Key Points
Question
Does broad-spectrum antibiotic treatment alter responsive to immune checkpoint inhibitors (ICIs) in routine practice?
Findings
In this observational study that included 196 patients with cancer treated with ICI therapy, antibiotic treatment administered within 30 days from commencement of ICI therapy was associated with significantly worse overall survival and a higher risk of disease refractory to treatment.
Meaning
Antibiotic therapy is associated with a reduced response to ICIs in routine practice, irrespective of tumor site; mechanistic studies exploring this relationship are warranted.
This cohort study assesses the clinical outcomes of patients with cancer treated with immune checkpoint inhibitors (ICIs) who received concurrent or prior broad-spectrum antibiotic treatment to determine whether an association exists between antibiotic therapy and overall survival and response to ICIs.
Abstract
Importance
Gut dysbiosis impairs response to immune checkpoint inhibitors (ICIs) and can be caused by broad-spectrum antibiotic (ATB) therapy.
Objective
To evaluate whether there is an association between ATB therapy administered concurrently (cATB) or prior (pATB) to ICI therapy and overall survival (OS) and treatment response to ICI therapy in patients with cancer treated with ICIs in routine clinical practice.
Design, Setting, and Participants
This prospective, multicenter, cohort study conducted at 2 tertiary academic referral centers recruited 196 patients with cancer who received ICI therapy between January 1, 2015, and April 1, 2018, in routine clinical practice rather than clinical trials.
Main Outcomes and Measures
Overall survival calculated from the time of ICI therapy commencement and radiologic response to ICI treatment defined using the Response Evaluation Criteria in Solid Tumors (version 1.1), with disease refractory to ICI therapy defined as progressive disease 6 to 8 weeks after the first ICI dose without evidence of pseudoprogression.
Results
Among 196 patients (137 men and 59 women; median [range] age, 68 [27-93] years) with non–small cell lung cancer (n = 119), melanoma (n = 38), and other tumor types (n = 39), pATB therapy (HR, 7.4; 95% CI, 4.3-12.8; P < .001), but not cATB therapy (HR, 0.9; 95% CI, 0.5-1.4; P = .76), was associated with worse OS (2 vs 26 months for pATB therapy vs no pATB therapy, respectively) (hazard ratio [HR], 7.4; 95% CI, 4.2-12.9) and a higher likelihood of primary disease refractory to ICI therapy (21 of 26 [81%] vs 66 of 151 [44%], P < .001). Overall survival in patients with non–small cell lung cancer (2.5 vs 26 months, P < .001), melanoma (3.9 vs 14 months, P < .001), and other tumor types (1.1 vs 11, P < .001) was consistently worse in those who received pATBs vs those who did not. Multivariate analyses confirmed that pATB therapy (HR, 3.4; 95% CI, 1.9-6.1; P < .001) and response to ICI therapy (HR, 8.2; 95% CI, 4.0-16.9; P < .001) were associated with OS independent of tumor site, disease burden, and performance status.
Conclusions and Relevance
Despite being limited by sample size, geographic origin, and the lack of correlative analyses on patients’ gut microbiota, this study suggests that pATB therapy but not cATB therapy is associated with a worse treatment response and OS in unselected patients treated with ICIs in routine clinical practice. Mechanistic studies are urgently required to investigate ATB-mediated alterations of gut microbiota as a determinant of poorer outcome following ICI treatment.
Introduction
Immune checkpoint inhibitors (ICIs) represent a novel treatment option for a widening range of malignant neoplasms. However, clinical benefit from this treatment modality is restricted to a proportion of patients across indications,1 a finding that has instigated the discovery of biomarkers capable of determining response to treatment to enable more precise delivery of immunotherapy in routine practice.
Exposure to broad-spectrum antibiotic (ATB) therapy may adversely influence outcomes of ICI therapy2 through modulation of intestinal microbiota,3 a key host determinant of response to ICIs known to precondition cancer-specific immunity.4 However, the clinical value of this observation is limited by its derivation from selected oncological indications, including renal and non–small cell lung cancer (NSCLC),2 for which external validation, especially outside clinical trials, is necessary.
