Abstract
Immune priming is defined as enhanced protection upon secondary exposure to a pathogen. Such enhanced resistance after prior exposure has been demonstrated for a number of insect species including the red flour beetle, Tribolium castaneum. In testing this phenomenon, the majority of studies have focused on introducing the pathogen into the insect's hemocoel via septic wounding through the cuticle. Although such septic injury can occur in nature, many pathogens enter their hosts via the oral route, i.e. by ingestion. Bacillus thuringiensis bacteria are well-known insect pathogens that infect their host orally. We found that T. castaneum larvae showed increased survival after oral exposure to B. thuringiensis, when they had been orally primed with filter-sterilized media in which spores of B. thuringiensis had been raised. Such priming was achieved only with a naturally pathogenic strain of B. thuringiensis and a strain that was made pathogenic by transfer of plasmids. Moreover, primed larvae were smaller in size 24 h after priming and had a longer developmental time, indicating that investment in such a response comes at a cost. However, the increased survival in primed larvae was not caused by larval size differences upon challenge.
Key Words: Innate immunity, Immune priming, Host-parasite coevolution, Red flour beetle, Tribolium castaneum, Bacillus thuringiensis
Introduction
Invertebrates, insects in particular, are being increasingly recognized as powerful models for studying basic principles in immunology [1]. Insect immune systems are no longer seen as hard-wired and inflexible. A number of studies on several insect species have provided evidence for phenomena that are reminiscent of immune memory, often called immune priming, which is enhanced resistance after prior exposure to pathogens (for review, see [2, 3, 4, 5, 6]). These studies have been conducive to the emerging awareness that the previous clear distinction between innate and adaptive immunity may need conceptual renewal [3, 7, 8, 9].
For example, priming with bacterial lipopolysaccharides provides protection against subsequent challenge with the entomopathogenic fungus Metarhizium anisopliae in the mealworm beetle Tenebrio molitor[10]. A low dose of live bacteria, heat-killed bacteria or fungal spores provides protection against a later challenge with potentially lethal doses of live pathogens in the bumblebee Bombus terrestris[11], the fruit fly Drosophila melanogaster[12] and the red flour beetle Tribolium castaneum[13]. These studies even provided evidence for a higher degree of specificity of such acquired protection, as survival was enhanced only when similar bacterial species or strains were used for priming and challenge.
In these and further studies, priming was generally achieved through septic wounding, i.e. by pathogen injection or pricking of the insect's cuticle. However, wounding itself may already have strong effects on the immune system and further components of the insect's physiology (e.g. [14, 15]). Moreover, many natural insect pathogens would usually enter their host by the oral route, e.g. through ingestion of spores or vegetative cells, often followed by penetration of the gut wall to get access to the insect's body cavity. An important question is, therefore, whether or not the oral uptake of pathogens, or components thereof, might likewise lead to enhanced resistance. Immune priming following oral infection with live parasites has been shown for Anopheles gambiae mosquitoes infected with Plasmodiummalariae parasites [16], and for crustacean species upon infection with live bacteria [17] or tapeworm larvae [2]. Oral exposure to yeast or peptidoglycans can stimulate the immune response of crustaceans, such as Artemia or shrimp [18] (for review, see [19]). In insect larvae, consumption of live bacteria or DNA viruses leads to increased immune activity and survival [20,][21]. However, to the best of our knowledge, there are currently no studies examining whether the oral uptake of components derived from bacteria (in the absence of the pathogen) can lead to increased resistance against this bacterial species. We therefore studied oral priming of resistance in the red flour beetle T. castaneum against the entomopathogenic Bacillus thuringiensis.
B. thuringiensis is a Gram-positive bacterium that forms highly resistant endospores. It produces plasmid-encoded crystalline inclusions (Cry proteins) during the sporulation phase, which are toxic to specific insect orders upon ingestion [22]. The ingestion of spores and the following infection process that takes place in the gut, is considered the natural infection route for B. thuringiensis [23]. We use a high-throughput oral infection protocol to expose individual T. castaneum larvae to coleopteran-specific B. thuringiensis bv. tenebrionis(Btt) bacteria [24].
To explore the potential of bacterium-derived substances to induce resistance in T. castaneum, we primed beetle larvae with supernatants derived from spore cultures of B. thuringiensis. We focused on spore supernatants based on a preliminary test for priming where we also included autoclaved spores and heat-killed vegetative cells (concentration of both 1 × 109 ml-1), which did not induce a clear priming effect as spore supernatants. Spore supernatants likely contain substances produced during bacterial growth, and might thus indicate a harmful situation to the insect when recognized in the gut or after consumption of infected larvae. Note that T. castaneum larvae are highly cannibalistic [25] and reinfection of larvae from infected cadavers could be demonstrated also under laboratory conditions [24].
We used different strains of B. thuringiensis to find out whether protection is a general phenomenon, or if it is achieved only by the Btt bacterial strain that is pathogenic to T. castaneum, and also included a strain that was made pathogenic to T. castaneum by transfer of the Cry-carrying plasmid [24]. Immune priming has been shown to come at a cost for the primed individual [26, 27] and it may also have an impact on development time [28, 29]. We therefore studied whether oral immune priming affected the growth and development of T. castaneum.
Materials and Methods
Insects
All experiments were done on a recently wild-collected Cro1 population of T. castaneum[24]. Insects were kept on heat-sterilized (75°C) organic wheat flour (Alnatura, type 550) with 5% Brewer's yeast. For rearing as well as for experimental treatments (priming and challenge, see below) the insects were kept at 30°C and 70% humidity with a 12-hour light/dark cycle.
