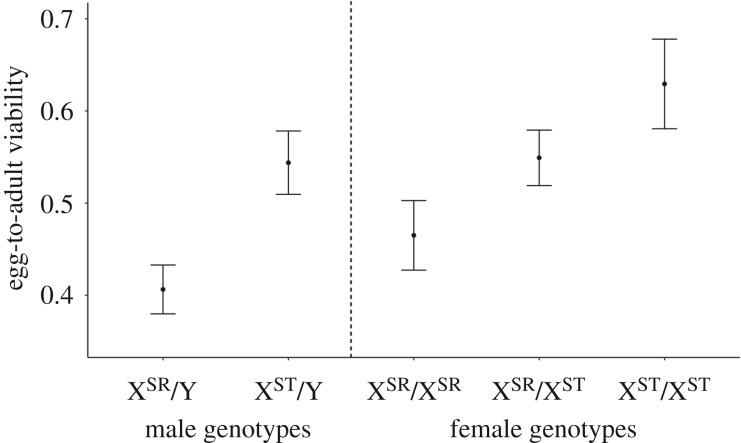Abstract
A number of species are affected by Sex-Ratio (SR) meiotic drive, a selfish genetic element located on the X-chromosome that causes dysfunction of Y-bearing sperm. SR is transmitted to up to 100% of offspring, causing extreme sex ratio bias. SR in several species is found in a stable polymorphism at a moderate frequency, suggesting there must be strong frequency-dependent selection resisting its spread. We investigate the effect of SR on female and male egg-to-adult viability in the Malaysian stalk-eyed fly, Teleopsis dalmanni. SR meiotic drive in this species is old, and appears to be broadly stable at a moderate (approx. 20%) frequency. We use large-scale controlled crosses to estimate the strength of selection acting against SR in female and male carriers. We find that SR reduces the egg-to-adult viability of both sexes. In females, homozygous females experience greater reduction in viability (sf = 0.242) and the deleterious effects of SR are additive (h = 0.511). The male deficit in viability (sm = 0.214) is not different from that in homozygous females. The evidence does not support the expectation that deleterious side effects of SR are recessive or sex-limited. We discuss how these reductions in egg-to-adult survival, as well as other forms of selection acting on SR, may maintain the SR polymorphism in this species.
Keywords: meiotic drive, selfish genetic element, sex ratio, sexual selection, stalk-eyed fly, Teleopsis
1. Introduction
Meiotic drivers are selfish genetic elements that subvert the standard mechanisms of gametogenesis to promote their own transmission [1]. During meiosis, a driver disables or prevents the maturation of gametes that contain the non-driving element [1,2]. In extreme cases, drive can reach 100% transmission to the next generation. In male heterogametic species, drivers are most frequently found on the X-chromosome [3], commonly known as Sex-Ratio or SR [4]. These drivers target developing sperm carrying the Y-chromosome, causing their dysfunction, which results in strongly female-biased broods.
SR is predicted to spread rapidly due to its transmission advantage. When homozygous female fitness is not greatly reduced, SR could potentially spread to fixation and cause population collapse and extinction through massive sex ratio imbalance [5,6]. Empirical evidence for this is limited to laboratory environments where drive causes extinction in small populations [7–9] and a single putative example under natural conditions [10]. More typically, studies in wild populations find that drive exists as a low-frequency polymorphism [10–12], with persistence that can span over a million years [13,14]. In order for SR to persist as a polymorphism, there must be frequency-dependent selection, allowing spread when rare but retarding further increases in frequency as drive becomes more common. The selective counter-forces that fulfil this requirement may act in males or females, but in general, they are not well understood. We discuss potential causes of selection first in males and then females in the following sections.
Selection on male viability may be associated with the drive chromosome. It is likely to operate in a frequency-independent manner and as a consequence will not have a stabilizing effect on the frequency of drive [15,16]. But it has been suggested that there will be negative frequency-dependent selection on male fertility [17]. This has intuitive appeal because the spread of SR causes the population sex ratio to become increasingly female-biased. In such a population, the average male mating rate will increase. If SR male fertility increases at a lower rate than non-drive (ST) male fertility when males mate many times (for instance, because SR males are sperm limited), then a polymorphism could be stabilized [17]. Decreased male fertility under multiple mating is a general feature observed in many drive systems [17–19]. However, for this effect alone to prevent SR fixation, SR male fertility must fall to less than half that of ST males as the mating rate increases [17], a condition not met in a number of species that nonetheless are found with stable SR polymorphism [16]. A related suggestion is that SR males may be outcompeted at higher mating rates, supported by some evidence that SR males are poor sperm competitors [20–22]. However, the strength of sperm competition weakens as SR spreads, as this reduces the number of competitor males in the population, which seems unlikely to exert a stabilizing effect on SR frequency. SR males may do poorly in other forms of male–male competition if SR is generally associated with poor performance. Such effects are likely to decrease as drive spreads and males become rare, again making it unlikely that this form of selection will stabilize drive. Models that combine the effects of decreased male fertility and reduced sperm competitive ability on SR frequency dynamics find they can lead to a stable polymorphism [23]. But this equilibrium can be destabilized by perturbations in either the population sex ratio or the frequency of SR. In particular, given a meta-population of small demes, slight fluctuations in SR frequency are likely to cause drive to spread to fixation, resulting in population extinction [24].
Suppressors are another selective force operating in males that limits the spread of drive alleles. Most obviously, selection favours the evolution of suppression on chromosomes targeted by drivers for dysfunction. In an SR system with complete drive, if resistance is linked to the Y-chromosome, it restores transmission to Mendelian levels, while non-resistant Y-chromosomes are not transmitted at all [25]. Y-linked suppressors are therefore expected to spread quickly even if they have deleterious side effects [26]. Unlinked suppressors will also be favoured because drive in males causes gamete loss and is often associated with dysfunction among the surviving, drive-carrying sperm. Reduced sperm number is likely to reduce organismal fertility. Additionally, as SR spreads, it causes the population sex ratio to become female-biased, providing a further advantage to suppressors as they increase the production of male offspring, which have higher reproductive value than female offspring [27,28]. The spread of suppressors reduces the advantage of drive and could lead to its loss. But both types of suppressors are under negative frequency-dependent selection, because a lower frequency of drive reduces selection in their favour. Under some circumstances, this could lead to a stable polymorphism at the drive locus. Y-linked and autosomal suppressors of SR drive have been detected in a number of species including Drosophila simulans, D. affinis, D. subobscura, D. quinara, D. mediopunctata, and Aedes aegypti [29]. The evolution of suppressors can be remarkably rapid. For example, in the Paris SR system of D. simulans, the increase in SR from less than 10% to more than 60% in a mere 5 years has been matched by a similar increase in suppressor frequency over the same time period [30]. While suppressors are common, they are not universal and have not been detected against SR drive in D. pseudoobscura, D. recens, and D. neotestacea [29]. In these systems, other factors are therefore necessary to explain extant SR polymorphism.
