Key Points
Question
Do patients who catastrophize pain, defined as patients who anticipate higher pain levels on the 11-point Numeric Rating Scale (score >4), receive equal benefit from a vibratory stimulus during local anesthetic injection as patient who do not catastrophize pain (Numeric Rating Scale score ≤4)?
Findings
In this randomized clinical trial of 87 patients who experienced 101 unique events, patients who catastrophized pain reported significantly higher Numeric Rating Scale scores during local anesthetic injection than patients who did not. The addition of vibration resulted in a 25.5% reduction of Numeric Rating Scale scores during local anesthetic injection in patients who catastrophized pain and a 79.4% reduction in patients who did not catastrophize pain.
Meaning
Patients who catastrophize pain can be identified in the clinical setting with assessment of anticipation of pain levels (Numeric Rating Scale score >4), and these patients benefit from the addition of vibration during local anesthetic injection.
Abstract
Importance
Vibration has been shown to decrease injection site pain in patients; however, to date, this effect has not been assessed for patients who catastrophize pain (ie, patients who anticipate a higher pain level). The anticipation of a pain score greater than 4 on the 11-point Numeric Rating Scale (NRS) has been associated with an increase in a patient’s perception of procedural pain.
Objective
To assess the efficacy of vibration during cutaneous anesthetic injection for dermatologic surgery for patients who catastrophize pain (NRS score >4) and patients who do not (NRS score ≤4).
Design, Setting, and Participants
Randomized, parallel-group clinical trial from June 19 to September 4, 2018, at a tertiary dermatologic surgery clinic among 87 adults undergoing cutaneous cancer removal surgery. Patients completed a preprocedural questionnaire detailing their baseline pain, anticipated pain, and drug use. Analysis was performed on an intent-to-treat basis.
Interventions
Use of a vibratory anesthetic device (VAD) on the treatment site prior to anesthetic injection in the on (VAD ON) or off (VAD OFF) mode.
Main Outcomes and Measures
Pain was reported using the 11-point NRS (where 0 indicates no pain and 11 indicates the worst pain imaginable). A minimum clinically important difference of 22% or more and a substantial clinically important difference of 57% or more were used to assess the efficacy of vibration in patient-reported NRS score during anesthetic injection (iNRS score).
Results
A total of 87 patients were included, with 101 unique events reported (among the unique events, 37 were reported in women and 64 were reported in men; mean [SD] age, 66.0 [11.3] years). The mean (confidence level [CL]) iNRS score for patients who catastrophized pain was 2.27 (0.66) compared with 1.44 (0.39) for patients who did not (P = .03). A 38.9% decrease in mean (CL) iNRS score was reported with VAD ON compared with VAD OFF in all participants (1.24 [0.38] vs 2.04 [0.54]). Patients who catastrophized pain reported a 25.5% decrease in mean (CL) iNRS score with VAD ON vs VAD OFF (1.91 [0.99] vs 2.57 [0.98]), and patients who did not reported a 79.4% decrease (1.02 [0.40] vs 1.84 [0.66]). VAD ON was the only statistically significant variable to affect iNRS score (F statistic, 2.741; P = .03).
Conclusions and Relevance
This trial demonstrates that those who catastrophize pain prior to a procedure report a higher perceived level of pain. The application of vibration during local anesthetic injection resulted in a minimum clinically important difference in pain level for patients who catastrophize pain and a substantial clinically important difference in pain level for patients who do not.
Level of Evidence
2.
Trial Registration
ClinicalTrials.gov identifier: NCT03467685
This randomized clinical trial assesses the effect of a vibratory anesthetic device during dermatologic surgery on patients who catastrophize pain as well as those who do not catastrophize pain.
Introduction
Local anesthetic injection for pain control is essential for most dermatologic surgical procedures. Preoperative procedures, such as venipuncture, may cause severe pain in certain individuals and is a primary concern for patients prior to their procedure.1,2,3,4,5 Approximately 40% of patients have a significant needle phobia, with 5% to 7% actively avoiding treatment as a result.6,7 This avoidant behavior may cause significant delays in the diagnosis and treatment of cutaneous conditions when patients are scheduling appointments for dermatologic procedures such as biopsies, excisions, electrodessication and curettage, and Mohs micrographic surgery.