Bacterial Strains
We tested 5 B. thuringiensis strains (online suppl. table 1; for all online suppl. material, see www.karger.com/doi/10.1159/000355211) for their ability to induce a priming response via ingestion. Bt morrisoni bv. tenebrionis(Btt) causes mortality to T. castaneum larvae, whereas Bt kurstaki(Btk), Bt thuringiensis(Bt 407cry-) and Bt thuringiensis Cry- (Bt 407gfpcry-) are nonpathogenic strains [24]. Bt thuringiensis Cry+ (Bt 407gfp-neocry+) was obtained via conjugation between the nonpathogenic Bt 407gfpcry-[30] and Btt in our previous study [24]. This conjugated strain received a large cry-carrying plasmid (together with a small plasmid with neomycin resistance) and gained the ability to successfully infect T. castaneum larvae. Btt and Btk were provided by the Bacillus Genetic Stock Center (BGSC, Ohio State University, Ohio, USA) and Bt 407cry- and Bt 407gfpcry- were kindly provided by Dr. Christina Nielsen-Leroux, Institut National de Recherche Agronomique, La Minière, 78285 Guyancourt Cedex, France.
Preparation of Priming and Challenge Diet
For oral priming, spore culture supernatants were added to the flour diet of T. castaneum larvae. Spore cultures were produced as previously described [24] and centrifuged at 4,000 rpm at room temperature for 12 min. The supernatants were transferred into a new tube, centrifuged again and subsequently filter-sterilized, first using a 0.45-µm and then a 0.2-µm cellulose acetate filter (Whatman GmbH). Such spore-free supernatants were mixed with 0.15 g of flour with yeast per milliliter of supernatant. Thirty microliters of this mixture were pipetted into each well of a 96-well plate (Sarstedt, Germany). Plates were covered with breathable sealing foil for culture plates (Kisker Biotech) and dried overnight at 36°C, to avoid heat-inactivation of potential priming substances. The diet for the control larvae was prepared in the same way by mixing flour with unconditioned culture media (i.e. media in which no spores had been grown). A medium control with neomycin (15 µg/ml) was used as the appropriate control for Bt 407gfp-neocry+, as spores of this strain are raised with neomycin as a selective medium. In addition, for a naïve control, flour with yeast was mixed with phosphate-buffered saline (PBS, Calbiochem®).
For oral challenge, the spores were prepared and added to the flour-with-yeast diet as previously described [24]. Briefly, spore concentration was adjusted to 5 × 109 ml-1 by adding PBS, and flour discs were prepared in the same way as for the priming diet. Plates with the challenge diet were placed in plastic boxes (Curver, New Grand Chef, 2.6 l) with caps plugged with foam stoppers (K-TK e.K., Germany) and dried at 50°C overnight.
Experimental Design
Priming and Challenge
Around 1,000 adults were allowed to lay eggs for 24 h in a plastic box plugged with foam stoppers containing approximately 300 g of flour, and the larvae were kept at 30°C and 70% humidity. At 15 days after oviposition (day 1 being the day when adults were placed on flour), similar-sized larvae were individually placed into 96-well plates containing the priming diet. Larvae of uniform size were chosen (online suppl. fig. 2: size before priming) because T. castaneum larvae hatch on different days [25, 31] and by the time of priming, they would differ in age otherwise. Plates were sealed with transparent adhesive tape (Tesa SE), 9 holes were punctured with a needle (0.3 mm diameter) and the plates were placed into plastic boxes (Curver, New Grand Chef, 2.6 liters) with caps plugged with foam stoppers to allow circulation of air. Investigators were blind with regard to the priming treatments, which were randomly assigned to the plates, such that each plate contained all treatments. Only in the third experiment were classes of small and large size placed on the same plate, while each plate contained one priming treatment, which was replicated on three plates. Larvae were kept on the priming diet for 24 h, and then transferred to new plates containing the naïve diet (flour with yeast in PBS) and kept for 4 days until challenge. For the challenge, the larvae were transferred to plates containing Btt spores prepared as described above, and the survival was checked on the 1st, 2nd and 3rd day after the start of the exposure.
Oral Priming with Spore Culture Supernatants Increases Survival of T. castaneum Larvae
In this experiment, we tested for the properties of spore culture media conditioned by the pathogenic Btt strain and the non-pathogenic Bt 407gfpcry- strain to induce priming, and compared their effects to the unconditioned medium control. In the same experiment, larvae were primed with the culture medium of the pathogenic Bt 407gfp-neocry+ which was raised with neomycin and were compared to the unconditioned medium control with neomycin. The diet for the naïve larvae was mixed with PBS and 96 larvae were primed per treatment. Only very few individuals died or escaped between priming and challenge, leading to the following sample sizes for the challenge: Btt: 91, Bt 407gfpcry-: 94, medium control: 93, naïve: 93, Bt 407gfp-neocry+: 92 and medium control with neomycin: 95.
Costs of Priming
To test whether oral priming has an effect on development, 192 larvae of each treatment were initially primed as described above, with the spore culture media conditioned by the Btt, Bt 407gfpcry- and Bt 407gfp-neocry+ strain and the corresponding control treatments. After 24 h, larvae were transferred to the naïve diet and their development was monitored daily until day 50 after oviposition (i.e. day 36 after priming). As some individuals died or escaped between priming and transfer to plates with the naïve diet, sample size for development observations were as follows: Btt: 177, Bt 407gfpcry-: 183, medium control: 176, naïve: 173, Bt 407gfp-neocry+: 175 and medium control with neomycin: 181.
