Abstract
Pazopanib (PAZ), a tyrosine kinase inhibitor used in the treatment of soft tissue sarcoma (STS), should not be administered with acid‐suppressive medications (ASMs) due to decreased drug solubility. Common practice for patients requiring ASM with PAZ is to separate administration by 12 hours; however, there is little real‐world evidence describing clinical outcomes using this strategy. The aim of this study was to determine whether concomitant ASM impacted efficacy and adverse event rates in patients with STS receiving PAZ. Medical records were retrospectively reviewed for patients with STS who received PAZ from June 2011 to July 2017. Patients were stratified into two groups, PAZ with or without ASM (PAZ + ASM or PAZ only). The primary objective was to determine whether progression‐free survival (PFS) differed between groups. Secondary objectives were to determine overall survival (OS) and occurrence of grade 3/4 toxicities. Ninety‐one patients were included in the study, 42 patients in the PAZ + ASM group and 49 in the PAZ only group. Median PFS was significantly shorter in the PAZ + ASM group than the PAZ only group (5.3 vs. 6.7 months). The PAZ + ASM group also had a 74% higher relative risk of progression or death than the PAZ only group, but there was no difference in OS. Regarding adverse events, the PAZ + ASM group trended toward lower levels of grade 3/4 hypertension (19% vs. 37%). These results suggest that ASM should be avoided in patients with STS receiving PAZ. Larger studies are needed to further elucidate the impact of ASM use with PAZ in clinical practice.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ The use of acid‐suppressive medications (ASMs) with pazopanib (PAZ) has been thought to reduce the efficacy of PAZ therapy.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ We currently counsel patients who have a difficult time reducing their ASM usage to space out or reduce their ASM usage in hopes of maintaining PAZ efficacy, but little is known about whether this strategy is effective.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ We show here that any ASM usage despite aggressive patient education and attempts to reduce/space out ASM usage reduces clinical PAZ efficacy.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ Patients who cannot discontinue their ASM use should be not be considered for PAZ usage.
Soft‐tissue sarcomas (STS) are rare, heterogeneous tumors that account for < 1% of all adult malignancies in the United States.1, 2 There are over 70 subtypes of sarcoma, each with their own unique pathologic characteristics and limited treatment options. In 2012, the US Food and Drug Administration (FDA) approved pazopanib (PAZ), an oral multitargeted tyrosine kinase inhibitor (TKI), for patients with advanced STS who failed standard anthracycline‐based chemotherapy. This approval was based on the results of the PALETTE trial, which demonstrated significantly improved progression‐free survival (PFS) and nonsignificantly improved overall survival (OS) in patients with nonadipocytic STS receiving PAZ compared with placebo.3
PAZ is taken by mouth once daily on an empty stomach. It is a hydrochloride salt, which is highly soluble in acidic environments (pH = 1) and practically insoluble above pH = 4 in aqueous media.4 Thus, gastrointestinal absorption of PAZ is dependent on the pH of the stomach. It has been hypothesized that there is a pharmacokinetic drug interaction between PAZ and acid‐suppressive medications (ASMs) due to alterations of stomach pH. In a small study of 13 patients, concomitant administration of PAZ with the ASM esomeprazole decreased the average area under the curve (AUC) of PAZ in patients with advanced solid tumors by 40%.5 This led the FDA label to suggest avoiding coadministration of PAZ with medications that increase gastric pH because of decreased drug solubility; however, there is little real‐world evidence to determine if decreased drug solubility leads to decreased drug efficacy.4
Unfortunately, patients with STS often require ASMs, such as proton pump inhibitors (PPIs) and histamine 2 receptor antagonists (H2RAs), for various diagnoses.6 Clinicians are, therefore, faced with the challenge of managing PAZ drug interactions in clinical practice. Options include separating the administration of PAZ from the ASM or to reduce the dose of ASM. Both strategies can be challenging for patients who benefit from twice‐daily ASM dosing regimens, and, even so, these strategies do not completely eliminate the interaction.7
To date, there have been no studies in peer‐reviewed literature assessing the effects of ASM and concomitant PAZ administration on STS survival outcomes. Other studies have evaluated the drug interaction of ASMs and TKIs with conflicting results. For instance, Lalani et al.8 reported that of 120 patients with metastatic renal cell carcinoma who concomitantly received PPIs with sunitinib, axitinib, or sorafenib, PPI users had similar OS compared with non‐PPI users. Conversely, Chu et al.9 showed that of 124 patients with stage IIIB/IV non‐small cell lung cancer who concomitantly received PPIs or H2RAs with erlotinib, the median PFS in the acid‐suppressive group was significantly lower than in the no acid‐suppressive group (1.4 vs. 2.3 months; P < 0.001). Therefore, the purpose of this study was to determine whether concomitant ASM impacted survival and/or safety outcomes in STS patients receiving PAZ. We hypothesized that ASM use would lower the efficacy of PAZ by decreasing its overall bioavailability. The primary objective of this study was to determine PFS in patients taking concomitant ASM vs. no ASM with PAZ. Secondary objectives were to determine OS and rate of grade 3 or 4 toxicities.