Methods
In this multicenter study of 196 patients with NSCLC (n = 119), melanoma (n = 38), and other tumor types (n = 39), patients were consecutively treated with ICI therapy in routine clinical practice rather than clinical trials at 2 academic medical centers between January 1, 2015, and January 4, 2018 (eMethods in the Supplement). The present study was conducted after approval by the Imperial College London Tissue Bank Institutional Review Board and patient written informed consent was obtained. We investigated whether ATB therapy administered up to 30 days prior (pATB) to or concurrent (cATB) with ICI therapy until cessation was associated with overall survival (OS) and response to ICI therapy. Survival analysis was conducted using univariate and multivariate Cox regression models to evaluate the association of pATB therapy with OS independent of other clinicopathologic factors. Statistical significance was indicated by P < .05.
Results
In this cohort study of 137 men and 59 women (median [range] age, 68 [27-93] years) with cancer, most patients (165 [84%]) had disseminated disease when ICI therapy commenced, a performance status (PS) of 0 to 1 (159 [81%]), and had received anti–programmed cell death 1/programmed cell death ligand 1 (PD-1/PD-L1) ICIs (189 [96%]) as first-line metastatic therapy (120 [62%]) (Table 1 and eTable 1 in the Supplement). Median OS was 14.6 months (95% CI, 5.3-23.8 months). Median ICI therapy duration was 3.3 months (interquartile range, 6 months), and the disease control rate was 46%, with 2 of 195 patients had a complete response (1%), 65 had a partial response (33%), and 23 had stable disease (12%). Twenty-two of 29 patients (75%) received β-lactam–based pATBs, with most (n = 26, 89%) receiving an ATB course of 7 days or fewer. Thirty-nine of 68 patients (57%) received β-lactam–based cATBs for up to 7 days. Respiratory tract infections were the most common indication for both pATBs (16 patients [55%]) and cATBs (38 patients [85%]) (eTable 2 in the Supplement). The use of pATBs was not associated with corticosteroid use up to 30 days prior to (Pearson χ2, 0.47; P = .69) or during ICI therapy (Pearson χ2, 0.27; P = .64) nor with the occurrence or severity of immune-related adverse events (Pearson χ2, 4.69; P = .10). Three of 7 patients who received pATBs and ICI monotherapy discontinued ICI therapy owing to grade 3 or 4 toxic effects.
Table 1. Patient Characteristics.
| Characteristic | No. (%) (n = 196) |
|---|---|
| Sex | |
| Male | 137 (70) |
| Female | 59 (30) |
| Median age, y (range) | 68 (27-93) |
| Tumor type | |
| Primary lung | |
| Adenocarcinoma | 80 (40) |
| Squamous cell carcinoma | 32 (16) |
| Carcinoma—other tumor types | 6 (4) |
| Clear cell renal cell carcinoma | 11 (6) |
| Primary head and neck squamous cell carcinoma | 10 (5) |
| Malignant melanoma | 38 (20) |
| Transitional cell carcinoma | 16 (8) |
| PD-L1 expression, <1%/>1%/uncharacterized, No. | |
| Non–small cell lung cancer | 14/84/21 |
| Malignant melanoma | 1/0/37 |
| Other tumor types | 3/1/35 |
| Performance status (ECOG) | |
| 0 | 67 (35) |
| 1 | 92 (49) |
| 2 | 26 (14) |
| 3 | 4 (2) |
| No. of metastatic sites, median (range) | 2 (0-7) |
| Sites of metastases | |
| Liver | 26 (13) |
| Lung | 80 (41) |
| Bone | 44 (22) |
| Adrenal gland | 21 (11) |
| Central nervous system | 30 (15) |
| Other sites | 51 (26) |
| Cause of ICI discontinuation | |
| Progressive disease | 96 (82) |
| Toxic effects | 16 (14) |
| Completion of treatment plan | 3 (2) |
| Withdrawal of consent | 2 (2) |
| Corticosteroids use | |
| No corticosteroid use | 145 (74) |
| Prednisone ≥10 mg/d prior to ICI | 12 (6) |
| Prednisone ≥10 mg/d concurrent with ICI | 33 (17) |
| Prednisone <10 mg/d throughout the study period | 5 (3) |
| Immune-related adverse events, maximum CTCAE grade | |
| 0 | 129 (66) |
| 1-2 | 51 (26) |
| 3-4 | 16 (8) |
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events, version 5.0; ECOG, Eastern Cooperative Oncology Group; ICI, immune checkpoint inhibitor; PD-L1, programmed cell death ligand 1.
