Abstract
A highly selective copper-catalyzed trifluoromethylation of indoles is reported with the assistance of a removable directing group. This protocol provides an easy and rapid method to various 2-position-selective trifluoromethylated heteroarenes including indoles, pyrroles, benzofuran, and acetanilide. What is more, the reaction takes place at ambient conditions without any external ligand or additive.
Keywords: C-H functionalization, trifluoromethylation, indoles, copper, radical reaction
Introduction
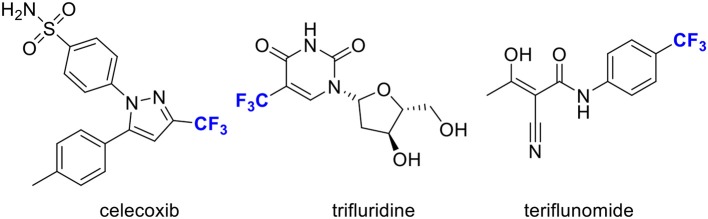
The introduction of trifluoromethyl (CF3) groups into heteroarenes enjoys a privileged role in medicinal chemistry, since it can substantially alter their properties, such as metabolic stability, lipophilicity and ability to penetrate the blood-brain barrier (Shimizu and Hiyama, 2005; Schlosser, 2006; Hagmann, 2008; Boechat and Bastos, 2010; Nie et al., 2011; Wang et al., 2014; Gouverneur and Seppelt, 2015). It has a great potential in the development of new pharmaceutical chemicals (Scheme 1). Thus, the trifluoromethylation of heteroarenes has received tremendous attentions (Sato et al., 2010; Furuya et al., 2011; Tomashenko and Grushin, 2011; Liu et al., 2013; Le et al., 2018). On the other hand, indoles represent ubiquitous structural motifs found in biologically active natural products and pharmaceutical compounds (Lee et al., 2015; Chripkova et al., 2016; Sravanthi and Manju, 2016; Goyal et al., 2018; Kaur et al., 2019). In this regard, direct trifluoromethylation of indoles offers an attractive alternative to the workers in the field of medicinal chemistry and biochemistry.
Scheme 1.
Several examples of pharmaceuticals with a trifluoromethylation group.
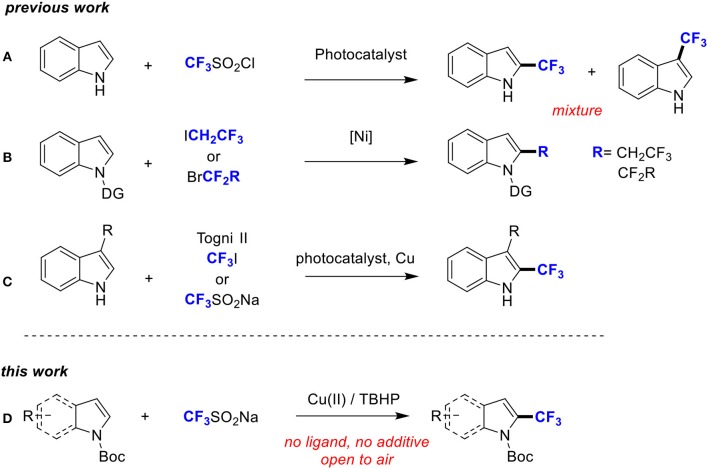
However, direct trifluoromethylation at the C2-position of indoles under radical trifluoromethylation conditions is quite difficult because of the lack of reaction selectivity at the C2/C3-position and the high reactivity of the CF3 radical (Scheme 2A; Nagib and MacMillan, 2011). Recently, directing group (DG) has emerged as a powerful tool to achieve regioselective C2-H functionalization of indoles (Nishino et al., 2012; Zhou et al., 2013; Yu et al., 2014). For example, Shi group and Punji group, respectively, achieved trifluoroethylation and difluoroalkylation of indoles at C2 position by introducing a directing group at the N-center of indoles (Scheme 2B; Yan et al., 2017; Soni et al., 2018). Also, Sodeoka group, Cho group, and Shi group accomplished trifluoromethylation of indoles at the C2 position with Togni's reagent, CF3I and CF3SO2Na respectively. However, a substituent at C3 was identified as a crucial factor for the selective trifluoromethylation at C2 (Scheme 2C; Shimizu et al., 2010; Iqbal et al., 2012; Shi et al., 2018).
Scheme 2.
Different approaches to C2 functionalized indole derivatives (A–D).
In this context, we envisioned that with the aid of a readily removable N-protecting group, trifluoromethyl group can be introduced to C2 position, which is complementary to the previous work. Herein, we report a copper-catalyzed C2-H trifluoromethylation of N-Boc (t-butyloxy) indoles with CF3SO2Na under ambient conditions in the absence of any external ligand or additive (Scheme 2D). Notably, the key to our success is the installation of a suitable Boc director on the indole nitrogen atom.
Results and Discussion
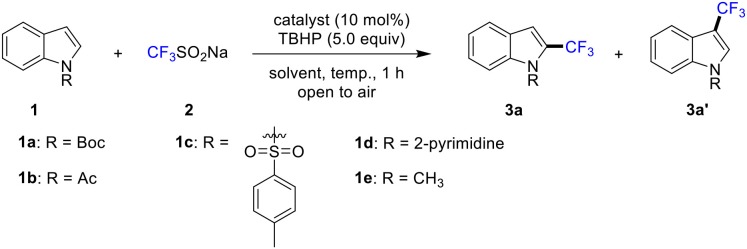
To begin, we chose N-Boc indole (1a) as model substrate. To our delight, when the reaction mixture of N-Boc indole (1a, 0.50 mmol), 2 (1.5 mmol), TBHP (t-butyl hydroperoxide, 70% solution in H2O, 2.5 mmol) and CuSO4 (10 mol%) in DMA (dimethylacetamide, 3 mL) was stirred at 85°C in air for 1 h, 22% yield of C2-trifluoromethylation product 3a was obtained (Table 1, entry 1). Trace amount of product could be detected with other solvents, such as DCM (dichloromethane), toluene and THF (tetrahydrofuran) (entries 2-4). The yield could be increased to 46% when acetone was used, and it was elevated to 54% by using MeCN (acetonitrile) (entries 5-6). Subsequently, various metal catalysts were selected. To our delighted, the yield was increased to 65% by employing CuSO4•5H2O as catalyst (entry 7). Meanwhile, other catalysts such as FeCl3, FeCl2, Cu(OTf)2, and CuCl were screened. Unfortunately, no positive results were obtained (entries 8-11). In addition, the reaction was completely shut down in the absence of metal catalysts (entry 12). Finally, the desired product 3a was obtained in 86% isolated yield when the solvent was reduced to 1 mL (entry 13). The reaction showed low reactivity at room temperature (entry 14). Afterward, the efficiency of different directing groups was investigated. And no desired product was achieved when Ac, Ts and 2-pym were tried (entries 15-17). In addition, the use of the methyl group resulted in a marked decreased in selectivity and yield (entry 18).
