs of human origin were not detected in the oxidized plasma, whereas miRNAs of plant origin such as osa-mir5837.2 (currently osa-mir5837) or aly-miR774a-3p.2 (currently aly-miR774a) were present in both the oxidized and non-oxidized samples (Data S1).
To further confirm that plant-derived miRNAs can transfer horizontally into the circulation, we utilized an in vivo murine model in which pollen was intranasally delivered for three consecutive days. Intranasal exposure led to the presence of pine miRNA in the platelets of mice exposed to pollen but not in the saline controls (Figure 1D; pde-mir1314); plasma presence of pollen miRNA in mice was rare (one of five mice). Interestingly, murine platelets isolated from the pollen-exposed mice at the same time point showed decreased aggregation potential in the presence of thrombin suggesting attenuated platelet function (Figure 1E). Pollen exposure did not lead to significant changes in platelet count (Figure 1F). However, similar intranasal delivery of pollen (with the additional challenge 7 days before first delivery) showed compromised lung function as evidenced by an increased bronchoconstriction, airway resistance, and dramatic leukocyte infiltration (Figure S1).
Our results suggest that the miRNA detected in the circulation of the FHS cohort is of plant origin, it can transfer horizontally into the circulation in vivo, and prolonged pollen exposure leads to compromised lung function even in the small airways.
Platelets Can Acquire Pine Pollen-derived miRNA from Endothelial or Epithelial Cells In Vitro
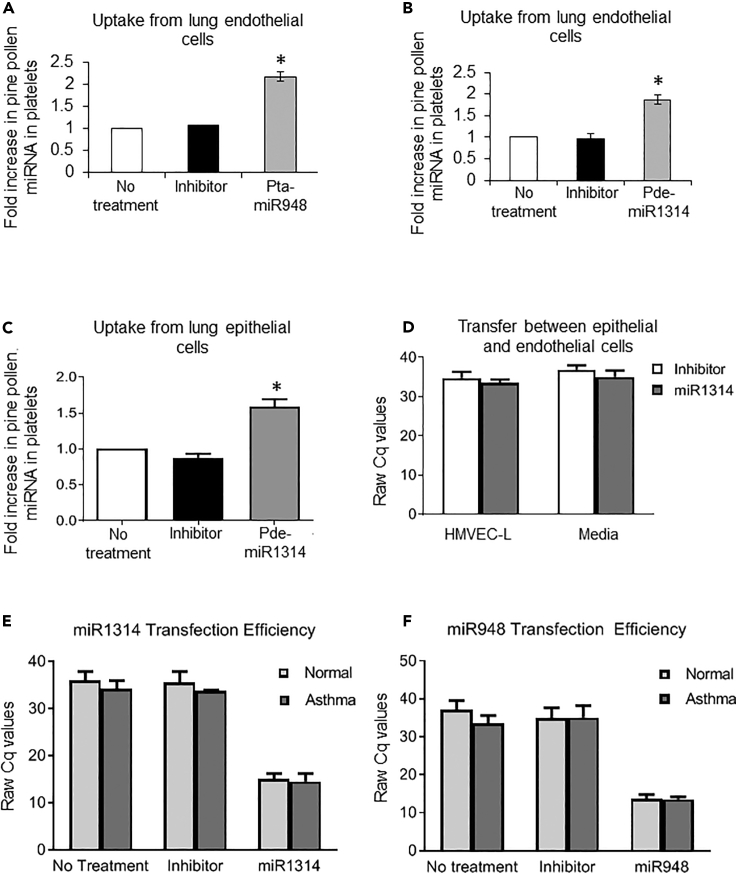
Platelets have the ability to take up and deliver miRNA or mRNA from cells or plasma to endothelial cells or circulating monocytes (Koupenova et al., 2018a, Clancy et al., 2017, Risitano et al., 2012, Laffont et al., 2013, Gidlof et al., 2013). Since pollen proteases can break down tight junctions between epithelial cells, we sought to evaluate if pollen particles can reach endothelial cells and if platelets can pick up pollen miRNA from endothelial cells (Runswick et al., 2007). For that purpose, we transfected primary human lung microvascular endothelial cells with synthetic pine-pollen miRNAs and circulated human platelets over the transfected cells (Clancy et al., 2017). By this model, platelets were able to take up the pine-pollen miRNA within 10 min of exposure to the transfected cells (Figures 2A and 2B).
Figure 2.
Transfer of Pollen miRNA between Platelets and Relevant Pulmonary and Vascular Cells
(A–D) Endothelial or epithelial cells were transfected with the respective synthetic pollen miRNA, and platelets were circulated over the cells for 10 min. miRNA presence after incubation was analyzed by RT-qPCR. Uptake of (A) pta-miR948 (raw Cq values of no treatment 32.9 ± 7; inhibitor 31.9 ± 5; miR1314 16.5 ± 1) and (B) pde-miR1314 (raw Cq values of no treatment 30.5 ± 4; inhibitor 30.4 ± 5; miR1314 16.3 ± 2) by platelets from endothelial cells (HMVEC-L) and of (C) pde-miR1314 (raw Cq values of no treatment 31.6 ± 1; inhibitor 33.2 ± 2; miR1314 18.3 ± 1) from epithelial cells (NHBE). Platelets in (A) and (B) were isolated from three donors (F, age 23 years; M, age 49 years; F, age 22 years) and in (C) also from three donors (F age 35 years; F age 43 years; M age 42 years). (D) Transfer of pde-miR1314 from transfected epithelial cells to endothelial cells in co-culture transwell system was not significant. Each condition was normalized to the baseline pollen pde-miR1314 content in each person's platelets without treatment.
