Abstract
Living in groups affords individuals many benefits, including the opportunity to reduce stress. In mammals, such ‘social buffering’ of stress is mediated by affiliative relationships and production of the neuropeptide oxytocin, but whether these mechanisms facilitate social buffering across vertebrates remains an open question. Therefore, we evaluated whether the social environment influenced the behavioural and physiological recovery from an acute stressor in a group-living cichlid, Neolamprologus pulcher. Individual fish that recovered with their social group displayed lower cortisol levels than individuals that recovered alone. This social buffering of the stress response was associated with a tendency towards lower transcript abundance of arginine vasotocin and isotocin in the preoptic area of the brain, suggesting reduced neural activation of the stress axis. Individuals that recovered with their social group quickly resumed normal behaviour but received fewer affiliative acts following the stressor. Further experiments revealed similar cortisol levels between individuals that recovered in visual contact with their own social group and those in visual contact with a novel but non-aggressive social group. Collectively, our results suggest that affiliation and familiarity per se do not mediate social buffering in this group-living cichlid, and the behavioural and physiological mechanisms responsible for social buffering may vary across vertebrates.
Keywords: affiliation, aggression, arginine vasotocin, cortisol, isotocin, social groups
1. Introduction
There are numerous benefits to living in a social group, including improved food acquisition [1,2], increased vigilance [1,3] and workload sharing [4,5]. Group living can also provide direct physiological benefits. For example, individuals that are with a social partner while recovering from a stressor often mount a reduced glucocorticoid response compared with individuals that recover alone (e.g. pair-bonded mates [6,7]; offspring and parents [8,9]). However, most studies of such ‘social buffering’ of stress have been conducted in mammals (reviewed in [10–12])—particularly rodents (e.g. prairie voles and guinea pigs) and primates (e.g. chimpanzees and humans)—and we know very little about how social relationships modulate stress in other animals. Additionally, the few studies that have examined social buffering of stress responses in non-mammalian animals, such as fishes [13,14] and birds [15], have not assessed the mechanisms responsible for these effects.
Glucocorticoid production in response to a stressor is stimulated by adrenocorticotropic hormone (ACTH) from the anterior pituitary [16,17]. Although ACTH release is largely regulated by corticotropin-releasing factor (CRF) [18], other neuropeptides, including arginine vasopressin (AVP) and oxytocin (OXT), can also play a role [19,20]. The OXT system has emerged as a key neural mediator of social buffering in mammals [6,21,22], as it reduces the ACTH-releasing actions of CRF [23,24]. In addition, OXT often promotes affiliation and social contact between social partners [25–27], which can further reduce stress [28–30]. Arginine vasopressin and CRF also have been implicated in the regulation of social behaviours [31–33], including affiliation [34,35]. Thus, the integrated actions of these neuropeptides in glucocorticoid production and social behaviour appear to be conserved across vertebrates [31,33,36], but their roles in the social buffering of stress have only been studied previously in mammals.
In the present study, we investigated social buffering of stress in a group-living cichlid fish, Neolamprologus pulcher. These fish live in social groups consisting of a dominant breeding pair and 1–20 subordinate helpers that assist breeders by performing brood care, as well as territory defence and maintenance [37–39]. Group composition fluctuates as individuals are lost to predation [38] and move between groups [37,40,41]. Consequently, relatedness among group members, particularly large helpers and breeders, typically is low [42,43]. Group members regularly engage in affiliative exchanges [44,45] and prefer familiar group members over size-matched groups of unfamiliar individuals [46], suggesting that affiliation among group members plays an important role in the rich social life of N. pulcher. Recently, isotocin (IT; the teleost homologue of OXT) has been implicated as a key regulator of affiliative behaviours in teleost fishes [47,48], including N. pulcher [45,49], suggesting the potential for IT to contribute to social buffering of stress in fishes through both behavioural and physiological effects.
We predicted that the glucocorticoid response of N. pulcher individuals that recovered from an acute stressor on their own would be greater than that of individuals that recovered within their social group. We focused on subordinate helpers because they are most likely to benefit from the protection of a social group; dominant breeding pairs are larger [39] and typically provide greater levels of territory defence [50]. To investigate how the social environment influenced neural regulators of behaviour and glucocorticoid synthesis, we measured transcript abundances of isotocin (it), arginine vasotocin (avt; the teleost homologue of AVP) and corticotropin-releasing factor (crf) in the preoptic area of the brain (POA). In fishes, the POA regulates ACTH release [51] and is widely implicated in the regulation of social behaviour [52,53]. To further disentangle whether the social buffering of stress is mediated by the presence of familiar social partners, or by conspecifics more generally, we also allowed individuals to recover in view of, but physically separated from, either their own social group or a novel social group. We predicted that if social buffering is dependent on the presence of known social partners, then buffering should not be observed in individuals that recovered in view of a novel social group.
2. Material and methods
(a). Experimental animals
Experiments took place between March and August 2018 at McMaster University in Hamilton, Ontario, Canada. All fish were F2 or F3 laboratory-reared descendants of wild-caught N. pulcher collected from Lake Tanganyika, Africa. Social groups consisting of a dominant breeding pair, 1–3 large helpers (standard length, SL > 4 cm) and up to 2 small helpers (SL < 4 cm) were held in 189 l aquaria under standard housing conditions (see [54,55]). Each fish in a group received a unique dorsal fin clip for identification; these marks do not adversely affect behaviour [56]. All social groups (n = 39) had been together for at least two months and had produced young in the month preceding experimentation.
(b). Experiment 1: recovery following a stressor with own group versus alone
Nineteen social groups were used in this experiment. Prior to each trial, groups were video recorded (Canon VIXIA HF S200) once per day for 3 days to assess behaviour (see §2d) of focal large helpers that were to be exposed to acute stress via air exposure. Focal individuals (n = 29) were quickly netted and held out of the water within the damp net for 3 min. Following the stressor, individuals were returned to their home tanks to recover with their social group (own group; n = 14) or by themselves (alone; n = 15). Recovery treatment was randomly assigned to each individual prior to the experiment. In trials where individuals recovered by themselves, the social group of that individual was removed from the home tank during the time the individual was subjected to air exposure, and social groups were placed into an identical empty tank that was visually isolated from the recovering focal individual. Thus, focal individuals always recovered in their home tanks and were video recorded throughout the recovery period (25 or 90 min) to assess behavioural changes across time.
We selected 25 and 90 min post-stressor to investigate how the social environment influenced peak and recovering cortisol levels, respectively. A separate preliminary study showed that circulating cortisol levels in N. pulcher peaked 15–30 min after a 3 min air exposure stressor, and were returning to baseline levels by 90 min post-stressor (electronic supplementary material, figure S1). Thus, focal individuals were euthanized after 25 or 90 min and sampled (see §2e). At the end of each trial, the remaining members of each social group were returned to their original home tank.
