Abstract
INTRODUCTION: An increase in detection of early-stage asymptomatic lung tumors could increase the overall survival rate of lung cancer patients. A new approach to cancer (pre-)screening focusses on detecting field cancerization instead of the tumor itself. The objective of this study was to investigate the use of optical spectroscopy to detect field cancerization in the buccal mucosa of lung cancer patients. METHODS: Optical buccal mucosa measurements were performed in lung cancer patients and controls using multidiameter single-fiber reflectance spectroscopy. We analyzed whether the measured optical parameters could distinguish lung cancer patients from controls. RESULTS: Twenty-three lung cancer patients, 24 chronic obstructive pulmonary disease (COPD) control patients, and 36 non-COPD controls were included. The majority of tumors were non-small-cell lung carcinomas (96%) and classified as stage I (48%). The tissue scattering properties μs' and γ at 800 nm and the tissue bilirubin concentration were all near-significantly different (P = .072, 0.058, and 0.060, respectively) between the lung cancer and COPD group. μs' at 800 nm had a sensitivity of 74% and a specificity of 63%. The microvascular blood oxygen saturation of the lung cancer patients was also higher than the COPD patients (78% vs. 62%, P = .002), this is probably a consequence of the systemic effect of COPD. CONCLUSIONS: We have demonstrated that μs' at 800 nm is increased in the buccal mucosa of patients with lung cancer compared to controls with COPD. This might be an indication of field cancerization in the oral cavity of patients with lung cancer.
Introduction
Lung cancer is a major public health problem because of its high incidence and high mortality. It is the worldwide leading cause of cancer related death. [1] This high mortality is partly caused by the fact that early-stage lung cancer often causes no clinical symptoms. [2] As a result lung cancer is commonly diagnosed in more advanced stages of development with regional and distant metastases. [1] Patients with early-stage lung tumors can benefit from complete surgical resection or curative radiotherapy, whereas treatment of patients with high-stage tumors is often not curative. [2] This results in a substantially higher 5-year survival of 52% for patients with early-stage tumors than the 5-year survival of 15% of the total lung cancer population. [1]
Early detection by screening asymptomatic high-risk patients holds the potential to substantially increase the survival rate of lung cancer patients. At present, most scientific research has focused on low-dose computed tomography (LDCT). [3] The largest randomized controlled trial on the effectiveness of LDCT-screening for lung cancer showed a 20% lung cancer mortality reduction compared to using chest radiography. [4] The awaited mortality outcome results of the Dutch–Belgian randomized lung cancer screen trial (NELSON trial) are thought to replicate this reduction. [5] A recent systematic review recommended to LDCT-screen adults between 55 and 74 years who are at high risk for lung cancer. [6] However, they also warn of the potential harm of screening: false-positive results, adverse effects of invasive follow-up testing, and overdiagnosis.
A novel strategy for lung cancer (pre-)screening is focused on field cancerization (FC). The goal of this type of screening is not to detect the tumor itself but instead detect local tissue changes caused by FC. These superficial tissue changes are caused by accumulating exposure to carcinogens and include alterations in the microvasculature and the tissue nanoscale architecture, such as the organization of the cytoskeleton and the size and structure of cell nuclei and organelles. [7], [8] An alternative theory states that multiple fields arise due to the migration of dysplastic and altered cells. Either by migration of malignant cells through the saliva (micro metastasis) or intra-epithelial migration of the progeny of initially transformed malignant cells. [9], [10] The FC of lung cancer is assumed to consist of the entire upper airway including the main bronchi, trachea and even the, easily accessible, oral cavity. [11] Most research on FC lung cancer screening is done on airway tissue gene expression. [3] However, this is an expensive and time consuming method. Optical reflectance spectroscopy has been proposed as a fast and easy-to-use alternative technique to detect FC and possibly use for cancer screening. [7]
The approach to pre-screen for lung cancer has been investigated by Roy et al. by optical measurements of the buccal mucosa. [12], [13] In a first ex vivo study they showed the proof of concept that buccal optical spectroscopy may potentially work as a pre-screening tool for lung cancer. [12] In a second in vivo study an optical fiber was used to interrogate the buccal mucosa with the aim to detect FC changes. [13] Their optical biomarker was able to predict the presence of lung cancer with a sensitivity of 79% and a specificity of 83%. However, 50% of the control patients in the validation set were non-smokers, which may have influenced the study outcome.
