Abstract
Over 600 Myr of evolutionary divergence between vertebrates and invertebrates is associated with considerable neuroanatomical variation both across and within these lineages. By contrast, valence encoding is an important behavioural trait that is evolutionarily conserved across vertebrates and invertebrates, and enables individuals to distinguish between positive (potentially beneficial) and negative (potentially harmful) situations. We tested the hypothesis that social interactions of positive and negative valence are modularly encoded in the honeybee brain (i.e. encoded in different cellular subpopulations) as in vertebrate brains. In vertebrates, neural activation patterns are distributed across distinct parts of the brain, suggesting that discrete circuits encode positive or negative stimuli. Evidence for this hypothesis would suggest a deep homology of neural organization between insects and vertebrates for valence encoding, despite vastly different brain sizes. Alternatively, overlapping localization of valenced social information in the brain would imply a ‘re-use' of circuitry in response to positive and negative social contexts, potentially to overcome the energetic constraints of a tiny brain. We used immediate early gene expression to map positively and negatively valenced social interactions in the brain of the western honeybee Apis mellifera. We found that the valence of a social signal is represented by distinct anatomical subregions of the mushroom bodies, an invertebrate sensory neuropil associated with social behaviour, multimodal sensory integration, learning and memory. Our results suggest that the modularization of valenced social information in the brain is a fundamental property of neuroanatomical organization.
Keywords: neurogenomics, brain evolution, valence encoding, behavioural neurobiology, social behaviour, immediate early genes
1. Introduction
Roughly 600 Myr of evolutionary divergence has given rise to tremendous structural variation in vertebrate and invertebrate nervous systems. While it remains unclear if all animal brains evolved from a common neural substrate, there is deep functional and genetic homology nevertheless [1–3]. In addition, similar encoding strategies underlying the processing of specific environmental stimuli have been identified. For example, in both the insect antennal lobe and mammalian olfactory bulb, olfactory information activates anatomically distinct glomeruli to form ‘odour maps' in the neural architecture [4,5]. By contrast, the neural encoding of social behaviour is less well understood.
Animals navigate complex social environments, and salient social information must be encoded in the nervous system and used to generate appropriate behavioural responses. Social interactions can be dichotomized as affiliative or agonistic, representing positive and negative valence, respectively [6,7]. Affiliative interactions like caregiving [8], food sharing [9] and other prosocial behaviours [10–12] represent social opportunities and are positively valenced, desirable situations. By contrast, agonistic encounters, in which threat or territorial intrusion release aggression [13], have a negative valence as they may confer harm or fitness reduction to an individual. Assigning valence to a social context is critical for an animal to make adaptive decisions and predict future interactions in a changing environment [14]. Therefore, specific neural mechanisms to encode positive and negative valence may be broadly conserved across taxa.
The brain coordinates adaptive decision-making in response to social stimuli for all animals, but the extent to which organizational strategies underlying valence encoding have been conserved across phylogenetically distinct species is poorly understood. Considering invertebrates, particularly social insects, leads to a provocative question in the evolution of neural mechanisms for valence encoding: how do tiny nervous systems represent the valence of social stimuli despite selective pressure to reduce both neuroenergetic costs and brain size? Due to the high metabolic demands of maintaining and activating neural tissue, pressure to expand the functionalities of neurons or neuronal circuits across multiple behavioural domains may be greater in invertebrates than in vertebrates [15,16]. A lower degree of regional modularization or an increased level of overlap or ‘re-use' of neuronal functionality across positive and negative valences has been predicted [4,15,16]. As an organizational strategy, re-use involves a dynamic restructuring of neural circuits such that specific subregions or populations of neurons are activated across multiple contexts [15–17].
In the fruit fly Drosophila melanogaster, numerous studies have reported a modular encoding of odour cues that signify positive or negative associations. Aso et al. [18] demonstrated that 34 mushroom body output neurons (MBONs) encode odour valence as an ensemble via combinatorial firing. Furthermore, optogenetic activation of distinct MBONs, which are classified by neurotransmitter profile, was sufficient to drive either avoidance or approach behaviour [18]. In another example, Perisse et al. [19] demonstrated that the anatomically distinct surface and core subdivisions of αβ MB neurons are vital for aversive and appetite olfactory conditioning, respectively. Both of these studies indicate a modular encoding of valence in the context of unimodal, non-social stimuli.
At the level of multimodal social interactions, the strategy for encoding attractive and aversive cues in distinct regions of the D. melanogaster brain is less clear. Certel et al. showed that expression of the gene fruitless (fru) determined whether suboesophageal octopamine/tyramine neurons governed aggression or courtship, suggesting that valenced social information is handled by overlapping circuits in the fly brain [20]. Similarly, a single population of doublesex+ neurons, the pC1 cluster, was shown to induce either courtship or aggression depending on fru expression in activated neurons [21]. These examples show less anatomical distinction in the processing of valenced social stimuli and instead suggest that gene expression within a specific brain region modulates a neuron's responsiveness to positive and negative scenarios. Therefore, modular organization of valence may apply to unimodal learning and memory in invertebrates, but it is presently unclear if it underlies social behaviour as well.
In other invertebrate nervous tissues, neuronal re-use seems to predominate. For example, the pyloric and gastric neurons of the crab stomatogastric ganglion, which are primarily associated with rhythmic chewing and digestion, respectively, can change firing patterns to participate in either rhythmic behaviour [22–24]. Similarly, in C. elegans, a single set of chemosensory interneurons regulate both appetitive and aversive behavioural responses [25]. Interestingly, in Neohelice crabs, strong behavioural evidence from assays that simulate naturalistic behavioural conditions suggests independent storage of rewarding and stressful memories [26]. In cephalopods, considerable structural evidence has indicated numerous components of the nervous system that are similar to the vertebrate brain and therefore may confer similar valence-encoding functions as seen in vertebrates [27]. Thus, invertebrate behavioural and anatomical findings are provocative and indicate a need for mapping naturalistic social interactions to the brain in a diversity of species.
Numerous studies have localized valence in the vertebrate brain, highlighting specific regions that differentially respond to positive and negative experiences, with specific circuits implicated therein [6,28,29]. For example, Kim et al. [30] proposed that two spatially segregated populations of excitatory neurons process valence-specific behavioural stimuli in the basolateral amygdala (BLA). Beyeler et al. [29] demonstrated a gradient of positive- and negative-coding neurons in the BLA, distributed across an anterior–posterior axis. Similarly, attraction and aversion to social cues were found to be controlled by separate circuits in limbic brain areas in rats [31]. Distinct regions of the avian medial bed nucleus of the stria terminalis were also shown to be differentially sensitive to affiliative or agonistic social stimuli, a feature that was found to be conserved across five species of songbirds [32]. These studies show varying degrees of segregation of valenced information in the vertebrate brain, in which distinct modules encode positive and negative experiences. More recently, Mukherjee et al. [33] used expression analyses of five immediate early genes (IEGs) in mice to demonstrate that stimulus valence could be consistently ‘decoded' in distinct brain regions in response to a variety of rewarding or aversive experiences. These results demonstrate that modular networks that broadly separate positive and negative valence exist in the vertebrate brain and likely detect valence across a range of stimuli [33].
