Abstract
The evolution of chemistries and instrument platforms for next‐generation sequencing has led to sequencing of genomic variants in both tumor biopsies as well as in circulating tumor cells (CTCs) and cell‐free DNA liquid biopsies. The transition of these analytical platforms into clinical ones has led to challenges in product development as well as regulatory strategies for the approval of diagnostic products with these platforms. Regulatory strategies for liquid biopsy diagnostics depend on a framework that has been developed over the past few years by the US Food and Drug Administration (FDA). This framework includes both guidances that cover enrichment biomarkers and companion diagnostics, as well as regulatory approval precedents, which can be used to design regulatory strategies for new liquid biopsy diagnostic products. However, the regulatory paths for these liquid biopsy diagnostics can also be tortuous, as is the example of CTC—platform liquid biopsies. The ultimate success of regulatory pathways of liquid biopsy diagnostics has been driven by the incremental value of FDA approval for Clinical Laboratory Improvement Amendment (CLIA)‐developed tests and by the inherent complexity of these diagnostics, which are practical barriers for the widespread replication of these tests throughout CLIA laboratories. The framework for FDA approval of sequence information from these liquid biopsies has been focused on single‐site approvals of diagnostics where sequencing information is considered at different diagnostic risk levels, ranging from novel or follow‐on companion diagnostics to variant calls in genomic targets considered independently valuable for therapeutic decision making.
Liquid biopsies1 are powerful platforms for therapeutic decision making and early cancer detection. Their potential for reducing the need for tissue biopsies and their analytical development over the last 2 decades are transforming the practice of personalized medicine and opening the technology for early cancer detection and disease monitoring. Most of these tests have been laboratory developed tests (LDTs),2 run in Clinical Laboratory Improvement Amendment (CLIA)‐certified clinical laboratories. The regulatory pathways at the US Food and Drug Administration (FDA) for these tests are still under development, but diagnostic developers and the FDA are optimizing, often through ad hoc regulatory pathways, the regulatory framework for these products. The groundwork at the FDA for these regulatory pathways started with the original Critical Path for Innovation document set drafted in 2004 to support the development of personalized medicine.3 Table 1 shows the lexicon associated with the development and regulatory pathways of liquid biopsies.
Table 1.
Lexicon for scientific, clinical, and regulatory concepts in liquid biopsies
| Term | Definition |
|---|---|
| Biomarker | Characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or biological responses to a therapeutic intervention.4 |
| Enrichment biomarker | Prospective use of a biomarker to select a study population in which detection of a drug effect (if one is in fact present) is more likely than it would be in an unselected population.5 |
| In vitro diagnostic | Tests done on samples such as blood or tissue that have been taken from the human body. In vitro diagnostics can detect diseases or other conditions and can be used to monitor a person's overall health to help cure, treat, or prevent diseases.6 |
| Companion diagnostic | Medical device, often an in vitro device, which provides information that is essential for the safe and effective use of a corresponding drug or biological product. The test helps a healthcare professional determine whether a particular therapeutic product's benefits to patients will outweigh any potential serious side effects or risks.7 |
| Follow‐on companion diagnostic | Companion diagnostic developed and approved after the initial version of this product.8 |
| 510k | Document containing information required under 21 CFR 807 Subpart E. All 510(k)s are based on the concept of substantial equivalence to a legally marketed (predicate) device. All 510(k)s provide a comparison between the device to be marketed and the predicate device or devices.9 |
| Class III device | Devices that support or sustain human life, are of substantial importance in preventing impairment of human health, or which present a potential, unreasonable risk of illness or injury.10 |
| PMA | FDA process of scientific and regulatory review to evaluate the safety and effectiveness of class III medical devices.11 |
| Clinical utility | Elements that need to be considered when evaluating the risks and benefits in diagnosing or predicting risk for an event (drug response, presence, or risk of a health condition).12 |
| IVDMIA | Combines the values of multiple variables using an interpretation function to yield a single, patient‐specific result (e.g., a “classification,” “score,” “index,” etc.) that is intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, and provides a result whose derivation is nontransparent and cannot be independently derived or verified by the end user.13 |
| OncotypeDx | Commercial diagnostic test that estimates the likelihood of disease recurrence in women with early‐stage hormone estrogen receptor positive only breast cancer. There is emerging evidence that such tests may also provide information about the likely benefit from chemotherapy.14 |
| Liquid biopsy | Testing for tumor DNA using a blood sample. Tumor DNA may be accessed in a liquid biopsy either from CTCs in blood or cell‐free DNA in plasma.15 |
| MRD | MRD in patients with ALL or multiple myeloma is a measure of the amount of cancer cells remaining in a person's bone marrow.16 |
ALL, acute lymphoblastic leukemia; CTC, circulating tumor cell; FDA, US Food and Drug Administration; IVDMIA, In Vitro Diagnostic Multivariate Index Assay; MRD, minimal residual disease; PMA, premarket approval.
Regulatory pathways and guidance in the background for liquid biopsy diagnostic evaluation and approval
Regulatory pathways are framed by regulatory guidance and regulatory precedent. Liquid biopsies entered the regulatory landscape through guidance from Center for Drug Evaluation and Research (CDER) addressing enrichment biomarkers selecting specific patient populations and from the Center for Devices and Radiological Health (CDRH) addressing the companion diagnostics, which will select these populations.5, 7 Companion diagnostics represent the basic regulatory layer for liquid biopsy diagnostics.
The regulatory pathways for approval of many liquid biopsy products map tightly to regulatory pathways for approval of companion diagnostics, because many of these products have companion diagnostic claims associated with them. However, we do not have a regulatory pathway yet specifically associated with liquid biopsies. We have, instead, evolving regulatory pathways supported by a number of key regulatory guidances issued over the past decade. Many of these guidances are focused on the regulatory application and acceptance of biomarkers.