Another force that may prevent SR fixation is reduced fitness of female carriers [31]. As male X-linked drive causes defects in spermatogenesis, there is no obvious mechanistic carry-over to female oogenesis. Likewise, examples of meiotic drive in female gametogenesis, which affect the biased segregation of chromosomes into the egg or polar bodies, show no carry-over to segregation bias in male gamete production [2]. For selection to act against female carriers, the drive locus must either have direct pleiotropic fitness effects or be in linkage with alleles that impact fitness. Linkage is a plausible explanatory factor, given that drive systems are often located in genomic regions with low recombination rates, such as in inversions [32–35]. If the inversion is at low frequency, it will rarely be homozygous and the recombination rate among SR chromosomes will be low. Inversions also severely limit the exchange of genes with the homologous region on the standard chromosome (as this requires a double cross-over within the inverted region [36,37]). The consequence is that low-frequency inversions will be subject to weak selection and suffer the accumulation of a greater mutation load [34,38]. Recessive viability and sterility effects are expected as they will not be evident in females until the frequency of drive is high enough for homozygotes to be common. By contrast, hemizygosity in males means recessive and dominant effects are always expressed and will be more strongly selected against. In general, SR inversions are expected to be enriched for sexually antagonistic alleles that benefit the sex in which drive occurs [39]. This means that we expect that the loss of fitness will be greater in females and likely to be recessive. These effects produce relevant frequency dependence that restricts the fixation of drive. Severe reductions in female viability and fertility in SR homozygotes, along with SR heterozygotes, have been reported in several Drosophila species [31,34,40]. But it is surprising how rarely viability effects of drive in either sex have been studied, compared to fertility effects in males [41]. These deleterious consequences are likely to build up and lead to a reduction in SR frequency through time [34].
Large-scale chromosomal inversions are not a universal feature of SR, however. Inversions are not present in the Paris SR system in D. simulans [29]. Despite this, SR must be weakly deleterious in this species as it is rapidly declining in frequency in populations that have recently become completely suppressed [42]. The deleterious effects of the Paris SR chromosome must arise due to the drive genes themselves or a tightly linked region. The genetically distinct Winters SR system in the same species also lacks association with an inversion [43]. It persists despite having been completely suppressed for thousands of years, suggesting it does not cause any pleiotropic fitness deficit [43]. These are the only well-characterized examples of meiotic drive not being associated with inversions, so this feature may be a rarity.
Another aspect operating in females concerns behavioural resistance to the spread of SR. Laboratory experiments suggest that increased levels of polyandry can be selected as a defence mechanism against SR [22]. This benefit arises when drive male sperm are weak competitors against wild-type male sperm [41]. Recent modelling work shows that polyandry helps prevent invasion of SR, but alone cannot prevent fixation of drive [44]. As drive spreads, additional matings have a lower probability of involving wild-type males, so the disadvantage to drive sperm declines. There needs to be positive frequency-dependent costs to achieve a stable polymorphism [44], for instance, when homozygous females have lower viability than heterozygotes. If a stable polymorphism can evolve, the frequency of drive should decline with the rate of female remating. There is evidence in favour of this idea in D. neotestacea which exhibits a stable cline in SR frequency that correlates negatively with the frequency of polyandry [10], and a similar pattern has been reported in D. pseudoobscura [11]. Alternatively, females may simply avoid mating with SR males [45,46]. In stalk-eyed flies, females prefer to mate with males with large eyespan [47,48], a trait that is reduced in SR males [47,49,50]. Sexual selection may therefore be acting in this species to limit the spread of SR. However, this form of selection against drive is likely to be restricted to a subset of species with drive, as it requires the linkage of SR with a conspicuous trait subject to mate choice [46]. Another potential example is the autosomal t-locus system in mice which is proposed to be detectable in mate choice through olfaction [51], but this preference has not been confirmed [52]. A counterexample is in D. pseudoobscura, where females do not avoid mating with SR males, though there would be considerable benefit to doing so [53].
In this study, we determine the effect of SR meiotic drive on viability in the Malaysian stalk-eyed fly, Teleopsis dalmanni. Our objective was to assess whether SR causes higher developmental mortality before adult eclosion. Populations of this species carry SR at a moderate level of approximately 20% but with considerable variation among populations [14,54,55]. SR resides within a large paracentric inversion (or inversions) that covers most of the X-chromosome [49]. There is no recombination between SR and ST haplotypes [14] and the lower frequency of SR in the wild means SR homozygous recombination events are relatively rare (at 20%, the recombination rate of SR is a quarter that of ST). SR is absent from a cryptic species of T. dalmanni estimated to have diverged approximately 1 Mya [14]. X-linked meiotic drive is also present in the more distantly related species T. whitei, which diverged 2–3.5 Mya ago [14,56]. But to what extent the mechanism or genetic basis is conserved remains to be established.
The ancient origin of the XSR chromosome and limited recombination across the XSR chromosome are predicted to have led to the accumulation of deleterious alleles. Consistent with a lack of recombination, there are 955 fixed sequence differences between transcripts linked to XSR and XST [35]. The main evidence for a deleterious effect of XSR on fitness is the reduced eyespan of SR males [47,50]. Male eyespan is an exaggerated, highly condition-dependent trait used in female mate choice [47,57], as well as signalling between males [58,59], which reflects male genetic and phenotypic quality [57,60,61]. However, in a series of experiments, Wilkinson et al. [62] found little direct evidence that SR reduces fitness components. Although larval viability was not directly assessed, progeny production showed no difference between SR and ST homozygous females [62]. Another study compared offspring genotypes of heterozygous females mated to ST males, and reported little deviation from expected, assuming no viability selection differences [49]. Adult survival did not vary with genotype in either males or females [62]. There was no evidence for a deleterious effect of XSR on female fecundity, rather heterozygotes were more productive, suggesting overdominance [62]. However, sample size in these experiments was small, and fecundity/fertility results were based on progeny counts which are confounded by genotype effects on larval survival. The only significant detriment reported was in SR male fertility which was reduced when males were allowed to mate with large numbers of females (eight) for 24 h [62]. However, a further experiment that measured male fertility through counts of fertile eggs (avoiding any confounding impact of larval survival) failed to show any difference between SR and ST male fertility [63].
To better understand these previous results, we were motivated to explicitly test for differences in larval survival. Our experimental design was similar to that used in early investigations of D. pseudoobscura [31,40]. Controlled crosses were carried out to produce eggs with all possible SR and ST male and female genotypes. These were reared together to ensure exposure to similar environmental variation. The sample size was large to maximize our power to detect genotypic survival differences. Offspring were genotyped at adult eclosion, yielding observed genotype ratios in order to estimate the selection coefficients operating against drive in both sexes. Our principal aims were to test whether the SR-drive chromosome causes viability loss during egg-to-adult development, and whether fitness effects are recessive or sex-limited.
