Key Points
Question
Can a shortened course of adjuvant oxaliplatin-based chemotherapy reduce peripheral sensory neuropathy (PSN) without compromising efficacy in patients with stage III colon cancer?
Findings
In this phase 3 randomized clinical trial of 1313 patients, 3 months of adjuvant therapy significantly reduced the rate of any grade of PSN at 3 years, compared with 6 months of treatment. The incidence of any grade of PSN lasting for 3 years was significantly lower for the chemotherapy drug capecitabine plus oxaliplatin (CAPOX) than for the drug modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6); these treatment outcomes were consistent with those of the International Duration Evaluation of Adjuvant Chemotherapy (IDEA) Collaboration.
Meaning
A 3-month chemotherapy regimen of CAPOX may be the most appropriate treatment option for colon cancer, particularly in low-risk patients.
This phase 3 randomized clinical trial evaluates the outcomes and the incidence of peripheral sensory neuropathy of 3 months vs 6 months of adjuvant oxaliplatin-based chemotherapy for colon cancer.
Abstract
Importance
Oxaliplatin-based chemotherapy is associated with debilitating peripheral sensory neuropathy (PSN) for patients with stage III colon cancer.
Objective
To assess disease-free survival (DFS) and long-lasting PSN in patients treated with 3 vs 6 months of adjuvant oxaliplatin-based chemotherapy.
Design, Setting, and Participants
An open-label, multicenter, phase 3 randomized clinical trial of 1313 Asian patients with stage III colon cancer was conducted investigating the noninferiority of 3 vs 6 months of adjuvant oxaliplatin-based chemotherapy. From August 1, 2012, to June 30, 2014, participants were randomized to the 2 treatment groups. Data were analyzed from July 2017 to June 2018.
Interventions
Patients were randomized to receive 3 or 6 months of adjuvant chemotherapy. The choice of chemotherapy regimen, with the drugs modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or capecitabine plus oxaliplatin (CAPOX), was at the discretion of the treating physician.
Main Outcomes and Measures
The primary outcome was DFS. Secondary end points included the evaluation of PSN for up to 3 years and overall survival.
Results
Of the 1313 patients (651 were women and mean age was 66 [range, 28-85] years) enrolled and randomized, 22 were not treated because 10 were unable to begin treatment within 2 weeks of enrollment, 7 withdrew their consent, and 5 were not treated for various other reasons. Of 1291 patients treated (650 in the 3-month arm and 641 in the 6-month arm), 969 (75%) received the chemotherapy drug CAPOX. The hazard ratio (HR) for DFS of the 3-month arm compared with the 6-month arm was 0.95 (95% CI, 0.76-1.20). Hazard ratios were 1.07 (95% CI, 0.71-1.60) and 0.90 (95% CI, 0.68-1.20) for the drugs mFOLFOX6 and CAPOX, and 0.81 (95% CI, 0.53-1.24) and 1.07 (95% CI, 0.81-1.40) for patients with low-risk disease (TNM classification stages T1-3 and N1) and high-risk disease (stages T4 or N2), respectively. The rates of any grade of PSN lasting for 3 years in the 3-month vs 6-month treatment arms were 9.7% vs 24.3% (P < .001). Incidence of PSN lasting for 3 years was significantly lower for patients treated with CAPOX than for patients treated with mFOLFOX6 in both the 3-month (7.9% vs 15.7%; P = .04) and 6-month arms (21.0% vs 34.1%; P = .02).
Conclusions and Relevance
The incidence of long-lasting PSN was significantly lower for 3 months than for 6 months of therapy, and significantly lower for treatment with the drug CAPOX than with mFOLFOX6. Since the shortened therapy duration did not compromise outcomes, a 3-month course of CAPOX may be the most appropriate treatment option, particularly for patients with low-risk disease.
Trial Registration
UMIN Clinical Trials Registry: UMIN000008543
Introduction
Adjuvant oxaliplatin-based fluorouracil, leucovorin, and oxaliplatin (FOLFOX) or capecitabine plus oxaliplatin (CAPOX) chemotherapy is the standard of care for patients with stage III colon cancer following surgery, as recommended by several treatment guidelines.1,2,3
The FOLFOX4 regimen has proven efficacy not only in the treatment of metastatic colorectal cancer4,5,6 but also in the adjuvant treatment of resected stage III colon cancers, as demonstrated by the pivotal MOSAIC7,8 and MASCOT9 trials conducted in Western European and Asian patient populations, respectively. More recently, the modified FOLFOX6 (mFOLFOX6) regimen has been substituted for the FOLFOX4 regimen in the adjuvant setting.10,11 The mFOLFOX6 regimen is easier to administer than FOLFOX4 and has been shown to be tolerable in the adjuvant treatment of Japanese patients with resected stage II and III colon cancer in the JOIN trial.12 The oral fluoropyrimidine capecitabine also has proven efficacy in the adjuvant setting13,14 and has been shown in a randomized phase 3 trial to have equal therapeutic efficacy to mFOLFOX6 when combined with oxaliplatin (CAPOX).15
Many patients, however, develop peripheral sensory neuropathy (PSN) during the standard 6-month administration of adjuvant therapy, leading to treatment modification or discontinuation.16,17,18,19,20 As an adverse event, PSN can be troublesome in daily life, and there have been few reports on patient recovery from PSN following the completion of treatment.8,16,18 In the key trials of adjuvant oxaliplatin-based therapy, the incidences of grade 3 or higher PSN due to FOLFOX4 therapy during treatment were 12.4% and 5.7% for the MOSAIC and Asian MASCOT trials, respectively.7,8,9 The incidences of grade 3 or higher PSN at the end of the studies due to adjuvant mFOLFOX6 therapy were 14.4% and 5.8% for the NSABP C-08 trial10 and JOIN trial,12 respectively. In turn, the cumulative treatment completion rates were 67.0%, 74.7%, and 81.8% for the JOIN, MOSAIC, and MASCOT trials, respectively.