Biomarkers in this case are mostly enrichment biomarkers, and their tests are approved concurrently with therapies developed for the specific patient subpopulations selected by the enrichment biomarkers. Guidances for enrichment biomarkers and companion diagnostics were issued for enrichment biomarkers in 20195 and companion diagnostics in 20147 to cover the application of enrichment biomarkers in drug development and their testing with in vitro diagnostics.
The basis of this regulatory landscape also maps closely to the basis of liquid biopsy product development. Enrichment biomarkers are integrated into clinical trials to select patient subpopulations, which are expected to receive therapeutic benefit in the study. The selection of these populations with companion diagnostics often requires tissue biopsies from patients. Where these tissue biopsies are difficult or impossible to access, liquid biopsies represent attractive alternatives as samples for companion diagnostic testing, and for accurate therapeutic decision making. The success of this approach, however, does depend on the sample and detection platforms used. Liquid biopsies are ultimately challenging assays to use as companion diagnostics.
The Companion Diagnostic Guidance addressed a major regulatory policy question: should an independent clinical utility be confirmed for a companion diagnostic? The risk level for a patient depending on the results from a companion diagnostic test absolutely requires comprehensive proof for analytical and clinical validity. Clinical utility for a companion diagnostic, however, is inherently linked to the therapeutic success of the therapy it is tested with, so is there a clinical utility which is inherently linked to a companion diagnostic?
There is no unique answer to this question. When the initial drafts of the Companion Diagnostic Guidance were drafted jointly by the CDER and the CDRH around 2005–2006, the proposal20 was focused on the clinical value of the therapy to justify the joint approval of the therapy and its companion diagnostic. Under this proposal, the companion diagnostic regulatory review would focus on its analytical and clinical validity, and the therapeutic product review would assess the clinical utility of the therapeutic product. Development costs for the companion diagnostic under this regulatory policy would be relatively modest, and, perhaps just as importantly, the cost barrier for entry of follow‐on companion diagnostics would have also remained modest and independent from the cost of clinical trials for the therapy.
However, the final version of the Companion Diagnostic Guidance in 2014 claimed an independent requirement for the clinical utility of the companion diagnostic. This decision led to some expected—and other unexpected—consequences. Clinical trials leading to approval of targeted therapies and their companion diagnostics are funded by companies developing the therapies. Biopsy samples from these trials required to confirm clinical utility for companion diagnostics are owned by therapeutic companies. Development of any follow‐on companion diagnostic for the same targeted therapy requires either access to these samples in independent clinical studies with the therapeutics (not possible if the therapies were successfully approved in the original trial) or access to other biopsy samples from patients treated with the approved therapy. The last option is fairly difficult, but viable, for solid tumor biopsies but unlikely for liquid biopsies.
Finally, the costs and complexity in the development follow‐on companion diagnostics for FDA approval have also contributed to encouraging development of “home brew” companion diagnostics in CLIA laboratories. Except perhaps for assays as complex as those that include next‐generation sequencing (NGS), the gap between development costs for companion diagnostics approved by the FDA and those developed in CLIA laboratories is still wide. After the initial approval of targeted therapies and their companion diagnostics, results from tests run in CLIA laboratories are the primary source for clinical applications of companion diagnostics.21
Fda Guidance Documents in The Development of Companion Diagnostics
The main policy framework for the regulation of in vitro diagnostics (IVDs) has been developed through regulatory guidance. The FDA regulates IVDs in the United States. Novel targeted therapies and companion diagnostic tests required to select patients who are likely to benefit from them, have both been reviewed under existing guidance, as well as “pushed the envelope” in the development of future regulatory policy. Key guidance documents in this area are summarized in Table 2.
Table 2.
Guidance documents in the development of regulatory approval pathways for liquid biopsies
| Guidance | Issued | Summary |
|---|---|---|
| Enrichment Strategies for Clinical Trials to Support Determination of Effectiveness of Human Drugs and Biological Products Guidance for Industry | 03/2019 | Develop enrichment strategies that can be used in clinical investigations intended to demonstrate the effectiveness of drug and biological products.5 |
| In Vitro Companion Diagnostic Devices Guidance for Industry and Food and Drug Administration Staff | 08/2014 | Medical device, often an in vitro device, which provides information essential for the safe and effective use of a corresponding drug or biological product. The test helps a healthcare professional determine whether a particular therapeutic product's benefits to patients will outweigh any potential serious side effects or risks.7 |
| Guidance for Industry Pharmacogenomic Data Submissions | 03/2005 | Recommendations to sponsors holding IND applications, NDAs, and BLAs on when to submit pharmacogenomic data to the Agency during the drug or biological drug product development and review processes.17 |
| Guidance for Industry and FDA Staff Pharmacogenetic Tests and Genetic Tests for Heritable Markers | 06/2007 | Recommendations to sponsors and FDA reviewers in preparing and reviewing PMA applications and premarket notification (510(k)) submissions for pharmacogenetic and other human genetic tests.18 |
| Draft Guidance for Industry, Clinical Laboratories, and FDA Staff IVDMIAs | 07/2007 | Definition and regulatory status of a class of in vitro diagnostic devices referred to as IVDMIAs.13 |
| Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only Guidance for Industry and Food and Drug Administration Staff | 11/2013 | Clarify the requirements applicable to RUO and IUO IVD products, including that RUO and IUO labeling must be consistent with the manufacturer's intended use of the device.19 |
BLA, biologics license application; FDA, US Food and Drug Administration; IND, investigational new drug; IUO, Investigational Use Only; IVD, in vitro diagnostic; IVDMIA, In Vitro Diagnostic Multivariate Index Assay; NDA, new drug application; RUO, Research Use Only.
The start of this guidance framework was the FDA Pharmacogenomics Guidance in 2005. This guidance anticipated a second document to cover companion diagnostics. Guidance documents following this one defined a continuous expansion of the regulatory space claimed by the FDA CDRH in the United States. The 2007 Guidance on Pharmacogenetic Tests and Genetic Tests for Heritable Markers set up the framework for the information needed for regulatory review and approval of pharmacogenetic and genetic tests for heritable markers.