The anticipation of pain and subsequent fear of pain can cause psychological distress that many patients experience before a procedure. Pain catastrophizing (where a patient anticipates a higher level of pain) is an overemphasis of negative consequences pertaining to noxious stimuli.8 It has been described as a maladaptive attempt to cope with present or predicted pain.9,10,11 Many studies have shown that patients who have catastrophic thoughts prior to a procedure, regardless of the type of procedure, exhibit higher levels of perceived pain, anxiety, depression, and disability.8,9,10,12,13,14,15,16,17,18 This characteristic is not reserved only for those undergoing extensive and complicated surgery; pain catastrophizing has also been shown to affect outcomes in patients within the setting of dermatologic surgery.19
A patient’s perceived pain during a procedure can be accurately predicted from their anticipated pain from 1 week to 6 months prior to the event.9 Surgical patients who anticipated pain greater than 40 mm on the visual analog scale (range, 0-100 mm, where 100 mm represents the worst possible pain) reported a significant increase in acute postsurgical pain compared with those who did not (visual analog scale ≤40 mm) on all postoperative days.20 Analogously, a reduction in patient catastrophizing leads to a reduction in perceived pain and disability.8,13,21,22
The use of nonnoxious tactile stimulation, specifically vibration, has been shown to significantly decrease pain perceived by patients in many clinical settings.23,24,25,26,27,28,29,30,31,32,33,34,35,36,37 Generalizability of these previous studies are limited owing to small sample size, the use of volunteers, and unreported and/or unperformed participant training for accurate pain score reporting. This study aims to promote generalizability by including a typical dermatologic surgery patient population, without exclusion of previous condition(s), who are undergoing scheduled skin cancer removal surgical procedures. Thorough training on appropriate pain reporting prior to the procedure was carried out to ensure test-retest accuracy of pain score reporting. Pain reporting is largely subjective; however, a recent study by Treister et al38 showed that pain reporting accuracy can be improved with training. Studies to date have also not considered the role of catastrophizing pain prior to a procedure and its influence on the efficacy of vibration during anesthetic injection. Our objective was to assess if the previously reported benefit of cutaneous nonnoxious vibration during cutaneous anesthetic injection was equally beneficial in both patients who anticipated greater pain, defined as a pain score on the 11-point Numeric Rating Scale (NRS) of more than 4, and those who did not (NRS score ≤4). It has been previously reported that a minimum clinically important difference of 22% reduction or more and a substantial clinically important difference of 57% reduction or more in acute pain score is used to assess the efficacy of vibration in a patient-reported NRS score during anesthetic injection (iNRS score).39
We hypothesized that patients who anticipate a pain score of more than 4 on an 11-point NRS will exhibit greater actual pain than those who anticipate a pain score of 4 or less. We expected that the use of nonnoxious vibration during local anesthetic injection would reduce pain in all patients, regardless of their anticipatory pain score.
Methods
Study Design
A randomized, parallel-group clinical study of the perception and interpretation of a nonnoxious vibration stimulus compared with placebo during syringe-mediated local cutaneous infiltration of anesthetic was performed. The protocol (Supplement 1) was approved by the University of Pittsburgh institutional review board (PRO17110134) and conforms to the ethical guidelines of the Declaration of Helsinki.40 All patients provided written informed consent. We followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Participants
All adults (≥18 years) presenting for Mohs micrographic surgery, surgical excision, and/or other cutaneous cancer removal surgery at Falk Dermatologic Surgery Clinic affiliated with the University of Pittsburgh Medical Center in Pennsylvania were eligible for inclusion. Enrollment occurred from June 19 to September 4, 2018. Eligible patients were informed of the study 24 to 72 hours before treatment via telephone call. Data analysis was performed from September 5, 2018, to March 1, 2019.