Note that while individuals that recovered by themselves were alone in their tank, they were able to see and interact (across the glass) with familiar neighbouring groups in nearby aquaria. As such, recovering fish were never completely socially isolated. Additionally, if more than one individual in a social group were used in this experiment, then trials were separated by 7 days to allow behavioural group dynamics to re-stabilize. Group behaviours were always assessed over the 3 days preceding each trial.
(c). Experiment 2: recovery following a stressor in view of own versus a novel social group
Twenty social groups were used in this experiment. This experiment was conducted as described for experiment 1, except that following air exposure, individuals (n = 33) recovered either in view of their own social group (own group; n = 16) or in view of a novel social group (novel group; n = 17). During the recovery period, focal individuals occupied one-quarter of the tank, and were separated by a non-perforated, clear barrier from the other three-quarters of the tank containing either their own social group or a novel social group. Neolamprologus pulcher are a highly visual species and are capable of individual recognition using only visual information [57–59]. Novel social groups were matched in number and composition of individuals to each individual's own social group and were exchanged with the original social groups during the air exposure of the focal individual.
(d). Behavioural analyses
The behaviour of individuals prior to experimentation was assessed from 15 min video recordings of social groups (rates of social and locomotor behaviours were similar across treatment groups prior to the experiments; see electronic supplementary material, tables S1 and S2). The initial 10 min of each 15 min recording served as an acclimation period following placement of the camera in front of a tank. During the final 5 min of each recording, the number of aggressive acts received (chases, bites, rams, opercular flares, aggressive postures and lateral displays) and affiliative behaviours (follows, parallel swims and soft touches) performed or received by the focal individual were scored (see [60] for a species-specific ethogram detailing all behaviours recorded). The time that focal fish spent within a body length (approx. 6 cm) of a groupmate was also measured and was reported as ‘time near group members'. To assess locomotor activity, tanks were visually split into 32 quadrats (11.5 × 12.5 cm) using a grid and the number of times a focal fish crossed quadrat boundaries was counted. To estimate the territory usage of each focal fish [61,62], the number of unique quadrats that focal fish entered during the observation period was counted and expressed as a proportion of the total number of possible quadrats. Additionally, we quantified the relative amount of time that individuals spent in preferred areas (near substrate and shelters) of the tank [63]. This time was calculated as the difference in time that fish spent in the bottom quarter of the tank (more preferred) versus the top quarter of the tank (least preferred). Pre-stressor behaviours are reported as the mean values over the three observations.
Following the acute stressor, behaviours were again assessed for three 5 min intervals during the recovery period. Specifically, individuals sampled 25 min after the stressor were observed at 0, 10 and 20 min, and individuals sampled 90 min after the stressor were observed at 0, 40 and 85 min. The behaviours that were assessed pre-stressor were also scored during recovery, with the exception that social behaviours could not be scored for fish that recovered alone. In addition, because all focal individuals in experiment 2 were confined to only one-quarter of their tank, changes in locomotor behaviour, territory usage, and time in preferred areas of the tank were not calculated.
(e). Tissue sampling
At the end of the recovery period, fish were rapidly netted and euthanized via terminal anaesthesia (0.5 g l−1 ethyl-p-aminobenzoate; Sigma-Aldrich, Oakville, Ontario, Canada). Blood was collected in heparinized micro-hematocrit capillary tubes via caudal severance (within 2 min of approaching the tank) and centrifuged (4750 g for 3 min). Plasma was collected, flash frozen in liquid nitrogen and stored at −80°C for later analysis of cortisol concentrations (see §2f). The POA and head kidney were dissected out, flash frozen and stored at −80°C for later analysis of transcript abundance (see §2g). All fish were sampled between 09.00 and 11.00 h.
(f). Cortisol quantification
Circulating cortisol levels were measured using an enzyme-linked immunosorbent assay (EIA; Neogen, Lexington, KY, USA) following the manufacturer's protocol; the assay was previously validated for N. pulcher [64]. Plasma samples were diluted 100× with Milli-Q water (EMD Millipore, Etobicoke, Ontario, Canada) prior to analysis. Samples were assayed in duplicate with intra-assay variation of 3.0% and inter-assay variation of 10.5% (% CV). We were unsuccessful in collecting blood from five individuals (one that recovered alone; two that recovered with their own group; and two that recovered in view of a novel group), and therefore, plasma cortisol could not be measured for these fish.
(g). Transcript abundance analysis by real-time polymerase chain reaction
Changes in transcript abundance were assessed by semi-quantitative real-time polymerase chain reaction using gene-specific primers (electronic supplementary material, table S3), as described previously [64]. In addition to the targets in the POA (avt, crf, and it), we also assessed transcript abundances of steroidogenic acute regulatory protein (star) and cytochrome P450 side-chain cleavage enzyme (p450scc) in the head kidney (analogous to the adrenal gland) because they mediate the rate-limiting step in glucocorticoid synthesis [65,66]. Transcript abundance was calculated according to the modified ΔΔCt method [67]. Data were normalized to mRNA abundance of the reference genes 18S (POA) or β-actin (head kidney), which did not vary among groups. For experiment 1, data are expressed relative to individuals that recovered by themselves for 25 min. For experiment 2, data are expressed relative to individuals that recovered in sight of a novel group for 25 min.
(h). Statistical analysis
Statistical analyses were performed using R (v. 3.4.4, [68]) and a significance level (α) of 0.05 was used for all tests. When data did not meet the assumptions of normality and/or equal variance, data were log-transformed, or if the data could not be transformed to meet the assumptions, then equivalent non-parametric analyses were performed. All models were fit using the lmer function in the ‘lme4’ package [69], and when overall differences were detected using the Anova function in the ‘car’ package [70], Tukey's HSD post hoc analysis was performed using the ‘emmeans’ package. For all models, group id was included as a random factor to account for non-independence of animals that were sampled from the same group.
To assess how plasma cortisol and transcript abundance varied between recovery environments, general linear mixed models (LMMs) that included recovery time (25 or 90 min), recovery environment (own group or alone; own group or novel group) and their interaction term as fixed factors were fit. To investigate how the behaviour of individuals changed during recovery, LMMs were fit which included the observation period (Pre, 0–5, 10–15, 20–25, 40–45 or 85–90 min) as a fixed factor, as well as individual id as a random factor to account for repeated observations of the same animal across time. The major findings of these analyses are described below; however, a detailed description of the statistical results in full is included in the electronic supplementary material.
