Abstract
Animal acoustic communication systems can be built upon co-opted structures that become specialized for sound production or morphological novelties. The ghost crab, Ocypode quadrata, evolved a novel stridulation apparatus on the claws that is used during agonistic interactions, but they also produce a rasping sound without their claw apparatus. We investigated the nature of these sounds and show that O. quadrata adopted a unique and redundant mode of sound production by co-opting the gastric mill (grinding teeth of the foregut). Acoustic characteristics of the sound are consistent with stridulation and are produced by both male and female crabs during aggressive interactions. Laser Doppler vibrometry localized the source of maximum vibration to the gastric region and fluoroscopy showed movement of the gastric mill that coincided with stridulation. The lateral teeth of the gastric mill possess a series of comb-like structures that rub against the median tooth to produce stridulation with dominant frequencies below 2 kHz. This previously undescribed gastric stridulation can be modulated and provide a means of assessment during aggressive interactions, similar to the use of the claw stridulation apparatus. This functional redundancy of stridulation in crabs offers unique insights into the mechanisms of evolution of acoustic communication systems.
Keywords: bioacoustics, Crustacea, Ocypode, animal behaviour, functional morphology
1. Introduction
The co-option of existing structures and emergence of morphological novelties are considered key routes for evolutionary diversification [1,2]. Both mechanisms have contributed to the broad repertoire of acoustic communication systems across the animal kingdom. Structures as diverse as swim bladders [3], wings [4], feathers [5], respiratory tracts [6], and walking legs [4] have become co-opted for acoustic communication, but less diversified are novel morphologies, such as the rattle of rattlesnakes [7], stridulatory structures in arthropods [8,9], and tymbals in insects [10]. Despite the pre-adaptive nature of so many structures for sound production, redundant morphologies with similar function are uncommon. An intriguing example of acoustic redundancy is the coevolution of vocal, wing feather, and tail feather mechanisms in male hummingbirds, which all produce similar acoustics [11]. Vocalizations are used for both defensive and courtship functions, but the feather mechanisms are specific to males and are likely driven by sexual selection. Here, we present a compelling example in an invertebrate, wherein both novel and co-opted structures are used in duplicative function, with neither being driven by sexual selection. Our primary goal is to characterize a previously undescribed form of acoustic communication in crabs that resulted from the co-option of the gastric teeth. This acoustic signal is similar in structure and is elicited in a context consistent with a similarity in function as that produced by the specialized stridulation morphology on the claws, but the gastric mechanism has the advantage of freeing the claws for display and aggressive actions.
Crustaceans have evolved a diversity of mechanisms for communicating with sound during courtship, agonistic interactions, and predator deterrence [12–14]. Sound production is not easily observed, particularly in aquatic species, but its importance is signified by the evolution of specialized sound-producing morphology in more than 30 genera of Brachyura [4,15]. Most crab species have specialized, external sound-producing structures on their exoskeleton that, when rubbed together, generate a rasping sound known as stridulation [15]. Stridulation is just one of multiple acoustic mechanisms used by crabs, which also produce sounds by percussion (drumming of body parts against each other or the substrate), tremulation of carapace or appendages, and bubbling of fluids from the buccal cavity [16–19]. Of these various forms of sound production, only stridulation requires special morphological structures: a plectrum (scraper) and a pars stridens (file). In crabs, the pars stridens is typically a series of tubercles that rubs against a ridge-like plectrum. Their locations on the cheliped, walking legs, and cephalothorax suggest that these acoustic structures are novel morphologies rather than co-opted structures [4,15].
Most of what is known about crab acoustic communication stems from studies on the semi-terrestrial fiddler and ghost crabs (Ocypodidae). Ghost crabs can be heard stridulating from inside their burrows [20] and seen stridulating with their claws at the burrow entrance when intruders approach [21,22]. Stridulation is used in territorial or other agonistic interactions, whereas claw waving and dancing displays with drumming are used by males during courtship [17,20,21,23,24]. The importance of claw stridulation for defence is indicated by the evolution of specialized claw stridulation apparatus in both males and females of all but one species of ghost crab [15]. In the Atlantic ghost crab, Ocypode quadrata, the stridulation apparatus is located on the major claw of both male and female crabs (figure 4d). To stridulate, crabs must flex their large claw so that the inner surface of the propodus, where the pars stridens is located, rubs against the plectrum on a basal segment (ischium) of the same cheliped using a lateral movement [13] (figure 4d). During close agonistic interactions, such as those that occur when defending burrows or food, the chelae are usually held outstretched either laterally or forward, presenting a visual display that is sometimes accompanied by pouncing [25–27]. In the laboratory, we observed that O. quadrata amends this aggressive behaviour with a rasping sound, sometimes accompanied by bubbling (electronic supplementary material, video S1). Yet, the outstretched claws preclude stridulation by the specialized claw apparatus. Here, we characterize this obscure acoustic signal as well as determine its origin and mechanism. Sound production and communication using the specialized stridulation apparatus on the claw is already well documented in ghost crabs [12,13,17,18,22–24,28], and it has been assumed that all stridulation sounds are produced by the claws. We have compelling observations, however, that there is another key sound production mechanism, the gastric mill, which appears to provide functional redundancy in ghost crab communication.
