Abstract
Mobile pastoralists are thought to have facilitated the first trans-Eurasian dispersals of domesticated plants during the Early Bronze Age (ca 2500–2300 BC). Problematically, the earliest seeds of wheat, barley and millet in Inner Asia were recovered from human mortuary contexts and do not inform on local cultivation or subsistence use, while contemporaneous evidence for the use and management of domesticated livestock in the region remains ambiguous. We analysed mitochondrial DNA and multi-stable isotopic ratios (δ13C, δ15N and δ18O) of faunal remains from key pastoralist sites in the Dzhungar Mountains of southeastern Kazakhstan. At ca 2700 BC, Near Eastern domesticated sheep and goat were present at the settlement of Dali, which were also winter foddered with the region's earliest cultivated millet spreading from its centre of domestication in northern China. In the following centuries, millet cultivation and caprine management became increasingly intertwined at the nearby site of Begash. Cattle, on the other hand, received low levels of millet fodder at the sites for millennia. By primarily examining livestock dietary intake, this study reveals that the initial transmission of millet across the mountains of Inner Asia coincided with a substantial connection between pastoralism and plant cultivation, suggesting that pastoralist livestock herding was integral for the westward dispersal of millet from farming societies in China.
Keywords: pastoralism, millet, ancient DNA, isotope analysis, steppe archaeology
1. Introduction
Mounting archaeological research in the Eurasian steppes demonstrates that pastoralists associated with diverse Bronze Age cultures and later nomadic empires engaged in farming to a far greater degree than previously thought [1–6]. By ca 1600 BC, archaeobotanical evidence links Inner Asian herding communities to the cultivation of southwest Asian domesticates of wheat (Triticum spp.) and barley (Hordeum spp.), alongside broomcorn millet (Panicum miliaceum) and foxtail millet (Setaria italica) domesticated in northern China. Around the same time, these key crops were also widely adopted by societies at opposite ends of Eurasia [7–10]. Interestingly, the earliest indication of people exchanging crops through Inner Asia is represented by carbonized seeds of wheat, barley and broomcorn millet in human cremation cists (ca 2500–2200 BC) in two small pastoralist settlements, Begash and Tasbas, in the Dzhungar Mountain foothills of southeastern Kazakhstan [5,11,12]. Without direct evidence for local cultivation or subsistence use, the mortuary context of these seeds raises the possibility that domesticated cereals initially spread to Inner Asia as traded goods for symbolic purposes, rather than as staple foods [13]. To date, an untested dispersal model is that crops were cultivated and transmitted as part of strategies tightly linked to herding sheep, goats and cattle. However, the general paucity of faunal remains of domesticated bovids dating to the Early Bronze Age in Inner Asia or northern China obscures the timing of the spread of pastoralist subsistence through this continental crossroad [14].
Here, we examined the early transmission of bovid domesticates of sheep, goat and cattle in the Dzhungar Mountains and the potential integration of pastoralist herding with plant cultivation during the initial Eurasian interchange of plant and animal domesticates. We performed genetic and isotopic analyses of faunal bones and teeth from the newly discovered Early Bronze Age settlement site of Dali dating to ca 2700 BC, in addition to faunal remains from younger strata at nearby Begash and Tasbas containing millet remains (ca 2345 BC to AD 30; figure 1). These analyses, respectively, provide concrete taxonomic identifications of faunal remains and resolve dietary intake of livestock at multi-year and seasonal scales in order to assess when and how pastoralists facilitated early processes of food globalization in Eurasia.
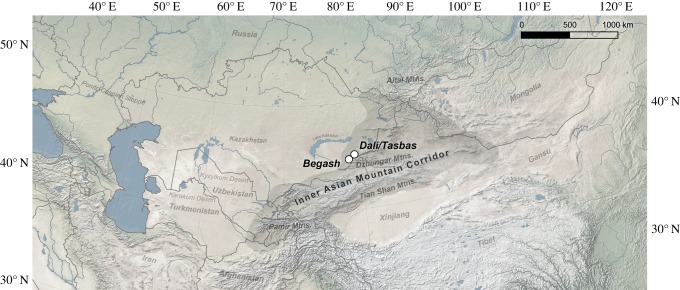
Figure 1.
Locations of Dali, Tasbas and Begash within the IAMC. (Online version in colour.)
2. Archaeological background
Pastoralism using sheep, goats and cattle has been a core subsistence strategy of Inner Asian societies for arguably more than 5000 years [15]. This mobile lifeway is thought to have spread eastward across the Eurasian steppe during the Eneolithic/Early Bronze Age as part of migrations of Yamnaya herders (3500–2700 BC) along a route from the Pontic-Caspian western steppe to the Altai mountains [16], giving rise to the Afanasievo culture (3100–2500 BC) [17]. Human genomic data showing indistinguishable genetic variation between Early Bronze Age communities of the western steppe and the Altai region support this purported migration [18–21]. However, Neolithic Altaic communities have chronological and cultural overlap with ‘Afanasievo’ groups [22], for which little is known about food production strategies using purported domesticated plants or animals. An alternative theory to pastoralism being translocated across the steppe is that pastoralist livestock spread to Inner Asia via limited human mobility and cultural transmissions along foothill ecozones linking the Iranian Plateau, Altai Mountains and western China, known as the Inner Asian Mountain Corridor (IAMC) [23].
Based on the macrobotanical record, one of the most important domesticated plants at human settlements in the central IAMC spanning the Early Bronze Age (2300 BC) to the Mongol period (AD 1200–1400) was broomcorn millet [3,5,12], a unique crop characterized by rapid seeding time and drought tolerance compared to Near Eastern wheat and barley [24]. Nevertheless, the early chronology of local cereal cultivation at quantities that would sustain human subsistence in this region remains hotly debated [1,2,5,11,12,25–27]. Unlike settlements of sedentary communities that generate considerable quantities of food waste in discrete middens, pastoralist occupations are often ephemeral and dispersed and yield little cultural material [15], such as the crop-processing debris that some consider to be unambiguous evidence for local cultivation [25]. Documenting human millet intake expected with the adoption of this isotopically distinct C4 crop using stable carbon isotope analysis has been severely impeded by the paucity of human remains dated to the third millennium BC.
3. Material and methods
(a). Excavation, radiocarbon dating and sample selection
Dali is located at 1500 m.a.s.l. in the Bayan-Zhurek range of the Dzhungar Mountains of Kazakhstan, at the heart of the IAMC and approximately 1 km southwest from Tasbas (figure 1). Previous excavations at Tasbas uncovered a small area dating to the Early Bronze Age (2655–2480 cal BC; electronic supplementary material, table S1), revealing a stone cremation cist containing wheat seeds (n = 5) and microliths (n = 3); no ceramics and only fragmented and burned animal bones identifiable to genus (n = 12) were recovered [11]. At Begash (2345–2080 cal BC; electronic supplementary material, table S1), a similar mortuary practice included seeds of wheat (n = 13), barley (n = 1) and broomcorn millet (n = 59) [5,12], and faunal remains more clearly indicate exploitation of pastoralist livestock based on less fragmented specimens identifiable to genus [28]. Excavations of the Dali settlement were conducted in 2011, 2012, 2014,1 following established methods [11,29]. New and previously published radiocarbon dates from Dali, Begash and Tasbas were modelled according to occupational layers in OxCal v. 4.3.2 with the INTCAL13 calibration curve [30], providing start and end dates of phases (electronic supplementary material, table S1). Faunal specimens for aDNA and isotope analyses were sampled from securely dated strata.
