Abstract
Equivocal knowledge of the phase-specific drivers of natal dispersal remains a major deficit in understanding causes and consequences of dispersal and thus, spatial dynamics within and between populations. We performed a field experiment combining partial cross-fostering of nestlings and nestling food supplementation in little owls (Athene noctua). This approach disentangled the effect of nestling origin from the effect of the rearing environment on dispersal behaviour, while simultaneously investigating the effect of food availability in the rearing environment. We radio-tracked fledglings to quantify the timing of pre-emigration forays and emigration, foray and transfer duration, and the dispersal distances. Dispersal characteristics of the pre-emigration phase were affected by the rearing environment rather than by the origin of nestlings. In food-poor habitats, supplemented individuals emigrated later than unsupplemented individuals. By contrast, transfer duration and distance were influenced by the birds' origin rather than by their rearing environment. We found no correlation between timing of emigration and transfer duration or distance. We conclude that food supply to the nestlings and other characteristics of the rearing environment modulate the timing of emigration, while innate traits associated with the nestling origin affect the transfer phases after emigration. The dispersal behaviours of juveniles prior and after emigration, therefore, were related to different determinants, and are suggested to form different life-history traits.
Keywords: Athene noctua, cross-fostering, food supplementation, little owl, natal dispersal, radio-tracking
1. Introduction
Natal dispersal is a fundamental life-history stage in many species [1–3], and is considered to be under strong selective pressure [4–6]. Although natal dispersal is pivotal in linking animal population dynamics to large spatial scales by redistributing individuals [7,8], limited and equivocal knowledge of the drivers of the spatio-temporal patterns of dispersal at the individual level remains a major deficit in understanding the causes and functions of natal dispersal at the population level.
Life-history theory posits that natal dispersal is ultimately driven by avoidance of inbreeding, avoidance of competition for resources or mates, or combinations thereof [9–11]. Thus, selection can shape dispersal traits that allow flexible adjustments of the dispersal behaviour in relation to changing environmental contexts [5,12]. Proximate drivers of dispersal are plastic, multi-causal, and context-dependent [13–16], and thus, cause complex dispersal patterns [17]. Plasticity of dispersal can occur in each of the three distinct phases of dispersal: emigration, transfer, or settlement [18]. On the one hand, proximate factors can be related to intrinsic prenatal factors, such as maternal effects [12], genetics [19,20], or epigenetics [21]. On the other hand, the environment in which offspring develop, such as habitat characteristics [22], parental performance, or population density [23–25], can have important extrinsic effects on an individual's dispersal decisions [15]. Phenotypic traits [26] or early dispersal decisions associated with intrinsic or extrinsic factors can correlate with decisions in later dispersal phases, and thus can form a behavioural syndrome predictive of the outcome of dispersal [15,27].
Among environmental factors in the rearing environment, food resources are thought to play a major role in modulating dispersal rates and distance through intraspecific competition [13,28]. Food availability also affects pre-dispersal body condition [29–31], potentially resulting in condition-dependent dispersal. Also, food availability may affect a trade-off that individuals face in the timing of emigration: early dispersers may encounter a higher availability of vacant potential breeding sites, while late dispersers may profit from improved energy reserves, experience, or competitive abilities [32,33]. However, the effects of food availability in the rearing environment on dispersal characteristics can be confounded by intrinsic prenatal factors and by correlations with other factors in the rearing environment [28,34,35]. To our knowledge, approaches to disentangle these effects experimentally have been very limited so far.
Herein, we present a field experiment in little owls (Athene noctua) combining partial cross-fostering of nestlings to control for nestling origin, and food supplementation to manipulate nestling food supply in a landscape with a natural gradient of habitat suitability. We radio-tracked the juveniles from fledging to their first settlement. We characterized dispersal phases—exploratory forays, permanent emigration, transfer, and settlement—based on movement modes obtained from the individual trajectories [36]. In the food supplementation experiment, we experimentally disentangled the effect of food availability from other factors related to the rearing environment while controlling for habitat suitability as experimental effects might differ in relation to natural food availability [37]. We tested two alternative hypotheses of the effect of food supplementation on dispersal behaviour simultaneously controlling for natural habitat suitability. Under a ‘delayed emigration hypothesis’, we predict a later emigration in food-supplemented broods compared to unsupplemented broods [32,33]. By contrast, under an ‘advanced emigration hypothesis’, we predict an earlier emigration in food-supplemented broods compared to unsupplemented broods [38,39]. At the same time, the partial cross-fostering experiment disentangled the effects of the rearing environment including food availability from the potential effect of nestling origin on dispersal characteristics. This also allowed investigating the relative importance of the effects of origin and rearing environment in the course of natal dispersal. These experimental insights clarify the determination of timing and duration of different phases of natal dispersal, and show how factors determining early pre-emigration dispersal phases carry-over to later post-emigration dispersal phases.
2. Material and methods
(a). Study area and study species
The little owl is a territorial, nocturnal, generalist avian predator of about 200 g and lives in various open habitats. We studied natal dispersal in a nest-box population of little owls in the Ludwigsburg District in Baden-Württemberg, southwest Germany (48° 53.6′ N; 9° 11.6′ E; 250 km2) in 2009–2012 [29–31,37,40,41]. In recent studies in the same population, experimental food supplementation positively affected nestlings' growth, body condition, and survival [29,30], and adults' reproductive success was shown to be positively related to habitat quality in terms of food availability [31,37]. Little owls have a monogamous mating system with biparental care and obligate dispersal [42]. Moreover, little owls being non-migratory, dispersal decisions are not confounded by seasonal migration. Ring recovery studies found a female-bias in net dispersal distances [42].
(b). Brood monitoring and tagging
From the beginning of April to mid-July, we checked occupied nest-boxes weekly until clutches were complete. From the earliest expected hatching date until hatching or brood loss, nests were visited every 3–5 days. We visually estimated hatching dates of nestlings through a spyhole using developmental illustrations (commented photographs of nestling for every second day post-hatching starting with day 1, given in [42]). We ringed chicks at ca 14 days old. We determined nestlings' sex genetically using feather samples [30,43]. At ca 4 weeks old, normally a few days before fledging, we tagged the chicks with a VHF radio-transmitter of our own construction mounted with a backpack figure-8-harness (total ca 7 g; 4.5% average adult body mass) [29–31]. Tag range was ca 40 km, and life expectancy was ca 400 days. We tracked each owl by ‘homing-in' [44,45] at least three times weekly at night and at least once weekly during the day: for each location, a single person followed the signal until the tagged individual could be exactly located without chasing it away. During the main dispersal period in September and October, we recorded 4–5 relocations per individual per week at night. During each tracking event, we were able to identify whether an individual was alive based on its activity.