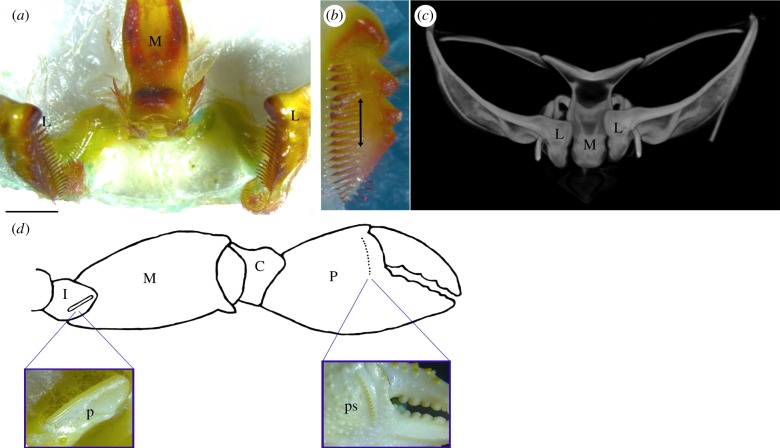
Figure 4.
Ghost crab gastric (a–c) and claw (d) stridulation apparatus. (a) Microscope image showing lateral teeth in open position, apart from the medial tooth. (b) Lateral tooth with 17 small comb-like teeth and arrow showing direction of movement along the medial tooth. (c) CT scan of intact gastric mill showing mineralized ossicles and closed position of the lateral teeth against the medial tooth (antero-ventral view). L, lateral tooth; M, medial tooth. Scale bar, 2.5 mm. (d) Major claw showing the location of novel stridulation structures. To stridulate, crabs flex the cheliped to rub the pars stridens of the propodus across the plectrum on the ischium. Enlarged images show the ridge-like plectrum (p) and the 14 tubercles of the pars stridens (ps). I, ishium; M, merus; C, carpus; P, propodus.
2. Material and methods
(a). Animals and care
Live Atlantic ghost crabs Ocypode quadrata (Decapoda, Ocypodidae) from the Florida panhandle were purchased from a supplier (Gulf Specimen Marine Lab, FL, USA). A total of 30 juvenile and adult crabs were used in this study (carapace width range 26.4–46.6 mm; 16 male and 14 female). Measurements of crabs were conducted at the University of California, Berkeley, and the Scripps Institution of Oceanography at the University of California, San Diego (UCSD). Crabs were maintained in an environmental room set at 28°C and a 12 h day, 12 h night cycle. Humidifiers kept the room at a minimum of 80% humidity. Crabs were held in individual plastic storage containers (43 cm × 30 cm × 16.5 cm) with a thick layer of sand moistened with seawater (35 ppt) and a water dish large enough for crabs to soak in. Crabs were fed carrots, lettuce, and cat food three times per week. Water was changed and sand was cleaned daily.
(b). Behaviour and acoustic analysis
Observations of sound production and associated behaviours were made on 15 crabs, 5 trials each. Testing was performed in an acoustic chamber at UC Berkeley, where individual crabs were placed in a large glass aquarium (55 gal) with a layer of sand on the bottom. A multi-directional microphone (6.3 Hz–20 kHz, Type 4189, Bruel & Kjaer, Naerum, Denmark) connected to a preamplifier (Type 2671, Bruel & Kjaer) and a digital audio recorder (48 kHz sample rate, maximum 20 kHz frequency response, PMD670, Marantz, NJ, USA) was placed in the middle of the tank. Aggression was elicited by approaching the crab closely with a rod, and each trial where rasping was heard was noted along with the corresponding behaviour. In addition, the following stimuli were presented to a subset of five crabs to determine a range of stimuli that induces the rasping sound: dead and live ghost crabs, plastic toy crab, and a remote control robotic toy (Hexbug spider). This series of stimuli was presented to individual crabs in random order, over the course of 4 days (one stimulus per day).
Audio recordings of crabs producing rasping sounds were analysed for acoustic characteristics using Raven software (v. 1.5, Cornell Lab of Ornithology, Ithaca, NY, USA). A bout of stridulation was defined as a sequence of rasps. Individual rasps were considered distinct when a pause of duration longer than the pulse rate occurred between consecutive rasps or there was an abrupt shift in pulse rate. Rasp rate was calculated as the number of rasps in a sequence divided by the duration of the sequence (from the start of the first rasp to the end of the last rasp). Within an individual rasp, the number of pulses were counted from the oscillogram and divided by the duration of the rasp to determine pulse frequency. Ranges and means of pulse frequency were determined from five individual rasps for each crab.
(c). Laser Doppler vibrometry
We used laser Doppler vibrometry on five crabs to locate the sound source by measuring exoskeletal regions that may vibrate from moving internal structures. The laser Doppler vibrometer (LDV, PDV 100, Polytec Inc., Irvine, CA, USA) was set for a peak velocity measurement range of ±20 mm s−1 with a low-pass filter at 22 kHz and was focused on multiple regions of the crab body. Small reflective stickers (less than 5 × 5 mm) were placed on the following exoskeleton locations to focus the laser: gastric, branchial, cardiac, and frontal regions of the carapace, maxilliped, and merus of the cheliped (see figure 3 for locations). Vibrations were digitized at a sampling rate of 48.1 kHz. An individual crab was strapped to the LDV platform using Velcro and aggressive behaviour was induced by prodding with a rod. Clear vibration impulses were viewed and analysed for RMS amplitude using Raven software (v. 1.5, Cornell Lab of Ornithology, Ithaca, NY, USA).
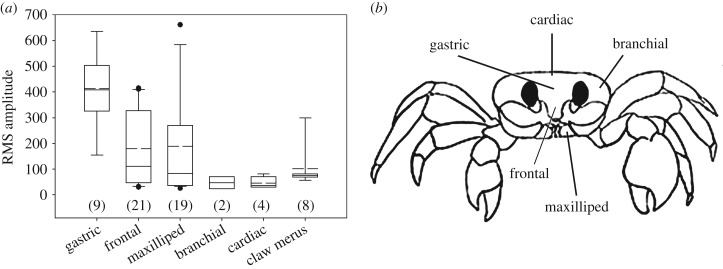
Figure 3.