Relationship between Priming and Larval Size
In this experiment, larvae were primed (n = 96 per treatment) with the spore culture media conditioned by the Btt, Bt 407cry- and Btk strains. To additionally test for potential effects of larval size differences on priming efficiency, the priming experiment was done with 2 groups of larvae: a small-size class was obtained from larvae at 14 days after oviposition, and a large-size class at 15 days after oviposition. We monitored larval growth during the experiment by measuring the larval size as the total area of the larvae. For this, we took pictures of larvae immediately before transfer to the priming diet, or in the case of naïve larvae immediately before transfer to the diet mixed with PBS, (denoted ‘pre priming’), after priming (i.e. 24 h after the initial exposure to priming diet, denoted ‘post priming’) and immediately before challenge (denoted ‘pre challenge’). We used the total area as a size measure instead of, for example, head-capsule width, because it would only change upon moulting to another larval instar, which was not normally the case within the short time frame studied here. For measuring size, larvae were placed on a black background (stereomicroscope stage-plate) and pictures were taken using a Canon EOS 2D Mark II camera, with a lens Canon EF 24-105 mm. We then measured total area of larvae using Acapella® High Content Imaging and Analysis Software (PerkinElmer®, Waltham, USA). As some individuals died or escaped between priming and challenge, sample size for the challenge was as follows: small-size class - Btt: 94, Btk: 88, Bt 407cry-: 91, medium control: 92 and naïve: 89 and the large-size class - Btt: 94, Btk: 94, Bt 407cry-: 91, medium control: 95 and naïve: 89.
Statistics
All experiments were analyzed with JMP statistical program, version 8.0.1 for Windows. Generally, for each experiment, a generalized linear model (GLM) was fitted and for significant terms, contrasts were used to test for differences between the treatments. We used GLM instead of survival analysis because survival curves do not provide additional information in our system, as mortality usually occurs within the first 24 h with any later mortality being rare (see survival curves in online suppl. fig. 3, 4). In the first and second experiment (influence of spore culture supernatants on the induction of priming and costs of priming) the model was fitted using survival as a response variable for the first experiment (1 = alive and 0 = dead) and the proportion of different developmental stages on day 20 after priming for the second experiment (1 for adult and 0 for larva/pupa). In addition to the priming treatment, plate was used as model effect, to account for potential differences among experimental plates (table 1, 2). As only the treatment had an influence on survival in both experiments, contrasts were used to compare treatments Btt, Bt 407gfpcry- and the naïve group to the medium control (Bonferroni correction, p = 0.0166). A separate, similar model was fitted for the Bt 407gfp-neocry+ and the corresponding medium control group with neomycin. In the third experiment, three models were fitted. First, to test whether larvae of different size classes differ in their survival, a model was fitted with survival as response variable (1 = alive and 0 = dead) and the factors: treatment, size class, treatment *size-class interaction and plate nested within the treatment. As only the treatment had a significant influence on survival (table 3), contrasts were used to compare priming treatments and the naïve group to the medium control group (Bonferroni correction, p = 0.0125). In the second model, we tested whether the priming treatment affected larval growth. We fitted a similar model, but using growth measures (difference between size prechallenge and prepriming) as the response variable. Contrasts were made separately for size class and treatment. In the third model, we tested whether larval size before challenge affected the survival. A model was fitted using survival as the response variable (1 = alive and 0 = dead) and treatment, size before the challenge and its interaction as factors (table 3). As only treatment had an effect, contrasts were taken to test which treatment differs from the medium control (Bonferroni correction, p = 0.0125).
Table 1.
Oral priming with spore culture supernatants increases survival ofT. castaneum larvae
| χ2 | DF | p | |
|---|---|---|---|
| A Medium control | |||
| Overall model – survival | 21.0211 | 8 | 0.0071 |
| Treatment | 12.110233 | 3 | 0.0070 |
| Plate | 9.044753 | 5 | 0.1073 |
| Contrasts | |||
| Medium vs. naïve (PBS) | 2.5891038924 | 0.1076 | |
| Medium vs.Btt SN | 9.7002479438 | 0.0018 | |
| Medium vs.Bt 407gfpcry− | 0.049230117 | 0.8244 | |
| B Medium neo control | |||
| Overall model – survival | 3.4117 | 2 | 0.1816 |
| Treatment | 3.4041662 | 1 | 0.0650 |
| Plate | 0.0188121 | 1 | 0.8909 |
GLM testing for the effect of priming treatment on survival; experimental plate was also included in the model.
Model for the priming treatments naïve (PBS),Btt, Bt 407gfpcry− and the corresponding medium control.
Model for the priming treatmentBt 407gfp-neocry+ and the corresponding medium control with neomycin.
Table 2.
Costs of priming
| χ2 | DF | p | |
|---|---|---|---|
| A Medium control | |||
| Overall model – development | 119.8951 | 14 | <0.0001 |
| Treatment | 85.422811 | 3 | <0.0001 |
| Plate | 38.329492 | 11 | <0.0001 |
| Contrasts | |||
| Medium vs. naïve (PBS) Medium vs.Btt SN Medium vs.Bt 407gfpcry− |
51.076242868 20.86734176 0.7513286303 |
<0.0001 <0.0001 0.3861 |
|
| B Medium neo control | |||
| Overall model – development | 61.5928 | 12 | <0.0001 |
| Treatment | 33.415784 | 1 | <0.0001 |
| Plate | 30.753963 | 11 | 0.0012 |
GLM testing for the effect of priming treatment on development (adult stage vs. larval/pupal stage); experimental plate was also included into the model.
Model for the priming treatments naïve (PBS),Btt,Bt 407gfpcry− and the corresponding medium control.
Model for the priming treatmentBt 407gfp-neocry+ and the corresponding medium control with neomycin.
Table 3.