Peripheral sensory neuropathy is regarded as the major toxic adverse event related to oxaliplatin-based therapy, but few studies have addressed the optimal duration of oxaliplatin-based adjuvant therapy, which is typically 6 months. Therefore, we conducted the present phase 3 ACHIEVE (adjuvant chemotherapy for colon cancer with high evidence) trial of 3 vs 6 months of mFOLFOX6 or CAPOX adjuvant chemotherapy to investigate the possible implications for Asian patients of reducing the duration of adjuvant therapy to 3 months. The primary objective of the ACHIEVE trial was to provide data on Asian patients for the International Duration Evaluation of Adjuvant Chemotherapy (IDEA) pooled analysis study to investigate the noninferiority of 3 vs 6 months of FOLFOX or CAPOX adjuvant chemotherapy.21 The IDEA pooled analysis study21 additionally collected the data from 5 other phase 3 trials (SCOT [United Kingdom, Denmark, Spain, Sweden, Australia, New Zealand],22 TOSCA [Italy],23 CALGB/SWOG 80702 [United States and Canada],21 IDEA-France [France],24 and HORG [Greece]21) to compare the therapeutic effect (noninferiority) of 3 months vs 6 months of adjuvant treatment in patients with stage III colon cancer worldwide.
The results of the ACHIEVE trial for toxic events during the course of treatment and for treatment compliance have been reported previously.25 Herein, we report the results for efficacy and long-term PSN for the ACHIEVE trial and address the incidence of long-lasting PSN according to both treatment duration (3 vs 6 months) and treatment regimen (CAPOX or FOLFOX drugs).
Methods
Study Design
The ACHIEVE trial was an open-label, multicenter randomized phase 3, noninferiority trial conducted in Japan. Patients who had undergone potentially curative resection for stage III colon cancer were randomly assigned 1:1 to receive either 6 months or 3 months of adjuvant oxaliplatin-based chemotherapy (mFOLFOX6 or CAPOX).
Patient inclusion and exclusion criteria as well as protocol amendment are described in the eMethods of Supplement 1. The institutional review board at each study center approved the protocol (Supplement 2), and the study was conducted by the Japanese Foundation for Multidisciplinary Treatment of Cancer (JFMC), a noncommercial organization for investigator-initiated cancer trials, in accordance with the principles expressed in the Declaration of Helsinki. All patients provided written informed consent prior to enrollment. This study is registered with the UMIN Clinical Trial Registry (Trial Identifier, UMIN000008543).
Treatment
Patients were randomized to receive either a 6-month or 3-month treatment regimen. Choice of mFOLFOX6 or CAPOX was at the discretion of the treating physician. Treatment with mFOLFOX6 involved the administration of 85 mg/m2 of oxaliplatin as a 2-hour intravenous infusion, bolus fluorouracil (400 mg/m2), and l-leucovorin (200 mg/m2) on day 1, followed by an infusion of 2400 mg/m2 of fluorouracil administered over 46 hours during days 1 to 3. This treatment regimen was repeated every 2 weeks (14 days), for a maximum of 12 cycles or 6 cycles for patients in the 6-month and 3-month treatment groups, respectively. Treatment with CAPOX included a 2-hour intravenous infusion of 130 mg/m2 of oxaliplatin on day 1 and oral capecitabine, 2000 mg/m2/d, from the evening of day 1 to the morning of day 15. Capecitabine was administered twice daily at a dose of 1000 mg/m2 within 30 minutes of the patients’ morning and evening meals. Treatment with CAPOX was repeated every 3 weeks (21 days), with a maximum of 8 cycles or 4 cycles being administered to patients in the 6-month and 3-month treatment groups, respectively. If continuing mFOLFOX6 or CAPOX therapy was considered to be difficult owing to oxaliplatin-related adverse events, mFOLFOX6 therapy was switched to fluorouracil/l-leucovorin therapy and CAPOX therapy was switched to capecitabine monotherapy, as outlined in the protocol (Supplement 2). Subsequent dose escalations of fluorouracil or capecitabine were not allowed. After administration of the study regimens was completed, no further adjuvant chemotherapy was permitted.