Table 1.
Optimization of reaction conditionsa.
 | |||||
|---|---|---|---|---|---|
| Entry | 1 | Cat. | Solvent | Yieldb | |
| 3a | 3a' | ||||
| 1 | 1a | CuSO4 | DMA | 22 | n.d. |
| 2 | 1a | CuSO4 | DCM | trace | n.d. |
| 3 | 1a | CuSO4 | toluene | trace | n.d. |
| 4 | 1a | CuSO4 | THF | trace | n.d. |
| 5 | 1a | CuSO4 | MeCN | 54 | 6 |
| 6 | 1a | CuSO4 | acetone | 46 | 8 |
| 7 | 1a | CuSO4•5H2O | MeCN | 65 | <5 |
| 8 | 1a | FeCl3 | MeCN | 21 | n.d. |
| 9 | 1a | FeCl2 | MeCN | 23 | n.d. |
| 10 | 1a | Cu(OTf)2 | MeCN | trace | n.d. |
| 11 | 1a | CuCl | MeCN | 12 | n.d. |
| 12 | 1a | - | MeCN | trace | n.d. |
| 13 | 1a | CuSO4•5H2O | MeCNc | 89(86) | <5 |
| 14 | 1a | CuSO4•5H2O | MeCNc,d | 58 | 7 |
| 15 | 1b | CuSO4•5H2O | MeCN | n.d. | n.d. |
| 16 | 1c | CuSO4•5H2O | MeCN | n.d. | n.d. |
| 17 | 1d | CuSO4•5H2O | MeCN | n.d. | n.d. |
| 18 | 1e | CuSO4•5H2O | MeCN | 12 | 9 |
Conditions: 1a (0.5 mmol), 2 (1.5 mmol), catalyst (10 mol %), solvent (3.0 mL), 85°C, 1 h, in air.
Reported yields were based on 3a and determined by 1H NMR using CH2Br2 as an internal standard.
MeCN (1 ml).
room temperature, 12 h.
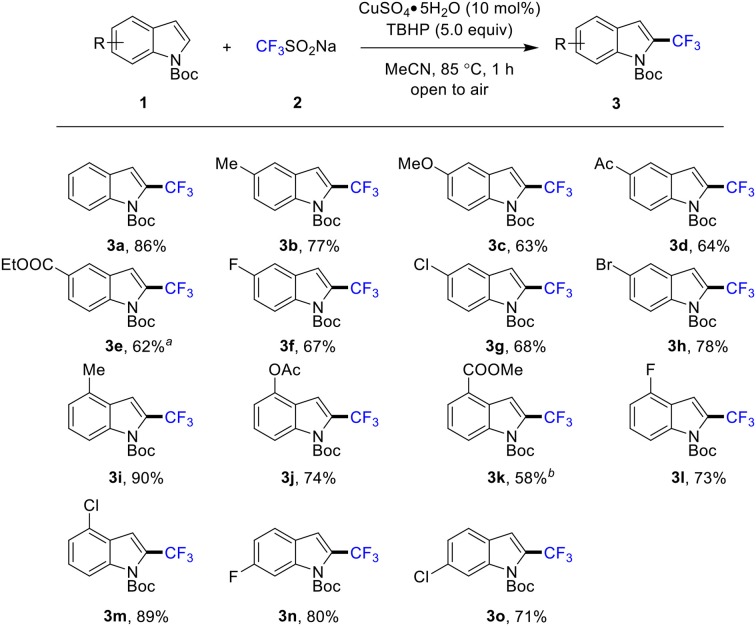
With an optimized protocol in hand, the scope and limitation of the title reaction was explored (Scheme 3). With respect to the various indole derivatives, the reaction was found quite general and tolerated by various functional groups. A wide range of 2-trifluoromethylated products with substituent groups such as methyl (3b, 3i), methoxy (3c), acetyl (3d), esters (3e, 3j, 3k), and halogen (3f-3h, 3l-3o) at 4-, 5- and 6-position of indole were produced in moderate to good yields. In particular, halides, such as F, Cl, and Br, were well tolerated, affording the desired 2-trifluoromethylated products (3f-3h and 3l-3o) in good yields of 67–89%. However, C7-substituted indoles are not reactive under the optimized reaction conditions, which is presumed due to the steric hindrance. In addition, owing to the strong electron-withdrawing property, the indoles containing cyanide and nitro are not reactive.
Scheme 3.
Scope of indoles. Conditions: 1 (0.5 mmol), 2 (1.5 mmol), CuSO4•5H2O (10 mol %), MeCN (1.0 mL), 85°C, 1 h, in air. Isolated yield. a 12 h. b 5 h.
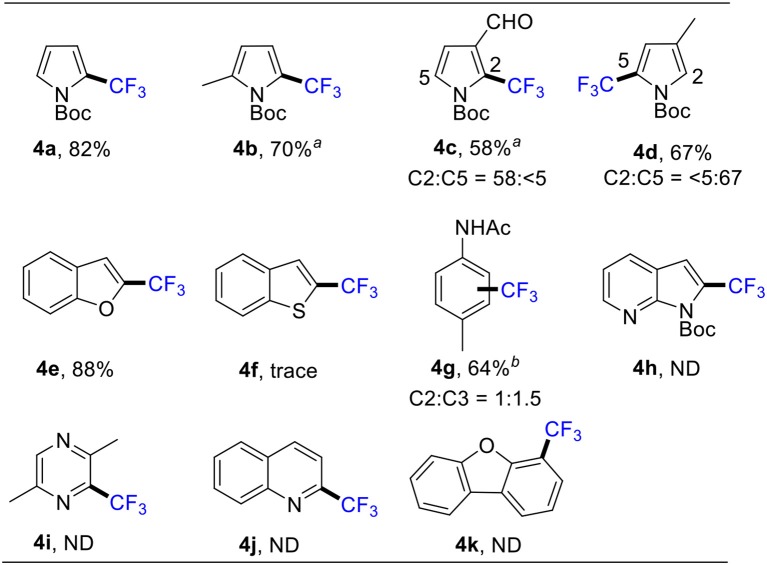
To extend the substrate scope of the above reaction, we proceeded to study the trifluoromethylation of other aromatics under the optimized reaction conditions. As shown in Scheme 4, pyrroles reacted smoothly to afford the corresponding 2-trifluoromethylated pyrroles (4a-4d) in good yields. Conventionally, direct deprotonation of benzofuran takes place at the most acidic C2 position (Larbi et al., 2017; Wang et al., 2018). Following C2 deprotonation, we obtained 2-trifluoromethylated benzofuran 4e in 88% through a radical addition mechanism. Notably, benzothiophene was also examined, but only a trace amount of product 4f was detected. Acetanilide, a drug to relieve pain or reduce fever, was also used for the synthesis of 4g. Additionally, we tried other “indole-like” compounds, but the products (4h-4k) were not gained.
