Abstract
Objective:
We investigated a large measles outbreak that occurred in 2009 in Burkina Faso in order to describe the epidemic, assess risk factors associated with measles, and estimate measles vaccine effectiveness.
Methods:
We reviewed national surveillance and measles vaccine coverage data, and conducted a case–control study in three geographic areas. Case-patients were randomly selected from the national case-based measles surveillance database or, when a case-patient could not be traced, were persons in the same community who experienced an illness meeting the WHO measles clinical case definition. Controls were matched to the same age stratum (age 1–14 years or age 15–30 years) and community as case-patients. Risk factors were assessed using conditional logistic regression.
Results:
Lack of measles vaccination was the main risk factor for measles in all three geographic areas for children aged 1–14 years (adjusted matched odds ratio [aMOR] [95% confidence interval (CI)], 19.4[2.4–155.9], 5.9 [1.6–21.5], and 6.4 [1.8–23.0] in Bogodogo, Zorgho, and Sahel, respectively) and persons aged 15–30 years (aMOR [95% CI], 3.2 [1.1–9.7], 19.7 [3.3–infinity], 8.0 [1.8–34.8] in Bogodogo, Zorgho, and Sahel, respectively). Among children aged 1–14 years, VE of any measles vaccination prior to 2009 was 94% (95% CI, 45–99%) in Bogodogo, 87% (95% CI, 37–97%) in Zorgho, and 84% (95% CI, 41–96%) in Sahel. Main reasons for not receiving measles vaccination were lack of knowledge about vaccination campaigns or need for measles vaccination and absence during vaccination outreach or campaign activities.
Conclusion:
These results emphasize the need for improved strategies to reduce missed opportunities for vaccination and achieve high vaccination coverage nationwide in order to prevent large measles outbreaks and to continue progress toward measles mortality reduction.
Keywords: Measles, Vaccination, Burkina Faso, Outbreak investigation, Risk factors
1. Introduction
Measles is a highly contagious disease associated with complications such as pneumonia, diarrhea, or encephalitis in approximately 30% of cases and case fatality ratios (CFRs) as high as 5–10% in developing countries [1–5]. In 2000, despite the availability of a safe and effective vaccine, measles caused an estimated 733,000 deaths worldwide; 371,000 (51%) of these were in Africa [6]. In 2001, the World Health Organization (WHO) Africa Region joined the global initiative to reduce measles deaths by 50% during 1999–2005 [6]. Strategies for measles mortality reduction recommended by WHO and United Nations Children’s Fund (UNICEF) include (1) providing a first dose of measles vaccine to all children at age nine months or shortly after; (2) providing every child with a second opportunity to receive measles vaccine, either through a campaign or routine immunization; (3) establishing effective measles surveillance; and (4) improving measles case management [7]. Implementation of these strategies led to a significant decline in measles mortality globally and in Africa, where estimated measles deaths decreased 92%, from 371,000 to 28,000 during 2000–2008 [6].
However, further declines have been threatened by multiple outbreaks in Africa during 2009–2010, including a large outbreak in Burkina Faso in 2009 [8]. Burkina Faso, formerly Upper Volta, was one of the first countries to introduce measles vaccine and has conducted intermittent mass measles vaccination campaigns for children since the 1960s [9–16]. Following the country’s adoption of the WHO/UNICEF measles mortality reduction strategies, a nationwide “catch-up” campaign was conducted targeting children aged 9 months to 14 years in 2001 [17], and nationwide “follow-up” campaigns were conducted targeting children aged 9–59 months in 2004 and 2007. Based on post-campaign surveys, estimated coverage during these campaigns ranged from 96% to 97% nationwide [18,19]. In addition to campaigns, routine measles immunization in Burkina Faso began in 1980 through the Expanded Programme on Immunization and consists of one dose of measles vaccine at age 9–11 months. WHO/UNICEF estimates of routine measles vaccination coverage have increased from 38% in 1985 to 75% during 2005–2008 (Fig. 1) [20].
Fig. 1.
Clinically diagnosed measles cases reported to the measles surveillance system by week and measles vaccination coverage, Burkina Faso, 1996–2009. aSource: World Health Organization (WHO)/United Nations Children’s Fund (UNICEF) estimates [20]. bSource: Post-campaign surveys [14–16]. cSource: Administrative data, Burkina Faso Ministry of Health.
Despite these immunization activities, Burkina Faso continued to experience periodic measles outbreaks, with a large outbreak in 2009. During May–August 2009, we conducted an investigation to describe the 2009 outbreak and outbreak response campaign, and a case–control study to determine risk factors for measles during the outbreak, estimate vaccine effectiveness (VE), and identify reasons for not receiving measles vaccination.
2. Methods
2.1. Field investigation
Measles vaccination coverage.
Administrative estimates of vaccination coverage (number of doses administered by health care workers divided by the census-projected number of eligible children in the population) during routine immunization, previous immunization campaigns, and the 2009 outbreak response campaign were calculated using data provided by the Burkina Faso Ministry of Health (MOH).
Measles surveillance.
Clinically diagnosed measles cases have been reportable to the Burkina Faso MOH since 1984; we obtained national aggregate measles surveillance data from 1996 to 2009. Case-based measles surveillance was introduced in 2000; we reviewed the 2009 case-based data that were available as of February 2010 and described the age distribution and reported vaccination status of included cases.
Laboratory testing.
The case-based measles surveillance system includes routine laboratory testing of suspected measles case-patients for confirmation of measles virus infection. Laboratory testing is performed at the National Measles Reference Laboratory (NMRL) at Centre Hospitalier National Pédiatrique Charles de Gaulle using standard protocols [21] and commercial indirect enzyme-linked immunosorbent assay (Enzygnost for IgM™, Siemens, Munich, Germany) for detection of measles-specific IgM antibody. Measles IgM antibody-negative specimens were tested for rubella-specific IgM antibody.
Following WHO guidelines, laboratory confirmation stopped in March 2009, when an outbreak was confirmed (defined as ≥3 confirmed cases within 1 month) [22]. In May 2009, oral fluid specimens from a convenience sample of suspected measles case-patients at health centers in the Centre, Plateau Central, and Sahel regions were collected. Specimens were collected using Oracol™ swabs (Malvern Medical Devices) and shipped on dry ice to the CDC Measles, Mumps, Rubella, and Herpesvirus Laboratory in Atlanta for real time reverse transcriptase polymerase chain reaction (RT-PCR) testing and inoculation onto Vero/hSLAM cells. Standard RT-PCR was used for sequence analysis and genotyping.