2. Methods
(a). Fly stocks and maintenance
A standard stock population was obtained from Ulu Gombak in Malaysia (3°19′ N 101°45′ E) in 2005 (by Sam Cotton and Andrew Pomiankowski). Stock flies are reared in high-density cage culture (cage size approx. 30 × 20 × 20 cm) at 25°C on a 12 : 12 h light : dark cycle, and fed puréed corn ad libitum. Fifteen minute artificial dawn and dusk phases are created by illumination from a single 60 W bulb at the start and end of each light phase. Meiotic drive is absent from the standard stock population.
A meiotic drive stock was created using flies collected from the same location in 2012 [50]. Meiotic drive is maintained in this stock by following a standard protocol [54,64]. Females heterozygous for the drive chromosome are mated to males from the standard stock. It is expected that half of their male offspring will inherit the drive chromosome. All male offspring are crossed to three females from the standard stock and the sex ratio of their progeny scored. Males that sire all-female broods of at least 15 individuals are considered to be carriers of meiotic drive. In the meiotic drive stock, drive strength is 100%, and no males are produced by XSR/Y males carrying the drive chromosome [64]. Progeny from drive males are female heterozygotes for the drive chromosome. They are subsequently mated to standard males, and the process is repeated.
(b). Experimental crosses
To generate the five possible genotypes of both females (XST/XST, XSR/XST, XSR/XSR) and males (XST/Y, XSR/Y), two crosses were performed (figure 1). In Cross A, drive males (XSR/Y) were mated to heterozygous females (XSR/XST). This cross produces XSR/XSR and XSR/XST female zygotes in equal proportions. In Cross B, standard males (XST/Y) were mated to heterozygous females (XSR/XST). This cross produces XST/Y and XSR/Y male, and XST/XST and XSR/XST female zygotes in equal proportions. Experimental males were collected from the drive stock that were approximately 50 : 50 XST/Y and XSR/Y males. They were crossed to standard stock females (XST/XST) and one larva per male was genotyped to define the paternal genotype. Experimental females heterozygous for drive (XSR/XST) were collected from crosses between drive males and females from the standard stock.
Figure 1.
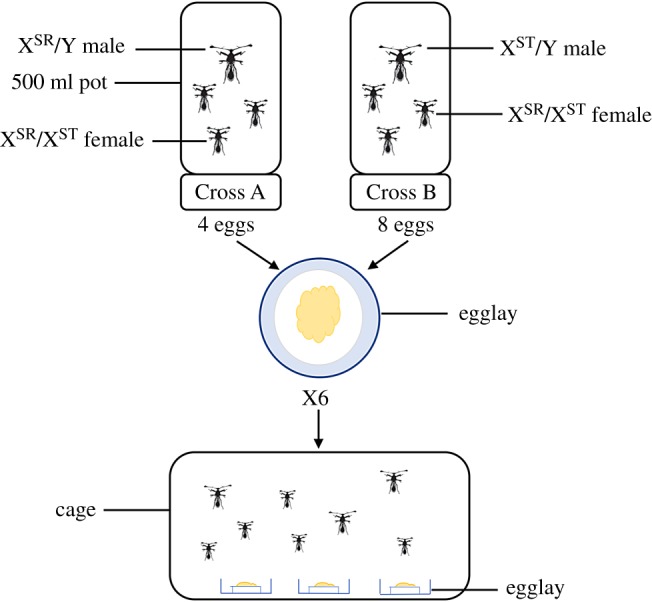
Experimental protocol. Individual males of known genotype were crossed with three heterozygous females in 500 ml pots. Cross A produces no males and XSR/XSR and XSR/XST females, in equal proportions. Cross B produces XSR/Y and XST/Y males and XST/XST and XSR/XST females, in equal proportions. Four eggs from Cross A and eight eggs from Cross B were added to each egglay—a Petri dish containing a moistened cotton pad and food. At pupation, six egglays were placed into a population cage and their lids were removed so as to allow the adult flies to eclose. (Online version in colour.)
Individual males were placed with three virgin females in 500 ml pots. Females that died during the experiment were replaced, but males were not. Twenty-five Cross A and 50 Cross B pots were set-up. The base of each pot was lined with moistened cotton wool covered with blue tissue paper to aid egg visualization. The cotton bases were removed for egg collection and replaced three times per week. Fertilized eggs were identified under light microscopy as those that showed signs of development (e.g. segmental striations, development of mouthparts; [65]) and transferred to a 90 mm Petri dish containing a large cotton pad moistened with 15 ml of water and 2.5 ml of food. Three different food conditions were used that varied in their corn content: 25% corn, 50% corn, and 75% corn. In each mixture, the remainder was made up with a sucrose solution (25% sucrose/water w/w). To ensure the sucrose solution had a similar viscosity to puréed corn, an indigestible bulking agent was added (methylcellulose, 3% w/w; [66]). Four eggs from Cross A and 8 eggs from Cross B were transferred to each Petri dish. This gives the five possible genotypes (XST/XST, XSR/XST, XSR/XSR, XST/Y, XSR/Y) in an expected 1 : 2 : 1 : 1 : 1 ratio (table 1). Prior to the end of development, six Petri dishes were placed inside a large cage and all eclosing adult flies were collected. The cage was used as a level of analysis of the relative egg-to-adult viability of different genotypes in the subsequent analyses.
Table 1.
Relative egg-to-adult viability. The five genotypes were drawn from crosses between heterozygous females and drive males (Cross A) or standard males (Cross B). The selection parameters, sf and sm, measure drive egg-to-adult viability relative to wild-type females and males, respectively. The dominance coefficient of drive is denoted h.
|
females |
males |
||||
|---|---|---|---|---|---|
| XSR/XSR | XSR/XST | XST/XST | XSR/Y | XST/Y | |
| Cross A XSR/XST × XSR/Y |
1 – sf | 1 – hsf | |||
| Cross B XSR/XST × XST/Y |
1 – hsf | 1 | 1−sm | 1 | |
(c). Genotyping
DNA was extracted in 96-well plates using a modification of a standard isopropanol precipitation protocol ([67]; see electronic supplementary material, S1 Methods for full protocol). DNA was PCR-amplified in 96-well plates, using forward and reverse primers for comp162710, an indel marker with small alleles (201 bp) indicating the presence of the drive chromosome and large alleles (286 bp) indicating the presence of the standard chromosome (GS Wilkinson 2017, personal communication; [64]).
(d). Statistical analysis
We used two approaches to estimate the egg-to-adult viability costs of the XSR chromosome. The first estimated the relative egg-to-adult viability cost of each genotype. The second estimated the strength of selection against drive in males and females, as well as the dominance coefficient. Model outputs are given in detail in the electronic supplementary material, tables S1–S7.