Our research group has developed a novel optical technique: multidiameter single-fiber reflectance (MDSFR) spectroscopy. [14] It enables fast, non-invasive measurements of how much light has been absorbed and scattered in tissue. Spectral deconvolution of the tissue absorption coefficient yields measurements of four physiological parameters. Repeated measurements with different diameters enables the quantification of two scattering parameters: the reduced scattering coefficient μs' and the phase function parameter γ. These scattering parameters are closely related to the nanoscale architecture of tissue and thus to FC changes. In two previous studies, our group used MDSFR spectroscopy to detect FC in the buccal mucosa of patients with esophageal and laryngeal cancer. [15], [16] These results show the promise of the use of MDSFR spectroscopy as a cancer pre-screening tool. In laryngeal cancer patients, the blood oxygen saturation and blood volume fraction were lowered in the buccal mucosa of the oncologic patients. [16] The combined parameter α, encompassing StO2 and BVF, was able to predict the presence of a laryngeal tumor with a sensitivity of 78% and a specificity of 74%. In esophageal cancer patients the μs' at 450 and 800 nm was increased in the buccal mucosa of the oncologic group, indicating changes in the nanoscale architecture possibly related to FC. [15]
The present study reports the first attempt to use MDSFR spectroscopy in the buccal mucosa of lung cancer patients to investigate if FC changes can be detected. This was accomplished by comparing the buccal mucosa optical properties of patients with and without lung cancer. We hypothesize that the values of the optical parameters will be different between these groups. This could indicate the presence of FC and thus a, distant, lung tumor. If proven feasible, this technique might be used as a pre-screening tool for a high-risk population and possible reduce lung cancer mortality by diagnosing more early-stage tumors.
Materials and Methods
Subjects and Examination Procedure
This prospective study was approved by the Medical Ethics Committees of the Erasmus MC Cancer Institute (MEC-2015-256) and the Franciscus Gasthuis & Vlietland. Patients were recruited from the outpatient clinic of the Pulmonology department of the Franciscus Gasthuis & Vlietland and the outpatient clinic of the Thoracic Surgery department of the Erasmus MC Cancer Institute between November 2016 and February 2018. Clinical parameters such as: sex, age, medical history, smoking (never/past/current and pack-years) and TNM-stage of tumor were collected using the electronic medical record (CSC-iSOFT, Virginia, USA). The oncologic group of patients consisted of patients with primary and untreated lung cancer. Patients with non-small cell lung cancer (NSCLC) and patients with small cell lung cancer (SCLC), tumor stages (I-IV), were included. The lung tumors were confirmed by imaging techniques or histopathology. The two non-oncologic control groups consisted of patients with chronic obstructive pulmonary disease (COPD) and non-COPD smoking patients with a variety of other non-oncologic diseases (e.g., chronic rhinosinusitis, cholesteatoma, gastro-esophageal reflux, dysphagia, and abdominal pain). The absence of an occult, unexpected malignancy in the lungs was confirmed by CT imaging <1 year prior to inclusion in the study in the COPD group. Patients with a medical history of head and neck or esophageal cancer were excluded from this study. Informed consent forms were signed before inclusion in this study by all patients.
The multi diameter single fiber reflectance in vivo measurements of the buccal mucosa were performed at the outpatient clinic (Figure 1). A single investigator (OB) performed all measurements. After disinfecting the fiber bundle with Tristel Trio (Tristel Solutions Ltd., Snailwell, UK), the probe tip was gently placed in contact with the buccal mucosa. Five consecutive MDSFR measurements were performed with the probe-tip on the same place on the mucosa. In total, the measurements take approximately 40 seconds.