Relationship between priming and larval size
| χ2 | DF | p | |
|---|---|---|---|
| A | |||
| Overall model – survival | 44.3514 | 19 | 0.0008 |
| Treatment | 27.9444 | 4 | <0.0001 |
| Size class | 1.2688 | 1 | 0.2600 |
| Plate, treatment | 11.5185 | 10 | 0.3186 |
| Treatment*size class Contrasts |
3.3722 | 4 | 0.4976 |
| Medium vs. naïve (PBS) | 1.8233 | 0.1769 | |
| Medium vs.Btt SN | 26.7661 | <0.0001 | |
| Medium vs.Btk SN | 2.5733 | 0.1087 | |
| Medium vs.Bt 407cry− | 3.8637 | 0.0493 | |
| B | |||
| Overall model – growth | 311.1827 | 19 | <0.0001 |
| Treatment | 264.9723 | 4 | <0.0001 |
| Size class | 7.0428 | 1 | 0.0080 |
| Plate, treatment | 49.0640 | 10 | <0.0001 |
| Treatment*size class Contrasts |
8.6540 | 4 | 0.0704 |
| Medium vs. naïve (PBS) | 174.9389 | <0.0001 | |
| Medium vs.Btt SN | 68.4564 | <0.0001 | |
| Medium vs.Btk SN | 4.4715 | 0.0345 | |
| Medium vs.Bt 407cry− | 0.4229 | 0.5155 | |
| C | |||
| Overall model – survival | 35.0160 | 9 | <0.0001 |
| Treatment | 24.3653 | 4 | <0.0001 |
| Size prechallenge | 1.1885 | 1 | 0.2756 |
| Treatment*size prechallenge Contrasts |
5.5757 | 4 | 0.2332 |
| Medium vs. naive (PBS) | 1.4213 | 0.2331 | |
| Medium vs.Btt SN | 22.7727 | <0.0001 | |
| Medium vs.Btk SN | 1.8990 | 0.1681 | |
| Medium vs.Bt 407cry− | 1.9463 | 0.1629 | |
GLM testing for the effect of priming treatment and size class on survival; experimental plate (nested within treatment) was also included into the model.
GLM testing for the effect of priming treatment and size class on growth; experimental plate (nested within treatment) was also included into the model.
GLM testing for the effect of priming treatment and size before challenge on survival.
Results
Oral Priming with Spore Culture Supernatants Increases Survival of T. castaneum Larvae
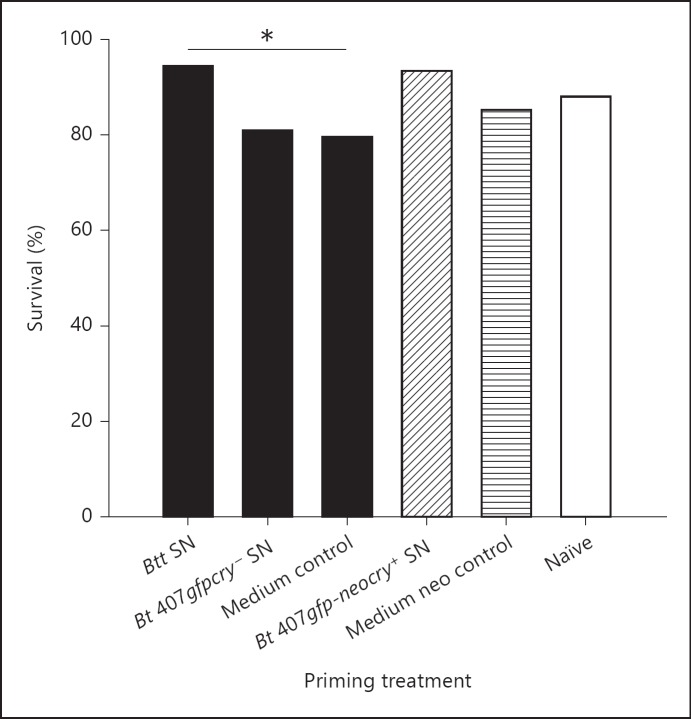
In this experiment, priming response was induced in larvae exposed to filter-sterilized medium, which was used to obtain spore suspensions (spore supernatants). Larvae exposed to the spore supernatant of a pathogenic Bt strain (Bt tenebrionis, [24]) in their diet, survived significantly better than larvae which were kept on a medium control (fig. 1, table 1, p = 0.0018). By contrast, no priming effect was observed in larvae exposed to the supernatant of a nonpathogenic Bt strain (Bt 407gfpcry-) which has a different chromosomal background and does not carry a cry toxin gene. We previously demonstrated that the transfer of a cry-carrying plasmid (together with a plasmid for neomycin resistance) from Bt tenebrionis to the same nonpathogenic strain enables the strain to effectively kill the larvae [24]. Compared to the corresponding media control with neomycin, the supernatant of the same conjugated strain (Bt 407gfp-neocry+) showed a trend for priming induction in larvae (p = 0.065), suggesting possible localization of the genes for the priming-inducing antigens on one of the two plasmids. Culture medium itself had a slightly negative effect on survival upon challenge because the larvae that were left naïve upon priming had greater survival than both media control groups, even though this effect was not significant.
Fig. 1.
Oral priming with spore culture supernatants increases survival of T. castaneum larvae. The figure shows the survival of T. castaneum larvae 3 days after constant exposure to 5 × 109 ml-1 of live Btt spores in the flour in relation to the different priming treatments and the corresponding media controls. Larvae primed with supernatants of Btt and Bt 407gfpcry- were compared to larvae exposed to the plain (unconditioned) medium without supernatant as control (black bars). In addition, the medium control for Bt 407gfp-neocry+ supernatant primed larvae contained neomycin, since this strain of bacteria was raised with this antibiotic (striped bars). Naïve group (white bar) larvae were exposed to diet mixed with buffer (PBS) only. Significant differences are indicated with asterisks. Sample size at the time point of challenge was as follows: Btt: 91, Bt 407gfpcry-: 94, medium control: 93, naïve: 93, Bt 407gfp-neocry+: 92 and medium control with neomycin (neo): 95. * p < 0.01.
