Abstract
Tremelimumab, an anti‐cytotoxic T‐lymphocyte antigen‐4 monoclonal antibody that enhances T‐cell activation, was evaluated in a randomized, double‐blind, placebo‐controlled, phase IIb study (NCT01843374) in patients with unresectable malignant mesothelioma. The study demonstrated no clinically meaningful differences in overall survival (OS). The objective of this analysis was to evaluate the relationship of exposure with OS. A population pharmacokinetic (PK) model adequately described the PK data. Three factors (sex, C‐reactive protein, and baseline tumor size) were identified as statistically significant PK predictors (P < 0.05 on clearance). A positive association between exposure and OS was observed. However, an association between key baseline factors with OS (regardless of treatment) and imbalances in prognostic factors favoring patients with higher exposure (upper vs. lower PK quartile) was seen. Taken together, these results suggest that the exposure OS relationship observed for tremelimumab in mesothelioma is likely spurious rather than a true association of exposure with efficacy.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Tremelimumab is a human monoclonal antibody that binds to CTLA‐4 and blocks its interaction with its ligands. Tremelimumab single‐agent was investigated in melanoma and in unresectable pleural and peritoneal malignant mesothelioma but did not provide sufficient efficacy to warrant approval. However, tremelimumab is currently investigated in a number of malignancies in combination with another checkpoint monoclonal antibody inhibitor, durvalumab, which targets programmed cell death ligand 1.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ This work presents a post hoc analysis evaluating the relationship of tremelimumab exposure with overall survival ( OS). In addition, an analysis to further evaluate the potential association among disease factors, exposure, and OS was performed.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ The OS exposure–response relationship for tremelimumab has not yet been described in malignant mesothelioma. This study proposes a pragmatic but systematic approach to data analysis, coupled with deductive reasoning, to decipher the OS exposure response through integrated analysis of trial data, including risk factors.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCES?
☑ A simplistic empirical approach to exposure–response analysis, when complemented by a review of baseline factors associated with OS, population pharmacokinetic analysis and review of baseline factors across exposure groups, can delineate the multivariate factors underlying an apparent trend in OS exposure response. Our pragmatic analysis provides an alternative approach to case control, especially in oncology, where generally no adequately powered dose‐response‐finding trials with comparator arm can be conducted.
Tremelimumab is an anti‐cytotoxic T‐lymphocyte‐associated protein‐4 (CTLA‐4) human monoclonal antibody investigated as a cancer immunotherapeutic agent. Tremelimumab binds specifically to CTLA‐4, a cell receptor primarily expressed on the surface of activated T lymphocytes. Binding of CTLA‐4 to its target ligands (B7.1 and B7.2) provides a negative regulatory signal, which limits T‐cell activation. Tremelimumab antagonizes binding of CTLA‐4 to B7 ligands and enhances human T‐cell activation as demonstrated by increased cytokine (interleukin‐2 and gamma interferon) production in vitro in whole blood or peripheral blood mononuclear cell cultures.1 In addition, blockade of B7 binding to CTLA‐4 by anti‐CTLA‐4 antibodies results in markedly enhanced T‐cell activation and antitumor activity in animal models, including killing of established murine solid tumors and induction of protective antitumor immunity. Therefore, it is expected that treatment with tremelimumab will lead to activation of the human immune system, increasing antitumor immunity in patients with solid tumors.
Although phase II and phase III studies of tremelimumab in metastatic melanoma did not meet the primary end points of response rate and overall survival (OS), respectively, the data suggested clinical activity.2, 3, 4 In a large phase III randomized study (NCT00257205), tremelimumab 15 mg/kg administered by i.v. infusion every 12 weeks (q12w) failed to demonstrate survival benefit compared with first‐line standard of care treatment with a reported median OS of 12.6 months for tremelimumab vs. 10.7 months for chemotherapy (hazard ratio = 0.88, P = 0.1274). Opportunistically, mesothelioma was considered to have an unmet medical need that could potentially be addressed by anti‐CTLA‐4 therapy. This was motivated by a promising sign of efficacy from two phase II investigator‐sponsored studies evaluating tremelimumab in second‐line unresectable metastatic malignant mesothelioma.5, 6 Mesothelioma is a lethal disease that has one of the worse prognoses among solid tumors, with < 5% of patients surviving 5 years. The estimated median survival time in untreated cases ranged from 6−9 months from the date of diagnosis.7 For advanced disease, cisplatin and pemetrexed combination therapy is standard of care for first‐line treatment for pleural mesothelioma, with an approximate 3‐month increase in median OS in patients treated with pemetrexed and cisplatin vs. cisplatin alone (12.1 months vs. 9.3 months, respectively).8 There is no approved treatment for peritoneal mesothelioma; however, pemetrexed and cisplatin are commonly used as first‐line treatment. In second‐line treatment, no therapies have shown survival benefits,9 and no agents are currently approved for pleural or peritoneal mesothelioma after progression from first‐line treatment.