2.2. Case–control study
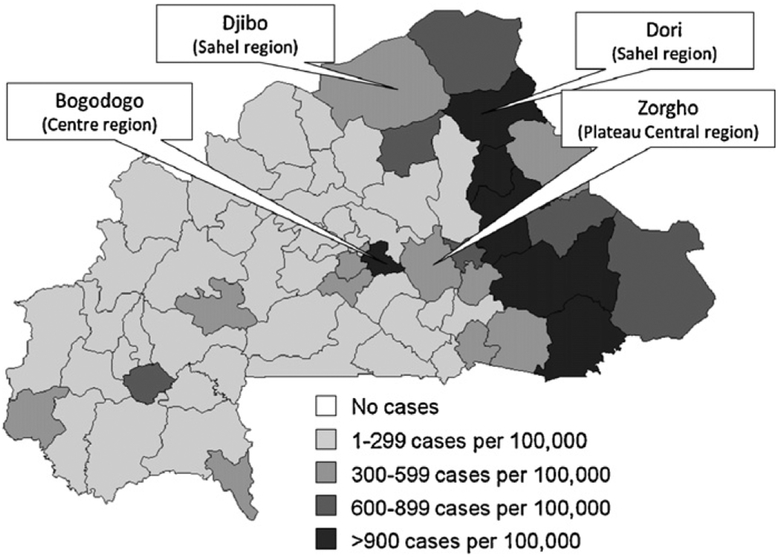
A matched case–control study was conducted during August 7–30, 2009 using two age strata, age 1–14 years and age 15–30 years, in four districts in three regions: Bogodogo district (Centre region), Zorgho district (Plateau Central region), and Djibo and Dori districts (Sahel region). Districts were selected based on high numbers of reported cases, laboratory-confirmed cases, and attack rates derived from national surveillance data reported during January–June 2009, and because they represented geographically and demographically diverse regions of Burkina Faso. Due to low population density and co-location in Sahel, Djibo and Dori districts were analyzed together as “Sahel”.
Sample size.
Based on estimated measles vaccination coverage in controls of 70%, alpha 0.05, and 1:1 matching of case-patients and controls, a sample size of 70 case-patients and 70 controls in each of the two age strata was required for each geographic area to have 93% power to detect an odds ratio (OR) of 0.3 or vaccine effectiveness of 70% or higher.
Case definition.
A case was either a measles case reported to the MOH case-based surveillance database or an illness meeting the WHO measles clinical case definition of fever, generalized rash, and ≥1 of the following: cough, conjunctivitis, or coryza [22]. Case-patients were included if aged 1–30 years at study enrollment and rash onset was during January 1–July 30, 2009.
Selection of case-patients.
Case-patients were randomly selected from the MOH case-based surveillance database. When a case-patient could not be traced, teams found a replacement case-patient who reported an illness meeting the WHO measles clinical case definition and who was from the same age stratum and community by searching successive households in a randomly chosen direction from the community center (rural areas) or from a randomly selected sub-section of the community (urban areas). If the household of a case-patient contained >1 persons meeting the case definition, the case-patient in the age stratum of interest who developed rash first was selected. Case-patients subsequently found not to meet the case definition based on their responses to the questionnaire and those whose age was ≥1 year outside the age stratum were excluded from analyses.
Selection of controls.
One control was selected for each case-patient, and controls were matched to the same age stratum (1–14 years or 15–30 years) and neighborhood as the case-patient. These wide age strata for matching were chosen so that age group within a stratum could be assessed as a risk factor for measles. Controls were selected from households without any measles cases since January 1, 2009 surrounding the case-patient’s household using a randomly chosen direction, and were randomly selected from eligible household members.
Data collection.
Trained data collectors from the study districts administered a questionnaire to case-patients and controls, or their caregivers, in the local language. The questionnaire collected information on clinical symptoms, demographic characteristics, education, employment, and travel and migration history. For children aged 1–14 years, detailed vaccination information from vaccination cards or caregiver recall and, if applicable, reasons for not receiving measles vaccination were collected.
Analysis of risk factors for measles.
Risk factors for each age stratum in each geographic area were evaluated using conditional logistic regression. Covariates with Wald chi-squared p-value <0.1 in univariate analysis were tested in multivariable models; final models were created using backwards elimination of covariates with p-value >0.05. Subjects who reported receiving measles vaccine prior to January 1, 2009 were considered vaccinated; those whose only reported measles vaccination was during 2009 were considered unvaccinated for the purposes of analysis. Vaccination status was included in all multivariable models, and age category was included in models for the 1–14 years age strata, regardless of p-value. Subjects with unknown vaccination status were not included in analyses of vaccination status as a risk factor.
Age categories for analysis were defined based on eligibility for various measles vaccination campaigns. Among children, age categories were: 12–28 months (not eligible for any campaigns before 2009), 29–79 months (eligible for the 2007 campaign), and 80 months to 14 years (eligible for ≥1 campaign before 2007). Among persons aged 15–30 years, categories were: 15.0–22.6 years (eligible for the 2001 campaign), 22.7–25.3 years (not eligible for any campaigns), and 25.4–30.9 years (eligible for the 1984 campaign).
Estimation of VE.
VE was estimated using the formula VE = 1 − aMOR, where aMOR was the adjusted matched odds ratio for receiving ≥1 dose of measles vaccine compared with no doses prior to 2009 [23]. Because attack rates in the study districts were <1%, the rare event assumption is satisfied, the odds ratio approximates relative risk, and this formula provides an acceptable estimate of VE. Subjects with a history of measles and subjects who were ineligible for vaccination prior to 2009 were excluded from VE analyses; in addition, because subjects with unknown or missing data for a variable included in the multivariable model were not included in the final model, subjects with unknown vaccination status were also excluded from estimation of VE.
Outbreak response campaign and reasons for not receiving measles vaccination.
Vaccination status during the June 2009 outbreak response campaign was calculated among controls aged 1–14 years with known vaccination status. Reasons for not receiving measles vaccination were explored in descriptive analysis.
3. Results
3.1. Measles epidemiology, 1996–2008
Before 2009, the largest documented measles epidemic in Burkina Faso occurred in 1996, with 32,415 reported cases (Fig. 1). During 1997–2005, annual outbreaks of 1077–8920 cases occurred, with peak transmission during January–June each year. In 2007, cases decreased to 150, an historic low. However, in 2008, cases increased to 1762 and transmission continued throughout the year and into 2009.
3.2. Measles outbreak, 2009
In total, 54,111 measles cases and 367 measles deaths were reported in 2009 through the aggregated surveillance system (Fig. 1). Cases peaked in epidemiological week 17 (April 26–May 2); 52,581 (97%) cases occurred during the typical transmission season, January–June. All 63 districts in Burkina Faso reported ≥1 measles case. Attack rates by district were 2–2422 cases per 100,000 persons (Fig. 2).
Fig. 2.
Number of measles cases per 100,000 persons by district, Burkina Faso, January–December 2009 (a total of 54,111 suspected measles cases were reported to the Burkina Faso Ministry of Health during January–December 2009), and location of districts included in the case–control study, 2009.