3. Results
(a). Social and locomotor behaviours
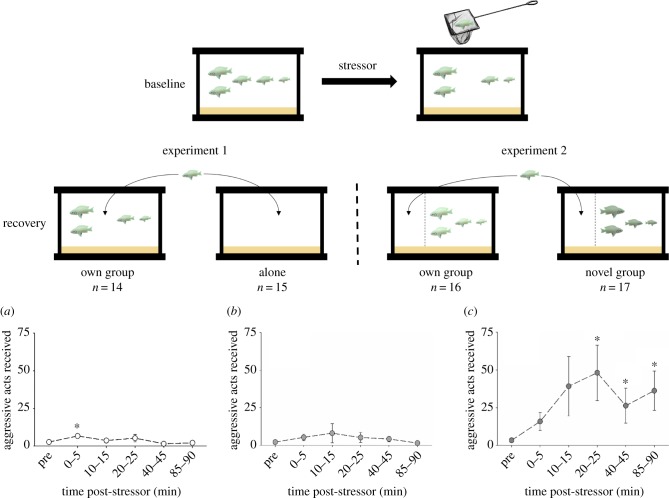
Individuals recovering within their own social group in experiment 1 received more aggression immediately following air exposure than prior to the stressor, but the rates of aggression quickly declined (figure 1a; χ 2 = 21.05, p < 0.001). In experiment 2, aggression received did not change compared to pre-stressor values when individuals recovered in visual contact with, but physically separated from, their own social group (figure 1b; χ 2 = 3.01, p = 0.70). By contrast, individuals in experiment 2 that recovered in view of a novel social group (but physically separated from it) encountered higher rates of aggression than they had received from their own social group prior to the stressor (figure 1c; χ 2 = 26.14, p < 0.001). Overall, individuals in experiment 1 that recovered within their own social group received fewer affiliative acts following the stressor compared to before the stressor, but did not adjust their own performance of affiliative acts nor did they change the amount of time spent near their groupmates following the stressor (table 1).
Figure 1.
Visual representation of the experiments. Following air exposure, N. pulcher recovered either with their own social group or alone (experiment 1) or they recovered in view of their own social group or a novel social group (experiment 2). The number of aggressive acts (tallied over a 5 min period) received by individuals during recovery is illustrated for individuals that recovered with their own social group (a); in visual contact with their own social group (b); or in visual contact with an unfamiliar social group (c). Values are means ± s.e.m. An asterisk indicates a significant difference from the pre-stressor value (see text for additional details). (Online version in colour.)
Table 1.
Affiliative behaviour before and after exposure of N. pulcher to an acute stressor for individuals that recovered with their own social group in experiment 1 (n = 14). Values for an individual fish represent the average of three 5 min observation periods. Behaviours are reported as means ± s.e.m. Significant differences (p < 0.05) are indicated by italic font. Rates of affiliative behaviours following air exposure did not change across time when individuals recovered with their own social group (electronic supplementary material, table S4). Therefore, post-stressor values of affiliation for each focal fish were averaged across the three observation periods during recovery and were compared to pre-stressor values.
| behaviour | pre-stressor | post-stressor | χ 2 | p-value | |
|---|---|---|---|---|---|
| affiliative acts | received | 0.4 ± 0.2 | 0.1 ± 0.1 | 4.94 | 0.02 |
| performed | 0.4 ± 0.2 | 0.2 ± 0.1 | 1.74 | 0.19 | |
| time near group members (s) | 32.4 ± 5.8 | 23.2 ± 4.5 | 2.09 | 0.15 |
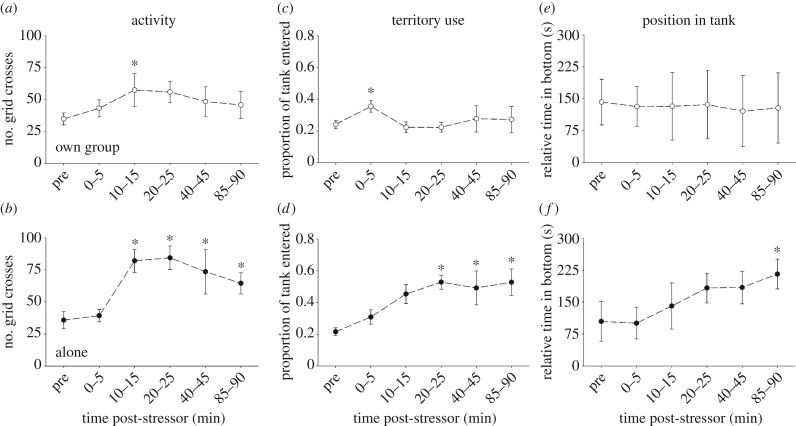
Relative to pre-stressor values, individuals in experiment 1 that recovered within their social group displayed a transient increase in locomotor activity (figure 2a; χ 2 = 14.36, p = 0.01) and visited a greater proportion of the territory (figure 2c; Χ2 = 22.35, p < 0.001) during the initial stages of recovery, but they did not significantly adjust the relative amount of time spent in more preferred, lower areas of the tank (figure 2e; χ 2 = 9.00, p = 0.11). By contrast, compared with pre-stressor values, fish in experiment 1 that recovered by themselves showed sustained increases in both locomotor activity (figure 2b; χ 2 = 66.56, p < 0.001) and the proportion of the territory visited (figure 2d; χ 2 = 46.42, p < 0.001), as well as steadily increasing the time that they spent in the preferred, lower tank areas near shelter (figure 2f; χ2 = 13.03, p = 0.02).
Figure 2.
Activity levels (a,b), territory usage (c,d) and relative time in the bottom of the tank (e,f) for individual N. pulcher that recovered from a stressor in experiment 1. Fish either recovered with their own social group (a,c,e) or alone (b,d,f). Values are means ± s.e.m. An asterisk indicates a significant difference from the pre-stressor value (see text for additional details).
(b). Cortisol concentrations and transcript abundances
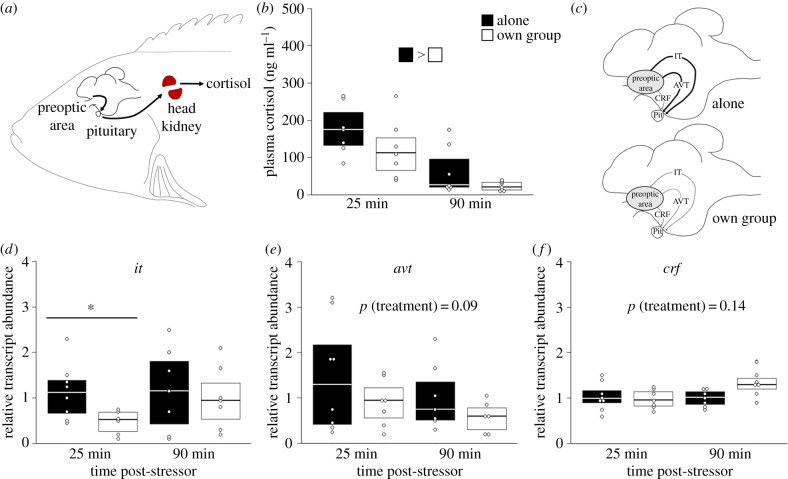
In experiment 1, individuals that recovered by themselves had higher circulating cortisol concentrations relative to individuals that recovered within their social group (figure 3b; LMM: χ2 = 4.98, p = 0.03). Preoptic area transcript abundance of it tended to be higher in individuals that recovered by themselves compared with those that recovered with their social group (figure 3c; LMM: χ2 = 2.75, p = 0.09), particularly 25 min post-stressor. Post hoc comparison of it transcript abundance 25 min post-stressor confirmed that abundance was higher in individuals that recovered by themselves compared to fish that recovered with their social group (p = 0.01). Transcript abundance of avt in the POA also tended to be higher in individuals that recovered alone relative to individuals that recovered with their social group (figure 3d; LMM: χ2 = 2.83, p = 0.09). Individuals that recovered with their social group versus alone did not differ in transcript abundance of crf (figure 3e; LMM: χ2 = 4.55, p = 0.14), but individuals that recovered within their social group tended to have higher crf transcript levels following 90 min of recovery compared to 25 min of recovery (p = 0.08). Transcript abundance of the steroidogenic enzyme p450scc in the head kidney was higher in individuals that recovered with their social groups compared to individuals that recovered alone, but only at 25 min post-stressor (electronic supplementary material, table S5; p = 0.02). No differences in transcript levels of star were detected in the head kidney (electronic supplementary material, table S5; p = 0.13).
