Abstract
Objective:
The current supply of acceptable donor lungs is not sufficient for the number of patients awaiting transplantation. We hypothesized that ex vivo lung perfusion (EVLP) with targeted drug therapy would allow successful rehabilitation and transplantation of donation after circulatory death (DCD) lungs exposed to 2-hours of warm ischemia.
Methods:
Donor porcine lungs were procured after 2-hours of warm ischemia post-cardiac arrest, and subjected to 4-hours of cold preservation or EVLP. ATL802, an adenosine A2B receptor antagonist, was administered to select groups. Four groups (n = 4/group) were randomized: cold preservation (Cold), cold preservation with ATL802 during reperfusion (Cold+ATL802), EVLP (EVLP), and EVLP with ATL802 during ex vivo perfusion (EVLP+ATL802). Lungs were subsequently transplanted, reperfused, and assessed by measuring dynamic lung compliance and oxygenation capacity.
Results:
EVLP+ATL802 significantly improved dynamic lung compliance compared with EVLP (25.0±1.8 vs 17.0±2.4 mL/cmH2O, p=0.04), and compared with cold preservation (Cold: 12.2±1.3, p=0.004; Cold+ATL802: 10.6±2.0 mL/cmH2O, p=0.002). Oxygenation capacity was highest in EVLP (440.4±37.0 vs Cold: 174.0±61.3 mmHg, p=0.037). No differences in oxygenation or pulmonary edema were observed between EVLP and EVLP+ATL802. A significant decrease in IL-12 expression in tissue and bronchoalveolar lavage was identified between groups EVLP and EVLP+ATL802, along with less neutrophil infiltration.
Conclusions:
Severely injured DCD lungs subjected to 2-hours of warm ischemia are successfully transplanted after enhanced EVLP with targeted drug therapy. Increased utilization of lungs following uncontrolled donor cardiac death and prolonged warm ischemia may be possible and may improve transplant wait list times and mortality.
Graphical Abstract
INTRODUCTION
Lung transplantation is a life-saving operation for patients with end-stage lung disease, but is accompanied by significant hurdles that must be overcome to ensure a positive result. Central to the discussion is the shortage of acceptable donor lungs, given the conservative acceptance criteria used by most surgeons and the increased risk of primary graft dysfunction (PGD) with transplantation of marginal lungs. [1] Considering outcomes after lung transplantation are the worst of any solid organ, strategies to optimize lung utilization and increase the likelihood of a successful outcome are needed. [2]
The expansion of donor acceptance criteria to include donation after circulatory death (DCD) lungs is one such approach that may substantially increase the pool of available organs. [3] Currently, DCD lungs are used in less than 2% of lung transplants per year due to the increased risk of ischemia-reperfusion (IR) injury, a major cause of PGD and a risk factor for the development of chronic graft rejection. [2, 4] The use of ex vivo lung perfusion (EVLP) prior to transplantation may help alleviate the resultant IR injury. [5] Using EVLP to perform donor lung assessment followed by targeted therapeutic rehabilitation may allow for successful transplantation of otherwise unacceptable lungs.
Targeting various cellular receptors in the lung, such as adenosine and sphingosine-1-phosphate receptors, our laboratory has shown improved outcomes and attenuation of IR injury after transplantation. [6, 7] Adenosine, a bioactive nucleoside, plays a significant role in purinergic signaling during inflammation through its interaction with four G-protein-coupled receptors (A1, A2A, A2B, A3). [8] Altering the level of adenosine receptor signaling with targeted drug delivery during EVLP may help improve outcomes after lung transplantation. The role of adenosine A2B receptor (A2BR) activation has been found to be both pro-inflammatory and anti-inflammatory in different experimental models of lung injury. [9–12] Our laboratory demonstrated that it is pro-inflammatory and that selective antagonism of A2BR attenuates IR injury. [13]
The objective of the current study was to determine if lungs procured following donor cardiac death and prolonged warm ischemia could be rehabilitated with EVLP and successfully transplanted, using a porcine model of lung transplantation. We hypothesized that use of EVLP with addition of an A2BR antagonist, as targeted drug therapy during the 4-hour ex vivo perfusion period, would allow for successful rehabilitation and transplantation of DCD lungs exposed to 2-hours of warm ischemia. The use of lungs for transplantation following uncontrolled donor cardiac death in the field could increase the pool of available organs, increase the number of lung transplants per year, and decrease wait list times and mortality.
MATERIALS AND METHODS
Animals and Study Groups
This study complied with the 1996 Guide for the Care and Use of Laboratory Animals as recommended by the US National Institutes of Health. The University of Virginia Animal Care and Use Committee approved the study protocol and all animals received humane care.
Mature domestic swine of both sexes (19–42 kg) underwent hypoxic cardiac arrest, followed by 2-hours of warm ischemia prior to cold preservation flush and procurement of the lungs. Two hours of warm ischemia time reflects the estimated amount of time necessary to procure lungs following uncontrolled donor cardiac death in the field. Donor lungs were randomized to four groups (n=4/group): 4-hours of cold preservation at 4°C (Cold), 4-hours of cold preservation at 4°C with the addition of ATL802 during the reperfusion period (Cold+ATL802), 4-hours of normothermic EVLP with Steen Solution™ (XVIVO Perfusion Inc, Englewood, CO) (EVLP), or 4-hours of EVLP supplemented with ATL802 (EVLP+ATL802) (Figure 1). In all groups, the left lung was subsequently transplanted into a size-matched recipient and reperfused for 4 hours in vivo. ATL802 (Lewis and Clark Pharmaceuticals, Charlottesville, VA), a selective A2BR antagonist, was given to groups Cold+ATL802 (via external jugular vein at start of reperfusion period) and EVLP+ATL802 (added to Steen at start of ex vivo perfusion period) as targeted anti-inflammatory drug therapy at a bolus dose of 1 mg/kg, based on previous studies. [13, 14] ATL802 is currently being used for preclinical studies only to determine the efficacy of A2BR antagonism.
Figure 1.