(b). DNA analysis
Morphological identifications of domesticated sheep and goat skeletal specimens from sites located in the IAMC are vulnerable to a high degree of taxonomic uncertainty due to pronounced fragmentation of skeletal material and overlapping diagnostic criteria with endemic wild taxa, including argali (Ovis ammon), urial (Ovis vignei), Siberian ibex (Capra sibirica) and markhor (Capra falconeri) [31]. Collagen peptide mass fingerprinting (Zooarchaeology by Mass Spectrometry, aka ‘ZooMS’), previously used to identify purported early livestock in Central Asia [32] and elsewhere, is unsuitable because of coarse taxonomic resolution limited to genus-level, which does not distinguish between domesticated and wild lineages. As a sensitive method for identifying Ovis and Capra species, a 110-bp stretch of the mitochondrial cytochrome b gene (MT-CYB) from suspected sheep and goat remains was amplified using primers CapFC1 (5′-CTCTGTAACTCACATTTGTC-3′) and CapRB1b (5′-GTTTCATGTTTCTAGAAAGGT-3′) [33]. Two independent DNA extractions for each specimen were conducted following established protocols [34]. See electronic supplementary material, text S1 for PCR and analytical methods.
(c). Stable isotope analysis
Millets use the C4 photosynthetic pathway and have substantially higher δ13C values by approximately 14‰ relative to C3 plants [35]. Intra-annual records of herbivore dietary intake were recovered through incremental measurement of carbon and oxygen isotopes in tooth enamel bioapatite (δ13Capa and δ18Oapa), which archives the proportion of C4 or C3 plants consumed in whole diet [36] and cycles of seasonal environmental inputs via body water [37], respectively. We analysed second and third mandibular molars, forming during the first and second years of life, respectively [38,39]. Enamel powder was collected from 1 mm wide bands oriented perpendicular to the tooth growth axis on the buccal side from crown to cervix [39,40]. Enamel pre-treatment followed reference [38]. Bioapatite carbonates were analysed at the Leibniz Labor, Kiel University with a Finnigan MAT 253 mass spectrometer coupled to a Kiel IV device using reactions with 100% orthophosphoric acid at 75°C. Duplicates were run for every approximately 10 samples, and two in-house enamel standards were run for every approximately five samples; analytical precision was 0.05‰ for carbon and 0.07‰ for oxygen. Measurements were calibrated using the carbonate standard NBS 19 (δ13C = +1.95‰ and δ18O = −2.2‰) and expressed relative to the international Vienna PeeDee Belemnite (VPDB) standard in delta notation.
We analysed the carbon and nitrogen isotopic composition of bone collagen (δ13Ccol and δ15Ncol), reflecting dietary protein at lifetime scales derived from plant ingesta [41] and nitrogen inputs from farming and pasturing activity [42], respectively. Collagen was extracted following Tuross et al. [43] using 0.5 M EDTA with a defatting step for modern samples and mass spectrometry was performed using a EuroVector Euro EA elemental analyser coupled to a GVI IsoPrime in continuous flow mode at the Boston University Stable Isotope Laboratory. Analytical error was 0.1‰ for δ13C and 0.2‰ for δ15N using peptone and glycine standards run for every approximately 15 samples. Isotopic values are expressed relative to VPDB for δ13C and atmospheric nitrogen for δ15N in standard delta notation. Collagen samples with elemental C : N ratios from 2.9 to 3.6 and also %C and %N within established ranges were accepted for analysis [41]. We also analysed previously published human δ13Ccol values from directly radiocarbon dated human individuals across the wider steppe region [27,44–47] (n = 174; dataset S5).
(d). Modelling livestock dietary intake
Statistical analyses were performed in R v. 3.4.1 [48]. We estimated time of year for δ13Capa values using an approach adapted from Balasse et al. [49], which normalizes differences in inter-individual tooth eruption and enamel maturation rates. Over annual rhythms of δ18Oapa values, which represent seasonal cycles of temperature reflected in meteoric water [49] and rainfall amount to a lesser extent [50], we modelled cosine periods as 365 ‘Julian days’ with January 15th as day zero. The strong seasonal climate in Inner Asia imparts regular oscillation in the oxygen isotopic composition of open and leaf water sources reflected in herbivore tooth enamel [38], overriding transient fluxes in environmental and biological systems [37,50]. We used MixSIAR to generate Bayesian estimates of C4 plant intake as dietary percentages [51,52]. We minimized temporal error by calculating means of δ13Capa sequences by two-month intervals for each tooth within archaeological periods at each site as fixed effects.
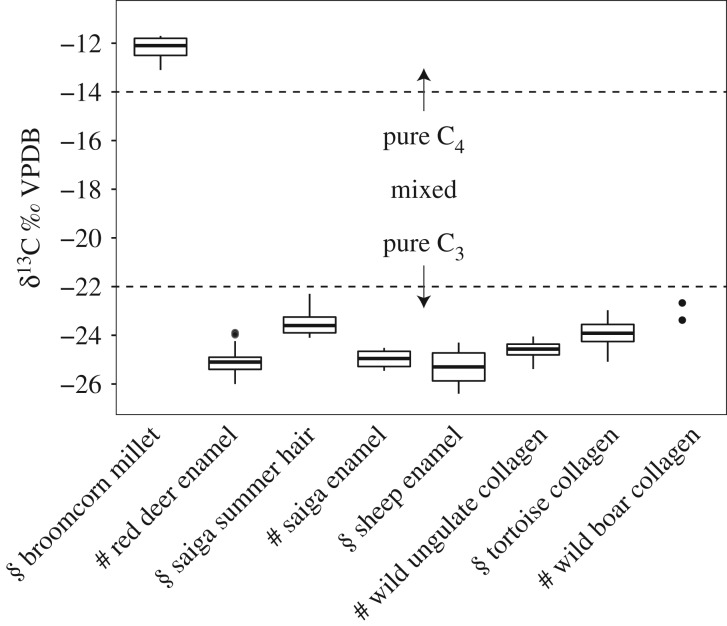
Critically, the carbon isotopic composition of local steppe vegetation was estimated from dietary δ13C values derived from (1) tooth enamel bioapatite of ancient red deer (Cervus elaphus), saiga (Saiga tatarica) and modern sheep herded in semi-arid lowlands near the sites, (2) bone collagen of ancient red deer, ancient Siberian ibex (Capra sibirica), ancient argali (Ovis ammon), ancient boar (Sus scrofa) and modern steppe tortoise (Agrionemys horsfieldii) and (3) summertime-forming hair of modern saiga [53]. These taxa substantially share dietary niches with pastoralist livestock and thus serve to detect wild C4 vegetation in local ecosystems that would confound isolation of millet foddering. Unlike their European counterparts, Central Asian red deer graze throughout the year and seasonally range into arid lowland pastures [54]. Migratory saiga antelope are voracious grazers in deserts and semi-arid steppes [53]. Bones of modern steppe tortoise, which are generalist feeders [55], were collected from the Saryesik-Atryan Desert enclosed by Lake Balkhash (figure 1). Central Asian wild boar consume a broad range of small and medium animals and herbaceous plants, including summer grasses [56]. See electronic supplementary material, text S2 for MixSIAR parameters and diet-tissue spacing factors used to derive dietary δ13C values from δ13C values of tissues from various taxa shown in figure 2.