(a,b) LDV signals from ghost crabs. (a) Amplitude of the vibration signal was significantly greater in the gastric region compared to all other regions. Number of signals analysed, N, noted in parentheses. Solid line, mean; dashed line, median. (b) Regions of crab measured with LDV.
(d). Fluoroscopy
Attempts were made to visualize movement of the gastric mill during aggressive behaviour using fluoroscopy. Five live crabs were imaged (Hologic 9900 OEC c-arm) in the Radiology unit at Hillcrest Medical Center, UCSD. Oral contrast (dilute barium) was administered to crabs through the buccal region using a syringe. To permit transmission of X-rays, crabs were recorded in a clear acrylic box (39 × 23 × 23 cm) placed on a table directly under the X-ray c-arm. X-ray imaging was processed at a rate of 15 frames s−1. Aggressive behaviour of crabs was induced by either approaching the crab with a net handle or adding a second live crab to the box. Video frames were examined for gastric mill activity using MicroDicom viewer (v. 2.8.3, Sofia, Bulgaria).
(e). Gastric mill morphology
The morphology of the gastric mill was examined to determine correlation of structure with the observed acoustic characteristics. Crabs (N = 18) were anaesthetized and euthanized by placement in a −20°C freezer. The gastric mill was dissected, cleaned of extra tissue and placed in 70% ethanol. The medial and lateral teeth of the gastric mill were examined and imaged with a digital camera (Leica DFC290, Buffalo Grove, 206 IL, USA) attached to a stereomicroscope (Leica M165 C, Buffalo Grove, Illinois, USA). From the digital images, the number of small comb-like teeth was counted on each gastric mill. The widths of five comb-like teeth from each lateral tooth were measured using microscope software. In addition, one complete gastric mill was carefully cleaned and imaged using Micro-Computed Tomography scanning (Micro-CT; Skyscan 1076, Kontich, Belgium) at the Cartilage Tissue Engineering Lab at UCSD. The gastric mill was scanned in humidified air under high resolution (9 µm voxel size) and processed for 3D rendering using CTVox software (Skyscan, Kontich, Belgium).
(f). Statistics
Acoustic data were tested for normality using the Shapiro–Wilk test and homogeneity using Spearman tests. Rasp and pulse characteristics were compared between sexes using two-tailed t-tests and with carapace width using linear regression. Signal RMS amplitudes were compared among exoskeleton regions using the Kruskal–Wallis and pairwise comparisons were made using pairwise Wilcoxon with BH correction. Statistics were performed using SigmaPlot v. 12.5 and R (v. 3.0.2).
3. Results
(a). Acoustic characteristics
Rasping sounds produced by the hypothesized gastric mechanism were heard in 13 of the 15 crabs tested. Spectrograms and oscillographs showed a series of homogeneous impulses, or oscillations, that are characteristic of stridulation [29] (figure 1). Specifically, stridulation consists of a series of rasps, each of which contain evenly spaced pulses that represent individual tubercles of the plectrum striking the ridge of the pars stridens.
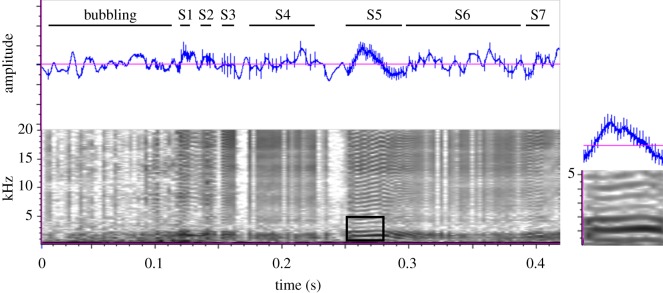
Figure 1.
Acoustic recording of a ghost crab bubbling and stridulating. Oscillogram (top) shows consistently spaced pulses within each rasp of stridulation but not during bubbling. Spectrogram (bottom) shows seven stridulations, or rasps, with most of the energy below 2 kHz and visible harmonics at high pulse rates. Rasp duration, pulse number, and pulse rates are highly variable within an individual (S1–S7). The boxed region of a rasp is magnified on the right to show harmonics with greatest energy.
A bout of stridulation ranged in duration from 0.48 to 0.95 s (0.68 ± 0.16, mean ± s.d.), with the number of rasps ranging from 5 to 13 (N = 13; 7.8 ± 2.2) (figure 2). Rasp rate (11.6 ± 1.8 Hz) was correlated with crab carapace width (linear regression: slope = 0.17, R2 = 0.37, N = 13, p = 0.03), revealing that larger crabs had higher rasp rates. Within a rasp, pulses, which reflect individual tubercles rubbing against a ridge, ranged from 4 to 31 (6.7 ± 1.3), but the pulse rate (149 ± 32 Hz) did not correlate with crab size (linear regression: slope = 0.62, R2 = 0.02, N = 13, p = 0.69). Female and male crabs had similar rasp (t-test: t = 1.469, d.f. = 11, N = 5, 8, p = 0.17) and pulse rates (t-test: t = −0.405, d.f. = 11, N = 5, 8, p = 0.69). Variation within individuals was high for all stridulation characteristics (figure 1), indicating signal modulation.