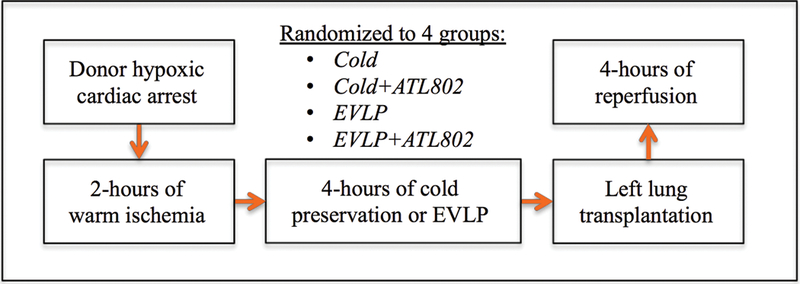
Schematic of experiment. Cold, transplantation after 4-hours of cold preservation; Cold+ATL802, transplantation after 4-hours of cold preservation with administration of ATL802 during reperfusion; EVLP, transplantation after 4-hours of EVLP; EVLP+ATL802, transplantation after 4-hours of EVLP with administration of ATL802 during ex vivo perfusion.
Donor Procurement Procedure
Procurement of donor lungs was performed as previously described. [15] Ketamine (50 mg/kg) and xylazine (5 mg/kg) were used for induction of anesthesia and intubation. Isoflurane (3%) and 1.0 fraction of inspired oxygen (FiO2) were used to maintain anesthesia. Animals were ventilated (tidal volume 8 mL/kg, respiratory rate 16–20 breaths/min, positive end-expiratory pressure 5 cmH2O) and an initial arterial blood gas (ABG) sample was analyzed. All donor animals received systemic heparin (200 U/kg, Hospira Inc., Lake Forest, IL) and had continuous electrocardiogram monitoring in place prior to clamping the endotracheal tube and inducing hypoxic cardiac arrest. Donor animals underwent 2-hours of warm ischemia from the time asystole was confirmed on the cardiac monitor.
A median sternotomy was then performed and a cardioplegia cannula (Terumo Heart Inc., Ann Arbor, MI) was inserted into the main pulmonary artery (PA) to deliver Prostaglandin-E1 (500 μg, Pfizer Inc., New York, NY) and cold Perfadex® (XVIVO Perfusion Inc., Englewood, CO). A total of 1.5 liters of Perfadex supplemented with 15,000 IU of heparin was flushed through the lungs after venting the left atrial (LA) appendage and ligating the vena cava. Ice slush was introduced into the thorax to rapidly cool the lungs. The trachea was clamped mid-inspiration and the heart-lung bloc was explanted.
For groups Cold and Cold+ATL802, the heart and right-lung were removed on the back-table and preparation of the left bronchus, PA, and LA cuff was completed. Retrograde flush with an additional 500 mL of cold Perfadex with heparin was completed and the lungs were placed in a protective plastic bag and stored at 4°C for 4-hours. For groups EVLP and EVLP+ATL802, the heart was removed on the back-table and the trachea, main PA, and LA cuff were prepared to allow for placement of the EVLP cannulas.
EVLP
Lungs randomized to groups EVLP and EVLP+ATL802 underwent 4-hours of normothermic EVLP. [16, 17] A yellow cannula (XVIVO Perfusion Inc., Englewood, CO) was placed in the main PA, a green cannula (XVIVO Perfusion Inc., Englewood, CO) was sutured to the LA cuff, and a 7–0 endotracheal tube was placed into the trachea. An additional 500 mL of cold Perfadex was flushed retrograde.
EVLP was initiated on both lungs as previously described. [18] The circuit was primed with 2L Steen supplemented with cefazolin (500 mg, APP Pharmaceuticals, Schaumburg, IL), methylprednisolone (500 mg, Pfizer Inc., New York, NY), and heparin (10,000 IU). ATL802 was added to the ex vivo perfusate for lungs randomized to EVLP+ATL802 and dimethyl sulfoxide (vehicle) was added for group EVLP. Surgeons were blinded as to whether the perfusate was supplemented with the treatment drug or vehicle. Flow was initiated (0.2 mL/min) and LA pressures maintained between 0–5 mmHg. The perfusate was warmed to 37°C over 30 minutes. Flow was titrated up to 40% of estimated cardiac output (100 mL/kg donor body weight) and when the perfusate reached 32°C, ventilation was started (tidal volume 8 mL/kg, respiratory rate 8 breaths/minute, positive end-expiratory pressure 5.0 cmH2O, FiO2 0.21). A tri-gas mixture (86% nitrogen, 8% carbon dioxide, 6% oxygen) was used to deoxygenate the perfusate.
Samples from the PA inflow and LA outflow were collected hourly following a 15-minute challenge with 1.0 FiO2 to measure the partial pressure of oxygen (PaO2). Airway pressures were measured hourly and used to calculate dynamic compliance. After 4-hours of EVLP, lungs were flushed anterograde with cold Perfadex (500 mL) and the left lung was separated off and prepared for transplantation.
Lung Transplant Procedure
Recipient animals were anesthetized and ventilated similar to donors. A multi-access cathether with PA cathether and an arterial catheter were placed. The animal was administered lidocaine (50 mg) and heparin (5000 IU). The transplant procedure was performed as previously described: left lateral thoracotomy, left pneumonectomy, left lung transplant (running sutures for anastomoses: end-to-end bronchial, end-to-end PA, and LA cuff to recipient LA appendage). [7] At the start of reperfusion, ATL802 was administered via external jugular vein injection for lungs randomized to group Cold+ATL802.
Post-transplant Reperfusion
In vivo reperfusion was maintained for 4-hours. Airway pressure measurements and systemic ABGs were performed every 30-minutes. Low-pressure lung recruitment was performed prior to each set of measurements. Superior and inferior pulmonary vein blood samples were obtained after 2-hours and at the completion of 4-hours of reperfusion for direct left lung PaO2 measurements. Adequate hemodynamics and acid/base status were maintained with use of normal saline, epinephrine, and sodium bicarbonate to meet the following goals: pH 7.35–7.45, base excess > −5, and mean arterial pressure > 55 mmHg. The lung was explanted after 4-hours of reperfusion and the animal euthanized.