Figure 2.
δ13C values of broomcorn millet versus dietary δ13C values of reference herbivores from study region. Symbol § denotes modern samples, including saiga antelope hair (n = 22), sheep tooth enamel (n = 51), steppe tortoise bone collagen (n = 6). Symbol # denotes ancient samples, including red deer enamel (n = 49), saiga tooth enamel (n = 6), red deer and ibex bone collagen (n = 21) and wild boar bone collagen (n = 2). See electronic supplementary material, text S2 for diet-tissue spacing factors and dataset S2 for raw data.
4. Results
(a). The Dali settlement complex
Phase 1 occupation levels at Dali date to 2705–2545 cal BC (electronic supplementary material, figure S1 and table S1) and include pit house architecture not previously described for the region. Ceramics from phase 1 resemble those from Altaic cultures of the third millennium BC (electronic supplementary material, figure S3). A human parietal bone recovered from the pit house exhibits a genetic composition most similar to Eurasian hunter–gatherers, including Eneolithic horse herders at Botai, with approximately 20% admixture from Neolithic populations from the northeastern Iranian plateau, while there was not a contribution of western steppe Yamnaya or Afanasievo ancestry [57]. Phase 2 of Dali dates to 1645–1520 cal BC (electronic supplementary material, figure S2 and table S1) and is characterized by a new cultural pattern (electronic supplementary material, figure S4). Architecture radically changes during this phase to rectilinear stone foundations, similar to structures recovered at contemporaneous sites in the region, such as nearby Begash [29] and Adunqiaolu in Xinjiang [58]. Human individuals (n = 3) excavated from Dali's mortuary complex are contemporaneous with phase 2 and prominently exhibit admixed western steppe ancestry [44], which spread over central Asia throughout the mid-second millennium BC [18–20].
(b). Ancient DNA of livestock
We recovered MT-CYB sequences from 79 of 104 samples tested, indicating good preservation of ancient mtDNA (electronic supplementary material, table S2). Seventy-seven samples representing a cultural sequence from the Early Bronze Age to the medieval period yielded haplotypes that were identical to MT-CYB sequences specific to domesticated taxa, while two sequences were from Capra sibirica (electronic supplementary material, figure S2, dataset S1). We also found four conflicts between genetic species determinations and previously published [28] morphological identifications of Siberian ibex and domesticated sheep and goat. One domesticated sheep specimen each from Dali phase 1 and Begash phase 1a were directly 14C dated, which tightly cluster with the other dates from their respective occupational strata (electronic supplementary material, tables S1 and S2). This demonstrates the earliest presence of domesticated sheep and goat in the central IAMC, since the first occupation of Dali (ca 2700 cal BC).
(c). Isotopic reconstruction of steppe vegetation
Wild animal taxa from the study region exhibit dietary δ13C values from between −26 and −22‰, demonstrating the predominance of C3 floral taxa in the regional floral biome (electronic supplementary material, dataset S2) and contrasting starkly with δ13C values of broomcorn millet, which cluster at −12‰ (figure 2). Low δ13C values from migratory saiga antelope and steppe tortoise indicate that drier environments, plausibly accessible to ancient livestock via transhumance to these areas, did not support detectable amounts of C4 plants or regularly water-stressed C3 plants enriched in 13C [59,60]. These results indicate a C3 environment in both the wetter mountains and drier open steppe, which reflect the present-day distribution of C3 plants in the study region, consisting of mostly grasses (Stipa and Festuca spp.), followed by sedges (Carex spp.), forbs (Artemesia spp.) and shrubs (Spiraea spp. and Caragana spp.) [61].
(d). Isotopic analysis of ancient pastoralist livestock
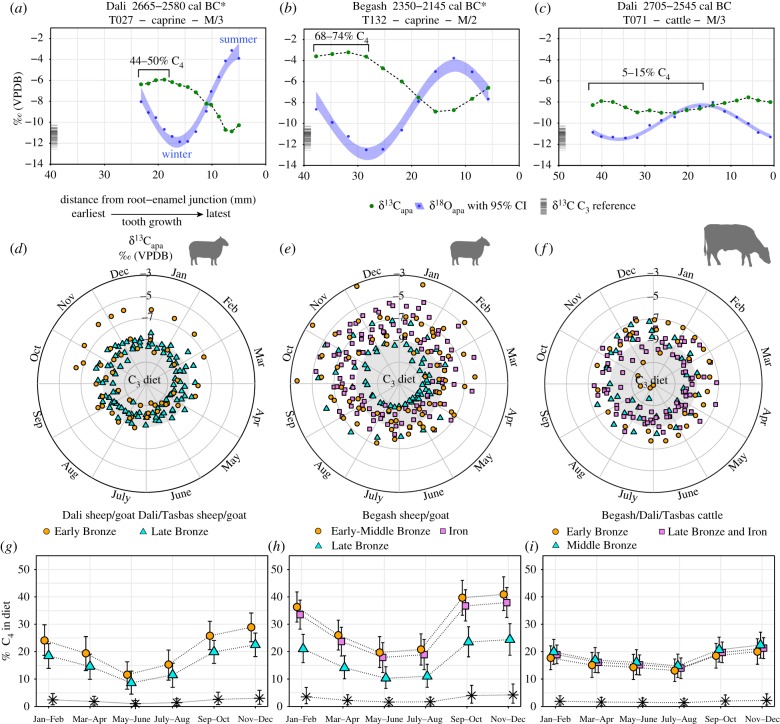
Intra-tooth isotopic sequences from Early Bronze Age domesticated sheep and cattle teeth exhibit high δ13Capa values from −7 to −3.2‰ that are notably coincident with low δ18Oapa values associated with winter-season environmental inputs (figure 3a–c). Diverse seasonal δ13Capa values indicate that animal management practices were applied non-uniformly across herds (figure 3d–f). Overall, decreasing δ13Capa values leading into summer months after peak C4 intake during winter strongly suggest a spring–summer dietary transition for sheep and goats to C3 steppe plants present in montane and steppe vegetation communities. MixSIAR dietary models provide estimates of the seasonal relative importance of C4 vegetation in livestock diets for the Early Bronze Age to Iron Age (figure 3g–i).