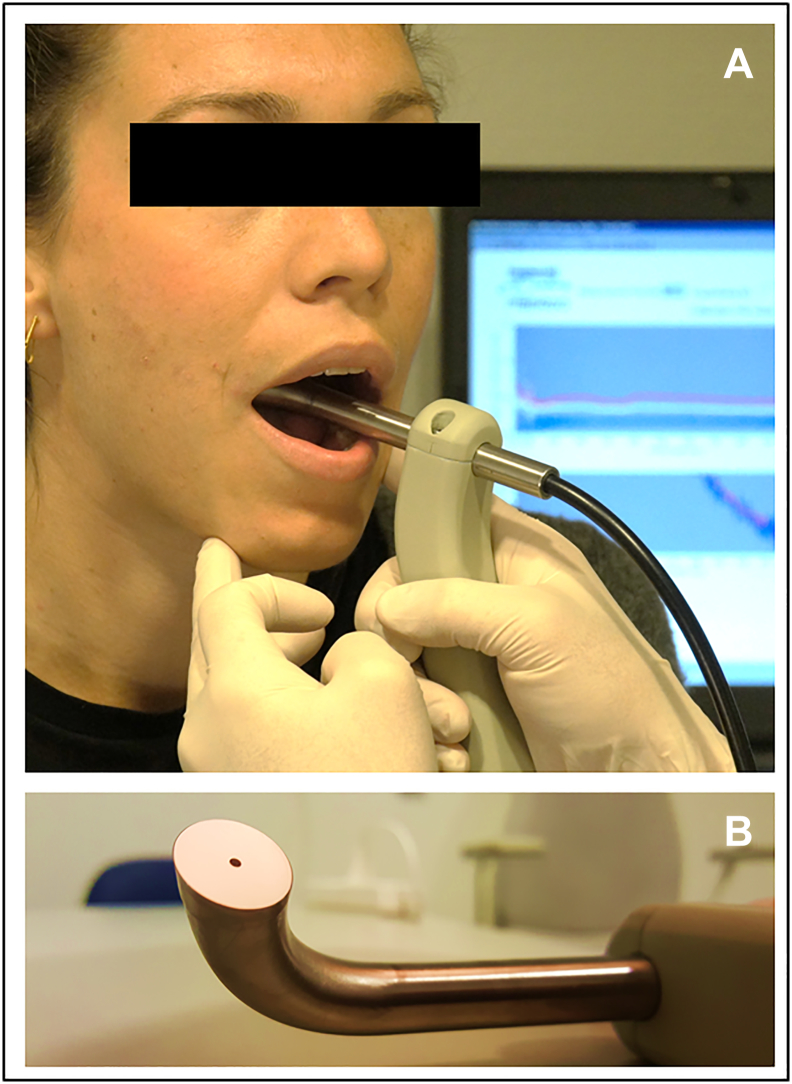
Figure 1.
Multidiameter single-fiber reflectance spectroscopy probe on the buccal mucosa. (A) Overview picture with in the background spectra on laptop. (B) Detail of probe tip angled at 15 degrees.
Study Samples
Eighty-three patients were included in this study: 23 patients with lung cancer, 24 control patients with COPD, and 36 non-COPD control patients (Table 1). The percentage of males was 56.5% in the lung cancer group, 41.7% in the COPD group (P = .308), and 75.0% in the non-COPD control group (P = .138). The median age of the patients was 69.1 (IQR 64.3–73.7) years in the lung cancer group. This was higher than the COPD control group (62.0 [IQR 51.8–65.6], P = .001) and the non-COPD control group (65.0 [IQR 58.3–69.1], P = .042). There was no significant difference in smoking status or smoking pack years between the lung cancer and both control groups.
Table 1.