Pulmonary Edema
Left lungs were weighed just prior to transplant and immediately after explant. Percent gross weight change from pre-transplantation to post-reperfusion was calculated to determine amount of pulmonary edema.
Cytokine Measurements
After explantation, two fresh tissue samples were obtained (upper and lower portion of lower lobe), flash frozen in liquid nitrogen, and stored at −80°C. FastPrep®−24 (MP Biomedicals, Santa Ana, CA) was used to homogenize tissue. Bicinchoninic acid protein assay (Pierce, Rockford, IL) was used to determine total protein concentration in the supernatant of each homogenized tissue sample. Bronchoalveolar lavage (BAL) of the upper lobe was performed with 30 mL of normal saline, centrifuged, and stored at −80°C. A commercially available multiplex immunosorbent assay (EMD Millipore, Billerica, MA) was used to measure levels of interleukin (IL)-1α, 1β, 4, 6, 8, 10, 12, 18, and TNF-α in tissue supernatant (normalized to equal protein concentrations) and BAL.
Histopathologic Assessment
The lower lobe was instilled with 10% buffered formalin via the bronchus after all fresh tissue sampling was complete, and then submerged in formalin. After storage overnight, peripheral lung tissue samples (n=4/lung) were obtained, paraffin-embedded and sectioned. One histology slide from each sample was stained with hematoxylin-eosin and two histology slides were used for immunohistochemistry evaluation of neutrophil infiltration.
The H&E stained slides were assessed by a masked pathologist for presence of lung injury. Each slide was scored based on the following three components: polymorphonuclear cells per 40X high-powered field (HPF) (0=<5, 1=6–10, 2=11–20, 3=>20), alveolar edema (0=<5%, 1=6–25%, 2=26–50%, 3=>50%), and interstitial inflammation (0=none, 1=minimal, 2=moderate, 3=severe), as previously described. [7]
For neutrophil immunohistochemistry staining, the primary antibody used was mouse monoclonal anti-porcine neutrophil antibody (MBA Biomedicals, Augst, Switzerland) and secondary antibody used was donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories Inc., West Grove, PA). After addition of avidin-biotin complex, slides were incubated for 30-minutes at room temperature, followed by incubation with 3,3-diaminobenzidine tetrahydrochloride (Dako Inc., Carpinteria, CA) to produce a brown precipitate, and finally counterstained with hematoxylin. [12] Five microscopic photographs of each slide were taken at 40X magnification. The number of neutrophils per HPF was counted by a masked reviewer and averaged per tissue sample to account for potential injury heterogeneity.
Statistical Analysis
One-way analysis of variance with Bonferroni’s multiple comparisons test and Student’s t-test were used to determine statistical significance between groups. Prism 6 (GraphPad Software Inc., La Jolla, CA) was used to perform statistical calculations and all data were reported as mean ± standard error of the mean, with p-value for significance of 0.05.
RESULTS
Lung Function
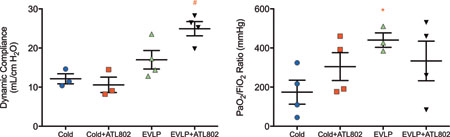
After transplantation and 4-hours of in vivo reperfusion, dynamic compliance was significantly higher in group EVLP+ATL802 compared with the vehicle EVLP group (25.0±1.8 vs. 17.0±2.4 mL/cmH2O, p=0.04), and compared to both non-EVLP groups (Cold: 12.2±1.3, p=0.004 and Cold+ATL802: 10.6±2.0 mL/cmH2O, p=0.002) (Figure 2A). Oxygenation capacity of the transplanted lung after 4-hours of reperfusion was highest in group EVLP (440.4±37.0 mmHg) and significant compared to Cold (174.0±61.3 mmHg, p=0.037) (Figure 2B). There were no significant differences in final PaO2/FiO2 ratios between groups Cold, Cold+ATL802, and EVLP+ATL802.
Figure 2.
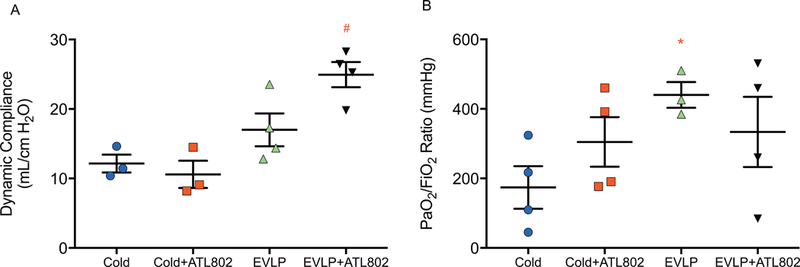
(A) Final dynamic compliance and (B) oxygenation (PaO2/FiO2) at the completion of 4-hours of reperfusion. Cold, transplantation after 4-hours of cold preservation; Cold+ATL802, transplantation after 4-hours of cold preservation with administration of ATL802 during reperfusion; EVLP, transplantation after 4-hours of EVLP; EVLP+ATL802, transplantation after 4-hours of EVLP with administration of ATL802 during ex vivo perfusion; PaO2/FiO2, partial pressure of oxygen/fraction of inspired oxygen. # p=0.04 vs EVLP, p=0.002 vs Cold+ATL802, and p=0.004 vs Cold, * p=0.037 vs Cold.
Prior to inducing hypoxic cardiac arrest, donor animals from all groups had similar PaO2/FiO2 ratios (Cold: 501.7±28.9, Cold+ATL802: 510.2±29.9, EVLP: 454.8±17.0, EVLP+ATL802: 490.3±39.8 mmHg, p=0.59) with an overall mean donor PaO2/FiO2 ratio of 489.3±14.5 mmHg. After clamping the endotracheal tube, the mean time to death for all donor animals was 22.6±2.0 minutes, with no difference between groups (p=0.2).