Scheme 4.
Scope of other heteroarenes. Conditions: 1 (0.5 mmol), 2 (1.5 mmol), CuSO4•5H2O (10 mol %), MeCN (1.0 mL), 85°C, 1 h, in air. Isolated yield. a NMR yield. b Using 6 equiv of 2 and 10 equiv of TBHP, 12h.
Mechanism
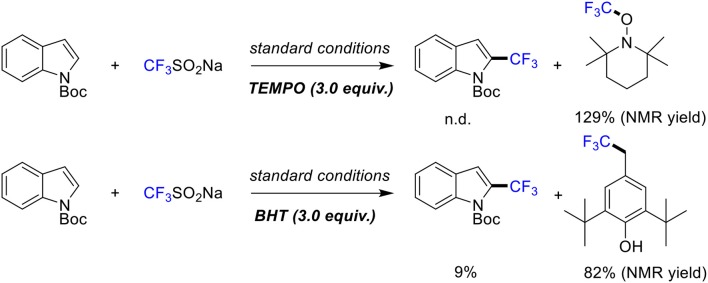
The radical scavenger experiments were conducted to gain some insights into the mechanism of this reaction (Scheme 5). When radical inhibitors such as TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy) and BHT (butylated hydroxytoluene) were added, the reaction was suppressed to a great extent. Also, 19F NMR analysis showed that radical trapping product TEMPO/BHT-CF3 was formed dominantly. Therefore, we speculated that the high C2 selective is due to the formation of a five membered metallacycle at the C2 position through N-Boc-directed C-H activation (Sandtorv, 2015; Yang et al., 2016).
Scheme 5.
Mechanistic study.
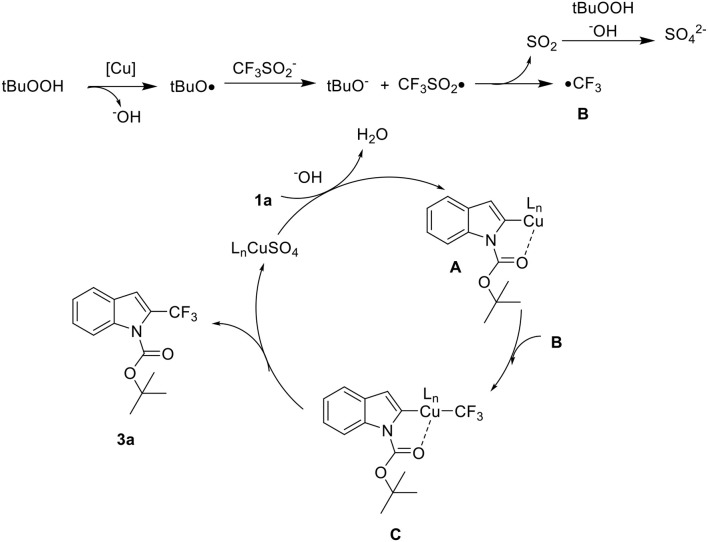
Based on the analysis of the aforementioned results and previous reports, a plausible mechanism was proposed in Scheme 6 (Langlois et al., 1991; Ji et al., 2011; Zhang et al., 2018; Khan et al., 2019). Initially, the t-butoxy radical, generated from copper metal, reacts with CF3 to provide •CF3SO2. This transient intermediate disproportionates, releasing SO2 and •CF3 B. Meanwhile, the copper catalyst was introduced to ensure the formation of a five membered metallacycle A at the C2 position. Subsequently, chelation-assisted C-H metalation of 1a A reacts with B to form C as a key intermediate. After reductive elimination the product 3a was formed and copper(II) catalyst regenerated.
Scheme 6.
Proposed reaction mechanism. L = H2O, O2, or solvent.
Conclusion
In conclusion, we have developed a direct C2-H trifluoromethylation of indoles with the assistance of a removable directing group under ambient conditions. This transformation exhibits high regioselectivity, functional group tolerance and provide a practical method to various trifluoromethylated heteroarenes including indoles, pyrroles, benzofuran, and acetanilide. What is more, control experiments testified that a radical mechanism may be involved in the reaction.
Materials and Methods
General
1H NMR spectra were recorded on Bruker 500 MHz spectrometer and the chemical shifts were reported in parts per million (δ) relative to internal standard TMS (0 ppm) for CDCl3. The peak patterns are indicated as follows: s, singlet; d, doublet; dd, doublet of doublet; t, triplet; m, multiplet; q, quartet. The coupling constants, J, are reported in Hertz (Hz). 13C NMR spectra were obtained at Bruker 126 MHz and referenced to the internal solvent signals (central peak is 77.0 ppm in CDCl3). The NMR yield was determined by 1H NMR using CH2Br2 as an internal standard. APEX II (Bruker Inc.) was used for ESI-HRMS. Flash column chromatography was performed over silica gel 200–300. All reagents were weighed and handled in air at room temperature. All chemical reagents were purchased from Alfa, Acros, Aldrich, and TCI, J&K and used without further purification.
General Procedure and Characterization Data for Product 3, 4
To a mixture of N-Boc indole 1 (0.5 mmol), CF3SO2Na 2 (1.5 mmol) and CuSO4•5H2O (10 mol%), MeCN (1.0 mL) was added in air at room temperature. tert-Butyl hydroperoxide (TBHP, 70% solution in H2O, 2.5 mmol) was dropped into the mixture in air at room temperature. The resulting mixture was stirred at 85°C in air for 1 h. Once the mixture was cooled to room temperature, the solvent was removed under reduced pressure. The crude product was purified by flash column chromatography on silica gel (petroleum ether/CH2Cl2) to give product 3 or 4 as colorless oil. The NMR spectra of synthesized compounds are depicted in Supplementary Material.