Baseline Characteristics of Lung Cancer, COPD Control and Non-COPD Control Patients
| Lung Cancer | COPD Control | P-value | Non-COPD control | P-value | |
|---|---|---|---|---|---|
| N | 23 | 24 | 36 | ||
| Male sex, n (%) | 13 (56.5) | 10 (41.7) | .308 | 27 (75.0) | .138 |
| Age, median (IQR) | 69.1 (64.3–73.68) | 62.0 (51.8–65.6) | .001∗ | 65.0 (58.3–69.1) | .042∗ |
| Smoking, n (%) | .555 | .266 | |||
| Never | 1 (4.3) | 0 (0.0) | 0 (0.0) | ||
| Past | 13 (56.5) | 13 (54.2) | 26 (72.2) | ||
| Current | 9 (39.1) | 11 (45.8) | 10 (27.8) | ||
| Smoking PY, median (IQR) | 36.0 (20.0–50.0) | 37.5 (25.5–45.8) | .594 | 30.0 (15.0–49.0) | .539 |
PY = pack years. P-values calculated with chi-square test (sex and smoking status) and Mann–Whitney U test (age and smoking pack years). ∗P < .005.
Table 2 shows the tumor stage and type of the lung cancer group. Most tumors were stage I (12, 52.2%). Four tumors were stage II (17.4%), 4 tumors were stage III (17.4%) and 3 tumors were stage IV (13.1%). The majority of tumors were NSCLC of which 12 were squamous cell carcinomas, 8 were adenocarcinomas and 2 were undifferentiated large cell carcinoma. One patient (4.3%) had a SCLC.
Table 2.
Tumor stage and type of lung cancer group
| Tumor stage, n (%) | |
|---|---|
| I | 12 (52.2) |
| II | 4 (17.4) |
| III | 4 (17.4) |
| IV | 3 (13.0) |
| Tumor type, n (%) | |
| NSCLC | 22 (95.7) |
| - Squamous cell carcinoma | - 12 (52.2) |
| - Adenocarcinoma | - 8 (34.8) |
| - Unknown | - 2 (8.7) |
| SCLC | 1 (4.3) |
NSCLC = non-small cell lung cancer; SCLC = small cell lung cancer.
Multidiameter Single-Fiber Reflectance Device
The buccal mucosa in vivo measurements were performed with a custom made MDSFR spectroscopy device, which was described in detail in a previous paper. [14] In summary, MDSFR spectroscopy uses a bundle of 19 fibers for both light delivery and collection. Each fiber of 200 μm in the bundle is trifurcated at the proximal end to enable light delivery from a halogen lamp, light delivery from a 365 nm and 405 nm LED, and light collection to the spectrometer. The fibers are bundled into three concentric groups of 1, 6 and 12 fibers. They are polished at an angle of 15 degrees at the fiber tip to avoid collection of specular reflection. The last 10 cm of the fiber bundle towards the fiber tip is encased in a 12 mm diameter curved metal housing, for optimal application on buccal mucosa (Figure 1). Three computer-controlled shutters and a series of fiber-optic interconnects enable illumination and spectroscopic detection of independent fiber groups. This allows single fiber reflectance (SFR) measurement of 200, 600 and 1000 μm without moving the probe. The sampling diameter (over which parameters are averaged) is 1000 μm. The sampling depth is of the order of 500 μm (half the maximum fiber diameter). The MDSFR spectroscopy device is easily portable and has been approved to be used in the clinic. A detailed description of the system calibration and validation has been described previously. [14]
The maximal sampling depth of MDSFR spectroscopy is approximately 500 μm. For the buccal mucosa this seems to be well matched for the superficial occurrence of FC. The epithelial layer of the buccal mucosa is 250–350 μm thick and the, vascularized, lamina propria is 300–350 μm thick. [17], [18]
Spectral Analysis
The complete analysis of spectra is described in detail in a previous paper by our group. [19] First, the tissue absorption properties were calculated using the individual SFR spectra of the 200, 600, and 1000 μm fibers. Next, the tissue scattering properties μs' (mm−1) and γ (−), that are influenced by the angular scattering probability (phase function), were determined by combining the absorption-corrected spectra of multiple fiber diameters. Finally, four physiological parameters were extracted from the 1000 μm SFR fit: microvascular blood oxygen saturation (StO2 [%]), blood volume fraction (BVF [%]), mean vessel diameter (VD [mm]) and tissue bilirubin concentration ([BIL]tis [μmol/L]).