Figure 3.
(a–c) Time series of δ13Capa and δ18Oapa values from enamel bioapatite of second and third mandibular molars (n = 42). Symbol * denotes directly dated specimen. (d–f) δ13Capa values plotted as modelled annual cycles. (g–i) MixSIAR models showing relative proportions of C4 plant consumption plotted in two-month periods with 90% credible intervals. Black asterisks represent herbivores with a C3 diet. Individuals (electronic supplementary material, table S3; dataset S3): Dali caprines Early Bronze Age (n = 4); Dali and Tasbas caprines Late and Final Bronze Age (n = 6); Begash caprines Early-Middle Bronze Age (n = 6), Late Bronze Age (n = 5) and Iron Age (n = 7); Dali cattle Early Bronze Age (n = 3); Begash cattle Middle Bronze Age (n = 3). Dali, Begash, Tasbas cattle: Late and Final Bronze Age (n = 8). (Online version in colour.)
Sheep and goats from Dali demonstrate dietary intake with a substantial C4 component by 2700 cal BC. Specifically, high δ13Capa values of ca −6‰ visible in some animals during winter months correspond to around 44–50% dietary intake of C4 plants (figure 3a,d). Cattle from Dali exhibiting winter δ13Capa values of ca −7 to −8‰ correspond to about 5–15% C4 dietary intake that changes little throughout the year, a pattern that persisted at the sites for millennia (figure 3f,i). Cattle require up to six times more feed by weight than caprines [15], so even a 10% C4 diet represents a sizeable amount of C4 biomass that must have been sourced from sufficiently large stockpiles regularly accessible to cattle.
The relative contribution of C4 plants to sheep and goat diets subsequently increased during phase 1a of Begash (2345–2080 cal BC), indicated by winter δ13Capa values that reflect up to 68–74% C4 diet (figure 3b,e). Over lifetime scales, ingestion of C4 plants by livestock at Begash is also more pronounced than that observed at Dali during the third millennium BC (figure 3a,b). Over two-thirds of Begash livestock exhibit δ13Ccol values greater than −18‰, including a prominent subset of caprine values between ca −13 and −12‰ that reflect approximately 50–60% C4 intake (figure 4b). As a point of reference, the domesticated sheep with the highest δ13Capa values in the dataset exhibited a δ13Ccol value of −16‰ that modelled to approximately 20% lifetime C4 intake (figure 4b), demonstrating that animals with higher δ13Ccol values could have been provided with exclusively C4 diets during winter.
Figure 4.
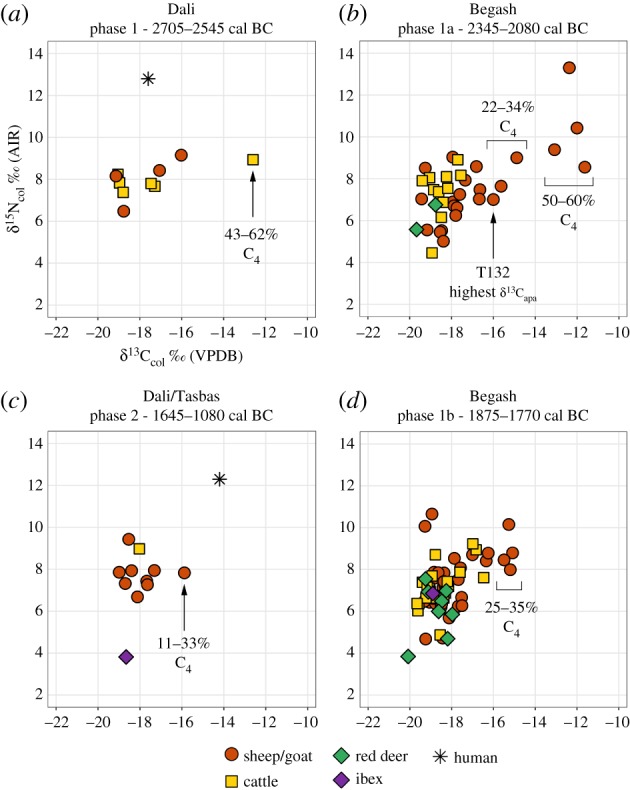
(a–d) δ13Ccol and δ15Ncol values of livestock, wild herbivores and humans from sampled sites. Isotopic data from Late Bronze Age and Iron Age periods are displayed in electronic supplementary material, figure S4. See electronic supplementary material, table S4 for sample summary and yields and dataset S4 for raw data. (Online version in colour.)
For the earliest occupations of Begash, livestock consuming the most C4 vegetation also exhibited high δ15Ncol values above 9‰ (figure 4b,c), indicated by significant positive correlations between δ13Ccol and δ15Ncol values for the Early and Middle Bronze Age phases (Pearson's r = 0.6589; p < 0.0001; n = 34 and Pearson's r = 0.4610; p = 0.0006; n = 52, respectively). In later phases at Begash, livestock with high δ15Ncol values above 9‰ tend to have low δ13Ccol values ca −19‰ (electronic supplementary material, figure S5). From all sampled phases at Begash, most livestock exhibit higher δ15Ncol values than that of wild herbivores by approximately 4‰ (Mann–Whitney U = 2690; p < 0.0001; nlivestock = 153; nwild = 21). This demonstrates not only long-term modifications of local ecosystems, due to pasturing and farming practices involving manure deposition, thus enriching vegetation in 15N, but also shows shared ecological niches between wild herbivores and livestock both exhibiting low δ13Ccol and δ15Ncol values.
A conspicuous decline in C4 dietary intake for caprines took place from the middle to late second millennium BC (figures 3g,h and 4c,d; electronic supplementary material, S5). By this time, however, direct human dietary intake of millet is common in the IAMC and adjacent regions, evidenced by a conspicuous rise in human δ13Ccol values first visible at approximately 2100 cal BC in an individual from Kanai in the Kazakh Altai with a δ13Ccol value of −14.5‰ (electronic supplementary material, figures S6 and S7). Unfortunately, human samples from the third millennium BC are too few to know whether early C4 plant intake by livestock at Dali or Begash coincided with direct human millet consumption (electronic supplementary material, figures S6 and S7). Strikingly, winter levels of livestock C4 dietary intake during the Iron Age at Begash are equally elevated compared to that of the site's Early and Middle Bronze Age occupations (figure 3h).
5. Discussion
(a). Millet foddering of Early Bronze Age livestock
While pastoralist livestock are known to alter the relative abundance of plant taxa in grasslands [62] and local nitrogen cycles [42], we are confident that wild C4 plants are not contributing to high δ13C values in livestock based on (1) site locations in the mountains where wild C4 plants do not grow [60] and (2) consistently low δ13C values in wild herbivorous taxa with dietary niches overlapping the more arid lowlands where wild C4 plants might be expected. Thus, relatively high δ13C values in ancient livestock are the direct outcome of pastoralists foddering animals with cultivated millet, which would have greatly enhanced the efficiency and resiliency of food production in the harsh winters of Inner Asia.