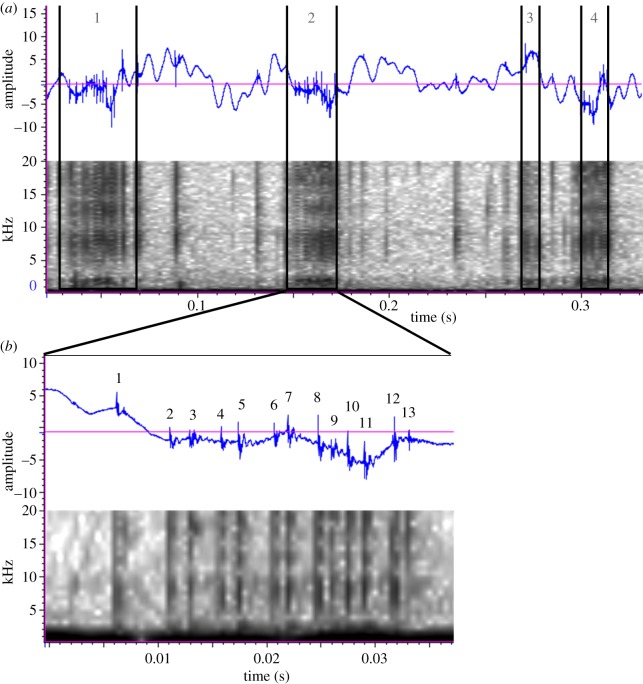
Figure 2.
Characteristics of gastric mill stridulation. A bout of stridulation (a) composed of four rasps, each of different duration (noted by width of the column). Rasp frequency was calculated as the number of rasps divided by the duration of stridulation bout (11.4 Hz in this example). Magnification of an individual rasp (b) revealing individual pulses. Each pulse represents a ridge of the comb-like structures on the lateral tooth rubbing against the medial tooth. Pulse rate was calculated as the number of pulses divided by the duration of the rasp (481 Hz in this example).
(b). Behavioural context
Rasping sounds by the hypothesized gastric mechanism were produced by both males (N = 7) and females (N = 6), and were emanated as part of a consistent behaviour during aggressive interactions. Of the trials where rasps were produced (35 total), crabs held their claws outstretched in an aggressive posture during 100% of the trials (electronic supplementary material, video S1). Rasping occurred with lunging attacks in the majority (77%) of those trials. Attacks were characterized by the chelae being outstretched and thrusting forward to grasp as the crab lunges forward. In all of the remaining trials with rasps (23%), crabs held the chelae outstretched, but without lunging. This aggressive behaviour with rasping sounds was consistently observed when other crabs or objects were placed within close range of the crab (less than 10 cm). Three of five crabs responded aggressively with rasping to a toy crab, hexbug, and live conspecific, and all crabs responded to dead conspecifics frozen with their chelipeds spread laterally in a threat display. These aggressive behaviours are consistent and characterized by spreading of the chelipeds, lunging, and snapping the chelae, often with a rasping sound that was sometimes accompanied by bubbling (electronic supplementary material, video S1).
(c). Sound source
Laser Doppler vibrometry detected clear exoskeletal vibrations from each target location, with vibration signal detected primarily in the anterior part of the crab (figure 3). The vibration signal varied in amplitude among locations, with the greatest amplitude occurring in the gastric region of the carapace (Kruskal–Wallis, χ2 = 17.9, d.f. = 5, p = 0.003, pairwise Wilcoxon, p < 0.05). The vibration signal was also strong in the buccal region. In a species of freshwater crab, the second and third maxillipeds have small tubercles that produce stridulation when rubbed together [30], but these appendages do not move when O. quadrata produces pure stridulation (without bubbling) nor do they have structures that could function as a plectrum or pars stridens. Regardless, we immobilized the maxillipeds of one crab with a small drop of cyanoacrylate glue, which did not prevent it from stridulating, thereby confirming that these structures are not the sound source.
Fluoroscopy enabled visualization of gastric mill activity in six instances of aggressive behaviour. In only one brief sequence, however, did the crab remain stationary to provide sufficient resolution for two full sequences of gastric movement (electronic supplementary material, video S2). Opening, closing, and grinding movements of the lateral teeth were observed, each occurring within one frame (0.067 s), which is within the same timescale of an individual rasp (0.048 ± 0.010 s). Video resolution was insufficient to calculate the precise duration of the lateral teeth rubbing against the medial tooth. An estimated pulse frequency based on the duration of one frame (0.067 s) and the range of individual pulses detected in rasps (4–31) provides a range of 60–463 Hz. This range is congruent with the range determined from the acoustic recordings.
(d). Gastric mill morphology
The medial tooth is 5.49 ± 1.04 mm (mean ± s.d.) in length, which is more than twice the length of the lateral teeth (2.54 ± 0.51 mm). Each of the lateral teeth possesses four large denticles and a series of small comb-like teeth (figure 4). The number of comb-like teeth on each lateral tooth ranged from 14 to 19 (17 ± 1) and was not correlated with crab size (linear regression: slope = 0.038, R2 = 0.027, N = 18, p = 0.51). The length (0.60 ± 0.24 mm) and width (0.14 ± 0.02 mm) of the small comb-like teeth were also consistent. All of the comb-like teeth, the denticles and the medial tooth are lined with a brown pigment that is typical of hard mastication structures.
4. Discussion
The ghost crab, O. quadrata, communicates with sounds that are characteristic of stridulation, but not with their specialized claw apparatus. Laser Doppler vibrometry helped to pinpoint the sound source to the foregut, where the gastric mill is located. The gastric mill is the only viable internal structure, with its intricate system of more than 40 ossicles and 1 large medial tooth and paired lateral teeth that work together to grind up food [31]. It is supported by a complex musculature and controlled by the stomatogastric ganglion (STG), allowing the lateral and medial teeth to move independently, in multiple planes and at different frequencies [32]. Motions of the gastric mill that are needed to stridulate stem from the pre-existing repertoire of movements used during mastication. We propose that ghost crabs stridulate by using the grinding motions of the medial tooth against the surface of the small comb-like structures on the lateral teeth. The congruence of morphology and movement with the observed acoustic characteristics further corroborate the gastric mill as a previously unidentified mechanism of sound production.