During the 4-hour EVLP period, no significant differences in oxygenation capacity or dynamic compliance were observed between groups EVLP and EVLP+ATL802, although the hourly mean values for both parameters were consistently higher in EVLP+ATL802 (Figure 3). By hour 1 of EVLP, the mean PaO2/FiO2 ratios for both groups were above 300 mmHg (EVLP: 375±23.3, EVLP+ATL802: 427.2±83.6 mmHg) and remained elevated at the completion of 4-hours of EVLP (EVLP: 376.7±61.0, EVLP+ATL802: 384.2±102.0 mmHg).
Figure 3.
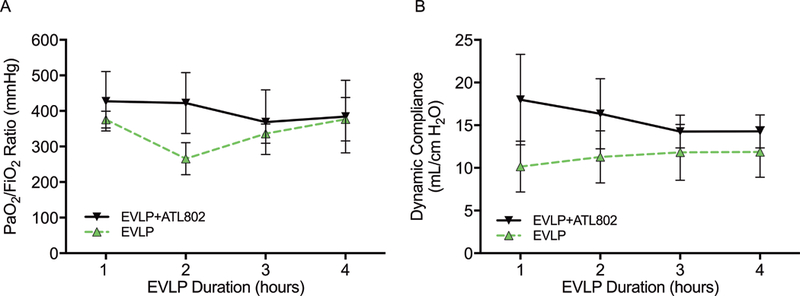
(A) Oxygenation (PaO2/FiO2) and (B) dynamic compliance recorded hourly during EVLP. Cold, transplantation after 4-hours of cold preservation; Cold+ATL802, transplantation after 4-hours of cold preservation with administration of ATL802 during reperfusion; EVLP, transplantation after 4-hours of EVLP; EVLP+ATL802, transplantation after 4-hours of EVLP with administration of ATL802 during ex vivo perfusion; PaO2/FiO2, partial pressure of oxygen/fraction of inspired oxygen.
The mean PaO2/FiO2 ratios from the start of the experiment to conclusion of the 4-hour reperfusion period for the two untreated groups (Cold and EVLP) are shown in Figure 4. The Cold group decreased significantly from an initial donor PaO2/FiO2 ratio of 501.7±28.9 mmHg to a post-reperfusion mean of 174.0±61.3 mmHg (p=0.003), while the final post-reperfusion PaO2/FiO2 ratio for the EVLP group was not significantly different compared with the donor pre-hypoxia ratio (440.4±37.0 vs. 454.8±17.0, p=0.71).
Figure 4.
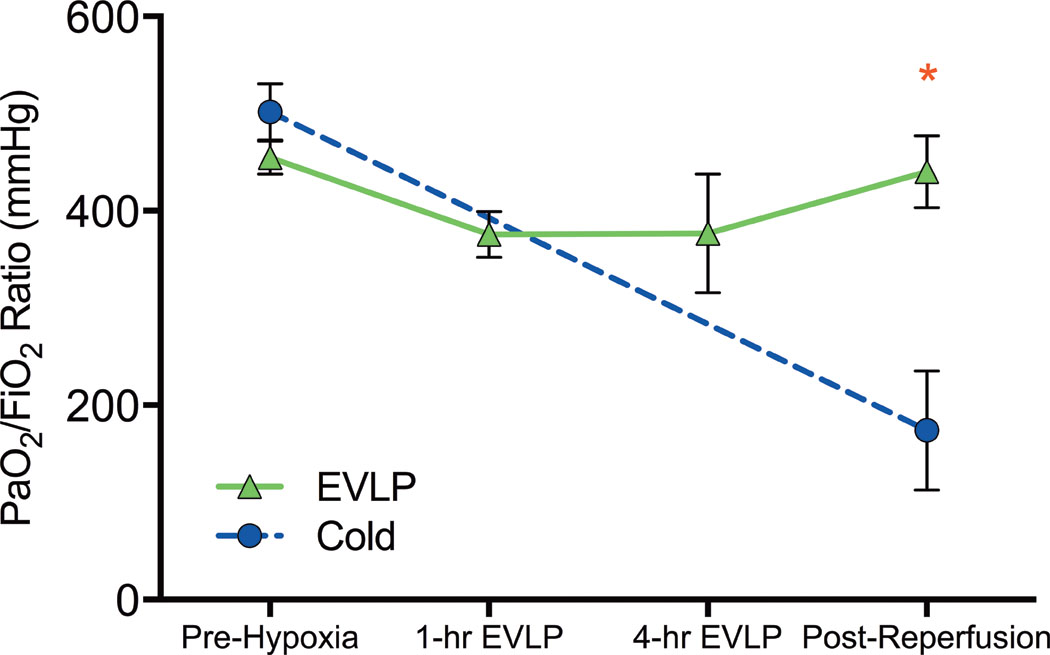
Oxygenation capacity (PaO2/FiO2) of groups without drug treatment recorded at four time points: donor animal prior to hypoxia (Pre-hypoxia), 1-hour into EVLP (1-hour EVLP), at the completion of EVLP (4-hour EVLP), and recipient after 4-hours of reperfusion (Post-Reperfusion). Cold, transplantation after 4-hours of cold preservation; EVLP, transplantation after 4-hours of EVLP; PaO2/FiO2, partial pressure of oxygen/fraction of inspired oxygen. * p=0.037.
Hemodynamic Support
During the reperfusion period, intravenous normal saline, epinephrine, and sodium bicarbonate were used judiciously to maintain adequate hemodynamics and appropriate acid/base status. Recipient animals in EVLP+ATL802 were the most stable, requiring the least amount of fluids (0.7 ± 0.1 L), epinephrine (1.2 ± 0.4 mg), and sodium bicarbonate (1.0 ± 0.0 amps) during the reperfusion period (Table 1).