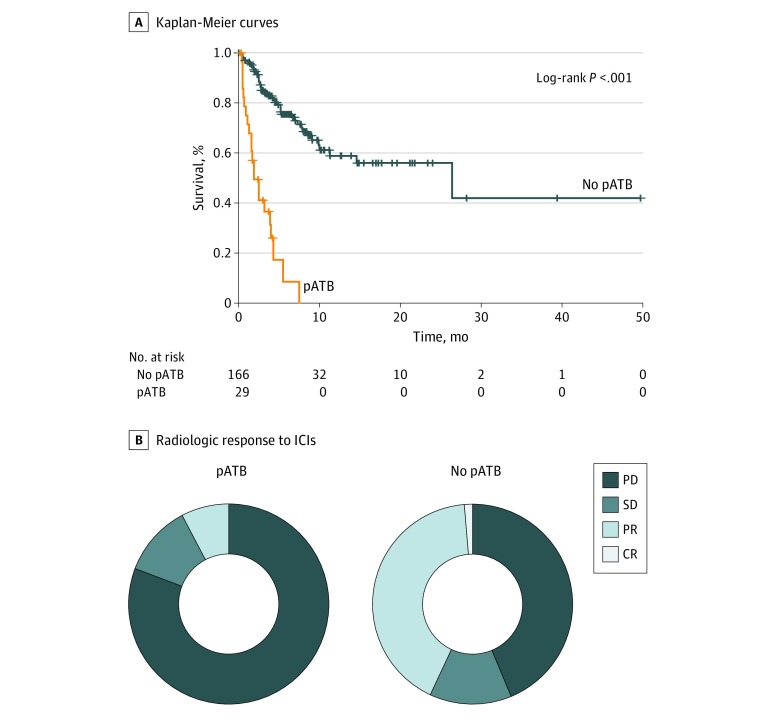
Univariate survival analyses confirmed that pATB therapy (HR, 7.4; 95% CI, 4.3-12.8; P < .001) but not cATB therapy (HR, 0.9; 95% CI, 0.5-1.4; P = .65) was associated with worse OS in those who received pATBs vs those who did not (2 vs 26 months) and found that those who received pATBs had nearly double the likelihood of poor response to ICI treatment (66 of 151 [44%] vs 21 of 26 [81%]; P < .001) (Figure). Patients treated with pATBs also were more likely to discontinue ICI therapy due to disease progression (24 of 29 pATB group vs 71 of 167 no pATB group; P < .001) and die of progressive disease while on ICI therapy (Pearson χ2, 7.44; P = .03, Table 2). Because the use of antibiotics might reflect patient comorbidities (eg, chronic airway disease in lung cancer) and may have a potential confounding effect on survival, we evaluated the association between pATB therapy and OS across cancer types and confirmed that pATB therapy vs no pATB therapy was associated with worse OS in the setting of NSCLC (2.5 vs 26 months; HR, 9.3; 95% CI, 4.3-19.0; P < .001), melanoma (3.9 vs 14 months; HR, 7.5; 95% CI, 1.7-30.4; P < .001), and other tumors (1.1 vs 11 months; HR, 7.8; 95% CI, 2.4-25.0; P < .001) (eFigure 1 in the Supplement). The association of pATB therapy with worse OS and poor response to ICI therapy was independent of the class of antibiotic used, patients’ PS, and corticosteroid use (eFigure 2, eFigure 3, eFigure 4, eFigure 5, and eFigure 6 in the Supplement). Multivariate analyses confirmed that pATB therapy (HR, 3.4; 95% CI, 1.9-6.1; P < .001) and response to ICIs (HR, 8.2; 95% CI, 4.0-16.9; P < .001) were independently associated with OS (eTable 3, eTable 4, and eTable 5 in the Supplement), a finding that was validated in propensity score analyses (eTable 6 in the Supplement).