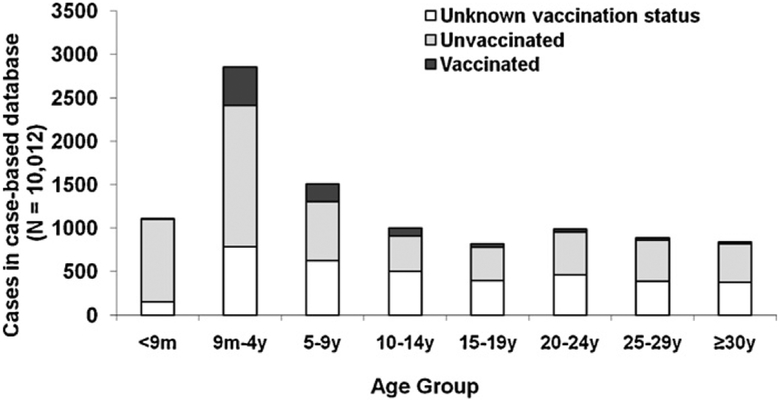
During January 1–May 20, 2009, 10,076 measles cases from 11 districts were reported in the case-based surveillance database. Of 10,012 (99%) case-patients with known age, 3971 (40%) were aged <5 years and 3534 (35%) were aged ≥15 years (Fig. 3). Among 5365 (54%) case-patients aged 9 months to 14 years, 2712 (51%) were unvaccinated and 1910 (36%) had unknown vaccination status. Among 3534 (35%) case-patients aged ≥15 years, 1799 (51%) were unvaccinated and 1624 (46%) had unknown vaccination status.
Fig. 3.
Age and vaccination status of measles case-patients in case-based database, Burkina Faso, January–May 2009 (N = 10,012) (as of February 2010, a total of 10,076 measles case-patients with onset January 1–May 20, 2009 from 11 districts were entered in the country’s 2009 case-based database; 64 case-patients with unknown age are not shown).
During January 1–March 28, 2009, the NMRL tested specimens from 748 suspected case-patients from 55 (87%) of 63 districts for measles IgM antibody. Measles was confirmed in 532 (71%) case-patients; 149 (20%) were measles IgM negative, and 67 (9%) were indeterminate. Of the negative specimens, 73 (49%) were collected within 2 days of rash onset, when ELISA sensitivity is decreased [24], and 10 (7%) were positive for rubella IgM antibody.
In May 2009, measles was confirmed in 7 (78%) of 9 patients with suspect measles by either RT-PCR or virus isolation from oral fluid specimens. Two lineages of genotype B3, the most commonly detected measles virus genotype in Western and Central Africa [25], were detected.
3.3. Outbreak response campaign
During June 17–21, 2009, an outbreak response campaign targeting children aged 6 months to 14 years was conducted in 31 (49% of 63 districts) with the highest and/or increasing measles attack rates as of May 2009. The administrative estimate of vaccination coverage during this campaign was 104%.
3.4. Case–control study
In total, 428 case–control pairs were eligible for the study. Of 426 (99.5%) case-patients with known outcome, 11 (2.6%) died. Eight deaths were attributed to measles (occurred within 30 days of rash onset and not due to trauma) for an overall CFR of 1.9%. Age-specific CFRs were 3.2% (4/125) for children aged 1–4 years, 1.1% (1/90) for children aged 5–14 years, and 1.4% (3/211) for persons aged 15–30 years.
Of 428 case-patients included in the study, 42 (9.8%), 331(77.3%), and 55 (12.9%) had onset of rash during January–February, March–April, and May–June, respectively. This distribution mirrored the epidemic curve for the study districts, where, of the 12,579 cases reported from the study districts in 2009, 1345 (10.7%), 7920 (63.0%), and 3213 (25.5%) were reported during January–February, March–April, and May–June, respectively.
3.4.1. Risk factors for measles
Among children aged 1–14 years, in all study areas, case-patients were more likely than controls to be unvaccinated (matched odds ratio [MOR] [95% confidence interval (CI)] 29.0[4.0–212.9], 7.0 [2.1–23.5], and 9.3 [2.8–30.7] in Bogodogo, Zorgho, and Sahel, respectively) and to be in the youngest age group, age 12–28 months (MOR [95% CI] 16.7 [3.4–82.5], 4.0 [1.5–10.8], and 4.0[1.3–12.6] in Bogodogo, Zorgho, and Sahel, respectively) (Table 1). Additional risk factors included male sex in Bogodogo; Muslim religion in Bogodogo and Zorgho; having a father whose highest level of education was alternative schooling, such as Koranic school, in Bogodogo; and Peul/Fulani ethnicity in Sahel. Of those vaccinated, 20 (56%) case-patients and 36 (55%) controls in Bogodogo, 7 (23%) case-patients and 18 (30%) controls in Zorgho, and 3 (20%) case-patients and 2 (5%) controls in Sahel had documentation of vaccination by vaccination card; the remainder were vaccinated according to caregiver recall.
Table 1.
Characteristics associated with measles in children aged 1–14 years stratified by geographic area, case–control study of measles outbreak in Burkina Faso, 2009.