Table 1:
Intravenous fluid, epinephrine, and sodium bicarbonate requirements during 4-hour reperfusion period (mean ± SE)
| Intravenous Fluid (L) | Epinephrine (mg) | Sodium Bicarbonate (amp) | |
|---|---|---|---|
| Cold | 1.2 ± 0.3 | 7.9 ± 4.2 | 1.0 ± 0.4 |
| Cold+ATL802 | 2.2 ± 0.6 | 14.1 ± 6.3 | 3.5 ± 1.0 |
| EVLP | 1.0 ± 0.3 | 2.1 ± 0.3 | 0.8 ± 0.5 |
| EVLP+ATL802 | 0.7 ± 0.1 | 1.2 ± 0.4 | 1.0 ± 0.0 |
Cold, transplantation after 4-hours of cold preservation; Cold+ATL802, transplantation after 4-hours of cold preservation with administration of ATL802 during reperfusion; EVLP, transplantation after 4-hours of EVLP; EVLP+ATL802, transplantation after 4-hours of EVLP with administration of ATL802 during ex vivo perfusion.
Pulmonary Edema
Compared to Cold, lungs that underwent 4-hours of EVLP without ATL802 had significantly less weight gain from pre-transplantation to post-reperfusion (Cold: 120±23% vs. EVLP: 20±20%, p=0.037) and trended towards significance for lungs in EVLP+ATL802 (28±10%, p=0.056) (Figure 5A). No significant differences were observed between Cold and Cold+ATL802 (116±41%) or between EVLP and EVLP+ATL802.
Figure 5.
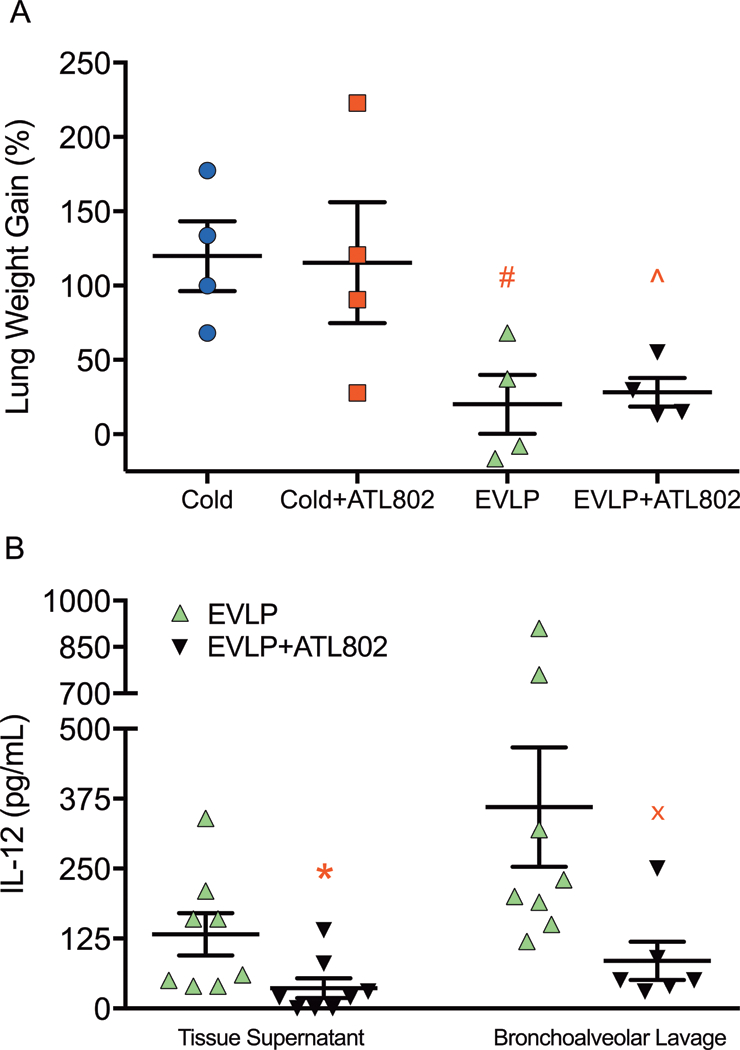
(A) Percentage increase in gross lung weight from pre-transplant to post-reperfusion and (B) tissue supernatant and bronchoalveolar lavage IL-12 levels after reperfusion. Cold, transplantation after 4-hours of cold preservation; Cold+ATL802, transplantation after 4-hours of cold preservation with administration of ATL802 during reperfusion; EVLP, transplantation after 4-hours of EVLP; EVLP+ATL802, transplantation after 4-hours of EVLP with administration of ATL802 during ex vivo perfusion. # p=0.037 vs Cold, ^ p =0.056 vs Cold, * p=0.007, x p=0.046.
Cytokine Expression
Proinflammatory cytokines were assessed in homogenized tissue supernatant and BAL fluid after the reperfusion period. A significant decrease was identified in levels of IL-12 when comparing EVLP to EVLP+ATL802 (tissue: 132.5±37.7 vs. 36.3±17.5 pg/mL, p=0.007; BAL: 360.0±106.7 vs. 85.0±34.0 pg/mL, p=0.046) (Figure 5B). No other significant differences were identified.
Lung Injury Severity Score
Although EVLP+ATL802 had the lowest mean composite lung injury severity score (comprised of neutrophil infiltration, alveolar edema, and interstitial inflammation), there were no significant differences observed between groups (Cold: 4.31±0.70, Cold+ATL802: 3.81±0.89, EVLP: 5.94±0.90, EVLP+ATL802: 2.94±1.35, p=0.24).
Neutrophil Infiltration
Immunohistochemistry stained slides were compared between all four groups using samples from two distinct lung regions, with five HPF counts per sample. Significantly less neutrophils were seen in group EVLP+ATL802 compared with Cold (56.0±6.7 vs. 122.8±16.2, p=0.002), and compared with EVLP (56.0±6.7 vs. 111.7±10.0, p=0.012) (Figure 6).
Figure 6.