Figure. Association Between pATB Therapy and Survival and Response to ICIs.
A, Kaplan-Meier curves illustrating the association of prior broad-spectrum antibiotic (pATB) therapy administered up to 30 days prior to immunotherapy with adverse survival in the study population (n = 196). B, The Figure shows the association between pATB and the best radiologic response to immune checkpoint inhibitors (ICIs) according to RECIST v 1.1 criteria. In 26 patients who received pATB therapy, 2 (8%) had a partial response (PR) to ICIs, 21 [81%] had progressive disease (PD), and 3 [11%] had stable disease (SD). In 151 patients who did not receive pATB therapy, 2 [1%] had a complete response to ICIs, 63 [42%] had PR, 20 [13%] had SD, and 66 [44%] had PD.
Table 2. Association Between Broad-Spectrum Antibiotic Use Prior to vs During ICI Therapy and Key Clinicopathologic Features Reflective of Disease Burden and Response.
| Characteristic | pATB | cATB | ||||
|---|---|---|---|---|---|---|
| No | Yes | P Value | No | Yes | P Value | |
| Tumor type | .97 | .38 | ||||
| NSCLC | 101 | 6 | 78 | 11 | ||
| Malignant melanoma | 32 | 17 | 27 | 40 | ||
| Other | 33 | 6 | 22 | 17 | ||
| ECOG PS | .09 | .84 | ||||
| 0-1 | 138 | 21 | 109 | 55 | ||
| 2-3 | 22 | 8 | 19 | 11 | ||
| Stage | .58 | .30 | ||||
| Locoregional | 28 | 3 | 23 | 8 | ||
| Metastatic | 136 | 26 | 103 | 59 | ||
| Tumor burden | .07 | .73 | ||||
| <2 metastases | 124 | 17 | 93 | 48 | ||
| >2 metastases | 40 | 22 | 33 | 19 | ||
| Age, y | .31 | .54 | ||||
| <65 | 69 | 15 | 57 | 27 | ||
| >65 | 97 | 14 | 70 | 41 | ||
| Response to ICIs | <.001a | .87 | ||||
| Primary progression | 66 | 21 | 54 | 33 | ||
| Objective response | 85 | 5 | 57 | 33 | ||
| ICI course | <.001a | .27 | ||||
| Ongoing | 75 | 4 | 52 | 27 | ||
| Discontinued due to progression | 71 | 24 | 58 | 37 | ||
| Discontinued due to other causes | 19 | 1 | 16 | 4 | ||
| Death while on ICIs | .03a | .61 | ||||
| Yes | 12 | 6 | 113 | 63 | ||
| No | 153 | 23 | 13 | 5 | ||
Abbreviations: cATB, concurrent antibiotic therapy; ECOG PS, Eastern Cooperative Oncology Group performance status; ICIs, immune checkpoint inhibitors; NSCLC, non–small cell lung cancer; pATB, prior antibiotic therapy.
Statistically significant (P < .05).
Discussion
In this study, we confirmed that pATB therapy vs no pATB therapy was associated with poor treatment response and worse OS in unselected, consecutive patients treated with ICI in routine clinical practice. The use of broad-spectrum antibiotics 30 days prior to ICI therapy was associated with poor outcomes across multiple oncologic indications in patients treated with immunotherapy.