Statistical Analysis
The optical parameters were calculated by averaging the five buccal mucosa measurements taken per patient. Ten parameters were analyzed: StO2, BVF, VD, [BIL]tis, μs' at 450 and 800 nm, γ at 450 and 800 nm, and average γ. Our sample size was calculated based on a study that tried to differentiate lung cancer patients from controls with ex vivo optical measurements of buccal mucosa cells. The difference in mean of their optical parameter was 2.3 with a standard deviation of 1.0. It was hypothesized that standard deviation of our measurements would be higher (2.1) because they were performed in a heterogeneous in vivo environment. The number of patients required in each group would therefore be ≥23 (power = 0.8 and alpha = 0.01). Continuous data were reported as median value and interquartile range (IQR) (non-normally distributed data and n < 30 per group) and differences between two groups were analyzed using a binary logistic regression, with age at measurement as a covariate. Categorical data were reported as counts and percentages, and differences between groups were analyzed using the chi-squared test or the Fisher's exact test when appropriate. Binary logistic regression (with age as covariate) was used to investigate if the outcome parameters were significantly different between the two groups. The sensitivity and specificity of optical parameters to predict patients with lung cancer were calculated using an ROC-curve. There were no missing data. Statistical analysis was performed using SPSS version 21 (IBM Co., Armonk, NY, USA) and the cut off point for significance was P < .05.
Results
Table 3 presents the results of the buccal mucosa in vivo measurements of the lung cancer patients and the two control groups. The StO2 in the buccal mucosa of the lung cancer patients was 77.5% (IQR 70.8–82.1), which was significantly higher than in the COPD control group (62.3% [IQR 57.6–68.3], P = .002). The [BIL]tis, μs' at 800 nm, and γ at 800 nm parameters were all near-significantly different between the lung cancer and COPD control group. μs' at 800 nm was also significantly lower in the non-COPD control group (1.00 [IQR 0.93–1.05] vs. 1.04 [IQR 0.98–1.13], P = .015). On the other hand, the BVF was higher in the non-COPD control group (3.25 [IQR 2.73–3.61]) than in the lung cancer group (2.50 [IQR 1.70–3.30]).
Table 3.
Optical properties of buccal mucosa measurements
| Parameter | Lung cancer | COPD control | P-value | Non-COPD control | P-value |
|---|---|---|---|---|---|
| (Median [IQR]) | (n = 23) | (n = 24) | (n = 36) | ||
| StO2 (%) | 77.5 (70.8–82.1) | 62.3 (57.6–68.3) | .002∗ | 75.9 (70.3–81.3) | .553 |
| BVF (%) | 2.50 (1.70–3.30) | 2.30 (1.73–3.68) | .701 | 3.25 (2.73–3.61) | .043∗ |
| VD (mm) | 0.04 (0.03–0.06) | 0.05 (0.04–0.08) | .651 | 0.05 (0.04–0.06) | .579 |
| [BIL]tis (μmol/L) | 7.30 (4.89–9.88) | 9.59 (6.69–11.6) | .060† | 6.70 (4.70–8.39) | .523 |
| μs' at 800 nm (mm−1) | 1.04 (0.98–1.13) | 0.96 (0.90–1.04) | .072† | 1.00 (0.93–1.05) | .015∗ |
| μs' at 450 nm (mm−1) | 1.82 (1.61–2.10) | 1.84 (1.65–2.11) | .968 | 1.84 (1.69–2.06) | .790 |
| γ at 800 nm (−) | 1.64 (1.60–1.74) | 1.63 (1.57–1.68) | .058† | 1.65 (1.60–1.71) | .784 |
| γ at 450 nm (−) | 1.65 (1.59–1.72) | 1.68 (1.56–1.74) | .585 | 1.68 (1.61–1.73) | .064† |
| γ average (−) | 1.65 (1.60–1.75) | 1.66 (1.60–1.72) | .157 | 1.67 (1.62–1.74) | .880 |
IQR = interquartile range, StO2 = blood oxygen saturation, BVF = blood volume fraction, VD = vessel diameter, [BIL]tis = tissue bilirubin concentration, μs' = reduced scattering coefficient, γ = phase function parameter. P values were calculated with binary logistic regression, with age at measurement as a covariate. ∗P < .05, †P < .10.