Preliminary data from study NCT016490245 that evaluated single‐arm tremelimumab at 15 mg/kg i.v. q12w in patients with malignant mesothelioma showed signs of efficacy. Although the study missed its primary end point of response rate in a small number of patients (n = 29), a promising median time of OS of 10.7 months was observed (95% confidence interval (CI): 0–21.9 months). A second study (NCT01655888) of similar design (open‐label, single‐arm) was later conducted by the same investigator6 with a higher dose intensity (10 mg/kg q4w for up to week 24 followed by q12w until disease progression or unacceptable toxicity). The higher dose intensity was motivated by a retrospective exposure–response (E‐R) analysis of data from melanoma failed trials suggesting that higher exposure in patients was associated with higher response rate,10 coupled with in vitro data showing an interleukin‐2 release enhancement proportional to tremelimumab concentrations from whole blood samples of nine cancer donors.11 Study NCT01655888 enrolled 29 patients and showed similar efficacy, with a median survival time of 11.3 months (95% CI: 3.4–19.2 months). Although suffering from the same limitations as the previous study (low number of patients, lacking a control group, and conducted at a single site), these results corroborated the former outcomes without dismissing or confirming the hypothesis that higher exposure translated to higher efficacy. A subgroup analysis of NCT01655888 further substantiated the efficacy potential of tremelimumab because seven patients with biphasic or sarcomatoid histology of mesothelioma had a median survival time of 15.8 months (95% CI: 13.2–18.4 months). However, the low sample size did not allow the appraisal of the clinical significance of this observation. A decision was then made to investigate tremelimumab as single‐agent in the DETERMINE trial (NCT01843374), a phase IIb, multicenter, randomized, double‐blind, placebo‐controlled study in patients with unresectable pleural or peritoneal malignant mesothelioma following one or two previous systemic treatments, including a platinum‐based regimen. Patients were randomized (2:1) to receive either tremelimumab (n = 382) at 10 mg/kg q4w (seven doses) followed by q12w i.v. or placebo (n = 189). However, the study demonstrated no clinically meaningful differences in OS12, 13 and motivated an in‐depth analysis, including tremelimumab pharmacokinetics (PK) and its relationship with efficacy. Currently, tremelimumab is investigated in various indications in combination with another checkpoint monoclonal antibody inhibitor, durvalumab, that targets programmed cell death ligand 1.
Tremelimumab PK properties were previously reported based on a population PK modeling approach combining data from phase I, II, and III studies (n = 654) in subjects with metastatic melanoma using nonlinear mixed‐effects modeling.10 A two‐compartment linear PK model, consistent with a natural immunoglobulin G2 molecule, adequately described the plasma concentrations of tremelimumab following various dosing regimens. The population estimates for clearance (CL) and central volume of distribution (V1) were 0.26 L/day and 3.97 L, respectively, with modest interindividual variability (31.8% and 20.4%, respectively). CL was higher in men, subjects with higher values of creatinine clearance and endogenous immunoglobulin G, and subjects with relatively poor baseline prognostic factors: Eastern Cooperative Oncology Group (ECOG) status (higher CL for ECOG > 0), lactate dehydrogenase (LDH) levels (higher LDH resulted in higher CL), and C‐reactive protein (CRP) levels (higher CRP resulted in higher CL). Central volume of distribution was higher in men and subjects with higher body weight. No dose adjustment was needed based on the magnitude of the change in PK. Similar to other monoclonal antibodies without target‐mediated drug disposition, tremelimumab is likely to be cleared from circulation by endothelial cell uptake and proteolysis.14 Hence, no impact of renal/hepatic functions is expected on tremelimumab elimination.
The same model structure fitted equally well the PK data from the two investigator‐initiated studies (NCT01649024/NCT01655888) in 40 patients with malignant mesothelioma15 (18 patients of the pooled analysis did not have PK data), confirming similar PK properties of tremelimumab across the two indications (CL and V1 were 0.2 L/day and 3.5 L, respectively, with body weight and ECOG performance status impacting exposure levels).
The primary objective of this analysis was to provide a systematic evaluation of the relationship of PK exposure with OS of the DETERMINE trial to demonstrate that tremelimumab dosing regimen fully tested the mechanism of action in patients with mesothelioma. Specifically, we aimed to derive individual PK metrics by PK modeling to evaluate potential relationship between exposure and OS. Subsequently, to further test any observed E‐R relationship, we assessed any potential factors other than exposure on OS and investigated any imbalance of disease status in each exposure subset. The statistical significance of potential confounders found in E‐R investigation was then tested by population PK covariate analysis.
Methods
The methodology applied in this work was performed in a three‐step process that involved empirical evaluation through graphical analysis of OS data and the use of population PK analysis to confirm determinants of tremelimumab PK. The first step consisted of obtaining a reliable representation of steady‐state exposure levels for each patient who received at least one dose of tremelimumab in the DETERMINE trial. The intent was to obtain a standardized PK metric to evaluate the degree of association of PK with OS data from this trial. In order to circumvent the limitations of observed concentration data (data handling issues associated with missing data, PK data obtained prior to achieving steady state for some patients, measurement errors, and other sources of residual variability in exposure measurements), use of model‐derived PK exposure metrics, such as the area under the exposure time‐course curve at steady‐state (AUCss) was considered as a robust and unbiased approach. To this aim, a population PK model of tremelimumab was developed based on the DETERMINE trial observed PK data and was used to predict steady‐state exposure metrics for each patient.
The second step of this analysis consisted of a graphical evaluation using a Kaplan–Meier (KM) plot of OS split by tremelimumab exposure levels. AUCss were ranked into quartiles and used to identify any potential underlying relationship between steady‐state PK exposure and OS data. An analysis was then performed using KM plots of OS split by baseline disease status in both the treatment group and the control group to evaluate any trend on the OS profile suggestive of potential prognostic or predictive factors. Given the presence of time‐dependent predictive factors (i.e., exposure) and the degree of collinearity between predictors, which are likely to confound interpretation, a multivariate Cox regression model has not been conducted.
The last step of the analysis consisted of evaluating a potential correlation between disease status and PK exposure in an attempt to identify any important factors that may explain the relationship found in step 2 of this analysis. This was performed by means of covariate analysis of the population PK model.
Step 1: Base population PK model development
Population PK of tremelimumab data was based on nonlinear mixed‐effects modeling methodology, implemented in the computer program NONMEM, version 7.3.16 Model development started with the base model (i.e., the best description of the data without considering the effect of covariates). The modeling details are provided in Supplementary Materials S1 .