Outcomes
The primary efficacy endpoint was disease-free survival (DFS) in accordance with the requirements of the IDEA Collaboration study, and was defined as the time from randomization to relapse or death from any cause, whichever occurred first. Secondary colorectal cancers were regarded as DFS events, whereas noncolorectal tumors were to be disregarded in the analysis. Secondary end points included incidence and longitudinal pattern of PSN, overall survival, time to treatment failure, toxic events, treatment completion rate, dose intensity, and evaluation of the association between clinical outcome and the number of involved and dissected lymph nodes. The results for time to treatment failure and toxic events have been presented elsewhere.25 In this article, we report results for DFS and the incidence and longitudinal pattern of PSN.
Procedures
Patients were evaluated for disease recurrence with chest, abdominal, and pelvic computed tomography scans taken every 6 months from the time of enrollment for a planned period of 6 years. Colonoscopies were to be performed at the end of the first and third years following enrollment. Investigators’ assessments for disease recurrence have been documented in this study. Evaluation of adverse events was performed in accordance with the Common Terminology Criteria for Adverse Events Version 4.0 and was carried out at the end of each treatment cycle and 4 weeks after the completion of 8 cycles and 12 cycles of CAPOX and mFOFOX6 therapy, respectively, for patients in the 6-month therapy arm, and after 4 cycles and 6 cycles of CAPOX and mFOFOX6 therapy, respectively, in the 3-month therapy arm. Hand-foot syndrome (HFS) and PSN were to be evaluated every 3 months in all patients for the first 3 years from the initiation of the study and every 6 months thereafter for years 4 to 6.
Randomization and Masking
Patients were enrolled by study investigators. Eligible patients were randomized 1:1 to 6 months or 3 months of adjuvant oxaliplatin-based chemotherapy (mFOLFOX6 or CAPOX) via a dynamic allocation method with a web-response system. The e-Trial Co Ltd (Tokyo) developed the randomization system and the JFMC data center maintained it. Randomization was stratified by (1) the number of lymph nodes (<3 vs >3); (2) treatment center; (3) chemotherapy regimen (mFOLFOX6 vs CAPOX); (4) primary tumor site (colon vs rectosigmoid vs multiple); and (5) age (<70 vs ≥70 years). There was no masking of patients or investigators after assignment to treatment groups.
Statistical Analysis
The principal aim of the study was to provide data on Asian patients for the pooled analysis of data for the IDEA Collaboration (IDEA-France, SCOT, CALGB/SWOG C80702, TOSCA, and HORG) investigation of the noninferiority of 3 vs 6 months of FOLFOX or CAPOX adjuvant chemotherapy.21 The noninferiority margin, significance level, type 1 error rate, and power were not defined for the ACHIEVE study and accordingly a sample-size calculation based on hypothesis testing was not planned. The targeted patient accrual was determined to be more than 1200 patients (600 patients per group), during the 3-year registration period, based on expected accrual rate and the IDEA Collaboration study request that more than 1000 patients would be required for the analysis of regional differences.
The frequency of PSN at 3 years was compared between the treatment groups using χ2 tests. The DFS curves were derived by Kaplan-Meier estimation. The hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using the Cox proportional hazard model as summary measures of the difference in therapeutic effect between the 6-month and 3-month treatment groups. The DFS analyses were performed according to preplanned subgroups including risk (low or high) and regimen (mFOLFOX6 or CAPOX).
In alignment with the IDEA Collaboration, all analyses were conducted on the modified intention-to-treat (mITT) population, which included patients who were randomized and received at least 1 dose of treatment. All P values were reported as 2-sided and P < .05 was considered to be statistically significant. No multiple comparison analysis was performed. All analyses were performed using SAS version 9.4. The trial protocol, history of protocol changes, and the statistical analysis plans are provided in Supplement 2. Data were analyzed between July 2017 and June 2018.
Results
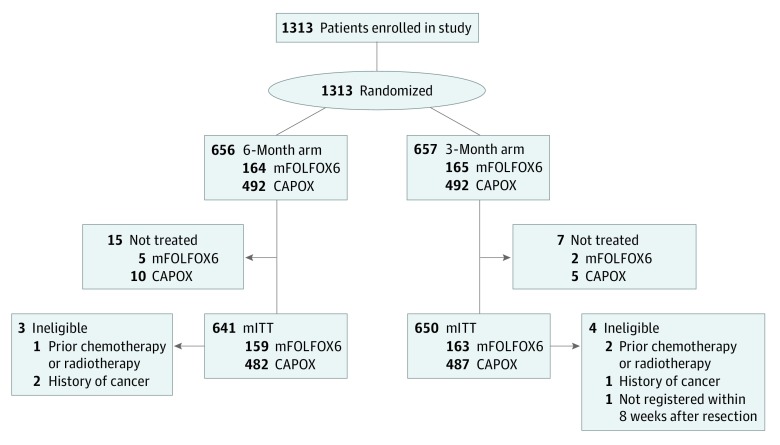
Between August 1, 2012, and June 30, 2014, 1313 patients were enrolled at 244 centers across Japan and randomly assigned to receive either 6 months or 3 months of adjuvant oxaliplatin-based mFOLFOX6 or CAPOX therapy at the discretion of the treating physician as outlined in the CONSORT diagram (Figure 1). Of 1313 patients enrolled, 22 were not treated: 10 patients were unable to begin the study treatments within 2 weeks of enrollment, 7 patients withdrew their consent, and 5 patients were not treated for various other reasons. Thus, the mITT patient population for the current analyses comprised 1291 patients. After of treatment courses had begun, 7 patients were found to be ineligible, including 3 patients with prior chemotherapy or radiation therapy before enrollment, 3 with a history of malignant disease, and 1 patient who was longer than 8 weeks postresection. Median follow-up period was 39 months.