The In Vitro Diagnostic Multivariate Index Assay Guidance, also in 2007, attempted to set up the regulation of tests that require algorithmic components to reach a result. In oncology, an example of an In Vitro Diagnostic Multivariate Index Assay is the OncotypeDx test. This draft guidance was not issued in a final version. This guidance was an important step in attempts to regulate LDTs at the FDA.
The Research Use Only (RUO; 2013) and companion diagnostic (2014) guidances should be considered jointly as two parts of a coherent regulatory policy for the development of companion diagnostics. The frequently asked questions in this guidance discuss limits on use of RUO or Investigational Use Only diagnostic tests leading to clinically actionable data. Frequently asked questions B5 and B8 in this guidance specifically addressed the interest of the FDA to regulate LDTs. The RUO Guidance was issued concurrently with white papers aimed at developing guidance for the regulation of LDT. Although a draft of an LDT Guidance22 was issued in 2014, no final version of the LDT Guidance is anticipated in the near future, and the issue of LDT regulation remains an open—and contentious—issue23 for the application of companion diagnostics.
The Companion Diagnostic Guidance anticipated in 2005 by the Pharmacogenomics Guidance was issued in 2014. The companion diagnostic guidance addressed three main issues in companion diagnostic regulatory policy:
Clinical utility: Clinical utility is directly applicable to the companion diagnostic used to determine patient selection markers.
Premarket approval vs. a 510(k): Most companion diagnostics will be premarket approvals because their testing will also identify which patients will—or will not—receive a novel therapy. Diagnostic risk is closely linked to diagnostic context. Minimal residual disease to monitor therapeutic response, for example, could be premarket approvals as companion diagnostics or 510(k) tests as accelerated end points.
Development path goal: The goal of a phase III trial is to establish the clinical utility of the companion diagnostic independently of the confirmation of therapeutic efficacy for the targeted therapy.
These guidance documents do not directly address regulatory pathways for liquid biopsies. They are behind by at least two regulatory policy layers from what would be needed for liquid biopsy guidances. These two layers would be needed to address the specific liquid biopsy platform (circulating tumor cells vs. cell‐free DNA (cfDNA)) and the specific analytical genomic platform (quantitative polymerase chain reaction (qPCR) vs. NGS). The final complexity of these regulatory permutations will be reached with the specific indication (companion diagnostic, minimal residual disease, and early disease detection) for the test.24
In the absence of guidance, the second driver for regulatory policy is regulatory precedent. In 2013, the FDA cleared the MiSeqDx NGS instrument from Illumina for the Cystic Fibrosis 139‐Variant Assay25 and their Cystic Fibrosis Clinical Sequencing Assay.26 These genotyping tests were reviewed as 510k clearances, but their review and clearance led to guidance for germline NGS panels. This guidance may be considered a guide for at least some of the content expected in an NGS onco panel guidance. The guidance on Considerations for Design, Development, and Analytical Validation of NGS‐Based IVDs Intended to Aid in the Diagnosis of Suspected Germline Diseases27 has sections on:
Test design considerations
Indications for Use Statement(s) of the Test
Specific User Needs for the Test
Specimen Type
Interrogated Regions of the Genome
Performance Needs
Test Elements and Methods
Test performance characteristics
Accuracy
Precision (Reproducibility and Repeatability)
Limit of Detection
Analytical Specificity
Test run quality metrics
Coverage (Read Depth and Completeness)
Test Run Metrics and Performance Thresholds
It is reasonable to expect many similarities among these sections for germline variants and the corresponding ones that will be eventually drafted for onco panels. However, a fluid regulatory documentation status, such as the current one for NGS onco panels, requires that submissions be supported both with available regulatory documents as well as with available regulatory precedents. Table 3 below shows useful regulatory precedents for NGS onco panel submissions.
Table 3.
Recent regulatory precedents for NGS onco panels
| Product | Document | Class | Approval date | Comment |
|---|---|---|---|---|
| OncomineDx28 | P160045 | III | June 22, 2017 | Selection of patients with NSCLC for treatment with targeted therapies |
| FoundationFocus CDxBRCA29 | P160018 | III | December 19, 2016 | Identification of patients with ovarian cancer for whom treatment with Rubraca (rucaparib) is considered |
| FoundationOne CDx30 | P170019 | III | November 30, 2017 | Illumina pan‐cancer panel covering most follow‐on CDx variants |
| MSK‐IMPACT31 | DEN170058 | II | November 15, 2017 | Targeted NGS of formalin‐fixed paraffin‐embedded tumor tissue matched with normal specimens from patients with solid malignant neoplasms to detect tumor gene alterations in a broad multigene panel |
CDxBRCA, companion diagnostics breast cancer; NGS, next‐generation sequencing; NSCLC, non‐small cell lung cancer.
Table 3 shows multiple product development and regulatory pathways for the development of onco panels. OncomineDx is a kit designed for use with the Ion Torrent PGM Dx System. The kit was developed and manufactured by the same company (Thermo‐Fisher) that developed the instrument. However, the other tests on this table were developed to be run at specific CLIA laboratories that received single‐site approval from the FDA in each case. Single‐site approvals have become critical for regulatory approvals when the company developing the test does not have access to FDA cleared instrument versions for that test platform. Finally, MSK‐IMPACT broke new ground as a class II test for tumor characterization, without specific actionable results and as the first NGS onco panel approved through third‐party review at the New York State Department of Health.
Evolution of tumor biopsy NGS assays into liquid biopsy NGS assays
Tumor biopsy specimens have been essential for the development of precision medicine. Therapeutic decision making with sequencing data from these samples has included both sequencing results for genes and variants of novel and follow‐on companion diagnostics, as well as for other genes and variants applicable to therapeutic decision making. Some tissues (such as lung tissue), however, may be challenging for tumor biopsy isolation,32 with incremental discomfort for the patient. Tumor biopsy specimens are also difficult to use to monitor therapeutic response and tumor recurrence in oncology.