Costs of Priming
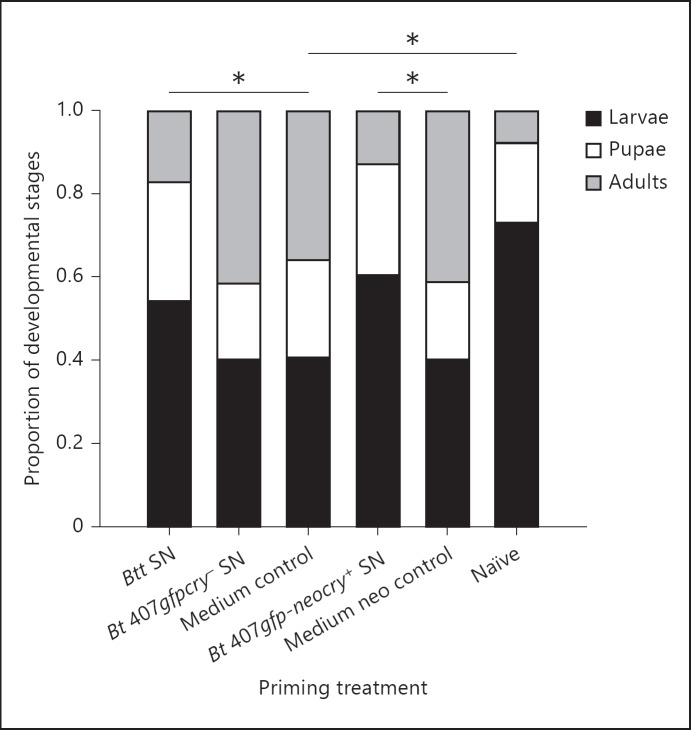
A primed immune response may come with a cost for the larvae, such as delayed development. We thus analyzed the proportion of larvae, pupae and adults following priming, using the same priming treatments as above, but without a challenge. Larvae exposed to the supernatants of Btt (p < 0.0001) and the conjugated Bt 407gfp-neocry+ strain (p < 0.0001) showed delayed development compared to the media control group (table 2), i.e. less larvae had reached the adult stage 20 days after priming (fig. 2). However, the development of the larvae that were not exposed to media at all (naïve) was even slower (table 2, p < 0.0001), which suggests a generally positive influence of media on development (online suppl. fig 1, 2).
Fig. 2.
Costs of priming. Proportion of different developmental stages of T. castaneum 20 days after exposure to the priming diet (36 days after oviposition). Treatment groups and media controls are as described in figure 1. Samples size at the time point of transfer to naïve diet (see Materials and Methods section) was as follows: Btt: 177, Bt 407gfpcry-: 183, medium control: 176, naïve: 173, Bt 407gfp-neocry+: 175 and medium control with neomycin (neo): 181. * p < 0.0001.
Relationship between Priming and Larval Size
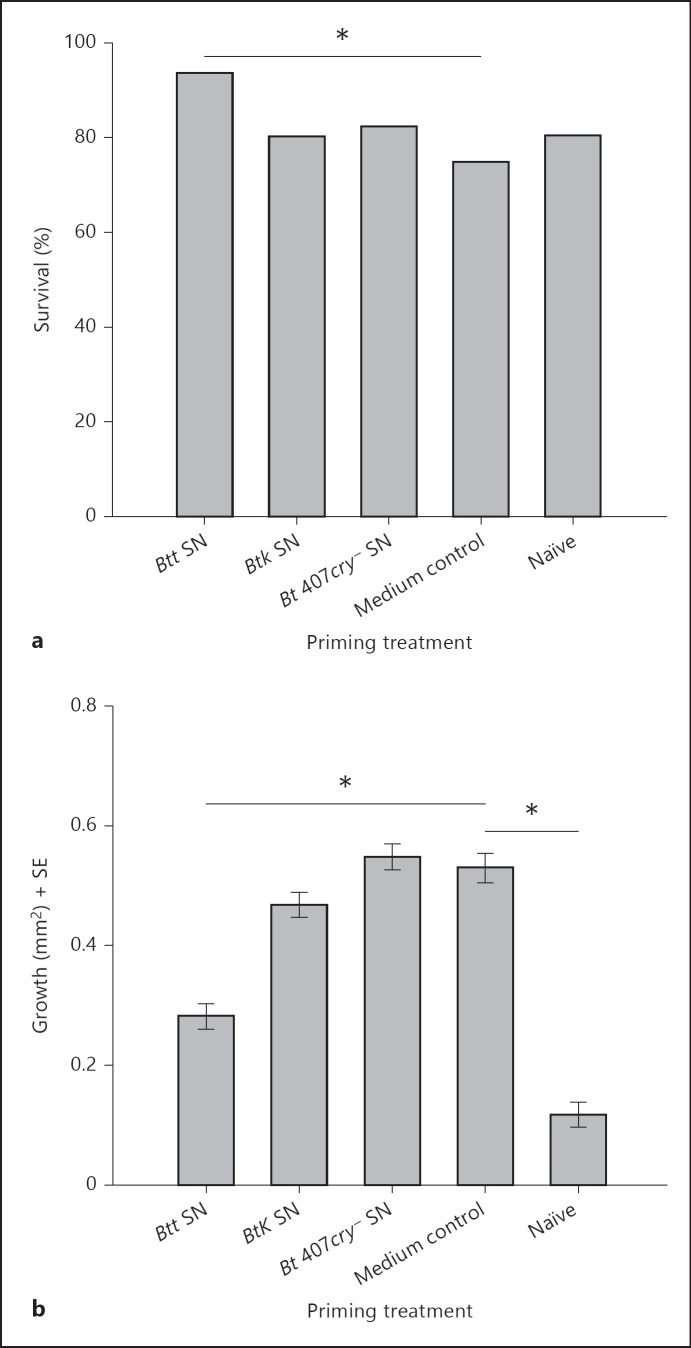
In the previous experiment, we observed that primed larvae needed more time to develop. We thus explored the relationship between priming and larval growth in more detail. We monitored larval growth during the experiment by taking measurements of larval size (the total area of larvae, see the Materials and Methods section) before and after priming and before challenge (online suppl. fig. 2). As the priming effect and survival upon challenge might differ for differently sized larvae, we conducted this experiment with two size classes of larvae. Survival data confirmed the priming effect of the pathogenic Btt. The experiment showed that larval size classes were equally able to mount a primed response when exposed to the spore supernatant of the pathogenic Btt strain [fig. 3; table 3, p (size class) < 0.260, p (Btt) < 0.0001]. In this experiment, we did not include the Bt 407gfp-neocry+ strain, but another Bt strain, Bt kurstaki. As expected, this strain, which is not pathogenic for T. castaneum, did not lead to a priming effect. Mounting a primed response reduced larval growth, such that Btt-primed larvae grew less during the experiment (table 3, p < 0.0001; fig. 3; online suppl. fig. 2) than the medium control and larvae exposed to the Bt strains that did not lead to priming. Even though a growth reduction upon priming was seen directly after priming, it mainly disappeared until challenge (fig. 3; online suppl. fig. 2) and larval size at challenge did not significantly influence survival (table 3, p < 0.275). Note that, after priming, larvae even show a reduction in size. This reduction is normal for certain instars of T. castaneum[25], but might also be due to stress experienced during the experimental procedure.