Broad-spectrum ATB use can cause prolonged disruption of the gut ecosystem5 and impair the effectiveness of the cytotoxic T-cell response against cancer,6 strengthening the biologic plausibility underlying the adverse effect of ATB therapy on immunotherapy outcomes.
Limitations
Our observations are limited by the small sample size, geographic origin, and the lack of correlative analyses between antibiotic use and dynamic changes in the gut microflora, a point that should be investigated in prospective studies. Despite these limitations, the prevalence of antibiotic exposure and its association with survival and response to ICI therapy observed in the present study are not dissimilar from those of contemporary cohorts of patients treated with ICIs,2,7 which suggests that these findings are externally valid. Enrichment of stool microbiota with specific subtypes of commensal bacteria including Bifidobacteria, Akkermansia, and Ruminococcus have been associated with altered sensitivity to ICI therapy in patients with advanced cancer,8,9 and future studies should establish whether ATB therapy can specifically modulate the composition of these bacterial species.
One might argue that comorbidities could have dictated the use of antibiotics in patients with concomitant medical conditions and therefore exerted an effect on outcomes independent of any true immune-modulatory effect of antibiotic therapy. However, a number of objective findings from the current study argue against this hypothesis. First, more than 80% of patients had a PS of 0 or 1 at ICI commencement and neither pATB therapy (Pearson χ2, 3.46; P = .32) or cATB therapy (Pearson χ2, 2.38; P = .46) were associated with PS, suggesting that the provision of ATB therapy is unrelated to baseline functional status. Second, the use of cATBs, a potential proxy for patients’ worsening clinical status while on ICI treatment, was not associated with response to ICI therapy or survival. In addition, frequency and severity of immune-related adverse events were unrelated to pATB therapy in patients who died while on ICI treatment (eTable 3 in the Supplement), ruling out the confounding effect of toxic effects.
Interestingly, our study suggests that the timing of ATB exposure is crucial in dictating its interaction with response to immunotherapy, leading us to postulate a potential “priming” effect of pATBs on patients’ anticancer immunity. Moreover, we found pATBs to be not only associated with worse OS but also strongly associated with radiologic responses to ICI, an association that is independent from any potential association with comorbidities and corroborates the important link between antibiotic use and response to ICI therapy. In this study, we could not adjust our estimates of response and survival for patients’ tumor mutational burden, an important tumor-intrinsic indication of outcome. We also did not take into account prior radiotherapy, a factor recently suggested to alter responsive to ICI therapy.10 Although these associations warrant testing in prospective studies, it is unlikely that the provision of ATBs would have varied as a function of tumor mutational burden or prior radiotherapy in this cohort, therefore limiting the chances of systematic bias.
Conclusions
We conclude that antibiotic use prior to ICI therapy is associated with worse response rates and survival in patients receiving ICI therapy. Although provision of cATB therapy appears to be safe in the context of immunotherapy, clinicians should carefully the weigh the pros and cons of prescribing broad-spectrum ATBs prior to ICI treatment. Mechanistic studies are urgently required to investigate ATB-mediated gut dysbiosis not solely as a prognostic marker but as a therapeutically actionable driver of the anticancer immune response in the context of ICI therapy.
e Methods
eTable 1. Supplementary clinical characteristics of patients receiving ICPI.
eTable 2. Indications and characteristics of antibiotic therapy in patients receiving ICPI.
eTable 3. The relationship between broad spectrum antibiotics use prior to (pATB) and during ICPI therapy (cATB) and key clinico-pathologic features reflective of disease burden and response.
eTable 4. The relationship between prior antibiotic treatment (pATB) and the incidence of immunerelated adverse events (irAE) in patients who died whilst on ICPI treatment (n=18).
eTable 5. The relationship between survival and response to ICPI and class of antibiotic received prior to immunotherapy.
eTable 6. Multivariable Cox regression using propensity score weights.