Liquid biopsies have been used for prognostic applications in oncology over 2 decades. Figure 1 shows the different permutations for indications, signal sources, and platforms for liquid biopsies.
Figure 1.
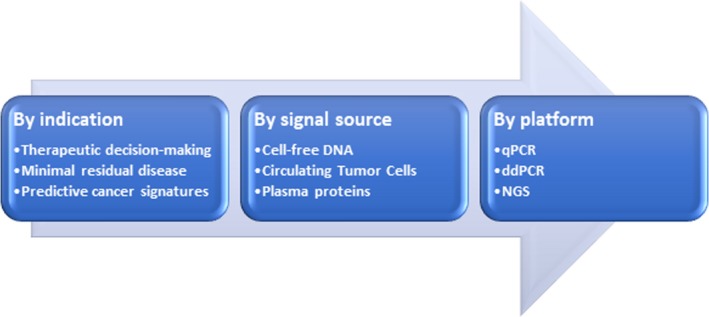
Product design permutations in liquid biopsies. ddPCR, droplet digital PCR; NGS, next‐generation sequencing; qPCR, quantitative polymerase chain reaction.
These permutations have led to two divergent product development and FDA regulatory approval pathways for two different platforms33, 34 over a decade apart from each other.
Circulating tumor cells in liquid biopsy platforms
Circulating tumor cells (CTCs) are tumor cells that originate from either primary tumor or metastatic sites and can be isolated from the peripheral blood of patients with solid tumors. The CTC research field was very active over the last decade of the 20th century, with many studies in a variety of tumor types.
CTCs may be enumerated and also characterized through molecular characterization and functional analysis. Enumeration data provide prognostic and predictive information, whereas molecular characterization and functional analysis of CTCs report on the biology of the tumor cells. This information is helpful in the design of personalized therapies for patients with cancer. Genomic testing of CTCs from each patient can be performed once or over multiple sampling throughout the course of treatment to identify therapeutic targets and guide the treatment for patients or to monitor the prognosis and molecular evolution of the disease.
CTC Platform Technology Development
The evolution of CTC technologies over the past 2 decades has led to a broad diversity of approaches to label, isolate, purify, and characterize these cells. The evolution of DNA sequencing and genotyping has been concurrent with the evolution of CTC technologies. NGS data for CTCs have only become available over the past 5 years. This timeline disparity is important: although CTCs have been available for at least 2 decades, analyses by NGS have been around as long for CTCs as for cfDNA samples. Table 4 summarizes different CTC technology platforms developed over the past 20 years.
Table 4.
CTC platform evolution
| Platform | Operational principles and challenges |
|---|---|
| CellSearch System35 | Focused on CTC detection and enumeration; multistep, labor‐intensive |
| CTC‐iChip,36 GEDI,37 Adnagen,38 EPIC 39 | Negative depletion step to remove leukocytes, or a positive selection step with markers, such as Epithelial Cell Adhesion Molecule (EpCAM), or other specific surface markers to capture the CTCs |
| Label‐free methods40 | Separation by taking advantage of larger size of tumor cells of epithelial origin compared with RBCs and WBCs |
| Clearbridge technology41 | Hydrodynamic sorting of cells by size in a specially designed microfluidic chip (Dean flow fractionation) |
| Use of microfilters to remove cells below a certain size cutoff,42 size exclusion filter on a syringe43 | Isolation by size |
| Parsortix device44 | Sorting cells by both deformability and size |
| Vortex Chip45, 46 | Microfluidic device |
CTC, circulating tumor cell; RBCs, red blood cells; WBCs, white blood cells.
The Challenging Path of CTC Liquid Biopsies Through the FDA
The CellSearch Circulating Tumor Cell Kit was approved by the FDA with an indication for detection of epithelial cells in the circulation. This test was initially indicated for the prognosis of patients with advanced breast cancer. Presence of CTCs in the blood, as detected by the CellSearch Circulating Tumor Cell Kit, was associated with decreased progression‐free survival and decreased overall survival in patients treated for metastatic breast cancer.47, 48 This prognostic indication was expanded to tumors in other tissues throughout the decade after the initial CDRH approval. Expansion of the prognostic indication into a predictive one, however, was not approved by the CDRH for this product. Prospective applications, where detection of more than a handful of CTCs is relatively straightforward, have been accepted by the FDA. Predictive applications, however, which require absence of detection of CTCs, have been rejected by the FDA. These applications could have been eventually developed as surrogates for therapeutic efficacy to facilitate future clinical study designs in oncology. However, the FDA review concluded that the limit of detection of this assay is not low or reproducible enough to guarantee absence of CTCs. This limitation makes this specific CTC technology insufficiently sensitive as a surrogate for clinical studies in oncology.
Clinical trials49, 50 have shown that the number of CTCs is associated with progression‐free and overall survival in advanced metastatic castration‐resistant prostate cancer. A parallel regulatory path has been attempted over the past decade by Memorial Sloan‐Kettering Cancer Center, Medivation, and Janssen Diagnostics,35 who, in 2002, obtained the prostate cancer prognostic indication at the FDA for the CellSearch Circulating Tumor Cell Kit. These sponsors submitted one of the earliest biomarker qualification requests to the FDA in 2008, seeking a surrogacy claim for CTC enumeration. This request has lingered for over a decade,51 through multiple CTC technology platforms, which went from novel to legacy,52 multiple requests for additional clinical data,53 and the recent conversion of the Biomarker Qualification Process to the “507” Qualification Process required by the 21st Century Cures legislation of 2016.54 Biomarker Qualification as a regulatory pathway for CTC tests is an important example of the long and unusually futile exercise for the academic, industrial, and government scientists who have attempted its completion.