(E and F) Transfection efficiency for synthetic pollen miRNAs used in this study. Epithelial cells were transfected with pollen miRNA and tested for mRNA expression by qPCR. Transfection efficiency was established for (E) pde-miR1314 and (F) pta-miR948 pollen miRNAs using primary human cells derived from normal (NHBE) and asthmatic (D-HBE-As) sources. Similar results were observed for HMVEC-L cells.
Data in each graph are representative of means ±SEM for each condition; significance in (A), (B), and (C) was assessed by one-way ANOVA, followed by Bonferroni post-test; significance in (D), (E), and (F) was assesed by two-way ANOVA and star symbol (*) indicates p < 0.05.
Pollen allergens can be internalized by airway epithelial cells and are recognized by surface epithelial receptors (TLR4, PPR2) leading to chemotaxis signals and crossover of blood cells (Lambrecht and Hammad, 2014, Blume et al., 2009). To evaluate if platelets can acquire pollen miRNA directly from epithelial cells, we transfected primary airway epithelial cells from normal and asthmatic donors. Using a similar setup as described for endothelial cells, platelets also acquired pollen miRNA from flow-based exposure to epithelial cells (Figure 2C). Transfection efficiency was assessed by qPCR (Figures 2E and 2F). To assess the possibility of direct transfer of pollen miRNA between epithelial and endothelial cells we utilized transwell co-culture of transfected epithelial cells in the presence of endothelial cells. There was no transfer of pollen miRNA to the endothelial cells nor was pollen miRNA present in the media (Figure 2D). Our results indicate that platelets can directly acquire pollen miRNA from either of the cells that compose the epi-endothelial barrier in lungs.
Platelets Are Found in BAL but Uptake Pollen DNA under Physiological Conditions
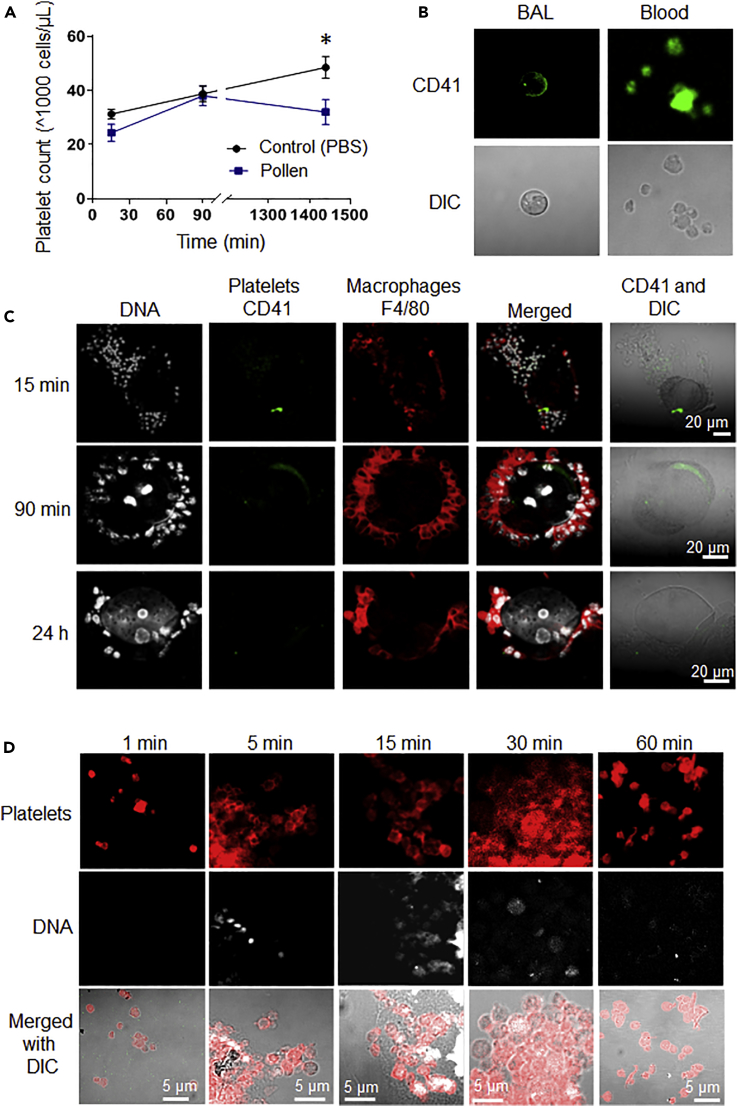
In the alveolar capillaries, platelets are instrumental in keeping the endothelial barrier intact. Thrombocytopenic patients have a destabilized pulmonary vascular endothelial barrier, asthmatic patients have dysfunctional platelets (Weyrich and Zimmerman, 2013, Gresele et al., 1987), and our results show decreased aggregation potential of platelets in pollen-exposed mice. Since platelets can be found in the broncho-alveolar fluid after lavage (BAL) in asthmatic patients (Metzger et al., 1987), we sought to evaluate if pollen increased platelet presence in BAL in mice.