One unlikely scenario to explain anticorrelation of δ13Capa and δ18Oapa values in caprines would be herding over 1000 km to the west of the Dzhungar Mountains to the Kyzylkum Desert (figure 1), where wild C4 plants are present in low densities [63]. However, our finding of steady and low levels of seasonal C4 dietary intake (approx. 10–20%) in cattle suggests that pastoralists primarily managed cattle near millet fodder reserves at settlements, where abundant mountain run-off provides ample water for these obligate-drinking livestock, especially if exploited for milk. A far more likely scenario is that caprines, compared to cattle, received more millet fodder in winter as a dietary percentage and in summer were herded over a wider local range away from millet resources to lowlands or to alpine pastures, causing large seasonal shifts in δ13Capa values for caprines (figure 3g,h).
Similar intensities of millet winter foddering between Begash's Early Bronze and Iron Age occupations suggests that third millennium BC agro-pastoral strategies reached high levels of local production, despite different social conditions. The Iron Age throughout the foothill zone of southeastern Kazakhstan is well characterized by abundant seed remains of broomcorn millet [3], which benefits from this region's adequate summer heat for productive cultivation [25,64]. Mixed herding and multi-crop farming in the IAMC during the Iron Age are associated with diverse communities commonly classified as Scythian/Saka and Wusun agro-pastoralists [6,12,65], exhibiting amplified social hierarchies and protracted cultural and political networks that arguably involved excess agricultural output [3,15]. For Early Bronze Age pastoralists in the IAMC, however, millet would have been initially perceived as an attractive fodder resource offering hardy and versatile properties, especially compared to wheat and barley, which might have already been locally cultivated. The subsequent decline in millet foddering during the Late Bronze Age is likely due to C3 cultigens arriving via secondary crop dispersals [5], especially landraces of barley adapted to high altitudes [66]. From ca 1750–1000 cal BC, this diversification in cropping across Asia is also linked to reducing risks during climatic shifts towards cooler conditions [25]. However, improved palaeo-climate records are needed to characterize earlier periods.
Our finding that not all livestock individuals exhibited high δ13C values corresponds with ethnographic accounts of steppe pastoralists exercising adaptable strategies of food production combining mixed herding and plant cultivation to balance graze and fodder availability with goals for dairy and fibre production, meat and fat harvesting, herd security and wealth generation [15,67–69]. Versatility in animal management practices is especially pronounced when considering the variation in δ15Ncol values of caprines from the first phase of Begash (figure 4b), which also points towards the recycling of nutrients in millet cultivation plots using manure. However, contemporaneous and later livestock with low δ13Ccol values also exhibit a wide ranging δ15Ncol values (figure 4b,d), consistent with variable growing conditions for C3 crops and/or irregular stocking rates on C3 steppe pastures characterized by uneven nitrogen pools [42,70].
Given the case for millet foddering at Begash during its earliest occupation, millet cultivation at Dali by ca 2700 cal BC emerges as a credible scenario. High δ13Capa values reflecting winter dietary intake of livestock from phase 1 Dali strongly contrast against the carbon isotopic composition of surrounding C3 steppe vegetation. That early millet foddering at Dali was followed by more prominent millet foddering of caprines at Begash several hundred years later suggests a rapid process of integrating pastoralism and millet agriculture, while herding strategies sustained a component of grazing livestock on C3 steppe vegetation. Taken together, our data illustrate that communities in the IAMC since the Early Bronze Age were engaged in ecologically dynamic subsistence strategies using mixed livestock herding and intermittent millet cultivation, likely involving flexible herd mobility patterns of seasonal transhumant moves and prolonged settlement residence.
(b). Domesticated bovids enhanced millet transmission
Early genetic evidence for domesticated sheep and goat and a human individual with admixed northeastern Iranian and Eurasian hunter–gatherer ancestries at Dali allow us to consider the transmission of pastoralism to Inner Asia as the combined effects of mobility, communication and exchange with agro-pastoralists in the southern reaches of the IAMC [23,44,71]. However, we cannot rule out simultaneous or earlier transmissions of livestock to the Dzhungar and Tian Shan mountains from Afanasievo or other Eneolithic communities in the Altai. Nevertheless, the integration of millet cultivation with livestock management at Dali and Begash implies that Early Bronze Age pastoralists were already well connected throughout the foothills of western China, perhaps as far east as Gansu, and accelerated the transmission of millet westward. The addition of millet agriculture to pastoralism likely facilitated new labour divisions and expanded social networks, reflecting an important medium for the inter-regional transfer of food technologies.
Prior to the arrival of pastoralist subsistence, millet may have been dispersed slowly across northern China by settled agriculturalists, who were without indigenously domesticated herbivores but instead had domesticated pigs, which are managed close to settlements and do not afford easy mobility as do sheep, goat and cattle. It would be unsurprising if future research in western China recovered millet remains associated with pastoralist livestock at least several centuries earlier than 2700 cal BC. Current human palaeogenetic studies in Eurasia invoke the so-called massive migrations of archaeologically defined populations to explain changes in the genetic composition of ancient peoples and shifts in their food production [18–21]. Our finding of swift millet transmission at Dali and Begash prior to influxes of people with western steppe ancestry (Yamnaya or Afanasievo) emphasizes a subsistence foundation for extensive social connectivity as part of more localized interactions and mobility, underscoring the substantial time depth and multi-directionality of cultural transmissions via pastoralist societies.
6. Conclusion
Combined genetic and stable isotopic analyses of faunal skeletal remains from settlement sites in the IAMC reveal the earliest evidence in Inner Asia (ca 2700 cal BC) for domesticated sheep, goat, and cattle and their intensive management by way of foddering with cultivated millet. In the context of contemporaneous mortuary rituals using domesticated plants [11–13], our findings suggest that Early Bronze Age pastoralists in the IAMC were immortalizing the economic significance of plant cultivation for enhancing livestock production, as they also contributed to the spread of millet and knowledge about its production westward. Early and precocious use of millet to fodder livestock by IAMC pastoralists suggests there may be a fundamentally fast pace to transmissions of domesticated plants by people exploiting sheep, goat and cattle. Our methodological approach focused on faunal remains provides a critical means to test this model for millets in other regions of Eurasia and for other C4 crops, such as sorghum in Africa, at both local and continental scales.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We greatly appreciate the support of B. Baitanayev, director of the Margulan Institute of Archaeology, and B. Kakabaev, former deputy director of the Central State Museum of the Republic of Kazakhstan; archaeological specimens were sampled from these institutions under permit nos. 54/20-202 and 1-10-224, respectively. We thank D. Voyakin for access to ancient saiga specimens. We also thank N. Andersen (Leibniz Labor, Kiel University) for assistance with carbon and oxygen mass spectrometry of bioapatite carbonates and R. Michener (Boston University Stable Isotope Laboratory) for assistance with carbon and nitrogen mass spectrometry of bone collagen. We thank the three anonymous reviewers for constructive criticism.