While some studies have successfully used neurophysiology, electromyography, and even endoscopy to study the control and movement of the gastric mill [32–35], they were carried out on much larger Cancer crabs and lobsters that were immobilized. Such methods are intractable for observing in vivo movement of the gastric mill in small, lunging ghost crabs. With fluoroscopy, we were able to observe gastric mill activity, including the opening, closing, and grinding movements of the lateral teeth. Though the resolution was insufficient to directly correlate with acoustic characteristics, the time course for the observed movements was consistent with the stridulation characteristics we recorded. Specifically, each movement occurred within a single frame (0.067 s), which corresponds to the average duration of an individual rasp (0.048 ± 10 s). Ghost crabs have a relatively high gastric mill rhythm, with a complete cycle occurring in as little as 0.2 s. By contrast, spontaneous chewing in the California spiny lobster involves gastric mill cycles as long at 27 s [32].
In addition to sharing the standard characteristics of stridulation, there are other similarities between claw stridulation and the gastric stridulation observed in this study. Both mechanisms show modulation, which results in high variability of the acoustic signal within an individual crab. During gastric stridulation, crabs can modulate the duration of the grinding stroke and thus the number of comb-like structures that the medial tooth rubs against. The number of pulses observed in the spectrograms of an individual rasp is therefore highly variable, ranging from 4 to 31, but always fewer than the total number of comb-like structures on the two lateral teeth. Similarly, in the ghost crab Ocypode jousseamuei, the pars stridens on the claw comprises 18 tubercles, but individual rasps contain only one to six pulses [22]. Both forms of stridulation have dominant frequencies that are generally low, below 2–3 kHz [18,22,28]. The claw stridulation apparatus varies among species of ghost crabs, resulting in species-specific acoustic signals [22]. Variation in gastric mill morphology may likewise produce acoustic signals that carry species identification. The primary distinguishing characteristic between the two forms of stridulation is that the pulse frequency is much higher when using the gastric apparatus (149 ± 32 Hz) than the claw apparatus (approximately 30–40 Hz) [18,22], at least for O. quadrata in this first description of gastric stridulation
Gastric stridulation appears to be of an aggressive or defensive nature and is used by juvenile and mature crabs of both sexes during close agonistic or otherwise threatening interactions. This acoustic signal is within the audible frequency range of common predators, such as raccoons (0.14–37 kHz) [36] and birds (1–5 kHz) [37], as well as conspecifics, which respond to airborne sounds of the same frequency, 3 kHz [18]. The observed behaviours of ghost crabs when stridulating with the gastric mill are consistent with field observations of burrow and food defence. Rather than constructing new burrows, O. quadrata prefers to annex those of other crabs [38], such that agonistic encounters are frequent. Gastric stridulation may have originated from physiological changes associated with aggression. A variety of neurotransmitters and hormones are known to affect the rhythmic output of the STG [39]. Seratonin, for instance, controls and modulates the STG [40,41], and is also associated with aggression in crustaceans [42,43]. Elevated levels of serotonin during agonistic interactions could stimulate action of the gastric mill in the absence of digestion processes.
A key advantage of using gastric stridulation over the claw apparatus is that it provides signal while freeing up the chelae for postural display and attack readiness. Generally, the acoustic characteristics of gastric stridulation are variable (e.g. pulse number, pulse frequency), potentially modulated by aggression levels. Rasp rate, however, was correlated with body mass and may thus provide a means of assessment during aggressive interactions. Ghost crabs may convey information about size or intent during aggressive gastric stridulation in a similar way that canines use growling as an aggressive warning when protecting food or territory [44,45] or red deer use roaring for assessment during contests [46]. The utility of the gastric mill for acoustic communication poses interesting questions about the evolution of the claw stridulation apparatus, considered to be a morphological novelty in some crabs.
The co-option of structures for sound production is common among animal lineages because sound is a byproduct of movement [47]. Most familiar are vertebrate vocalizations co-opted from respiratory structures that vibrate during breathing, but the vast majority of acoustic signals are co-opted from moving external structures. These mechanisms are remarkably diverse and include, for example, castanets on the forewings of moths that percuss during flight [48], anal oars of caterpillars that scrape and stridulate during crawling [49], thickened feather shafts of manakins that resonate after wing contact [50], and the specialized claw of snapping shrimp that produces cavitation during closure [51]. There are many other examples to demonstrate that evolutionary co-option is a common origin of novel signals. This newly described internal gastric stridulation by ghost crabs presents another novel mechanism to probe the evolution and diversification of acoustic communication systems.
While gastric stridulation appears to be unique, food grinding structures are inherent sound producers. Even the grinding of gizzards can be heard when chickens are feeding [52]. Considering the repeated convergence of sound-producing mechanisms in lineages, it is intriguing that similar mastication structures have not been co-opted for acoustic communication in other organisms, or at least not yet discovered. The only other known examples are fish that have co-opted their pharyngeal teeth for acoustic communication by rubbing the upper and lower teeth together [3]. Gastric mills are omnipresent in crustaceans and most other arthropods, but similar food grinding machinery exists in the stomachs of animals as diverse as birds [53], molluscs [54], worms [55], and dinosaurs [56]. We therefore pose the possibility that this sound production mechanism lays undetected in other animals as well, as it has only now been described even in a well-studied crab.