Step 2: Exposure−efficacy (OS) analysis and confounding analysis
Once a base model had been established, individualized PK exposure metrics were estimated (specifically, AUCss and CL) for E‐R analysis of OS data. An exposure−OS relationship was evaluated by a KM plot of OS by quartiles of the AUCss distribution performed in R (version 3.3.1 or higher17).
A similar exploratory evaluation on OS data was done based on several risk factors measured at baseline. Instead of using exposure as a potential predictor of OS, as is typically done for E‐R analysis, the KM plot of OS was presented based on patients’ characteristics at baseline. A pool of 13 potential baseline confounders was defined a priori based on mechanistic plausibility, scientific interest, and prior knowledge. It consisted of the three stratification factors of the trial that are considered prognostic in mesothelioma (European Organisation for Research and Treatment of Cancer (EORTC) status, anatomic site, and line of therapy), as well as the additional factors of histology, ECOG performance status, race, sex, age, body weight, inflammatory status as measured by CRP and serum albumin, LDH levels, and baseline tumor burden. Categorical covariates were split based on subgroups defined in Maio et al.13 For continuous covariates, the median was used as a cutoff to dichotomize patients into high or low levels. Because the DETERMINE trial included a placebo comparator arm, the KM plot of OS was split by treatment arm (tremelimumab and placebo) for each of the 13 risk factors to evaluate their prognostic/predictive value. Risk factors for which a trend was visible on visual exploration of survival were then evaluated for potential imbalance in each AUCss exposure quartile.
Step 3: Covariate analysis to confirm potential PK predictors
After completion of base population PK model development, each of the 13 covariates (evaluated in step 2 as potential risk factors on OS) was tested for their explanatory power on PK model parameters by means of likelihood ratio statistical testing with a type‐I error of 5%. The effect of antidrug antibodies (ADAs) on tremelimumab exposure was also examined. The clinical or physiological relevance for each covariate effect was ultimately evaluated for its significance to establish the final population PK model. More details are provided in Supplementary Materials S1 .
Finally, a presentation of the multidimensional relationship among PK, disease factors, and OS was attempted using the iGraph package version 1.1.2 in R.17
Results
PK modeling
The PK analysis data set from the DETERMINE study included 376 patients with mesothelioma and 1,328 evaluable PK concentrations (six patients had no PK evaluable data). Tremelimumab PK (Table 1) was consistent with previous reports. A two‐compartment linear model adequately described the data. CL and V1 estimates from the final PK model were 310 mL/day and 3.85 L, with moderate variability of 38.0% and 32.5%, respectively, vs. 260 mL/day (31.8% between‐subject variability) and 3.97 L (20.4%) in melanoma.10 Fifteen (4%) of 377 patients with ADA evaluable data were ADA‐positive postbaseline in the treatment arm, which was comparable to the placebo arm (3.2%, 6 ADA‐positive postbaseline of 188 patients). The ADA incidence was consistent with previous melanoma studies (< 6%). ADA had no apparent effect on PK.
Table 1.
Final population PK model parameter estimates of tremelimumab from the DETERMINE trial
| Model parameter | Θ (Median) | RSE (Θ) (%) | BSV (Ω) (%) | RSE (Ω) (%) |
|---|---|---|---|---|
| CL (L/day) | 0.310 | 4.62 | 38.0 | 8.54 |
| V1 (L) | 3.85 | 1.91 | 32.5 | 16.5 |
| V2 (L) | 1.72 | 19.4 | 25.2 | 84.6 |
| Q (L/day) | 0.273 | 42.8 | ||
| CV% (proportional ε) | 37.7% | 4.92 |
BSV, between‐subject variability of parameter with random effect assumed normally‐distributed with mean 0 and variance Ω2; CL, clearance; CV%, coefficient of variation percentage; PK, pharmacokinetic; RSE, relative standard error obtained from $COVARIANCE step in NONMEM; V, volume of distribution.
Higher baseline tumor size, higher CRP levels, and men were associated with significantly lower tremelimumab exposure levels (P < 0.05; Table 2). These results were consistent with previous findings10 but were not found clinically relevant with respect to differences in exposure (< 30% effect on AUCss; Figure S1 ).
Table 2.
Population PK covariate analysis of tremelimumab from the DETERMINE trial
| Hierarchical model | Covariate‐PK relationship | Covariate‐PK relationship strength | OFV likelihood | Reference OFV | P value |
|---|---|---|---|---|---|
| 1. Base model | None | – | 10,428 | – | – |
| 2. Intermediate model 1 | CL % reduction for female patients vs. male | −0.19 |
10,414 (Δ = −14) |
1 | 0.00018 |
| 3. Intermediate model 2 | Serum albumin on CL | 0.00094 |
10,413 (Δ = −1) |
2 | 0.32 |
| 4. Intermediate model 3 | CRP effect on CL | 0.0034 |
10,370 (Δ = −44) |
2 | < 10−10 |
| 5. Intermediate model 4 | ECOG effect on CL | 0.019 |
10,368 (Δ = −1.4) |
4 | 0.24 |
| 6. Intermediate model 5 | EORTC effect on CL | 0.068 |
10,368 (Δ = −2.0) |
4 | 0.16 |
| 8. Intermediate model 6 | LDH effect on CL | −2.1 × 10−04 |
10,364 (Δ = −1.3) |
7 | 0.26 |
| 5. Intermediate model 7 | Histology (epithelioid) % increase on CL | 0.084 |
10,363 (Δ = −1.7) |
7 | 0.19 |
| 7. Final model | Baseline tumor size effect on CL | 0.00058 |
10,365 (Δ = −4.3) |
4 | 0.039 |
Continuous covariates were entered in the model assuming proportional linear effect of the covariate on PK from median cutoff (median is 31 g/L for albumin, 33 mg/L for CRP, and 97 mm for baseline tumor size). Sex, CRP, and baseline tumor size were significant PK predictors and explained 20% of the interindividual variability on CL, reducing coefficient of variation from 42% to 38%. ΔOFV was computed for nested models according to the order displayed in the “Reference OFV” column, where the reference OFV is taken as the previous model with the statistically significant covariate‐PK relationship included.