Endnote
Dzhungar Mountain Archaeology Project, Co-PIs: M.D.F. and A.M.
Data accessibility
Genetic data are available in electronic supplementary material, dataset S1. Isotopic data are available in electronic supplementary material, datasets S2–S5.
Authors' contributions
T.R.H., M.D.F., P.N.D.D., and A.M. designed and performed archaeological research at Dali. T.R.H. designed and performed genetic and isotopic research. T.R.H. and C.A.M. interpreted isotopic data. T.R.H. and A.N. interpreted genetic data. T.R.H. wrote the manuscript with guidance from C.A.M. and M.D.F. All authors contributed critically to subsequent drafts and gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by the doctoral fellowship of T.R.H. at the Graduate School ‘Human Development in Landscapes' (Supervisors: C.A.M. and A.N.; German Research Foundation: GSC 208). Excavations of Dali were funded by Washington University in St Louis (M.D.F.) and the U.S. National Science Foundation (no. 1132090; P.N.D.D. and M.D.F.). This research is part of a project that has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (Grant agreement no. 772957; ‘ASIAPAST’ held by C.A.M.).
References
- 1.Motuzaite Matuzeviciute G, Abdykanova A, Kume S, Nishiaki Y, Tabaldiev K. 2018. The effect of geographical margins on cereal grain size variation: case study for highlands of Kyrgyzstan. J. Archaeol. Sci. Rep. 20, 400–410. ( 10.1016/j.jasrep.2018.04.037) [DOI] [Google Scholar]
- 2.Spengler RN. 2015. Agriculture in the Central Asian Bronze Age. J. World Prehistory 28, 215–253. ( 10.1007/s10963-015-9087-3) [DOI] [Google Scholar]
- 3.Spengler RN, Miller NF, Neef R, Tourtellotte PA, Chang C. 2017. Linking agriculture and exchange to social developments of the Central Asian Iron Age. J. Anthropol. Archaeol. 48, 295–308. ( 10.1016/j.jaa.2017.09.002) [DOI] [Google Scholar]
- 4.Spengler RN, Frachetti MD, Doumani PN. 2014. Late Bronze Age agriculture at Tasbas in the Dzhungar Mountains of eastern Kazakhstan. Quat. Int. 348, 147–157. ( 10.1016/j.quaint.2014.03.039) [DOI] [Google Scholar]
- 5.Spengler RN, Frachetti M, Doumani P, Rouse L, Cerasetti B, Bullion E, Mar'yashev A. 2014. Early agriculture and crop transmission among Bronze Age mobile pastoralists of Central Eurasia. Proc. R. Soc. B 281, 20133382 ( 10.1098/rspb.2013.3382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spengler RN, Chang C, Tourtellotte PA. 2013. Agricultural production in the Central Asian mountains: Tuzusai, Kazakhstan (410–150 b.c.). J. Field Archaeol. 38, 68–85. ( 10.1179/0093469012Z.00000000037) [DOI] [Google Scholar]
- 7.Jones M, Hunt H, Lightfoot E, Lister D, Liu X, Motuzaite-Matuzeviciute G. 2011. Food globalization in prehistory. World Archaeol. 43, 665–675. ( 10.1080/00438243.2011.624764) [DOI] [Google Scholar]
- 8.Liu X, et al. 2019. From ecological opportunism to multi-cropping: mapping food globalisation in prehistory. Quat. Sci. Rev. 206, 21–28. ( 10.1016/j.quascirev.2018.12.017) [DOI] [Google Scholar]
- 9.Motuzaite-Matuzeviciute G, Staff RA, Hunt HV, Liu X, Jones MK. 2013. The early chronology of broomcorn millet (Panicum miliaceum) in Europe. Antiquity 87, 1073–1085. ( 10.1017/S0003598X00049875) [DOI] [Google Scholar]
- 10.Long T, Leipe C, Jin G, Wagner M, Guo R, Schröder O, Tarasov PE.. 2018. The early history of wheat in China from 14C dating and Bayesian chronological modelling. Nat. Plants 4, 272–279. ( 10.1038/s41477-018-0141-x) [DOI] [PubMed] [Google Scholar]
- 11.Doumani PN, Frachetti MD, Beardmore R, Schmaus TM, Spengler RN, Mar'yashev AN. 2015. Burial ritual, agriculture, and craft production among Bronze Age pastoralists at Tasbas (Kazakhstan). Archaeol. Res. Asia 1–2, 17–32. ( 10.1016/j.ara.2015.01.001) [DOI] [Google Scholar]
- 12.Frachetti MD, Spengler RN, Fritz GJ, Mar'yashev AN. 2010. Earliest direct evidence for broomcorn millet and wheat in the central Eurasian steppe region. Antiquity 84, 993–1010. ( 10.1017/S0003598X0006703X) [DOI] [Google Scholar]
- 13.Frachetti MD. 2014. Seeds for the soul: ideology and diffusion of domesticated grains across inner Asia. In Reconfiguring the silk road: new research on east–west exchange in antiquity (eds Mair VH, Hickman J), pp. 41–53. Philadelphia, PA: University of Pennsylvania Press. [Google Scholar]
- 14.Vigne J-D. 2015. Early domestication and farming: what should we know or do for a better understanding? Anthropozoologica 50, 123–151. ( 10.5252/az2015n2a5) [DOI] [Google Scholar]
- 15.Frachetti MD. 2008. Pastoralist landscapes and social interaction in Bronze Age Eurasia. Berkeley, CA: University of California Press. [Google Scholar]
- 16.Anthony D. 2007. The horse, the wheel, and language: how bronze-age riders from the Eurasian steppes shaped the modern world. Princeton, NJ: Princeton University Press. [Google Scholar]
- 17.Pogozheva AP. 2006. Afanas'evskaya Kul'tura. In Epokha eneolita i bronzy gornogo altaya: chast’ 1 (eds Pogozheva AP, Rykun MP, Stepanova NF, Tup SS), pp. 18–48. Barnaul, Russia: Azbuka. [Google Scholar]
- 18.Allentoft ME, et al. 2015. Population genomics of Bronze Age Eurasia. Nature 522, 167–172. ( 10.1038/nature14507) [DOI] [PubMed] [Google Scholar]
- 19.Damgaard P de B, et al. 2018. The first horse herders and the impact of early Bronze Age steppe expansions into Asia. Science 360, eaar7711 ( 10.1126/science.aar7711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damgaard P de B, et al. 2018. 137 ancient human genomes from across the Eurasian steppes. Nature 557, 369–374. ( 10.1038/s41586-018-0094-2) [DOI] [PubMed] [Google Scholar]
- 21.Haak W, et al. 2015. Massive migration from the steppe was a source for Indo-European languages in Europe. Nature 522, 207–211. ( 10.1038/nature14317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiryushin YuF, Kiryushin KYu. 2005. K voprosu o roli neoliticheskogo komponenta v slozhenii afanas'evskoi kul'tury gornogo altaya. In Arkheologiya yuzhnoi sibiri: idei, metody, itkrytiya (ed. Korol’ GG.), pp. 26–28. Krasnoyarsk: Krasnoyarsk gosudarstvennyi pedagogicheskii universitet. [Google Scholar]