Patients who expressed interest in the study were given more information on the day of study, including the risks and benefits of participating. Any subsequent questions were answered at this time. All participants were given a preprocedural questionnaire. A follow-up telephone call was conducted 4 to 6 hours after the procedure to assess patients’ recollection of the study as well as their current pain level.
Participant Training
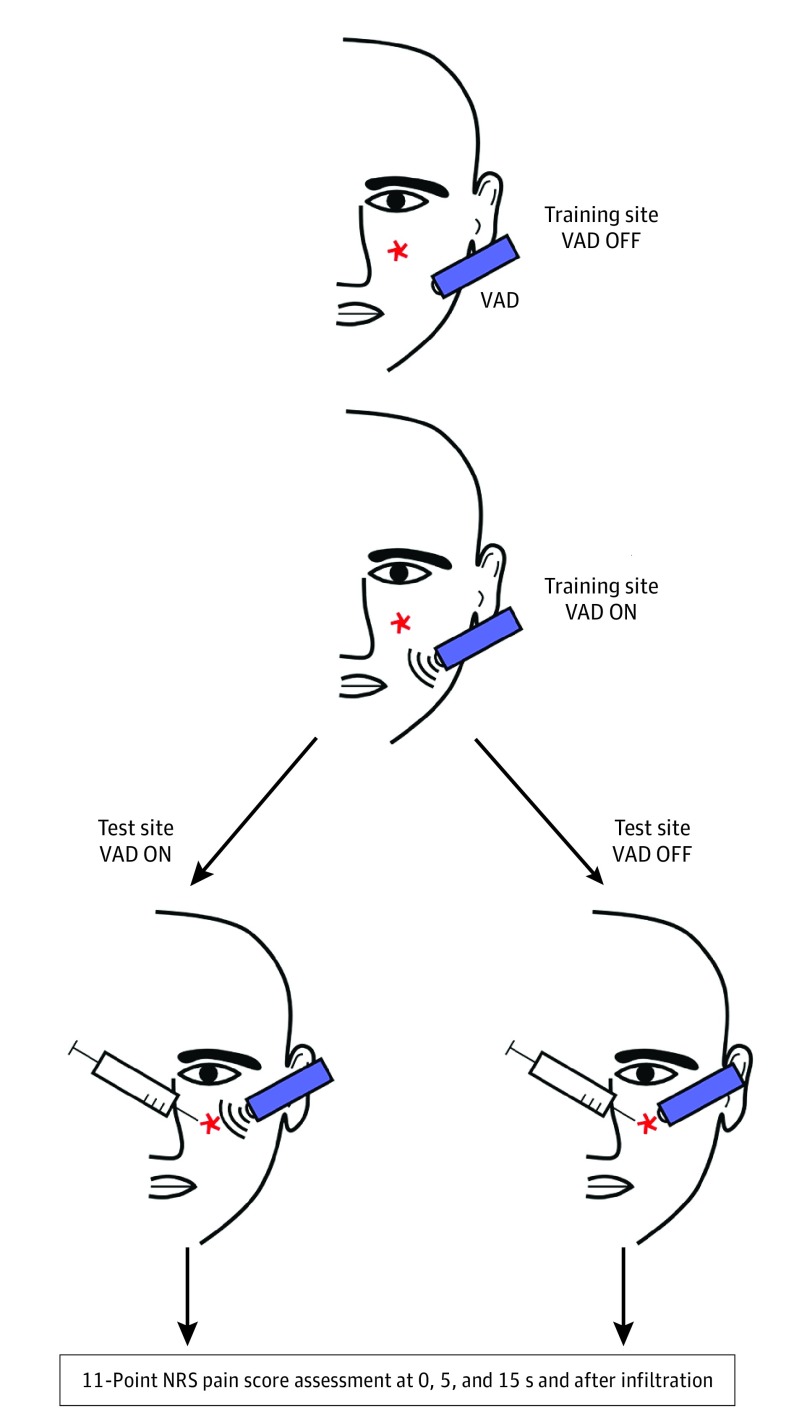
Participants underwent a training session to appropriately answer trial questions using the 11-point NRS (“What is your pain level?”) and descriptive adjectives of sensation (“Can you describe the sensation you felt on your skin?”). The vibratory anesthetic device (VAD) was placed on a training site approximately 5 cm away from the treatment site in the off mode (VAD OFF) for 15 seconds (Figure 1). Participants were then asked to describe the sensation they felt on their skin as well as the NRS score pain level experienced. This process was repeated with the VAD in the on mode (VAD ON). During this time, patients who were unable to discern between the VAD ON and VAD OFF modes, expressed NRS score pain levels greater than zero during the training session, could not communicate effectively, had a language barrier, or were unable to follow instructions were excluded.
Figure 1. Depiction of Participant Training.
Participants underwent a training session to appropriately answer trial questions using the 11-point Numeric Rating Scale (NRS) score and descriptive adjectives of sensation. The vibratory anesthetic device (VAD) was placed on a training site approximately 5 cm away from their treatment site in the off mode (VAD OFF) for 15 seconds. Participants were then asked to describe the sensation they felt on their skin as well as the NRS score pain level experienced. This process was then repeated with the VAD in the on mode (VAD ON).
Intervention
The VAD is a 10-cm, handheld, battery-operated device (Finever Inc). In the VAD ON mode, the device oscillates at a continuous frequency of approximately 100 Hz. It is nonexperimental and currently available for purchase over the counter. Prior to use, the device was customized by positioning a cotton ball at the tip with subsequent placement into a nitrile glove.28 The above modification was replicated for every study participant.
The VAD, anesthetic injection and infiltration, and surgical procedure were all performed by 1 board-certified dermatologic surgeon (B.T.C.). The anesthetic used in all cases was lidocaine hydrochloride, 1%, with epinephrine, 1:100 000, and buffered with bicarbonate, 8.4%, manufactured by Hospira Inc (ratio of 1:10). The buffered anesthetic was prepared in a 3-mL syringe with a 30-gauge needle.
Outcome Measures
Participants’ pain levels were assessed using the validated 11-point NRS score (0 indicates no pain, and 10 indicates the worst pain imaginable). The NRS score was evaluated at 4 different time points prior to (0 and 5 seconds), during (15 seconds), and after (5 seconds after infiltration) the first local anesthetic injection. Time began once the VAD was placed onto the testing site in the on or off mode (0 and 5 seconds) prior to injection. After 15 seconds of VAD application, the needle was advanced quickly with local anesthetic infused slowly for approximately 5 seconds; this time point was defined as iNRS score. The VAD and needle were removed directly after injection and NRS score was evaluated again 5 seconds after infiltration without VAD. Patients communicated their NRS score verbally at all time points.
Anticipated NRS (aNRS) pain score thresholds (>4 and ≤4) were adapted from the results of Sommer et al.20 The minimum clinically important difference for pain relief was defined as a relative reduction of 22% or more from patient’s baseline pain, and the substantial clinically important difference for pain relief was defined as a relative reduction of 57% or more from patient’s baseline pain (iNRS score, VAD OFF vs iNRS score, VAD ON).39
Sample Size
We aimed for a power of 0.90 with α = .05, for a 2-sided analysis. Previously reported data using VAD during dermatologic procedures shows a mean (SD) NRS score of 2.7 (0.9).29 If we use our minimum clinically important difference of 22% or greater, it results in a calculated sample size of 98 participants. This calculation was done using a statistical sample size calculator (http://clincalc.com/stats/samplesize.aspx).29 Per Ferreira-Valente et al,41 parameters of power = 0.80, α = .05, and SD of 2.2 were defined, and a sample size of 5 was calculated to detect differences in the NRS score. Similar numbers are calculated for our study using those parameters.