Methods
Patients
This retrospective cohort study included patients with STS, aged 18−89 years, who had received at least one dose of PAZ at The Ohio State University Comprehensive Cancer Center between June 2011 and July 2017. Patients were excluded if they were incarcerated, pregnant, or had received PAZ for a clinical trial, gastrointestinal stromal tumor, or primary bone sarcoma. This study was approved by the local institutional review board (The Ohio State University Institutional Review Board Protocol Approval: 2017C0172). Data were collected from electronic medical records for patients with electronically generated prescriptions for PAZ and International Classification of Disease‐version 9 or International Classification of Disease–version 10 codes for STS. Data collected included patient demographics, STS histological subtypes, prescription dates and dosing for PAZ and ASM, grade 3 or 4 drug‐related toxicities, ASM indications, and survival end points. For survival end points, given the heterogeneity of tumor types and of treatments post‐PAZ, follow‐up was limited to patients during their exposure to PAZ.
Patients were stratified into two groups: (i) PAZ with concomitant ASM (PAZ + ASM) and (ii) PAZ without ASM (PAZ only). In the PAZ + ASM group, patients could have received a PPI alone, an H2RA alone, or both a PPI and an H2RA. Toxicities were graded utilizing the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.10
The recommended starting dose of PAZ for STS is 800 mg once daily; however, for some patients, our practice is to initiate at a lower dose per physician discretion, typically 200–600 mg once daily and titrating to the target dose within 2–4 weeks after initiation based on tolerability. Patients unable to discontinue ASM for medical reasons were educated to separate administration of PAZ and ASM by 10–12 hours. Patients requiring twice‐daily dosing of H2RAs were educated to take the first dose of H2RA 2 hours after administration of PAZ with the second dose 10–12 hours after PAZ.
Statistical analysis
PFS was calculated as the time from the start of PAZ treatment to death, progression, or censoring. Patients were considered censored at the time they discontinued PAZ, even if they later experienced death or disease progression. If they remained on PAZ for the entirety of follow‐up without death or progression, censoring occurred at either the date of the last provider visit or last scan. OS was calculated as the time from the start of PAZ treatment to either death or censoring. The follow‐up period for the survival analysis covered only the duration of when the patients were treated by PAZ.
The log rank test and unadjusted Cox proportional hazard models were used to assess the unadjusted association between concurrent acid suppressive therapy and the survival outcomes. A Cox proportional hazards model adjusting for the potential confounding factor Eastern Cooperative Oncology Group (ECOG) performance status was also fit to the PFS outcome. As three patients had missing data for their ECOG status, multiple imputation of the missing data was performed, conditioned on age, sex, stage, prior surgery, prior radiation, and prior chemotherapy. The hazard ratios were combined across the 30 imputed data sets using the MIANALYZE procedure in SAS version 9.4. The low event count for OS precluded any adjustment for potential confounding variables.
Toxicity data were compared between patients with and without concurrent acid suppressive therapy using Fisher's exact test. The ASM used was described and the time overlapping with PAZ administration was also calculated. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Baseline patient demographics
A total of 148 patients with STS who had an electronically generated prescription for PAZ from June 2011 until July 2017 were evaluated, with 57 patients meeting exclusion criteria, thus leaving 91 of these patients available for analysis (Figure 1 ). Of these patients, 42 were included in the concomitant PAZ + ASM group, and 49 patients were included in the PAZ only group. The baseline patient characteristics were statistically balanced between the two groups, with the exceptions of ECOG performance status and sarcoma subtype (Table 1 ).