Utilizing the Hemavet HV950 system, designed specifically to measure the blood differential for mice, we were able to detect a modest rise of platelet number in BAL over the 24 h following the last exposure (Figure 3A). Platelet influx in BAL, however, was not as great as the influx of lymphocytes or basophils (Figure S2), and immunofluorescence of BAL showed singular platelets with barely identifiable CD41 expression and resting morphology (Figure 3B). Reduced CD41 expression in the alveolar fluid was consistent with the BAL pH which is around 6.92. At this pH platelets have a reduced aggregation ability and velocity (Scharbert et al., 2011). Interestingly, pine pollen was identified, clearly surrounded by numerous amounts of macrophages some of which contained platelet-CD41 (Figure 3C). Occasionally, platelet CD41 was found on the pollen particle outside of macrophages (Figure 3C). Pollen particles were detectable 24 h post the last exposure, although the DNA no longer was concentrated as in the first 15 min (Figure 3C). Lack of activation of platelets in BAL, significant interaction of platelets with pollen, and overwhelming pulmonary macrophage presence suggest that platelets are not likely to pick up genetic material directly from pollen in BAL.
Figure 3.
Platelet Function in Plasma and Interaction with Pollen in BAL and under Physiological Conditions
(A and B) Twelve-week-old male C57BL/6J mice were challenged intranasally with pollen for 3 consecutive days. Platelet presence in murine BAL was assessed by (A) Blood counter (n = 4 mice/group/time point) and by (B) Immunofluorescence (in PBS challenged mice) and compared with the immunofluorescence in blood. Platelet number measured in BAL at the indicated time points post last delivery of pollen by blood counter (Hemavet 950HV).
(C) Immunofluorescence of BAL collected at the indicated time points shows overwhelming macrophage presence and platelets rarely found to interact with pollen particles; scale bar is 20 μm.
(D) Washed human platelets were incubated with 10 ng/μL of pine pollen in HEPES-buffered Tyrode solution for the indicated times (representative of n = 5 mice per group is shown). (D) Platelets, anucleated cells, are able to take up pollen DNA (DAPI, bright and concentrated 5 min) and digest it to RNA (DAPI low fluorescence and spotty, 30 min to 1 h). Images are representative of n = 3 human donors (2 F and 1 M); scale bar is 5 μm. In all cases, data are represented as mean ± SEM for each condition; significance was assessed by two-way ANOVA, followed by Bonferroni post-test, and star symbol (*) indicates p < 0.05.
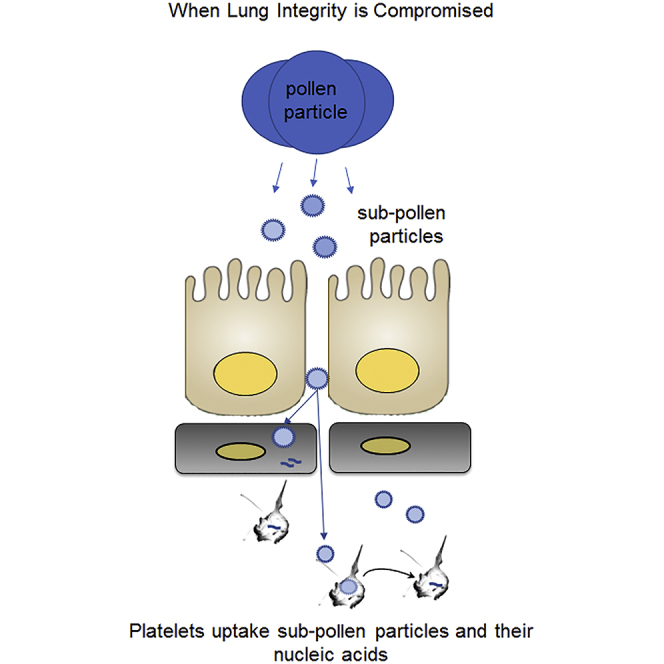
Since pollen particles (70 μm) in the presence of water can spontaneously form sub-pollen particles (Bacsi et al., 2006) of much smaller size (1–10 μm, Figure S2) and these particles can possibly enter the capillary circulation owing to the compromised endothelial barrier because of impaired platelet function, we assessed whether platelets can acquire pollen nucleic acids under physiological conditions (re-calcified HEPES Buffered Tyrode Solution, pH 7.4). Incubating platelets in vitro with pollen (10 ng/μL) led to uptake of pollen DNA starting at 5 min post initial mixing (Figure 3D). Platelets are anucleate, and the bright DAPI stain inside of them indicates DNA uptake. Platelets began digestion of DNA at 15 min post initial mixing, and the DNA was completely digested by 60 min. Overall our results suggest that, as a function of pollen exposure, there is a compromised epithelial-endothelial barrier, consistent with impaired platelet function. The compromised epithelial-endothelial barrier may result in pollen sub-particles leaking into the circulation with their genetic material subsequently taken up by platelets and digested to RNA.