Figure 1. CONSORT Flow Diagram Showing Patient Disposition.
CAPOX indicates capecitabine plus oxaliplatin; mFOLFOX6, modified fluorouracil, l-leucovorin, and oxaliplatin; mITT, modified intention to treat.
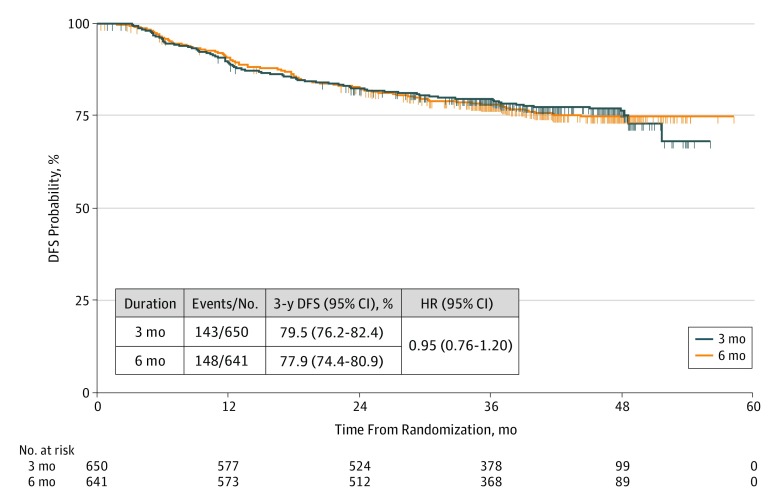
Baseline characteristics for the patients in each arm of the mITT population were well balanced across the 2 treatment groups and across the 2 therapy regimens (Table 1). Seventy-five percent of patients in the mITT population (969/1291) received CAPOX therapy. At the time of analysis, 291 events had been observed (148 in the 6-month arm and 143 in the 3-month arm). The 3-year DFS for patients in the 3-month arm was 79.5% (95% CI, 76.2%-82.4%), and in the 6-month arm it was 77.9% (95% CI, 74.4%-80.9%), yielding an HR of 0.95 (95% CI, 0.76-1.20) (Figure 2). Analysis of 3-year DFS rates according to risk group showed that for patients with low-risk disease (TNM classifications T1-3 and N1), the 3-year DFS rate was 90.5% (95% CI, 87.0%-93.1%) for 3 months of treatment and 87.3% (95% CI, 83.3%-90.5%) for 6 months of treatment (HR, 0.81; 95% CI, 0.53-1.24).For patients with high-risk disease (TNM classifications T4 or N2), DFS rates were 65.4% (95% CI, 59.6%-70.7%) for 3 months of treatment and 66.5% (95% CI, 60.6%-71.7%) for 6 months of treatment (HR, 1.07; 95% CI, 0.81-1.40) (eFigure 1 in Supplement 1). Analysis of DFS according to regimen showed a 3-year DFS rate of 73.9% (95% CI, 66.4%-80.0%) for 3 months of mFOLFOX6 treatment vs 72.3% (95% CI, 64.5%-78.7%) for 6 months of treatment (HR, 1.07; 95% CI, 0.71-1.60), and a 3-year DFS rate of 81.4% (95% CI, 77.6%-84.6%) for 3 months of CAPOX treatment vs 79.7% (95% CI, 75.8%-83.1%) for 6 months of CAPOX treatment (HR, 0.90; 95% CI, 0.68-1.20) (eFigure 2 in Supplement 1). Analysis of 3-year DFS according to both patient risk group (T stage and N stage) and regimen showed 3 months of mFOLFOX6 treatment to have worse outcomes than 6 months of mFOLFOX6 treatment in patients with low-risk (T1-3 and N1) disease (HR, 1.24; 95% CI, 0.59-2.6), but 3 months of CAPOX therapy to be better than 6 months of CAPOX therapy in patients with low-risk disease (HR, 0.64; 95% CI, 0.38-1.08) (Figure 3). For patients with high-risk (T4 or N2) disease, the HRs were 1.09 (95% CI, 0.67-1.78) and 1.06 (95% CI, 0.76-1.48) for mFOLFOX6 and CAPOX therapies, respectively (Figure 3). We note that the HRs in various subgroup analyses were consistent with those seen in the 5 other studies in the IDEA Collaboration (data not shown).21
Table 1. Patient Characteristics in the mITT Populationa,b.