While anatomical modularity is clearly established in the insect brain [34–36], neural re-use in the context of complex social behaviour may blur the boundaries between modules and emerge as a more efficient organization strategy in a tiny brain [15,16]. Evidence of one strategy for encoding the valence of social interactions over another (re-use versus modularity) would offer a comparative framework for the neuroanatomical architecture of social information across brains of different sizes.
The western honeybee (Apis mellifera) is an ideal model for exploring valence encoding in social contexts in the invertebrate brain. Honeybees have tiny brains of approximately 1 000 000 neurons, roughly one-third of which are dedicated to higher-order sensory processing, learning and memory in a region termed the mushroom bodies (MBs) [37,38]. Shpigler et al. [39] demonstrated with transcriptomic analyses that unique profiles of gene expression in the MBs are associated with behavioural responses to social affiliation or agonism, which invoke caregiving and aggression, respectively. These are behavioural responses to social interactions of positive and negative survival value, respectively, and are therefore representative of opposing biological valences [39]. These previous analyses have been fruitful in elucidating the neurogenomic correlates of positive and negative social interactions. Moving from brain region analyses to a finer-grained spatial localization of gene expression could reveal encoding strategies for social experiences in tiny brains.
To explore whether social information activates dissociated or overlapping, intermingled neuronal populations in the honeybee brain, we performed in situ hybridization to localize and quantitate the transcriptional activity of two highly conserved IEGs, hr38 [40,41] and egr1 [42,43], in the brains of adult worker bees following an encounter with an affiliative or agonistic stimulus. IEGs, usually the first genes to respond to neural activity, are rapidly and transiently upregulated in activated neurons, and thus are excellent targets in general for tracing valence encoding with spatio-temporal cellular resolution [32,44]. Furthermore, using IEGs to map the neural correlates of honeybee behaviour is well-established [42,45]. We chose hr38 and egr1 for exploring valence encoding in the brain because each was shown to be among the most responsive genes in the MBs following exposure to an affiliative or agonistic interaction [39]. Both IEGs are conserved in vertebrates [43,46] and invertebrates [40,41], probably serving to initiate transcriptomic cascades following neuronal activity [47].
We tested the hypothesis that distinct regions of the honeybee brain will be differentially sensitive to a social stimulus depending on its valence. Support for this hypothesis would imply, at least in part, a modular partitioning of social information similar to findings in vertebrates [6,16], and would support a conserved neuroarchitecture across animals of varying neuroenergetic constraints and brain sizes. With one positive and one negative social stimulus tested here, we present this study as steps towards a comprehensive understanding of the evolution of circuit organization underlying valence encoding.
2. Material and methods
(a). Honeybees
Frames of honeycomb containing capped pupae were collected from apiaries maintained according to standard beekeeping practices at the University of Illinois Bee Research Facility in Urbana, IL. Frames were stored in the dark in an incubator that mimics the interior conditions of a beehive (33 ± 1°C, 50 ± 10% relative humidity). Upon emergence, groups of 10 adult bees (1–18 h old) were individually paint-marked with a unique colour applied to the dorsal thorax (Testors Paint, Rockford, IL, USA) and placed in a vertically oriented Petri dish (100 × 20 mm, Thermo Fisher Scientific, Waltham, MA, USA) containing a section of beeswax foundation (Mann Lake Ltd, Hackensack, MN, USA). Dishes were provided a tube of honey (approx. 1.2 ml), 50% sucrose solution (2 ml) and a pollen ball. We used bees from two colonies per experiment, each headed by a naturally mated queen, for experiment 1 (June–July 2016) and experiment 2 (July–September 2017). All bees were reared in identical conditions and left undisturbed in the incubator for 7 days prior to behavioural assays.
(b). Behavioural response to affiliative and agonistic stimuli
To examine region-specific neural activity following social stimuli of opposite valence, we used two established behavioural assays that expose groups of honeybees either to a positive (affiliative) or negative (agonistic) stimulus [39,48,49]. For experiments 1 and 2, we used an established laboratory-based intruder assay which evokes an aggressive response in focal honeybees [48,50]. Dishes of 7-day-old bees were placed in a room that simulated the conditions of the hive entrance (28°C, 30% relative humidity) where colony defence normally occurs [51,52]. To avoid capturing the IEG response to the new environment or light exposure [53], bees were left undisturbed for at least 60 min. To evoke an agonistic interaction, an unrelated bee (intruder) was introduced to each experimental group for 5 min, and aggressive interactions between resident bees and the intruder were quantified based on frequency and duration of biting and stinging events, as adapted from previous reports [39,46]. In experiment 1, groups were left undisturbed for 5, 15 or 30 min post-assay to capture the dynamics of IEG expression following the encounter [40,54], after which the most aggressive bee (greater than 30 s of biting and stinging, a ‘guard' [50]) was collected for IEG analysis. Control dishes received a novel inanimate object that induces both minimally aggressive and investigative behaviours. For experiment 2, the intruder assay described in experiment 1 was used, except bees were only collected at 15 min post-assay (see Results from experiment 1).
To represent positive valence, a laboratory-based social affiliation assay was used [39,49]. To simulate in-hive conditions where nursing occurs [49,51], this assay was performed in the rearing incubator (33 ± 1°C, 50 ± 10% relative humidity), and groups of bees were similarly allowed to acclimate to light undisturbed for at least 60 min. Briefly, groups were exposed to a 4-day-old queen larva in a natural wax ‘cell' to evoke affiliative caregiving behaviour. Affiliative behaviour was determined by the frequency and duration of prosocial events leading up to and including the regurgitation of larval food (nursing), as previously described [39,49]. An identical inanimate object to the intruder assay was used as a negative control. After 5 min, the queen larva or control was removed and groups of bees were left undisturbed for 15 min. Bees that showed high levels of affiliative caregiving (greater than 30 s of assay spent in queen cell, ‘nurses' [39]) were collected for IEG analysis. For both assays, we only collected controls that interacted with the inanimate object as a means of controlling for gene expression related to stimulus novelty [55,56].