It is unusual that O. quadrata evolved specialized stridulation morphology on the claws and co-opted the gastric mill when both produce stridulation sounds with apparently similar defensive function. While it is not uncommon for animals to use multiple modes of acoustic communication, they are typically partitioned into mating and defence functions. For example, hummingbirds of both sexes use vocalizations for a variety of purposes, but males also co-opted wings and tails to produce sounds as part of their courtship display [5,11]. Fiddler and ghost crabs use stridulation via the claws for warning signals, whereas males use either claw stridulation or percussion (drumming) for courtship, the latter of which does not require any specialized morphology. In O. quadrata, burrow defence is critical for mating and general survival, yielding high selection pressure on sound-producing mechanisms in aggressive interactions such that redundant acoustic systems can evolve. Claw stridulation may be most effective at a distance, whereas gastric stridulation is effective during close contact because it permits animals to continue conveying size and aggression information acoustically while their weapons are in use.
Sequential, functionally redundant signalling is known to occur in the visual courtship displays of one group of animals (fiddler crabs) [57], but we know of no examples in acoustic communication systems. Redundancy based on novel and co-opted morphologies that are not under sexual selection is to our knowledge rare and may reflect a time point in evolutionary transition or diversification. The evolution of novel signals allows for the expansion of signalling space and confers advantages across many contexts, such as defence and social interactions. The diverse brachyura may, therefore, hold unique insights into the evolutionary processes of co-option and emergence of novelties.
Supplementary Material
Supplementary Material
Acknowledgements
We are grateful to Sheila Patek for insights into crustacean acoustics and Jessica Garcia and Aislynn Valdez from the ENLACE program for dissecting and imaging the gastric mills. Ryan Brunsing, Emily Kelly, and Rebecca Rivera helped with fluoroscopy scanning of the crabs. Robert Sah and Beatrice Tierra performed the Micro-CT scan.
Data accessibility
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.11n81vh [58].
Authors' contributions
J.R.A.T. conceived of the study, carried out the acoustic, behavioural, LDV, and fluoroscopy analyses, supervised the morphology analysis and wrote the manuscript. D.O.E. helped carry out LDV data collection and analysis and edited the manuscript. M.S.d.V. aided in fluoroscopy scanning and acoustic recordings of crabs and edited the manuscript.
Competing interests
The authors declare no competing interests.
Funding
This research was supported by a National Science Foundation (NSF) Minority Postdoctoral Fellowship (to J.R.A.T.) and the Marine Biology Research Division at Scripps Institution of Oceanography.
References
- 1.Gould SJ, Vrba ES. 1982. Exaptation—a missing term in the science of form. Paleobiology 8, 4–15. ( 10.1017/S0094837300004310) [DOI] [Google Scholar]
- 2.McLennan DA. 2008. The concept of co-option: why evolution often looks miraculous. Evol. Educ. Outreach 1, 247–258. ( 10.1007/s12052-008-0053-8) [DOI] [Google Scholar]
- 3.Parmentier E, Diogo R, Fine ML. 2017. Multiple exaptations leading to fish sound production. Fish Fish. 18, 958–966. ( 10.1111/faf.12217) [DOI] [Google Scholar]
- 4.Dumortier B. 1963. Morphology of sound emission apparatus in Arthropoda. In Acoustic behaviour of animals (ed. Busnel R-G.), pp. 277–345. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 5.Clark CJ, Elias DO, Prum RO. 2011. Aeroelastic flutter produces hummingbird feather songs. Science 333, 1430–1433. ( 10.1126/science.1205222) [DOI] [PubMed] [Google Scholar]
- 6.Lieberman P. 1984. The biology and evolution of language. Cambridge, MA: Harvard University Press. [Google Scholar]
- 7.Allf BC, Durst PAP, Pfennig DW. 2016. Behavioral plasticity and the origins of novelty: the evolution of the rattlesnake rattle. Am. Nat. 188, 475–483. ( 10.1086/688017) [DOI] [PubMed] [Google Scholar]
- 8.Gerhardt HC, Huber F. 2002. Acoustic communication in insects and anurans: common problems and diverse solutions. Chicago, IL: University of Chicago Press. [Google Scholar]
- 9.Uhl G, Elias DO. 2011. Communication. In Spider behavior: flexibility and versatility (ed. Herberstein ME.), pp. 127–190. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 10.Bennet-Clark H. 1999. Resonators in insect sound production: how insects produce loud pure-tone songs. J. Exp. Biol. 202, 3347–3357. [DOI] [PubMed] [Google Scholar]
- 11.Clark CJ, McGuire JA, Bonaccorso E, Berv JS, Prum RO. 2018. Complex coevolution of wing, tail, and vocal sounds of courting male bee hummingbirds. Evolution 72, 630–646. ( 10.1111/evo.13432) [DOI] [PubMed] [Google Scholar]