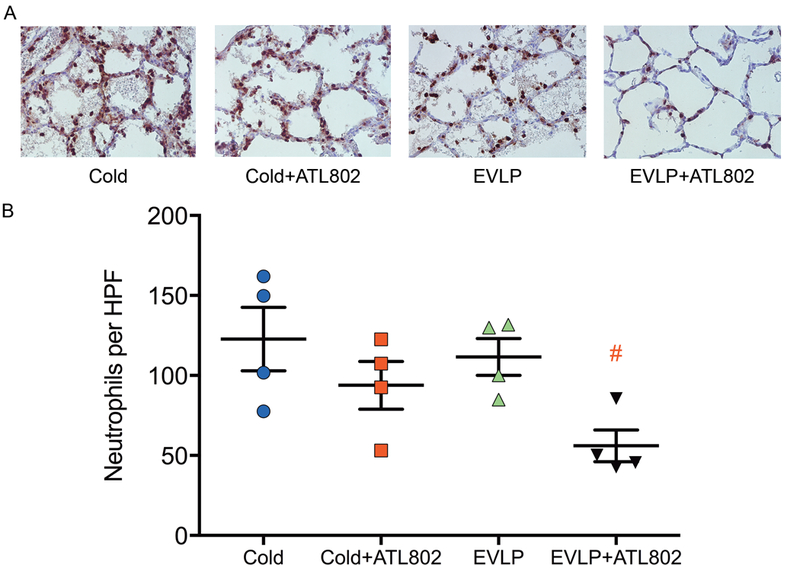
Immunohistochemistry staining for neutrophils. (A) Representative sections (40X magnification). (B) Neutrophils per high-powered field (HPF). Cold, transplantation after 4-hours of cold preservation; Cold+ATL802, transplantation after 4-hours of cold preservation with administration of ATL802 during reperfusion; EVLP, transplantation after 4-hours of EVLP; EVLP+ATL802, transplantation after 4-hours of EVLP with administration of ATL802 during ex vivo perfusion. # p=0.002 vs Cold and p=0.012 vs EVLP.
DISCUSSION
Using a porcine model of left lung transplantation, the present study sought to determine the ability of EVLP, with and without the addition of targeted drug therapy, to rehabilitate DCD lungs exposed to 2-hours of warm ischemia. Dynamic compliance 4-hours after reperfusion was improved in lungs treated with 4-hours of EVLP supplemented with an A2BR antagonist (ATL802), and oxygenation was improved in lungs treated with EVLP alone compared with cold preservation. After the reperfusion period, lungs that received 4-hours of EVLP, with or without ATL802, demonstrated less percent weight gain compared with lungs that underwent 4-hours of cold preservation prior to transplant. The combination of EVLP plus ATL802 significantly reduced neutrophil infiltration after 4-hours of reperfusion, and a decrease in the amount of IL-12 expression was observed with the addition of ATL802 compared with EVLP alone. Collectively, DCD lungs exposed to 2-hours of warm ischemia can be transplanted successfully after 4-hours of EVLP as opposed to cold preservation, and targeted drug therapy during the ex vivo perfusion period may further improve outcomes.
Increasing the amount of warm ischemia time acceptable for donor lung procurement would allow for inclusion of uncontrolled DCD lungs from patients who experience cardiac arrest outside of a medical facility, with heparin administration as the only intervention possibly needed before procurement. Further investigation may show that heparin administration is not necessary if lungs are flushed with a fibrinolytic agent prior to EVLP. This strategy for increasing the pool of available donor lungs may shorten wait list times and improve mortality for deteriorating patients with end-stage pulmonary disease, and is gaining support clinically as the supply-demand chasm continues to widen. [19] A case report of successful transplantation after EVLP of human DCD lungs exposed to 4-hours of warm ischemia has been reported. [20] Unfortunately, longer ischemic times increase the risk for IR injury and PGD, but EVLP has been shown to be protective and beneficial when transplanting marginal donor lungs. [21] Our results support the use of EVLP compared with cold preservation alone, as the majority of the benefit seen in this study can be attributed to EVLP and not the treatment drug.
The present study employed the use of ATL802, an A2BR antagonist, as targeted drug therapy to reduce inflammation and IR injury after transplantation. Both activation and inhibition of A2BRs have been shown to decrease lung inflammation. [9–12] Previous work from our lab implicated resident pulmonary cells as opposed to bone-marrow derived cells as the location of A2BR signaling, with a resultant decrease in cytokine expression and neutrophil activation. [9] In a mouse model of acute lung injury, Hogel, S et al. identified alveolar epithelial cells as the location of A2BR signaling, which supports the findings from our lab showing attenuation of mouse lung IR with A2BR antagonism. [13, 22] The present study translated these findings into a large animal transplant model for the first time.
IL-12 is a proinflammatory cytokine secreted by tissue-resident macrophages and dendritic cells, which leads to increased production of interferon-γ by T-helper cells. [23] IL-12, in conjunction with IL-18, an important component of inflammasome activation, has been implicated in the up-regulation of matrix degrading enzymes and T-cell infiltration in lung injury. [24] Additionally, adenosine signaling has been shown to inhibit IL-12 production, which may further support the use of adenosine receptor-targeted therapies to prevent inflammation. [25] Two IL-12 antagonists, ustekinumab and briakinumab, are currently being tested in clinical trials as targeted drug therapy for immune-mediated inflammatory disease, including graft-vs-host disease. [23] Targeted treatment to down-regulate IL-12 expression may be a possible adjunctive therapy, delivered during the EVLP period, to attenuate IR injury and improve outcomes after lung transplantation.
Neutrophil activation and infiltration is dependent on various signaling pathways related to both innate and adaptive immunity. We have previously shown that A2AR activation significantly attenuates neutrophil infiltration in a mouse model of lung IR injury, as well as after transplantation in a porcine model. [15, 26] Additionally, neutrophil activation has been shown to worsen acute lung injury through the involvement of A3Rs. [27] The present study shows evidence for a decrease in neutrophil infiltration with ATL802 treatment during EVLP. No significant difference was observed between groups Cold and Cold+ATL802 when ATL802 was administered during reperfusion, as opposed to 4 hours prior to reperfusion (EVLP+ATL802). This timing difference may impact the drug’s ability to alter signaling pathways in alveolar epithelial cells and future studies are needed to better understand this effect. The resuscitative requirements of Cold+ATL802 may be due to hemodynamic effects of systemic drug administration.