Tert-Butyl 2-(Trifluoromethyl)-1H-Indole-1-Carboxylate (3a) (Xu et al., 2011; Arimori and Shibata, 2015) (123 mg, 86%)
Isolated by flash column chromatography (petroleum ether/CH2Cl2 = 50:1, Rf = 0.3). 1H NMR (500 MHz, CDCl3) δ 8.28 (d, J = 8.5 Hz, 1H), 7.62 (d, J = 7.9 Hz, 1H), 7.45 (t, J = 7.9 Hz, 1H), 7.30 (t, J = 7.5 Hz, 1H), 7.14 (s, 1H), 1.68 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 148.57, 137.70, 126.98, 126.93 (q, J = 38.9 Hz), 126.46, 123.51, 122.77 (q, J = 266.6 Hz), 121.99, 116.04, 113.43 (q, J = 5.0 Hz), 85.43, 27.86. 19F NMR (470 MHz, CDCl3) δ−58.15. HRMS (ESI) caculated for C9H5NF3 [M-Boc]−, 184.0374; found: 184.0380.
Tert-Butyl 5-Methyl-2-(Trifluoromethyl)-1H-Indole-1-Carboxylate (3b) (115 mg, 77%)
Isolated by flash column chromatography (petroleum ether/CH2Cl2 = 50:1, Rf = 0.3). 1H NMR (500 MHz, CDCl3) δ 8.14 (d, J = 8.7 Hz, 1H), 7.39 (s, 1H), 7.27 (d, J = 7.5 Hz, 1H), 7.06 (s, 1H), 2.45 (s, 3H), 1.67 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 148.61, 135.95, 133.07, 128.52, 126.83 (q, J = 40.4 Hz), 126.65, 121.63, 120.78 (q, J = 266.1 Hz), 115.67, 113.19 (q, J = 5.1 Hz), 85.20, 27.86, 21.17. 19F NMR (470 MHz, CDCl3) δ−58.15. HRMS (ESI) caculated for C10H7NF3 [M-Boc]−, 198.0531; found: 198.0536.
Tert-Butyl 5-Methoxy-2-(Trifluoromethyl)-1H-Indole-1-Carboxylate (3c) (99 mg, 63%)
Isolated by flash column chromatography (petroleum ether/CH2Cl2 = 50:1, Rf = 0.3). 1H NMR (500 MHz, CDCl3) δ 8.17 (d, J = 9.2 Hz, 1H), 7.08 – 7.05 (m, 2H), 7.03 (d, J = 2.4 Hz, 1H), 3.86 (s, 3H), 1.66 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 156.25, 148.52, 132.44, 127.24 (q, J = 38.6 Hz), 127.17, 120.68 (q, J = 266.4 Hz), 116.98, 116.53, 113.14 (q, J = 5.1 Hz), 103.45, 85.26, 55.63, 27.86. 19F NMR (470 MHz, CDCl3) δ−58.24. HRMS (ESI) caculated for C10H7ONF3 [M-Boc]−, 214.0480; found: 214.0485.
Tert-Butyl 5-Acetyl-2-(Trifluoromethyl)-1H-Indole-1-Carboxylate (3d) (105 mg, 64%)
Isolated by flash column chromatography (petroleum ether/ ethyl acetate = 50:1, Rf = 0.3). 1H NMR (500 MHz, CDCl3) δ 8.34 (d, J = 8.9 Hz, 1H), 8.25 (d, J = 1.2 Hz, 1H), 8.06 (dd, J = 9.0, 1.7 Hz, 1H), 7.22 (s, 1H), 2.67 (s, 3H), 1.68 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 197.39, 148.14, 140.21, 132.93, 128.42 (q, J = 38.9 Hz), 126.85, 126.23, 123.23, 122.52 (q, J = 266.9 Hz), 116.06, 113.85 (q, J = 5.0 Hz), 86.31, 27.78, 26.68. 19F NMR (470 MHz, CDCl3) δ−58.38. HRMS (ESI) caculated for C11H7ONF3 [M-Boc]−, 226.0480; found: 226.0485.
1-(Tert-Butyl) 5-Ethyl 2-(Trifluoromethyl)-1H-Indole-1,5-Dicarboxylate (3e) (111 mg, 62%)
Isolated by flash column chromatography (petroleum ether/ ethyl acetate = 50:1, Rf = 0.3). 1H NMR (500 MHz, CDCl3) δ 8.36 (d, J = 0.9 Hz, 1H), 8.32 (d, J = 8.9 Hz, 1H), 8.13 (dd, J = 8.9, 1.5 Hz, 1H), 7.20 (s, 1H), 4.41 (q, J = 7.1 Hz, 2H), 1.68 (s, 9H), 1.43 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 166.43, 148.21, 140.16, 128.23 (q, J = 39.3 Hz), 127.97, 126.15, 125.99, 124.32, 120.45 (q, J = 266.5 Hz), 115.83, 113.70 (q, J = 5.0 Hz), 86.18, 61.08, 27.79, 14.36. 19F NMR (470 MHz, CDCl3) δ−58.35. HRMS (ESI) caculated for C12H9F3NO2 [M-Boc]−, 256.0585; found: 256.0591.
Tert-Butyl 5-Fluoro-2-(Trifluoromethyl)-1H-Indole-1-Carboxylate (3f) (102 mg, 67%)
Isolated by flash column chromatography (petroleum ether/CH2Cl2 = 50:1, Rf = 0.3). 1H NMR (500 MHz, CDCl3) δ 8.26 (dd, J = 9.2, 4.5 Hz, 1H), 7.28 – 7.25 (m, 1H), 7.18 (td, J = 9.2, 2.5 Hz, 1H), 7.09 (s, 1H), 1.67 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 160.30, 158.38, 148.34, 134.09, 128.30 (q, J = 39.1 Hz), 120.47 (q, J = 266.5 Hz), 117.41 (d, J = 8.9 Hz), 115.22 (d, J = 25.0 Hz), 112.84 (q, J = 4.9 Hz), 107.07 (d, J = 23.8 Hz), 85.80, 27.81. 19F NMR (470 MHz, CDCl3) δ−58.42,−119.41 (td, J = 8.5, 4.7 Hz). HRMS (ESI) caculated for C9H4NF4 [M-Boc]−, 202.0280; found: 202.0285.