(c). Sample preparation
Guards, nurses and controls were rapidly decapitated and the head was submerged in ice-cold 0.1% diethylpyrocarbonate (DEPC)-treated insect saline (in mM: NaCl 130; KCl 5; MgCl2 4, HEPES 15; glucose 25; sucrose 160). The antennae were removed, a window was cut in the cuticle, and the intact brain was gently lifted out of the head capsule. Brains were placed in a small plastic mould, covered with Optimal Cutting Temperature medium (OCT, Tissue-Tek, Sakura Finetek USA, Torrance, CA, USA), and fresh-frozen on half a Petri dish floating on liquid nitrogen, which allowed for rapid, even freezing of the OCT. All dissections were conducted in RNase-free conditions. Plastic moulds containing brains in OCT were wrapped in foil, vacuum-sealed in a plastic bag, and stored in a −80°C freezer until processing. Before cryosectioning, samples were allowed to incubate at least 1 h at −18°C in an HM550 cryostat (Microm, Walldorf, Germany) chamber. OCT blocks were individually mounted on a cryostat chuck, serially sectioned with a low profile microtome blade (#DT315N50, CL Sturkey, Lebanon, PA, USA) on to two series at 10 µm, and thaw-mounted onto Superfrost Plus Gold slides (Thermo Fisher Scientific). Slides were allowed to dry at room temperature (RT) in an RNase-free container.
(d). Hr38 and egr1 primer design and riboprobe development
Using the sequenced genome of the honeybee (v. OGS 3.2 [57]), we designed riboprobes targeted against intronic mRNA, allowing us to pinpoint the first neurons that are activated, and avoiding the detection of constitutively expressed mature mRNA [54,58]. We designed probes to target intronic regions of hr38 (GB40074) and egr1 (GB50091). (Primers for hr38: 5′-ATCGTGCACCATCTGCATTC-3′ and 5′-ATTCTTCGATAACTCGAGCC-3′; egr1: 5′-TACACACCTCGACGAGATCGAA-3′ and 5′-GTCGCCCCTATCTGTCGTCTAC-3′.) The fragments were amplified from genomic DNA extracted from frozen honeybee brains. PCR products were then cloned into the pGEM-T vector (A3600, Promega, Madison, WI, USA). After sequencing, the correct clones were linearized by restriction enzymes and antisense probes were transcribed using the MegaScript SP6 kit (AM1330, Invitrogen, Carlsbad, CA, USA) supplemented with digoxigenin (DIG)-labelled UTPs (3359247910, Roche Diagnostics, Basel, Switzerland). Probes were stored in 50% formamide at −20°C.
(e). In situ hybridization for IEGs hr38 and egr1
In situ hybridization was performed on honeybee brains following existing protocols [42,59]. Briefly, tissue was fixed on-slide in 4% paraformaldehyde and hybridized overnight at 55°C with DIG-labelled hr38 or egr1 riboprobes diluted to 500–1000 ng ml−1 in hybridization buffer (50% formamide, 200 mg ml−1 RNase-free tRNA, 1x Denhardt's solution, 10% dextran sulfate, 600 mM NaCl, 0.25% sodium dodecyl sulfate, 1 mM ethylenediaminetetraacetic acid). Next, slides were incubated overnight at 4°C with an anti-DIG antibody conjugated to alkaline phosphatase (Roche Diagnostics). Probe signal was then developed using one tablet of NBT/BCIP (Roche Diagnostics) dissolved in 10 ml water and applied on-slide to cover each brain section for 2–3 h, at which point optimum colour development was achieved.
(f). Imaging and IEG transcriptional activity quantitation
Slides were imaged on a Nanozoomer 2.0HT slide scanning system (Hamamatsu, Skokie, IL, USA), which automates the imaging of slides at 40× resolution, and analysis was performed using the associated NDP.view software [60]. To assure consistent sampling of brain regions across samples, we only analysed brain sections that contained the antennal lobe and each calyx of the MBs. Following published neuroanatomical data [61], we outlined regions of interest including the AL and subregions of the MBs (figure 1); IEG transcriptional activity was not consistently detected outside of these components of the olfactory pathway for either probe. The number of hr38+ or egr1+ cells were manually counted by an investigator who was blind to experimental conditions, as has been previously described [40,45], and we used the number of IEG+ cells divided by unit area for statistical analyses. Because of previous findings suggesting that neuromolecular activity is lateralized in the honeybee AL in the context of odour processing [62,63], we performed counts on both brain hemispheres for all analyses.
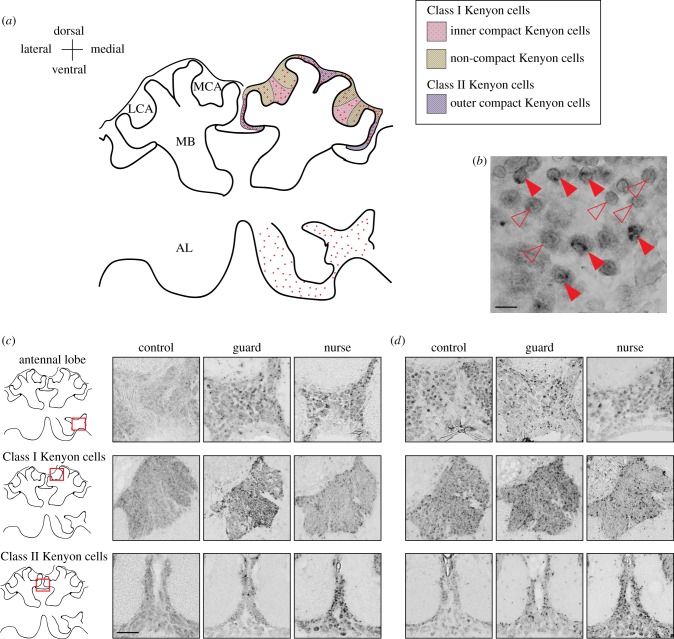
Figure 1.
Neural activity map of immediate early gene (IEG) localization following agonistic and affiliative contexts. (a) Generalized anatomical organization of the honeybee olfactory pathway, which transfers environmental information from the antennal lobe (AL) to the mushroom bodies (MBs), regions of higher-order sensory processing, learning and memory. Olfactory receptor neurons are housed in antennal sensilla and project axons to glomeruli in the AL. After local processing, the AL projects axons via antennocerebral tracts to the MBs and lateral horn (LH, not shown). The MBs are composed of neurons called Kenyon cells (KCs), which are divided into three subpopulations: the inner compact cells, outer compact cells and non-compact KCs. Left side of brain diagram shows generalized anatomical structures in the olfactory pathway; right side shows detailed anatomy and representative labelling. Red dots on right side of brain diagram show regions of high hr38 and egr1 transcriptional activity at 15 min following guarding or nursing behaviour, relative to controls; IEG activity in these regions was bilaterally distributed. We analysed both sides of the bee brain and only detected consistent IEG transcriptional activity in the AL and MBs. LCA: lateral calyx; MCA: medial calyx. Class I KCs include inner (pink) and non-compact (yellow) KCs; Class II KCs contain outer compact KCs (purple). (b) Representative magnified portion of AL showing clear outline of neurons and distinction between IEG+ (solid arrowheads) and IEG− (hollow arrowheads) cell. Scale bar, 10 µm. Coronal sections showing representative patterns of hr38 (C) and egr1 (D) induction in the AL and MBs at 15 min following exposure to an inanimate object (control), intruder bee (guard) or queen larva (nurse). Coronal sections are rendered in greyscale. Scale bar, 50 µm. (Online version in colour.)