| Bogodogo | Zorgho | Sahel | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases (na = 72) n (%) |
Controls (n = 72) n (%) |
MOR (95% CI)b | Cases (n = 73) n (%) |
Controls (n = 73) n (%) |
MOR (95% CI) | Cases (n = 70) n (%) |
Controls (n = 70) n (%) |
MOR (95%CI) | |
| Demographic characteristics | |||||||||
| Age group | |||||||||
| 12–28 months | 27 (38) | 8 (11) | 16.7 (3.4–82.5) | 25 (34) | 9 (12) | 4.0 (1.5–10.8) | 24 (34) | 9 (13) | 4.0 (1.3–12.6) |
| 29–79 months | 27 (38) | 30 (42) | 2.2 (0.8–5.7) | 24 (33) | 30 (41) | 1.2 (0.5–2.6) | 21 (30) | 31 (44) | 0.7 (0.3–1.7) |
| 80 months to 14 years | 18 (25) | 34 (47) | Reference | 24 (33) | 34 (47) | Reference | 25 (36) | 30 (43) | Reference |
| Sex | |||||||||
| Male | 44 (62) | 32 (44) | 2.1 (1.05–4.1) | 34 (47) | 43 (59) | 0.6 (0.3–1.2) | 37 (53) | 34 (49) | 1.2 (0.6–2.3) |
| Female | 27 (38) | 40 (56) | Reference | 39 (53) | 30 (41) | Reference | 33 (47) | 36 (51) | Reference |
| Ethnicity | |||||||||
| Peul/Fulani | 2 (3) | 1 (1) | >999.9 (<0.1->999.9) | 4 (6) | 5 (7) | 0.7 (0.1–4.0) | 47 (67) | 35 (50) | 3.8 (1.03–14.3) |
| Other | 5 (7) | 14 (19) | 0.3 (0.1–0.9) | 0 (0) | 2 (3) | <0.1 (<0.1->999.9) | 15 (21) | 20 (29) | 1.8 (0.4–8.2) |
| Mossi | 65 (90) | 57 (79) | Reference | 68 (94) | 65 (90) | Reference | 8 (11) | 15 (21) | Reference |
| Religion | |||||||||
| Muslim | 53 (74) | 35 (49) | 4.0 (1.6–9.8) | 62 (86) | 48 (67) | 4.3 (1.4–12.6) | 65 (94) | 66 (94) | 1.0 (0.2–5.0) |
| Not Muslim | 19 (26) | 37 (51) | Reference | 10 (14) | 24 (33) | Reference | 4 (6) | 4 (6) | Reference |
| Mother’s education | |||||||||
| None | 42 (59) | 44 (62) | 0.9 (0.5–1.9) | 60 (83) | 50 (69) | 2.0 (0.1–4.4) | 60 (87) | 64 (91) | 0.9 (0.1–6.4) |
| Alternative schooling | 3 (4) | 1 (1) | >999.9 (<0.1->999.9) | 2 (3) | 5 (7) | 0.7 (0.8–5.0) | 7 (10) | 4 (6) | 1.5 (0.2–13.7) |
| Any standard schooling | 26 (37) | 26 (37) | Reference | 10 (14) | 17 (24) | Reference | 2 (3) | 2 (3) | Reference |
| Father’s education | |||||||||
| None | 27 (38) | 31 (45) | 1.0 (0.5–2.3) | 50 (69) | 49 (68) | 1.1 (0.5–2.4) | 54 (77) | 48 (69) | 2.6 (0.6–10.3) |
| Alternative schooling | 17 (24) | 5 (7) | 5.1 (1.4–18.4) | 6 (8) | 5 (7) | 1.3 (0.3–5.4) | 13 (19) | 15 (21) | 1.9 (0.4–8.3) |
| Any standard schooling | 27 (38) | 33 (48) | Reference | 16 (22) | 18 (25) | Reference | 3 (4) | 7 (10) | Reference |
| Travel and migration | |||||||||
| Lived outside ofarea (>20 km away) in last year | |||||||||
| Yes | 7 (10) | 8 (11) | 0.9 (0.3–2.6) | 4 (6) | 6 (8) | 0.7 (0.2–2.4) | 3 (4) | 2 (3) | 1.5 (0.3–9.0) |
| No | 65 (90) | 64 (89) | Reference | 68 (94) | 67 (92) | Reference | 67 (96) | 68 (97) | Reference |
| Lived outside of Burkina Faso in last year | |||||||||
| Yes | 0 (0) | 1 (1) | <0.1 (<0.1->999.9) | 0 (0) | 0 (0) | - | 0 (0) | 0 (0) | - |
| No | 72 (100) | 71 (99) | Reference | 72 (100) | 73 (100) | 70 (100) | 70 (100) | ||
| Household member lived outside area in last year | |||||||||
| Yes | 8 (11) | 11 (15) | 0.7 (0.3–1.8) | 12 (16) | 15 (21) | 0.8 (0.3–1.8) | 7 (10) | 8 (11) | 0.9 (0.3–2.4) |
| No | 64 (89) | 60 (85) | Reference | 61 (84) | 58 (79) | Reference | 63 (90) | 62 (89) | Reference |
| Had visitor stay in household in last year | |||||||||
| Yes | 15 (21) | 11 (16) | 1.5 (0.6–3.7) | 11 (15) | 8 (11) | 1.4 (0.5–3.8) | 4 (6) | 8 (11) | 0.4 (0.1–1.7) |
| No | 57 (79) | 59 (84) | Reference | 62 (85) | 65 (89) | Reference | 66 (94) | 62 (89) | Reference |
| Vaccination status | |||||||||
| Vaccinated prior to January 2009 | |||||||||
| No | 33 (48) | 4 (6) | 29.0 (4.0–212.9) | 26 (46) | 8 (12) | 7.0 (2.1–23.5) | 52 (78) | 27 (40) | 9.3 (2.8–30.7) |
| Yesc | 36 (52) | 65 (94) | Reference | 30 (54) | 61 (88) | Reference | 15 (22) | 41 (60) | Reference |
Discrepancies between sum of cases or controls listed for a risk factor and the total number of cases or controls reflect unknown/missing data which were not included in analysis.
MOR, matched odds ratio, and 95% CI, 95% confidence interval, from conditional logistic regression.
Of those vaccinated, 20 (56%) case-patients and 36 (55%) controls in Bogodogo, 7 (23%) case-patients and 18 (30%) controls in Zorgho, and 3 (20%) case-patients and 2 (5%) controls in Sahel had documentation of vaccination by vaccination card; the remainder were vaccinated according to caregiver recall.
Among persons aged 15–30 years, case-patients were more likely than controls to be unvaccinated (MOR [95% CI] 4.8[1.8–12.6], 19.7 [3.3–infinity], and 8.0 [1.8–34.8] in Bogodogo, Zorgho, and Sahel, respectively) and to be employed outside the home (MOR [95% CI] 7.8 [2.7–22.0], 12.0 [1.6–92.3], and 10.0[1.3–78.1] in Bogodogo, Zorgho, and Sahel, respectively) (Table 2). Additional risk factors included Muslim religion in Bogodogo, no or alternative education in Bogodogo and Zorgho, and having a mother or father with no or alternative education in Bogodogo. Vaccination status in persons aged 15–30 years was obtained by recall only.
Table 2.
Characteristics associated with measles in persons aged 15–30 years stratified by geographic area, case–control study of measles outbreak in Burkina Faso, 2009.