CL, clearance; CRP, C‐reactive protein; ECOG, Eastern Cooperative Oncology Group; EORTC, European Organisation for Research and Treatment of Cancer; LDH, lactate dehydrogenase; OFV, objective function value; PK, pharmacokinetic.
Given that the population model adequately described the PK of tremelimumab and that no covariate was found to be clinically relevant, the final PK model was equivalent to the base model (with no covariate) used to derive individual PK metrics (AUCss). The distribution of predicted AUCss values representative of individuals’ exposure level used for investigation of E‐R based on observed OS is displayed in Figure S2 .
Exposure−OS analysis
Similar OS data were observed for tremelimumab (median OS time = 7.72 months) compared with placebo (7.29 months) based on the intention‐to‐treat population (Figure 1 left panel). The hazard ratio from stratified Cox regression model (including the two stratification factors of EORTC status and line of therapy) was 0.92 (95% CI: 0.76−1.12, P = 0.408).12, 13
Figure 1.
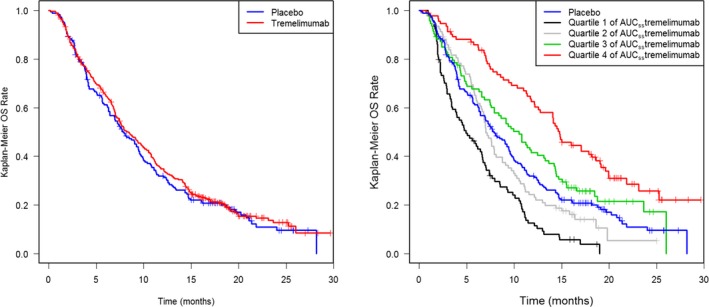
Left panel: Kaplan–Meier analysis of overall survival (OS) by treatment group (intention‐to‐treat population). Right panel: Kaplan–Meier analysis of OS by quartiles of exposure at area under the exposure time‐course curve at steady‐state (AUC ss) for the tremelimumab‐treated group with overlaid placebo group OS profile.
When assessed, based on tremelimumab PK exposure (AUCss), a monotonic relationship with OS was observed, with the all‐comers curves centered between the interquartile range (Q2 and Q3; Figure 1 right panel and Table 3). The median survival in the tremelimumab group for patients with the highest quartile of exposure was 14.9 months (95% CI: 12.5–18.6). A twofold increase in median AUCss exposure level between the most extreme exposure quartiles (Q1 and Q4) resulted in a threefold improvement in median OS time (Table 3). However, although increasing exposure seemed to yield a better OS profile, the lowest AUCss quartile (Q1) median survival was worse than placebo (4.93 vs. 7.29 months), with its 95% CI (3.86–6.68) not including the placebo point estimate; this suggests that some confounders are likely affecting the E‐R relationship. A sensitivity analysis based on observed peak plasma concentration (Cmax) after the first cycle of treatment led to a similar trend (Figure S3 ).
Table 3.
Estimated median overall survival time for 376 patients with PK (out of 382 in the tremelimumab arm), derived from a Kaplan‐Meier plot of OS split by quartile of AUCss distribution
| Subgroup | n | No. events | Median OS time (95% CI) | Median AUCss exposure (min‐max) |
|---|---|---|---|---|
| Quartile 1 of AUCss (low exposure) | 94 | 88 | 4.93 months (3.86–6.68) | 1629 mg/L.day (783–1942) |
| Quartile 2 of AUCss | 94 | 79 | 7.04 months (6.64–8.79) | 2118 mg/L.day (1945–2328) |
| Quartile 3 of AUCss | 94 | 72 | 10.5 months (7.89–13.3) | 2548 mg/L.day (2331–2852) |
| Quartile 4 of AUCss (high exposure) | 94 | 63 | 14.9 months (12.5–18.6) | 3404 mg/L.day (2860–19751) |
Potential confounders of the tremelimumab E‐R in OS of patients with mesothelioma were assessed by evaluating imbalance of patients’ characteristics in the extreme quartiles of exposure. Analysis of OS data based on baseline patient characteristics other than exposure revealed a number of factors as important, regardless of treatment. Higher OS was observed in patients with lower CRP levels, in patients with ECOG performance status = 0 compared with ECOG = 1, and in patients with EORTC low risk compared with EORTC high risk (Figure 2). Specifically, when assessing by performance status across treatment arms, OS data show ECOG = 0 patients survive longer than ECOG = 1 patients (median OS = 11–13 vs. 5–7 months) irrespective of treatment received. Review of baseline characteristics across AUC groups indicated that there were more ECOG = 0 patients in Q4 of AUCss (highest tremelimumab exposure) than Q1 (lowest exposure; Figure 3). Longer OS was also observed in patients with low‐risk (EORTC = 1) compared with high‐risk status (EORTC = 2), with a median OS by EORTC status across treatment groups of 10 vs. 6 months, respectively (Figure 2). Conversely, an imbalanced distribution of low‐risk/high‐risk EORTC patients was visible across the exposure quartiles, with more low risk (as assessed by EORTC) patients in Q4 (highest exposure) than Q1 (lowest exposure) (Figure 3). Similarly, data suggested that high tumor burden (tumor size at baseline ≥ median = 97 mm) was associated with shorter survival (median OS = 11‐9 vs. 7 months), although this is more visible in the tremelimumab treatment group (Figure 2). Patients with high tumor burden at baseline demonstrated lower PK exposure (Q1 of AUCss; Figure 3). Longer OS was observed in patients with lower inflammatory biomarker levels (CRP < median = 33 mg/L; i.e., ~ 11‐fold the upper limit of normal) compared with patients with higher inflammatory biomarker levels (Figure 2). Patients with high CRP were more common in the lower PK exposure group (Q1 of AUCss; Figure 3). Last, longer OS was observed in women than in men, albeit the tails of the OS KM plot converge at the later timepoints (Figure 2). This finding is aligned with a recent publication that found women had a 28% lower mortality rate than men in pleural mesothelioma.18 Men had higher CL than women (Q4 of CL) and, thus, had lower PK exposure (Q1 of AUCss; Figure 3).