Pine Pollen miRNA Targets Mammalian Gene Expression
Although plant miRNAs have a methyl group on the ribose of the last nucleotide instead of a hydroxyl group as in mammalian miRNAs, they still have the potential to target mammalian mRNA levels by shared 7-mer seed sequences. Using the miRNA nucleotide sequence for the pine miRNAs (mirbase.org) and the “custom prediction” function in miRDB database (mirdb.org) we found many possible human mRNA targets. Pde-miR946 had 329 human mRNAs predicted, including prostaglandin E receptor 4 and fibronectin type III domain. Pta-miR948 had 763 predicted human gene targets, including insulin-like growth factor binding protein and coagulation factor II (thrombin) receptor. Pde-miR1314 had only 36 human mRNA targets, one of which was matrix metallopeptidase (MMP24). Epithelial or endothelial cells transfected with pine pollen miRNA and gene expression from transcripts with the highest predicted targets was measured using epithelial cells derived from normal and asthmatic sources. Interestingly, epithelial cells from normal and asthmatic patients, or endothelial cells, showed differences in regulation of gene expression according to cell type or pathology (Table 2). Overall pollen miRNA can affect the expression of certain human mRNAs, although regulation differs according to cell type and asthmatic origin.
Table 2.
Fold Change of Gene Targets in Primary Human Lung Cells Transfected with Synthetic Pollen miRNAs
| Cells | pta-miR948 |
pta-miR1314 |
||||
|---|---|---|---|---|---|---|
| Normal Epithelial | Asthmatic Epithelial | Endothelial (HMLEC) | Normal Epithelial | Asthmatic Epithelial | Endothelial (HMLEC) | |
| Gene Target | ||||||
| HIVEP3 | 1.32 ± 0.2a | 0.92 ± 0.3a | 0.59 ± 0.001b | 1.66 ± 0.4b | 1.09 ± 0.2b | 0.85 ± 0.1b |
| p=0.03 | p = n.s. | p=0.00005 | p = n.s. | p = n.s. | p = n.s. | |
| PIK3AP1 | 0.80 ± 0.4a | 0.54 ± 0.3a | 2.24 ± 3.6b | 0.44 ± 0.03b | 0.53 ± 0.07b | 2.03 ± 4.9b |
| p = n.s. | p=0.02 | p = n.s. | p = n.s. | p = n.s. | p = n.s. | |
| PRR11 | 1.07 ± 0.6b | 1.39 ± 0.7b | 1.84 ± 0.8b | 0.72 ± 0.7b | 1.46 ± 0.7b | 1.75 ± 1.0b |
| p = n.s. | p = n.s. | p = n.s. | p = n.s. | p = n.s. | p = n.s. | |
| MMP24 | 1.29 ± 0.3b | 0.64 ± 0.05b | 0.75 ± 0.1b | 0.89 ± 0.3a | 1.40 ± 0.6a | 1.08 ± 0.01b |
| p = n.s. | p = n.s. | p=0.02 | p = n.s. | p = n.s. | p = n.s. | |
| PAD5 | 0.70 ± 0.2b | 0.64 ± 0.1b | 1.28 ± 0.1b | 0.46 ± 0.3b | 1.11 ± 0.4b | 1.19 ± 1.2b |
| p = n.s. | p = n.s. | p = n.s. | p= 0.02 | p = n.s. | p = n.s. | |
Gene targets were chosen based on the highest scores predicted using http://mirdb.org/and the sequence of each pollen miRNA listed in http://mirbase.org.
HIVEP3-Human Immunodeficiency Virus Type 1 Enhancer Binding Protein; PIK3AP1-Phospho-inositide 3-Kinase Adaptor Protein 1; PRR11-poly(A) polymerase D5; MMP24-Matrix Metallopeptidase 24; PAD5-Proline Rich 11.
Expression at 6 h post transfection
Expression at 24 h post transfection.
Discussion
Limitations in bioinformatic analyses of RNA sequencing have precluded broad assessment of non-human RNAs from large numbers of samples. Using a specialized pipeline developed by the NIH Common Fund Extracellular RNA Consortium, we re-analyzed the sequencing data and identified many small RNAs mapped to non-human origins. Using these data, we found that plant RNAs appear to be present in a subgroup of people in variable amounts. We re-analyzed each plant miRNA individually using stringency analysis to eliminate sequence homology to human miRNAs. It is highly relevant that even when examining pollen miRNA expression in a large human cohort, no clear association with disease was observed.
Once thought to be static, platelets are now known to interact and communicate in broader ways. The platelet, a cell central in hemostasis and thrombosis, has seen its biological roles expanded exponentially over the last decade to include immunity, inflammation, and mediation of oncogenesis (Clancy and Freedman, 2015). Platelets, although anucleate, contain a wealth of transcriptomic information with distinct expression profiles as compared with white cells (Freedman et al., 2010). Platelets' ability to participate in diverse systemic responses is noted by our growing understanding of their contents and the revelation of their capacity to share these contents (Risitano et al., 2012, Clancy et al., 2017).