(c). Experimental treatment
About two weeks post-hatching, at the day of ringing and two weeks before VHF-tagging, we paired 88 synchronous broods as partner broods (44 pairs of broods). We exchanged half of the chicks in each partner brood keeping brood sizes constant (see a timeline of events in electronic supplementary material, figure S1). Depending on brood size, we selected one or two nestlings of similar age and body weight at ca 14 days old for the exchange. In 30 of the 44 pairs of broods, we randomly assigned one brood to experimental food supplementation, while no food supplementation was applied in the remaining 14 of the pairs of broods due to time constraints in applying the food supplementation treatment. For monitoring and food supplementation after the exchange, all supplementary fed and control partner broods were visited every second day over 36 days until ca three weeks after fledging. Food supplementation started immediately after the exchange, and a total of 480 g of dead laboratory mice per nestling was deposited inside the nest-box of each supplemented brood: 20 g per visit and per nestling for the first six visits, 30 g per visit and per nestling thereafter [29,30]. This experimental approach created four groups of individuals: (1) individuals reared in their original parental environment, unsupplemented, (2) individuals reared in their original parental environment, food supplemented, (3) individuals reared in a foster parental environment, unsupplemented, and (4) individuals reared in a foster parental environment, food supplemented. After the exchange, brood members always shared the same rearing environment including the food supplementation treatment while differing in their origin. Three additional broods for which no synchronous partner brood was available were food supplemented and the nestlings treated as food-supplemented individuals reared in their original environment. Furthermore, 146 untreated broods were monitored and VHF-tagged at fledging, and entered the analyses as unsupplemented individuals reared in their original environment.
A standard protocol for partial cross-fostering is the exchange of eggs or hatchlings. However, as little owls tend to desert clutches and newly hatched broods after disturbance, we conducted the exchanges around day 14 after hatching. Since energy requirements of nestlings in the first two weeks after hatching are smaller than later in the nestling period and the total period of presence in the rearing environment after the exchange was much longer than the two weeks, we consider the effect of the delay of the exchange to be small. We, therefore, assume that the delayed cross-fostering treatment only marginally biased the results, and reliably separates the factors related to the nestlings' origin from the factors related to the nestlings' rearing environment.
(d). Habitat suitability
To test for the effect of habitat quality on natal dispersal characteristics, we used a scale-integrated habitat suitability index [46]. We integrated three order-specific resource selection functions to account for conditional dependencies across scales in a single model. For the purpose of the present analyses, we calculated the average habitat suitability score of each breeding home range. Because not every parental pair was radio-tracked, we defined natal ranges as the area within a 300 m radius around the nest-boxes based on average adult home range size estimates in this population [31].
(e). Body condition
The nestlings' origin, as well as their rearing environment, may affect body condition at fledging, potentially resulting in condition-dependent dispersal. To test for potential effects of differential body condition we used the fledglings' body mass. We weighted 152 fledglings at an average age of 29.2 days ± 3.6 (s.d.). We used the residuals of a linear relationship between body mass and age (β = 0.968; 95% CI [0.339, 1.598]; p < 0.001) as the age-corrected variable for body condition in the analyses.
(f). Dispersal timing, duration, and distance
We defined exploratory forays as temporary moves of fledglings beyond a 300 m radius from the nest-box, which represents the average size of an adult home range, followed by a return into the natal range. We defined the foray period as the time from the first foray to permanent emigration, i.e. when an individual permanently exited the 300 m radius of the natal range. Starting with permanent emigration, we fitted a hidden Markov model (HMM) to the post-emigration dispersal trajectories using the R package ‘moveHMM' [47] to identify three distinct movement modes: (i) directional movement (transfer), (ii) encamped movement indicating home-ranging (settlement), and (iii) an intermediate mode of encamped movement within a temporarily visited area (stopover). Specified starting values for the initial parameters for gamma and von Mises distributions are reported in the electronic supplementary material, S2. We then used the change points between successive movement modes to define the timing and the duration of the dispersal phases [48]. Herein, we restricted our analysis to the timing and duration of the exploratory and transfer phase until first settlement. We measured the net dispersal distance as the Euclidean distance between the nest-box of fledglings, and their first settlement location.
(g). Statistical analyses
Only 160 birds that survived to the first exploratory foray entered the analyses. We analysed the effects of experimental and control variables on five response variables: age at first foray (days), duration of foray (days), age at emigration (days), duration of transfer (days), and net dispersal distance (km) using linear mixed-effects models (LMM). We fitted the LMMs in a Bayesian framework using the R package ‘rstanarm’ [49]. We square-root-transformed all response variables to meet the assumption of normal distribution of the error residuals. In all models, we included as fixed-effects food supplementation (categorical; supplemented, unsupplemented), average habitat suitability (continuous; score range 0–1), and their interaction as our focal explanatory variables, and we controlled for sex (categorical; female, male) and hatching date (continuous; Julian day). Where food supplementation or its interaction with habitat suitability was an important predictor of the dependent variable, we tested in a second step for a possible indirect effect of food availability mediated by body condition by adding residuals of mass prior to fledging (g) to the fixed-effect structure of the model. For every model, we included the timing (Julian date) and duration (days) of the preceding phase. To disentangle pre-exchange contexts (nestling origin) from post-exchange contexts (rearing environment), we included the identity of both the original and the fostering pair as two random intercepts in all models. Thus, exchanged nestlings showed different pair identities for the two contexts while unexchanged nestlings showed the same pair identities. We also included year as a random intercept to control for year-to-year variations.