Figure 2.
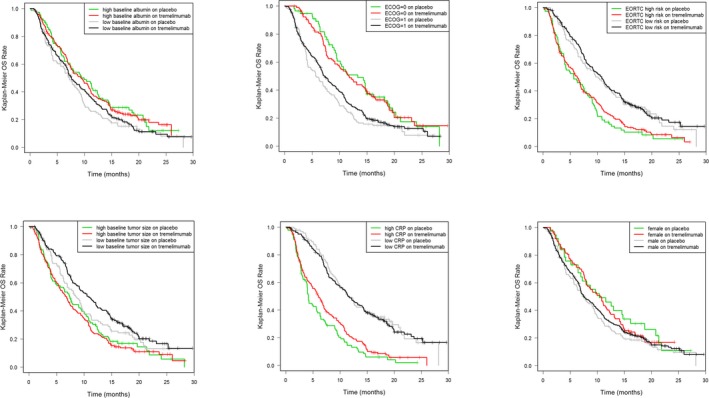
Kaplan–Meier analyses of overall survival (OS) split by baseline patient factors in each treatment arm. Kaplan–Meier plots of the key factors indicating differentiation in OS are presented for reference. All other Kaplan–Meier plots showed little difference (data on file). CRP, C‐reactive protein; ECOG, Eastern Cooperative Oncology Group ; EORTC, European Organisation for Research and Treatment of Cancer.
Figure 3.
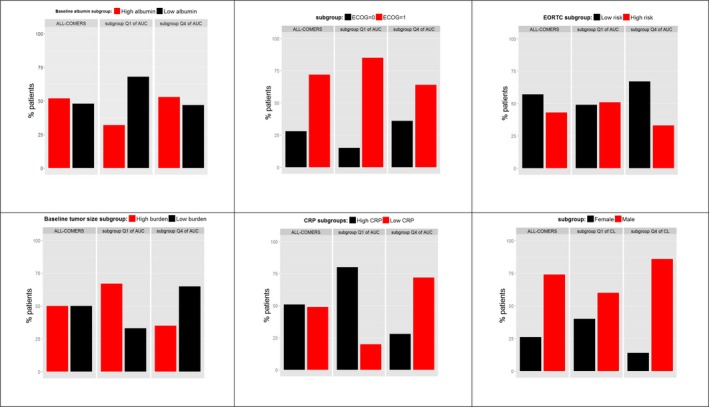
Proportion of patients with each baseline risk factor indicating differentiation in overall survival in the overall population (all‐comers) and in each extreme exposure quartile of area under the exposure time‐course curve at steady‐state (AUC ss) or clearance (CL; Q1 and Q4). Continuous covariates (serum albumin, C‐reactive protein (CRP), and tumor burden) were dichotomized into high and low levels based on their respective median cutoff. Tremelimumab predicted exposure at steady‐state (AUC ss) was used for all comparison apart for SEX for which CL was used instead to prevent body weight confounding due to the tremelimumab weight‐based dosing scheme (AUC ss = dose/CL). Q1, lowest quartile; Q4, highest quartile. ECOG, Eastern Cooperative Oncology Group; EORTC, European Organisation for Research and Treatment of Cancer.
Based on these findings, a map of risk factors identified for OS and of the covariates impacting PK was built (Figure 4). This map was adapted based on the original mapping of confounding factors by Skelly et al.19
Figure 4.
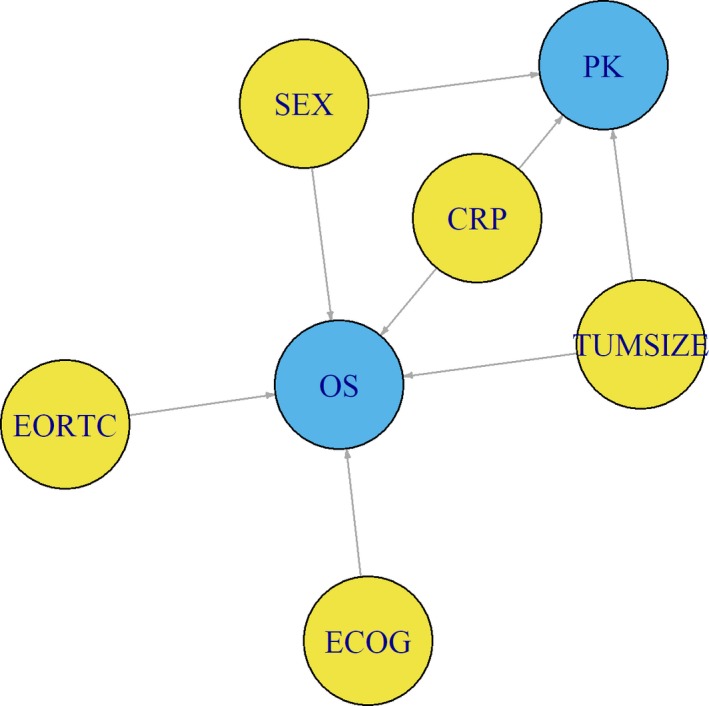
Deductive map of the putative relationships among overall survival (OS), pharmacokinetics (PK) of tremelimumab, and patients’ baseline characteristics in second‐line unresectable malignant mesothelioma. The key variables (OS and PK) for exposure–response (E‐R) analysis are coded in blue, whereas variables impacting OS and/or PK are coded in yellow. Single direction arrow (→) represents a putative causal path, based on either clinical judgment or observed correlation between risk factors and key variables. Correlations among risk factors were ignored for simplicity. Sex (SEX), tumor burden (TUMSIZE), and C‐reactive protein (CRP) appear as confounders of tremelimumab E‐R analysis, because these factors simultaneously affect both the dependent and independent variables (OS and PK, respectively), thus preventing the direct application of standard approaches to E‐R analysis. These relationships have variable strength, with the longer length of the line linkers of TUMSIZE and SEX with OS representing weaker link than the relationship of CRP with OS as well as the positioning of variables in the map; CRP relationship with PK and OS is further reinforced by placing it in the middle of the diagram, to reflect its relative larger effect on PK and OS compared with TUMSIZE and SEX. ECOG, Eastern Cooperative Oncology Group; EORTC, European Organisation for Research and Treatment of Cancer.