Platelets are now known to horizontally transfer ribonucleic acids (RNAs), traffic pathogens, and regulate physiological and pathophysiological processes far beyond hemostasis (Koupenova et al., 2014, Risitano et al., 2012). Platelets can transfer transcripts, including messenger RNA (mRNA) and small noncoding microRNA, to recipient cells or extracellular vesicles (including exosomes and microparticles) with functional implications (Risitano et al., 2012, Gidlof et al., 2013). The observation that platelets possess the capacity to transfer cytosolic RNA suggested a new function for platelets in the regulation of vascular homeostasis (Risitano et al., 2012). The processes involved in this horizontal cellular transfer of RNA are not fully understood; however, it is believed to be multifactorial with some contribution of platelet exosome release and uptake by other platelets or vascular cells. Platelet-specific exosomes have been investigated and found to be functional, mediating vascular processes. For example, platelets from patients with myocardial infarction exhibit loss of specific miRNAs, and activated platelets shed miRNAs that can regulate endothelial cell gene expression (Gidlof et al., 2013).
Recently, the field of plasma-derived RNA or extracellular RNAs (exRNAs) has rapidly and greatly expanded, and it is thought that the most abundant microparticle subtype in circulation is platelet derived. Previous investigations have demonstrated an association of plasma miRNA with cardiovascular disease and studied the relevance of platelet miRNA to platelet function (Nagalla et al., 2011, Ward et al., 2013). It has been previously shown that platelet miRNAs can repress expression of platelet proteins, and miRNA profiles are associated with platelet reactivity (Nagalla et al., 2011). Platelets and their response to antiplatelet therapy may be important to the circulating miRNA pool (Willeit et al., 2013). It is known that platelet microparticles, rich in miRNAs, can modify the transcriptome of macrophages and reprogram their function toward a phagocytic phenotype (Laffont et al., 2015).
There are several possible mechanisms of transfer within the pulmonary vasculature. Grass pollen exposure of lung epithelial cells affects immunological barrier properties by modulating release of mediators such as CXC chemokine ligand (CXCL) 8/interleukin 8 (IL-8) (Leino et al., 2013) and MMPs that can degrade the basement membrane and destroy the intraepithelial/interendothelial junctions, disrupting the alveolar-capillary barrier (Aschner et al., 2014). Once the epithelial cells are “activated” by the pollen, the epithelial cell may (1) release MMPs, which can degrade the collagen in the basal membrane, and (2) simultaneously the epithelial barrier can become leaky because of a reduction in adhesion proteins at the junctions. Pollen particles may then reach the endothelium and affect the endothelial gap junctions to transfer to platelets. Another possibility is that epithelial cells release vesicles/vacuoles after engaging the pollen, and this could lead to the formation of vesicles with processed pollen miRNA. Additionally, the epithelial cells may release IL-8 as a chemo-attractant for neutrophils. Platelets bind neutrophils during inflammatory processes, and this interaction could bring them closer to the epithelium. Also, direct treatment of endothelial cells with pollen extract causes an increase of E-selectin and VCAM-1 protein levels as well as an increase of IL-8 production (Taverna et al., 2008). Here we report that platelets are able to uptake pollen miRNA from both endothelial and epithelial cells in vitro. This process can be of particular importance in the capillary beds of the alveoli where the epithelial and endothelial cells are very close in proximity. Evidence of crossover of platelets is seen in the BAL of pollen-treated mice described in our study and in human asthmatic patients described previously (Metzger et al., 1987).
Our study, together with previous work, outlines another plausible mechanism by which pollen can affect the epithelial-endothelial pulmonary barrier and lead to the presence of pollen nucleic acids in the circulation. As pollen enters the lung, certain pollen particles can spontaneously form smaller pollen sub-particles. Pollen destabilizes the epithelial-endothelial barrier leading to crossover of pollen sub-particles in the circulation. Platelets encounter these sub-particles full of pollen DNA and quickly take them up. Platelets completely digest the pollen DNA within an hour. Chronic exposure to pollen (in a process similar to allergens or asthmatic patients) leads to dysfunctional platelets as evident by the reduced aggregation potential in our murine model and in asthmatic patients (Gresele et al., 1987). Reduced aggregation potential of platelets in asthmatic patients has been explained as platelets becoming exhausted and losing their contents, which can be found in plasma (Gresele et al., 1987). Dysfunctional platelets in turn can no longer stabilize the epithelial-endothelial barrier leading to the possibility of a larger influx of pollen sub-particles in the circulation. As platelets become exhausted, their ability to get rid of pollen nucleic acids may be reduced and certain pollen RNA material can become detected in the circulation. There is a possibility that, since mice have approximately four-times more platelets per microliter than humans, we were only able to detect pollen miRNA in platelets.
In summary, we demonstrate that, with the assistance of platelet uptake, pollen miRNA can transfer into the circulation and platelets appear to play a previously undescribed role in pollen nucleic acid clearance. Although these data suggest mechanistic plausibility for pulmonary-mediated plant-derived miRNA transfer into the human circulation, our large observational cohort data do not implicate disease or risk factor association.
Limitations of the Study
We have utilized various databases to analyze our results and confirmed that the miRNAs found in the human circulation are of plant origin. Although none of the miRNAs that we have used throughout this study aligned with any human miRNA that can be detected by sequencing, we are relying on what is currently available in the mirbase.org and we cannot account for any future discoveries. By utilizing various methods in addition to sequencing and our animal studies our data suggest that pollen miRNA indeed can be transferred in the circulation.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by the American Heart Association (USA) grant 16SDG30450001 (to M.K.) and National Institutes of Health (USA) grants: N01-HC 25195 (to FHS), U01HL126495, UH3TR000921-04, and a supplement to UH3TR000921-04 provided by the National Institutes of Health (USA) Common Fund (to J.E.F.). The authors thank Dr. Lea Beaulieu for helping with the collection of the initial pollen experiments.