An alternative regulatory pathway for CTC technologies could have been as a patient selection companion diagnostic product, instead of as a biomarker. The original choice a decade ago to proceed with biomarker qualification as the regulatory pathway for CTCs was driven by the marketing strategy of the diagnostic company and by the context of use for CTCs driven by this marketing strategy. As predictive markers, CTCs were being proposed a decade ago as surrogates for therapeutic efficacy. The evidentiary standards required for acceptance of a therapeutic efficacy surrogate by the FDA throughout the past decade were in flux, as new clinical studies requested by the FDA led to additional questions and study requests by the FDA. Absence of a clear definition by the FDA of a surrogate context of use for these biomarkers and of the evidentiary standards required to prove this claim made the investment in multiple clinical studies by several academic institutions really futile.55 The strong biological mechanistic rationale for CTCs was insufficient throughout the past decade to support their acceptance as surrogates but would have sufficed to propose CTCs as patient selection markers. At this point, a viable regulatory pathway for CTCs in clinical trials would be as patient selection companion diagnostic products, similar to the path shown below for cfDNA platforms.
CfDNA in liquid biopsy platforms
CfDNA has been broadly used in liquid biopsies over the past 5 years. A major goal for the indication of cfDNA liquid biopsies has been the detection of variants that guide therapeutic decisions in oncology, either as companion diagnostics or as variants, which may be used by molecular tumor boards for additional decisions beyond initial treatment triggered by a companion diagnostic result. However, cfDNA detection is itself dependent on:
Total cfDNA plasma concentration
Percentage of tumor‐sourced cfDNA in total cfDNA
Specificity for detection in tumor‐sourced cfDNA
Heterogeneity of variants detected from primary vs. metastasized tumors
Detection of other variants from tissue sources other than tumors
These analytical variables are also affected by the location of the primary tumor, the stage of the disease, and other genotypic and phenotypic variables. These sources of variability have at times been proposed as due to real clinical variability.56 However, the specificity of each of these analytical variability metrics as clinical biomarkers has not been established.
There are several approaches to mitigate the clinical impact of this analytical variability into viable clinical biomarkers. The first approach is to improve the analytical sensitivity of the sequencing platform, to detect lower total cfDNA plasma concentrations, and even more important, to detect lower percentage of tumor‐sourced cfDNA in total cfDNA. The sequencing chemistry can be optimized to improve the analytical sensitivity of NGS. However, the challenge with this approach is that the ultimate analytical sensitivity of NGS is ultimately limited by the performance of instrument platforms available today for short‐read sequencing (Illumina and Ion Torrent).57
These analytical constraints can also be mitigated when the focus of liquid biopsies is on tumor mutational burden58 or microsatellite instability.59 In these cases, the goal of detection for individual variants is replaced by the enumeration of variants detected. Finally, the restriction of variant detection to patients with advanced stages of cancer also restricts samples to relatively high and homogeneous total cfDNA and percentage of tumor‐sourced cfDNA.
Cfdna Liquid Biopsies at The FDA
The first liquid biopsy cfDNA test was approved by the FDA in 2017 for Epidermal Growth Factor Receptor (EGFR)‐targeted therapeutic decision making using qPCR on the Roche COBAS instrument.60 This product shows excellent specificity but only 77% sensitivity when compared with its indication with tumor biopsy specimens. Its label recommends EGFR therapy for positive results with this test and tumor biopsy follow‐up testing for negative results from the test. This labeling language addresses the sensitivity challenge for this (and similar) tests. A shift to NGS adds complexity to this language, with sensitivity that will not necessarily match that of qPCR for a specific single nucleotide polymorphism.
There have been several other LDTs developed for therapeutic decision making using cfDNA and NGS.61 Some have also received Breakthrough Designation61, 62, 63 at the FDA. However, the path from Breakthrough Designation at the FDA to regulatory approval may not be as smooth as the Breakthrough Designations at the FDA may suggest. A recent paper64 comparing results for two of these LDT liquid biopsies concluded:
Very low congruence for same patient‐paired samples
Cannot determine which test is more accurate
Reported gene alterations will not be the same across different platforms
Patients could receive different treatments depending on the cfDNA platform
A follow‐up 2018 report from the American Society of Clinical Oncology on Liquid Biopsy Tests in People with Cancer65 concluded that:
There is not enough evidence, at this time, to know whether use of the majority of circulating tumor DNA tests in advanced cancer is justified, outside of screening for participation in, or during, a clinical trial
There is not enough evidence, at this time, to support the routine use of circulating tumor DNA tests for early‐stage cancer, making treatment decisions, monitoring how well a treatment is working, finding remaining cancer cells, or for cancer screening, except screening for participation in, or during, a clinical trial
There are inconsistent findings when testing with liquid biopsies vs. testing with tumor tissue, so negative liquid biopsy results should be confirmed with tumor tissue genotyping
This is an instance where the performance of a clinical platform is dependent on the performance of an analytical platform, which may be at the limit of its analytical performance capability. There are challenges in the accurate detection of all possible variant types in tissue biopsies.66 These are exacerbated by the uncertainty and bias in different pipeline software products67 for calling these variants. Performance requirements for liquid biopsies represent a major analytical challenge for NGS chemistries and hardware. As companion diagnostics, liquid biopsies require somatic variant detection in <30 ng total DNA and tumor fractions < 0.1%.68
Sequencing chemistries developed for liquid biopsies can reach nominal specifications in this range for single‐nucleotide variations but may fail to reach them for other variant types.69 These analytical challenges are superimposed on a biological and clinical variability, which make consistent diagnostic detection of specific variants in oncology patients a difficult goal. There are gaps between the experimental description of the capabilities for different liquid biopsy platforms and their performance as diagnostic platforms.
Can cfDNA liquid biopsies develop as accurate diagnostic tools? There are several applications for liquid biopsies where the analytical specifications for a biological measurement are consistent with those for liquid biopsies. These include tumor mutational burden and microsatellite instability. Tumor mutational burden and microsatellite instability applications are closely linked with the successful Breakthrough Designation of some liquid biopsy platforms.