Figure 1.
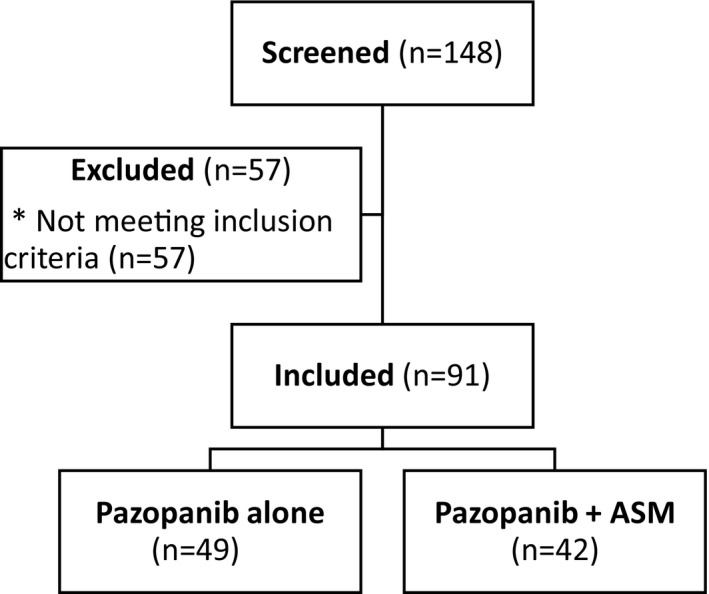
Consort flow diagram. ASM, acid suppressive medication. *Reasons for not meeting inclusion criteria were: patients who did not take at least one dose of pazopanib (n = 36), pazopanib indication not STS (n = 14), patients received pazopanib in the adjuvant setting (n = 4), patients received pazopanib for a clinical trial (n = 2), and patients followed locally by oncologist (n = 1). ASM, acid suppressive medication; STS, soft tissue sarcoma.
Table 1.
Baseline patient characteristics
| Characteristic | Pazopanib without ASM (n = 49) | Pazopanib with ASM (n = 42) |
|---|---|---|
| Age – no. (%) | ||
| <65 years old | 36 (74) | 27 (64) |
| ≥65 years old | 13 (26) | 15 (36) |
| Sex – no. (%) | ||
| Male | 25 (51) | 18 (43) |
| Female | 24 (49) | 24 (57) |
| Race – no. (%) | ||
| White | 40 (82) | 38 (91) |
| African American | 8 (16) | 4 (9) |
| Asian | 0 | 0 |
| Latin American | 0 | 0 |
| Other | 1 (2) | 0 |
| ECOGa – no. (%) | ||
| 0 | 22 (45) | 12 (29) |
| 1 | 17 (35) | 17 (41) |
| 2 | 7 (14) | 8 (19) |
| 3 | 1 (2) | 4 (10) |
| 4 | 0 | 0 |
| Sarcoma subtypeb – no. (%) | ||
| Leiomyosarcoma | 19 (39) | 11 (26) |
| Synovial | 4 (8) | 5 (12) |
| UPS | 6 (12) | 11 (26) |
| Liposarcoma | 3 (6) | 6 (14) |
| Other | 17 (35) | 9 (22) |
| Stage – no. (%) | ||
| I | 0 | 0 |
| II | 2 (4) | 1 (2) |
| III | 4 (8) | 1 (2) |
| IV | 43 (88) | 40 (96) |
| Prior chemotherapy – no. (%) | ||
| Yes | 37 (76) | 34 (81) |
| No | 12 (24) | 8 (19) |
ASM, acid suppressive medication; ECOG, Eastern Oncology Cooperative Group; UPS, undifferentiated pleomorphic sarcoma.aTwo patients in the pazopanib without ASM group and one patient in the pazopanib with ASM group did not have ECOG documented at baseline. bOther histological subtypes of sarcoma were spindle cell, epithelioid fibrosarcoma, myxofibrosarcoma, sarcomatoid, alveolar soft part sarcoma, angiosarcoma, large paraspindle cell, clear cell, and desmoplastic small round cell.