(g). Statistical analyses
Raw behavioural and molecular data with accompanying code for statistical analysis can be found at https://doi.org/10.6084/m9.figshare.7996196. For experiment 1, normalized IEG counts in guards and controls were compared with a Welch's two-sample t-test (n = 5–7 individuals per group per time point). To compare controls, guards and nurses in experiment 2, we performed an ANOVA for each brain region or subregion with assay date as a blocking factor to control for day-to-day variation in behavioural or molecular procedures (n = 9–15 individuals per group). Model residuals were assessed via the Shapiro–Wilk test and normal quantile plots, and no deviations from statistical assumptions were detected. ANOVA was followed with a Tukey–Kramer's honest significant difference (HSD) test to check for differences between specific groups post hoc. To further evaluate treatment group differences, principal component analysis (PCA) was performed on matrices containing hr38 or egr1 normalized cell counts per sample in four regions of the honeybee olfactory pathway: the AL, and the inner compact, outer compact and non-compact Kenyon cells (figure 1 for anatomical depiction). PCA plots were generated using the R packages FactoMineR [64] and factoextra [65]. All data analysis was performed with R v. 3.5.2.
3. Results
(a). Experiment 1: time-course analysis of IEG transcriptional activity in the brain
We first performed a time-course analysis to explore bilateral IEG expression in the honeybee brain following an agonistic encounter with an intruder bee (figure 2). Hr38 expression was not distinguishable in guards compared to controls at 5 min post assay in the antennal lobe (AL, p = 0.44; figure 2a) or the mushroom bodies (MBs, p = 0.69; figure 2c) but was dramatically higher in guards than in controls at 15 min post-assay in both regions (AL: p = .00093; MBs: p = 0.0039) before dropping to levels slightly higher than controls at 30 min post-assay (AL: p = 0.15; MBs: p = 0.029). Similarly, egr1 transcriptional activity in guards was similar to controls at 5 min post-assay (AL: p = 0.63; MBs: p = 0.16; figure 2b,d) before rising at 15 min (AL: p = 0.078; MBs: p = 0.0049) and falling back to baseline levels at 30 min post-assay (AL: p = 0.79; MBs: p = 0.33). Intronic probes for both IEGs revealed the most intense increase in transcriptional activity at 15 min post-assay (figure 2), consistent with previous studies demonstrating that mature transcripts of hr38 and egr1 peaked at 30 min following guarding or nursing behaviour before dropping [39]. We therefore decided to exclusively use 15 min post-assay for our collection strategy to compare guards and nurses in experiment 2.
Figure 2.
Immediate early gene (IEG) transcription is transiently induced following an aggressive interaction in an agonistic context. Time-course analysis shows peak activity of hr38 and egr1 transcription in the (a,b) antennal lobe and (c,d) mushroom bodies at 15 min following a display of guarding behaviour that included biting and stinging an unfamiliar intruder bee. Median values divide boxes, ends of box show lower and upper quartiles (25% and 75%, respectively), extreme lines show the minimum and maximum values excluding outliers, and extreme values (points 1.5 times the interquartile range above or below the upper or lower quartile, respectively) are represented by hollow circles. n.s.p > 0.05, *p < 0.05, **p < 0.005, ***p < 0.001, Welch's t-test, n = 5–7 bees per group per time point. Brain regions analysed here are highlighted in figure 1. (Online version in colour.)
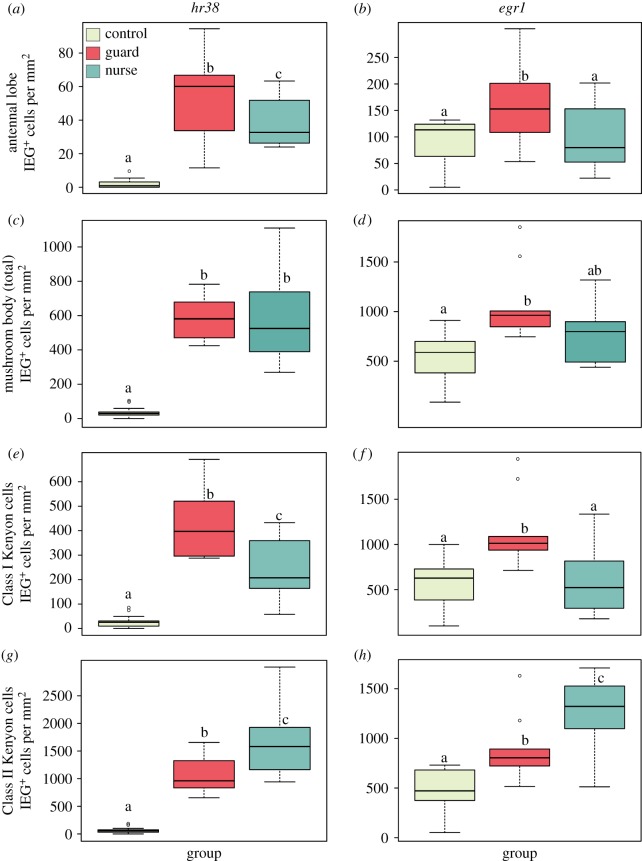
(b). Experiment 2: analysis of IEG transcriptional activity in guards and nurses
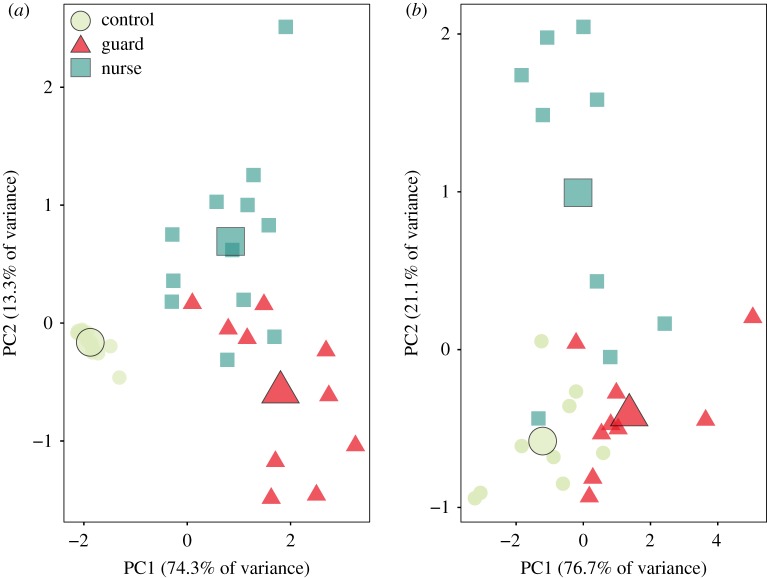
We tested the hypothesis that the valence of positive and negative social interactions would activate distinct regions of the honeybee brain (figure 1a). We examined bilateral, region-specific differences in IEG transcriptional activity between the three treatment groups (figure 3). In the AL, the primary sensory neuropil, we found significantly more IEG+ cells in guards compared to nurses for hr38 (figure 3a, Tukey HSD post hoc test following ANOVA, p = 0.024) and egr1 (figure 3b, p = 0.018). Despite there being no difference in overall MB IEG transcriptional activity between guards and nurses for either IEG (figure 3c, hr38: p = 0.99; figure 3d, egr1: p = 0.069), we found significantly higher levels of hr38 transcription (figure 3e, p = 0.0014) and egr1 (figure 3f, p = 0.003) in the Class I Kenyon cells (KCs) of guards compared to nurses. Conversely, IEG transcriptional activity was significantly higher in the Class II KCs of nurses compared to guards (figure 3g, hr38: p = 0.0026; figure 3h, egr1: p = 0.016). The first two principal components of a PCA separated controls, guards and nurses by normalized IEG+ cells in the AL and three subregions of the MBs: the inner compact, non-compact and outer compact KCs (figure 4, anatomical regions highlighted in figure 1a). There was no difference in the duration of contact between guards and nurses and their respective stimuli (Welch's t-test, p = 0.96, data not shown). In addition, we compared IEG transcriptional activity in controls from the two different behavioural testing environments and found no evidence of temperature-induced effects on baseline gene expression in any brain region (Welch's t-test, p > 0.1 for all comparisons, data not shown).