(e). Egg-to-adult viability of each genotype
In the first analysis, the number of eclosed adult flies of each genotype was compared to the number expected at the level of the cage. Each cage contained six Petri dishes with 12 eggs, producing a maximum of 72 flies. Genotyping effort varied across cages and sexes. The expected number of each genotype was determined with respect to the genotyping effort of the relevant sex for a particular cage. For example, if 24 males were collected from a given cage, and 75% of these males were genotyped, then the expected number of XSR/Y individuals is (24 × 0.75)/2 = 9. Owing to the nature of the experimental design, we expected twice as many XSR/XST females compared with XSR/XSR and XST/XST females. For example, in a cage with 36 genotyped females, we expected 18 XSR/XST females and 9 each of the remaining two female homozygotes. We then divided the observed number of flies of a given genotype by the expectation for that genotype to obtain the cage estimate of egg-to-adult viability. We split the data by sex and analysed the relationship between egg-to-adult viability and genotype using linear mixed-effect modelling with lme4 [68] in R [69]. Genotype and food condition were modelled as fixed effects and cage ID and collection date as random effects. Significance of model terms was determined using the lmerTest R package [70]. The mean viability measures were estimated using model terms.
(f). Estimating the strength of selection against drive
In the second analysis, we estimated the strength of selection against drive using Bayesian inference, separately for males and females. Cage survival frequencies for each genotype were pooled. The probability of drawing the male genotype distribution was calculated for values of the selection coefficient taken from a uniform prior distribution for sm = 0–1, in 0.001 increments. We then used a binomial model to determine the likelihood of drawing the observed number of XST/Y and XSR/Y males for each value of sm. As we used a uniform prior, the posterior probability simplifies to the likelihood. The 95 and 99% credible intervals were determined from the probability density. The probability of observing the distribution of the three female genotypes was estimated under a multinomial where the values of sf and h (table 1) were taken from a uniform prior distribution for every combination of values of sf and h ranging from 0 to 1, in 0.001 intervals. The 95 and 99% credible intervals were determined in the same way as in males, and displayed as a two-dimensional contour. Note that the probability of drawing XSR/XST females was multiplied by two because the experimental design was expected to generate twice as many heterozygote eggs compared to all other genotypes. To determine if sm and sf were of different strength, 1000 random samples each of sm and sf (taking h equal to its mode) were drawn from the posterior distributions with probability of drawing a value equal to its likelihood. A distribution of differences was obtained by subtracting the randomly drawn sf values from the randomly drawn sm values. A z-score was calculated to determine if this distribution is different from zero.
We also estimated the difference in the strength of selection between female genotypes. To compare egg-to-adult viability between wild-type (XST/XST) and heterozygous (XSR/XST) females, the likelihood of observing the counts of these two genotypes was determined under a binomial as above, but shrinking h and sf to a single term with a uniform prior. The process was repeated to compare drive heterozygotes (XSR/XST) and homozygotes (XSR/XSR).
3. Results
(a). Effect of food condition
Food condition had no overall effect on the egg-to-adult viability of males (F2,72 = 0.1085, p = 0.8973) or females (F2,54 = 0.1552, p = 0.8566), nor did it alter the genotype response (genotype-by-condition interaction, males F2,79 = 0.8026, p = 0.4518; females F4,116 = 0.2044, p = 0.9355). So, offspring counts were pooled across food conditions within sexes in the following analyses.
(b). Egg-to-adult viability of each genotype
From a total of 96 cages, each containing 72 eggs, we collected a total of 1065 males and 2500 females, of which 798 and 1272 were genotyped, respectively. Male genotype had a significant effect on egg-to-adult viability, with XSR/Y males showing reduced viability (F1,81 = 11.7296, p < 0.001). XST/Y males had a mean viability of 0.5412, and XSR/Y males had a mean viability of 0.4036 (figure 2). Genotype also had a significant effect on egg-to-adult viability in females (F2,120 = 4.7593, p = 0.0103). The mean viability was 0.6294 in XST/XST females, 0.5491 in XSR/XST females and 0.4650 in XSR/XSR individuals. A Tukey's post hoc comparison test revealed that the viability of XST/XST females was greater than XSR/XSR females (p = 0.0104), while XSR/XST females had intermediate viability, but not different from either homozygote (XSR/XST–XSR/XSR comparison: p = 0.2949; XSR/XST–XST/XST comparison: p = 0.3293; figure 2).
Figure 2.
Male and female genotype mean ± s.e. egg-to-adult viability. Values were determined from the fraction of a given genotype observed in replicate cages. (Online version in colour.)
(c). Estimating the strength of selection against drive
The posterior probability of each value of the male selection parameter sm is given in figure 3. The mode of sm = 0.214 with a 95% credible interval 0.097–0.316 and a 99% credible interval 0.056–0.346. The probability of the modal value compared to the null hypothesis of no viability selection against drive males has a Bayes factor BF10 = 321.79.
Figure 3.
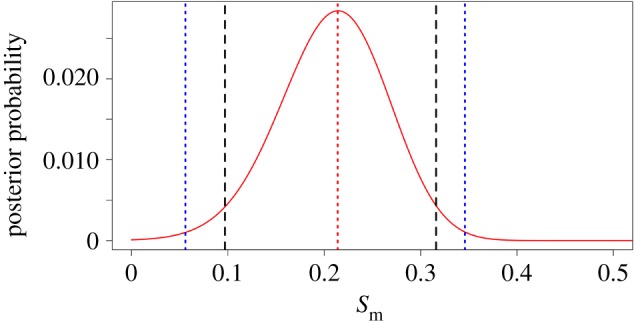
The posterior probability density of the strength of selection against drive in males (sm). The mode is shown as a dotted red line. The dashed black lines indicate the 95% credible interval. The dotted blue lines indicate the 99% credible interval. (Online version in colour.)
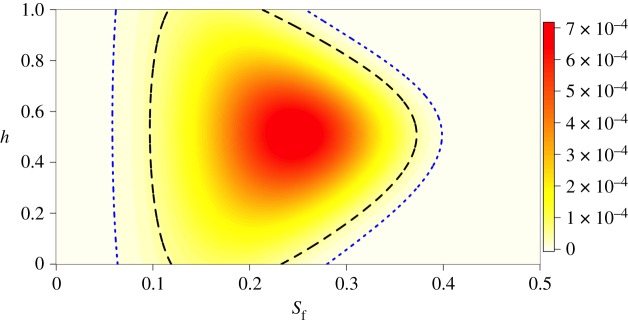
The posterior probability of each combination of the female selection parameters sf and h values is shown in figure 4. The modal values are sf = 0.242 and h = 0.511, with the bivariate 95 and 99% credible intervals displayed as a two-dimensional contour (figure 4). The probability of the modal sf value compared to the null hypothesis of no viability selection against drive in females has a Bayes factor BF10 = 572.89. The strength of selection against drive in males and females (sf and sm; setting h to its modal value) did not differ between the sexes (|z| = 0.3785, α = 0.01, p = 0.7047).
Figure 4.
The posterior probability density of the strength of selection against drive in females (sf) and the dominance coefficient (h). Colour indicates probability density, with darker colours indicating higher likelihood. The black dashed contour shows the 95% credible interval and the blue dotted line shows the 99% credible interval. (Online version in colour.)