Author Contributions
All authors assisted with the preparation and editing of the manuscript. M.K., K.T., and J.E.F. designed and M.K., K.T., H.A.C., A.S., S.E.T., O.V., A.M.K., M.E.M., and M.K.E. executed all experiments. E.M. performed the statistical analysis for the FHS participants. J.E.F. is the lead author and oversaw the entire study.
Declaration of Interests
The authors declare no competing interests.
Published: September 27, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.08.035.
Contributor Information
Milka Koupenova, Email: milka.koupenova@umassmed.edu.
Jane E. Freedman, Email: jane.freedman@umassmed.edu.
Supplemental Information
Cq after miScript RT-qPCR after oxidation of plasma-derived RNA with NaIO4 confirming that the pollen miRNA reads are of plant origin. Plant miRNAs have a methyl group at the 3′ end that cannot be oxidized; the miRNA then can be transcribed and detected by miScript chemistry.
References
- Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107:823–826. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Ambros V. The evolution of our thinking about microRNAs. Nat. Med. 2008;14:1036–1040. doi: 10.1038/nm1008-1036. [DOI] [PubMed] [Google Scholar]
- Ambros V. MicroRNAs and developmental timing. Curr. Opin. Genet. Dev. 2011;21:511–517. doi: 10.1016/j.gde.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambros V., Lee R.C., Lavanway A., Williams P.T., Jewell D. MicroRNAs and other tiny endogenous RNAs in C. elegans. Curr. Biol. 2003;13:807–818. doi: 10.1016/s0960-9822(03)00287-2. [DOI] [PubMed] [Google Scholar]
- Argyropoulos C., Wang K., Mcclarty S., Huang D., Bernardo J., Ellis D., Orchard T., Galas D., Johnson J. Urinary microRNA profiling in the nephropathy of type 1 diabetes. PLoS One. 2013;8:e54662. doi: 10.1371/journal.pone.0054662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner Y., Zemans R.L., Yamashita C.M., Downey G.P. Matrix metalloproteinases and protein tyrosine kinases: potential novel targets in acute lung injury and ARDS. Chest. 2014;146:1081–1091. doi: 10.1378/chest.14-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacsi A., Choudhury B.K., Dharajiya N., Sur S., Boldogh I. Subpollen particles: carriers of allergenic proteins and oxidases. J. Allergy Clin. Immunol. 2006;118:844–850. doi: 10.1016/j.jaci.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier S.R., Nguyen C., Xie F., Wood J.R., Zempleni J. MicroRNAs are absorbed in biologically meaningful amounts from nutritionally relevant doses of cow milk and affect gene expression in peripheral blood mononuclear cells, HEK-293 kidney cell cultures, and mouse livers. J. Nutr. 2014;144:1495–1500. doi: 10.3945/jn.114.196436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume C., Foerster S., Gilles S., Becker W.M., Ring J., Behrendt H., Petersen A., Traidl-Hoffmann C. Human epithelial cells of the respiratory tract and the skin differentially internalize grass pollen allergens. J. Invest. Dermatol. 2009;129:1935–1944. doi: 10.1038/jid.2008.459. [DOI] [PubMed] [Google Scholar]
- Calin G.A., Ferracin M., Cimmino A., Di Leva G., Shimizu M., Wojcik S.E., Iorio M.V., Visone R., Sever N.I., Fabbri M. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N. Engl. J. Med. 2005;353:1793–1801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- Carrington J.C., Ambros V. Role of microRNAs in plant and animal development. Science. 2003;301:336–338. doi: 10.1126/science.1085242. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Tan N., Yang J., Liu X., Cao X., He P., Dong X., Qin S., Zhang C. A translational study of circulating cell-free microRNA-1 in acute myocardial infarction. Clin. Sci. (Lond.) 2010;119:87–95. doi: 10.1042/CS20090645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy L., Beaulieu L.M., Tanriverdi K., Freedman J.E. The role of RNA uptake in platelet heterogeneity. Thromb. Haemost. 2017;117:948–961. doi: 10.1160/TH16-11-0873. [DOI] [PubMed] [Google Scholar]
- Clancy L., Freedman J.E. The role of circulating platelet transcripts. J. Thromb. Haemost. 2015;13(Suppl 1):S33–S39. doi: 10.1111/jth.12922. [DOI] [PubMed] [Google Scholar]
- Dickinson B., Zhang Y., Petrick J.S., Heck G., Ivashuta S., Marshall W.S. Lack of detectable oral bioavailability of plant microRNAs after feeding in mice. Nat. Biotechnol. 2013;31:965–967. doi: 10.1038/nbt.2737. [DOI] [PubMed] [Google Scholar]