- 23.Frachetti MD. 2012. Multiregional emergence of mobile pastoralism and nonuniform institutional complexity across Eurasia. Curr. Anthropol. 53, 2–38. ( 10.1086/663692) [DOI] [Google Scholar]
- 24.Nesbitt M. 2005. Grains. In The cultural history of plants (eds Prance G, Nesbitt M), pp. 45–60. London, UK: Routledge. [Google Scholar]
- 25.d'Alpoim Guedes JA, Bocinsky RK. 2018. Climate change stimulated agricultural innovation and exchange across Asia. Sci. Adv. 4, eaar4491 ( 10.1126/sciadv.aar4491) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Reid RE, Lightfoot E, Matuzeviciute GM, Jones MK. 2016. Radical change and dietary conservatism: mixing model estimates of human diets along the Inner Asia and China's mountain corridors. The Holocene 26, 1556–1565. ( 10.1177/0959683616646842) [DOI] [Google Scholar]
- 27.Motuzaite Matuzeviciute G, Lightfoot E, O'Connell TC, Voyakin D, Liu X, Loman V, Svyatko S, Usmanova E, Jones MK. 2015. The extent of cereal cultivation among the Bronze Age to Turkic period societies of Kazakhstan determined using stable isotope analysis of bone collagen. J. Archaeol. Sci. 59, 23–34. ( 10.1016/j.jas.2015.03.029) [DOI] [Google Scholar]
- 28.Frachetti M, Benecke N. 2009. From sheep to (some) horses: 4500 years of herd structure at the pastoralist settlement of Begash (south-eastern Kazakhstan). Antiquity 83, 1023–1037. ( 10.1017/S0003598X00099324) [DOI] [Google Scholar]
- 29.Frachetti MD, Mar'yashev AN. 2007. Long-term occupation and seasonal settlement of Eastern Eurasian Pastoralists at Begash, Kazakhstan. J. Field Archaeol. 32, 221–242. ( 10.1179/009346907791071520) [DOI] [Google Scholar]
- 30.Reimer PJ, Bard E, Bayliss A, Beck JW. 2013. IntCal13 and Marine13 radiocarbon age calibration curves 0–50,000 years cal BP. Radiocarbon 55, 1869–1887. ( 10.2458/azu_js_rc.55.16947) [DOI] [Google Scholar]
- 31.Singh NJ, Milner-Gulland EJ. 2011. Monitoring ungulates in Central Asia: current constraints and future potential. Oryx 45, 38–49. ( 10.1017/S0030605310000839) [DOI] [Google Scholar]
- 32.Taylor W, et al. 2018. Early pastoral economies along the Ancient Silk Road: biomolecular evidence from the Alay Valley, Kyrgyzstan. PLoS ONE 13, e0205646 ( 10.1371/journal.pone.0205646) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernández H, Hughes S, Vigne J-D, Helmer D, Hodgins G, Miquel C, Hänni C, Luikart G, Taberlet P. 2006. Divergent mtDNA lineages of goats in an Early Neolithic site, far from the initial domestication areas. Proc. Natl Acad. Sci. USA 103, 15 375–15 379. ( 10.1073/pnas.0602753103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krause-Kyora B, et al. 2018. Ancient DNA study reveals HLA susceptibility locus for leprosy in medieval Europeans. Nat. Commun. 9, 1569 ( 10.1038/s41467-018-03857-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farquhar GD, Ehleringer JR, Hubick KT. 1989. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 40, 503–537. ( 10.1146/annurev.pp.40.060189.002443) [DOI] [Google Scholar]
- 36.Sullivan CH, Krueger HW. 1981. Carbon isotope analysis of separate chemical phases in modern and fossil bone. Nature 292, 333–335. ( 10.1038/292333a0) [DOI] [PubMed] [Google Scholar]
- 37.Bryant DJ, Koch PL, Froelich PN, Showers WJ, Genna BJ. 1996. Oxygen isotope partitioning between phosphate and carbonate in mammalian apatite. Geochim. Cosmochim. Acta 60, 5145–5148. ( 10.1016/S0016-7037(96)00308-0) [DOI] [Google Scholar]
- 38.Makarewicz CA, Pederzani S. 2017. Oxygen (δ18O) and carbon (δ13C) isotopic distinction in sequentially sampled tooth enamel of co-localized wild and domesticated caprines: complications to establishing seasonality and mobility in herbivores. Palaeogeogr. Palaeoclimatol. Palaeoecol. 485, 1–15. ( 10.1016/j.palaeo.2017.01.010) [DOI] [Google Scholar]
- 39.Balasse M. 2002. Reconstructing dietary and environmental history from enamel isotopic analysis: time resolution of intra-tooth sequential sampling. Int. J. Osteoarchaeol. 12, 155–165. ( 10.1002/oa.601) [DOI] [Google Scholar]
- 40.Zazzo A, Balasse M, Passey BH, Moloney AP, Monahan FJ, Schmidt O. 2010. The isotope record of short- and long-term dietary changes in sheep tooth enamel: implications for quantitative reconstruction of paleodiets. Geochim. Cosmochim. Acta 74, 3571–3586. ( 10.1016/j.gca.2010.03.017) [DOI] [Google Scholar]
- 41.DeNiro MJ. 1985. Postmortem preservation and alteration of in vivo bone collagen isotope ratios in relation to palaeodietary reconstruction. Nature 317, 806–809. ( 10.1038/317806a0) [DOI] [Google Scholar]
- 42.Szpak P. 2014. Complexities of nitrogen isotope biogeochemistry in plant–soil systems: implications for the study of ancient agricultural and animal management practices. Front. Plant Sci. 5, 1–19. ( 10.3389/fpls.2014.00288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tuross N, Fogel ML, Hare PE. 1988. Variability in the preservation of the isotopic composition of collagen from fossil bone. Geochim. Cosmochim. Acta 52, 929–935. ( 10.1016/0016-7037(88)90364-X) [DOI] [Google Scholar]
- 44.Narasimhan VM, et al. 2018 doi: 10.1101/292581. The Genomic Formation of South and Central Asia. bioRxiv . ( ) [DOI] [Google Scholar]
- 45.Motuzaite-Matuzeviciute G, Kiryushin YF, Rakhimzhanova SZ, Svyatko S, Tishkin AA, O'Connell TC. 2016. Climatic or dietary change? Stable isotope analysis of Neolithic–Bronze Age populations from the Upper Ob and Tobol River basins. The Holocene 26, 1711–1721. ( 10.1177/0959683616646843) [DOI] [Google Scholar]