The median PAZ starting dose was 400 mg daily and the median PAZ dose at discontinuation was 600 mg daily. The most common indication for ASM use was gastrointestinal reflux disease followed by high‐dose steroids. The most common ASM used was a PPI (n = 35, 83%) with esomeprazole being used most often. All PPIs were dosed once daily. The most common H2RA used was famotidine. Seven of the 10 patients who received H2RAs received twice‐daily dosing. The mean overlap for PAZ and ASM regimens was calculated on a scale from 0−100%, with 100% being complete concomitant administration of the ASM for all doses of PAZ. For patients receiving a PPI, the mean overlap was 88% (range 3–100). The mean overlap for PAZ and H2RAs was 85% (range 33–100; Table 1 , Table S1 ).
Concomitant ASM usage decreased the PFS of patients on PAZ
Disease progression while on PAZ occurred in 29 of 42 patients (69%) in the concomitant PAZ + ASM group and in 25 of 49 patients (51%) in the PAZ only group. The median PFS was 5.3 months in the PAZ + ASM group (95% confidence interval (CI): 2.6–8.0) and 6.7 months in the PAZ only group (95% CI: 3.6–9.5; P = 0.041). These results were not substantially different in patients on PPI only (n = 32; PFS 5.3 months; 95% CI: 2.5–10.2) or H2RA only (n = 8; PFS 5.6 months; 95% CI: 2.3–6.2). There was a 74% higher short‐term risk of progression or death in the PAZ + ASM group than in the PAZ only group (hazard ratio: 1.74; 95% CI: 1.02–2.97; P = 0.044). After multiple imputation and adjustment for ECOG performance status, the adjusted hazard ratio for progression or death was 1.46 and no longer statistically significant (95% CI: 0.83–2.58; P = 0.19). These results were similar when the three patients with missing ECOG status were excluded from the adjusted analysis (data not shown).
A total of 63 deaths occurred during the study period, 33 deaths (79%) in the concomitant PAZ + ASM group and 30 deaths (61%) in the PAZ only group. However, only three patients (7.1%) in the PAZ + ASM group died while being treated with PAZ compared with one patient (2%) in the PAZ only group. The short‐term risk of death during the follow‐up period was 3.9 times higher for the PAZ + ASM group, although this was not significantly different (95% CI: 0.41–37.6; P = 0.24) due to the low number of deaths overall during the follow‐up period (Figure 2 ).
Figure 2.
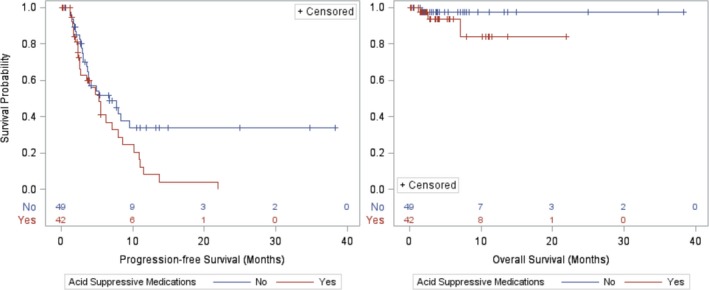
Kaplan–Meier Curves of progression‐free survival and overall survival.
Fewer hypertension adverse events were seen in the ASM cohort
Grade 3 or 4 adverse events were reported in 13 of 42 patients (31%) in the PAZ + ASM group and in 22 of 49 patients (45%) in the PAZ only group (P = 0.20; Table 2 ). Grade 3 or 4 hypertension occurred in 19% of patients in the PAZ + ASM group vs. 37% in the PAZ only group, a marginally significant difference (P = 0.069). Other grade 3 or 4 toxicities, such as increased bilirubin, increased alanine aminotransferase, increased aspartate aminotransferase, fatigue, nausea, anorexia, and diarrhea, were low overall and did not differ significantly between groups.
Table 2.