- Freedman J.E., Gerstein M., Mick E., Rozowsky J., Levy D., Kitchen R., Das S., Shah R., Danielson K., Beaulieu L. Diverse human extracellular RNAs are widely detected in human plasma. Nat. Commun. 2016;7:11106. doi: 10.1038/ncomms11106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman J.E., Larson M.G., Tanriverdi K., O'donnell C.J., Morin K., Hakanson A.S., Vasan R.S., Johnson A.D., Iafrati M.D., Benjamin E.J. Relation of platelet and leukocyte inflammatory transcripts to body mass index in the Framingham heart study. Circulation. 2010;122:119–129. doi: 10.1161/CIRCULATIONAHA.109.928192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidlof O., Van Der Brug M., Ohman J., Gilje P., Olde B., Wahlestedt C., Erlinge D. Platelets activated during myocardial infarction release functional miRNA, which can be taken up by endothelial cells and regulate ICAM1 expression. Blood. 2013;121:3908–3917. doi: 10.1182/blood-2012-10-461798. S1-26. [DOI] [PubMed] [Google Scholar]
- Gresele P., Ribaldi E., Grasselli S., Todisco T., Nenci G.G. Evidence for platelet activation in allergic asthma. Agents Actions Suppl. 1987;21:119–128. doi: 10.1007/978-3-0348-7451-9_9. [DOI] [PubMed] [Google Scholar]
- Heintz-Buschart A., Yusuf D., Kaysen A., Etheridge A., Fritz J.V., May P., De Beaufort C., Upadhyaya B.B., Ghosal A., Galas D.J., Wilmes P. Small RNA profiling of low biomass samples: identification and removal of contaminants. BMC Biol. 2018;16:52. doi: 10.1186/s12915-018-0522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koupenova M., Clancy L., Corkrey H.A., Freedman J.E. Circulating platelets as mediators of immunity, inflammation, and thrombosis. Circ. Res. 2018;122:337–351. doi: 10.1161/CIRCRESAHA.117.310795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koupenova M., Mick E., Corkrey H.A., Huan T., Clancy L., Shah R., Benjamin E.J., Levy D., Kurt-Jones E.A., Tanriverdi K., Freedman J.E. Micro RNAs from DNA viruses are found widely in plasma in a large observational human population. Sci. Rep. 2018;8:6397. doi: 10.1038/s41598-018-24765-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koupenova M., Vitseva O., Mackay C.R., Beaulieu L.M., Benjamin E.J., Mick E., Kurt-Jones E.A., Ravid K., Freedman J.E. Platelet-TLR7 mediates host survival and platelet count during viral infection in the absence of platelet-dependent thrombosis. Blood. 2014;124:791–802. doi: 10.1182/blood-2013-11-536003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffont B., Corduan A., Ple H., Duchez A.C., Cloutier N., Boilard E., Provost P. Activated platelets can deliver mRNA regulatory Ago2*microRNA complexes to endothelial cells via microparticles. Blood. 2013;122:253–261. doi: 10.1182/blood-2013-03-492801. [DOI] [PubMed] [Google Scholar]
- Laffont B., Corduan A., Rousseau M., Duchez A.C., Lee C.H., Boilard E., Provost P. Platelet microparticles reprogram macrophage gene expression and function. Thromb. Haemost. 2015;115:311–323. doi: 10.1160/TH15-05-0389. [DOI] [PubMed] [Google Scholar]
- Lambrecht B.N., Hammad H. Allergens and the airway epithelium response: gateway to allergic sensitization. J. Allergy Clin. Immunol. 2014;134:499–507. doi: 10.1016/j.jaci.2014.06.036. [DOI] [PubMed] [Google Scholar]
- Laterza O.F., Lim L., Garrett-Engele P.W., Vlasakova K., Muniappa N., Tanaka W.K., Johnson J.M., Sina J.F., Fare T.L., Sistare F.D., Glaab W.E. Plasma MicroRNAs as sensitive and specific biomarkers of tissue injury. Clin. Chem. 2009;55:1977–1983. doi: 10.1373/clinchem.2009.131797. [DOI] [PubMed] [Google Scholar]
- Leino M.S., Loxham M., Blume C., Swindle E.J., Jayasekera N.P., Dennison P.W., Shamji B.W., Edwards M.J., Holgate S.T., Howarth P.H., Davies D.E. Barrier disrupting effects of Alternaria alternata extract on bronchial epithelium from asthmatic donors. PLoS One. 2013;8:e71278. doi: 10.1371/journal.pone.0071278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia J., Caja S., Strano Moraes M.C., Couto N., Costa-Silva B. Exosome-based cell-cell communication in the tumor microenvironment. Front. Cell Dev. Biol. 2018;6:18. doi: 10.3389/fcell.2018.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger W.J., Sjoerdsma K., Richerson H.B., Moseley P., Zavala D., Monick M., Hunninghake G.W. Platelets in bronchoalveolar lavage from asthmatic patients and allergic rabbits with allergen-induced late phase responses. Agents Actions Suppl. 1987;21:151–159. doi: 10.1007/978-3-0348-7451-9_13. [DOI] [PubMed] [Google Scholar]
- Michel F.B., Marty J.P., Quet L., Cour P. Penetration of inhaled pollen into the respiratory tract. Am. Rev. Respir. Dis. 1977;115:609–616. doi: 10.1164/arrd.1977.115.4.609. [DOI] [PubMed] [Google Scholar]