| Characteristic | Treatment Arm | |||
|---|---|---|---|---|
| 6-month (n = 641) | 3-month (n = 650) | |||
| mFOLFOX6 (n = 159) | CAPOX (n = 482) | mFOLFOX6 (n = 163) | CAPOX (n = 487) | |
| Age, median (range), y | 67 (34-82) | 65 (28-85) | 69 (31-85) | 65 (29-83) |
| Sex | ||||
| Male | 81 (51) | 239 (50) | 77 (47) | 252 (52) |
| Female | 78 (49) | 243 (50) | 86 (53) | 235 (48) |
| ECOG PS | ||||
| 0 | 155 (97) | 467 (97) | 156 (96) | 467 (96) |
| 1 | 4 (3) | 15 (3) | 7 (4) | 20 (4) |
| Tumor size, median (range), mm | 45 (6-110) | 42 (8-160) | 48 (8-120) | 42 (1-145) |
| T stage | ||||
| T1-3 | 111 (70) | 352 (73) | 112 (69) | 353 (72) |
| T4 | 48 (30) | 130 (27) | 51 (31) | 134 (28) |
| N stage | ||||
| N1 | 113 (71) | 362 (75) | 120 (74) | 364 (75) |
| N2 | 46 (29) | 120 (25) | 43 (26) | 123 (25) |
| No. of nodes examined, median (range) | 21 (2-75) | 20 (1-122) | 20 (1-123) | 21 (4-104) |
| No. of positive nodes, median (range) | 2 (1-31) | 2 (1-25) | 2 (1-26) | 2 (1-23) |
| Days from surgery to chemotherapy, median (range) | 41 (15-65) | 40 (15-69) | 39 (16-67) | 40 (10-70) |
Abbreviations: CAPOX, capecitabine plus oxaliplatin; ECOG PS, Eastern Cooperative Oncology Group Performance Status; mFOLFOX6, modified fluorouracil, l-leucovorin, and oxaliplatin; mITT, modified intention to treat.
Unless otherwise noted, data are expressed as number (percentage) of patients.
The mITT population was derived as previously described.21
Figure 2. Overall Disease-Free Survival (DFS) According to Duration of Adjuvant Treatment.
HR indicates hazard ratio; No., number of patients.
Figure 3. Disease-Free Survival (DFS) Rates According to Disease Risk and Regimen Used.
Three-year DFS rates in patients with low-risk (A and B) and high-risk (C and D) disease treated with mFOLFOX6 (A and C) or CAPOX (B and D) regimens for 3 or 6 months. CAPOX indicates capecitabine plus oxaliplatin; HR, hazard ratio; mFOLFOX6, modified fluorouracil, l-leucovorin, and oxaliplatin; No., number of patients.
During treatment, 13% (84/650) and 0.9% (6/650) of patients in the 3-month arm experienced grade 2 or 3 PSN, respectively, compared with 30% (195/641) and 6% (38/641) of patients in the 6-month arm (Table 2). At 3 years (36 months) after the start of treatment, grade 1, 2, and 3 PSN were observed in 10% (37/380), 0% (0/380), and 0% (0/380) of patients in the 3-month arm, and in 21% (78/363), 2% (9/363), and 0.3% (1/363) of patients in the 6-month arm.
Table 2. Time Course of Occurrence and Recovery From Peripheral Sensory Neuropathy (PSN)a.
| PSN Grade | No. (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAPOX | FOLFOX | Total | ||||||||||
| During Treatment | ≤12 mo | ≤24 mo | ≤36 mo | During Treatment | ≤12 mo | ≤24 mo | ≤36 mo | During Treatment | ≤12 mo | ≤24 mo | ≤36 mo | |
| 3-Month Treatment | ||||||||||||
| Patients, No. | 487 | 476 | 353 | 291 | 163 | 158 | 107 | 89 | 650 | 634 | 460 | 380 |
| Grade 3 | 5 (1.0) | 1 (0.2) | 0 | 0 | 1 (0.6) | 0 | 0 | 0 | 6 (0.9) | 1 (0.2) | 0 | 0 |
| Grade 2 | 65 (13.0) | 2 (0.4) | 1 (0.3) | 0 | 19 (12.0) | 2 (1.0) | 0 | 0 | 84 (13.0) | 4 (0.6) | 1 (0.2) | 0 |
| Grade 1 | 282 (58.0) | 108 (23.0) | 45 (13.0) | 23 (8.0) | 102 (63.0) | 44 (28.0) | 18 (17.0) | 14 (16.0) | 384 (59.0) | 152 (24.0) | 63 (14.0) | 37 (10.0) |
| Grade 0 | 135 (28.0) | 365 (77.0) | 307 (87.0) | 268 (92.0) | 41 (25.0) | 112 (71.0) | 89 (83.0) | 75 (84.0) | 176 (27.0) | 477 (75.0) | 396 (86.0) | 343 (90.0) |
| 6-Month Treatment | ||||||||||||
| Patients, No. | 482 | 437 | 321 | 272 | 159 | 152 | 106 | 91 | 641 | 589 | 427 | 363 |
| Grade 3 | 30 (6.0) | 2 (0.5) | 1 (0.3) | 0 | 8 (5.0) | 2 (1.0) | 1 (0.9) | 1 (1.0) | 38 (6.0) | 4 (0.7) | 2(0.5) | 1 (0.3) |
| Grade 2 | 146 (30.0) | 25 (6.0) | 5 (2.0) | 4 (1.0) | 49 (31.0) | 10 (7.0) | 6 (6.0) | 5 (6.0) | 195 (30.0) | 35 (6.0) | 11 (3.0) | 9 (2.0) |
| Grade 1 | 208 (43.0) | 161 (37.0) | 95 (30.0) | 53 (20.0) | 85 (54.0) | 80 (53.0) | 40 (38.0) | 25 (28.0) | 293 (46.0) | 241 (41.0) | 135 (32.0) | 78 (21.0) |
| Grade 0 | 98 (20.0) | 249 (5.0) | 220 (69.0) | 215 (79.0) | 17 (11.0) | 60 (40.0) | 59 (56.0) | 60 (66.0) | 115 (18.0) | 309 (53.0) | 279 (65.0) | 275 (76.0) |
Abbreviations: CAPOX, capecitabine plus oxaliplatin; mFOLFOX6, modified fluorouracil, l-leucovorin, and oxaliplatin; mITT, modified intention to treat.