3. Results
(a). General patterns of dispersal
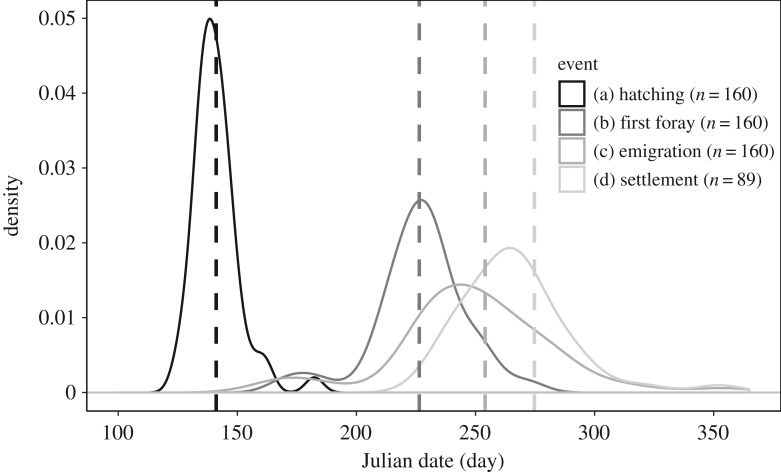
We radio-tracked 160 little owl fledglings surviving to the first exploratory foray during the pre-dispersal exploratory phase and dispersal transfer until first settlement, or until they died or we lost contact with them. Sample sizes at the first foray until emigration were 67 fledglings from 26 supplemented broods (36 females, 31 males), and 93 fledglings from 47 unsupplemented broods (55 females, 38 males). In this sample of surviving juveniles, we found no significant relationship between body mass residuals at fledging and food supplementation (β = 1.9; 95% CrI [−2.5, 6.3], n = 152), or body mass residuals and habitat suitability (β = −3.6; 95% CrI [−22.2, 16.9], n = 152). After permanent emigration, 71 individuals did not complete the transfer (we lost contact with 47 individuals, and 24 were found dead). We calculated transfer duration and dispersal distance in the remaining 89 individuals from 20 supplemented broods (22 females, 17 males), and from 33 unsupplemented broods (30 females, 20 males). The variation of hatching date was comparatively small and most hatching occurred within a month. Although the variability in the timing increased from hatching via fledging to dispersal events, a major proportion of the population reached each event within a narrow time window of ca 50 days (figure 1). Characteristics of the dispersal phases in the population are presented in table 1.
Figure 1.
Density distribution of hatching date (black), date of first foray (darkest grey), date of emigration (grey), and date of first settlement (lightest grey) in little owl fledglings. The vertical dashed lines of corresponding grey shading indicate the median value for each event.
Table 1.
Sample size, mean, and median of individual traits and natal dispersal characteristics in little owl fledglings.
| variable | N | mean ± s.d. | median [range] |
|---|---|---|---|
| hatching date (Julian day) | 160 | 141a ± 10 | 140b [125–183] |
| habitat suitability index | 160 | 0.85 ± 0.12 | 0.86 [0.55–1.00] |
| age of first foray (day) | 160 | 85.2 ± 19.4 | 86 [30–129] |
| foray duration (day) | 160 | 28.3 ± 35.9 | 19 [0–195] |
| age of emigration (day) | 160 | 112.9 ± 42.7 | 109 [26–310] |
| transfer duration (day) | 89 | 22.6 ± 32.6 | 10 [1–175] |
| net dispersal distance (km) | 89 | 9.7 ± 7.3 | 7.6 [0.5–30.8] |
aMay 21 ± 10 days.
bMay 20 (5 May–2 July).
(b). Exploratory forays
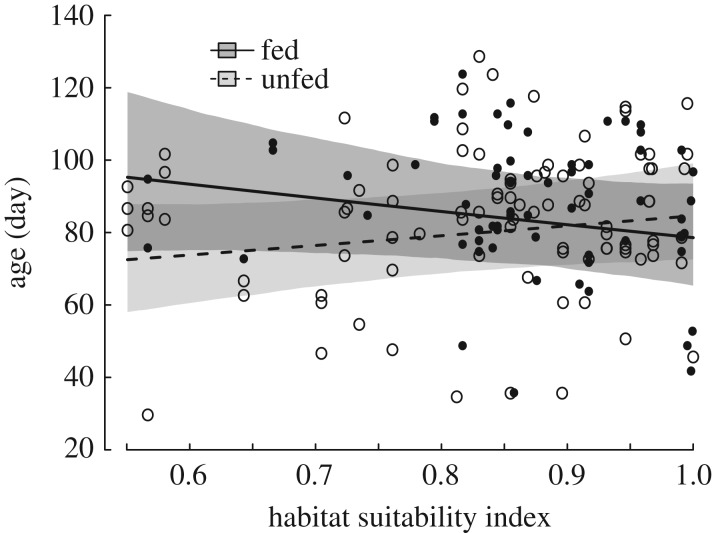
We found a significant interaction between the food supplementation treatment and the average habitat suitability index of the natal range in predicting the timing of first foray. In poor habitats, supplemented individuals emigrated out of their natal range 22 days later than unsupplemented individuals (figure 2). By contrast, in rich habitats, supplemented and unsupplemented individuals started exploratory forays at about the same age (85 days old). Unsupplemented fledglings (n = 93) tended to conduct forays later in rich habitats than in poor habitats (β = 1.706; 95% CrI [−0.595, 4.167]). No other fixed-effect control variable influenced the age at first foray (table 2). Contrary to age at first foray, foray duration was not associated with experimental food supplementation or habitat suitability, and none of the control fixed-effect variables were an important predictor of foray duration (table 2).
Figure 2.
Predicted age at first foray in relation to habitat suitability for 67 little owl fledglings from 26 food-supplemented broods (black dots, solid line, 95% CrI dark grey shaded area), and 93 fledglings from 47 unsupplemented broods (open circles, broken line, 95% CrI light grey shaded area).
Table 2.