| Bogodogo | Zorgho | Sahel | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases (na = 74) n (%) |
Controls (n = 74) n (%) |
MOR (95% CI)b | Cases (n = 68) n (%) |
Controls (n = 68) n (%) |
MOR (95% CI) | Cases (n = 71) n (%) |
Controls (n = 71) n (%) |
MOR (95% CI) | |
| Demographic characteristics | |||||||||
| Age group | |||||||||
| 15–22.6 years | 34 (46) | 41 (55) | 0.8 (0.3–1.8) | 26 (38) | 30 (44) | 1.0 (0.4–2.2) | 40 (56) | 36 (51) | 1.2 (0.5–2.7) |
| 22.7–25.3 years | 18 (24) | 10 (14) | 1.9 (0.7–5.1) | 16 (24) | 9 (13) | 2.4 (0.8–7.3) | 11 (15) | 14 (20) | 0.8 (0.3–2.2) |
| 25.4–30 years | 22 (30) | 23 (31) | Reference | 26 (38) | 29 (43) | Reference | 20 (28) | 21 (30) | Reference |
| Sex | |||||||||
| Male | 37 (51) | 25 (34) | 1.9 (0.97–3.6) | 40 (60) | 32 (48) | 1.7 (0.8–3.4) | 46 (65) | 41 (58) | 1.4 (0.7–3.0) |
| Female | 36 (49) | 49 (66) | Reference | 27 (40) | 35 (52) | Reference | 25 (35) | 30 (42) | Reference |
| Ethnicity | |||||||||
| Peul/Fulani | 5 (7) | 1 (1) | >999.9 (<0.1->999.9) | 7 (10) | 1 (1) | >999.9 (<0.1->999.9) | 53 (75) | 47 (66) | 2.8 (0.7–10.9) |
| Other | 10 (14) | 17 (23) | 0.6 (0.3–1.4) | 1 (2) | 2 (3) | 0.5 (0.0–5.5) | 14 (20) | 15 (21) | 2.1 (0.4–10.3) |
| Mossi | 59 (80) | 56 (76) | Reference | 60 (88) | 65 (96) | Reference | 4 (6) | 9 (13) | Reference |
| Religion | |||||||||
| Muslim | 57 (78) | 45 (61) | 2.9 (1.2–6.8) | 42 (62) | 37 (55) | 1.3 (0.6–2.8) | 68 (96) | 67 (94) | 2.0 (0.2–22.1) |
| Not Muslim | 16 (22) | 29 (39) | Reference | 26 (38) | 30 (45) | Reference | 3 (4) | 4 (6) | Reference |
| Education | |||||||||
| None | 37 (50) | 23 (31) | 5.3 (1.9–14.7) | 54 (81) | 36 (53) | 6.0 (2.1–17.5) | 63 (89) | 52 (73) | >999.9 (<0.1->999.9) |
| Alternative schooling | 16 (22) | 8 (11) | 5.7 (1.7–18.8) | 6 (9) | 5 (7) | 5.5 (1.2–26.4) | 7 (10) | 10 (14) | >999.9 (<0.1->999.9) |
| Any standard schooling | 21 (28) | 43 (58) | Reference | 7 (10) | 27 (40) | Reference | 1 (1) | 9 (13) | Reference |
| Mother’s education | |||||||||
| None | 64 (86) | 56 (76) | 13.3 (1.8–101.7) | 66 (97) | 62 (94) | >999.9 (<0.1->999.9) | 65 (92) | 63 (90) | Reference |
| Alternative schooling | 8 (11) | 3 (4) | 40.7 (3.2–524.9) | 2 (3) | 3 (5) | >999.9 (<0.1->999.9) | 6 (8) | 7 (10) | 0.8 (0.2–3.4) |
| Any standard schooling | 2 (3) | 15 (20) | Reference | 0 (0) | 1 (2) | Reference | 0 (0) | 0 (0) | - |
| Father’s education | |||||||||
| None | 52 (70) | 45 (61) | 4.7 (1.3–16.2) | 62 (91) | 62 (93) | 4.0 (0.4–35.8) | 57 (81) | 58 (85) | 1.1 (0.1–16.7) |
| Alternative schooling | 14 (19) | 9 (12) | 10.2 (1.7–60.3) | 5 (7) | 1 (1) | >999.9 (<0.1->999.9) | 12 (17) | 8 (12) | 2.8 (0.2–35.2) |
| Any standard schooling | 8 (11) | 20 (27) | Reference | 1 (1) | 4 (6) | Reference | 1 (1) | 2 (3) | Reference |
| Occupation | |||||||||
| Employed outside the home | 49 (66) | 22 (30) | 7.8 (2.7–22.0) | 66 (97) | 55 (81) | 12.0 (1.6–92.3) | 66 (93) | 57 (80) | 10.0 (1.3–78.1) |
| Unemployed | 25 (34) | 52 (70) | Reference | 2 (3) | 13 (19) | Reference | 5 (7) | 14 (20) | Reference |
| Travel and migration | |||||||||
| Lived outside ofarea (>20 km away) in last year | |||||||||
| Yes | 9 (12) | 8 (11) | 1.1 (0.4–3.2) | 6 (9) | 6 (9) | 1.0 (0.2–5.0) | 10 (14) | 6 (8) | 1.7 (0.6–4.6) |
| No | 64 (88) | 66 (89) | Reference | 62 (91) | 62 (91) | Reference | 61 (86) | 65 (92) | Reference |
| Lived outside of Burkina Faso in last year | |||||||||
| Yes | 1 (1) | 1 (1) | 1.0 (0.1–16.0) | 0 (0) | 0 (0) | - | 1 (1) | 1 (1) | 1.0 (0.1–16.0) |
| No | 72 (99) | 73 (99) | Reference | 68 (100) | 68 (100) | 70 (99) | 70 (99) | Reference | |
| Household member lived outside area in last year | |||||||||
| Yes | 13 (18) | 6 (8) | 2.4 (0.8–6.8) | 7 (10) | 5 (7) | 1.5 (0.4–5.3) | 6 (8) | 6 (8) | 1.0 (0.3–4.0) |
| No | 61 (82) | 68 (92) | Reference | 61 (90) | 63 (93) | Reference | 65 (92) | 65 (92) | Reference |
| Had visitor stay in household in last year | |||||||||
| Yes | 11 (15) | 12 (16) | 0.9 (0.3–2.3) | 3 (4) | 2 (3) | 1.5 (0.3–9.0) | 3 (4) | 5 (7) | 0.6 (0.1–2.5) |
| No | 63 (85) | 62 (84) | Reference | 65 (96) | 66 (97) | Reference | 68 (96) | 66 (93) | Reference |
| Vaccination statusc | |||||||||
| Vaccinated prior to January 2009 | |||||||||
| No | 33 (57) | 12 (19) | 4.8 (1.8–12.6) | 27 (71) | 14 (29) | 19.7 (3.3-infinity)d | 54 (87) | 39 (59) | 8.0 (1.8–34.8) |
| Yes | 25 (43) | 52 (81) | Reference | 11 (29) | 35 (71) | Reference | 8 (13) | 27 (41) | Reference |
Discrepancies between sum of cases or controls listed for a risk factor and the total number of cases or controls reflect unknown/missing data which were not included in analysis.
MOR, matched odds ratio, and 95% CI, 95% confidence interval, from conditional logistic regression.
Vaccination status in persons aged 15–30 years was obtained by recall only.
Exact test used due to small cell frequencies: 30 (44%) case-patients and 19 (28%) controls were omitted due to unknown vaccination status.
In multivariable analysis, lack of vaccination remained significantly associated with measles in both age strata in all areas (adjusted MOR [aMOR] [95% CI] 19.4 [2.4–155.9], 5.9 [1.6–21.5], and 6.4 [1.8–23.0] in children aged 1–14 years, and 3.2 [1.1–9.7],19.7 [3.3–infinity], and 8.0 [1.8–34.8] in persons aged 15–30 years in Bogodogo, Zorgho, and Sahel, respectively) (Table 3). Among children, other independent risk factors associated with measles included age 12–28 months in Bogodogo and Sahel, Muslim religion in Bogodogo, and Peul/Fulani ethnicity in Sahel. Among persons aged 15–30 years, employment was independently associated with measles in Bogodogo.