Discussion
The understanding of confounding factors in E‐R analysis is evolving in oncology.20, 21 This is primarily motivated by two factors: because of trial design issues specific to oncology, PK information is usually limited and encompassing a narrow dose–response range; and, especially for biotherapeutics, cancer progression and patient health status may affect PK.22, 23 This exacerbates the issue of having to decide whether observed differences in OS between standard of care and experimental treatment are due to imbalances in confounding risk factors at baseline or differences in drug exposure. When a single‐dose experimental arm fails to demonstrate superiority over standard of care (as in the tremelimumab confirmatory trial in melanoma4), post hoc analysis of E‐R10 is generally conducted to delineate whether a higher dose could have resulted in survival benefit, provided that the maximum tolerated dose was not yet tested. When the single‐dose experimental arm shows a clinically relevant benefit over standard of care, as in the case of trastuzumab in gastric cancer24 or trastuzumab‐DM1 in breast cancer,25 a post hoc analysis of E‐R26, 27 was also conducted to decide whether nonresponders could have benefited from a higher dose. In both cases, this can result in further confirmatory trials with a higher dose (as in tremelimumab in mesothelioma13) or in postapproval commitment trials, as exemplified by the HELOISE trial evaluating higher dose of trastuzumab28 and by the TH3RESA trial for trastuzumab‐DM1.29 For both tremelimumab and trastuzumab, results demonstrated no additional benefit of higher doses and, thus, put into question the conclusions drawn from such E‐R post hoc analyses.
Case‐control E‐R analysis has been suggested26, 27 to ascertain whether baseline risk factors known to affect OS and associated with drug exposure have a role in changes in OS. Case‐matched control comparison allows for the definition of matched subgroups that minimize differences in patient characteristics, thus resulting in balanced distributions of measured risk factors that allow the role of differences in exposure to be ascertained. However, this methodology may lack robustness, as demonstrated by the negative outcome of the HELOISE trial that was predicated from such an analysis. One possible reason is that it does not account for the dynamic nature of the intricate interactions among risk factors, survival, and exposure, which can change relatively rapidly with time, especially when PK is reaching steady state (the most relevant exposure metric when conducting E‐R). As a workaround, Wang et al.20 suggests to use an early metric of exposure (e.g., AUC at the end of the first cycle). The reasoning behind this is that early assessment of PK metrics may be seen as less affected by longitudinal changes in therapeutic antibody clearance over time driven by disease status.22, 23 However, the effect of disease status on PK at baseline is not considered, nor the change of exposure within individuals over time; therefore, more sophisticated approaches that account for longitudinal changes in both disease markers and PK are preferable.20
The E‐R post hoc analysis of OS data from the DETERMINE study suggested a trend in patients exposed to tremelimumab, with higher exposure levels resulting in longer survival. Specifically, this translated into the highest quartile of the AUCss group with a reasonable sample size (n = 94) showing a large difference of ~ 8 months in median OS over the all‐comers control arm (n = 189). Such effect size could be considered a breakthrough therapy for unresectable malignant mesothelioma, a disease with a dismal outcome and no approved treatment option beyond first‐line therapy. Conversely, the lowest quartile of the AUCss subgroup did worse than the all‐comers control arm (4.9 vs. 7.3 months median survival). This raised suspicion about a confounding bias induced by the E‐R categorization because no biologically plausible hypothesis could be proposed for why low tremelimumab exposure would cause a worse survival outcome than placebo. A sensitivity analysis using an early metric of exposure found similar E‐R as observed when using steady‐state PK. Instead of conducting a case‐control E‐R analysis that would have further reduced the sample size, potential confounding factors were initially explored to assess their impact. Through graphical evaluation of OS using KM plots, we identified five potential risk factors impacting OS: sex, ECOG performance status, EORTC prognostic status, inflammatory status (CRP), and tumor burden at baseline. Based on the biology of malignant tumors, these factors are expected to relate to OS regardless of tremelimumab exposure, and any imbalance in these baseline factors when grouping by exposure status may result in a confounding effect, making the E‐R only “apparent.” Interestingly, in the DETERMINE trial, patients in the highest exposure quartile (Q4 AUCss or Q1 CL) exhibited more favorable prognostic factors (low CRP, female, low tumor burden, ECOG = 0, and low‐risk EORTC) compared with patients in the lowest exposure quartile (Q1 AUCss or Q4 CL). Likewise, more patients with poor prognostic factors (high CRP, male, high tumor burden, ECOG = 1, and high‐risk EORTC) were belonging to the lowest exposure quartile than to the highest exposure quartile.