- Mick E., Shah R., Tanriverdi K., Murthy V., Gerstein M., Rozowsky J., Kitchen R., Larson M.G., Levy D., Freedman J.E. Stroke and circulating extracellular RNAs. Stroke. 2017;48:828–834. doi: 10.1161/STROKEAHA.116.015140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P.S., Parkin R.K., Kroh E.M., Fritz B.R., Wyman S.K., Pogosova-Agadjanyan E.L., Peterson A., Noteboom J., O'briant K.C., Allen A., Lin D.W. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. U S A. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagalla S., Shaw C., Kong X., Kondkar A.A., Edelstein L.C., Ma L., Chen J., Mcknight G.S., Lopez J.A., Yang L. Platelet microRNA-mRNA coexpression profiles correlate with platelet reactivity. Blood. 2011;117:5189–5197. doi: 10.1182/blood-2010-09-299719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risitano A., Beaulieu L.M., Vitseva O., Freedman J.E. Platelets and platelet-like particles mediate intercellular RNA transfer. Blood. 2012;119:6288–6295. doi: 10.1182/blood-2011-12-396440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozowsky J., Kitchen R.R., Park J.J., Galeev T.R., Diao J., Warrell J., Thistlethwaite W., Sabramanian S.L., Milosavljevic A., Gerstein M. exceRpt: a comprehensive analytic platform for extracellular RNA profiling. Cell Syst. 2019;8:1–6. doi: 10.1016/j.cels.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runswick S., Mitchell T., Davies P., Robinson C., Garrod D.R. Pollen proteolytic enzymes degrade tight junctions. Respirology. 2007;12:834–842. doi: 10.1111/j.1440-1843.2007.01175.x. [DOI] [PubMed] [Google Scholar]
- Scharbert G., Franta G., Wetzel L., Kozek-Langenecker S. Effect of pH levels on platelet aggregation and coagulation: a whole blood in vitro study. Crit. Care. 2011;15:P446. [Google Scholar]
- Shah R., Murthy V., Pacold M., Danielson K., Tanriverdi K., Larson M.G., Hanspers K., Pico A., Mick E., Reis J. Extracellular RNAs are associated with insulin resistance and metabolic phenotypes. Diabetes Care. 2017;40:546–553. doi: 10.2337/dc16-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow J.W., Hale A.E., Isaacs S.K., Baggish A.L., Chan S.Y. Ineffective delivery of diet-derived microRNAs to recipient animal organisms. RNA Biol. 2013;10:1107–1116. doi: 10.4161/rna.24909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna S., Flugy A., Colomba P., Barranca M., De Leo G., Alessandro R. Effects of Parietaria judaica pollen extract on human microvascular endothelial cells. Biochem. Biophys. Res. Commun. 2008;372:644–649. doi: 10.1016/j.bbrc.2008.05.118. [DOI] [PubMed] [Google Scholar]
- Timmons L., Court D.L., Fire A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 2001;263:103–112. doi: 10.1016/s0378-1119(00)00579-5. [DOI] [PubMed] [Google Scholar]
- Vinhas R., Cortes L., Cardoso I., Mendes V.M., Manadas B., Todo-Bom A., Pires E., Verissimo P. Pollen proteases compromise the airway epithelial barrier through degradation of transmembrane adhesion proteins and lung bioactive peptides. Allergy. 2011;66:1088–1098. doi: 10.1111/j.1398-9995.2011.02598.x. [DOI] [PubMed] [Google Scholar]
- Ward J.A., Esa N., Pidikiti R., Freedman J.E., Keaney J.F., Tanriverdi K., Vitseva O., Ambros V., Lee R., Mcmanus D.D. Circulating cell and plasma microRNA profiles differ between non-ST-segment and ST-segment-elevation myocardial infarction. Fam. Med. Med. Sci. Res. 2013;2:108. doi: 10.4172/2327-4972.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyrich A.S., Zimmerman G.A. Platelets in lung biology. Annu. Rev. Physiol. 2013;75:569–591. doi: 10.1146/annurev-physiol-030212-183752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willeit P., Zampetaki A., Dudek K., Kaudewitz D., King A., Kirkby N.S., Crosby-Nwaobi R., Prokopi M., Drozdov I., Langley S.R. Circulating microRNAs as novel biomarkers for platelet activation. Circ. Res. 2013;112:595–600. doi: 10.1161/CIRCRESAHA.111.300539. [DOI] [PubMed] [Google Scholar]
- Witwer K.W., Mcalexander M.A., Queen S.E., Adams R.J. Real-time quantitative PCR and droplet digital PCR for plant miRNAs in mammalian blood provide little evidence for general uptake of dietary miRNAs: limited evidence for general uptake of dietary plant xenomiRs. RNA Biol. 2013;10:1080–1086. doi: 10.4161/rna.25246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Hou D., Chen X., Li D., Zhu L., Zhang Y., Li J., Bian Z., Liang X., Cai X. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA. Cell Res. 2012;22:107–126. doi: 10.1038/cr.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cq after miScript RT-qPCR after oxidation of plasma-derived RNA with NaIO4 confirming that the pollen miRNA reads are of plant origin. Plant miRNAs have a methyl group at the 3′ end that cannot be oxidized; the miRNA then can be transcribed and detected by miScript chemistry.