Figure 3.
Visual representation of the endocrine stress axis (hypothalamic–pituitary–interrenal axis) in N. pulcher (a), and changes in neuroendocrine regulation of the stress axis in the brains of individuals that recovered alone or with their own social group, where darker lines indicate increased activation (c). Circulating plasma cortisol levels (b); and relative transcript abundance of it (d), avt (e) and crf (f) in the preoptic area of individuals that either recovered alone or with their own social group (experiment 1). Values are presented as medians and 1st and 3rd quartiles; points represent individual values. Differences between groups are indicated using the bar fills or by an asterisk (see text for further details). AVT, arginine vasotocin; CRF, corticotropin-releasing factor; IT, isotocin; Pit, pituitary.
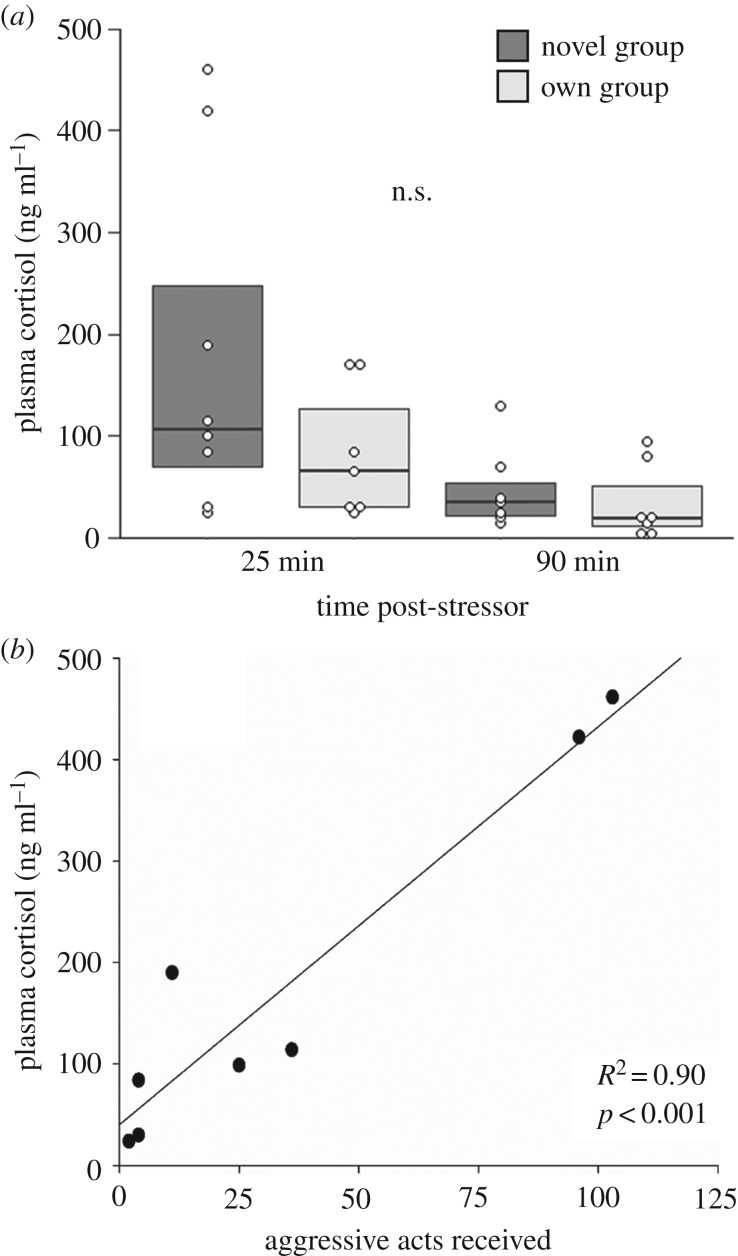
In experiment 2, cortisol levels of individuals that recovered while physically isolated from (but still in visual contact with) their own social group did not differ from levels observed in individuals that recovered in view of a novel social group (figure 4a: Χ2 = 2.55, p = 0.11). However, peak cortisol levels of individuals that recovered in view of a novel group were highest when the members of the novel group were decidedly aggressive (figure 4b; Pearson's correlation: R2 = 0.90, p < 0.001). No differences in transcript levels were detected between individuals that recovered in view of their own social group and those that recovered in view of a novel social group in either the POA or the head kidney (electronic supplementary material, table S6).
Figure 4.
Plasma cortisol levels of individual N. pulcher that recovered in view of a novel social group or in view of their own social group in experiment 2 (a), and the correlation between peak circulating cortisol levels and the number of aggressive acts received from members of a novel social group (b). Aggressive acts were tallied over a 5 min period. Values in (a) are presented as medians and 1st and 3rd quartiles; points represent individual values.
4. Discussion
The presence of an individual's social group reduced post-stressor cortisol levels in the group-living cichlid, N. pulcher. This social buffering of the cortisol response was associated with a tendency towards reduced transcript abundance of IT (the teleost homologue of oxytocin) and AVT (the teleost homologue of arginine vasopressin) in the POA, changes that are consistent with reduced neural activation of the glucocorticoid stress axis. Interestingly, individuals received less affiliative behaviour from their groupmates following the stressor, and even novel social groups appeared to function as a social buffer to stress when these groups behaved non-aggressively towards the focal recovering individual. Peak cortisol levels of recovering individuals correlated positively with the number of aggressive acts received from unfamiliar group members; when individuals recovered in sight of non-aggressive novel groups, cortisol levels were similar to individuals that recovered in sight of their own social group.