Grade 3 or 4 adverse events
| Toxicity | Pazopanib without ASM (n = 49) n (%) | Pazopanib with ASM (n = 42) n (%) | P value |
|---|---|---|---|
| Blood pressure | 18 (37) | 8 (19) | 0.069 |
| Blood bilirubin | 0 | 0 | NA |
| Alanine aminotransferase | 5 (10) | 3 (7) | 0.72 |
| Aspartate aminotransferase | 5 (10) | 2 (5) | 0.44 |
| Fatigue | 1 (2) | 2 (5) | 0.59 |
| Nausea | 0 | 1 (2) | 0.46 |
| Anorexia | 2 (4) | 1 (2) | 1.0 |
| Diarrhea | 0 | 1 (2) | 0.46 |
| Any grade 3 or 4 toxicity | 22 (45) | 13 (31) | 0.20 |
ASM, acid‐suppressive medication.
Discussion
In this retrospective cohort study involving patients with STS who received PAZ, concomitant ASM with PAZ resulted in significantly decreased efficacy, as evidenced by shorter PFS compared with no ASM (5.3 months vs. 6.7 months) despite efforts to minimize and/or separate medication administration. OS did not differ between groups, and importantly, patients in this study had similar PFS and OS compared with the patients receiving PAZ in the PALETTE trial (PFS of 4.6 months and OS of 12.5 months), which led to PAZ approval by the FDA in 2012.3
We hypothesized that adverse events would be lower in the concomitant PAZ + ASM group due to decreased drug absorption. Accordingly, there was a marginally significant difference, with less frequent grade 3 or 4 hypertension in the concomitant PAZ + ASM group compared with the group without ASM (19% vs. 37%). Unfortunately, due to the study's sample size, there was not enough power to confirm this finding. The study was underpowered to detect overall differences in grade 3 or 4 toxicity (45% no ASM vs. 31% concomitant ASM).
In patients with STS in particular, Mir et al.11 reported in abstract form that of 333 European patients with STS retrospectively analyzed from the European Organization for Research and Treatment of Cancer (EORTC) 62043/62072 trials, 117 patients received concomitant ASMs with PAZ, and median PFS was shorter in the concomitant acid suppressive group vs. the no ASM group (2.8 months vs. 4.6 months; P = 0.008). Our study supports these findings, but is also the first to describe the real‐world use of PAZ with concomitant ASM and counseling as to appropriate timing in a non‐European cohort.
Other studies defined a threshold for duration of clinically meaningful overlap between ASMs and TKIs ranging from 20−80%.9, 11 In this study, we did not set a predefined threshold for percent overlap and instead included any duration of concomitant ASM use. The mean percent overlap in the combination ASM and PAZ group was high overall, with 88% overlap in the PPI‐containing group and 85% in the H2RA group. Our mean overlap of ASM and PAZ was higher compared with other studies, which may have contributed to the decreased PFS seen in the concomitant ASM group.
Although reduced PFS was seen in the concomitant ASM group, the use of an ASM in patients with STS may be unavoidable depending on the indication. Because the pharmacokinetic drug interaction cannot be fully eliminated, for patients who absolutely require an ASM, some potential strategies have been evaluated to attempt to minimize this interaction. One strategy has been to separate administration times by at least several hours between TKIs and ASMs.12 Specifically, PAZ has been recommended to be taken at least 2 hours before or 10 hours after a dose of an H2RA.12 Our data demonstrate that even separation of ASMs compromised the efficacy of the PAZ.
Alternative strategies that were not implemented at our institution include that of changing twice‐daily PPI regimens to once‐daily regimens, scheduling the PPI to be taken at the same time or 2 hours after the TKI,7 and adding acidic beverages to overcome this pharmacokinetic drug interaction. Van Leeuwen et al.13 reported that in patients with non‐small cell lung cancer who took erlotinib with the beverage cola while on esomeprazole, administering erlotinib with cola led to increased erlotinib bioavailability. Coadministration of other acidic beverages with TKIs, including PAZ, may have similar effects on increasing absorption; however, this has not yet been proven in other studies. Ultimately, none of these strategies have been proven to be superior over others to overcome the drug–drug interaction between TKIs and ASMs.
Limitations of this study include its retrospective, single‐center design, small sample size, and incomplete toxicity reporting. Potential confounding factors that could have affected survival outcomes were more conservative starting PAZ doses than recommended in the package insert, discrepancies in PAZ and ASM start dates, other drug–drug interactions with PAZ, and lack of methodology for determining patient adherence.4
As more TKIs are approved and utilized for various oncologic indications, a standardized, evidence‐based approach to managing drug–drug interactions is needed to ensure optimal medication efficacy.