- 12.Salmon M. 1983. Acoustic ‘calling’ by fiddler and ghost crabs. Mem. Austral. Mus. 18, 63–76. ( 10.3853/j.0067-1967.18.1984.372) [DOI] [Google Scholar]
- 13.Clayton D. 2005. Substrate (acoustic/vibrational) communication and ecology of the ghost crab Ocypode jousseaumei (Brachyura: Ocypodidae). Mar. Fresh. Physiol. 38, 53–70. ( 10.1080/10236240500057952) [DOI] [Google Scholar]
- 14.Patek SN. 2001. Spiny lobsters stick and slip to make sound. Nature 411, 153–154. ( 10.1038/35075656) [DOI] [PubMed] [Google Scholar]
- 15.Guinot-Dumortier D, Dumortier B. 1960. La stridulation chez les crabes. Crustaceana 1, 117–155. ( 10.1163/156854060X00168) [DOI] [Google Scholar]
- 16.Taylor J, Patek S. 2010. Crustacean seismic communication: heard but not present. In The use of vibrations in communication: properties, mechanisms and function across taxa (ed. O'Connell-Rodwell CE.), pp. 9–23. Trivandrum, Kerala: Research Signpost. [Google Scholar]
- 17.von Hagen HO. 1975. Klassifikation und phylogenetische Einordnung der Lautausse rungen von Ocypodiden und Grapsiden (Crustacea, Brachyura). Zeitschrift Zool. Syst. 13, 300–316. ( 10.1111/j.1439-0469.1975.tb00511.x) [DOI] [Google Scholar]
- 18.Horch KW, Salmon M. 1969. Production, perception and reception of acoustic stimuli by semiterrestrial crabs (genus Ocypode and Uca, family Ocypodidae). Forma Functio 1, 1–25. [Google Scholar]
- 19.Patek SN, Caldwell RL. 2006. The stomatopod rumble: sound production in Hemisquilla californiensis. Mar. Fresh. Behav. Physiol. 39, 99–111. ( 10.1080/10236240600563289) [DOI] [Google Scholar]
- 20.Hughes D. 1966. Behavioural and ecological investigations of the crab Ocypode ceratophthalmus (Crustacea: Ocypodidae). J. Zool. 150, 129–143. ( 10.1111/j.1469-7998.1966.tb03000.x) [DOI] [Google Scholar]
- 21.Barrass R. 1963. The burrows of Ocypode ceratophthalmus (Pallas) (Crustacea, Ocypodidae) on a tidal wave beach at Inhaca Island, Mocambique. J. Anim. Ecol. 32, 73–85. ( 10.2307/2518) [DOI] [Google Scholar]
- 22.Clayton D. 2001. Acoustic calling in four species of ghost crabs: Ocypode jousseaumei, O. platytarsus, O. rotundata and O. saratan (Brachyura: Ocypodidae). Bioacoustics 12, 37–55. ( 10.1080/09524622.2001.9753477) [DOI] [Google Scholar]
- 23.Clayton D. 2008. Singing and dancing in the ghost crab Ocypode platytarsus (Crustacea, Decapoda, Ocypodidae). J. Nat. Hist. 42, 141–155. ( 10.1080/00222930701840530) [DOI] [Google Scholar]
- 24.Imafuku M, Habu E, Nakajima H. 2001. Analysis of waving and sound-production display in the ghost crab, Ocypode stimpsoni. Mar. Fresh. Behav. Physiol. 34, 197–211. ( 10.1080/10236240109379074) [DOI] [Google Scholar]
- 25.Evans S, Cram A, Eaton K, Torrance R, Wood V. 1976. Foraging and agonistic behaviour in the ghost crab Ocypode kuhlii de Haan. Mar. Fresh. Behav. Physiol. 4, 121–135. ( 10.1080/10236247609386946) [DOI] [Google Scholar]
- 26.Lucrezi S, Schlacher TA. 2014. The ecology of ghost crabs. Ocean. Mar. Biol. 52, 201–256. [Google Scholar]
- 27.Schöne H. 1968. Agonistic and sexual display in aquatic and semi-terrestrial brachyuran crabs. Am. Zool. 8, 641–654. ( 10.1093/icb/8.3.641) [DOI] [Google Scholar]
- 28.Horch K. 1975. The acoustic behaviour of the ghost crab Ocypode cordimana Latreille 1818 (Decapoda, Brachyura). Crustaceana 29, 193–205. ( 10.1163/156854075X00207) [DOI] [Google Scholar]
- 29.Dumortier B. 1963. The physical characteristics of sound emissions in Arthropoda. In Acoustic behaviour of animals (ed. Busnel R-G.), pp. 346–373. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 30.Abele LG, Robinson MH, Robinson B. 1973. Observations on sound production by two species of crabs from Panama (Decapoda, Gecarcinidae and Pseudothelphusidae). Crustaceana 25, 147–152. ( 10.1163/156854073X00795) [DOI] [Google Scholar]
- 31.Brosing A. 2010. Recent developments on the morphology of the brachyuran foregut ossicles and gastric teeth. Zootaxa 2510, 1–44. ( 10.11646/zootaxa.2510.1.1) [DOI] [Google Scholar]
- 32.Heinzel H-G. 1988. Gastric mill activity in the lobster. I. Spontaneous modes of chewing. J. Neurophsyiol. 59, 528–550. ( 10.1152/jn.1988.59.2.528) [DOI] [PubMed] [Google Scholar]
- 33.Heinzel H-G, Weimann JM, Marder E. 1993. The behavioral repertoire of the gastric mill in the crab, Cancer pagurus: an in situ endoscopic and electrophysiological examination. J. Neurosci. 13, 1793–1803. ( 10.1523/JNEUROSCI.13-04-01793.1993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heinzel H-G. 1988. Gastric mill activity in the lobster. II. Proctolin and octopamine initiate and modulate chewing. J. Neurophsyiol. 59, 551–565. ( 10.1152/jn.1988.59.2.551) [DOI] [PubMed] [Google Scholar]
- 35.Heinzel H-G, Selverston AI. 1988. Gastric mill activity in the lobster. III. Effects of proctolin on the isolated central pattern generator. J. Neurophsyiol. 59, 566–585. ( 10.1152/jn.1988.59.2.566) [DOI] [PubMed] [Google Scholar]