Figure 3.
Anatomical localization of immediate early gene (IEG) transcriptional activity following a social stimulus is dependent on the valence of the social interaction. Both hr38 (a,c,e,g) and egr1 (b,d,f,h) showed similar patterns of activation at 15 min following an interaction with an inanimate object (control), an unfamiliar, aggressive bee (guard) or a caregiving interaction with a queen larva in a wax cup (nurse). Boxplots show IEG transcriptional activity in the antennal lobe (a,b), total Kenyon cells (KCs) in the mushroom bodies (c,d), Class I KCs (e,f) and Class II KCs (g,h) using the same designation as in figure 2. Significantly different groups are denoted by different letters (p < 0.05, Tukey–Kramer's honestly significant difference test after ANOVA, n = 9–15 bees per group). Brain regions analysed here are highlighted in figure 1. (Online version in colour.)
Figure 4.
Principal component analysis (PCA) separates controls, guards and nurses based on normalized immediate early gene expression in the four regions of the honeybee olfactory pathway analysed in this study (figure 1 and Methods). We included normalized expression of (a) hr38 and (b) egr1 in the antennal lobe, Class I Kenyon cells (inner compact + non-compact) and Class II Kenyon cells (outer compact). Analyses were conducted at 15 min post-assay, where we found peak hr38 and egr1 expression. Largest shape denotes centroid for each group. n = 9–15 bees per group. (Online version in colour.)
4. Discussion
We demonstrated that IEG transcriptional activity in the MBs, a major processing centre for social information in the insect brain [34,66], localized to anatomically distinct subpopulations of neurons in a valence-dependent manner, implying a modular encoding of valence in the honeybee brain (figures 3 and 4). In vertebrates, considerable literature supports a similar modular encoding of valence, suggesting that the BLA, nucleus accumbens and other structures are composed of subregions that are sensitive to valence [6,28,67]. Moreover, Namburi et al. outlined the criteria for encoding valence, proposing that neurons must either (1) increase activity following exposure to one valence and decrease activity to a cue of the opposite valence or (2) modulate activity to a cue of one valence but not to the opposite valence [28]. It appears that the Class I and II KC populations of the MBs satisfy the first criterion, as the IEG transcriptional activity in each region reflects the valence of the stimulus (figure 3).
It has been argued that the modularization of a tiny brain into functionally discrete networks that correspond to positive and negative valence could theoretically overcome metabolic limitations by shortening distances between similarly functioning neurons [15,68], though few studies have examined this possibility. Until now, this phenomenon has only been shown for non-social behaviours. In fruit flies, analyses of non-social stimuli processing have indicated that valence-specific output neurons form topographical clusters in the AL and second-order projection neurons, corresponding to attractive and aversive cues [69]. Similarly, non-overlapping control of repulsion or attraction is segregated among MBONs, which receive afferents from multimodal sensory processing regions of the fly brain [18]. We suggest that this organizational strategy is ‘scaled up' to apply to complex, multimodal social interactions in the honeybee brain, and present evidence for this in the context of responding to affiliative and agonistic social stimuli.
This organizational strategy of functionally discrete networks that correspond to positive and negative valence may also be present in other arthropods. In crustaceans, specific biogenic amines differentially facilitate positive and negative memory formation in the crab Chasmagnathus, further implying the possibility of valence-sensitive modules in the brain [70,71]. While valence encoding has not been explicitly looked at in cephalopods, Fiorito & Chichery demonstrated impaired social learning but no impact on memory acquisition or recall following lesions to the vertical lobe in Octopus vulgaris, suggesting distinct brain regions process specific aspects of social stimuli [72]. With our results as a new reference point, further work mapping biological processes to the invertebrate brain will elucidate organizational strategies for the encoding of social interactions.
Modularity, as we suggest here, could capitalize on the denser neuronal packing prevalent in insect nervous systems by reducing the distance neural processes must travel [4]. Consistent with modular organization, the segregation of specific ‘types' of social information to distinct regions of the brain may be additionally favoured on account of low myelin levels in the insect brain [73], allowing for a higher degree of local connectivity instead of delayed signalling between long-distance connections [4]. A mechanism by which the bee brain may support valence encoding is through the actions of local interneurons, which have been shown in crickets to connect various parts of the brain with neuronal assemblies in the MBs via extensive branching patterns [74]. Local interneurons may therefore ‘tune' subregions of the MBs to have heightened sensitivity in response to specific combinations of sensory stimuli such that a threatening stimulus can be distinguished from an opportunity to provide caregiving. More comparative behavioural and anatomical analyses of arthropod brains will demonstrate the extent to which this emerging view of the MBs is unique to the honeybee.
The results of this study demonstrate an additional conserved organizational principle between vertebrate and invertebrate brains [28], and validate theoretical considerations regarding the partitioning of valenced information to different subregions of a tiny brain [15,75]. Recently, IEG mapping has implicated nodes of the vertebrate social decision-making network (SDMN [76]) that are differentially sensitive to aggressive encounters compared to reproductive attempts in brown anoles [77], and a similar segregation of positive and negative valence was found in songbirds [32]. While no SDMN has been proposed for invertebrates, our findings point to a similar system in invertebrate brains, which awaits further elucidation.