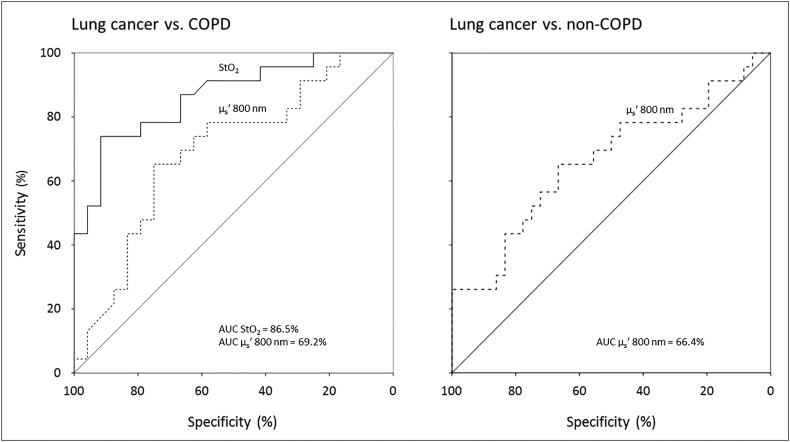
Figure 2 shows the discriminative power of two buccal mucosa parameters, StO2 and μs' at 800 nm, that differentiate the best between lung cancer patient and controls. Compared to the COPD group, StO2 could predict the presence of a lung tumor with a sensitivity of 78.3%, a specificity of 79.2% and an area under the curve (AUC) of 86.5% (95% CI 76.1–96.9). μs' at 800 nm had a lower discriminative power between the same two groups with a sensitivity of 73.9%, a specificity of 62.5% and an AUC of 69.2% (95% CI 53.9–84.6). The results of μs' at 800 nm compared to the non-COPD group were similar with a sensitivity of 65.2%, a specificity of 67.7% and an AUC of 66.4% (95% CI 51.6–81.2). Combining multiple parameters did not result in a higher discriminative power.
Figure 2.
ROC curves of blood oxygen saturation (solid line) and μs' at 800 nm (dashed lines) for predicting the presence of cancer between the lung cancer and the two control groups. AUC = area under the curve.
Discussion
In this study, we attempted to detect FC in the buccal mucosa of lung cancer patients. For this purpose we used MDSFR, which was hypothesized to be sensitive to the sub-diffraction length tissue changes caused by FC. Measurements were performed on oncologic patients and matched controls. Several buccal mucosa optical parameters showed significant differences between the lung cancer and control groups.
Our primary interest lay in the comparison of the lung cancer and COPD patients, since we hypothesized that the patients in these groups would be as homogeneous as possible. Also COPD is closely linked with lung cancer at a molecular level. [20] One notable result of the present study is that the buccal mucosa StO2 was significantly lower in the COPD patients, but not in the non-COPD control group. However, this is probably not the result of FC but a consequence of a systemic decrease in StO2 due to COPD. Therefore, we believe that measuring the buccal mucosa StO2 for screening purposes is not appropriate.
Interestingly, the scattering parameter μs' at 800 nm was also near-significantly higher in the lung cancer group than the COPD patients and significantly higher than in the, larger, non-COPD group. An increase in μs' means that the photons that enter the mucosa undergo more scattering events. This indicates that the buccal mucosa of lung cancer patients has undergone some form of transformation that might be the result of FC. [21] An increase of scatter events is correlated with an increase of the local density of macromolecules and changes in their organization. [22]
Our findings confirm the results of a study with a very similar study design that used low-coherence enhanced backscattering spectroscopy (LEBS). [13] The LEBS biomarker was also increased in lung cancer patients, indicating buccal mucosa transformation due to FC. However, the discriminative power of the LEBS power was higher than μs' at 800 nm with a sensitivity, specificity, and AUC of 79%, 83%, 89%, respectively. It is unclear how this difference might be explained. Two possible explanations are that their validation control group consisted of 33% non-smokers and that Radosevich et al. included 53% high-stage tumors (stage III and IV), compared to 30% in the present study.