Fig. 3.
Relationship between priming and larval size. a Survival in relation to priming treatment. Survival is shown 3 days after constant exposure to 5 × 109 ml-1 of live Btt spores in flour. Larvae were primed with supernatants of Btt, Btk, Bt 407cry-. Larvae exposed to the plain (unconditioned) medium and buffer (PBS) served as control. b Growth (difference in larval area before challenge and before priming) of T. castaneum larvae after exposure to the priming diet and the medium control. Since size class had no influence on priming, survival and growth data are shown pooled for small and large size class. Significant differences are indicated with asterisks. Pooled sample sizes at the time point of challenge were as follows: Btt: 188, Btk: 182, Bt 407cry-: 182, medium control: 187 and naïve: 178. * p < 0.0001.
Discussion
In this study, we found that oral priming leads to increased survival of T. castaneum larvae upon oral exposure to B. thuringiensis. To the best of our knowledge, this is the first demonstration of oral priming against a bacterial infection in insects. Moreover, priming was induced by sterile-filtered supernatants of spore cultures of B. thuringiensis which is, in itself, a novel method for testing such a phenomenon.
Our initial screening for priming-inducing agents showed that heat-killed Bt bacteria as well as autoclaved spores did not consistently induce a primed immune response. By contrast, heat-inactivated bacteria were efficient in priming when introduced via septic wounding through the cuticle [13]. The tissue damage through wounding may here provide a danger signal [32] that is absent when heat-inactivated pathogens are offered orally. From an evolutionary-ecological point of view, it may be possible that certain priming elicitors would only be effective when administered via the hemolymph or via the oral route of infection, and the gut and the hemolymph priming mechanisms could be substantially different. In the midgut, the peritrophic membrane lines the epithelial cells and prevents direct access of pathogens to the cells [33], which is in contrast to the hemolymph where pathogens come into direct contact with hemocytes. For this reason, heat-inactivated pathogens in the gut may not be recognized as a threat. In addition, heat treatment may render antigens undetectable by receptors in the gut. In this study, priming for increased survival was only induced by Bt tenebrionis, a strain that is infective to the beetle. A Bt strain that was made infective by the transfer of plasmids from the Btt strain also showed a trend for the induction of priming. Thus, a potential candidate substance for priming might be encoded by a gene on one of those plasmids. The Cry toxins are major, plasmid-encoded virulence factors of Bt, and the toxin itself might be responsible for the priming. However, Cry toxins form large crystals which would not remain in the supernatant after centrifugation, although protein monomers may still be retained to some extent. Cry toxins bind specifically to receptors in midgut epithelial cells and cause cell lysis [34]. Even though T. castaneum, in contrast to other insect species, cannot be killed by the spore-free toxin preparations [35], toxins could still damage epithelial cells. The damage induced by Cry toxins or other bacteria-derived substances might serve as a danger signal to the host [32]. Moreover, upon such damage, the access of gut-associated microbiota to the hemolymph could lead to priming, as reported in Plasmodium infections of mosquito hosts [16]. Causes of enhanced resistance after oral priming are likely immunological in a narrow sense, but might also include immunity in a broader sense, such as changes in gut microbiota or even increased avoidance behavior. However, these possibilities need further study.
In addition to the remnants of small bacterial cell wall components, culture supernatants may contain substances that are indicative of pathogen growth and replication, and a host that is able to use such cues of a potential pathogenic threat for the prophylactic increase of resistance would have a fitness benefit. A recent study showed that Drosophila midgut epithelial cells can recognize bacteria by detecting uracil, which is secreted only by pathogenic bacteria and can be abundantly found in the culture supernatants [36]. This could support the hypothesis that bacteria secrete substances into their environment that can be recognized by the gut epithelia, but this needs to be investigated further.
We also found evidence for the costs of oral priming when there was no subsequent challenge. Primed larvae were smaller and developed more slowly. Interestingly, these effects were again specifically observed only when priming with the beetle-pathogenic Bt strain. Fitness costs of immunity are generally assumed, based on life-history theory [37] and might, for example, be due to the expression of immune effectors that are costly to produce [38]. Similar costs were also found for septic, transgenerational immune priming [26, 27]. By contrast, septic bacterial priming accelerated development in T. castaneum[39], which might be an adaptive modulation of life history by earlier reproduction.