Supplementary Material
Acknowledgements
We thank Alison Sankey for honeybee management, Dr Amy Cash Ahmed for laboratory assistance, Drs Mayandi Sivaguru and Kingsley Boateng for assistance with brain imaging and analysis, and members of the Robinson Laboratory and three anonymous reviewers for thoughtful commentary that improved the manuscript.
Data accessibility
Raw data and code can be accessed at https://doi.org/10.6084/m9.figshare.7996196.
Authors' contributions
I.M.T. and G.E.R. conceived and designed the experiments; I.M.T. and V.B. performed fieldwork; I.M.T and Z.C. performed molecular analyses; I.M.T. and G.E.R wrote the paper.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by grant no. SFLife 291812 from the Simons Foundation (G.E.R. and L. Stubbs), grant no. R01GM117467 from the National Institute of General Medical Sciences (G.E.R. and N. Goldenfeld) and the Illinois Sociogenomics Initiative (G.E.R.).
References
- 1.Strausfeld NJ, Hirth F. 2013. Deep homology of arthropod central complex and vertebrate basal ganglia. Science 340, 157–161. ( 10.1126/science.1231828) [DOI] [PubMed] [Google Scholar]
- 2.Tomer R, Denes AS, Tessmar-Raible K, Arendt D. 2010. Profiling by image registration reveals common origin of annelid mushroom bodies and vertebrate pallium. Cell 142, 800–809. ( 10.1016/j.cell.2010.07.043) [DOI] [PubMed] [Google Scholar]
- 3.Farris SM. 2011. Are mushroom bodies cerebellum-like structures? Arthropod. Struct. Dev. 40, 368–379. ( 10.1016/J.ASD.2011.02.004) [DOI] [PubMed] [Google Scholar]
- 4.Chittka L, Niven J. 2009. Are bigger brains better? Curr. Biol. 19, R995–R1008. ( 10.1016/J.CUB.2009.08.023) [DOI] [PubMed] [Google Scholar]
- 5.Li Q, Liberles SD. 2015. Aversion and attraction through olfaction. Curr. Biol. 25, R120–R129. ( 10.1016/j.cub.2014.11.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tye KM. 2018. Neural circuit motifs in valence processing. Neuron 100, 436–452. ( 10.1016/J.NEURON.2018.10.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rumbaugh DM. et al 2012. Approach and avoidance motivation. In Encyclopedia of the sciences of learning, pp. 286–288. Boston, MA: Springer US. [Google Scholar]
- 8.Cardoso C, Orlando MA, Brown CA, Ellenbogen MA. 2014. Oxytocin and enhancement of the positive valence of social affiliation memories: an autobiographical memory study. Social Neurosci. 9, 186–195. ( 10.1080/17470919.2013.873079) [DOI] [PubMed] [Google Scholar]
- 9.Desmet PMA, Schifferstein HNJ. 2008. Sources of positive and negative emotions in food experience. Appetite 50, 290–301. ( 10.1016/J.APPET.2007.08.003) [DOI] [PubMed] [Google Scholar]
- 10.Boissy A, et al. 2007. Assessment of positive emotions in animals to improve their welfare. Physiol. Behav. 92, 375–397. ( 10.1016/J.PHYSBEH.2007.02.003) [DOI] [PubMed] [Google Scholar]
- 11.Marzouki Y, Gullstrand J, Goujon A, Fagot J. 2014. Baboons' response speed is biased by their moods. PLoS ONE 9, e102562 ( 10.1371/journal.pone.0102562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawes CT, Loewen PJ, Schreiber D, Simmons AN, Flagan T, McElreath R, Bokemper SE, Fowler JH, Paulus MP. 2012. Neural basis of egalitarian behavior. Proc. Natl Acad. Sci. USA 109, 6479–6483. ( 10.1073/pnas.1118653109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanogo YO, Band M, Blatti C, Sinha S, Bell AM. 2012. Transcriptional regulation of brain gene expression in response to a territorial intrusion. Proc. R. Soc. B 279, 4929–4938. ( 10.1098/rspb.2012.2087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmer CR, Kristan WB. 2011. Contextual modulation of behavioral choice. Curr. Opin Neurobiol. 21, 520–526. ( 10.1016/j.conb.2011.05.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niven JE, Chittka L. 2010. Reuse of identified neurons in multiple neural circuits. Behav. Brain Sci. 33, 285 ( 10.1017/S0140525X10001068) [DOI] [Google Scholar]
- 16.Anderson ML. 2016. Neural reuse in the organization and development of the brain. Dev. Med. Child Neurol. 58, 3–6. ( 10.1111/dmcn.13039) [DOI] [PubMed] [Google Scholar]
- 17.Stanley ML, et al. 2016. Modularity in network neuroscience and neural reuse. Behav. Brain Sci. 39, e133 ( 10.1017/S0140525X15001673) [DOI] [PubMed] [Google Scholar]
- 18.Aso Y, et al. 2014 Mushroom body output neurons encode valence and guide memory-based action selection in Drosophila. eLife 3, 4580 ( 10.7554/eLife.04580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perisse E, Yin Y, Lin AC, Lin S, Huetteroth W, Waddell S. 2013. Different Kenyon cell populations drive learned approach and avoidance in Drosophila. Neuron 79, 945–956. ( 10.1016/J.NEURON.2013.07.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Certel SSJ, et al. 2010. Octopamine neuromodulatory effects on a social behavior decision-making network in Drosophila males. PLoS ONE 5, e13248 ( 10.1371/journal.pone.0013248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koganezawa M, Kimura K, Yamamoto D. 2016. The neural circuitry that functions as a switch for courtship versus aggression in Drosophila males. Curr. Biol. 26, 1395–1403. ( 10.1016/J.CUB.2016.04.017) [DOI] [PubMed] [Google Scholar]
- 22.Weimann JM, Marder E. 1994. Switching neurons are integral members of multiple oscillatory networks. Curr. Biol. 4, 896–902. ( 10.1016/S0960-9822(00)00199-8) [DOI] [PubMed] [Google Scholar]
- 23.Marder E, Weimann JM. 1992. Modulatory control of multiple task processing in the stomatogastric nervous system. Neurobiol. Motor Program. Sel., 3–19. ( 10.1016/B978-0-08-041986-2.50006-0) [DOI] [Google Scholar]
- 24.Weimann JM, Meyrand P, Marder E. 1991. Neurons that form multiple pattern generators: identification and multiple activity patterns of gastric/pyloric neurons in the crab stomatogastric system. J. Neurophysiol. 65, 111–122. ( 10.1152/jn.1991.65.1.111) [DOI] [PubMed] [Google Scholar]