In the pairwise comparison of individual female genotypes, there was a difference between the egg-to-adult viability of XST/XST and XSR/XST females, with a selection coefficient mode = 0.126 with a 95% credible interval = 0.007–0.232 and a 99% credible interval = −0.017–0.261. A similar difference was observed in the comparison of XSR/XST and XSR/XSR, with a selection coefficient mode = 0.138 with a 95% credible interval of 0.008–0.252 and a 99% credible interval of −0.038 to 0.287.
4. Discussion
Owing to their twofold transmission advantage in males, X chromosomes that exhibit SR meiotic drive (XSR) potentially can spread to fixation and cause population extinction [5,6]. Despite this, several meiotic drive systems exist in broadly stable polymorphisms [10,11,55]. This suggests that there are costs of carrying the XSR chromosome. In the stalk-eyed fly system, the XSR chromosome contains a large inversion [49], which is expected to accumulate deleterious mutations as they are less efficiently removed by recombination than those on the XST chromosome. This mutation load is expected to lead to a decrease in fitness of the XSR chromosome. Here, controlled crosses were used to estimate one component of fitness, egg-to-adult viability, of meiotic drive genotypes. There was a reduction in viability linked to XSR in both males and females. In XSR hemizygous males, this was sm = 21% (figure 3) and in XSR homozygous females, sf = 24% (figure 4). The negative effect of XSR in females was largely additive (), with heterozygotes being intermediate in viability compared to homozygotes. The estimates of selection (sm and sf) do not differ between the sexes. This probably reflects a lack of sexual dimorphism in fitness at the larval stage. In Drosophila melanogaster, egg-to-adult viability measured for particular genotypes is strongly positively correlated across the sexes, whereas adult reproductive success is typically negatively correlated [71,72].
In the experiment, individual males of known genotype, either SR or ST, were crossed with heterozygous females. Eggs were collected and combined in groups of six Petri dishes each containing 12 eggs. The eggs were visually inspected for signs of development, so as to be able to exclude the possibility that differential fertility of the two paternal genotypes (i.e. SR or ST) affected the subsequent output of adult flies. In addition, a pilot experiment showed equal levels of SR and ST male fertility in conditions similar to those used here (electronic supplementary material, table S8). The combination of eggs from the two crosses was expected to generate all five genotypes in an even ratio, except for heterozygous females which were expected at double the number of the other genotypes. The objective was to standardize competition between genotypes. It is hard to estimate whether this objective was attained, as only surviving adults were genotyped. The observed adult genotype frequencies were compared to infer genotype-specific survival in the egg-to-adult stage. The number of flies genotyped was sufficiently large (Nm = 798, Nf = 1272) to give reasonable assurance of the accuracy of the estimates. Even with this sizeable sample, the bounds on the estimates of sm, sf, and h remain large (figures 3 and 4), but we can be confident that drive is associated with the loss of viability in both sexes. Our results contrast with a prior study showing that adult lifespan is independent of SR genotype in males and females [62], revealing a difference between larval and adult genotypic effects. This previous study also suggested that larval survival is independent of SR genotype [62]. The reasons for this difference are unclear; there could be differences that relate to food and housing, the mixture of genotypes undergoing larval competition, or the SR haplotype used as those in Wilkinson et al. [62] cause less than 100% transmission distortion. This suggests that further investigation is warranted in a number of directions.
This is the first study showing a reduction in SR viability in stalk-eyed flies. Similar methods have been applied previously in D. pseudoobscura [31,32,40]. Wallace [40] observed strong selection against XSR in both sexes. In high-density populations, Beckenbach [32] found a reduction in XSR/Y male viability, but no homozygous XSR female viability effect. By contrast, Curtsinger & Feldman [31] report stronger selection against homozygous XSR females. Comparisons of these three studies provide strong evidence to suggest that viability selection is density-dependent, as reduction in XSR viability was greatest under high density [40], and a lack of differential viability was observed in another experiment carried out at low density [32]. In the present study, stalk-eyed fly larvae were cultured under low density and provided with excess food. Future work will need to determine whether varying levels of food stress enhance or restrict the deleterious effect of the XSR chromosome.
Strong viability selection against the XSR chromosome, as found here under laboratory conditions, may play a key role in determining the equilibrium level of the SR polymorphism in the wild. There are several other factors that could be involved in determining SR frequency, such as suppressors, polyandry, and various forms of sexual behaviour which we discuss further here. First, in D. simulans, SR commonly co-occurs with suppressors which restrict the transmission advantage [43,73]. Although early work on the stalk-eyed fly drive system suggested that there were suppressors [47], this has not been sustained by further work, either on the autosomes or Y-chromosomes [14]. Second, polyandry may evolve to limit the spread of SR [22]. Polyandry is the norm in T. dalmanni [55,65], and there is evidence that SR male sperm does less well under sperm competition [62] and may suffer from interactions with non-sperm ejaculate components produced by standard males (though this has only been shown in the related species T. whitei, [21]). But it has not been shown whether variation in the degree of polyandry correlates with SR frequency in natural populations of stalk-eyed flies.
Third, it has long been suggested that mate choice may play a role in determining the frequency of drive [51]. This may be important in stalk-eyed flies as they are canonical examples of sexual selection driven by mate choice [74,75]. In T. dalmanni, drive males are expected to attract fewer females as they have reduced eyespan, and hence mate less often [47,50]. However, there is as yet no evidence in stalk-eyed flies that the strength of female mate preference has been enhanced in populations subject to drive. Nor has there been investigation of whether females that carry SR show alterations in their mating behaviour. A related consideration is male mate preference [76] which has been shown to be an important behavioural adaptation in T. dalmanni favouring male matings with fecund females [77]. A recent study reported that SR had no direct effect on male mate choice [78]. However, the strength of male mate preference positively covaries with male eyespan. As drive males have smaller eyespan [50], we expect they will be less discriminating in their mate choice [78].
Finally, measurements of sperm number per mating report that SR males deliver as many sperm as ST males, and a single mating with an SR male results in the same female fertility as a mating with an ST male [64]. Whether this pattern carries over to situations where a male can mate with multiple females is less clear. One experiment showed no difference between SR and ST males [63], whereas another experiment found lower fertility in SR males [62] when multiple females were allowed to mate freely with a single male for a day. The cause of this difference is unclear, but drive males have been shown to have lower mating rates compared to standard males [63], and this could conceivably have contributed to lower fertility in females mated to SR males. As mentioned previously, SR males are poor sperm competitors compared to ST males, which must arise from reasons other than numerical sperm transfer from the male [62].
The number of different factors set out above make it difficult to predict whether they are sufficient to explain SR's observed frequency of approximately 20% [14,55]. Many could act as stabilizing forces which restrict the spread of drive in a frequency-dependent manner. Future work should aim to examine these factors, in combination with the intensity of egg-to-adult viability selection measured here, in a modelling framework in order to predict the evolutionary outcomes. This needs to be coupled to better estimation of ecological and demographic parameters across local populations of T. dalmanni in which SR frequency is known to be highly variable [50].