Fixed and random coefficients and credibility intervals of generalized linear mixed-models investigating factors affecting (i) age at first foray out of the natal range, (ii) foray duration, and (iii) age at emigration in 160 little owl fledglings. Coefficients with 95% credibility interval not overlapping zero are denoted significant effects.
| age at first foraya |
foray durationb |
age at emigrationc |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| fixed-effect variables | β | lower 95% CrI | upper 95% CrI | β | lower 95% CrI | upper 95% CrI | β | lower 95% CrI | upper 95% CrI |
| (intercept) | 13.468 | 9.317 | 17.638 | −1.288 | −13.349 | 10.927 | 3.181 | 1.972 | 4.401 |
| food supplementation | 3.099 | 0.128 | 6.036 | 0.446 | −0.895 | 1.756 | 0.006 | −0.128 | 0.138 |
| habitat suitability index (HSI) | −1.925 | −4.615 | 0.888 | −0.168 | −5.163 | 4.986 | −0.047 | −0.564 | 0.452 |
| food supplementation X HSI | −3.423 | −6.801 | −0.015 | — | — | — | — | — | — |
| males | 0.131 | −0.183 | 0.443 | 0.312 | −0.648 | 1.264 | −0.068 | −0.170 | 0.030 |
| hatching date | −0.019 | −0.042 | 0.005 | 0.037 | −0.034 | 0.108 | 0.009 | 0.002 | 0.016 |
| age at first foray | — | — | — | 0.012 | −0.016 | 0.040 | 0.058 | 0.055 | 0.060 |
| foray duration | — | — | — | — | — | — | 0.042 | 0.040 | 0.043 |
aRandom-effect variance [95% credibility interval]: pre-exchange 0.101 [0.000, 0.457]; post-exchange 0.419 [0.028, 0.880]; year 0.200 [0.000, 1.330].
bRandom-effect variance [95% credibility interval]: pre-exchange 1.265 [0.002, 4.614]; post-exchange 2.591 [0.031, 6.462]; year 4.328 [0.021, 26.175].
cRandom-effect variance [95% credibility interval]: pre-exchange 0.008 [0.000, 0.033]; post-exchange 0.034 [0.006, 0.068]; year 0.028 [0.000, 0.420].
(c). Emigration and transfer
The age at emigration was positively related to both the age at first foray and the foray duration (table 2). Emigration was postponed by one day for each day an individual started the exploratory phase later. Emigration was postponed by another day for each day the exploratory phase lasted longer. Age at emigration also correlated positively with hatching date (table 2), indicating that the duration of the stages from hatching to emigration was stable, modulated by factors affecting the start of exploratory forays. Duration of transfer was not related to any of the focal or control variables (table 3; electronic supplementary material table S3). Finally, females (10.6 ± 8.4 km) tended to disperse farther than males (7.1 ± 7.6 km; β = 0.584; 95% CrI [−0.557, 1.658]; table 3; electronic supplementary material, figure S4).
Table 3.
Fixed and random coefficients and credibility intervals of generalized linear mixed-models investigating factors affecting (i) transfer duration and (ii) net dispersal distance in 89 little owl fledglings. Coefficients with 95% credibility interval not overlapping zero are denoted significant effects.
| transfer durationa |
net dispersal distanceb |
|||||
|---|---|---|---|---|---|---|
| fixed-effect variables | β | lower 95% CrI | upper 95% CrI | β | lower 95% CrI | upper 95% CrI |
| (intercept) | −1.401 | −12.504 | 9.244 | 4.060 | −1.521 | 9.426 |
| food supplementation | 0.596 | −0.491 | 1.707 | 0.102 | −0.419 | 0.613 |
| habitat suitability index (HSI) | −0.666 | −5.384 | 3.929 | −0.260 | −2.483 | 1.992 |
| males | 0.268 | −0.788 | 1.285 | −0.429 | −0.943 | 0.079 |
| hatching date | 0.046 | −0.021 | 0.112 | −0.006 | −0.038 | 0.028 |
| age at emigration | −0.003 | −0.029 | 0.022 | −0.001 | −0.012 | 0.011 |
| transfer duration | — | — | — | 0.004 | −0.003 | 0.012 |
aRandom-effect variance [95% credibility interval]: pre-exchange 0.724 [0.002, 2.712]; post-exchange 0.277 [0.000, 1.288]; year 2.045 [0.007, 13.051].
bRandom-effect variance [95% credibility interval]: pre-exchange 0.235 [0.001, 0.699]; post-exchange 0.098 [0.000, 0.441]; year 0.260 [0.000, 1.870].
(d). Pre-exchange versus post-exchange effects
We found that the explained variance in the age at first foray was 4.1 times higher for the nestlings' rearing environment after the exchange than for the shared origin before the exchange (table 4). The variance ratio was 2.6 for foray duration, and 3.1 for age at emigration. These variance ratios show that juveniles sharing the rearing environment after the exchange were more similar in the age at first foray, duration of forays, and age at emigration than juveniles sharing the same origin before the exchange. Conversely, the rearing/origin variance ratios were 0.4 and 0.3 for transfer duration and dispersal distance, respectively (table 4). Therefore, in dispersal characteristics following emigration the explained variance of shared origin before the exchange was 2.3 and 3.9 times higher than the explained variance of shared rearing environment after the exchange. Relative to the variances of shared origin before and rearing environment after the exchange, the year-to-year variance was larger for the duration of exploratory forays and transfer than for the timing events of foray start and permanent emigration (table 4). In summary, individual dispersal behaviour until emigration was mainly determined by the nestlings' rearing environment rather than by the nestlings' origin. Conversely, post-emigration dispersal characteristics were mainly determined by the nestlings' origin.
Table 4.