The discriminative power of our optical method was lower for patients with lung cancer than for patients with head and neck or esophageal squamous cell carcinoma, which we have investigated in two earlier studies. [15], [16] This might be explained by the distance from the buccal mucosa to the tumor, which is the longest for lung cancer. To our knowledge, there are no studies that investigated optical methods to detect FC closer to the lung tumor (e.g., larynx, trachea, or bronchi). However, this would increase the complexity of the screening method, since it would require endoscopy.
There are some limitations of our study design that should be considered. One is the relative small number of patients per group. This might have led to an underestimation of the significance of the differences between groups (p-value) and it prevented us from testing the discriminative power of our optical parameters on an independent training set. Another limitation is that the different groups were not optimally matched. Age at measurement proved to be lower in both control groups than the lung cancer group. However, we corrected for this difference in our statistical analysis.
Conclusions
In conclusion, we have demonstrated that μs' at 800 nm is increased in the buccal mucosa of patients with lung cancer compared to controls with and without COPD. This increase could be an indication of FC in the oral cavity of patients with lung cancer. A study with a larger study population is needed to investigate whether MDSFR spectroscopy of the buccal mucosa could function as a (pre)screening tool for lung cancer.
Funding
The Dutch Cancer Society funded this study [TNO 2014–7074]. It had no role in study conception, design, data collection, analysis, data interpretation, or the writing of the manuscript. The corresponding author had full access to all data and final responsibility for the decision to submit for publication.
Footnotes
Funding: The Dutch Cancer Society funded this study [TNO 2014–7074]. It had no role in study conception, design, data collection, analysis, data interpretation, or the writing of the manuscript. The corresponding author had full access to all data and final responsibility for the decision to submit for publication.
Conflict of Interest: All authors declare that there is no conflict of interest regarding the publication of this manuscript.
References
- 1.Siegel RL, Miller KD. Jemal A (2015). Cancer statistics. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Horeweg N, de Koning H. The importance of screening for lung cancer. Expert Rev Respir Med. 2014;8:597–614. doi: 10.1586/17476348.2014.937428. [DOI] [PubMed] [Google Scholar]
- 3.Spira A, Halmos B, Powell CA. Update in Lung Cancer 2014. Am J Respir Crit Care Med. 2015;192:283–294. doi: 10.1164/rccm.201504-0756UP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Lung Screening Trial Research T, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, et al. (2011). Reduced lung-cancer mortality with low-dose computed tomographic screening N Engl J Med 365, 395–409. [DOI] [PMC free article] [PubMed]
- 5.Ru Zhao Y, Xie X, de Koning HJ, Mali WP, Vliegenthart R, Oudkerk M. 2011. NELSON lung cancer screening study Cancer Imaging11 Spec No A; pp. S79–S84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewin G, Morissette K, Dickinson J, Bell N, Bacchus M, Singh H, Tonelli M, Jaramillo Garcia A. Canadian Task Force on Preventive Health C. Recommendations on screening for lung cancer. Cmaj. 2016;188:425–432. [Google Scholar]