Table 3.
Results of multivariable analyses of risk factors associated with measles in children aged 1–14 years and persons aged 15–30 years, stratified by geographic area, case–control study of measles outbreak in Burkina Faso, 2009.
| Children aged 1–14 years | Adults aged 15–30 years | |||||
|---|---|---|---|---|---|---|
| Bogodogoa aMOR (95% Cl)d |
Zorghob aMOR (95% Cl) |
Sahelc aMOR (95% Cl) |
Bogodogoe aMOR (95% Cl) |
Zorghof aMOR (95% Cl) |
Sahelg aMOR (95% Cl) |
|
| Vaccinated prior to January 2009 | ||||||
| No | 19.4 (2.4–155.9) | 5.9 (1.6–21.5) | 6.4 (1.8–23.0) | 3.2 (1.1–9.7) | 19.7 (3.3-infinity)h | 8.0 (1.8–34.8) |
| Yes | Reference | Reference | Reference | Reference | Reference | Reference |
| Age group | ||||||
| 12–28 months | 9.9 (1.1–86.7) | 2.2 (0.7–7.6) | 4.6 (1.03–20.3) | - | - | - |
| 29–79 months | 2.1 (0.6–7.1) | 1.7 (0.6–4.5) | 1.0 (0.3–3.5) | - | - | - |
| 80 months to 14 years | Reference | Reference | Reference | - | - | - |
| Religion | ||||||
| Muslim | 4.4 (1.2–15.9) | - | - | - | - | - |
| Not Muslim | Reference | - | - | - | - | - |
| Ethnicity | ||||||
| Peul/Fulani | - | - | 4.0 (1.2–13.2) | - | - | - |
| Not Peul/Fulani | - | - | Reference | - | - | - |
| Occupation | ||||||
| Employed outside the home | - | - | - | 9.0 (2.0–39.6) | - | - |
| Unemployed | - | - | - | Reference | - | - |
Analysis included 66 matched case–control pairs with complete data for variables included in the model.
Analysis included 54 matched case–control pairs with complete data for variables included in the model.
Analysis included 65 matched case–control pairs with complete data for variables included in the model.
aMOR, adjusted matched odds ratio, and 95% CI, 95% confidence interval, from multivariable conditional logistic regression.
Analysis included 52 matched case–control pairs with complete data for variables included in the model.
Analysis included 28 matched case–control pairs with complete data for variables included in the model.
Analysis included 59 matched case–control pairs with complete data for variables included in the model.
Exact test used due to small cell frequencies.
A previous history of measles was reported in 4 (1.9%) of 215 controls aged 1–14 years and 31 (14.5%) of 213 controls aged 15–30 years. When these controls were excluded, age group was no longer significantly associated with measles in the 1–14 year age stratum in Bogodogo; otherwise, factors significantly associated with measles in multivariable analysis did not change.
3.4.2. Vaccine effectiveness (VE)
VE of any measles vaccination prior to 2009 was 94% (95% confidence interval [CI], 45–99%) in Bogodogo, 87% (95% CI, 37–97%) in Zorgho, and 84% (95% CI, 41–96%) in Sahel among children aged 1–14 years, and was 73% (95% CI, 11–92%) in Bogodogo, 95% (95% CI, 70–100%) in Zorgho, and 86% (95% CI, 27–97%) in Sahel among persons aged 15–30 years.
3.4.3. Vaccination status and reasons for not receiving measles vaccination
Measles vaccination status varied between geographic areas; reported vaccination prior to 2009 was lower among controls from Sahel (41/68 [60%] and 27/66 [41%] in children aged 1–14 and adults aged 15–30 years, respectively) than controls from Bogodogo (65/69 [94%] in children and 52/64 [81%] in adults) or Zorgho (61/69 [88%] in children and 35/49 [71%] in adults). Similarly, among controls with vaccination information for the 2009 outbreak response campaign, 65% (45/69) reported vaccination in Sahel, compared with 94% (66/70) in Bogodogo and 89% (65/73) in Zorgho.
Among eligible subjects who provided reasons for not receiving routine measles vaccination, the most common reasons were “didn’t know vaccine was needed” (60/140 [43%]) and absence of caregiver or child during routine outreach activities (26/140 [19%]). During campaigns, the most common reasons were “not informed about campaign” (53/133 [40%]) and absence of caregiver or child during campaign (24/133 [18%]).
4. Discussion
Despite implementation of WHO-UNICEF measles mortality reduction strategies since 2001, including three nationwide immunization campaigns with >95% estimated coverage during 2001–2007 and increasing routine measles vaccination coverage, Burkina Faso experienced its largest measles outbreak on record in 2009. This outbreak was notable for its size and age distribution, with 35% of cases occurring in persons aged ≥15 years. The main risk factor for measles was lack of vaccination, and measles vaccine was effective in reducing risk of measles. Additionally, the proportion of vaccinated controls in our study was lower than reported national estimates.
Historically, measles has been a disease of childhood [2]. The increased proportion of older case-patients observed in this outbreak reflects Burkina Faso’s long history of suboptimal measles vaccination coverage through routine immunization and intermittent campaigns since the 1960s [9–16], which reduced measles incidence, but created cohorts of older children and adults never exposed to measles. Similar shifts in age distribution of measles cases were observed previously in Burkina Faso [17] and in multiple outbreaks throughout Africa during 2009–2010 [26,27].
The WHO measles mortality reduction strategy calls for ≥90% measles vaccination coverage nationally and ≥80% in each dis trict [28]. Vaccination coverage in study areas, particularly Sahel, was likely lower than national estimates and did not meet WHO-recommended targets. Unvaccinated children and adults, who had also escaped measles virus infection during previous outbreaks, existed throughout Burkina Faso and played a significant role in sustaining virus transmission.
Expected measles VE is approximately 85% for 1 dose of measles vaccine received at age 9–11 months, 95% for 1 dose received at age ≥12 months, and 98% for ≥2 doses with the second dose at age ≥12 months [29–31]. Due to the proportion of subjects in our study with incomplete data on number of doses of measles vaccine received or age at which vaccine was received, we were not able to calculate reliable VE estimates for 1 dose at age 9–11 months, 1 dose at age ≥12 months, and ≥2 doses with the second dose at age ≥ 12 months. While inferences from this study’s VE point estimates are limited by wide confidence intervals, some of our VE estimates were slightly lower than expected and observed during previous outbreak investigations elsewhere [32–36]. The prevalence of HIV infection in Burkina Faso is relatively low (1.2% among adults) [37] and is unlikely to have significantly affected VE estimates. Given that lack of vaccination was the main risk factor for measles and the low measles vaccination rates described in this study, widespread poor performance of measles vaccine does not appear to be a major cause of the outbreak.