Tert-Butyl 5-Chloro-2-(Trifluoromethyl)-1H-Indole-1-Carboxylate (3g) (109 mg, 68%)
Isolated by flash column chromatography (petroleum ether/CH2Cl2 = 50:1, Rf = 0.3). 1H NMR (500 MHz, CDCl3) δ 8.22 (d, J = 9.0 Hz, 1H), 7.58 (d, J = 2.0 Hz, 1H), 7.39 (dd, J = 9.0, 2.0 Hz, 1H), 7.06 (s, 1H), 1.67 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 148.22, 136.05, 129.18, 128.10 (q, J = 39.3 Hz), 127.49, 127.28, 121.36, 120.43 (q, J = 266.5 Hz), 117.28, 112.45 (q, J = 5.1 Hz), 85.98, 27.80. 19F NMR (470 MHz, CDCl3) δ−58.39. HRMS (ESI) caculated for C9H4NClF3 [M-Boc]−, 217.9984; found: 217.9990.
Tert-Butyl 5-Bromo-2-(Trifluoromethyl)-1H-Indole-1-Carboxylate (3h) (142 mg, 78%)
Isolated by flash column chromatography (petroleum ether/CH2Cl2 = 50:1, Rf = 0.3). 1H NMR (500 MHz, CDCl3) δ 8.17 (d, J = 9.0 Hz, 1H), 7.75 (d, J = 1.7 Hz, 1H), 7.53 (dd, J = 9.0, 1.9 Hz, 1H), 7.06 (s, 1H), 1.67 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 148.20, 136.41, 129.92, 128.03, 127.95 (q, J = 39.2 Hz), 124.48, 120.39 (q, J = 266.5 Hz), 117.62, 116.75, 112.32 (q, J = 5.0 Hz), 86.01, 27.80. 19F NMR (470 MHz, CDCl3) δ−58.37. HRMS (ESI) caculated for C9H4NBrF3 [M-Boc]−, 261.9479; found: 261.9485.
Tert-Butyl 4-Methyl-2-(Trifluoromethyl)-1H-Indole-1-Carboxylate (3i) (135 mg, 90%)
Isolated by flash column chromatography (petroleum ether/CH2Cl2 = 50:1, Rf = 0.3). 1H NMR (500 MHz, CDCl3) δ 8.10 (d, J = 8.5 Hz, 1H), 7.34 (t, J = 7.9 Hz, 1H), 7.18 (s, 1H), 7.09 (d, J = 7.2 Hz, 1H), 2.54 (s, 3H), 1.67 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 148.63, 137.60, 131.57, 127.10, 126.31 (q, J = 38.9 Hz), 126.22, 123.81, 120.85 (q, J = 266.3 Hz), 113.51, 111.86 (q, J = 5.1 Hz), 85.33, 27.85, 18.23. 19F NMR (470 MHz, CDCl3) δ−57.97. HRMS (ESI) caculated for C10H7NF3 [M-Boc]−, 198.0531; found: 198.0536.
Tert-Butyl 4-Acetoxy-2-(Trifluoromethyl)-1H-Indole-1-Carboxylate (3j) (127 mg, 74%)
Isolated by flash column chromatography (petroleum ether/ ethyl acetate = 100:1, Rf = 0.3). 1H NMR (500 MHz, CDCl3) δ 8.16 (d, J = 8.6 Hz, 1H), 7.43 (t, J = 8.2 Hz, 1H), 7.07 (dd, J = 7.9, 0.6 Hz, 1H), 7.05 (s, 1H), 2.40 (s, 3H), 1.67 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 168.91, 148.30, 144.04, 138.98, 127.46, 127.17 (q, J = 39.0 Hz), 120.46 (q, J = 266.4 Hz), 120.07, 115.80, 113.96, 109.74 (q, J = 5.3 Hz), 85.88, 27.80, 20.96. 19F NMR (470 MHz, CDCl3) δ−58.27. HRMS (ESI) caculated for C11H7O2NF3 [M-Boc]−, 242.0429; found: 242.0434.
1-(Tert-Butyl) 4-Methyl 2-(Trifluoromethyl)-1H-Indole-1,4-Dicarboxylate (3k) (100 mg, 58%)
Isolated by flash column chromatography (petroleum ether/ ethyl acetate = 50:1, Rf = 0.3). 1H NMR (500 MHz, CDCl3) δ 8.54 (d, J = 8.5 Hz, 1H), 8.04 (dd, J = 7.6, 0.8 Hz, 1H), 7.85 (s, 1H), 7.50 (t, J = 8.2 Hz, 1H), 4.00 (s, 3H), 1.67 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 166.68, 148.35, 138.28, 128.38 (q, J = 38.9 Hz), 126.41, 126.34, 126.14, 123.10, 120.72, 120.59 (q, J = 266.8 Hz), 113.77 (q, J = 5.4 Hz), 86.04, 52.13, 27.79. 19F NMR (470 MHz, CDCl3) δ−58.20. HRMS (ESI) caculated for C11H7O2NF3 [M-Boc]−, 242.0429; found: 242.0434.
Tert-Butyl 4-Fluoro-2-(Trifluoromethyl)-1H-Indole-1-Carboxylate (3l) (111 mg, 73%)
Isolated by flash column chromatography (petroleum ether/CH2Cl2 = 50:1, Rf = 0.3). 1H NMR (500 MHz, CDCl3) δ 8.06 (d, J = 8.6 Hz, 1H), 7.38 (td, J = 8.3, 5.6 Hz, 1H), 7.24 (s, 1H), 6.97 (t, J = 9.0, 1H), 1.67 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 157.23, 155.23, 148.34, 139.54, 127.84 (d, J = 7.5 Hz), 127.01 (q, J = 39.1 Hz), 120.42 (q, J = 266.5 Hz), 112.11 (d, J = 4.0 Hz), 108.91 (q, J = 5.4 Hz), 108.51 (d, J = 17.9 Hz), 86.02, 27.80. 19F NMR (470 MHz, CDCl3) δ−58.31,−120.93 (q, J = 9.4 Hz). HRMS (ESI) caculated for C9H4NF4 [M-Boc]−, 202.0280; found: 202.0285.
Tert-Butyl 4-Chloro-2-(Trifluoromethyl)-1H-Indole-1-Carboxylate (3m) (142 mg, 89%)
Isolated by flash column chromatography (petroleum ether/CH2Cl2 = 50:1, Rf = 0.3). 1H NMR (500 MHz, CDCl3) δ 8.19 (d, J = 8.5 Hz, 1H), 7.36 (t, J = 8.1 Hz, 1H), 7.29 (dd, J = 7.8, 0.6 Hz, 1H), 7.27 (s, 1H), 1.67 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 148.23, 138.29, 127.62, 127.46 (q, J = 35.8 Hz), 127.28, 125.45, 123.27, 120.46 (q, J = 266.3 Hz), 114.66, 111.37 (q, J = 5.3 Hz), 86.10, 27.80. 19F NMR (470 MHz, CDCl3) δ−58.30. HRMS (ESI) caculated for C9H4NClF3 [M-Boc]−, 217.9984; found: 217.9990.