Social buffering most commonly occurs between individuals that share strong social relationships, such as pair-bonded mates [6,7] or offspring-and-parents [8,9]. The intimate nature of these long-term social relationships appears critical for social buffering, as surrogate social partners typically confer less effective buffering [9,71]. However, social relationships can be much more transient in nature (e.g. aggregations), and even unfamiliar conspecifics appear capable of buffering stress under some circumstances [10,72]. We found that subordinate N. pulcher that were allowed to recover within the confines of their own social group displayed lower cortisol levels than individuals that recovered alone. Additionally, even novel social groups appeared to buffer stress in recovering individuals as long as the members of the novel group were non-aggressive. This breadth of social buffering capacity probably reflects the social structure in groups of N. pulcher. Individuals can emigrate and immigrate between groups [37,40,41], and individual group members typically are not close relatives [42,43]. As such, social buffering in N. pulcher appears to rely less on genetic relatedness, and may instead be driven by the general presence of non-aggressive conspecifics—especially the dominant breeders, as they provide high levels of defence [50] and serve as the social glue for groups [54,73]. A similar finding was reported for aggregations of juvenile lake sturgeon (Acipenser fulvescens), where cortisol responses following an acute stressor were lower than those of isolated fish [13]. The authors suggested that buffering in these sturgeon stems from a lack of aggression among juveniles of this species. Therefore, the suppression of aggressive tendencies appears to be a key catalyst for social buffering in fishes (among the limited species tested to date), although further work is clearly necessary to ascertain whether this generalization holds across a broader spectrum of species and social relationships.
Social contact and affiliation play key roles in the social buffering of stress in mammals [74], with social partners often increasing the performance of grooming and contact communication behaviours towards stressed companions. Such affiliative behaviours appear to console and protect the individual from the physiological and psychological harm imposed by stressors [27,28]. Similarly, tactile stimulation was found to reduce glucocorticoid responses in fish [29,75], but we found that individuals recovering from stress received fewer affiliative acts (including affiliative touches) from their groupmates. Although N. pulcher individuals commonly engage in affiliative interactions with groupmates, subordinates typically receive far fewer affiliative acts than breeders [44,64]. Even following a territory intrusion by an unfamiliar conspecific (a stressful event that can destabilize a social group), rates of affiliation directed towards subordinates did not change [44]. Only when territory intruders came from neighbouring groups—which more directly threatens group stability—did rates of affiliation towards subordinate helpers increase. As such, affiliation appears to act as a master regulator of social relationships, enhancing group cohesion during periods of social instability. However, as our experiment demonstrated, affiliation between group members is not strictly necessary for social buffering in N. pulcher.
Although OXT enhances affiliative behaviour in mammals [26,27], IT does not seem to promote affiliation in fishes [47,48,76]. Indeed, IT is associated with a reduction in affiliative tendencies in N. pulcher [45,49] and instead appears to enhance an individual's attention to social information [55,77], in line with the notion that OXT-related peptides promote the performance of socially salient behaviours [78]. In mammals, OXT is considered a key mediator of the social buffering of stress [6,21], reducing glucocorticoid production by promoting stress-reducing affiliative behaviours [26,27], and by reducing activation of CRF neurons in response to stress [23,24]. In our experiment, however, POA transcript abundance of it tended to be higher in N. pulcher that recovered alone compared to individuals that recovered with their social group—as were circulating cortisol levels—while preoptic area transcript abundance of crf in the POA did not differ. These parallel increases in IT and cortisol are consistent with the admittedly sparse data indicating that IT stimulates glucocorticoid production in fishes [19,51,79]. Collectively, these data suggest that OXT-related peptides may have opposing effects on glucocorticoid synthesis in mammals (inhibitory) versus fishes (stimulatory) during social buffering. Across vertebrates, AVP-related peptides (including the teleost homologue, AVT) activate the glucocorticoid stress axis [20,79], and avt transcript abundance also tended to be higher in the POA of N. pulcher that recovered by themselves. Therefore, the combined actions of AVT and IT probably modulated stress axis activity during social buffering in N. pulcher (figure 3c).
Additionally, AVT plays a key role in regulating transitions in social status in fishes [80,81]. Therefore, the tendency for higher avt transcript abundance in the POA of individuals that recovered by themselves may reflect in part alleviation of social suppression. Removal of the dominant breeding pair provides a rare opportunity for subordinates to ascend to dominance. In the current study, individuals that recovered by themselves quickly increased their locomotor activity and territory usage. Similar behavioural modifications also occur during ascension to dominance in N. pulcher [61], a transition that is associated with activation of the stress axis and elevated circulating cortisol levels [64]. Therefore, the release from social suppression by dominants also may have contributed to the behavioural and physiological responses of individuals that recovered by themselves.
In conclusion, social buffering of the cortisol response to an acute stressor in N. pulcher was associated with a tendency for reduced preoptic expression of the nonapeptides IT and AVT, and occurred in the absence of increases in affiliative behaviour. These findings constitute one of the first studies of the physiological and behavioural factors associated with social buffering of stress in a non-mammalian vertebrate, and suggest that social buffering is regulated by different behavioural and physiological mechanisms across vertebrates.
Supplementary Material
Acknowledgements
The authors thank Carol Best for assistance with real-time polymerase chain reaction and illustrations, and Denys deCatanzaro for allowing us access to his spectrophotometer.
Ethics
All experimental protocols were approved by the Animal Research Ethics Board of McMaster University (Animal Utilization Protocol No. 18-04-16), and were in compliance with the guidelines of the Canadian Council on Animal Care (CCAC).
Data accessibility
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.7v93210 [82].
Authors' contributions
B.M.C., K.M.G. and S.B. designed the study. B.M.C. performed the experiments, conducted the analyses and wrote the first draft of the manuscript. All authors discussed results and commented on the manuscript.
Competing interests
The authors declare no competing interests.
Funding
This work was supported by Natural Sciences and Engineering Research Council (NSERC) of Canada Discovery grants to K.M.G. (RGPIN-2017-05487) and S.B. (RGPIN-2016-05772). B.M.C. was supported by an NSERC Master's Canadian Graduate Scholarship (CGS-M) and is currently supported by an NSERC Doctoral CGS (CGS-D).