- 46.Svyatko SV, Polyakov AV, Soenov VI, Stepanova NF, Reimer PJ, Ogle N, Tyurina EA, Grushin SP, Rykun MP. 2017. Stable isotope palaeodietary analysis of the Early Bronze Age Afanasyevo Culture in the Altai Mountains, Southern Siberia. J. Archaeol. Sci. Rep. 14, 65–75. ( 10.1016/j.jasrep.2017.05.023) [DOI] [Google Scholar]
- 47.Wang T, Wei D, Chang X, Yu Z, Zhang X, Wang C, Hu Y, Fuller BT.. 2017. Tianshanbeilu and the Isotopic Millet Road: reviewing the late Neolithic/Bronze Age radiation of human millet consumption from north China to Europe. Natl. Sci. Rev. ( 10.1093/nsr/nwx015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (https://www.R-project.org/). [Google Scholar]
- 49.Balasse M, Obein G, Ughetto-Monfrin J, Mainland I. 2012. Investigating seasonality and season of birth in past herds: a reference set of sheep enamel stable oxygen isotope ratios. Archaeometry 54, 349–368. ( 10.1111/j.1475-4754.2011.00624.x) [DOI] [Google Scholar]
- 50.Higgins P, MacFadden BJ. 2004. ‘Amount Effect’ recorded in oxygen isotopes of Late Glacial horse (Equus) and bison (Bison) teeth from the Sonoran and Chihuahuan deserts, southwestern United States. Palaeogeogr. Palaeoclimatol. Palaeoecol. 206, 337–353. ( 10.1016/j.palaeo.2004.01.011) [DOI] [Google Scholar]
- 51.Moore JW, Semmens BX. 2008. Incorporating uncertainty and prior information into stable isotope mixing models. Ecol. Lett. 11, 470–480. ( 10.1111/j.1461-0248.2008.01163.x) [DOI] [PubMed] [Google Scholar]
- 52.Stock BC, Semmens BX. 2016. MixSIAR GUI user manual. Version 3.1. See https://github.com/brianstock/MixSIAR. [DOI] [PubMed]
- 53.Jürgensen J, Drucker DG, Stuart AJ, Schneider M, Buuveibaatar B, Bocherens H. 2017. Diet and habitat of the saiga antelope during the late Quaternary using stable carbon and nitrogen isotope ratios. Quat. Sci. Rev. 160, 150–161. ( 10.1016/j.quascirev.2017.01.022) [DOI] [Google Scholar]
- 54.Baskin L, Danell K.. 2003. Ecology of ungulates: a handbook of species in Eastern Europe and northern and Central Asia. Berlin, Germany: Springer Science & Business Media. [Google Scholar]
- 55.Bondarenko DA, Peregontsev EA. 2012. Itogi izucheniya pitaniya sredneaziatskoj cherepakhi (Agrionemys horsfieldii). Zool. Zhurnal 91, 1397–1410. [Google Scholar]
- 56.Sludskii AA. 1956. Kaban: ekologiya i khozyaistvennoe znacheniye. Alma-Ata: Izd-vo Akademii Nauka Kazakhskoj SSR. [Google Scholar]
- 57.Narasimhan VM. et al. 2019. The formation of human populations in South and Central Asia. Science 365, eaat7487 ( 10.1126/science.aat7487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jia PW, Betts A, Cong D, Jia X, Dupuy PD. 2017. Adunqiaolu: new evidence for the Andronovo in Xinjiang, China. Antiquity 91, 621–639. ( 10.15184/aqy.2017.67) [DOI] [Google Scholar]
- 59.Tieszen LL. 1991. Natural variations in the carbon isotope values of plants: implications for archaeology, ecology, and paleoecology. J. Archaeol. Sci. 18, 227–248. ( 10.1016/0305-4403(91)90063-U) [DOI] [Google Scholar]
- 60.Körner Ch, Farquhar GD, Wong SC. 1991. Carbon isotope discrimination by plants follows latitudinal and altitudinal trends. Oecologia 88, 30–40. ( 10.1007/BF00328400) [DOI] [PubMed] [Google Scholar]
- 61.Rachkovskaya EI, Bragina TM. 2012. Steppes of Kazakhstan: diversity and present state. In Eurasian steppes. Ecological problems and livelihoods in a changing world (eds Werger MJA, van Staalduinen MA), pp. 103–148. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 62.Zhang Q, Ding Y, Ma W, Kang S, Li X, Niu J, Hou X, Li X, Sarula. 2014. Grazing primarily drives the relative abundance change of C4 plants in the typical steppe grasslands across households at a regional scale. Rangel. J. 36, 565–572. ( 10.1071/RJ13050) [DOI] [Google Scholar]
- 63.Toderich K, Black C, Juylova E, Kozan O, Mukimov T, Matsuo N. 2007. C3/C4 plants in the vegetation of Central Asia, geographical distribution and environmental adaptation in relation to climate. In Climate change and terrestrial carbon sequestration in Central Asia, pp. 33–63. Taylor & Francis. [Google Scholar]
- 64.Miller NF, Spengler RN, Frachetti M. 2016. Millet cultivation across Eurasia: Origins, spread, and the influence of seasonal climate. The Holocene 26, 1566–1575. ( 10.1177/0959683616641742) [DOI] [Google Scholar]
- 65.Chang C, Benecke N, Grigoriev FP, Rosen AM, Tourtellotte PA. 2003. Iron Age society and chronology in South-east Kazakhstan. Antiquity 77, 298–312. ( 10.1017/S0003598X00092280) [DOI] [Google Scholar]
- 66.Lister DL, Jones H, Oliveira HR, Petrie CA, Liu X, Cockram J, Kneale CJ, Kovaleva O, Jones MK. 2018. Barley heads east: genetic analyses reveal routes of spread through diverse Eurasian landscapes. PLoS ONE 13, e0196652 ( 10.1371/journal.pone.0196652) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vainshtein SI. 1980. Nomads of South Siberia. London, UK: Cambridge University Press. [Google Scholar]
- 68.Khazanov AM. 1994. Nomads and the outside world, 2nd edn. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 69.Makarewicz C, Tuross N. 2006. Foddering by Mongolian pastoralists is recorded in the stable carbon (δ13C) and nitrogen (δ15N) isotopes of caprine dentinal collagen. J. Archaeol. Sci. 33, 862–870. ( 10.1016/j.jas.2005.10.016) [DOI] [Google Scholar]
- 70.Makarewicz CA. 2014. Winter pasturing practices and variable fodder provisioning detected in nitrogen (δ15N) and carbon (δ13C) isotopes in sheep dentinal collagen. J. Archaeol. Sci. 41, 502–510. ( 10.1016/j.jas.2013.09.016) [DOI] [Google Scholar]
- 71.Frachetti MD, Smith CE, Traub CM, Williams T. 2017. Nomadic ecology shaped the highland geography of Asia's Silk Roads. Nature 543, 193–198. ( 10.1038/nature21696) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Genetic data are available in electronic supplementary material, dataset S1. Isotopic data are available in electronic supplementary material, datasets S2–S5.