Tert-Butyl 6-Fluoro-2-(Trifluoromethyl)-1H-Indole-1-Carboxylate (3n) (121 mg, 80%)
Isolated by flash column chromatography (petroleum ether/CH2Cl2 = 50:1, Rf = 0.3). 1H NMR (500 MHz, CDCl3) δ 8.02 (dd, J = 10.6, 1.8 Hz, 1H), 7.55 (dd, J = 8.5, 5.5 Hz, 1H), 7.10 (s, 1H), 7.06 (td, J = 8.7, 1.7 Hz, 1H), 1.67 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 163.29, 161.36, 148.33, 122.93 (d, J = 10.1 Hz), 122.74, 120.51 (q, J = 266.1 Hz), 113.15 (q, J = 4.9 Hz), 112.38 (d, J = 24.6 Hz), 103.36 (d, J = 29.1 Hz), 102.90 (q, J = 28.5 Hz), 85.92, 27.81. 19F NMR (470 MHz, CDCl3) δ−58.26,−112.94 (td, J = 9.6, 5.6 Hz). HRMS (ESI) caculated for C9H4NF4 [M-Boc]−, 202.0280; found: 202.0285.
Tert-Butyl 6-Chloro-2-(Trifluoromethyl)-1H-Indole-1-Carboxylate (3o) (113 mg, 71%)
Isolated by flash column chromatography (petroleum ether/CH2Cl2 = 50:1, Rf = 0.3). 1H NMR (500 MHz, CDCl3) δ 8.35 (s, 1H), 7.53 (d, J = 8.4 Hz, 1H), 7.30 – 7.26 (m, 1H), 7.10 (s, 1H), 1.67 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 148.22, 138.03, 133.12, 127.48 (q, J = 39.1 Hz), 124.87, 124.37, 122.72, 120.48 (q, J = 266.5 Hz), 116.37, 113.03 (q, J = 5.0 Hz), 86.07, 27.80. 19F NMR (470 MHz, CDCl3) δ−58.30. HRMS (ESI) caculated for C9H4NClF3 [M-Boc]−, 217.9984; found: 217.9990.
Tert-Butyl 2-(Trifluoromethyl)-1H-Pyrrole-1-Carboxylate (4a) (Nagib and MacMillan, 2011; Du et al., 2017) (96 mg, 82%)
Isolated by flash column chromatography (petroleum ether/CH2Cl2 = 50:1, Rf = 0.3). 1H NMR (500 MHz, CDCl3) δ 7.44 (d, J = 1.9 Hz, 1H), 6.73 (s, 1H), 6.19 (t, J = 3.2 Hz, 1H), 1.61 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 125.78, 120.53 (q, J = 264.9 Hz), 117.76 (q, J = 4.6 Hz), 109.59, 85.62, 27.71. 19F NMR (470 MHz, CDCl3) δ−58.33. HRMS (ESI) caculated for C5H3NF3 [M-Boc]−, 134.0218; found: 134.0223.
Tert-Butyl 2-Methyl-5-(Trifluoromethyl)-1H-Pyrrole-1-Carboxylate (4b)
Isolated by flash column chromatography (petroleum ether/CH2Cl2 = 50:1, Rf = 0.3). 1H NMR (500 MHz, CDCl3) δ 6.59 (d, J = 3.5 Hz, 1H), 5.92 (d, J = 3.2 Hz, 1H), 2.44 (s, 3H), 1.60 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 148.43, 137.08, 121.58 (q, J = 39.3 Hz), 120.77 (q, J = 264.5 Hz), 116.00 (q, J = 4.8 Hz), 109.96, 85.39, 31.60, 27.63. 19F NMR (470 MHz, CDCl3) δ−57.19. HRMS (ESI) caculated for C6H5NF3 [M-Boc]−, 148.0374; found: 148.0380.
Tert-Butyl 3-Formyl-2-(Trifluoromethyl)-1H-Pyrrole-1-Carboxylate (4c)
Isolated by flash column chromatography (petroleum ether/ ethyl acetate = 50:1, Rf = 0.3). 1H NMR (500 MHz, cdcl3) δ 10.18 (s, 1H), 7.43 (d, J = 3.3 Hz, 1H), 6.72 (d, J = 3.4 Hz, 1H), 1.63 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 185.88 (q, J = 5.6 Hz), 171.10, 146.75, 125.86, 123.69 (q, J = 41.4 Hz), 120.46 (q, J = 267.8 Hz), 109.08, 87.39, 27.57. 19F NMR (470 MHz, CDCl3) δ−54.31. HRMS (ESI) caculated for C6H3ONF3 [M-Boc]−, 162.0167; found: 162.0172.
Tert-Butyl 4-Methyl-2-(Trifluoromethyl)-1H-Pyrrole-1-Carboxylate (4d) (83 mg, 67%)
Isolated by flash column chromatography (petroleum ether/CH2Cl2 = 50:1, Rf = 0.3). 1H NMR (500 MHz, CDCl3) δ 7.32 (s, 1H), 6.01 (s, 1H), 2.21 (s, 3H), 1.59 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 147.67, 128.96 (q, J = 2.6 Hz), 124.61, 121.68 (q, J = 266.0 Hz), 119.54 (q, J = 4.4 Hz), 117.06 (q, J = 38.3 Hz), 113.52, 85.09, 27.69. 19F NMR (376 MHz, CDCl3) δ−54.63. HRMS (ESI) caculated for C6H5NF3 [M-Boc]−, 148.0374; found: 148.0380.
2-(Trifluoromethyl)Benzofuran (4e) (Liu and Shen, 2011) (82 mg, 88%)
Isolated by flash column chromatography (petroleum ether, Rf = 0.3). 1H NMR (500 MHz, CDCl3) δ 7.67 (d, J = 7.8 Hz, 1H), 7.58 (d, J = 8.0 Hz, 1H), 7.45 (t, J = 7.4 Hz, 1H), 7.34 (t, J = 7.2 Hz, 1H), 7.18 (s, 1H). 13C NMR (126 MHz, CDCl3) δ 155.13, 143.48 (q, J = 41.9 Hz), 126.90, 125.99, 123.95, 122.46, 119.31 (q, J = 266.5 Hz), 112.09, 108.09 (q, J = 3.1 Hz). 19F NMR (470 MHz, CDCl3) δ -64.87.