References
- 1.Evans JC, Votier SC, Dall SRX. 2016. Information use in colonial living. Biol. Rev. 91, 658–672. ( 10.1111/brv.12188) [DOI] [PubMed] [Google Scholar]
- 2.Ward P, Zahavi A. 1973. The importance of certain assemblages of birds as ‘Information-Centres’ for food-finding. Ibis 115, 517–534. ( 10.1111/j.1474-919X.1973.tb01990.x) [DOI] [Google Scholar]
- 3.Roberts G. 1996. Why individual vigilance declines as group size increases. Anim. Behav. 51, 1077–1086. ( 10.1006/ANBE.1996.0109) [DOI] [Google Scholar]
- 4.Clutton-Brock TH, Russell AF, Sharpe LL, Young AJ, Balmforth Z, McIlrath GM. 2002. Evolution and development of sex differences in cooperative behavior in meerkats. Science 297, 253–256. ( 10.1126/science.1071412) [DOI] [PubMed] [Google Scholar]
- 5.Ulrich Y, Saragosti J, Tokita CK, Tarnita CE, Kronauer DJC. 2018. Fitness benefits and emergent division of labour at the onset of group living. Nature 560, 635–638. ( 10.1038/s41586-018-0422-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith AS, Wang Z. 2014. Hypothalamic oxytocin mediates social buffering of the stress response. Biol. Psychiatry 76, 281–288. ( 10.1016/j.biopsych.2013.09.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith TE, McGreer-Whitworth B, French JA. 1998. Close proximity of the heterosexual partner reduces the physiological and behavioral consequences of novel-cage housing in black tufted-ear marmosets (Callithrix kuhli). Horm. Behav. 34, 211–222. ( 10.1006/HBEH.1998.1469) [DOI] [PubMed] [Google Scholar]
- 8.Wiedenmayer CP, Magarinos AM, McEwen BS, Barr GA. 2003. Mother lowers glucocorticoid levels of preweaning rats after acute threat. Ann. N. Y. Acad. Sci. 1008, 304–307. ( 10.1196/annals.1301.038) [DOI] [PubMed] [Google Scholar]
- 9.Graves FC, Hennessy MB. 2000. Comparison of the effects of the mother and an unfamiliar adult female on cortisol and behavioral responses of pre- and postweaning guinea pigs. Dev. Psychobiol. 36, 91–100. () [DOI] [PubMed] [Google Scholar]
- 10.Kiyokawa Y, Hiroshima S, Takeuchi Y, Mori Y. 2014. Social buffering reduces male rats' behavioral and corticosterone responses to a conditioned stimulus. Horm. Behav. 65, 114–118. ( 10.1016/J.YHBEH.2013.12.005) [DOI] [PubMed] [Google Scholar]
- 11.DeVries AC, Glasper ER, Detillion CE. 2003. Social modulation of stress responses. Physiol. Behav. 79, 399–407. ( 10.1016/S0031-9384(03)00152-5) [DOI] [PubMed] [Google Scholar]
- 12.Hennessy MB, Kaiser S, Sachser N. 2009. Social buffering of the stress response: diversity, mechanisms, and functions. Front. Neuroendocrinol. 30, 470–482. ( 10.1016/J.YFRNE.2009.06.001) [DOI] [PubMed] [Google Scholar]
- 13.Allen PJ, Barth CC, Peake SJ, Abrahams MV, Anderson WG. 2009. Cohesive social behaviour shortens the stress response: the effects of conspecifics on the stress response in lake sturgeon Acipenser fulvescens. J. Fish Biol. 74, 90–104. ( 10.1111/j.1095-8649.2008.02112.x) [DOI] [PubMed] [Google Scholar]
- 14.Mommer BC, Bell AM. 2013. A test of maternal programming of offspring stress response to predation risk in threespine sticklebacks. Physiol. Behav. 122, 222–227. ( 10.1016/J.PHYSBEH.2013.04.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banerjee SB, Adkins-Regan E. 2011. Effect of isolation and conspecific presence in a novel environment on corticosterone concentrations in a social avian species, the zebra finch (Taeniopygia guttata). Horm. Behav. 60, 233–238. ( 10.1016/J.YHBEH.2011.05.011) [DOI] [PubMed] [Google Scholar]
- 16.Fridmanis D, Roga A, Klovins J. 2017. ACTH receptor (MC2R) specificity: what do we know about underlying molecular mechanisms? Front. Endocrinol. 8, 13 ( 10.3389/fendo.2017.00013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simpson ER, Waterman MR. 1988. Regulation of the synthesis of steroidogenic enzymes in adrenal cortical cells by ACTH. Annu. Rev. Physiol. 50, 427–440. ( 10.1146/annurev.ph.50.030188.002235) [DOI] [PubMed] [Google Scholar]
- 18.Aguilera G. 1998. Corticotropin releasing hormone, receptor regulation and the stress response. Trends Endocrinol. Metab. 9, 329–336. ( 10.1016/S1043-2760(98)00079-4) [DOI] [PubMed] [Google Scholar]
- 19.Fryer J, Lederis K, Rivier J. 1985. ACTH-releasing activity of urotensin I and ovine CRF: interactions with arginine vasotocin, isotocin and arginine vasopressin. Regul. Pept. 11, 11–15. ( 10.1016/0167-0115(85)90026-6) [DOI] [PubMed] [Google Scholar]
- 20.Aguilera G, Rabadan-Diehl C. 2000. Vasopressinergic regulation of the hypothalamic–pituitary–adrenal axis: implications for stress adaptation. Regul. Pept. 96, 23–29. ( 10.1016/S0167-0115(00)00196-8) [DOI] [PubMed] [Google Scholar]
- 21.Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. 2003. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol. Psychiatry 54, 1389–1398. ( 10.1016/S0006-3223(03)00465-7) [DOI] [PubMed] [Google Scholar]
- 22.Chen FS, Kumsta R, von Dawans B, Monakhov M, Ebstein RP, Heinrichs M. 2011. Common oxytocin receptor gene (OXTR) polymorphism and social support interact to reduce stress in humans. Proc. Natl Acad. Sci. USA 108, 19 937–19 942. ( 10.1073/pnas.1113079108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith AS, Tabbaa M, Lei K, Eastham P, Butler MJ, Linton L, Altshuler R, Liu Y, Wang Z. 2016. Local oxytocin tempers anxiety by activating GABAA receptors in the hypothalamic paraventricular nucleus. Psychoneuroendocrinology 63, 50–58. ( 10.1016/J.PSYNEUEN.2015.09.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jurek B, Slattery DA, Hiraoka Y, Liu Y, Nishimori K, Aguilera G, Neumann ID, van den Burg EH. 2015. Oxytocin regulates stress-induced CRF gene transcription through CREB-Regulated Transcription Coactivator 3. J. Neurosci. 35, 12 248–12 260. ( 10.1523/JNEUROSCI.1345-14.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodson JL, Schrock SE, Klatt JD, Kabelik D, Kingsbury MA. 2009. Mesotocin and nonapeptide receptors promote estrildid flocking behavior. Science 325, 862–866. ( 10.1126/science.1174929) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mooney SJ, Douglas NR, Holmes MM. 2014. Peripheral administration of oxytocin increases social affiliation in the naked mole-rat (Heterocephalus glaber). Horm. Behav. 65, 380–385. ( 10.1016/J.YHBEH.2014.02.003) [DOI] [PubMed] [Google Scholar]