Random-effect variance and credibility intervals of generalized linear mixed-models with identical additive fixed-effect structure for comparison of their relative importance during dispersal of little owl fledglings. Sample size for each dispersal variable are indicated.
| model | random-effect variables | variance | lower 95% CrI | upper 95% CrI |
|---|---|---|---|---|
| age at first foray (n = 160) | ||||
| pre-exchange | 0.101 | 0.000 | 0.457 | |
| post-exchange | 0.419 | 0.028 | 0.880 | |
| year | 0.200 | 0.000 | 1.330 | |
| foray duration (n = 160) | ||||
| pre-exchange | 1.131 | 0.003 | 4.435 | |
| post-exchange | 2.963 | 0.049 | 6.835 | |
| year | 4.414 | 0.028 | 25.795 | |
| age at emigration (n = 160) | ||||
| pre-exchange | 0.471 | 0.001 | 1.634 | |
| post-exchange | 1.476 | 0.250 | 2.986 | |
| year | 1.144 | 0.002 | 6.871 | |
| transfer duration (n = 89) | ||||
| pre-exchange | 0.656 | 0.001 | 2.641 | |
| post-exchange | 0.284 | 0.000 | 1.382 | |
| year | 2.275 | 0.009 | 13.619 | |
| net dispersal distance (n = 89) | ||||
| pre-exchange | 0.304 | 0.002 | 0.800 | |
| post-exchange | 0.077 | 0.000 | 0.372 | |
| year | 0.255 | 0.000 | 1.751 | |
4. Discussion
Our experiments enable disentangling various determinants of natal dispersal characteristics in little owls and support the hypothesis that juveniles delay dispersal when food is abundant in the natal patch (i.e. delayed emigration hypothesis). First, food supply to the growing juveniles was an important determinant of the timing of emigration: in low-quality habitats, experimentally supplemented juveniles explored and emigrated later than unsupplemented juveniles. This relationship disappeared in food-rich habitats where unsupplemented juveniles also dispersed later, and thus at the same time as food-supplemented individuals. Natural variation in habitat quality had a similar effect on timing of forays and emigration as had the experimental increase in food supply. Second, in addition to the food supplementation, the effect of the shared rearing environment on timing of forays and emigration was stronger than the effect of the shared origin. Conversely, shared origin of the birds was an important determinant of the duration and the distance of the transfer phase. Thus, after permanent emigration from the natal home range, innate individual factors associated with their origin mainly affected the transfer phase. These results, therefore, provide empirical support for theoretical considerations that causal factors strongly differ in their effect on successive dispersal phases [14].
(a). Effects of food supply
Food supplementation and high habitat suitability of the natal home range delayed the timing of first forays which carried over to a delayed timing of permanent emigration. This indicates that the quality of the breeding home range affects not only the nestlings' growth and fledging condition [30], but also the later development and behaviour until family break-up and emigration of the juveniles. We, therefore, experimentally identified food resources during rearing to be a main habitat factor modulating the timing of emigration. The fact that increasing habitat suitability also delayed exploration behaviour in unsupplemented individuals provides further correlative support, since habitat suitability is associated with food availability and breeding success [31]. Strategies of delaying dispersal under food-rich conditions have been shown to increase survival or inclusive fitness [32,33,50]. More abundant resources within the home range could reduce competition among parents or siblings, and thus, promote delayed dispersal [32,33]. In accordance with their own life-history trade-offs, the resident parents can stop parental care and thus, influence the emigration of offspring [51,52]. Earlier termination of parental care or tolerance in a food-poor home range can improve the energetic conditions for the parents themselves [52]. Moreover, another non-exclusive mechanism could explain the pattern of advanced dispersal in food-poor habitats: early emigration from poor patches may enable individuals to find better conditions early. This, in turn, could increase their fitness, in particular, if dispersers from food-poor home ranges can settle earlier than better-fed competitors [38,39].
(b). Other fostering environment effects
As in studies investigating the natal dispersal of eagle owls (Bubo bubo) [53,54], we found a stronger effect of the shared rearing environment than of the shared nestling origin on timing of emigration. However, our study controlled for the effects of food supplementation and habitat suitability in the natal habitat. Thus, the importance of the shared rearing environment for early phases of dispersal in our study suggests that other environmental factors than food availability also affect dispersal timing and the duration of the exploratory phase. Two possible non-exclusive mechanisms operating in the rearing environment can explain this effect. First, essential features of the habitat that were not captured by our habitat suitability model could influence timing of emigration, e.g. micro-structures affecting resource availability or mortality risk such as roost sites [41], food accessibility [40], or predator occurrence [55] during the post-fledging period. Second, the social context, including parental or sib-sib aggression, might have been important in affecting emigration timing [24,56,57].
(c). Importance of origin versus rearing environment
We disentangled the effects of a shared origin (before exchange) from the effects of the shared rearing environment (after exchange) using a partial cross-fostering experiment. We found that the timing of first forays and of emigration were determined by the rearing environment rather than by origin. By contrast, the shared origin was the more important determinant of the duration of the transfer phase and the distance dispersed. The effect of origin on dispersal behaviour can develop by different mechanisms: either the behaviour is determined genetically [19,20,58], affected by prenatal maternal effects [12], or by environmental effects transferred to offspring epigenetically [21]. However, since cross-fostering was not conducted at hatching, we cannot exclude that the effect of origin partly developed due to a shared environment early in the nestling period. Our results show that, as dispersal advanced, the importance of the rearing environment decreased while the importance of origin increased. The effects of the rearing environment affecting pre-emigration dispersal phases did not carry-over to the transfer phase. We, therefore, suggest that the transfer phase, i.e. the movement part of dispersal resulting in spatial dynamics within and between populations, is only marginally driven by the conditions juveniles experienced in the natal home range and more by innate dispersal phenotypes and conditions encountered during the transfer phase when moving around within and between populations.
The duration of the exploration and the transfer phase showed large between-year differences, potentially due to year-specific conditions encountered during explorations or transfer such as food availability [40], fluctuations in conspecific densities [23,25], or annual climatic variations [59]. Moreover, it is likely that transfer duration and distance are strongly influenced by the broader landscape conditions encountered post-emigration. We have shown elsewhere that suitable habitat influences little owl movement trajectories during dispersal [60]. Thus, strong effects operating during the transfer phase might even hide carry-over effects from the pre-emigration phase. This is consistent with the idea of the broader landscape and habitat encountered on the move being the main factor affecting natal dispersal trajectories during the transfer phase [14,22,48].