Child/caregiver absence during outreach vaccination activities or campaigns was a common reason provided for not receiving measles vaccination and might be due in part to the narrow window of opportunity to receive routine measles vaccination, which is generally available only at age 9–11 months. Additionally, measles vaccine is packaged in 10-dose vials which must be discarded ≤ 6 h of opening and adding diluents; in an effort to limit vaccine wastage, some health centers offer measles vaccine only 1–2 days per week. Policies limiting age of eligibility and number of days vaccine is available should be reconsidered. Also, new strategies are needed to trace and vaccinate absent children and to increase public awareness of the importance of vaccination.
This is the second outbreak investigation in Burkina Faso since the country adopted the WHO/UNICEF mortality reduction strategies in 2001. In a 2002 outbreak investigation, Yameogo and colleagues found 39% of case-patients aged 9 months to 14 years and 10% of case-patients aged ≥15 years had recently traveled from Côte d’Ivoire, and identified migration as an important risk factor for measles [17]. In contrast, <2% of our case-patients stayed anywhere outside Burkina Faso within the previous year, and migration was not associated with measles. As both studies took place during August–September, these differences cannot be explained by seasonal migration patterns; however, the studies took place in different regions, and geographic differences in migration patterns cannot be excluded. Most likely though, these differences are attributable to historical changes: migration from Côte d’Ivoire into Burkina Faso increased surrounding the 2002–2007 civil war in Côte d’Ivoire [38].
This study is subject to limitations. The case-based surveillance database was incomplete, and included data from 11 of 63 districts in Burkina Faso. Measles surveillance systems do not detect all measles cases due to incomplete reporting from communities and within health systems, and the 54,111 reported cases in 2009 in Burkina Faso represented only a portion of the measles cases that occurred during the outbreak. In the case–control study, the proportion of non-respondents is unknown, most findings are dependent on recall, and misclassification of vaccination status, as well as the relatively high proportion of subjects with unknown vaccination status, might have led to inaccurate VE estimates. If misclassification was non-differential, this would likely bias toward the null. In addition, because routine laboratory confirmation of suspected cases stopped in March 2009, other illnesses with fever and rash might have been misclassified as measles. To minimize misclassification of cases, we deliberately chose study districts with high attack rates, high number of laboratory-confirmed measles cases, and no laboratory-confirmed rubella. Findings from this study might not be applicable to other regions in Burkina Faso; in particular, vaccination coverage and reasons for not receiving measles vaccination may vary between regions. However, the finding that lack of measles vaccination was a significant risk factor in each of three study regions suggests lack of measles vaccination was an important factor throughout the country. Finally, we compared the proportion of controls that were vaccinated to administrative vaccination coverage estimates. Controls were selected based on absence of measles; therefore, selection might have been biased toward being vaccinated. If this bias occurred, then the discrepancy between the proportion vaccinated and administrative coverage estimates was likely greater than was suggested by our findings.
Multiple countries in the WHO Africa Region experienced a resurgence of measles in 2009–2010 [8]. To improve measles control and prevent future outbreaks throughout the region, accurate vaccination data, based on high-quality vaccination coverage surveys, will be essential. Reasons for not receiving measles vaccination should be identified, and strategies to improve access to vaccination should be implemented in each country. Improved strategies for identifying and reaching unvaccinated children and for reducing missed opportunities for vaccination are needed to continue the region’s progress in measles mortality reduction.
Acknowledgments
This work was supported by the Burkina Faso Ministry of Health, World Health Organization (WHO), United Nations Children’s Fund (UNICEF), and the United States Centers for Disease Control and Prevention (CDC). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. This investigation would not have been possible without the support of numerous people in Burkina Faso and elsewhere. The authors would particularly like to thank Dr. Sylvestre Tiendrebeogo and Dr. Mété Bonkoungou from the Burkina Faso Ministry of Health; Dr. Djamila Cabral and Dr. Ma Ouattara from WHO Burkina Faso; Dr. Annick Dosseh and Dr. Bokar Toure from WHO West Africa Intercountry Support Team; Dr. Peter Strebel from WHO Geneva; Dr. Maurice Hours and Dr. Fernand Toe from UNICEF Burkina Faso; Dr. Marie Therese Guigui-Zoundi from UNICEF West and Central Africa Regional Office; Dr. Robin Nandy from UNICEF New York; Dr. Kathleen Gallagher and Dr. Paul Rota from the CDC Division of Viral Diseases; the U.S. Embassy Ouagadougou; and the other Burkina Faso staff, health workers, data collectors, and study participants for their contributions to the investigation.
References
- [1].Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis 2004;189(Suppl. 1):S4–16. [DOI] [PubMed] [Google Scholar]
- [2].Strebel PM, Papania MJ, Dayan GH, Halsey NA. Measles vaccine In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. fifth ed. Saunders Elsevier; 2008. p. 353–98. [Google Scholar]
- [3].Nandy R, Handzel T, Zaneidou M, Biey J, Coddy RZ, Perry R, et al. Case-fatality rate during a measles outbreak in eastern Niger in 2003. Clin Infect Dis 2006;42:322–8. [DOI] [PubMed] [Google Scholar]
- [4].Wolfson LJ, Grais RF, Luquero FJ, Birmingham ME, Strebel PM. Estimates of measles case fatality ratios: a comprehensive review of community-based studies. Int J Epidemiol 2009;38:192–205. [DOI] [PubMed] [Google Scholar]
- [5].Wolfson LJ, Strebel PM, Gacic-Dobo M, Hoekstra EJ, McFarland JW, Hersh BS. Has the 2005 measles mortality reduction goal been achieved? A natural history modelling study. Lancet 2007;369:191–200. [DOI] [PubMed] [Google Scholar]
- [6].Centers for Disease Control and Prevention. Global measles mortality 2000–2008. MMWR 2009;58(December (47)):1321–6. [PubMed] [Google Scholar]