- 27.Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FBM, Young LJ.. 2016. Oxytocin-dependent consolation behavior in rodents. Science 351, 375–378. ( 10.1126/science.aac4785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fraser ON, Stahl D, Aureli F. 2008. Stress reduction through consolation in chimpanzees. Proc. Natl Acad. Sci. USA 105, 8557–8562. ( 10.1073/pnas.0804141105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soares MC, Oliveira RF, Ros AFH, Grutter AS, Bshary R. 2011. Tactile stimulation lowers stress in fish. Nat. Commun. 2, 534 ( 10.1038/ncomms1547) [DOI] [PubMed] [Google Scholar]
- 30.Taylor GT. 1981. Fear and affiliation in domesticated male rats. J. Comp. Physiol. Psychol. 95, 685–693. ( 10.1037/h0077817) [DOI] [Google Scholar]
- 31.Goodson JL. 2013. Deconstructing sociality, social evolution and relevant nonapeptide functions. Psychoneuroendocrinology 38, 465–478. ( 10.1016/j.psyneuen.2012.12.005) [DOI] [PubMed] [Google Scholar]
- 32.Godwin J, Thompson R. 2012. Nonapeptides and social behavior in fishes. Horm. Behav. 61, 230–238. ( 10.1016/j.yhbeh.2011.12.016) [DOI] [PubMed] [Google Scholar]
- 33.Hostetler CM, Ryabinin AE. 2013. The CRF system and social behavior: a review. Front. Neurosci. 7, 92 ( 10.3389/fnins.2013.00092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young LJ, Nilsen R, Waymire KG, MacGregor GR, Insel TR. 1999. Increased affiliative response to vasopressin in mice expressing the V1a receptor from a monogamous vole. Nature 400, 766–768. ( 10.1038/23475) [DOI] [PubMed] [Google Scholar]
- 35.DeVries AC, Guptaa T, Cardillo S, Cho M, Carter SC. 2002. Corticotropin-releasing factor induces social preferences in male prairie voles. Psychoneuroendocrinology 27, 705–714. ( 10.1016/S0306-4530(01)00073-7) [DOI] [PubMed] [Google Scholar]
- 36.Kelly AM, Goodson JL. 2014. Social functions of individual vasopressin–oxytocin cell groups in vertebrates: what do we really know? Front. Neuroendocrinol. 35, 512–529. ( 10.1016/J.YFRNE.2014.04.005) [DOI] [PubMed] [Google Scholar]
- 37.Taborsky M. 1984. Broodcare helpers in the cichlid fish Lamprologus brichardi: their costs and benefits. Anim. Behav. 32, 1236–1252. ( 10.1016/S0003-3472(84)80241-9) [DOI] [Google Scholar]
- 38.Balshine S, Leach B, Neat FC, Reid H, Taborsky M, Werner N. 2001. Correlates of group size in a cooperatively breeding cichlid fish (Neolamprologus pulcher). Behav. Ecol. Sociobiol. 50, 134–140. ( 10.1007/s002650100343) [DOI] [Google Scholar]
- 39.Wong MYL, Balshine S. 2011. The evolution of cooperative breeding in the African cichlid fish, Neolamprologus pulcher. Biol. Rev. 86, 511–530. ( 10.1111/j.1469-185X.2010.00158.x) [DOI] [PubMed] [Google Scholar]
- 40.Stiver KA, Fitzpatrick JL, Desjardins JK, Balshine S. 2006. Sex differences in rates of territory joining and inheritance in a cooperatively breeding cichlid fish. Anim. Behav. 71, 449–456. ( 10.1016/j.anbehav.2005.06.011) [DOI] [Google Scholar]
- 41.Balshine-Earn S, Neat FC, Reid H, Taborsky M. 1998. Paying to stay or paying to breed? Field evidence for direct benefits of helping behavior in a cooperatively breeding fish. Behav. Ecol. 9, 432–438. ( 10.1093/beheco/9.5.432) [DOI] [Google Scholar]
- 42.Stiver KA, Desjardins JK, Fitzpatrick JL, Neff B, Quinn JS, Balshine S. 2007. Evidence for size and sex-specific dispersal in a cooperatively breeding cichlid fish. Mol. Ecol. 16, 2974–2984. ( 10.1111/j.1365-294X.2007.03350.x) [DOI] [PubMed] [Google Scholar]
- 43.Hellmann JK, Sovic MG, Gibbs HL, Reddon AR, O'Connor CM, Ligocki IY, Marsh-Rollo SE, Balshine S, Hamilton IM. 2016. Within-group relatedness is correlated with colony-level social structure and reproductive sharing in a social fish. Mol. Ecol. 25, 4001–4013. ( 10.1111/mec.13728) [DOI] [PubMed] [Google Scholar]
- 44.Bruintjes R, Lynton-Jenkins J, Jones JW, Radford AN. 2016. Out-group threat promotes within-group affiliation in a cooperative fish. Am. Nat. 187, 274–282. ( 10.1086/684411) [DOI] [PubMed] [Google Scholar]
- 45.Reddon AR, O'Connor CM, Marsh-Rollo SE, Balshine S, Gozdowska M, Kulczykowska E. 2015. Brain nonapeptide levels are related to social status and affiliative behaviour in a cooperatively breeding cichlid fish. R. Soc. open sci. 2, 140072 ( 10.1098/rsos.140072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jordan LA, Wong MYL, Balshine S. 2010. The effects of familiarity and social hierarchy on group membership decisions in a social fish. Biol. Lett. 6, 301–303. ( 10.1098/rsbl.2009.0732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lindeyer CM, ELangen EMA, Swaney WT, Reader SM. 2015. Nonapeptide influences on social behaviour: effects of vasotocin and isotocin on shoaling and interaction in zebrafish. Behaviour 125, 897–915. ( 10.1163/1568539X-00003261) [DOI] [Google Scholar]
- 48.Thompson RR, Walton JC. 2004. Peptide effects on social behavior: effects of vasotocin and isotocin on social approach behavior in male goldfish (Carassius auratus). Behav. Neurosci. 118, 620–626. ( 10.1037/0735-7044.118.3.620) [DOI] [PubMed] [Google Scholar]
- 49.Reddon AR, Voisin MR, O'Connor CM, Balshine S. 2014. Isotocin and sociality in the cooperatively breeding cichlid fish, Neolamprologus pulcher. Behaviour 151, 1389–1411. ( 10.1163/1568539X-00003190) [DOI] [Google Scholar]
- 50.Desjardins JK, Stiver KA, Fitzpatrick JL, Balshine S. 2008. Differential responses to territory intrusions in cooperatively breeding fish. Anim. Behav. 75, 595–604. ( 10.1016/j.anbehav.2007.05.025) [DOI] [Google Scholar]
- 51.Bernier NJ, Flik G, Klaren PHM. 2009. Regulation and contribution of the corticotropic, melanotropic and thyrotropic axes to the stress response in Fishes. In Fish neuroendocrinology (eds Bernier NJ, Van Der Kraak G, Farrell AP, Brauner CJ), pp. 235–311. London, UK: Elsevier. [Google Scholar]
- 52.Goodson JL. 2005. The vertebrate social behavior network: evolutionary themes and variations. Horm. Behav. 48, 11–22. ( 10.1016/J.YHBEH.2005.02.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O'Connell LA, Hofmann HA. 2011. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J. Comp. Neurol. 519, 3599–3639. ( 10.1002/cne.22735) [DOI] [PubMed] [Google Scholar]