- 25.Guillermin ML, Carrillo MA, Hallem EA. 2017. A single set of interneurons drives opposite behaviors in C. elegans. Curr. Biol. 27, 2630–2639. ( 10.1016/J.CUB.2017.07.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klappenbach M, Nally A, Locatelli FF. 2017. Parallel memory traces are built after an experience containing aversive and appetitive components in the crab Neohelice. Proc. Natl Acad. Sci. USA 114, E4666–E4675. ( 10.1073/pnas.1701927114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shigeno S, Andrews PLR, Ponte G, Fiorito G. 2018. Cephalopod brains: an overview of current knowledge to facilitate comparison with vertebrates. Front. Physiol. 9, 952 ( 10.3389/fphys.2018.00952) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Namburi P, Al-Hasani R, Calhoon GG, Bruchas MR, Tye KM. 2016. Architectural representation of valence in the limbic system. Neuropsychopharmacology 41, 1697–1715. ( 10.1038/npp.2015.358) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beyeler A, Chang C-J, Silvestre M, Lévêque C, Namburi P, Wildes CP, Tye KM. 2018. Organization of valence-encoding and projection-defined neurons in the basolateral amygdala. Cell Rep. 22, 905–918. ( 10.1016/j.celrep.2017.12.097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J, Pignatelli M, Xu S, Itohara S, Tonegawa S. 2016. Antagonistic negative and positive neurons of the basolateral amygdala. Nature Neurosci. 19, 1636–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.House PK, Vyas A, Sapolsky R. 2011. Predator cat odors activate sexual arousal pathways in brains of Toxoplasma gondii infected rats. PLoS ONE 6, e23277 ( 10.1371/journal.pone.0023277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodson JL, Wang Y. 2006. Valence-sensitive neurons exhibit divergent functional profiles in gregarious and asocial species. Proc. Natl Acad. Sci. USA 103, 17 013–17 017. ( 10.1073/pnas.0606278103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukherjee D, et al. 2018. Salient experiences are represented by unique transcriptional signatures in the mouse brain. eLife 7, e31220 ( 10.7554/elife.31220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strausfeld NJ. 2012. Arthropod brains: evolution, functional elegance, and histroical significance. Cambridge, MA: Belknap Press of Harvard University Press. [Google Scholar]
- 35.Niven JE, Graham CM, Burrows M. 2008. Diversity and evolution of the insect ventral nerve cord. Annu. Rev. Entomol. 53, 253–271. ( 10.1146/annurev.ento.52.110405.091322) [DOI] [PubMed] [Google Scholar]
- 36.Burrows M. 1996. The neurobiology of an insect brain. Oxford, UK: Oxford University Press. [Google Scholar]
- 37.Menzel R, Giurfa M. 2001. Cognitive architecture of a mini-brain: the honeybee. Trends Cogn. Sci. 5, 62–71. ( 10.1016/S1364-6613(00)01601-6) [DOI] [PubMed] [Google Scholar]
- 38.Heisenberg M. 2003. Mushroom body memoir: from maps to models. Nat. Rev. Neurosci. 4, 266–275. ( 10.1038/nrn1074) [DOI] [PubMed] [Google Scholar]
- 39.Shpigler HY, Saul MC, Murdoch EE, Corona F, Cash-Ahmed AC, Seward CH, Chandrasekaran S, Stubbs LJ, Robinson GE. 2018. Honey bee neurogenomic responses to affiliative and agonistic social interactions. Genes , Brain Behav. 18, e12509 ( 10.1111/gbb.12509) [DOI] [PubMed] [Google Scholar]
- 40.Fujita N, Nagata Y, Nishiuchi T, Sato M, Iwami M, Kiya T. 2013. Visualization of neural activity in insect brains using a conserved immediate early gene, Hr38. Curr. Biol. 23, 2063–2070. ( 10.1016/j.cub.2013.08.051) [DOI] [PubMed] [Google Scholar]
- 41.Singh AS, Shah A, Brockmann A. 2018. Honey bee foraging induces upregulation of early growth response protein 1, hormone receptor 38 and candidate downstream genes of the ecdysteroid signalling pathway. Insect. Mol. Biol. 27, 90–98. ( 10.1111/imb.12350) [DOI] [PubMed] [Google Scholar]
- 42.Lutz CC, Robinson GE. 2013. Activity-dependent gene expression in honey bee mushroom bodies in response to orientation flight. J. Exp. Biol. 216, 2031–2038. ( 10.1242/jeb.084905) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mello CV, Vicario DS, Clayton DF. 1992. Song presentation induces gene expression in the songbird forebrain. Proc. Natl Acad. Sci. USA 89, 6818–6822. ( 10.1073/pnas.89.15.6818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyashita T, Kubik S, Lewandowski G, Guzowski JF. 2008. Networks of neurons, networks of genes: an integrated view of memory consolidation. Neurobiol. Learn. Mem. 89, 269–284. ( 10.1016/j.nlm.2007.08.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ugajin A, Kiya T, Kunieda T, Ono M, Yoshida T, Kubo T. 2012. Detection of neural activity in the brains of Japanese honeybee workers during the formation of a ‘hot defensive bee ball’. PLoS ONE 7, e32902 ( 10.1371/journal.pone.0032902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rittschof CC, Coombs CB, Frazier M, Grozinger CM, Robinson GE. 2015. Early-life experience affects honey bee aggression and resilience to immune challenge. Sci. Rep. 5, 15572 ( 10.1038/srep15572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clayton DF. 2000. The genomic action potential. Neurobiol. Learn. Mem. 74, 185–216. ( 10.1006/nlme.2000.3967) [DOI] [PubMed] [Google Scholar]
- 48.Rittschof CC, et al. 2014. Neuromolecular responses to social challenge: common mechanisms across mouse, stickleback fish, and honey bee. Proc. Natl Acad. Sci. USA 111, 17 929–17 934. ( 10.1073/pnas.1420369111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shpigler HY, Robinson GE. 2015. Laboratory assay of brood care for quantitative analyses of individual differences in honey bee (Apis mellifera) affiliative behavior. PLoS ONE 10, e0143183 ( 10.1371/journal.pone.0143183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shpigler HY, et al. 2017. Behavioral, transcriptomic and epigenetic responses to social challenge in honey bees. Genes, Brain Behav. 16, 579–5691. ( 10.1111/gbb.12379) [DOI] [PubMed] [Google Scholar]
- 51.Winston ML. 1991. The biology of the honey bee. Cambridge, MA: Harvard University Press. [Google Scholar]