In conclusion, the results of this study further suggest that any ASM usage should be avoided in patients with STS receiving concomitant PAZ therapy, as efficacy is likely to be compromised, despite efforts to minimize the interaction. Larger prospective studies, including evaluating co‐administration of PAZ with an acidic beverage or comparing different administration schedules of ASMs, are needed to further elucidate alternative strategies to minimize the effects of concomitant ASM use with PAZ in clinical practice.
Funding
No funding was received for this work.
Conflicts of Interest
J.L.C. has served on the speaker's bureau for Novartis. All other authors declared no competing interests for this work.
Author Contributions
S.G.P., S.E.H., C.S.L., J.L.C., E.M.M., and K.P. wrote the manuscript. S.G.P., S.E.H., C.S.L., and J.L.C. designed the research. S.G.P., S.E.H., C.S.L., and J.L.C. performed the research. S.G.P., S.E.H., C.S.L., J.L.C., E.M.M., and K.P. analyzed the data.
Supporting information
Table S1. Acid‐suppressive medication use.
References
- 1. National Comprehensive Cancer Network (NCCN) Guidelines . Soft Tissue Sarcoma, version 2.2017. <https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf>.
- 2. Pang, A. , Carbini, M. & Maki, R.G. Contemporary therapy for advanced soft‐tissue sarcomas in adults: a review. JAMA Oncol. 2, 941–947 (2016). [DOI] [PubMed] [Google Scholar]
- 3. van der Graaf, W.T. et al Pazopanib for metastatic soft‐tissue sarcoma (PALETTE): a randomized, double‐blind, placebo‐controlled phase 3 trial. Lancet 379, 1879–1886 (2012). [DOI] [PubMed] [Google Scholar]
- 4. Votrient® [package insert]. GlaxoSmithKline, Research Triangle Park, NC, 2016.
- 5. Tan, A.R. et al Effects of ketoconazole and esomeprazole on the pharmacokinetics of pazopanib in patients with solid tumors. Cancer Chemother. Pharmacol. 71, 1635–1643 (2013). [DOI] [PubMed] [Google Scholar]
- 6. Numico, G. , Fusco, V. , Franco, P. & Roila, F. Proton pump inhibitors in cancer patients: how useful they are? A review of the most common indications for their use. Crit. Rev. Oncol. Hematol. 111, 144–151 (2017). [DOI] [PubMed] [Google Scholar]
- 7. van Leeuwen, R.W.F. et al Tyrosine kinase inhibitors and proton pump inhibitors: an evaluation of treatment options. Clin. Pharmacokinet. 56, 683–688 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lalani, A.A. , Mckay, R.R. , Lin, X. , Simantov, R. , Kaymakcalan, M.D. & Choueiri, T.K. Proton pump inhibitors and survival outcomes in patients with metastatic renal cell carcinoma. Clin. Genitourin. Cancer. 15, 724–732 (2017). [DOI] [PubMed] [Google Scholar]
- 9. Chu, M.P. et al Gastric acid suppression is associated with decreased erlotinib efficacy in non‐small‐cell lung cancer. Clin. Lung Cancer 16, 33–39 (2015). [DOI] [PubMed] [Google Scholar]
- 10. National Cancer Institute . Common Terminology Criteria for Adverse Events v4.0 NCI, NIH, DHHS. NIH publication #09‐7473. (2009).
- 11. Mir, O. et al Impact on outcome of concomitant administration of gastric acid suppression therapy and pazopanib in soft tissue sarcoma patients treated within EORTC 62043/62072 trials. Poster presented at: The Connective Tissue Oncology Society Annual Meeting; November 9, 2016; Lisbon, Portugal.
- 12. van Leeuwen, R.W. , van Gelder, T. , Mathijssen, R.H. & Jansman, F.G. Drug‐drug interactions with tyrosine‐kinase inhibitors: a clinical perspective. Lancet Oncol. 15, e315–e326 (2014). [DOI] [PubMed] [Google Scholar]
- 13. van Leeuwen, R.W. et al Influence of the acidic beverage cola on the absorption of erlotinib in patients with non‐small‐cell lung cancer. J. Clin. Oncol. 34, 1309–1314 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Acid‐suppressive medication use.