- 54.Dey CJ, Reddon AR, O'Connor CM, Balshine S. 2013. Network structure is related to social conflict in a cooperatively breeding fish. Anim. Behav. 85, 395–402. ( 10.1016/J.ANBEHAV.2012.11.012) [DOI] [Google Scholar]
- 55.Reddon AR, O'Connor CM, Marsh-Rollo SE, Balshine S. 2012. Effects of isotocin on social responses in a cooperatively breeding fish. Anim. Behav. 84, 753–760. ( 10.1016/j.anbehav.2012.07.021) [DOI] [Google Scholar]
- 56.Stiver KA, Dierkes P, Taborsky M, Balshine S. 2004. Dispersal patterns and status change in a co-operatively breeding cichlid Neolamprologus pulcher: evidence from microsatellite analyses and behavioural observations. J. Fish Biol. 65, 91–105. ( 10.1111/j.0022-1112.2004.00427.x) [DOI] [Google Scholar]
- 57.Balshine-Earn S, Lotem A. 1998. Individual recognition in a cooperatively breeding cichlid: evidence from video playback experiments. Behaviour 135, 369–386. ( 10.1163/156853998793066221) [DOI] [Google Scholar]
- 58.Kohda M, Jordan LA, Hotta T, Kosaka N, Karino K, Tanaka H, Taniyama M, Takeyama T. 2015. Facial recognition in a group-living cichlid fish. PLoS ONE 10, e0142552 ( 10.1371/journal.pone.0142552) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saeki T, Sogawa S, Hotta T, Kohda M. 2018. Territorial fish distinguish familiar neighbours individually. Behaviour 155, 279–293. ( 10.1163/1568539X-00003489) [DOI] [Google Scholar]
- 60.Sopinka NM, Fitzpatrick JL, Desjardins JK, Stiver KA, Marsh-Rollo SE, Balshine S. 2009. Liver size reveals social status in the African cichlid Neolamprologus pulcher. J. Fish Biol. 75, 1–16. ( 10.1111/j.1095-8649.2009.02234.x) [DOI] [PubMed] [Google Scholar]
- 61.Culbert BM, Balshine S, Gilmour KM. 2019. Physiological regulation of growth during social ascension in a group-living fish. Physiol. Biochem. Zool. 92, 211–222. ( 10.1086/702338) [DOI] [PubMed] [Google Scholar]
- 62.Werner NY, Balshine S, Leach B, Lotem A. 2003. Helping opportunities and space segregation in cooperatively breeding cichlids. Behav. Ecol. 14, 749–756. ( 10.1093/beheco/arg067) [DOI] [Google Scholar]
- 63.Konings A. 2015. Tanganyika cichlids in their natural habitat, 3rd edn El Paso, TX: Cichlid Press. [Google Scholar]
- 64.Culbert BM, Gilmour KM, Balshine S. 2018. Stress axis regulation during social ascension in a group-living cichlid fish. Horm. Behav. 103, 121–128. ( 10.1016/j.yhbeh.2018.06.007) [DOI] [PubMed] [Google Scholar]
- 65.Payne AH, Hales DB. 2004. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 25, 947–970. ( 10.1210/er.2003-0030) [DOI] [PubMed] [Google Scholar]
- 66.Tokarz J, Möller G, Hrabě de Angelis M, Adamski J.. 2015. Steroids in teleost fishes: a functional point of view. Steroids 103, 123–144. ( 10.1016/j.steroids.2015.06.011) [DOI] [PubMed] [Google Scholar]
- 67.Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45 ( 10.1093/nar/29.9.e45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.R Core Team. 2019. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org/. [Google Scholar]
- 69.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 70.Fox J, Weisberg S. 2011. An R companion to applied regression, 2nd edn Thousand Oaks, CA: Sage Publications. [Google Scholar]
- 71.Hennessy MB, Zate R, Maken DS. 2008. Social buffering of the cortisol response of adult female guinea pigs. Physiol. Behav. 93, 883–888. ( 10.1016/J.PHYSBEH.2007.12.005) [DOI] [PubMed] [Google Scholar]
- 72.Stanton ME, Patterson JM, Levine S. 1985. Social influences on conditioned cortisol secretion in the squirrel monkey. Psychoneuroendocrinology 10, 125–134. ( 10.1016/0306-4530(85)90050-2) [DOI] [PubMed] [Google Scholar]
- 73.Schürch R, Rothenberger S, Heg D. 2010. The building-up of social relationships: behavioural types, social networks and cooperative breeding in a cichlid. Phil. Trans. R. Soc. B 365, 4089–4098. ( 10.1098/rstb.2010.0177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kikusui T, Winslow JT, Mori Y. 2006. Social buffering: relief from stress and anxiety. Phil. Trans. R. Soc. B 361, 2215–2228. ( 10.1098/rstb.2006.1941) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bshary R, Oliveira RF, Oliveira TSF, Canário AVM. 2007. Do cleaning organisms reduce the stress response of client reef fish? Front. Zool. 4, 21 ( 10.1186/1742-9994-4-21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ramsey ME, Fry D, Cummings ME. 2019. Isotocin increases female avoidance of males in a coercive mating system: assessing the social salience hypothesis of oxytocin in a fish species. Horm. Behav. 112, 1–9. ( 10.1016/J.YHBEH.2019.03.001) [DOI] [PubMed] [Google Scholar]
- 77.Hellmann JK, Reddon AR, Ligocki IY, O'Connor CM, Garvy KA, Marsh-Rollo SE, Hamilton IM, Balshine S. 2015. Group response to social perturbation: impacts of isotocin and the social landscape. Anim. Behav. 105, 55–62. ( 10.1016/J.ANBEHAV.2015.03.029) [DOI] [Google Scholar]
- 78.Shamay-Tsoory SG, Abu-Akel A. 2016. The social salience hypothesis of oxytocin. Biol. Psychiatry 79, 194–202. ( 10.1016/J.BIOPSYCH.2015.07.020) [DOI] [PubMed] [Google Scholar]
- 79.Fryer JN, Leung E. 1982. Neurohypophysial hormonal control of cortisol secretion in the teleost Carassius auratus. Gen. Comp. Endocrinol. 48, 425–431. ( 10.1016/0016-6480(82)90177-0) [DOI] [PubMed] [Google Scholar]
- 80.Huffman LS, Hinz FI, Wojcik S, Aubin-Horth N, Hofmann HA. 2015. Arginine vasotocin regulates social ascent in the African cichlid fish Astatotilapia burtoni. Gen. Comp. Endocrinol. 212, 106–113. ( 10.1016/j.ygcen.2014.03.004) [DOI] [PubMed] [Google Scholar]
- 81.Semsar K, Kandel FLM, Godwin J. 2001. Manipulations of the AVT system shift social status and related courtship and aggressive behavior in the bluehead wrasse. Horm. Behav. 40, 21–31. ( 10.1006/hbeh.2001.1663) [DOI] [PubMed] [Google Scholar]
- 82.Culbert BM, Gilmour KM, Balshine S. 2019. Data from: Social buffering of stress in a group-living fish Dryad Digital Repository. ( 10.5061/dryad.7v93210 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Culbert BM, Gilmour KM, Balshine S. 2019. Data from: Social buffering of stress in a group-living fish Dryad Digital Repository. ( 10.5061/dryad.7v93210 [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.7v93210 [82].