N-(4-Methyl-2-(Trifluoromethyl)Phenyl)Acetamide (4g) (Zou et al., 2019) (28 mg, 26%)
Isolated by flash column chromatography (petroleum ether/ ethyl acetate = 5:1, Rf = 0.3). 1H NMR (500 MHz, CDCl3) δ 7.95 (d, J = 8.1 Hz, 1H), 7.40 (s, 1H), 7.34 (d, J = 7.8 Hz, 2H), 2.36 (s, 3H), 2.19 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 168.48, 134.75, 133.28, 132.45, 126.33 (q, J = 4.9 Hz), 125.20, 124.00 (q, J = 271.5 Hz), 120.65 (q, J = 29.4 Hz), 24.43, 20.79. 19F NMR (470 MHz, CDCl3) δ−60.67. HRMS (ESI) caculated for C10H11ONF3 [M+H]+, 218.0793; found: 218.0787.
N-(4-Methyl-3-(Trifluoromethyl)Phenyl)Acetamide (4g) (41 mg, 38%)
Isolated by flash column chromatography (petroleum ether/ ethyl acetate = 5:1, Rf = 0.3). 1H NMR (500 MHz, CDCl3) δ 7.64 (d, J = 10.7 Hz, 2H), 7.57 (s, 1H), 7.21 (d, J = 8.1 Hz, 1H), 2.42 (s, 3H), 2.17 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 168.60, 135.70, 132.48, 132.26, 129.18 (q, J = 29.9 Hz), 124.13 (q, J = 272.4 Hz), 123.00, 117.40 (q, J = 5.9 Hz), 24.41, 18.70. 19F NMR (470 MHz, CDCl3) δ−61.97. HRMS (ESI) caculated for C10H11ONF3 [M+H]+, 218.0793; found: 218.0787.
Data Availability
All datasets generated for this study are included in the manuscript/Supplementary Files.
Author Contributions
XS, XianL, and DS constructed the workflow. XS synthesized and purified the compounds. XS and XiaoL performed the mass spectrometric analysis. XS completed the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by the Funded by National Natural Science Foundation of China (No. 81773586, 81703354), and Key research and development project of Shandong province (2016GSF201193, 2016ZDJS07A13, 2016GSF115002, 2016GSF115009), and Key Research Program of Frontier Sciences, CAS (QYZDB-SSW-DQC014), and the Project of Discovery, Evaluation and Transformation of Active Natural Compounds, Strategic Biological Resources Service Network Program of Chinese Academy of Sciences (ZSTH-026), and Shandong Provincial Natural Science Foundation for Distinguished Young Scholars (JQ201722), and National Program for Support of Top-notch Young Professionals, and Taishan scholar Youth Project of Shandong province.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2019.00613/full#supplementary-material
References
- Arimori S., Shibata N. (2015). Catalytic trifluoromethylation of aryl- and vinylboronic acids by 2-cyclopropyl-1-(trifluoromethyl)benzo[b]thiophenium triflate. Org. Lett. 17, 1632–1635. 10.1021/acs.orglett.5b00164 [DOI] [PubMed] [Google Scholar]
- Boechat N., Bastos M. M. (2010). Trifluoromethylation of carbonyl compounds. Curr. Org. Synth. 7, 403–413. 10.2174/157017910792246081 [DOI] [Google Scholar]
- Chripkova M., Zigo F., Mojzis J. (2016). Antiproliferative effect of indole phytoalexins. Molecules 21, 1626–1640. 10.3390/molecules21121626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Pearson R. M., Lim C.-H., Sartor S. M., Ryan M. D., Yang H., et al. (2017). Strongly reducing, visible-lightorganic photoredox catalysts as sustainable alternatives to precious metals. Chem. Eur. J. 23, 10962–10968. 10.1002/chem.201702926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya T., Kamlet A. S., Ritter T. (2011). Catalysis for fluorination and trifluoromethylation. Nature 473, 470–477. 10.1038/nature10108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouverneur V., Seppelt K. (2015). Introduction: fluorine chemistry. Chem. Rev. 115, 563–565. 10.1021/cr500686k [DOI] [PubMed] [Google Scholar]
- Goyal D., Kaur A., Goyal B. (2018). Benzofuran and indole: promising scaffolds for drug development in Alzheimer's disease. Chem. Med. Chem. 13, 1275–1299. 10.1002/cmdc.201800156 [DOI] [PubMed] [Google Scholar]
- Hagmann W. K. (2008). The many roles for fluorine in medicinal chemistry. J. Med. Chem. 51, 4359–4369. 10.1021/jm800219f [DOI] [PubMed] [Google Scholar]
- Iqbal N., Choi S., Ko E., Cho E. J. (2012). Trifluoromethylation of heterocycles via visible light photoredox catalysis. Tetrahedron Lett. 53, 2005–2008. 10.1016/j.tetlet.2012.02.032 [DOI] [Google Scholar]
- Ji Y., Brueckl T., Baxter R. D., Fujiwara Y., Seiple I. B., Su S., et al. (2011). Innate C-H trifluoromethylation of heterocycles. Proc. Natl Acad. Sci. U. S.A. 108, 14411–14415. 10.1073/pnas.1109059108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H., Singh J., Narasimhan B. (2019). Indole hybridized diazenyl derivatives: synthesis, antimicrobial activity, cytotoxicity evaluation and docking studies. BMC Chem. 13, 65–82. 10.1186/s13065-019-0580-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan R., Kumar M., Kant R., Koley D. (2019). Cu-catalyzed directed C7–H imidation of indolines via crossdehydrogenative coupling. Adv. Synth. Catal. 369, 3108–3113. 10.1002/adsc.201900166 [DOI] [Google Scholar]
- Langlois B. R., Laurent E., Roidot N. (1991). Trifluoromethylation of aromatic compounds with sodium trifluoromethanesulfinate under oxidative conditions. Tetrahedron Lett. 32, 7525–7528. 10.1016/0040-4039(91)80524-A [DOI] [Google Scholar]
- Larbi K. S., Djebbar S., Soul,é J.-F., Doucet H. (2017). Reactivity of benzofuran and benzothiophene in palladium-catalysed direct C2, C3-diarylations. J. Organomet. Chem. 843, 32–39. 10.1016/j.jorganchem.2017.05.029 [DOI] [Google Scholar]