- 52.Breed MD, Guzmán-Novoa E, Hunt GJ. 2004. Defensive behavior of honey bees: organization, genetics, and comparisons with other bees. Annu. Rev. Entomol. 49, 271–298. ( 10.1146/annurev.ento.49.061802.123155) [DOI] [PubMed] [Google Scholar]
- 53.Sommerlandt FMJ, Rössler W, Spaethe J. 2017. Impact of light and alarm pheromone on immediate early gene expression in the European honeybee, Apis mellifera. Entomol. Sci. 20, 122–126. ( 10.1111/ens.12234) [DOI] [Google Scholar]
- 54.Ugajin A, Kunieda T, Kubo T. 2013. Identification and characterization of an Egr ortholog as a neural immediate early gene in the European honeybee (Apis mellifera L.). FEBS Lett. 587, 3224–3230. ( 10.1016/j.febslet.2013.08.014) [DOI] [PubMed] [Google Scholar]
- 55.Gallitano-Mendel A, Izumi Y, Tokuda K, Zorumski CF, Howell MP, Muglia LJ, Wozniak DF, Milbrandt J. 2007. The immediate early gene early growth response gene 3 mediates adaptation to stress and novelty. Neuroscience 148, 633–643. ( 10.1016/J.NEUROSCIENCE.2007.05.050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montag-Sallaz M, Welzl H, Kuhl D, Montag D, Schachner M. 1999. Novelty-induced increased expression of immediate-early genes c-fos and arg 3.1 in the mouse brain. J. Neurobiol. 38, 234–246. () [DOI] [PubMed] [Google Scholar]
- 57.Consortium HGS. 2014. Finding the missing honey bee genes: lessons learned from a genome upgrade. BMC Genomics 15, 86 ( 10.1186/1471-2164-15-86) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kiya T, Kunieda T, Kubo T. 2008. Inducible- and constitutive-type transcript variants of kakusei, a novel non-coding immediate early gene, in the honeybee brain. Insect. Mol. Biol. 17, 531–536. ( 10.1111/j.1365-2583.2008.00821.x) [DOI] [PubMed] [Google Scholar]
- 59.Velarde RA, Robinson GE, Fahrbach SE. 2006. Nuclear receptors of the honey bee: annotation and expression in the adult brain. Insect. Mol. Biol. 15, 583–595. ( 10.1111/j.1365-2583.2006.00679.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng P-P, van der Weiden M, Kros JM.. 2014. Fast tracking of co-localization of multiple markers by using the nanozoomer slide scanner and NDPViewer. J. Cell. Physiol. 229, 967–973. ( 10.1002/jcp.24538) [DOI] [PubMed] [Google Scholar]
- 61.Strausfeld NJ. 2002. Organization of the honey bee mushroom body: representation of the calyx within the vertical and gamma lobes. J. Comp. Neurol. 450, 4–33. ( 10.1002/cne.10285) [DOI] [PubMed] [Google Scholar]
- 62.Guo Y, et al. 2016. Lateralization of gene expression in the honeybee brain during olfactory learning. Sci. Rep. 6, 34727 ( 10.1038/srep34727) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rigosi E, Haase A, Rath L, Anfora G, Vallortigara G, Szyszka P. 2015. Asymmetric neural coding revealed by in vivo calcium imaging in the honey bee brain. Proc. R. Soc. B 282, 20142571 ( 10.1098/rspb.2014.2571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lê S, Josse J, Husson F. 2008. FactoMineR: an R package for multivariate analysis. J. Stat. Softw. 25, 1–18. ( 10.18637/jss.v025.i01) [DOI] [Google Scholar]
- 65.Kassambara A, Mundt F. 2016. Package ‘factoextra’: extract and visualize the results of multivariate data analyses. See https://cran.r-project.org/web/packages/factoextra/index.html.
- 66.Heisenberg M. 1998. What do the mushroom bodies do for the insect brain? An introduction. Learn. Mem. 5, 1–10. ( 10.1101/lm.5.1.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nasser HM, McNally GP. 2013. Neural correlates of appetitive-aversive interactions in Pavlovian fear conditioning. Learning Memory (Cold Spring Harbor, N.Y.) 20, 220–228. ( 10.1101/lm.029744.112) [DOI] [PubMed] [Google Scholar]
- 68.Krashes MJ, et al. 2009. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell 139, 416–427. ( 10.1016/j.cell.2009.08.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Knaden M, Hansson BS. 2014. Mapping odor valence in the brain of flies and mice. Curr. Opin Neurobiol. 24, 34–38. ( 10.1016/J.CONB.2013.08.010) [DOI] [PubMed] [Google Scholar]
- 70.Kaczer L, Klappenbach M, Maldonado H. 2011. Dissecting mechanisms of reconsolidation: octopamine reveals differences between appetitive and aversive memories in the crab Chasmagnathus. Eu. J. Neurosci. 34, 1170–1178. ( 10.1111/j.1460-9568.2011.07830.x) [DOI] [PubMed] [Google Scholar]
- 71.Klappenbach M, Maldonado H, Locatelli F, Kaczer L. 2012. Opposite actions of dopamine on aversive and appetitive memories in the crab. Learning Memory (Cold Spring Harbor, N.Y.) 19, 73–83. ( 10.1101/lm.024430.111) [DOI] [PubMed] [Google Scholar]
- 72.Fiorito G, Chichery R. 1995. Lesions of the vertical lobe impair visual discrimination learning by observation in Octopus vulgaris. Neurosci. Lett. 192, 117–120. ( 10.1016/0304-3940(95)11631-6) [DOI] [PubMed] [Google Scholar]
- 73.Edwards TN, Meinertzhagen IA. 2010. The functional organisation of glia in the adult brain of Drosophila and other insects. Prog. Neurobiol. 90, 471–497. ( 10.1016/J.PNEUROBIO.2010.01.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schildberger K. 1983. Local interneurons associated with the mushroom bodies and the central body in the brain of Acheta domesticus. Cell Tissue Res. 230, 573–586. ( 10.1007/bf00216202) [DOI] [PubMed] [Google Scholar]
- 75.Niven JE, Laughlin SB. 2008. Energy limitation as a selective pressure on the evolution of sensory systems. J. Exp. Biol. 211, 1792–1804. ( 10.1242/jeb.017574) [DOI] [PubMed] [Google Scholar]
- 76.O'Connell LA, Hofmann HA. 2011. Genes, hormones, and circuits: an integrative approach to study the evolution of social behavior. Front. Neuroendocrinol. 32, 320–335. ( 10.1016/j.yfrne.2010.12.004) [DOI] [PubMed] [Google Scholar]
- 77.Kabelik D, Weitekamp CA, Choudhury SC, Hartline JT, Smith AN, Hofmann HA. 2018. Neural activity in the social decision-making network of the brown anole during reproductive and agonistic encounters. Horm. Behav. 106, 178–188. ( 10.1016/J.YHBEH.2018.06.013) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data and code can be accessed at https://doi.org/10.6084/m9.figshare.7996196.