In this study, the delayed development might also be interpreted as an adaptive response rather than as a cost, because the susceptibility to oral infection may be dependent on size or developmental stage [40]. We found no support for this interpretation in our experiment, however, when we included two size classes. First, we found a consistent priming effect for both size classes. Second, even though there was an effect of priming on size immediately after priming, the size difference was not that prominent closer to the challenge and did not influence survival. It was noted that completely naïve larvae were smaller than larvae kept on the relevant media controls. Media seemed to have a positive effect on larval growth, and showed an insignificant trend for a slightly negative effect on survival. The effect of media on growth might be due to additional nutrients that are present in the medium, or potentially by a direct effect of peptone on larval development [41].
Although many pathogens enter their hosts by ingestion, priming via the oral route has not been intensively investigated for insect pathogens. We showed that oral priming by pathogen-conditioned medium induces priming of resistance in the red flour beetle. The existence of this phenomenon may be explained by the ecology of T. castaneum. The larvae are highly cannibalistic and we were able to reinfect new larvae by offering them infected cadavers [24]. Thus, cannibalizing infected cadavers may confer resistance to the respective individuals and prevent further transmission of the pathogens in the population. It will be interesting to see whether oral priming might also play a role in other insect species, particularly against orally infecting pathogens. Pathogen-derived substances that are indicative of pathogen growth might be used by the host as danger signals, leading to the protective upregulation of defense traits. The observed oral priming of resistance in this study adds to the increasing evidence that insect immune defenses are plastically adjusted to various pathogens in their environment.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Acknowledgments
We would like to thank Swantje Prahl for writing the Acapella® script for image analysis, Sophie A.O. Armitage for helpful comments on the experiments, Gunther Jansen (Department of Evolutionary Genetics, CAU Kiel, Germany) and Christina Nielsen-Leroux (Institut National de Recherche Agronomique, La Minière, 78285 Guyancourt Cedex, France) for providing the Bt 407cry- and Bt 407gfpcry- strains. We are grateful for the useful comments by two anonymous reviewers, the editor Arne Egesten for handling our manuscript and the guest editor of the special issue on Training the Innate Immunity, Mihai Netea. The German Science Foundation (Deutsche Forschungsgemeinschaft) provided funding for the project (DFG-KU 1929/4-1 within SPP 1399).
References
- 1.Chambers MC, Schneider DS. Pioneering immunology: insect style. Curr Opin Immunol. 2011;24:1–5. doi: 10.1016/j.coi.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Kurtz J, Franz K. Evidence for memory in invertebrate immunity. Nature. 2003;425:37–38. doi: 10.1038/425037a. [DOI] [PubMed] [Google Scholar]
- 3.Kurtz J. Specific memory within innate immune systems. Trends Immunol. 2005;26:186–192. doi: 10.1016/j.it.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Little TJ, Kraaijeveld AR. Ecological and evolutionary implications of immunological priming in invertebrates. Trends Ecol Evol. 2004;19:58–60. doi: 10.1016/j.tree.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Schmid-Hempel P. Evolutionary ecology of insect immune defenses. Annu Rev Entomol. 2005;50:529–551. doi: 10.1146/annurev.ento.50.071803.130420. [DOI] [PubMed] [Google Scholar]
- 6.Little TJ, Hultmark D, Read AF. Invertebrate immunity and the limits of mechanistic immunology. Nat Immunol. 2005;6:651–654. doi: 10.1038/ni1219. [DOI] [PubMed] [Google Scholar]
- 7.Flajnik MF, Du Pasquier., L Evolution of innate and adaptive immunity: can we draw a line? Trends Immunol. 2004;25:640–644. doi: 10.1016/j.it.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Netea MG, Quintin J, van der Meer JWM. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9:355–361. doi: 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Criscitiello MF, de Figueiredo P. Fifty shades of immune defense. Plos Pathog. 2013;9:e1003110. doi: 10.1371/journal.ppat.1003110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moret Y, Siva-Jothy MT. Adaptive innate immunity? Responsive-mode prophylaxis in the mealworm beetle, Tenebrio molitor. P Roy Soc Biol Sci. 2003;270:2475–2480. doi: 10.1098/rspb.2003.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadd BM, Schmid-Hempel P. Insect immunity shows specificity in protection upon secondary pathogen exposure. Curr Biol. 2006;16:1206–1210. doi: 10.1016/j.cub.2006.04.047. [DOI] [PubMed] [Google Scholar]
- 12.Pham LN, Dionne MS, Shirasu-Hiza M, Schneider DS. A specific primed immune response in Drosophila is dependent on phagocytes. Plos Pathog. 2007;3:e26. doi: 10.1371/journal.ppat.0030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roth O, Sadd BM, Schmid-Hempel P, Kurtz J. Strain-specific priming of resistance in the red flour beetle, Tribolium castaneum. P Roy Soc Biol Sci. 2009;276:145–151. doi: 10.1098/rspb.2008.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altincicek B, Gross J, Vilcinskas A. Wounding-mediated gene expression and accelerated viviparous reproduction of the pea aphid Acyrthosiphon pisum. Insect Mol Biol. 2008;17:711–716. doi: 10.1111/j.1365-2583.2008.00835.x. [DOI] [PubMed] [Google Scholar]