The findings are limited by low sample size, donor heparin administration, inherent variability with large animals, and the dichotomy between compliance and oxygenation values in EVLP+ATL802. Improved oxygenation was not observed with ATL802 which may be the result of low sample size considering one animal had significantly low oxygenation (PaO2/FiO2 of 84.35). Additionally, post-transplant compliance values represent both lungs. Studies further evaluating the role of A2BRs in acute lung injury are needed prior to making conclusions about the benefit of A2BR antagonism as a treatment modality.
The use of EVLP as an assessment and rehabilitation platform to improve organ utilization may lead to a reduction in the critical shortage of donor lungs that meet acceptance criteria. Current functional measurements (oxygenation and compliance) used to assess lung quality may not be sufficient to fully predict success after transplantation, but measuring biomarkers, such as endothelin-1 or proinflammatory cytokines, during EVLP may improve our ability to correctly predict outcomes. [28, 29] Additionally, EVLP provides an opportunity to provided targeted rehabilitative strategies based on the needs of each individual set of donor lungs. The administration of inhaled β2-agonist and neutrophil elastase during EVLP has been shown to improve outcomes after transplantation. [30, 31] Potentially more beneficial is the opportunity to provide drug treatments, such as antibiotics, at concentration levels that would otherwise be detrimental if systemically administered to the recipient. [32]
In summary, the use of EVLP to rehabilitate uncontrolled DCD lungs subjected to prolonged warm ischemia is promising. Our findings of improved outcomes after transplantation with the use of EVLP and targeted drug therapy during the ex vivo perfusion period suggest that it may be possible to successfully transplant lungs procured from patients suffering cardiac death in the field. Expanding current acceptance criteria for donor lung procurement is needed to decrease wait list times and improve wait list mortality for patients with end-stage pulmonary disease. Using EVLP to assess lung quality and administer targeted drug therapies prior to recipient allocation will allow for maximal organ utilization and may improve outcomes after transplantation.
Supplementary Material
Video Legend University of Virginia Porcine Lung Procurement and Transplant Procedure: Deceased Donor Harvest (00:09), EVLP Cannulation (03:30), Left Lung Back-table Preparation (06:20), Recipient Pneumonectomy (08:09), Left Lung Transplant (11:50), Left Pulmonary Vein Sampling (12:59)
Central Message
Targeted drug therapy during ex vivo lung perfusion improves outcomes after transplantation of severely injured lungs.
Perspective Statement
A significant dichotomy exists between the number of acceptable donor lungs and patients needing a transplant. Increasing utilization of donation after circulatory death lungs subjected to prolonged warm ischemia may decrease wait list times and mortality. Enhanced ex vivo lung perfusion with targeted drug therapy may improve outcomes.
Central Picture Legend
Lung compliance and oxygenation capacity after transplant with and without enhanced EVLP.
ACKNOWLEDGEMENTS:
This work was supported by National Institutes of Health T32HL007849 (ILK) and R01HL119218 (ILK, VEL). The authors extended special appreciation to Tony Herring, Cindy Dodson, Sheila Hammond, and the UVA Histology Core for their support of this project.
This work was supported by National Institutes of Health T32HL007849 (Irving L Kron) and R01HL119218 (Irving L Kron, Victor E Laubach). The authors had full control of the design of the study, methods used, results, data analysis and production of the written manuscript.
Glossary of Abbreviations
- ABG
arterial blood gas
- A2BR
adenosine A2B receptor
- BAL
bronchoalveolar lavage
- DCD
donation after circulatory death
- EVLP
ex vivo lung perfusion
- FiO2
fraction of inspired oxygen
- HPF
high-powered field
- IL
interleukin
- IR
ischemia-reperfusion
- LA
left atrium
- PA
pulmonary artery
- PaO2
partial pressure of oxygen
- PGD
primary graft dysfunction
Footnotes
None of the authors report any financial conflicts of interest.
REFERENCES
- 1.Chaney J, Suzuki Y, Cantu E 3rd, van Berkel V. Lung donor selection criteria. J Thorac Dis. 2014;6:1032–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valapour M, Skeans MA, Heubner BM, Smith JM, Hertz MI, Edwards LB, et al. OPTN/SRTR 2013 Annual Data Report: lung. Am J Transplant. 2015;15 Suppl 2:1–28. [DOI] [PubMed] [Google Scholar]
- 3.Algahim MF, Love RB. Donation after circulatory death: the current state and technical approaches to organ procurement. Curr Opin Organ Transplant. 2015;20:127–32. [DOI] [PubMed] [Google Scholar]
- 4.Fiser SM, Tribble CG, Long SM, Kaza AK, Kern JA, Jones DR, et al. Ischemia-reperfusion injury after lung transplantation increases risk of late bronchiolitis obliterans syndrome. Ann Thorac Surg. 2002;73:1041–7; discussion 47–8. [DOI] [PubMed] [Google Scholar]
- 5.Cypel M, Keshavjee S. Extending the donor pool: rehabilitation of poor organs. Thorac Surg Clin. 2015;25:27–33. [DOI] [PubMed] [Google Scholar]