5. Conclusion
We found limited evidence that innate traits play an important role for the timing of emigration. We suggest that the proximate causes affecting emigration are likely in the context of the natal habitat conditions and the social environment. However, determinants related to the juveniles' origin and innate traits appeared to shape the dispersal behaviour during transfer and settlement. Our results, therefore, suggest that the timing of obligate natal dispersal movements in little owls is extrinsically affected by the rearing environment. By contrast, the duration and distance of natal dispersal depends more on intrinsic factors associated with origin, and with sex being a predictor of dispersal distance. A lack of association between timing of emigration, and duration and distance of transfer indicates a breakdown of initial behavioural correlations as the process advances [61]. Pre-emigration and post-emigration dispersal behaviours related each to a different suite of pre-emigration correlates [62]. Thus, causal factors differed greatly in their effect on different dispersal phases. This suggests that optimal timing and optimal duration of dispersal represent life-history traits that may have evolved independently. While the ultimate causes for the habitat-dependent timing of emigration might be kin competition within the natal home range, the ultimate causes for dispersal duration and distance are likely associated with the spatio-temporal variation in habitat quality and its interaction with population density outside of the parental home range. Such dispersal pattern likely occur in species with year-round territories where juveniles do not explore the surroundings of their parental home ranges during the post-fledging period, and have to share resources with their kin, but do not gain any information about quality of the broader habitat or population density before dispersal.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
Capture and tagging was permitted by the regional council of Baden-Württemberg, Germany (Permit no. 35-9185.81/0288), and radio-tracking was permitted by the Deutsche Bundesnetzagentur (licence no. 37 55 5413). F. Korner-Nievergelt gave statistical advice. Comments from M. Tschumi, G. Pasinelli, C. Camacho, and two anonymous reviewers helped improve the manuscript.
Data accessibility
The primary telemetry and dispersal parameters data have been submitted as electronic supplementary material.
Authors' contributions
B.N.-D. and M.U.G. conceived the ideas and designed methodology; M.P., B.N.-D., and M.U.G. collected the data; J.F. and M.P. analysed the data; J.F. and M.U.G. led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
The Swiss National Science Foundation (Grant no. 3100A 132951/1 to B.N.-D. and M.U.G.), the Hirschmann Foundation and the Karl Mayer Foundation funded this study.
References
- 1.Chin A, Heupel M, Simpfendorfer C, Tobin A. 2013. Ontogenetic movements of juvenile blacktip reef sharks: evidence of dispersal and connectivity between coastal habitats and coral reefs. Aquat. Conserv. Mar. Freshw. Ecosyst. 23, 468–474. ( 10.1002/aqc.2349) [DOI] [Google Scholar]
- 2.Ferrer M. 1993. Ontogeny of dispersal distances in young Spanish imperial eagles. Behav. Ecol. Sociobiol. 32, 259–263. ( 10.1007/BF00166515) [DOI] [Google Scholar]
- 3.Massot M, Clobert J, Lorenzon P, Rossi JM. 2002. Condition-dependent dispersal and ontogeny of the dispersal behaviour: an experimental approach. J. Anim. Ecol. 71, 253–261. ( 10.1046/j.1365-2656.2002.00592.x) [DOI] [Google Scholar]
- 4.Bonte D, et al. 2012. Costs of dispersal. Biol. Rev. 87, 290–312. ( 10.1111/j.1469-185X.2011.00201.x) [DOI] [PubMed] [Google Scholar]
- 5.Garrard GE, McCarthy MA, Vesk PA, Radford JQ, Bennett AF. 2012. A predictive model of avian natal dispersal distance provides prior information for investigating response to landscape change. J. Anim. Ecol. 81, 14–23. ( 10.1111/j.1365-2656.2011.01891.x) [DOI] [PubMed] [Google Scholar]
- 6.Waser PM, Nichols KM, Hadfield JD. 2013. Fitness consequences of dispersal: is leaving home the best of a bad lot? Ecology 94, 1287–1295. ( 10.1890/12-1037.1) [DOI] [PubMed] [Google Scholar]
- 7.Hanski I. 1998. Metapopulation dynamics. Nature 396, 41–49. ( 10.1038/23876) [DOI] [Google Scholar]
- 8.Benton TG, Bowler DE. 2012. Linking dispersal to spatial dynamics. In Dispersal ecology and evolution (eds Clobert J, Baguette M, Benton TG, Bullock JM), pp. 251–265. Oxford, UK: Oxford University Press. [Google Scholar]
- 9.Pedersen D, Thorup K, Sunde P, Jacobsen LB, Rahbek C. 2013. Post-fledging behaviour of juveniles in the little owl (Athene noctua). Ornis Fenn. 90, 117–128. ( 10.1111/j.1474-919x.2010.01046.x) [DOI] [Google Scholar]
- 10.Gandon S, Michalakis Y. 2001. Multiple causes of the evolution of dispersal. In Dispersal (eds Clobert J, Danchin A, Dhondt AA, Nichols JD), pp. 155–167. Oxford, UK: Oxford University Press. [Google Scholar]
- 11.Mabry KE, Shelley EL, Davis KE, Blumstein DT, van Vuren DH. 2013. Social mating system and sex-biased dispersal in mammals and birds: a phylogenetic analysis. PLoS ONE 8, 1–9. ( 10.1371/journal.pone.0057980) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tschirren B, Fitze PS, Richner H. 2007. Maternal modulation of natal dispersal in a passerine bird: an adaptive strategy to cope with parasitism? Am. Nat. 169, 87–93. ( 10.1086/509945) [DOI] [PubMed] [Google Scholar]
- 13.Bowler DE, Benton TG. 2005. Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol. Rev. 80, 205–225. ( 10.1017/S1464793104006645) [DOI] [PubMed] [Google Scholar]
- 14.Matthysen E. 2012. Multicausality of dispersal: a review. In Dispersal ecology and evolution (eds Clobert J, Baguette M, Benton TG, Bullock JM), pp. 3–18. Oxford, UK: Oxford University Press. [Google Scholar]
- 15.Ronce O, Clobert J. 2012. Dispersal syndromes. In Dispersal ecology and evolution (eds Clobert J, Baguette M, Benton TG, Bullock JM), pp. 119–138. Oxford, UK: Oxford University Press. [Google Scholar]
- 16.Cote J, Bestion E, Jacob S, Travis J, Legrand D, Baguette M. 2017. Evolution of dispersal strategies and dispersal syndromes in fragmented landscapes. Ecography (Cop.) 40, 56–73. ( 10.1111/ecog.02538) [DOI] [Google Scholar]