- 36.Echteler SW, Fay RR, Popper AN. 1994. Structure of the mammalian cochlea. In Comparative hearing: mammals (eds Fay RR, Popper AN), pp. 134–171. New York, NY: Springer. [Google Scholar]
- 37.Dooling RJ, Popper AN. 2000. Hearing in birds and reptiles: an overview. In Comparative hearing: birds and reptiles (eds Dooling RJ, Fay RR, Popper AN), pp. 1–12. New York, NY: Springer. [Google Scholar]
- 38.Fimpel E. 1972. Phänomene der landadaptation bei terrestrischen und semiterrestrischen Brachyura der brasilianischen Küste (Malacostraca, Decapoda). Zool. Jahrb. Syst. 102, 173–214. [Google Scholar]
- 39.Nusbaum MP, Blitz DM, Swensen AM, Wood D, Marder E. 2001. The roles of co-transmission in neural network modulation. Trends Neurosci. 24, 146–154. ( 10.1016/S0166-2236(00)01723-9) [DOI] [PubMed] [Google Scholar]
- 40.Beltz B, Eisen JS, Flamm R, Harris-Warrick R, Hooper SL, Marder E. 1984. Serotonergic innervation and modulation of the stomatogastric ganglion of three decapod crustaceans (Panulirus interruptus, Homarus americanus and Cancer irroratus). J. Exp. Biol. 109, 35–54. [DOI] [PubMed] [Google Scholar]
- 41.Grashow R, Brookings T, Marder E. 2009. Reliable neuromodulation from circuits with variable underlying structure. Proc. Natl Acad. Sci. USA 106, 11 742–11 746. ( 10.1073/pnas.0905614106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kravitz E. 2003. Serotonin and aggression: insights gained from a lobster model system and speculations on the role of amine neurons in a complex behavior. J. Comp. Physiol. A 186, 221–238. ( 10.1007/s003590050423) [DOI] [PubMed] [Google Scholar]
- 43.Kravitz EA, Huber R. 2003. Aggression in invertebrates. Curr. Opin. Neurobiol. 13, 736–743. ( 10.1016/j.conb.2003.10.003) [DOI] [PubMed] [Google Scholar]
- 44.Yeon SC. 2007. The vocal communication of canines. J. Vet. Behav. 2, 141–144. ( 10.1016/j.jveb.2007.07.006) [DOI] [Google Scholar]
- 45.Taylor AM, Reby D, McComb K. 2010. Size communication in domestic dog, Canis familiaris, growls. Anim. Behav. 79, 205–210. ( 10.1016/j.anbehav.2009.10.030) [DOI] [Google Scholar]
- 46.Clutton-Brock TH, Albon SD. 1979. The roaring of red deer and the evolution of honest advertisement. Behaviour 69, 145–170. ( 10.1163/156853979X00449) [DOI] [Google Scholar]
- 47.Clark CJ. 2016. Locomotion-induced sounds and sonations: mechanisms, communication function, and relationship with behavior. In Vertebrate sound production and acoustic communication (eds Suthers RA, Fitch WT, Fay RR, Popper AN), pp. 83–118. Cham, Switzerland: Springer International. [Google Scholar]
- 48.Bailey WJ. 1978. Resonant wing systems in the Australian whistling moth Hecatesia (Agarasidae, Lepidoptera). Nature 272, 444–446. ( 10.1038/272444a0) [DOI] [Google Scholar]
- 49.Scott JL, Kawahara AY, Skevington JH, Yen S-H, Sami A, Smith ML et al. 2010. The evolutionary origins of ritualized acoustic signals in caterpillars. Nat. Commun. 1, 4 ( 10.1038/ncomms1002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bostwick KS, Elias DO, Mason A, Montealegre-Z F. 2009. Resonating feathers produce courtship song. Proc. R. Soc. B 277, 835–841. ( 10.1098/rspb.2009.1576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Versluis M, Schmitz B, von der Heydt A, Lohse D. 2000. How snapping shrimp snap: through cavitating bubbles. Science 289, 2114–2117. ( 10.1126/science.289.5487.2114) [DOI] [PubMed] [Google Scholar]
- 52.Bunch GH, Adcock DF. 1939. Brief communications and case reports. Giant faceted calculus of the appendix. Ann. Surg. 109, 143–146. ( 10.1097/00000658-193901000-00014)17857305 [DOI] [Google Scholar]
- 53.Moore SJ. 1999. The comparative functional gizzard morphology of several species of birds. Aust. J. Zool. 46, 359–368. ( 10.1071/ZO94037) [DOI] [Google Scholar]
- 54.Morton JE. 1953. The functions of the gastropod stomach. Proc. Linn. Soc. Lond. 164, 240–246. ( 10.1111/j.1095-8312.1953.tb00689.x) [DOI] [Google Scholar]
- 55.Curry JP, Schmidt O. 2007. The feeding ecology of earthworms—a review. Pedobiologia 50, 463–477. ( 10.1016/j.pedobi.2006.09.001) [DOI] [Google Scholar]
- 56.Wings O, Sander PM. 2006. No gastric mill in sauropod dinosaurs: new evidence from analysis of gastrolith mass and function in ostriches. Proc. R. Soc. B 274, 635–640. ( 10.1098/rspb.2006.3763) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Christy JH, Salmon M. 1991. Comparative studies of reproductive behavior in mantis shrimps and fiddler crabs. Am. Zool. 31, 329–337. ( 10.1093/icb/31.2.329) [DOI] [Google Scholar]
- 58.Taylor JRA, deVries MS, Elias DO. 2019. Data from: Growling from the gut: co-option of the gastric mill for acoustic communication in ghost crabs. Dryad Digital Repository. ( 10.5061/dryad.11n81vh) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Taylor JRA, deVries MS, Elias DO. 2019. Data from: Growling from the gut: co-option of the gastric mill for acoustic communication in ghost crabs. Dryad Digital Repository. ( 10.5061/dryad.11n81vh) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.11n81vh [58].