- 7.Subramanian H, Roy HK, Pradhan P, Goldberg MJ, Muldoon J, Brand RE, Sturgis C, Hensing T, Ray D, Bogojevic A. Nanoscale cellular changes in field carcinogenesis detected by partial wave spectroscopy. Cancer Res. 2009;69:5357–5363. doi: 10.1158/0008-5472.CAN-08-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evers D, Hendriks B, Lucassen G, Ruers T. Optical spectroscopy: current advances and future applications in cancer diagnostics and therapy. Future Oncol. 2012;8:307–320. doi: 10.2217/fon.12.15. [DOI] [PubMed] [Google Scholar]
- 9.Mohan M, Jagannathan N. Oral field cancerization: an update on current concepts. Oncol Rev. 2014;8:244. doi: 10.4081/oncol.2014.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angadi PV, Savitha JK, Rao SS, Sivaranjini Y. Oral field cancerization: current evidence and future perspectives. Oral Maxillofac Surg. 2012;16:171–180. doi: 10.1007/s10006-012-0317-x. [DOI] [PubMed] [Google Scholar]
- 11.Kopelovich L, Henson DE, Gazdar AF, Dunn B, Srivastava S, Kelloff GJ, Greenwald P. Surrogate anatomic/functional sites for evaluating cancer risk: an extension of the field effect. Clin Cancer Res. 1999;5:3899–3905. [PubMed] [Google Scholar]
- 12.Roy HK, Subramanian H, Damania D, Hensing TA, Rom WN, Pass HI, Ray D, Rogers JD, Bogojevic A, Shah M. Optical detection of buccal epithelial nanoarchitectural alterations in patients harboring lung cancer: implications for screening. Cancer Res. 2010;70:7748–7754. doi: 10.1158/0008-5472.CAN-10-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radosevich AJ, Mutyal NN, Rogers JD, Gould B, Hensing TA, Ray D, Backman V, Roy HK. Buccal spectral markers for lung cancer risk stratification. PLoS One. 2014;9 doi: 10.1371/journal.pone.0110157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoy CL, Gamm UA, Sterenborg HJ, Robinson DJ, Amelink A. Method for rapid multidiameter single-fiber reflectance and fluorescence spectroscopy through a fiber bundle. J Biomed Opt. 2013;18 doi: 10.1117/1.JBO.18.10.107005. [DOI] [PubMed] [Google Scholar]
- 15.Bugter O, Spaander MCW, Bruno MJ, Baatenburg de Jong RJ, Amelink A, Robinson DJ. Optical detection of field cancerization in the buccal mucosa of patients with esophageal cancer. Clin Transl Gastroenterol. 2018;9:152. doi: 10.1038/s41424-018-0023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bugter O, Hardillo JA, Baatenburg de Jong RJ, Amelink A, Robinson DJ. Optical pre-screening for laryngeal cancer using reflectance spectroscopy of the buccal mucosa. Biomed Opt Express. 2018;9:4665–4678. doi: 10.1364/BOE.9.004665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prestin S, Rothschild SI, Betz CS, Kraft M. Measurement of epithelial thickness within the oral cavity using optical coherence tomography. Head Neck. 2012;34:1777–1781. doi: 10.1002/hed.22007. [DOI] [PubMed] [Google Scholar]
- 18.Kanick SC, Robinson DJ, Sterenborg HJ, Amelink A. Monte Carlo analysis of single fiber reflectance spectroscopy: photon path length and sampling depth. Phys Med Biol. 2009;54:6991–7008. doi: 10.1088/0031-9155/54/22/016. [DOI] [PubMed] [Google Scholar]
- 19.Middelburg TA, Hoy CL, Neumann HA, Amelink A, Robinson DJ. Correction for tissue optical properties enables quantitative skin fluorescence measurements using multi-diameter single fiber reflectance spectroscopy. J Dermatol Sci. 2015;79:64–73. doi: 10.1016/j.jdermsci.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Durham AL, Adcock IM. The relationship between COPD and lung cancer. Lung Cancer. 2015;90:121–127. doi: 10.1016/j.lungcan.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Backman V, Roy HK. Light-scattering technologies for field carcinogenesis detection: a modality for endoscopic prescreening. Gastroenterology. 2011;140:35–41. doi: 10.1053/j.gastro.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roy HK, Hensing T, Backman V. Nanocytology for field carcinogenesis detection: novel paradigm for lung cancer risk stratification. Future Oncol. 2011;7:1–3. doi: 10.2217/fon.10.176. [DOI] [PMC free article] [PubMed] [Google Scholar]