- [7].World Health Organization, United Nations Children’s Fund Measles mortality reduction and regional elimination strategic plan 2001–2005. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- [8].Centers for Disease Control and Prevention. Measles outbreaks and progress toward measles preelimination – African region, 2009–2010. MMWR 2011;60(12):374–8. [PubMed] [Google Scholar]
- [9].Meyer HM, Hostetler DD, Bernheim BC, Rogers NG, Lambin P, Chassary A, et al. Response of Volta children to jet inoculation of combined live measles, smallpox, and yellow fever vaccines. Bull World Health Organ 1964;30:783–94. [PMC free article] [PubMed] [Google Scholar]
- [10].Meyer HM, Hostetler DD, Bernheim BC, Rogers NG, Lambin P, Chassary A, et al. Response of Volta children to live attenuated measles virus vaccine. Bull World Health Organ 1964;30:769–81. [PMC free article] [PubMed] [Google Scholar]
- [11].Etheridge EW. Sentinel for health: a history of the Centers for Disease Control. Berkeley and Los Angeles, CA: University of California Press; 1992. p. 188–210. [Google Scholar]
- [12].Kalabus F, Sansarricq H, Lambin P, Proulx J, Hillman MR. Standardization and mass application of combined live measles-smallpox vaccine in Upper Volta. Am J Epidemiol 1967;86(1):93–111. [DOI] [PubMed] [Google Scholar]
- [13].Kessler S, Falaha F, Gonidec G, Parker D. Lessons learned Rapid assessment: vaccination commando Burkina Faso. New York, NY: UNICEF; 1986. [Google Scholar]
- [14].Zuber PLF, Conombo KSG, Traoré AD, Millogo JD, Ouattara A, Ouédraogo IB, et al. Mass measles vaccination in urban Burkina Faso, 1998. Bull World Health Organ 2001;79(4):296–300. [PMC free article] [PubMed] [Google Scholar]
- [15].Kambiré C, Konde MK, Yaméogo A, Tiendrébéogo SRM, Ouédraogo RT, Otten MW, et al. Measles incidence before and after mass vaccination campaigns in Burkina Faso. J Infect Dis 2003;187(Suppl. 1):S80–5. [DOI] [PubMed] [Google Scholar]
- [16].Yaméogo KR, Yaméogo A, Nacoulma SD, Zuber PLF. Measles vaccination coverage during poliomyelitis national immunization days in Burkina Faso, 1999. J Infect Dis 2003;197(Suppl 1):S74–9. [DOI] [PubMed] [Google Scholar]
- [17].Yaméogo KR, Perry RT, Yaméogo A, Kambiré C, Kondé MK, Nshimirimana D, et al. Migration as a risk factor for measles after a mass vaccination campaign, Burkina Faso, 2002. Int J Epidemiol 2005;34:556–64. [DOI] [PubMed] [Google Scholar]
- [18].Ministère de la Santé, Commission Nationale d’Organisation de la Campagne Rougeole et la Polio 2004 Evaluation de la campagne nationale de vaccination de masse contre la rougeole et la polio décembre 2004 au Burkina Faso. Ouagadougou: Ministère de la Santé; 2005. [Google Scholar]
- [19].Ministère de la Santé, Commission Nationale d’Organisation de la Campagne Rougeole-Vitamine A 2007 Rapport de l’évaluation nationale de vaccination de masse contre la rougeole et la supplementation en vitamine A de décembre 2007 au Burkina Faso. Ouagadougou: Ministère de la Santé; 2008. [Google Scholar]
- [20].World Health Organization, UNICEF. WHO/UNICEF review of national immunization coverage 1980–2008. Burkina Faso; 2009. Available from: http://www.who.int/immunization_monitoring/data/bfa.pdf [cited 2010 May10]. [Google Scholar]
- [21].World Health Organization. Manual for the laboratory diagnosis of measles virus infection. December 1999. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- [22].World Health Organization. Response to measles outbreaks in measles mortality reduction settings. Geneva, Switzerland: World Health Organization; 2009. [PubMed] [Google Scholar]
- [23].Orenstein WA. Field evaluation of vaccine efficacy. Bull World Health Organ 1985;63(6):1055–68. [PMC free article] [PubMed] [Google Scholar]
- [24].Helfand RF, Heath JL, Anderson LJ, Maes EF, Guris D, Bellini W. Diagnosis of measles with an IgM capture EIA: the optimal timing of specimen collection after rash onset. J Infect Dis 1997;175:195–9. [DOI] [PubMed] [Google Scholar]
- [25].Rota PA, Featherstone DA, Bellini WJ. Molecular epidemiology of measles virus In: Griffin D, Oldstone M, editors. Measles – pathogenesis and control. Berlin/Heidelberg: Springer-Verlag; 2009. p. 129–50. [DOI] [PubMed] [Google Scholar]
- [26].Masresha BG, Fall A, Eshetu M, Sosler S, Alleman M, Goodson JL, et al. Measles mortality reduction and pre-elimination in the African region, 2001–2009. J Infect Dis 2011;204(Suppl. 1):S198–204. [DOI] [PubMed] [Google Scholar]
- [27].Goodson JL, Masresha BG, Wannemuehler K, Uzicanin A, Cochi S. Changing epdiemiology of measles in Africa. J Infect Dis 2011;204(Suppl. 1):S205–14. [DOI] [PubMed] [Google Scholar]
- [28].World Health Organization. Measles vaccines: WHO position paper. Wkly Epidemiol Rec 2009;84(35):349–60. [PubMed] [Google Scholar]
- [29].Sudfeld CR, Navar AM, Halsey NA. Effectiveness of measles vaccination and vitamin A treatment. Int J Epidemiol 2010;39:i48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cutts F, Grabowsky M, Markowitz L. The effect of dose and strain of live attenuated measles vaccines on serological responses in young infants. Biologicals 1995;23:96–106. [DOI] [PubMed] [Google Scholar]
- [31].World Health Organization. The immunological basis for immunization series, module 7: measles update 2009. Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- [32].Hennessey KA, Ion-Nedelcu N, Craciun M- D, Toma F, Wattigney W, Strebel PM. Measles epidemic in Romania, 1996–998: assessment of vaccine effectiveness by case–control and cohort studies. Am J Epidemiol 1999;150(11):1250–7. [DOI] [PubMed] [Google Scholar]
- [33].McMorrow ML, Gebremedhin G, van dan Heever J, Kezaala R, Harris BN, Nandy R, et al. Measles outbreak in South Africa, 2003–2005. S Afr Med J 2009;99(5):314–9. [PMC free article] [PubMed] [Google Scholar]
- [34].Goodson JL, Perry RT, Mach O, Manyanga D, Luman ET, Kitambi M, et al. Measles outbreak in Tanzania, 2006–2007. Vaccine 2010;28(37):5979–85. [DOI] [PubMed] [Google Scholar]
- [35].Marin M, Nguyen HQ, Langidrik JR, Edwards R, Briand K, Papania MJ, et al. Measles transmission and vaccine effectiveness during a large outbreak on a densely populated island: implications for vaccination policy. Clin Infect Dis 2006;42:315–9. [DOI] [PubMed] [Google Scholar]
- [36].Uzicanin A, Zimmerman L. Field effectiveness of live attenuated measles-containing vaccines: a review of published literature. J Infect Dis 2011;204(Suppl. 1):S133–48. [DOI] [PubMed] [Google Scholar]
- [37].UNICEF. Children and AIDS. Fifth Stocktaking report, 2010. New York, NY: United Nations Children’s Fund; 2010. [Google Scholar]
- [38].International Organization for Migration. Migration en Côte d’Ivoire: Profil national 2009. Geneva, Switzerland: International Organization for Migration; 2009. [Google Scholar]