Supplementary Material
Acknowledgements
The authors thank Rebecca Finlay for her help in maintaining the laboratory stocks, Dr Lara Meade for advice on experimental design, and Prof. Mark Thomas for help with the statistical analysis.
Data accessibility
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.kc49jk1 [79].
Authors' contributions
The research project was conceived by S.R.F., N.J.W., K.F., and A.P. The experiment was carried out by S.R.F. and N.J.W., with genotyping by S.R.F., D.K., and M.F.C. The data were analysed by S.R.F., N.J.W., and A.P., and the paper written by S.R.F. and A.P.
Competing interests
We declare we have no competing interests.
Funding
S.R.F. is supported by a London NERC DTP PhD Studentship (NE/L002485/1). A.P. is supported by EPSRC grant nos (EP/ F500351/1, EP/I017909/1), and K.F. and A.P. by NERC grant nos (NE/G00563X/1, NE/R010579/1).
References
- 1.Lindholm AK, et al. 2016. The ecology and evolutionary dynamics of meiotic drive. Trends Ecol. Evol. 31, 315–326. ( 10.1016/j.tree.2016.02.001) [DOI] [PubMed] [Google Scholar]
- 2.Burt A, Trivers R. 2006. Genes in conflict. Cambridge, MA: Harvard University Press. [Google Scholar]
- 3.Hurst LD, Pomiankowski A. 1991. Causes of sex ratio bias may account for unisexual sterility in hybrids: a new explanation of Haldane's rule and related phenomena. Genetics 4, 841–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hurst GD, Werren JH. 2001. The role of selfish genetic elements in eukaryotic evolution. Nat. Rev. Genet. 2, 597–606. ( 10.1038/35084545) [DOI] [PubMed] [Google Scholar]
- 5.Hamilton WD. 1967. Extraordinary sex ratios. Science 156, 477–488. ( 10.1126/science.156.3774.477) [DOI] [PubMed] [Google Scholar]
- 6.Hatcher MJ, Taneyhill DE, Dunn AM, Tofts C. 1999. Population dynamics under parasitic sex ratio distortion. Theor. Popul. Biol. 56, 11–28. ( 10.1006/tpbi.1998.1410) [DOI] [PubMed] [Google Scholar]
- 7.Lyttle TW. 1977. Experimental population genetics of meiotic drive systems I. Pseudo-Y chromosomal drive as a means of elimination cage populations of Drosophila melanogaster. Genetics 86, 413–445. [PMC free article] [PubMed] [Google Scholar]
- 8.Price TAR, Hurst GDD, Wedell N. 2010. Polyandry prevents extinction. Curr. Biol. 20, 471–475. ( 10.1016/j.cub.2010.01.050) [DOI] [PubMed] [Google Scholar]
- 9.Galizi R, Doyle LA, Menichelli M, Bernardini F, Deredec A, Burt A, Stoddart BL, Windbichler N, Crisanti A. 2014. A synthetic sex ratio distortion system for the control of human malaria mosquito. Nat. Commun. 5, 1–8. ( 10.1038/ncomms4977) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinzone CA, Dyer KA. 2013. Association of polyandry and sex-ratio drive prevalence in natural populations of Drosophila neotestacea. Proc. R. Soc. B 280, 20131397 ( 10.1098/rspb.2013.1397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price TAR, Bretman A, Gradilla AC, Reger J, Taylor ML, Giraldo-Perez P, Campbell A, Hurst GDD, Wedell N. 2014. Does polyandry control population sex ratio via regulation of a selfish gene? Proc. R. Soc. B 281, 20133259 ( 10.1098/rspb.2013.3259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verspoor RL, Price TAR, Smith JML, Mannion NLM, Hurst GDD. 2018. Strong hybrid male incompatibilities impede the spread of a selfish chromosome between populations of a fly. Evol. Lett. 2, 169–179. ( 10.1002/evl3.55) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kovacevic M, Schaeffer SS. 2000. Molecular population genetics of X-linked genes in Drosophila pseudoobscura. Genetics 156, 155–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paczolt KA, Reinhardt JA, Wilkinson GS. 2017. Contrasting patterns of X-chromosome divergence underlie multiple sex-ratio polymorphisms in stalk-eyed flies. J. Evol. Biol. 30, 1772–1784. ( 10.1111/jeb.13140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards AWF. 1961. The population genetics of ‘sex-ratio’ in Drosophila pseudoobscura. Heredity 16, 291–304. ( 10.1038/hdy.1961.35) [DOI] [Google Scholar]
- 16.Carvalho AB, Vaz SC. 1999. Are Drosophila SR chromosomes always balanced? Heredity 83, 221–228. ( 10.1038/sj.hdy.6886100) [DOI] [PubMed] [Google Scholar]
- 17.Jaenike J. 1996. Sex-ratio meiotic drive in the Drosophila quinaria group. Am. Nat. 148, 237–254. ( 10.1086/285923) [DOI] [Google Scholar]
- 18.Beckenbach AT. 1978. The ‘Sex-Ratio’ trait in Drosophila pseudoobscura: fertility relations of males and meiotic drive. Am. Nat. 112, 97–117. ( 10.1086/283255) [DOI] [Google Scholar]
- 19.Atlan A, Joly D, Capillon C, Montchamp-Moreau C. 2004. Sex-ratio distorter of Drosophila simulans reduces male productivity and sperm competition ability. J. Evol. Biol. 17, 744–751. ( 10.1111/j.1420-9101.2004.00737.x) [DOI] [PubMed] [Google Scholar]
- 20.Wu CI. 1983. Virility deficiency and the sex-ratio trait in Drosophila pseudoobscura. I. Sperm displacement and sexual selection. Genetics 105, 651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilkinson GS, Fry CL. 2001. Meiotic drive alters sperm competitive ability in stalk-eyed flies. Proc. R. Soc. B 268, 2559–2564. ( 10.1098/rspb.2001.1831) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Price TAR, Hodgson DJ, Lewis Z, Hurst GDD, Wedell N. 2008. Selfish genetic elements promote polyandry in a fly. Science 322, 1241–1243. ( 10.1126/science.1163766) [DOI] [PubMed] [Google Scholar]
- 23.Taylor JE, Jaenike J. 2002. Sperm competition and the dynamics of X chromosome drive: stability and extinction. Genetics 160, 1721–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor JE, Jaenike J. 2003. Sperm competition and the dynamics of X chromosome drive in finite and structured populations. Ann. Zool. Fennici 40, 195–206. [Google Scholar]
- 25.Thomson GJ, Feldman MW. 1975. Population genetics of modifiers of meiotic drive: IV. On the evolution of sex-ratio distortion. Theor. Popul. Biol. 8, 202–211. ( 10.1016/0040-5809(75)90032-5) [DOI] [PubMed] [Google Scholar]