Randomization
Prior to participation, all eligible participants were randomly assigned to the treatment group (VAD ON) or the placebo group (VAD OFF) using a Gaussian random number generator (https://www.google.com/search?q=random+number). The randomization sequence and assignment of participants to the VAD ON vs VAD OFF groups were done by 1 of us (P.G.), while participant enrollment was shared between 2 of us (P.G. and R.M.S.).
Blinding
During the consent process, participants were informed that they would be randomized to receive “pressure” (VAD OFF) or “vibration and pressure” (VAD ON) during their local anesthetic injection. Prior to the application of VAD, the surgeon (B.T.C.) and study participants were unaware of study group allocation. Directly prior to anesthetic injection, participants were asked to keep their eyes closed. During this time, a coinvestigator (P.G. or R.M.S.) displayed the participant’s study group allocation (VAD ON or OFF) to the surgeon (B.T.C.), who applied the tool in the correct mode. Owing to the nature of vibration, it was impossible to keep participants and clinicians blinded to treatment arm (VAD ON or OFF).
Statistical Analysis
Analysis was performed on an intent-to-treat basis. Mean group comparisons were analyzed using a paired t test. P = .05 was considered statistically significant. All values collected were analyzed to report mean values and 95% CIs or SDs from the mean. Mean group comparisons (ie, relative change reduction) were calculated from full values. Multivariate analysis was used to compare categorical variables with iNRS score (eg, age, sex, aNRS, and VAD ON or OFF). All statistical analyses were calculated with Microsoft Excel, version 1808 (Microsoft Corp).
Results
Study Population
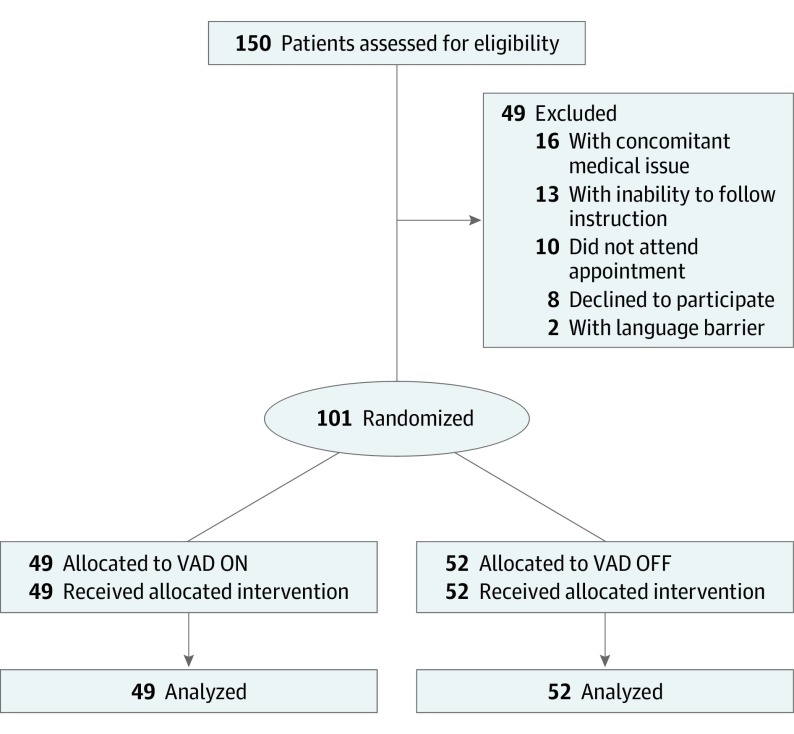
Participants were recruited from June 19 to September 4, 2018. A total of 150 participants were eligible for the study; 87 consenting patients were included with 101 unique events reported. A total of 49 patients were excluded for the following reasons: procedure was cancelled secondary to a concomitant medical problem (n = 16), inability to follow instructions (n = 13), nonattendance (n = 10), participation refusal during appointment (n = 8), and language barrier (n = 2) (Figure 2). No follow-up was performed.
Figure 2. CONSORT 2010 Flow Diagram.
VAD indicates vibratory anesthetic device, VAD ON indicates the device is in the on mode, and VAD OFF indicates the device is in the off mode.
Outcomes
Among the 101 unique events, 37 were reported in women and 64 were reported in men, with an overall mean (SD) age of 66.0 (11.3) years (Table 1). The most frequent testing site was the head and neck region (74 [73.3%]), followed by the extremities (22 [21.8%]) and the trunk (5 [5.0%]). Further patient demographics are depicted in Table 1.