Subsequently, we used nonlinear mixed‐effects modeling of the DETERMINE trial of tremelimumab longitudinal PK data to decipher whether any of these five baseline disease factors could also affect tremelimumab exposure. Conceptually, if an interaction between PK and a known risk factor of OS was identified, this could translate into an indirect relationship between PK and OS, the resultant being an apparent trend of E‐R when this is explored in a simplified framework, such as KM plots. Our analysis found that at least three baseline factors (sex, CRP, and baseline tumor size) were statistically significant predictors of tremelimumab PK (P < 0.05 for CL), indicating a multidimensional confounding effect. Thus, the observed apparent exposure−OS relationship of tremelimumab is likely due to the imbalance in key baseline factors across the tremelimumab PK groups and the association of baseline prognostic factors for OS with PK (CL), rather than a true association of exposure with efficacy.
In conclusion, this analysis supports that patients with higher tremelimumab exposure did not derive additional benefit from this treatment after chemotherapy, despite signs of biological activity of anti‐CTLA‐4 therapy as judged by the higher incidence of immune‐mediated adverse events (e.g., colitis) observed in tremelimumab‐treated patients.13 In light of the confounding risk factors affecting OS and the correlation of some risk factors with PK, we conclude that the apparent E‐R relationship observed for tremelimumab in mesothelioma is spurious. In this respect, the standard assumption used in E‐R analysis (i.e., considering PK as an independent predictor variable of the dependent outcome variable OS) is invalidated by the imbalance of risk factors across exposure groups and relationship of risk factors with both PK and OS. The deductive map shown in Figure 4 provides a simplistic view to cogently distinguish apparent proximate cause (PK) from root causes (disease risk factors) that govern mortality in mesothelioma. This implies that careful consideration should be given not to over‐interpret empirical E‐R results, and complementary analyses, such as dose–response and mixed‐effects modeling, should be relied upon to prevent misleading conclusions.
Funding
This analysis was funded by MedImmune.
Conflict of Interest
P.B., L.R., N.L., Paolo Vicini and R.N. are employees of MedImmune, a wholly owned subsidiary of AstraZeneca. P.S. and M. Taboada are employees of AstraZeneca. M. Tatipalli and P.V. are former employees of MedImmune, and K.H. is a former employee of AstraZeneca.
Author Contributions
P.B., L.R., P.V., and R.N. wrote the article. P.B., L.R., P.V., and R.N. designed the research. P.B., L.R., N.L., P.S., P.V., M. Tatipalli, M. Taboada, K.H., and R.N. performed the research. P.B. and P.V. analyzed the data. N.L. contributed new reagents/analytical tools.
Supporting information
Figure S1. Effect of baseline covariates on exposure parameter AUCss.
Figure S2. Distribution of tremelimumab predicted exposure at steady‐state (AUCss) in all‐comers and across each quartile (Q1 = lowest exposure, Q3‐Q2 = interquartile range, and Q4 = highest exposure).
Figure S3. Kaplan–Meier sensitivity analysis of OS by quartiles of observed exposure levels after the end of infusion of the first dose of tremelimumab (Cmax,1) for the tremelimumab‐treated group.
Supplementary Material S1. Methods.
Acknowledgments
The contents of this paper were partially presented at the AACR Annual Meeting 2017, held on April 1–5, 2017, (Paul Baverel, Lorin Roskos, Manasa Tatipalli, Nancy Lee, Paul Stockman, Maria Taboada, Paolo Vicini, Kevin Horgan, Rajesh Narwal) and published in abstract form in the conference proceedings in Cancer Research 77 (13 Supplement), 5046.
References
- 1. Tarhini, A.A. & Kirkwood, J.M. Tremelimumab (CP‐675,206): a fully human anticytotoxic T lymphocyte‐associated antigen 4 monoclonal antibody for treatment of patients with advanced cancers. Expert Opin. Biol. Ther. 8, 1583–1593 (2008). [DOI] [PubMed] [Google Scholar]
- 2. Ribas, A. et al Antitumor activity in melanoma and anti‐self responses in a phase I trial with the anti‐cytotoxic T lymphocyte‐associated antigen 4 monoclonal antibody CP‐675,206. J. Clin. Oncol. 23, 8968–8977 (2005). [DOI] [PubMed] [Google Scholar]
- 3. Kirkwood, J.M. et al Phase II trial of tremelimumab (CP‐675,206) in patients with advanced refractory or relapsed melanoma. Clin. Cancer Res. 16, 1042–1048 (2010). [DOI] [PubMed] [Google Scholar]
- 4. Ribas, A. et al Phase III randomized clinical trial comparing tremelimumab with standard‐of‐care chemotherapy in patients with advanced melanoma. J. Clin. Oncol. 31, 616–622 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Calabrò, L. et al Tremelimumab for patients with chemotherapy‐resistant advanced malignant mesothelioma: an open‐label, single‐arm, phase 2 trial. Lancet Oncol. 14, 1104–1111 (2013). [DOI] [PubMed] [Google Scholar]
- 6. Calabrò, L. et al Efficacy and safety of an intensified schedule of tremelimumab for chemotherapy‐resistant malignant mesothelioma: an open‐label, single‐arm, phase 2 study. Lancet Respir. Med. 3, 301–309 (2015). [DOI] [PubMed] [Google Scholar]
- 7. Edwards, J.G. , Abrams, K.R. , Leverment, J.N. , Spyt, T.J. , Waller, D.A. & O'Byrne, K.J. Prognostic factors for malignant mesothelioma in 142 patients: validation of CALGB and EORTC prognostic scoring systems. Thorax 55, 731–735 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vogelzang, N.J. et al Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J. Clin. Oncol. 21, 2636–2644 (2003). [DOI] [PubMed] [Google Scholar]