- 26.Wu CI. 1983. The fate of autosomal modifiers of the sex-ratio trait in Drosophila and other sex-linked meiotic drive systems. Theor. Popul. Biol. 24, 121–135. ( 10.1016/0040-5809(83)90036-9) [DOI] [PubMed] [Google Scholar]
- 27.Fisher RA. 1930. The genetical theory of natural selection. Oxford, UK: Clarendon Press. [Google Scholar]
- 28.Carvalho AB, Sampaio MC, Varandas FR, Klaczko LB. 1998. An experimental demonstration of Fisher's principle: evolution of sexual proportion by natural selection. Genetics 148, 719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaenike J. 2001. Sex chromosome meiotic drive. Annu. Rev. Ecol. Syst. 32, 25–49. ( 10.1146/annurev.ecolsys.32.081501.113958) [DOI] [Google Scholar]
- 30.Bastide H, Gérard PR, Ogereau D, Cazemajor M, Montchamp-Moreau C. 2013. Local dynamics of a fast-evolving sex-ratio system in Drosophila simulans. Mol. Ecol. 22, 5352–5367. ( 10.1111/mec.12492) [DOI] [PubMed] [Google Scholar]
- 31.Curtsinger JW, Feldman MW. 1980. Experimental and theoretical analysis of the ‘sex-ratio’ polymorphism in Drosophila pseudoobscura. Genetics 94, 445–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beckenbach AT. 1983. Fitness analysis of the ‘sex-ratio’ polymorphism in experimental populations of Drosophila pseudoobscura. Am. Nat. 121, 630–648. ( 10.1086/284091) [DOI] [Google Scholar]
- 33.Silver L. 1993. The peculiar journey of a selfish chromosome: mouse t haplotypes and meiotic drive. Trends Genet. 9, 250–254. ( 10.1016/0168-9525(93)90090-5) [DOI] [PubMed] [Google Scholar]
- 34.Dyer KA, Charlesworth B, Jaenike J. 2007. Chromosome-wide linkage disequilibrium as a consequence of meiotic drive. Proc. Natl Acad. Sci. USA 104, 1587–1592. ( 10.1073/pnas.0605578104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reinhardt JA, Brand CL, Paczolt KA, Johns PM, Baker RH, Wilkinson GS. 2014. Meiotic drive impacts expression and evolution of X-linked genes in stalk-eyed flies. PLoS Genet. 10, e1004362 ( 10.1371/journal.pgen.1004362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Navarro A, Betrán E, Barbadilla A, Ruiz A. 1997. Recombination and gene flux caused by gene conversion and crossing over in inversion heterokaryotypes. Genetics 146, 695–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Navarro A, Ruiz A. 1997. On the fertility effects of percentric inversions. Genetics 147, 931–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirkpatrick M. 2010. How and why chromosome inversions evolve. PLoS Biol. 8, e1000501 ( 10.1371/journal.pbio.1000501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rydzewski WT, Carioscia SA, Liévano G, Lynch VD, Patten MM. 2016. Sexual antagonism and meiotic drive cause stable linkage disequilibrium and favour reduced recombination on the X chromosome. J. Evol. Biol. 29, 1247–1256. ( 10.1111/jeb.12866) [DOI] [PubMed] [Google Scholar]
- 40.Wallace B. 1948. Studies on ‘sex-ratio’ in Drosophila pseudoobscura. I. Selection and ‘sex-ratio’. Evolution 2, 189–217. ( 10.1111/j.1558-5646.1948.tb02741.x) [DOI] [PubMed] [Google Scholar]
- 41.Price TAR, Wedell N. 2008. Selfish genetic elements and sexual selection: their impact on male fertility. Genetica 134, 99–111. ( 10.1007/s10709-008-9253-y) [DOI] [PubMed] [Google Scholar]
- 42.Bastide H, Cazemajor M, Ogereau D, Derome N, Hospital F, Montchamp-Moreau C. 2011. Rapid rise and fall of selfish sex-ratio X chromosomes in Drosophila simulans: spatiotemporal analysis of phenotypic and molecular data. Mol. Biol. Evol. 28, 2461–2470. ( 10.1093/molbev/msr074) [DOI] [PubMed] [Google Scholar]
- 43.Kingan SB, Garrigan D, Hartl DL. 2010. Recurrent selection on the winters sex-ratio genes in Drosophila simulans . Genetics 184, 253–265. ( 10.1534/genetics.109.109587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holman L, Price TAR, Wedell N, Kokko H. 2015. Coevolutionary dynamics of polyandry and sex-linked meiotic drive. Evolution 69, 709–720. ( 10.1111/evo.12595) [DOI] [PubMed] [Google Scholar]
- 45.Lande R, Wilkinson GS. 1999. Models of sex-ratio meiotic drive and sexual selection in stalk-eyed flies. Genet. Res. (Camb.) 74, 245–253. ( 10.1017/S0016672399004218) [DOI] [Google Scholar]
- 46.Pomiankowski A, Hurst LD. 1999. Driving sexual preference. Trends Ecol. Evol. 14, 425–426. ( 10.1016/S0169-5347(99)01701-2) [DOI] [PubMed] [Google Scholar]
- 47.Wilkinson GS, Presgraves DC, Crymes L. 1998. Male eye span in stalk-eyed flies indicates genetic quality by meiotic drive suppression. Nature 391, 276–279. ( 10.1038/34640) [DOI] [Google Scholar]
- 48.Cotton S, Small J, Hashim R, Pomiankowski A. 2010. Eyespan reflects reproductive quality in wild stalk-eyed flies. Evol Ecol. 24, 83–95. ( 10.1007/s10682-009-9292-6) [DOI] [Google Scholar]
- 49.Johns PM, Wolfenbarger LL, Wilkinson GS. 2005. Genetic linkage between a sexually selected trait and X chromosome meiotic drive. Proc. R. Soc. B 272, 2097–2103. ( 10.1098/rspb.2005.3183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cotton AJ, Földvári M, Cotton S, Pomiankowski A. 2014. Male eyespan size is associated with meiotic drive in stalk-eyed flies (Teleopsis dalmanni). Heredity 112, 363–369. ( 10.1038/hdy.2013.131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coopersmith CB, Lenington S. 1990. Preferences of female mice for males whose t-haplotype differs from their own. Anim. Behav. 40, 1179–1181. ( 10.1016/S0003-3472(05)80184-8) [DOI] [Google Scholar]
- 52.Sutter A, Lindholm AK. 2016. No evidence for female discrimination against male house mice carrying a selfish genetic element. Curr. Zool. 62, 675–685. ( 10.1093/cz/zow063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Price TAR, Lewis Z, Smith DT, Hurst GDD, Wedell N. 2012. No evidence of mate discrimination against males carrying a sex ratio distorder in Drosophila pseudoobscura. Behav. Ecol. Sociobiol. 66, 561–568. ( 10.1007/s00265-011-1304-1) [DOI] [Google Scholar]