Table 1. Participant Demographics.
| Characteristic | VAD ON and OFF (N = 101) | VAD OFF (n = 52) | VAD ON (n = 49) | ||
|---|---|---|---|---|---|
| aNRS Score ≤4 (n = 38) | aNRS Score >4 (n = 14) | aNRS Score ≤4 (n = 37) | aNRS Score >4 (n = 12) | ||
| Age, mean (SD) [range], y | 66.0 (11.3) [32-89] | 70.1 (9.8) [43-88] | 60.1 (10.1) [47-78] | 66.1 (11.4) [45-89] | 59.5 (11.6) [32-74] |
| Sex, No. (%) | |||||
| Male | 64 (63.4) | 26 (68.4) | 6 (42.9) | 26 (70.3) | 6 (50.0) |
| Female | 37 (36.6) | 12 (31.6) | 8 (57.1) | 11 (29.7) | 6 (50.0) |
| Location of surgery, No. (%) | |||||
| Head and neck | 74 (73.3) | 28 (73.7) | 13 (92.9) | 24 (64.9) | 9 (75.0) |
| Extremities | 22 (21.8) | 10 (26.3) | 1 (7.1) | 10 (27.0) | 1 (8.3) |
| Trunk | 5 (5.0) | 0 | 0 | 3 (8.1) | 2 (16.7) |
Abbreviations: aNRS, anticipated Numeric Rating Scale; VAD, vibratory anesthetic device.
A total of 87 consenting patients were included, with 101 unique events reported (26 patients who catastrophized pain [aNRS score >4] compared with 75 patients who did not catastrophize pain [NRS score ≤4]). The mean (confidence level [CL]) iNRS score for patients who catastrophized pain was 2.27 (0.66) compared with 1.44 (0.39) for patients who did not catastrophize pain (P = .03) (Table 2). A 38.9% decrease in mean (CL) iNRS score was reported with VAD ON vs VAD OFF for all participants (1.24 [0.38] vs 2.04 [0.54]) (Table 2). Patients who catastrophized pain reported a 25.5% decrease in mean (CL) iNRS score with VAD ON vs VAD OFF (1.91 [0.99] vs 2.57 [0.98]), while patients who did not catastrophize pain reported a 79.4% reduction (1.02 [0.40] vs 1.84 [0.66]) (Table 3).
Table 2. Anticipated and Injection Pain With or Without Application of VADa.
| Scale | VAD OFF (n = 52) | VAD ON (n = 49) |
|---|---|---|
| aNRS score, mean (CL) | 2.92 (0.63) | 2.67 (0.64) |
| iNRS score, mean (CL) | 2.04 (0.54) | 1.24 (0.38)b |
Abbreviations: aNRS, anticipated Numeric Rating Scale; CL, confidence level; iNRS, Numeric Rating Scale at Injection; VAD OFF and ON, vibratory anesthetic device off and on.
Mean (CL) iNRS score in patients with an aNRS score of 4 or less (n = 75), 1.44 (0.39); and mean (CL) iNRS score in patients with an aNRS score is higher than 4 (n = 26), 2.27 (0.66); P = .03 (paired t test); df = 45.
Substantial clinically importance difference.
Table 3. Anticipated and Injection Pain With or Without Application of VAD, by aNRS Score.
| Scale | VAD OFF | VAD ON | ||
|---|---|---|---|---|
| aNRS Score ≤4 (n = 38) | aNRS Score >4 (n = 14) | aNRS Score ≤4 (n = 37) | aNRS Score >4 (n = 12) | |
| aNRS score, mean (CL) | 1.79 (0.41) | 6.00 (0.72) | 1.67 (0.44) | 5.75 (0.86) |
| iNRS score, mean (CL) | 1.84 (0.66) | 2.57 (0.98) | 1.02 (0.40)a | 1.91 (0.99)b |
Abbreviations: aNRS, anticipated Numeric Rating Scale; CL, confidence level; iNRS, Numeric Rating Scale at Injection; VAD OFF and ON, vibratory anesthetic device off and on.
Substantial clinically importance difference.
Minimum clinically important difference.
Multivariate analysis was performed on categorical variables of age, sex, VAD ON or OFF, and aNRS score for the outcome (iNRS score). Analysis of variance was performed to test the reliability of our regression model (P = .03) (eTable in Supplement 2). In the regression analysis, the application of VAD ON was the only statistically significant variable to affect iNRS score (F statistic, 2.741; P = .03). Nonsignificant variables included age, sex, and aNRS score (eTable in Supplement 2). No harm or unintended effects were observed in either group.
Discussion
The anticipation and fear of operative pain can result in significant psychological distress in many patients. One study showed that a large proportion of both patients undergoing both inpatient and outpatient surgical procedures reported high levels of anxiety about surgery-related pain prior to their procedure.42 Numerous studies have shown that higher levels of anxiety prior to a surgical procedure correlated with higher levels of pain after surgery.8,9,10,13,14,15,16,17,19,42 In our study, we defined catastrophizing as an aNRS score of greater than 4. This definition was based on the previous results by Sommer et al,20 who showed that surgical patients who anticipated pain greater than 40 mm on the visual analog scale experienced significantly higher postoperative pain than their counterparts.20 Paralleling that study, our data show that patients undergoing dermatologic surgery who catastrophize pain had a significantly higher perceived iNRS score than those who did not (2.27 [0.66] vs 1.44 [0.39]; P = .03) (Table 2).