Ultimately, however, the success of cfDNA liquid biopsies will also depend on the specific clinical definitions for the patient populations that will be tested with cfDNA. The goal of a less‐invasive detection of biomarkers for therapeutic decision making, detection of minimal residual disease, or early cancer detection will need to be accurately calibrated to the analytical performance of the cfDNA liquid biopsy platforms.
Regulatory path for liquid biopsy diagnostic approvals
Although each liquid biopsy product in a regulatory submission is ultimately unique for specific review issues associated with it, there are some common threads presented here that emerge from the NGS onco panel approvals and liquid biopsy product breakthrough designations and are also likely to apply to future liquid biopsy regulatory submissions. These tools are shown in Table 5.
Table 5.
Elements for liquid biopsy regulatory submissions
| Tools | Characteristics |
|---|---|
| Single‐site approvals | Simplify regulatory review and approval for liquid biopsy diagnostics, which cannot be submitted for approval with FDA‐cleared instruments. Strategic decision early in development. Instinctive for product evolution from a CLIA laboratory to FDA approval. |
| Narrow contexts of use | Narrow clinical phenotype for populations that will benefit from the liquid biopsy test result in smaller and more accurate clinical studies for liquid biopsy tests. |
| Breakthrough designation | Requested before pre‐submission meetings. Enhance regulatory review communications for the liquid biopsy product. |
| Third‐party review | Novel approach, which in the case of the MSK‐IMPACT panel led to essentially simultaneous New York State Department of Health and FDA approvals. |
| CDx vs. other NGS genes and variants | PMA classifications and clinical utility demonstrations are expected for both novel as well as follow‐up CDx sequences. Other sequences of potential value in therapeutic decision making require only analytical validation to be included in the final reportable result. |
CDx, companion diagnostics; CLIA, Clinical Laboratory Improvement Amendment; FDA, US Food and Drug Administration; NGS, next‐generation sequencing; PMA, premarket approval.
Table 5 will be helpful in the development and regulatory submission of liquid biopsy diagnostic products.
Funding
No funding was received for this work.
Conflict of Interest
The authors declared no competing interests for this work.
References
- 1. Heitzer, E. , Haque, I.S. , Roberts, C.E.S. & Speicher, M.R. Current and future perspectives of liquid biopsies in genomics‐driven oncology. Nat. Rev. Genet. 20, 71–88 (2019). [DOI] [PubMed] [Google Scholar]
- 2. Gatter, K. FDA oversight of laboratory‐developed tests: where are we now? Arch. Pathol. Lab. Med. 141, 746–748 (2017). [DOI] [PubMed] [Google Scholar]
- 3. US Food and Drug Administration (FDA) . Challenge and opportunity on the critical path to new medical products. <http://wayback.archive-it.org/7993/20180125035500/https://www.fda.gov/downloads/ScienceResearch/SpecialTopics/CriticalPathInitiative/CriticalPathOpportunitiesReports/UCM113411.pdf> (2004). Accessed January 30, 2019.
- 4. Biomarkers Definition Working Group Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 69, 89–95 (2001). [DOI] [PubMed] [Google Scholar]
- 5. US Food and Drug Administration (FDA) . Enrichment strategies for clinical trials to support determination of effectiveness of human drugs and biological products guidance for industry. <https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM332181.pdf> (2019). Accessed March 31, 2019.
- 6. US Food and Drug Administration (FDA) . In vitro diagnostics. <https://www.fda.gov/medicaldevices/productsandmedicalprocedures/invitrodiagnostics/default.htm>. Accessed April 18, 2019.
- 7. US Food and Drug Administration (FDA) . In vitro companion diagnostic devices guidance for industry and food and drug administration staff. <https://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM262327.pdf> (2014). Accessed January 30, 2019.
- 8. Li, M. Statistical methods for clinical validation of follow‐on companion diagnostic devices via an external concordance study. Stat. Biopharm. Res. 8, 355–363 (2016). [Google Scholar]
- 9. US Food and Drug Administration (FDA) . How to prepare a traditional 510(k). <https://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/HowtoMarketYourDevice/PremarketSubmissions/PremarketNotification510k/ucm134572.htm>. Accessed April 18, 2019.
- 10. US Food and Drug Administration (FDA) . CFR – Code of Federal Regulations Title 21. <https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=860.3>. Accessed April 18, 2019.
- 11. US Food and Drug Administration (FDA) . Premarket approval (PMA). <https://www.fda.gov/medicaldevices/deviceregulationandguidance/howtomarketyourdevice/premarketsubmissions/premarketapprovalpma/>. Accessed April 18, 2019.
- 12. Hessler, K. Companion diagnostics: evolving FDA regulation and issues for resolution In In Vitro Diagnostics: The Complete Regulatory Guide (Chapter 8). (Covington & Burling LLP, 2010). Washington, DC: Food and Drug Law Institute. [Google Scholar]
- 13. US Food and Drug Administration (FDA) . Draft Guidance for Industry, Clinical Laboratories, and FDA Staff. In Vitro Diagnostic Multivariate Index Assays. <https://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm071455.pdf> (2007). Accessed January 30, 2019.
- 14. OncotypeDx . <https://www.oncotypeiq.com/en-US/breast-cancer/healthcare-professionals/oncotype-dx-breast-recurrence-score/about-the-test?gclxml:id=EAIaIQobChMIk-_Cstbb4QIVDNtkCh1uzgXoEAAYASAAEgIv__D_BwE>. Accessed April 19, 2019.
- 15. National Cancer Institute (NCI) . Definition of liquid biopsy. <https://www.cancer.gov/publications/dictionaries/cancer-terms/def/liquid-biopsy>. Accessed April 19, 2019.
- 16. US Food and Drug Administration (FDA) . MRD. <https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm622004.htm>. Accessed April 19, 2019.
- 17. US Food and Drug Administration (FDA) . Guidance for industry pharmacogenomic data submissions. <https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM079849.pdf> (2005). Accessed January 30, 2019.