This table shows the proportion of patients in the mITT population (n = 1291) with different PSN grades during and following 3 and 6 months of treatment. The rates of PSN lasting for 3 years for CAPOX vs FOLFOX treatments in the 3-month treatment arm were 8% vs 16% (P = .04) and for the 6-month treatment group were 21% vs 34% (P = .02).
The rate of any grade PSN lasting for 3 years in the 3- vs 6-month treatment arm was 8% vs 21% for CAPOX (P < .001) and 16% vs 34% for mFOLFOX6 (P < .001). Notably, the occurrence of PSN lasting for 3 years was significantly lower for the CAPOX regimen than for the FOLFOX regimen in both the 3-month (8% vs 16%; P = .04) and 6-month (21% vs 34%; P = .02) treatment groups. The time course of average PSN grade is shown in eFigure 3 in Supplement 1.
During treatment, 9% vs 5% and 2% vs less than 1% of patients in the 6- or 3-month treatment arms, respectively, experienced grade 2 or 3 HFS (eTable 1 in Supplement 1). No patient experienced grade 2 or 3 HFS in the 6- or 3-month treatment groups after 3 years of treatment (eTable 1 in Supplement 1). However, as shown previously,25 HFS was associated with the capecitabine/fluoropyrimidine chemotherapy backbone.
Discussion
The ACHIEVE study was 1 of the 6 prospective IDEA Collaboration trials and the only trial conducted in Asia. At the time the trial was initiated, little was known about any potential efficacy or safety differences for adjuvant chemotherapy for colon cancer between Asian and European/American patients because there was no worldwide collaborative study in which a substantive number of Asian patients had been enrolled. The present study, as part of the IDEA Collaboration, was the first such study to our knowledge and it is notable that no difference in terms of efficacy was observed. The HRs for DFS for 3 months vs 6 months of adjuvant chemotherapy in the mITT population and in various subgroup analyses were consistent with those seen in 5 other studies in the IDEA Collaboration.21
The ACHIEVE study shows 3 months of adjuvant treatment confers a 3-year DFS benefit of 1.6% vs 6 months of adjuvant treatment with an HR of 0.95 (95% CI, 0.76-1.20). Although this study did not define a noninferiority margin, the upper limit of the 95% CI exceeds the upper limit of 1.12 for noninferiority set by the IDEA Collaboration. Analysis of 3-year DFS according to patient disease risk (T stage and N stage) and regimen (mFOLFOX6 or CAPOX therapies) showed 3 months of mFOLFOX6 treatment to have worse outcomes than 6 months of mFOLFOX6 treatment in patients with low-risk disease, but 3 months of CAPOX therapy to have better outcomes than 6 months of CAPOX therapy in patients with low-risk disease.
As previously reported,25 for this study the 3 months of adjuvant chemotherapy significantly reduced the rate of occurrence of PSN during study treatment compared with 6 months of adjuvant chemotherapy. In this study, it was further observed that the incidence of patients experiencing any grade of PSN at 3 years after treatment was significantly lower for patients in the 3-month treatment arm than for those in the 6-month treatment arm. Also noteworthy was the observation that the percentage of patients with grade 1 PSN or higher at 3 years was significantly lower in patients receiving CAPOX therapy than in those receiving mFOLFOX6 therapy in both the 6-month (21% vs 34%) and 3-month (8% vs 16%) treatment groups. Little is known about the differences between CAPOX and FOLFOX drugs in terms of long-lasting neurotoxicity. Only 1 prospective observational study of 150 patients with colorectal cancer has previously reported that the cumulative PSN rate at the end of the treatment was significantly lower for patients receiving CAPOX than for those receiving FOLFOX.26 Although our exploratory analysis requires confirmation of the reproducibility of the results as part of the IDEA Collaboration as well as in a prospective randomized comparison, this is the first evidence that FOLFOX may be a more neurotoxic chemotherapy drug than CAPOX in terms of long-term PSN, despite comparable cumulative oxaliplatin doses.