- Le C., Chen T. Q., Liang T., Zhang P., MacMillan D. W. C. (2018). A radical approach to the copper oxidative addition problem: trifluoromethylation of bromoarenes. Science 360, 1010–1014. 10.1126/science.aat4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.-H., Wood T. K., Lee J. (2015). Roles of indole as an interspecies and interkingdom signaling molecule. Trends Microbiol. 23, 707–718. 10.1016/j.tim.2015.08.001 [DOI] [PubMed] [Google Scholar]
- Liu H., Gu Z., Jiang X. (2013). Direct trifluoromethylation of the C-H bond. Adv. Synth. Catal. 355, 617–626. 10.1002/adsc.201200764 [DOI] [Google Scholar]
- Liu T., Shen Q. (2011). Copper-catalyzed trifluoromethylation of aryl and vinyl boronic acids with an electrophilic trifluoromethylating reagent. Org. Lett. 13, 2342–2345. 10.1021/ol2005903 [DOI] [PubMed] [Google Scholar]
- Nagib D. A., MacMillan D. W. (2011). Trifluoromethylation of arenes and heteroarenes by means of photoredox catalysis. Nature 480, 224–228. 10.1038/nature10647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie J., Guo H.-C., Cahard D., Ma J.-A. (2011). Asymmetric construction of stereogenic carbon centers featuring a trifluoromethyl group from prochiral trifluoromethylated substrates. Chem. Rev. 111, 455–529. 10.1021/cr100166a [DOI] [PubMed] [Google Scholar]
- Nishino M., Hirano K., Satoh T., Miura M. (2012). Copper-mediated and copper-catalyzed cross-coupling of indoles and 1,3-azoles: double C-H activation. Angew. Chem. Int. Ed. 51, 6993–6997. 10.1002/anie.201201491 [DOI] [PubMed] [Google Scholar]
- Sandtorv A. H. (2015). Transition metal-catalyzed C-H activation of indoles. Adv. Synth. Catal. 357, 2403–2435. 10.1002/adsc.201500374 [DOI] [Google Scholar]
- Sato K., Tarui A., Omote M., Ando A., Kumadaki I. (2010). Trifluoromethylation of organic compounds and related reactions. Synthesis 11, 1865–1882. 10.1055/s-0029-1218745 [DOI] [Google Scholar]
- Schlosser M. (2006). CF3-bearing aromatic and heterocyclic building blocks. Angew. Chem. Int. Ed. 45, 5432–5446. 10.1002/anie.200600449 [DOI] [PubMed] [Google Scholar]
- Shi X., Li X., Ma L., Shi D. (2018). Cu(II)-catalyzed oxidative trifluoromethylation of indoles with KF as the base. Catalysts 9, 278–291. 10.3390/catal9030278 [DOI] [Google Scholar]
- Shimizu M., Hiyama T. (2005). Modern synthetic methods for fluorine-substituted target molecules. Angew. Chem. Int. Ed. 44, 214–231. 10.1002/anie.200460441 [DOI] [PubMed] [Google Scholar]
- Shimizu R., Egami H., Nagi T., Chae J., Hamashima Y., Sodeoka M. (2010). Direct C2-trifluoromethylation of indole derivatives catalyzed by copper acetate. Tetrahedron Lett. 51, 5947–5949. 10.1016/j.tetlet.2010.09.027 [DOI] [Google Scholar]
- Soni V., Sharma D. M., Punji B. (2018). Nickel-catalyzed regioselective C(2)-H difluoroalkylation of indoles with difluoroalkyl bromides. Chem. Asian. J. 13, 2516–2522. 10.1002/asia.201800504 [DOI] [PubMed] [Google Scholar]
- Sravanthi T. V., Manju S. L. (2016). Indoles — a promising scaffold for drug development. Eur. J. Pharm. Sci. 91, 1–10. 10.1016/j.ejps.2016.05.025 [DOI] [PubMed] [Google Scholar]
- Tomashenko O. A., Grushin V. V. (2011). aromatic trifluoromethylation with metal complexes. Chem. Rev. 111, 4475–4521. 10.1021/cr1004293 [DOI] [PubMed] [Google Scholar]
- Wang J., Sánchez-Rosell,ó M., Aceña J. L., Pozo C., Sorochinsky A. E., Fustero S., et al. (2014). Fluorine in pharmaceutical industry: fluorine-containing drugs introduced to the market in the last decade (2001–2011). Chem. Rev. 114, 2432–2506. 10.1021/cr4002879 [DOI] [PubMed] [Google Scholar]
- Wang Y., Yang Y., Jie K., Huang L., Guo S., Cai H. (2018). Copper-catalyzed C2 and C3 phosphonation of benzofuran and benzothiophene with trialkyl phosphites. ChemCatChem. 10, 716–717. 10.1002/cctc.201701361 [DOI] [Google Scholar]
- Xu J., Luo D.-F., Xiao B., Liu Z.-J., Gong T.-J., Fu Y., et al. (2011). Copper-catalyzed trifluoromethylation of aryl boronic acids using a reagent. Chem. Commun. 47, 4300–4302. 10.1039/c1cc10359h [DOI] [PubMed] [Google Scholar]
- Yan S.-Y., Zhang Z.-Z., Shi B.-F. (2017). Nickel-catalyzed direct C–H trifluoroethylation of heteroarenes with trifluoroethyl iodide. Chem. Commun. 53, 10287–10290. 10.1039/c7cc05532c [DOI] [PubMed] [Google Scholar]
- Yang Y., Qiu X., Zhao Y., Mu Y., Shi Z. (2016). Palladium-catalyzed C–H arylation of indoles at the C7 position. J. Am. Chem. Soc. 138, 495–498. 10.1021/jacs.5b11569 [DOI] [PubMed] [Google Scholar]
- Yu D.-G., Gensch T., Azambuja F., Vásquez-Céspedes S., Glorius F. (2014). Co(III)-Catalyzed C–H activation/formal SN-type reactions: selective and efficient cyanation, halogenation, and allylation. J. Am. Chem. Soc. 136, 17722–17725. 10.1021/ja511011m [DOI] [PubMed] [Google Scholar]
- Zhang H., Wang H.-L., Luo Y., Chen C., Cao Y., Chen P., et al. (2018). Regioselective palladium-catalyzed C-H Bond trifluoroethylation of indoles: exploration and mechanistic insight. ACS Catal. 8, 2173–2180. 10.1021/acscatal.7b03220 [DOI] [Google Scholar]
- Zhou B., Yang Y., Lin S., Li Y. (2013). Rhodium-catalyzed direct addition of indoles to N-sulfonylaldimines. Adv. Synth. Catal. 355, 360–364. 10.1002/adsc.201200909 [DOI] [Google Scholar]
- Zou L., Li P., Wang B., Wang L. (2019). Visible-light-induced Pd-catalyzed ortho-trifluoromethylation of acetanilides with CF3SO2Na under ambient conditions in the absence of an external photocatalyst. Chem. Commun. 55, 3737–3740. 10.1039/c9cc01014a [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated for this study are included in the manuscript/Supplementary Files.