- 54.Presgraves DC, Severance E, Wilkinson GS. 1997. Sex chromosome meiotic drive in stalk-eyed flies. Genetics 147, 1169–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilkinson GS, Swallow JG, Christensen SJ, Madden K. 2003. Phylogeography of sex ratio and multiple mating in stalk-eyed flies from southeast Asia. Genetica 117, 37–46. ( 10.1023/A:1022360531703) [DOI] [PubMed] [Google Scholar]
- 56.Swallow JG, Wallace LE, Christianson SJ, Johns PM, Wilkinson GS. 2005. Genetic divergence does not predict change in ornament expression among populations of stalk-eyed flies. Mol. Ecol. 12, 3787–3800. ( 10.1111/j.1365-294X.2005.02691.x) [DOI] [PubMed] [Google Scholar]
- 57.Cotton S, Fowler K, Pomiankowski A. 2004. Condition dependence of sexual ornament size and variation in the stalk-eyed fly Cyrtodiopsis dalmanni (Diptera: Diopsidae). Evolution 58, 1038–1046. ( 10.1111/j.0014-3820.2004.tb00437.x) [DOI] [PubMed] [Google Scholar]
- 58.Panhuis TM, Wilkinson GS. 1999. Exaggerated male eye span influences contest outcome in stalk-eyed flies (Diopsidae). Behav. Ecol. Sociobiol. 4, 221–227. ( 10.1007/s002650050613) [DOI] [Google Scholar]
- 59.Small J, Cotton S, Fowler K, Pomiankowski A. 2009. Male eyespan and resource ownership affect contest outcome in the stalk-eyed fly, Teleopsis dalmanni. Anim. Behav. 5, 1213–1220. ( 10.1016/j.anbehav.2009.08.009) [DOI] [Google Scholar]
- 60.David P, Bjorksten T, Fowler K, Pomiankowski A. 2000. Condition-dependent signalling of genetic variation in stalk-eyed flies. Nature 406, 186–188. ( 10.1038/35018079) [DOI] [PubMed] [Google Scholar]
- 61.Howie JM, Dawson HAD, Pomiankowski A, Fowler K. 2019. Limits to environmental masking of genetic quality in sexual signals. J. Evol. Biol. 32, 868–877. ( 10.1111/jeb.13491) [DOI] [PubMed] [Google Scholar]
- 62.Wilkinson GS, Johns PM, Kelleher ES, Muscadere ML, Lorsong A. 2006. Fitness effects of X chromosome drive in the stalk-eyed fly Cyrtodiopsis dalmanni. J. Evol. Biol. 19, 1851–1860. ( 10.1111/j.1420-9101.2006.01169.x) [DOI] [PubMed] [Google Scholar]
- 63.Meade L, Finnegan SR, Kad R, Fowler K, Pomiankowski A. 2019. Maintenance of fertility in the face of meiotic drive. bioXriv ( 10.1101/675108) [DOI] [PubMed]
- 64.Meade L, Dinneen D, Kad R, Lynch DM, Fowler K, Pomiankowski A. 2019. Ejaculate sperm number compensation in stalk-eyed flies carrying a selfish meiotic drive element. Heredity 122, 916–926. ( 10.1038/s41437-018-0166-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baker RH, Ashwell RIS, Richards TA, Fowler K, Chapman T, Pomiankowski A. 2001. Effects of multiple mating and male eye span on female reproductive output in the stalk-eyed fly Cyrtodiopsis dalmanni. Behav. Ecol. 14, 607–611. ( 10.1093/beheco/arg053) [DOI] [Google Scholar]
- 66.Rogers DW, Denniff M, Chapman T, Fowler K, Pomiankowski A. 2008. Male sexual ornament size is positively associated with reproductive morphology and enhanced fertility in the stalk-eyed fly Teleopsis dalmanni. BMC Evol. Biol. 8, 236 ( 10.1186/1471-2148-8-236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Green MR, Sambrook J. 2017. Precipitation of DNA with isopropanol. Cold Spring Harb. Protoc. 2017, pdb-rot093385 ( 10.1101/pdb.prot093385) [DOI] [PubMed] [Google Scholar]
- 68.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using {lme4}. J Stat Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 69.R Core Team. 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; http://www.R-project.org/ [Google Scholar]
- 70.Kuznetsova A, Brockhoff PB, Christensen RHB. 2017. lmerTest package: tests in linear mixed effects models. J. Stat. Softw. 82, 1–26. ( 10.18637/jss.v082.i13) [DOI] [Google Scholar]
- 71.Chippindale AK, Gibson JR, Rice WR. 2001. Negative genetic correlation for adult fitness between sexes reveals ontogenetic conflict in Drosophila . Proc. Natl Acad. Sci USA. 98, 1671–1675. ( 10.1073/pnas.98.4.1671) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arnqvist G, Tuda M. 2010. Sexual conflict and the gender load: correlated evolution between population fitness and sexual dimorphism in seed beetles. Proc. R. Soc. B 277, 1345–1352. ( 10.1098/rspb.2009.2026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Merçot H, Atlan A, Jacques M, Montchamp-Moreau C. 1995. Sex-ratio distortion in Drosophila simulans: co-occurrence of a meiotic drive and a suppressor of drive. J. Evol. Biol. 8, 283–300. ( 10.1046/j.1420-9101.1995.8030283.x) [DOI] [Google Scholar]
- 74.Burkhardt D, de la Motte I. 1985. Selective pressures, variability, and sexual dimorphism in stalk-eyed flies (Diopsidae). Naturwissenshaften 72, 204–206. ( 10.1007/BF01195763) [DOI] [Google Scholar]
- 75.Burkhardt D, de la Motte I. 1988. Big ‘antlers’ are favoured: female choice in stalk-eyed flies (Diptera, Insecta), field collected harems and laboratory experiments. J. Comp. Physiol. A 162, 649–652. ( 10.1007/BF01342640) [DOI] [Google Scholar]
- 76.Bonduriansky R. 2001. The evolution of mate choice in insects: a synthesis of ideas and evidence. Biol. Rev. 76, 305–339. ( 10.1017/S1464793101005693) [DOI] [PubMed] [Google Scholar]
- 77.Cotton AJ, Cotton S, Small J, Pomiankowski A. 2015. Male mate preference for female eyespan and fecundity in the stalk-eyed fly Teleopsis dalmanni . Behav. Ecol. 26, 376–385. ( 10.1093/beheco/aru192) [DOI] [Google Scholar]
- 78.Finnegan SR, Nitsche L, Mondani M, Camus F, Fowler K, Pomiankowski A. 2019. Does meiotic drive alter male mate preference? bioXriv ( 10.1101/736595) [DOI]
- 79.Finnegan S, White N, Koh D, Camus M, Fowler K, Pomiankowski A. 2019. Data from: Meiotic drive reduces egg-to-adult viability in stalk-eyed flies. Dryad Digital Repository. ( 10.5061/dryad.kc49jk1) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Finnegan S, White N, Koh D, Camus M, Fowler K, Pomiankowski A. 2019. Data from: Meiotic drive reduces egg-to-adult viability in stalk-eyed flies. Dryad Digital Repository. ( 10.5061/dryad.kc49jk1) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.kc49jk1 [79].