- 9. Ceresoli, G.L. et al Phase II study of pemetrexed and carboplatin plus bevacizumab as first‐line therapy in malignant pleural mesothelioma. Br. J. Cancer 109, 552–558 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang, E. , Kang, D. , Bae, K.‐S. , Marshall, M.A. , Pavlov, D. & Parivar, K. Population pharmacokinetic and pharmacodynamic analysis of tremelimumab in patients with metastatic melanoma. J. Clin. Pharmacol. 54, 1108–1116 (2014). [DOI] [PubMed] [Google Scholar]
- 11. Narwal, R. et al Tremelimumab, a fully human anti‐CTLA‐4 monoclonal antibody, optimal dosing regimen for patients with unresectable malignant mesothelioma (MM). ASCO. (2015) Abstract 3042. [Google Scholar]
- 12. Kindler, H.L. , Scherpereel, A. , Calabrò, L. , Aerts, J. , Cedres Perez, S. & Bearz, A. Tremelimumab as second‐ or third‐line treatment of unresectable malignant mesothelioma (MM): results from the global, double‐blind, placebo‐controlled DETERMINE study. ASCO. (2016) Abstract 8502. [Google Scholar]
- 13. Maio, M. et al Tremelimumab as second‐line or third‐line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, international, randomised, double‐blind, placebo‐controlled phase 2b trial. Lancet Oncol. 18, 1261–1273 (2017). [DOI] [PubMed] [Google Scholar]
- 14. Dirks, N.L. & Meibohm, B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin. Pharmacokinet. 49, 633–659 (2010). [DOI] [PubMed] [Google Scholar]
- 15. Calabro, L et al Pharmacokinetics of tremelimumab, a fully human anti‐CTLA‐4 monoclonal antibody, in subjects with unresectable malignant mesothelioma. AACR. 2014, Abstract 228. [Google Scholar]
- 16. Beal, S.L. , Sheiner, L.B. , Boeckmann, A.J. & Bauer, R.J. NONMEM Users Guides (Icon Development Solutions, Ellicott City, MD, 1989). [Google Scholar]
- 17. R Development Core Team . R: A Language and Environment for Statistical Computing, Vol. 3.3.1. (R Foundation for Statistical Computing, Vienna, Austria, 2013). [Google Scholar]
- 18. Shavelle, R. , Vavra‐Musser, K. , Lee, J. & Brooks, J. Life expectancy in pleural and peritoneal mesothelioma. Lung Cancer Int. 2017;(2):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Skelly, A. , Dettori, J. & Brodt, E. Assessing bias: the importance of considering confounding. Evid. Based Spine Care J. 3, 9–12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang, Y. , Booth, B. , Rahman, A. , Kim, G. , Huang, S.M. & Zineh, I. Toward greater insights on pharmacokinetics and exposure–response relationships for therapeutic biologics in oncology drug development. Clin. Pharmacol. Ther. 101, 582–584 (2017). [DOI] [PubMed] [Google Scholar]
- 21. Ryman, J.T. & Meibohm, B. Pharmacokinetics of monoclonal antibodies. CPT Pharmacometrics Syst. Pharmacol. 6, 576–588 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baverel, P. et al Population pharmacokinetics of durvalumab in cancer patients and association with longitudinal biomarkers of disease status. Clin. Pharmacol. Ther. 103, 631–642 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu, C. et al Association of time‐varying clearance of nivolumab with disease dynamics and its implications on exposure response analysis. Clin. Pharmacol. Ther. 101, 657–666 (2017). [DOI] [PubMed] [Google Scholar]
- 24. Bang, Y.J. et al Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2‐positive advanced gastric or gastro‐oesophageal junction cancer (ToGA): a phase 3, open‐label, randomised controlled trial. Lancet 376, 687–697 (2010). [DOI] [PubMed] [Google Scholar]
- 25. Verma, S. et al Trastuzumab emtansine for HER2‐positive advanced breast cancer. N. Engl. J. Med. 367, 1783–1791 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang, J. et al The combination of exposure‐response and case‐control analyses in regulatory decision making. J. Clin. Pharmacol. 53, 160–166 (2013). [DOI] [PubMed] [Google Scholar]
- 27. Wang, J. et al Exposure‐response relationship of T‐DM1: insight into dose optimization for patients with HER2‐positive metastatic breast cancer. Clin. Pharmacol. Ther. 95, 558–564 (2014). [DOI] [PubMed] [Google Scholar]
- 28. Shah, M.A. et al HELOISE: phase IIIb randomized multicenter study comparing standard‐of‐care and higher‐dose trastuzumab regimens combined with chemotherapy as first‐line therapy in patients with human epidermal growth factor receptor 2‐positive metastatic gastric or gastroesophageal junction adenocarcinoma. J. Clin. Oncol. 35, 2558–2567 (2017). [DOI] [PubMed] [Google Scholar]
- 29. Krop, I.E. et al Trastuzumab emtansine versus treatment of physician's choice in patients with previously treated HER2‐positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open‐label phase 3 trial. Lancet Oncol. 18, 743–754 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Effect of baseline covariates on exposure parameter AUCss.
Figure S2. Distribution of tremelimumab predicted exposure at steady‐state (AUCss) in all‐comers and across each quartile (Q1 = lowest exposure, Q3‐Q2 = interquartile range, and Q4 = highest exposure).
Figure S3. Kaplan–Meier sensitivity analysis of OS by quartiles of observed exposure levels after the end of infusion of the first dose of tremelimumab (Cmax,1) for the tremelimumab‐treated group.
Supplementary Material S1. Methods.