Limitations
The main limitation of the trial was that the primary aim, to provide Asian data for the IDEA Collaboration, meant that the sample size did not allow any results of the efficacy analyses to be conclusive. However, the results were entirely consistent with those of the SCOT trial,22 in which 67% of patients received CAPOX.
Overall, the efficacy results for the ACHIEVE trial were consistent with those of the IDEA pooled analysis of data from 12 834 patients21 and suggested that for patients with low-risk colon cancer, 3 months of oxaliplatin-based adjuvant chemotherapy, preferably CAPOX, may be adequate, but for patients with high-risk colon cancer 6 months of adjuvant therapy may be required. Three months of adjuvant therapy resulted in a reduction by 1/3 or 1/2 in the percentage PSN rate when compared with 6 months of adjuvant therapy. However, the results of this study should be interpreted within the IDEA combined analysis as well as in terms of the reproducibility of the results across all trials. Patient attitude, the choice of regimen, and whether a patient has high- or low-risk disease, should all form part of the treatment decision-making process for patients with resected stage III colon cancer.
Conclusions
This study revealed that the rate of long-lasting PSN was significantly lower for patients in the 3-month treatment group than for those in the 6-month treatment group, and the rate of long-lasting PSN was also significantly lower for patients treated with CAPOX than with mFOLFOX6. Since the shortened therapy duration did not compromise outcomes, 3 months of CAPOX therapy might be the most appropriate treatment option, especially in patients with low-risk stage III colon cancer, although our results need to be interpreted within the IDEA combined analysis as well as in terms of the reproducibility of results across all trials.
eMethods.
eFigure 1. DFS According to Whether Patients Were Classified as Having Low-risk or High-risk Disease
eFigure 2. DFS According to Regimen Used
eFigure 3. PSN by Treatment Duration
eTable 1. Time Course of Occurrence and Recovery from HFS
Trial Protocol and Statistical Analysis Plan
Data Sharing Statement.
References
- 1.Labianca R, Nordlinger B, Beretta GD, et al. ; ESMO Guidelines Working Group . Early colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi64-vi72. doi: 10.1093/annonc/mdt354 [DOI] [PubMed] [Google Scholar]
- 2.Watanabe T, Muro K, Ajioka Y, et al. ; Japanese Society for Cancer of the Colon and Rectum . Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018;23(1):1-34. doi: 10.1007/s10147-017-1101-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network . NCCN Guidelines for Patients: Colon Cancer. https://www.nccn.org/patients/guidelines/colon/index.html. Accessed August 6, 2019.
- 4.André T, Bensmaine MA, Louvet C, et al. Multicenter phase II study of bimonthly high-dose leucovorin, fluorouracil infusion, and oxaliplatin for metastatic colorectal cancer resistant to the same leucovorin and fluorouracil regimen. J Clin Oncol. 1999;17(11):3560-3568. doi: 10.1200/JCO.1999.17.11.3560 [DOI] [PubMed] [Google Scholar]
- 5.de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18(16):2938-2947. doi: 10.1200/JCO.2000.18.16.2938 [DOI] [PubMed] [Google Scholar]
- 6.Giacchetti S, Perpoint B, Zidani R, et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18(1):136-147. doi: 10.1200/JCO.2000.18.1.136 [DOI] [PubMed] [Google Scholar]
- 7.André T, Boni C, Mounedji-Boudiaf L, et al. ; Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators . Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350(23):2343-2351. doi: 10.1056/NEJMoa032709 [DOI] [PubMed] [Google Scholar]
- 8.André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27(19):3109-3116. doi: 10.1200/JCO.2008.20.6771 [DOI] [PubMed] [Google Scholar]
- 9.Lee P-H, Park Y-S, Ji J-F, Fu Y-T, Ratanatharathorn V. Safety and tolerability of FOLFOX4 in the adjuvant treatment of colon cancer in Asian patients: The MASCOT study. Asia Pac J Clin Oncol. 2009;5(2):101-110. doi: 10.1111/j.1743-7563.2009.01199.x [DOI] [Google Scholar]
- 10.Allegra CJ, Yothers G, O’Connell MJ, et al. Initial safety report of NSABP C-08: a randomized phase III study of modified FOLFOX6 with or without bevacizumab for the adjuvant treatment of patients with stage II or III colon cancer. J Clin Oncol. 2009;27(20):3385-3390. doi: 10.1200/JCO.2009.21.9220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allegra CJ, Yothers G, O’Connell MJ, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol. 2011;29(1):11-16. doi: 10.1200/JCO.2010.30.0855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotaka M, Yoshino T, Oba K, et al. Initial safety report on the tolerability of modified FOLFOX6 as adjuvant therapy in patients with curatively resected stage II or III colon cancer (JFMC41-1001-C2: JOIN trial). Cancer Chemother Pharmacol. 2015;76(1):75-84. doi: 10.1007/s00280-015-2757-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmoll HJ, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results of the NO16968 randomized controlled phase III trial. J Clin Oncol. 2015;33(32):3733-3740. doi: 10.1200/JCO.2015.60.9107 [DOI] [PubMed] [Google Scholar]