- 18. US Food and Drug Administration (FDA) . Pharmacogenetic tests and genetic tests for heritable markers. <https://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM071075.pdf> (2007). Accessed January 30, 2019.
- 19. US Food and Drug Administration (FDA) . Commercially distributed in vitro diagnostic products labeled for research use only or investigational use only. <https://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM376118.pdf> (2013). Accessed January 30, 2019.
- 20. Hinman, L.M. et al The drug diagnostic co‐development concept paper: commentary from the 3rd FDA‐DIA‐PWG‐PhRMA‐BIO Pharmacogenomics Workshop. Pharmacogenomics J. 6, 375–380 (2006). [DOI] [PubMed] [Google Scholar]
- 21. American Clinical Laboratory Association (ACLA) . Comments on March 21, 2017 Discussion Draft of the Diagnostic Accuracy and Innovation Act. <https://www.acla.com/acla-comments-on-march-21-2017-discussion-draft-of-the-diagnostic-accuracy-and-innovation-act/> (2017).
- 22. US Food and Drug Administration (FDA) . Framework for regulatory oversight of laboratory developed tests (LDTs). <https://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM416685.pdf> (2014). Accessed January 30, 2019.
- 23. US Food and Drug Administration (FDA) . Discussion paper on laboratory developed tests (LDTs). <https://www.fda.gov/downloads/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/LaboratoryDevelopedTests/UCM536965.pdf> (2017). Accessed January 30, 2019.
- 24. US Food and Drug Administration (FDA) . Public Workshop on Next Generation Sequencing‐Based Oncology Panels. In vitro companion diagnostic devices. www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm262327.pdf (2016). Accessed January 30, 2019.
- 25. US Food and Drug Administration (FDA) . Illumina MiSeqDx Cystic Fibrosis 139‐Variant Assay. <https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=K124006> (2013).
- 26. US Food and Drug Administration (FDA) . Illumina MiSeqDx Cystic Fibrosis Clinical Sequencing Assay 510k Clearance. <https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=K132750> (2013).
- 27. US Food and Drug Administration (FDA) . Considerations for design, development, and analytical validation of next generation sequencing (NGS) – based in vitro diagnostics (IVDs) intended to aid in the diagnosis of suspected germline diseases (2018). <https://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM509838.pdf>. Accessed January 30, 2019.
- 28. US Food and Drug Administration (FDA) . OnomineDx premarket approval. <https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?xml:id=p160045> (2017). Accessed January 30, 2019
- 29. US Food and Drug Administration (FDA) . FoundationFocus™ CDxBRCA. Premarket approval. <https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?xml:id=p160018> (2016). Accessed January 30, 2019.
- 30. US Food and Drug Administration (FDA) . FoundationOne CDx™. Premarket approval. <https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?xml:id=p170019> (2017). Accessed January 30, 2019.
- 31. US Food and Drug Administration (FDA) . MSK‐IMPACT. Device classification under Section 513(f)(2)(de novo). <https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/denovo.cfm?ID=DEN170058> (2017). Accessed January 30, 2019.
- 32. Deng, C.J. et al Clinical updates of approaches for biopsy of pulmonary lesions based on systematic review. BMC Pulm. Med. 18, 146 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stoecklein, N.H. , Fischer, J.C. , Niederacher, D. & Terstappen, L.W. Challenges for CTC‐based liquid biopsies: low CTC frequency and diagnostic leukapheresis as a potential solution. Expert Rev. Mol. Diagn. 16, 147–164 (2016). [DOI] [PubMed] [Google Scholar]
- 34. Polivka, J. Jr , Pesta, M. & Janku, F. Testing for oncogenic molecular aberrations in cell‐free DNA‐based liquid biopsies in the clinic: are we there yet? Expert Rev. Mol. Diagn. 15, 1631–1644 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. US Food and Drug Administration (FDA) . CellSearch System. <https://www.accessdata.fda.gov/cdrh_docs/pdf7/k073338.pdf> (2008). Accessed January 30, 2019.
- 36. Sequist, L.V. , Nagrath, S. , Toner, M. , Haber, D.A. & Lynch, T.J. The CTC‐chip: an exciting new tool to detect circulating tumor cells in lung cancer patients. J. Thorac. Oncol. 4, 281–283 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gleghorn, J.P. et al Capture of circulating tumor cells from whole blood of prostate cancer patients using geometrically enhanced differential immunocapture (GEDI) and a prostate‐specific antibody. Lab Chip 10, 27–29 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Andreopoulou, E. et al Comparison of assay methods for detection of circulating tumor cells in metastatic breast cancer: AdnaGen AdnaTest BreastCancer Select/Detect™ versus Veridex Cell Search™ system. Int. J. Cancer 130, 1590–1597 (2012). [DOI] [PubMed] [Google Scholar]
- 39. Werner, S.L. et al Analytical validation and capabilities of the Epic CTC platform: enrichment‐free circulating tumor cell detection and characterization. J. Circ. Biomark. 4, 3 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kang, Y.T. , Doh, I. , Byun, J. , Chang, H.J. & Cho, Y.H. Label‐free rapid viable enrichment of circulating tumor cell by photosensitive polymer‐based microfilter device. Theranostics 7, 3179–3191 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Khoo, B.L. et al Clinical validation of an ultra high‐throughput spiral microfluidics for the detection and enrichment of viable circulating tumor cells. PLoS One 9, e99409 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rawal, S. , Ao, Z. , Datar, R.H. & Agarwal, A. Microfilter‐based capture and release of viable circulating tumor cells. Methods Mol. Biol. 1634, 93–105 (2017). [DOI] [PubMed] [Google Scholar]