We hypothesized that vibration would reduce perceived pain at injection (iNRS) in both patients who catastrophized pain and patients who did not. Vibration has been previously shown to significantly decrease pain perceived by patients in multiple clinical settings, but, to our knowledge, had not been tested in those who catastrophize pain.23,24,25,26,27,28,29,30,31,32,33,34,35,36,37 In our study, we found that patients who catastrophized pain experience a minimum clinically important difference in relative change (≥22%) and patients who did not catastrophize pain experience a substantial clinically important difference (≥57%) with the use of vibration during anesthetic injection (Table 3).39 During multivariate analysis of the entire cohort, the only variable to significantly affect the perceived pain of patients during anesthetic injection was the presence of the VAD (F statistic, 2.741; P = .03) (eTable in Supplement 2). This result demonstrates that the use of vibration had a statistically significant effect on perceived pain during injection regardless of catastrophizing, age, or sex.
We showed that the benefit of vibration was more distinct in the cohort of patients who did not catastrophize pain (79.4% relative decrease in perceived pain). Vibration does not have the same efficacy for every patient. Previous studies have identified a subset of the population that does not benefit from the use of vibration, with a proportion of patients who have a negative response.23,24,25,27,29,33,35,36 It has been reported that as many as 23.8% of patients experienced more pain with the use of vibration during injection or pain than without vibration.36 Investigations into how to accurately determine which patients will not respond to vibration would be helpful to pursue. Clinically, we will continue to use vibration on our patients; however, we recognize that those who catastrophize may require further intervention for pain relief in addition to vibration alone. Our findings suggest that future studies should consider assessing the efficacy of other nonnoxious tactile stimulation devices, sensations, or techniques.
Limitations
This study has some limitations. The nature of vibration makes it impossible to completely blind both patient and clinician from the assigned intervention. By concealing allocation until the last possible moment, we attempted to minimize the resultant bias. Determining whether the anesthetic effect of vibration was directly attributable to the patient’s awareness of its presence would not decrease the meaningfulness of the reported clinically significant reduction in iNRS score.
No exclusions based on preexisting conditions were made, to ensure generalizability of our patient population to other dermatologic surgery practice populations; exclusion would limit the generalizability of our study. All study participants had a cutaneous biopsy performed prior to the surgical procedure and consequently had an expectation toward receiving local anesthetic injection. In addition, this was an elective study, and it is possible that the patients who catastrophized pain the most self-selected to not participate, representing further selection bias.
The generalizability of our study is potentially limited because of the use of a single operator at a single center. A multicenter site would be challenged by standardization of the assessment, but larger studies may reveal further demographic risk factors for uncontrolled pain. We used anticipation as defined by the NRS and not the Pain Catastrophizing Scale8 to evaluate the performance of an easily applied tool that could be incorporated into the common practices of most dermatologists. The Pain Catastrophizing Scale has been thoroughly validated and would produce a more precise understanding of patients’ experience or perceptions of vibratory pain sensation.
Conclusions
Our study recapitulates that vibration during local anesthetic injection works in patients undergoing cutaneous cancer removal procedures. An aNRS score greater than 4 is associated with worse perceived pain during anesthetic injection. Finally, we show that vibration is beneficial in both patients who catastrophize and those who do not.
Trial Protocol
eTable. ANOVA and Multivariate Regression Analysis
Data Sharing Statement
References
- 1.Agarwal A, Sinha PK, Tandon M, Dhiraaj S, Singh U. Evaluating the efficacy of the Valsalva maneuver on venous cannulation pain: a prospective, randomized study. Anesth Analg. 2005;101(4):1230-1232. doi: 10.1213/01.ane.0000167270.15047.49 [DOI] [PubMed] [Google Scholar]