- 17.van Overveld T, Careau V, Adriaensen F, Matthysen E. 2014. Seasonal- and sex-specific correlations between dispersal and exploratory behaviour in the great tit. Oecologia 174, 109–120. ( 10.1007/s00442-013-2762-0) [DOI] [PubMed] [Google Scholar]
- 18.Clobert J, Baguette M, Benton TG, Bullock JM. 2012. Dispersal ecology and evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 19.Saastamoinen M, et al. 2017. Genetics of dispersal. Biol. Rev. 358, 574–599. ( 10.1111/brv.12356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selonen V, Hanski IK. 2010. Condition-dependent, phenotype-dependent and genetic-dependent factors in the natal dispersal of a solitary rodent. J. Anim. Ecol. 79, 1093–1100. ( 10.1111/j.1365-2656.2010.01714.x) [DOI] [PubMed] [Google Scholar]
- 21.Van Petegem KHP, Pétillon J, Renault D, Wybouw N, Van Leeuwen T, Stoks R, Bonte D. 2015. Empirically simulated spatial sorting points at fast epigenetic changes in dispersal behaviour. Evol. Ecol. 29, 299–310. ( 10.1007/s10682-015-9756-9) [DOI] [Google Scholar]
- 22.Camacho C, Canal D, Potti J. 2016. Natal habitat imprinting counteracts the diversifying effects of phenotype-dependent dispersal in a spatially structured population. BMC Evol. Biol. 16, 158 ( 10.1186/s12862-016-0724-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fattebert J, Balme G, Dickerson T, Slotow R, Hunter L. 2015. Density-dependent natal dispersal patterns in a leopard population recovering from over-harvest. PLoS ONE 10, 1–15. ( 10.1371/journal.pone.0122355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cozzi G, Maag N, Börger L, Clutton-Brock T, Ozgul A. 2018. Socially informed dispersal in a territorial cooperative breeder. J. Anim. Ecol. 38, 42–49. ( 10.1111/1365-2656.12795) [DOI] [PubMed] [Google Scholar]
- 25.Maag N, Cozzi G, Clutton-Brock T, Ozgul A. 2018. Density-dependent dispersal strategies in a cooperative breeder. Ecology 99, 1932–1941. ( 10.1002/ecy.2433) [DOI] [PubMed] [Google Scholar]
- 26.van den Brink V, Dreiss AN, Roulin A. 2012. Melanin-based coloration predicts natal dispersal in the barn owl, Tyto alba. Anim. Behav. 84, 805–812. ( 10.1016/j.anbehav.2012.07.001) [DOI] [Google Scholar]
- 27.Sih A, Del Giudice M. 2012. Linking behavioural syndromes and cognition: a behavioural ecology perspective. Phil. Trans. R. Soc. B 367, 2762–2772. ( 10.1098/rstb.2012.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rémy A, Le Galliard JF, Gundersen G, Steen H, Andreassen HP. 2011. Effects of individual condition and habitat quality on natal dispersal behaviour in a small rodent. J. Anim. Ecol. 80, 929–937. ( 10.1111/j.1365-2656.2011.01849.x) [DOI] [PubMed] [Google Scholar]
- 29.Perrig M, Grüebler MU, Keil H, Naef-Daenzer B. 2017. Post-fledging survival of little owls Athene noctua in relation to nestling food supply. Ibis (Lond. 1859) 159, 532–540. ( 10.1111/ibi.12477) [DOI] [Google Scholar]
- 30.Perrig M, Grüebler MU, Keil H, Naef-Daenzer B. 2014. Experimental food supplementation affects the physical development, behaviour and survival of Little Owl Athene noctua nestlings. Ibis (Lond. 1859) 156, 755–767. ( 10.1111/ibi.12171) [DOI] [Google Scholar]
- 31.Michel VT, Naef-Daenzer B, Keil H, Grüebler MU. 2017. Reproductive consequences of farmland heterogeneity in little owls (Athene noctua). Oecologia 183, 1019–1029. ( 10.1007/s00442-017-3823-6) [DOI] [PubMed] [Google Scholar]
- 32.Baglione V, Canestrari D, Marcos JM, Ekman J. 2006. Experimentally increased food resources in the natal territory promote offspring philopatry and helping in cooperatively breeding carrion crows. Proc. R. Soc. B 273, 1529–1535. ( 10.1098/rspb.2006.3481) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dickinson JL, McGowan A. 2005. Winter resource wealth drives delayed dispersal and family-group living in western bluebirds. Proc. R. Soc. B 272, 2423–2428. ( 10.1098/rspb.2005.3269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Debeffe L, Morellet N, Cargnelutti B, Lourtet B, Bon R, Gaillard JM, Mark Hewison AJ. 2012. Condition-dependent natal dispersal in a large herbivore: heavier animals show a greater propensity to disperse and travel further. J. Anim. Ecol. 81, 1327–1327. ( 10.1111/j.1365-2656.2012.02014.x) [DOI] [PubMed] [Google Scholar]
- 35.Delgado MM, Penteriani V, Revilla E, Nams VO. 2010. The effect of phenotypic traits and external cues on natal dispersal movements. J. Anim. Ecol. 79, 620–632. ( 10.1111/j.1365-2656.2009.01655.x) [DOI] [PubMed] [Google Scholar]
- 36.Baguette M, Stevens VM, Clobert J. 2014. The pros and cons of applying the movement ecology paradigm for studying animal dispersal. Mov. Ecol. 2, 1–13. ( 10.1186/s40462-014-0013-6)25520812 [DOI] [Google Scholar]
- 37.Grüebler MU, Müller M, Michel VT, Perrig M, Keil H, Naef-Daenzer B, Korner-Nievergelt F. 2018. Brood provisioning and reproductive benefits in relation to habitat quality: a food supplementation experiment. Anim. Behav. 141, 45–55. ( 10.1016/j.anbehav.2018.05.009) [DOI] [Google Scholar]
- 38.Emlen ST. 1994. Benefits, constraints and the evolution of the family. Trends Ecol. Evol. 9, 282–285. ( 10.1016/0169-5347(94)90030-2) [DOI] [PubMed] [Google Scholar]
- 39.Hatchwell BJ, Komdeur J. 2000. Ecological constraints, life history traits and the evolution of cooperative breeding. Anim. Behav. 59, 1079–1086. ( 10.1006/anbe.2000.1394) [DOI] [PubMed] [Google Scholar]
- 40.Apolloni N, Grüebler MU, Arlettaz R, Gottschalk TK, Naef-Daenzer B. 2017. Habitat selection and range use of little owls in relation to habitat patterns at three spatial scales. Anim. Conserv. 21, 1–11. ( 10.1111/acv.12361) [DOI] [Google Scholar]
- 41.Bock A, Naef-Daenzer B, Keil H, Korner-Nievergelt F, Perrig M, Grüebler MU. 2013. Roost site selection by Little Owls Athene noctua in relation to environmental conditions and life-history stages. Ibis (Lond. 1859) 155, 847–856. ( 10.1111/ibi.12081) [DOI] [Google Scholar]
- 42.van Nieuwenhuyse D, Génot J-C, Johnson DH. 2008. The little owl: conservation, ecology and behaviour of Athene noctua. New York, NY: Cambridge University Press. [Google Scholar]
- 43.Tschumi M, et al. 2019. Parental sex allocation and sex-specific survival drive offspring sex ratio bias in little owls. Behav. Ecol. Sociobiol. 73, 85 ( 10.1007/s00265-019-2694-8) [DOI] [Google Scholar]
- 44.White GC, Garrott RA. 1990. Analysis of wildlife radio-tracking data. London, UK: Academic Press.