- 43. Mu, Z. et al Detection and characterization of circulating tumor associated cells in metastatic breast cancer. Int. J. Mol. Sci. 17, 1665–1676 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu, L. et al Optimization and evaluation of a novel size based circulating tumor cell isolation system. PLoS One 10, e0138032 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sollier, E. et al Size‐selective collection of circulating tumor cells using Vortex technology. Lab Chip 14, 63–77 (2014). [DOI] [PubMed] [Google Scholar]
- 46. Renier, C. et al Label‐free isolation of prostate circulating tumor cells using Vortex microfluidic technology. NPJ Precis. Oncol. 1, 15 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cristofanilli, M. et al Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 351, 781–791 (2004). [DOI] [PubMed] [Google Scholar]
- 48. Cristofanilli, M. et al Circulating tumor cells in metastatic breast cancer: biologic staging beyond tumor burden. Clin. Breast Cancer 7, 471–479 (2007). [PubMed] [Google Scholar]
- 49. Danila, D.C. et al Circulating tumour cell number and prognosis in progressive castration‐resistant prostate cancer. Clin. Cancer Res. 13, 7053–7058 (2007). [DOI] [PubMed] [Google Scholar]
- 50. Scher, H.I. , Warren, M. & Heller, G. The association between measures of progression and survival in castrate‐metastatic prostate cancer. Clin. Cancer Res. 13, 1488–1492 (2007). [DOI] [PubMed] [Google Scholar]
- 51. Shaffer, D.R. et al Circulating tumor cell analysis in patients with progressive castration‐resistant prostate cancer. Clin. Cancer Res. 13, 2023–2029 (2007). [DOI] [PubMed] [Google Scholar]
- 52. Serrano, M.J. , Sanchez‐Rovira, P. , Delgado‐Rodriguez, M. & Gaforio, J.J. Detection of circulating tumor cells in the context of treatment: prognostic value in breast cancer. Cancer Biol. Ther. 8, 671–675 (2009). [DOI] [PubMed] [Google Scholar]
- 53. Danila, D.C. et al TMPRSS2‐ERG status in circulating tumor cells as a predictive biomarker of sensitivity in castration‐resistant prostate cancer patients treated with abiraterone acetate. Eur. Urol. 60, 897–904 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. US Food and Drug Administration (FDA) .US FDA: Letter of Support for CTCs enumeration as a potential disease activity biomarker for use in clinical trials for metastatic castration resistant prostate cancer (mCRPC). <https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DrugDevelopmentToolsQualificationProgram/BiomarkerQualificationProgram/UCM605359.pdf> (2015). Accessed January 31, 2019.
- 55. Fiteni, F., Westeel, V. & Bonnetain, F. Surrogate endpoints for overall survival in lung cancer trials: a review. Expert Rev. Anticancer Ther. 17, 447–454 (2017). [DOI] [PubMed] [Google Scholar]
- 56. Cozar, J.M. et al Genetic markers a landscape in prostate cancer. Mutat. Res. 775, 1–10 (2018). [DOI] [PubMed] [Google Scholar]
- 57. Xu, T. et al Cross‐platform comparison of four leading technologies for detecting EGFR mutations in circulating tumor DNA from non‐small cell lung carcinoma patient plasma. Theranostics 7, 1437–1446 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Goodman, A.M. et al Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol. Cancer Ther. 16, 2598–2608 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Huang, C. , Liu, S. , Tong, X. & Fan, H. Extracellular vesicles and ctDNA in lung cancer: biomarker sources and therapeutic applications. Cancer Chemother. Pharmacol. 82, 171–183 (2018). [DOI] [PubMed] [Google Scholar]
- 60. US Food and Drug Administration (FDA) . Cobas EGFR Mutation Test v2, Cobas DNA and cfDNA Sample Preparation Kit Pre‐Market Approval. <https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?xml:id=P120019s019> (2018). Accessed January 31, 2019.
- 61. US Food and Drug Administration (FDA) . Foundation Medicine's New Liquid Biopsy Assay Granted Breakthrough Device Designation by the US Food and Drug Administration. <http://investors.foundationmedicine.com/news-releases/news-release-details/foundation-medicines-new-liquid-biopsy-assay-granted> (2018). Accessed January 31, 2019.
- 62. US Food and Drug Administration (FDA) . The Guardant360® Assay Receives Expedited Access Pathway Designation for Breakthrough Devices from the FDA. <http://investors.guardanthealth.com/news-releases/news-release-details/guardant360r-assay-receives-expedited-access-pathway-designation> (2018). Accessed January 31, 2019.
- 63. US Food and Drug Administration (FDA) . Personal Genome Diagnostics’ PGDx elio™ plasma resolve Receives Breakthrough Device Designation from the FDA. <http://www.personalgenome.com/wp-content/uploads/2018/07/Personal-Genome-Diagnostics-PGDx-elio-plasma-resolve-Receives-Breakthrough-Device-Designation-from-FDA.pdf> (2018). Accessed January 31, 2019.
- 64. Torga, G. & Pienta, K.J. Patient‐paired sample congruence between 2 commercial liquid biopsy tests. JAMA Oncol. 4, 868–870 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Merker, J.D. et al Circulating tumor DNA analysis in patients with cancer: American Society of Clinical Oncology and College of American Pathologists joint review. J. Clin. Oncol. 36, 1631–1641 (2018). [DOI] [PubMed] [Google Scholar]
- 66. Froyen, G. & Maes, B. Clinical validation of targeted solid tumor profiling. Methods Mol. Biol. 1908, 73–87 (2019). [DOI] [PubMed] [Google Scholar]
- 67. Allali, I. et al A comparison of sequencing platforms and bioinformatics pipelines for compositional analysis of the gut microbiome. BMC Microbiol. 17, 194 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rohanizadegan, M. Analysis of circulating tumor DNA in breast cancer as a diagnostic and prognostic biomarker. Cancer Genet. 228–229, 159–168 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gorgannezhad, L. , Umer, M. , Islam, M.N. , Nguyen, N.T. & Shiddiky, M.J.A. Circulating tumor DNA and liquid biopsy: opportunities, challenges, and recent advances in detection technologies. Lab Chip 18, 1174–1196 (2018). [DOI] [PubMed] [Google Scholar]