eFigure 1. The prognostic effect of prior antibiotic treatment (pATB) as a determinant of adverse survival in NSCLC (n=119, Panel A), Melanoma (n=38, Panel B) and other tumor types (n=38, Panel C).
eFigure 2. Comparison between standard and inverse probability of treatment weighted Kaplan Meier plots illustrating the prognostic role of pATB in the whole study population receiving ICPI (n=196).
eFigure 3. The relationship between timing of antibiotic exposure and overall survival of patients treated with ICPI (n=196).
eFigure 4. The prognostic effect of antibiotic subtypes in patients receiving antibiotic therapy prior to ICPI (pATB, N=29).
eFigure 5. Verification of the prognostic role of pATB in patients with ECOG PS 0-2.
eFigure 6. pATB exerts detrimental effect on the prognosis of patients treated with ICPI irrespective of corticosteroids use.
eReferences
References
- 1.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123-135. doi: 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Derosa L, Hellmann MD, Spaziano M, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol. 2018;29(6):1437-1444. doi: 10.1093/annonc/mdy103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber D, Hiergeist A, Weber M, et al. Detrimental effect of broad-spectrum antibiotics on intestinal microbiome diversity in patients after allogeneic stem cell transplantation: lack of commensal sparing antibiotics. Clin Infect Dis. 2019;68(8):1303-1310. doi: 10.1093/cid/ciy711 [DOI] [PubMed] [Google Scholar]
- 4.Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13(11):800-812. doi: 10.1038/nrc3610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon MY, Yoon SS. Disruption of the gut ecosystem by antibiotics. Yonsei Med J. 2018;59(1):4-12. doi: 10.3349/ymj.2018.59.1.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gui QF, Lu HF, Zhang CX, Xu ZR, Yang YH. Well-balanced commensal microbiota contributes to anti-cancer response in a lung cancer mouse model. Genet Mol Res. 2015;14(2):5642-5651. doi: 10.4238/2015.May.25.16 [DOI] [PubMed] [Google Scholar]
- 7.Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018;36(28):2872-2878. doi: 10.1200/JCO.2018.79.0006 [DOI] [PubMed] [Google Scholar]
- 8.Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):104-108. doi: 10.1126/science.aao3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97-103. doi: 10.1126/science.aan4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18(7):895-903. doi: 10.1016/S1470-2045(17)30380-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
e Methods
eTable 1. Supplementary clinical characteristics of patients receiving ICPI.
eTable 2. Indications and characteristics of antibiotic therapy in patients receiving ICPI.
eTable 3. The relationship between broad spectrum antibiotics use prior to (pATB) and during ICPI therapy (cATB) and key clinico-pathologic features reflective of disease burden and response.
eTable 4. The relationship between prior antibiotic treatment (pATB) and the incidence of immunerelated adverse events (irAE) in patients who died whilst on ICPI treatment (n=18).
eTable 5. The relationship between survival and response to ICPI and class of antibiotic received prior to immunotherapy.
eTable 6. Multivariable Cox regression using propensity score weights.
eFigure 1. The prognostic effect of prior antibiotic treatment (pATB) as a determinant of adverse survival in NSCLC (n=119, Panel A), Melanoma (n=38, Panel B) and other tumor types (n=38, Panel C).
eFigure 2. Comparison between standard and inverse probability of treatment weighted Kaplan Meier plots illustrating the prognostic role of pATB in the whole study population receiving ICPI (n=196).
eFigure 3. The relationship between timing of antibiotic exposure and overall survival of patients treated with ICPI (n=196).
eFigure 4. The prognostic effect of antibiotic subtypes in patients receiving antibiotic therapy prior to ICPI (pATB, N=29).
eFigure 5. Verification of the prognostic role of pATB in patients with ECOG PS 0-2.
eFigure 6. pATB exerts detrimental effect on the prognosis of patients treated with ICPI irrespective of corticosteroids use.
eReferences