- 2.Hambleton VL, Gómez IA, Andreu FAB. Venipuncture versus peripheral catheter: do infusions alter laboratory results? J Emerg Nurs. 2014;40(1):20-26. doi: 10.1016/j.jen.2012.03.014 [DOI] [PubMed] [Google Scholar]
- 3.Singer AJ, Taira BR, Chisena EN, Gupta N, Chipley J. Warm lidocaine/tetracaine patch versus placebo before pediatric intravenous cannulation: a randomized controlled trial. Ann Emerg Med. 2008;52(1):41-47. doi: 10.1016/j.annemergmed.2008.01.336 [DOI] [PubMed] [Google Scholar]
- 4.Basaranoglu G, Basaranoglu M, Erden V, Delatioglu H, Pekel AF, Saitoglu L. The effects of Valsalva manoeuvres on venepuncture pain. Eur J Anaesthesiol. 2006;23(7):591-593. doi: 10.1017/S0265021506000160 [DOI] [PubMed] [Google Scholar]
- 5.Glass JS, Hardy CL, Meeks NM, Carroll BT. Acute pain management in dermatology: risk assessment and treatment. J Am Acad Dermatol. 2015;73(4):543-560. doi: 10.1016/j.jaad.2015.04.050 [DOI] [PubMed] [Google Scholar]
- 6.Taddio A, Ipp M, Thivakaran S, et al. . Survey of the prevalence of immunization non-compliance due to needle fears in children and adults. Vaccine. 2012;30(32):4807-4812. doi: 10.1016/j.vaccine.2012.05.011 [DOI] [PubMed] [Google Scholar]
- 7.Jenkins K., II II, Needle phobia: a psychological perspective. Br J Anaesth. 2014;113(1):4-6. doi: 10.1093/bja/aeu013 [DOI] [PubMed] [Google Scholar]
- 8.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7(4):524-532. doi: 10.1037/1040-3590.7.4.524 [DOI] [Google Scholar]
- 9.Keefe FJ, Brown GK, Wallston KA, Caldwell DS. Coping with rheumatoid arthritis pain: catastrophizing as a maladaptive strategy. Pain. 1989;37(1):51-56. doi: 10.1016/0304-3959(89)90152-8 [DOI] [PubMed] [Google Scholar]
- 10.Sullivan MJL, Tripp DA, Santor D. Gender differences in pain and pain behavior: the role of catastrophizing. Cognit Ther Res. 2000;24(1):121-134. doi: 10.1023/A:1005459110063 [DOI] [Google Scholar]
- 11.Sullivan MJ, Thorn B, Haythornthwaite JA, et al. . Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17(1):52-64. doi: 10.1097/00002508-200103000-00008 [DOI] [PubMed] [Google Scholar]
- 12.Chaves JF, Brown JM. Spontaneous cognitive strategies for the control of clinical pain and stress. J Behav Med. 1987;10(3):263-276. doi: 10.1007/BF00846540 [DOI] [PubMed] [Google Scholar]
- 13.Sullivan MJL, Martel MO, Tripp D, Savard A, Crombez G. The relation between catastrophizing and the communication of pain experience. Pain. 2006;122(3):282-288. doi: 10.1016/j.pain.2006.02.001 [DOI] [PubMed] [Google Scholar]
- 14.Martin MY, Bradley LA, Alexander RW, et al. . Coping strategies predict disability in patients with primary fibromyalgia. Pain. 1996;68(1):45-53. doi: 10.1016/S0304-3959(96)03179-X [DOI] [PubMed] [Google Scholar]
- 15.Sullivan MJ, Stanish W, Waite H, Sullivan M, Tripp DA. Catastrophizing, pain, and disability in patients with soft-tissue injuries. Pain. 1998;77(3):253-260. doi: 10.1016/S0304-3959(98)00097-9 [DOI] [PubMed] [Google Scholar]
- 16.Keefe FJ, Lefebvre JC, Egert JR, Affleck G, Sullivan MJ, Caldwell DS. The relationship of gender to pain, pain behavior, and disability in osteoarthritis patients: the role of catastrophizing. Pain. 2000;87(3):325-334. doi: 10.1016/S0304-3959(00)00296-7 [DOI] [PubMed] [Google Scholar]
- 17.Sullivan MJL, Stanish WD. Psychologically based occupational rehabilitation: the Pain-Disability Prevention Program. Clin J Pain. 2003;19(2):97-104. doi: 10.1097/00002508-200303000-00004 [DOI] [PubMed] [Google Scholar]
- 18.Sobol-Kwapinska M, Bąbel P, Plotek W, Stelcer B. Psychological correlates of acute postsurgical pain: a systematic review and meta-analysis. Eur J Pain. 2016;20(10):1573-1586. doi: 10.1002/ejp.886 [DOI] [PubMed] [Google Scholar]
- 19.Chen AF, Landy DC, Kumetz E, Smith G, Weiss E, Saleeby ER. Prediction of postoperative pain after Mohs micrographic surgery with 2 validated pain anxiety scales. Dermatol Surg. 2015;41(1):40-47. doi: 10.1097/DSS.0000000000000224 [DOI] [PubMed] [Google Scholar]
- 20.Sommer M, de Rijke JM, van Kleef M, et al. . Predictors of acute postoperative pain after elective surgery. Clin J Pain. 2010;26(2):87-94. doi: 10.1097/AJP.0b013e3181b43d68 [DOI] [PubMed] [Google Scholar]
- 21.Sullivan MJ, Neish N. The effects of disclosure on pain during dental hygiene treatment: the moderating role of catastrophizing. Pain. 1999;79(2-3):155-163. doi: 10.1016/S0304-3959(98)00163-8 [DOI] [PubMed] [Google Scholar]
- 22.Adams H, Ellis T, Stanish WD, Sullivan MJL. Psychosocial factors related to return to work following rehabilitation of whiplash injuries. J Occup Rehabil. 2007;17(2):305-315. doi: 10.1007/s10926-007-9082-3 [DOI] [PubMed] [Google Scholar]