- 45.Janse van Rensburg J, McMillan M, Giżejewska A, Fattebert J. 2018. Rainfall predicts seasonal home range size variation in Nyala. Afr. J. Ecol. 56, 418–423. ( 10.1111/aje.12455) [DOI] [Google Scholar]
- 46.Fattebert J, Michel V, Scherler P, Naef-Daenzer B, Milanesi P, Grüebler MU. 2018. Little owls in big landscapes: informing conservation using multi-level resource selection functions. Biol. Conserv. 228, 1–9. ( 10.1016/j.biocon.2018.09.032) [DOI] [Google Scholar]
- 47.Michelot T, Langrock R, Patterson TA. 2016. moveHMM: an R package for the statistical modelling of animal movement data using hidden Markov models. Methods Ecol. Evol. 7(11), 1308–1315. ( 10.1111/2041-210X.12578) [DOI] [Google Scholar]
- 48.Fattebert J, Robinson HS, Balme G, Slotow R, Hunter L. 2015. Structural habitat predicts functional dispersal habitat of a large carnivore: how leopards change spots. Ecol. Appl. 25, 1911–1921. ( 10.1890/14-1631.1) [DOI] [PubMed] [Google Scholar]
- 49.Stan Development Team. 2016. rstanarm: Bayesian applied regression modeling via Stan. R package version 2.13.1. http://mc-stan.org/.
- 50.Tarwater CE, Brawn JD. 2010. Family living in a Neotropical bird: variation in timing of dispersal and higher survival for delayed dispersers. Anim. Behav. 80, 535–542. ( 10.1016/j.anbehav.2010.06.017) [DOI] [Google Scholar]
- 51.Naef-Daenzer B, Grüebler MU. 2016. Post-fledging survival of altricial birds: ecological determinants and adaptation. J. F. Ornithol. 87, 227–250. ( 10.1111/jofo.12157) [DOI] [Google Scholar]
- 52.Grüebler MU, Naef-Daenzer B. 2008. Postfledging parental effort in barn swallows: evidence for a trade-off in the allocation of time between broods. Anim. Behav. 75, 1877–1884. ( 10.1016/j.anbehav.2007.12.002) [DOI] [Google Scholar]
- 53.Penteriani V, Delgado MM. 2011. Birthplace-dependent dispersal: are directions of natal dispersal determined a priori? Ecography (Cop.) 34, 729–737. ( 10.1111/j.1600-0587.2010.06773.x) [DOI] [Google Scholar]
- 54.Bombieri G, Fasciolo A, Penteriani V, Illera JC, Chamberlain D, Delgado MdM. 2018. Disentangling the effects of genetic and environmental factors on movement behaviour. Ethology 124, 139–148. ( 10.1111/eth.12712) [DOI] [Google Scholar]
- 55.Michel VT, Jiménez-Franco MV, Naef-Daenzer B, Grüebler MU. 2016. Intraguild predator drives forest edge avoidance of a mesopredator. Ecosphere 7, 1–12. ( 10.1002/ecs2.1229) [DOI] [Google Scholar]
- 56.Galliard JL, Ferriere CJ. 2003. Mother–offspring interactions affect natal dispersal in a lizard. Proc. R. Soc. Lond. B 270, 1163–1169. ( 10.1098/rspb.2003.2360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Michelangeli M, Smith CR, Wong BBM, Chapple DG. 2017. Aggression mediates dispersal tendency in an invasive lizard. Anim. Behav. 133, 29–34. ( 10.1016/j.anbehav.2017.08.027) [DOI] [Google Scholar]
- 58.Pasinelli G, Schiegg K, Walters JR. 2004. Genetic and environmental influences on natal dispersal distance in a resident bird species. Am. Nat. 164, 660–669. ( 10.1086/424765) [DOI] [PubMed] [Google Scholar]
- 59.Paniw M, Maag N, Cozzi G, Clutton-Brock T, Ozgul A. 2019. Life history responses of meerkats to seasonal changes in extreme environments. Science 363, 631–635. ( 10.1126/science.aau5905) [DOI] [PubMed] [Google Scholar]
- 60.Hauenstein S, Fattebert J, Grüebler M, Naef-Daenzer B, Pe'er G, Hartig F. 2019. Calibrating an individual-based movement model to predict functional connectivity for little owls. Ecol. Appl. 29, 1–16. ( 10.1002/eap.1873) [DOI] [PubMed] [Google Scholar]
- 61.Class B, Brommer JE. 2015. A strong genetic correlation underlying a behavioural syndrome disappears during development because of genotype–age interactions. Proc. R. Soc. B 282, 20142777 ( 10.1098/rspb.2014.2777) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stevens VM, Trochet A, Blanchet S, Moulherat S, Clobert J, Baguette M. 2013. Dispersal syndromes and the use of life-histories to predict dispersal. Evol. Appl. 6, 630–642. ( 10.1111/eva.12049) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The primary telemetry and dispersal parameters data have been submitted as electronic supplementary material.