- 14.Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352(26):2696-2704. doi: 10.1056/NEJMoa043116 [DOI] [PubMed] [Google Scholar]
- 15.Pectasides D, Karavasilis V, Papaxoinis G, et al. Randomized phase III clinical trial comparing the combination of capecitabine and oxaliplatin (CAPOX) with the combination of 5-fluorouracil, leucovorin and oxaliplatin (modified FOLFOX6) as adjuvant therapy in patients with operated high-risk stage II or stage III colorectal cancer. BMC Cancer. 2015;15:384. doi: 10.1186/s12885-015-1406-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kidwell KM, Yothers G, Ganz PA, et al. Long-term neurotoxicity effects of oxaliplatin added to fluorouracil and leucovorin as adjuvant therapy for colon cancer: results from National Surgical Adjuvant Breast and Bowel Project trials C-07 and LTS-01. Cancer. 2012;118(22):5614-5622. doi: 10.1002/cncr.27593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mols F, Beijers T, Lemmens V, van den Hurk CJ, Vreugdenhil G, van de Poll-Franse LV. Chemotherapy-induced neuropathy and its association with quality of life among 2- to 11-year colorectal cancer survivors: results from the population-based PROFILES registry. J Clin Oncol. 2013;31(21):2699-2707. doi: 10.1200/JCO.2013.49.1514 [DOI] [PubMed] [Google Scholar]
- 18.Pachman DR, Qin R, Seisler DK, et al. Clinical course of oxaliplatin-induced neuropathy: results from the randomized phase III trial N08CB (Alliance). J Clin Oncol. 2015;33(30):3416-3422. doi: 10.1200/JCO.2014.58.8533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pietrangeli A, Leandri M, Terzoli E, Jandolo B, Garufi C. Persistence of high-dose oxaliplatin-induced neuropathy at long-term follow-up. Eur Neurol. 2006;56(1):13-16. doi: 10.1159/000094376 [DOI] [PubMed] [Google Scholar]
- 20.Tofthagen C, Donovan KA, Morgan MA, Shibata D, Yeh Y. Oxaliplatin-induced peripheral neuropathy’s effects on health-related quality of life of colorectal cancer survivors. Support Care Cancer. 2013;21(12):3307-3313. doi: 10.1007/s00520-013-1905-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grothey A, Sobrero AF, Shields AF, et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med. 2018;378(13):1177-1188. doi: 10.1056/NEJMoa1713709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iveson TJ, Kerr RS, Saunders MP, et al. 3 versus 6 months of adjuvant oxaliplatin-fluoropyrimidine combination therapy for colorectal cancer (SCOT): an international, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2018;19(4):562-578. doi: 10.1016/S1470-2045(18)30093-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sobrero A, Lonardi S, Rosati G, et al. ; TOSCA Investigators . FOLFOX or CAPOX in stage II to III colon cancer: efficacy results of the Italian three or six colon adjuvant trial. J Clin Oncol. 2018;36(15):1478-1485. doi: 10.1200/JCO.2017.76.2187 [DOI] [PubMed] [Google Scholar]
- 24.André T, Vernerey D, Mineur L, et al. ; for PRODIGE investigators, GERCOR, Fédération Française de Cancérologie Digestive, and UNICANCER . Three versus 6 months of oxaliplatin-based adjuvant chemotherapy for patients with stage III colon cancer: disease-free survival results from a randomized, open-label, international duration evaluation of adjuvant (IDEA) France, phase III trial. J Clin Oncol. 2018;36(15):1469-1477. doi: 10.1200/JCO.2017.76.0355 [DOI] [PubMed] [Google Scholar]
- 25.Kotaka M, Yamanaka T, Yoshino T, et al. Safety data from the phase III Japanese ACHIEVE trial: part of an international, prospective, planned pooled analysis of six phase III trials comparing 3 versus 6 months of oxaliplatin-based adjuvant chemotherapy for stage III colon cancer. ESMO Open. 2018;3(3):e000354. doi: 10.1136/esmoopen-2018-000354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Argyriou AA, Velasco R, Briani C, et al. Peripheral neurotoxicity of oxaliplatin in combination with 5-fluorouracil (FOLFOX) or capecitabine (XELOX): a prospective evaluation of 150 colorectal cancer patients. Ann Oncol. 2012;23(12):3116-3122. doi: 10.1093/annonc/mds208 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eFigure 1. DFS According to Whether Patients Were Classified as Having Low-risk or High-risk Disease
eFigure 2. DFS According to Regimen Used
eFigure 3. PSN by Treatment Duration
eTable 1. Time Course of Occurrence and Recovery from HFS
Trial Protocol and Statistical Analysis Plan
Data Sharing Statement.