- 23.Nanitsos E, Vartuli R, Forte A, Dennison PJ, Peck CC. The effect of vibration on pain during local anaesthesia injections. Aust Dent J. 2009;54(2):94-100. doi: 10.1111/j.1834-7819.2009.01100.x [DOI] [PubMed] [Google Scholar]
- 24.Park KY, Lee Y, Hong JY, Chung WS, Kim MN, Kim BJ. Vibration anesthesia for pain reduction during intralesional steroid injection for keloid treatment. Dermatol Surg. 2017;43(5):724-727. doi: 10.1097/DSS.0000000000001040 [DOI] [PubMed] [Google Scholar]
- 25.Kuwahara H, Ogawa R. Using a vibration device to ease pain during facial needling and injection. Eplasty. 2016;16:e9. [PMC free article] [PubMed] [Google Scholar]
- 26.Smith KC, Comite SL, Balasubramanian S, Carver A, Liu JF. Vibration anesthesia: a noninvasive method of reducing discomfort prior to dermatologic procedures. Dermatol Online J. 2004;10(2):1. [PubMed] [Google Scholar]
- 27.Fix WC, Chiesa-Fuxench ZC, Shin T, et al. . Use of a vibrating kinetic anesthesia device reduces the pain of lidocaine injections: a randomized split-body trial. J Am Acad Dermatol. 2019;80(1):58-59. doi: 10.1016/j.jaad.2018.08.011 [DOI] [PubMed] [Google Scholar]
- 28.Gresham KA, Carroll BT. A simple elastomer-pad vibratory dampener to maximize pain control of injections in patient’s undergoing dermatological surgery. Dermatol Surg. 2016;42(6):788-790. doi: 10.1097/DSS.0000000000000718 [DOI] [PubMed] [Google Scholar]
- 29.Mally P, Czyz CN, Chan NJ, Wulc AE. Vibration anesthesia for the reduction of pain with facial dermal filler injections. Aesthetic Plast Surg. 2014;38(2):413-418. doi: 10.1007/s00266-013-0264-4 [DOI] [PubMed] [Google Scholar]
- 30.Ungor C, Tosun E, Dayisoylu EH, Taskesen F, Senel FC. The effects of vibration on pain and anxiety during local anesthesia administration. JSM Dent. 2014;2(1):1022. [Google Scholar]
- 31.Eichhorn MG, Karadsheh MJ, Krebiehl JR, Ford DM, Ford RD. Vibration for pain reduction in a plastic surgery clinic. Plast Surg Nurs. 2016;36(2):63-68. doi: 10.1097/PSN.0000000000000134 [DOI] [PubMed] [Google Scholar]
- 32.Duplisea MJ, Flores K. Buzzing away the pain: using an electric toothbrush for vibration anesthesia during painful procedures. Pediatr Dermatol. 2019;36(3):414-415. doi: 10.1111/pde.13802 [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Dong W, Wang M, Xu N. Investigation of the efficacy and safety of topical vibration anesthesia to reduce pain from cosmetic botulinum toxin A injections in Chinese patients: a multicenter, randomized, self-controlled study. Dermatol Surg. 2017. doi: 10.1097/DSS.0000000000001349 [DOI] [PubMed] [Google Scholar]
- 34.Pedersen C, Miller M, Xu KT, Carrasco L, Smith C, Richman PB. Use of a dental vibration tool to reduce pain from digital blocks: a randomized controlled trial. Reg Anesth Pain Med. 2017;42(4):458-461. doi: 10.1097/AAP.0000000000000584 [DOI] [PubMed] [Google Scholar]
- 35.Sharma P, Czyz CN, Wulc AE. Investigating the efficacy of vibration anesthesia to reduce pain from cosmetic botulinum toxin injections. Aesthet Surg J. 2011;31(8):966-971. doi: 10.1177/1090820X11422809 [DOI] [PubMed] [Google Scholar]
- 36.Fayers T, Morris DS, Dolman PJ. Vibration-assisted anesthesia in eyelid surgery. Ophthalmology. 2010;117(7):1453-1457. doi: 10.1016/j.ophtha.2009.11.025 [DOI] [PubMed] [Google Scholar]
- 37.Nasehi A, Bhardwaj S, Kamath A-T, Gadicherla S, Pentapati K-C. Clinical pain evaluation with intraoral vibration device during local anesthetic injections. J Clin Exp Dent. 2015;7(1):e23-e27. doi: 10.4317/jced.51643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Treister R, Lawal OD, Shecter JD, et al. . Accurate pain reporting training diminishes the placebo response: Results from a randomised, double-blind, crossover trial. PLoS One. 2018;13(5):e0197844. doi: 10.1371/journal.pone.0197844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olsen MF, Bjerre E, Hansen MD, et al. . Pain relief that matters to patients: systematic review of empirical studies assessing the minimum clinically important difference in acute pain. BMC Med. 2017;15(1):35. doi: 10.1186/s12916-016-0775-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 41.Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain. 2011;152(10):2399-2404. doi: 10.1016/j.pain.2011.07.005 [DOI] [PubMed] [Google Scholar]
- 42.Gan TJ, Habib AS, Miller TE, White W, Apfelbaum JL. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin. 2014;30(1):149-160. doi: 10.1185/03007995.2013.860019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable. ANOVA and Multivariate Regression Analysis
Data Sharing Statement