- 6.Stone ML, Sharma AK, Zhao Y, Charles EJ, Huerter ME, Johnston WF, et al. Sphingosine-1-phosphate receptor 1 agonism attenuates lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2015;308:L1245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner CE, Pope NH, Charles EJ, Huerter ME, Sharma AK, Salmon MD, et al. Ex vivo lung perfusion with adenosine A2A receptor agonist allows prolonged cold preservation of lungs donated after cardiac death. J Thorac Cardiovasc Surg. 2016;151:538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med. 2012;367:2322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anvari F, Sharma AK, Fernandez LG, Hranjec T, Ravid K, Kron IL, et al. Tissue-derived proinflammatory effect of adenosine A2B receptor in lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2010;140:871–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pejman L, Omrani H, Mirzamohammadi Z, Shahbazfar AA, Khalili M, Keyhanmanesh R. The Effect of Adenosine A2A and A2B Antagonists on Tracheal Responsiveness, Serum Levels of Cytokines and Lung Inflammation in Guinea Pig Model of Asthma. Adv Pharm Bull. 2014;4:131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, Schneider DJ, Morschl E, Song L, Pedroza M, Karmouty-Quintana H, et al. Distinct roles for the A2B adenosine receptor in acute and chronic stages of bleomycin-induced lung injury. J Immunol. 2011;186:1097–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Y, LaPar DJ, Steidle J, Emaminia A, Kron IL, Ailawadi G, et al. Adenosine signaling via the adenosine 2B receptor is involved in bronchiolitis obliterans development. J Heart Lung Transplant. 2010;29:1405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huerter ME, Sharma AK, Zhao Y, Charles EJ, Kron IL, Laubach VE. Attenuation of Pulmonary Ischemia-Reperfusion Injury by Adenosine A2B Receptor Antagonism. Ann Thorac Surg. 2016;102:385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cagnina RE, Ramos SI, Marshall MA, Wang G, Frazier CR, Linden J. Adenosine A2B receptors are highly expressed on murine type II alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;297:L467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaPar DJ, Laubach VE, Emaminia A, Crosby IK, Hajzus VA, Sharma AK, et al. Pretreatment strategy with adenosine A2A receptor agonist attenuates reperfusion injury in a preclinical porcine lung transplantation model. J Thorac Cardiovasc Surg. 2011;142:887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cypel M, Yeung JC, Hirayama S, Rubacha M, Fischer S, Anraku M, et al. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2008;27:1319–25. [DOI] [PubMed] [Google Scholar]
- 17.Emaminia A, Lapar DJ, Zhao Y, Steidle JF, Harris DA, Laubach VE, et al. Adenosine A(2)A agonist improves lung function during ex vivo lung perfusion. Ann Thorac Surg. 2011;92:1840–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulloy DP, Stone ML, Crosby IK, Lapar DJ, Sharma AK, Webb DV, et al. Ex vivo rehabilitation of non-heart-beating donor lungs in preclinical porcine model: delayed perfusion results in superior lung function. J Thorac Cardiovasc Surg. 2012;144:1208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mason DP, Brown CR, Murthy SC, Vakil N, Lyon C, Budev MM, et al. Growing single-center experience with lung transplantation using donation after cardiac death. Ann Thorac Surg. 2012;94:406–11; discussion 11–2. [DOI] [PubMed] [Google Scholar]
- 20.Valenza F, Citerio G, Palleschi A, Vargiolu A, Fakhr BS, Confalonieri A, et al. Successful Transplantation of Lungs From an Uncontrolled Donor After Circulatory Death Preserved In Situ by Alveolar Recruitment Maneuvers and Assessed by Ex Vivo Lung Perfusion. Am J Transplant. 2016;16:1312–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boffini M, Ricci D, Bonato R, Fanelli V, Attisani M, Ribezzo M, et al. Incidence and severity of primary graft dysfunction after lung transplantation using rejected grafts reconditioned with ex vivo lung perfusion. Eur J Cardiothorac Surg. 2014;46:789–93. [DOI] [PubMed] [Google Scholar]
- 22.Hoegl S, Brodsky KS, Blackburn MR, Karmouty-Quintana H, Zwissler B, Eltzschig HK. Alveolar Epithelial A2B Adenosine Receptors in Pulmonary Protection during Acute Lung Injury. J Immunol. 2015;195:1815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teng MW, Bowman EP, McElwee JJ, Smyth MJ, Casanova JL, Cooper AM, et al. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med. 2015;21:719–29. [DOI] [PubMed] [Google Scholar]
- 24.Cero FT, Hillestad V, Loberg EM, Christensen G, Larsen KO, Skjonsberg OH. IL-18 and IL-12 synergy induces matrix degrading enzymes in the lung. Exp Lung Res. 2012;38:406–19. [DOI] [PubMed] [Google Scholar]
- 25.Hasko G, Kuhel DG, Chen JF, Schwarzschild MA, Deitch EA, Mabley JG, et al. Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J. 2000;14:2065–74. [DOI] [PubMed] [Google Scholar]
- 26.Sharma AK, Laubach VE, Ramos SI, Zhao Y, Stukenborg G, Linden J, et al. Adenosine A2A receptor activation on CD4+ T lymphocytes and neutrophils attenuates lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2010;139:474–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inoue Y, Chen Y, Pauzenberger R, Hirsh MI, Junger WG. Hypertonic saline up-regulates A3 adenosine receptor expression of activated neutrophils and increases acute lung injury after sepsis. Crit Care Med. 2008;36:2569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Machuca TN, Cypel M, Zhao Y, Grasemann H, Tavasoli F, Yeung JC, et al. The role of the endothelin-1 pathway as a biomarker for donor lung assessment in clinical ex vivo lung perfusion. J Heart Lung Transplant. 2015;34:849–57. [DOI] [PubMed] [Google Scholar]
- 29.Vasanthan V, Nagendran J. Compliance trumps oxygenation: Predicting quality with ex vivo lung perfusion. J Thorac Cardiovasc Surg. 2015;150:1378–9. [DOI] [PubMed] [Google Scholar]
- 30.Harada M, Oto T, Otani S, Miyoshi K, Okada M, Iga N, et al. A neutrophil elastase inhibitor improves lung function during ex vivo lung perfusion. Gen Thorac Cardiovasc Surg. 2015;63:645–51. [DOI] [PubMed] [Google Scholar]
- 31.Kondo T, Chen F, Ohsumi A, Hijiya K, Motoyama H, Sowa T, et al. beta2-Adrenoreceptor Agonist Inhalation During Ex Vivo Lung Perfusion Attenuates Lung Injury. Ann Thorac Surg. 2015;100:480–6. [DOI] [PubMed] [Google Scholar]
- 32.Biancosino C, Albert M, Linder A. Acute toxicity of irinotecan in the ex-vivo isolated perfused human lung model--high-dose therapy during isolated perfusion without acute toxic lung edema. Interact Cardiovasc Thorac Surg. 2007;6:583–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video Legend University of Virginia Porcine Lung Procurement and Transplant Procedure: Deceased Donor Harvest (00:09), EVLP Cannulation (03:30), Left Lung Back-table Preparation (06:20), Recipient Pneumonectomy (08:09), Left Lung Transplant (11:50), Left Pulmonary Vein Sampling (12:59)