- 15.Freitak D, Knorr E, Vogel H, Vilcinskas A. Gender- and stressor-specific microRNA expression in Tribolium castaneum. Biol Letters. 2012;8:860–863. doi: 10.1098/rsbl.2012.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodrigues J, Brayner FA, Alves LC, Dixit R, Barillas-Mury C. Hemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science. 2010;329:1353–1355. doi: 10.1126/science.1190689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Little TJ, O'Connor B, Colegrave N, Watt K, Read AF. Maternal transfer of strain-specific immunity in an invertebrate. Curr Biol. 2003;13:489–492. doi: 10.1016/s0960-9822(03)00163-5. [DOI] [PubMed] [Google Scholar]
- 18.Soltanian S, François J-M, Dhont J, Arnouts S, Sorgeloos P, Bossier P. Enhanced disease resistance in Artemia by application of commercial beta-glucans sources and chitin in a gnotobiotic Artemia challenge test. Fish Shellfish Immunol. 2007;23:1304–1314. doi: 10.1016/j.fsi.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Rowley AF, Pope EC. Vaccines and crustacean aquaculture - a mechanistic exploration. Aquaculture. 2012:334–337. 1–11. [Google Scholar]
- 20.Freitak D, Wheat CW, Heckel DG, Vogel H. Immune system responses and fitness costs associated with consumption of bacteria in larvae of Trichoplusia ni. BMC Biol. 2007;5:56. doi: 10.1186/1741-7007-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tidbury HJ, Pedersen AB, Boots M. Within and transgenerational immune priming in an insect to a DNA virus. P Roy Soc Biol Sci. 2011;278:871–876. doi: 10.1098/rspb.2010.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frankenhuyzen KV. Insecticidal activity of Bacillus thuringiensis crystal proteins. J Inv Path. 2009;101:1–16. doi: 10.1016/j.jip.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Raymond B, Johnston PR, Nielsen-LeRoux C, Lereclus D, Crickmore N. Bacillus thuringiensis: an impotent pathogen? Trends Microbiol. 2010;18:189–194. doi: 10.1016/j.tim.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Milutinović B, Stolpe C, Peuβ R, Armitage SAO, Kurtz J. The red flour beetle as a model for bacterial oral infections. PloS ONE. 2013;8:e64638. doi: 10.1371/journal.pone.0064638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sokoloff A, The Biology of Tribolium, vol 2. Oxford, Clarendon Press, 1974, p 131 (cannibalism), pp 57-59 (hatching rate), p 48 (decrease in size)
- 26.Sadd BM, Schmid-Hempel P. A distinct infection cost associated with trans-generational priming of antibacterial immunity in bumble-bees. Biol Lett. 2009;5:798–801. doi: 10.1098/rsbl.2009.0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zanchi C, Troussard JP, Moreau J, Moret Y. Relationship between maternal transfer of immunity and mother fecundity in an insect. P Roy Soc Biol Sci. 2012;279:3223–3230. doi: 10.1098/rspb.2012.0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roth O, Joop G, Eggert H, Hilbert J, Daniel J, Schmid-Hempel P, Kurtz J. Paternally derived immune priming for offspring in the red flour beetle, Tribolium castaneum. J Anim Ecol. 2010;79:403–413. doi: 10.1111/j.1365-2656.2009.01617.x. [DOI] [PubMed] [Google Scholar]
- 29.Mukherjee K, Fischer R, Vilcinskas A. Histone acetylation mediates epigenetic regulation of transcriptional reprogramming in insects during metamorphosis, wounding and infection. Front Zool. 2012;9:25. doi: 10.1186/1742-9994-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daou N, Buisson C, Gohar M, Vidic J, Bierne H, Kallassy M, Lereclus D, Nielsen-LeRoux C. IlsA, a unique surface protein of Bacillus cereus required for iron acquisition from heme, hemoglobin and ferritin. Plos Pathog. 2009;5:e1000675. doi: 10.1371/journal.ppat.1000675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang Y, Lam SL, Ho SH. Bioactivities of essential oil from Elletaria cardamomum (L.) Maton to Sitophilus zeamais Motschulsky and Tribolium castaneum (Herbst) J Stored Prod Res. 2000;36:107–117. [Google Scholar]
- 32.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 33.Lehane MJ. Peritrophic matrix structure and function. Annu Rev Entomol. 1997;42:525–550. doi: 10.1146/annurev.ento.42.1.525. [DOI] [PubMed] [Google Scholar]
- 34.Soberon M, Gill SS. Signaling versus punching hole: how do Bacillus thuringiensis toxins kill insect midgut cells? Cell Mol Life Sci. 2009;66:1337–1349. doi: 10.1007/s00018-008-8330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oppert B, Morgan TD, Kramer KJ. Efficacy of Bacillus thuringiensis Cry3Aa protoxin and protease inhibitors against coleopteran storage pests. Pest Manag Sci. 2011;67:568–573. doi: 10.1002/ps.2099. [DOI] [PubMed] [Google Scholar]
- 36.Lee KA, Kim SH, Kim EK, Ha EM, You H, Kim B, Kim MJ, Kwon Y, Ryu JH, Lee WJ. Bacterial-derived uracil as a modulator of mucosal immunity and gut-microbe homeostasis in Drosophila. Cell. 2013;153:797–811. doi: 10.1016/j.cell.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Sheldon BC, Simon V. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. [DOI] [PubMed] [Google Scholar]
- 38.Moret Y. Survival for immunity: The price of immune system activation for bumblebee workers. Science. 2000;290:1166–1168. doi: 10.1126/science.290.5494.1166. [DOI] [PubMed] [Google Scholar]
- 39.Roth O, Kurtz J. The stimulation of immune defence accelerates development in the red flour beetle (Tribolium castaneum) J Evol Biol. 2008;21:1703–1710. doi: 10.1111/j.1420-9101.2008.01584.x. [DOI] [PubMed] [Google Scholar]
- 40.Blaser M, Schmid-Hempel P. Determinants of virulence for the parasite Nosema whitei in its host Tribolium castaneum. J Inv Path. 2005;89:251–257. doi: 10.1016/j.jip.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Millert SC, Bottema CDK, Stathis PA, Tokes LG, Feldman D. Unexpected presence of estrogens in culture medium supplements: subsequent metabolism by the yeast. Endocrinology. 1986;119:1362–1369. doi: 10.1210/endo-119-3-1362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data