Abstract
Eosinophilic gastrointestinal disorders are a set of conditions with a wide range of clinical manifestations and treatment modalities. The disorders are suspected to result from an abnormal inflammatory response to allergen(s), and individuals may develop a relapsing or chronic disease, if the allergen is not eliminated. Mechanisms of disease pathogenesis, including the humoral immune response, need to be fully elucidated. A variety of therapies are used, though there is a lack of well-defined randomized, prospective studies. Other therapeutic options are needed as the current treatments have potential concerns; elimination diets may impair a child’s quality of life, and corticosteroids have adverse risks with long-term use. We review what is known about non-esophageal eosinophilic gastrointestinal disorders, and discuss research investigations which need to be conducted to facilitate diagnosis and enhance treatment methods.
Introduction
Eosinophilic gastrointestinal disorders (EGIDs) are conditions with an elevated number of eosinophils in any segment of the GI tract. Eosinophilic esophagitis (EoE), the most described and encountered of EGIDs, is a chronic, immune-mediated disease which causes esophageal dysfunction and histologically demonstrates ≥15 eosinophils per high-power field (eos/HPF) on esophageal biopsies. About a third of patients with esophageal eosinophilia will respond to high-dose proton pump inhibitor (PPI) and are considered to have proton pump inhibitor-responsive esophageal eosinophilia (PPI-REE) [1]; non-responders require dedicated treatment for EoE.
Non-esophageal EGIDs (neEGIDs) present with diverse symptomology and increased intestinal eosinophilia without a known secondary etiology. Eosinophilic gastritis (EG) causes upper GI symptoms, including abdominal pain, vomiting, and failure to gain weight. Eosinophilic gastroenteritis (EGE) typically involves the stomach and small bowel to produce a multitude of upper and lower intestinal symptoms. Eosinophils may be elevated in segments or throughout the colon in eosinophilic colitis (EC). In addition, eosinophils can be present at varying depths of the intestinal wall, from the mucosa to the serosa. Due to the wide-spectrum of symptoms, neEGIDs have a broad differential diagnosis (Table 1).
Table 1:
Clinical presentation of non-esophageal eosinophilic gastrointestinal disease
| Eosinophilic Gastritis | Eosinophilic Gastroenteritis | Eosinophilic Colitis | |
|---|---|---|---|
| Age of Prevalence | increases with age, 7th decade | less than 5 years | similar for all ages |
| Gender | Female > male | Female > male | Female > male |
| Symptoms | epigastric pain, dyspepsia, vomiting, early satiety, oral aversion, failure to gain weight | abdominal pain, dyspepsia, vomiting, hematemesis, bloating, diarrhea | abdominal pain, diarrhea and/or constipation, lower gastrointestinal bleeding, tenesmus |
| Mucosal eosinophilia | malabsorption weight loss, ulcerations, | malabsorption, anemia, protein-losing enteropathy | protein-losing enteropathy, bleeding |
| Muscularis mucosa eosinophilia | gastric outlet obstruction | intussusception, dysmotility | intussusception, dysmotility |
| Serosal eosinophilia | bloating | bloating, ascites | ascites, edema |
| Differential Diagnosis | peptic ulcer disease, infection, drug injury, toxins, neoplasm, vasculitis, hypereosinophilic syndrome, Langerhan cell histiocytosis | food protein-induced enterocolitis, allergic proctocolitis, inflammatory bowel disease | |
While there remains much to be learned about the pathophysiology and management, we aim to increase clinicians’ awareness of neEGIDs in order to make a timely diagnosis, provide appropriate long-term care, and foster research to advance the field.
Prevalence
EG, EGE, and EC have a reported prevalence of 6.3, 8.4, and 3.3/100,000 people, respectively. In children, the prevalence is 4.4, 10.7, and 4.3/100,000, respectively [2]. In comparison, EoE has a prevalence of 1/2,000 children [3]. The prevalence of EG increases with age, being highest in the 7th decade of life, while the prevalence of EGE is highest in children under age five years. The prevalence of EC does not significantly differ by age (Table 1) [2].
There is a baseline female predominance in all neEGIDs but particularly in EG with 7.9/100,000 cases in females versus 5.4/100,000 cases in males [2]. This female preponderance is in stark contrast to EoE where the gender ratios are reversed.
neEGIDs may coexist with other allergic and/or atopic conditions, food sensitivities, and/or EoE [5]. The reported co-incidence of EoE with EG, EGE, and EC is 10.6%, 12.0%, and 10.9%, respectively. About a third of neEGID patients have concomitant atopy with similar incidences in children and adults [2].
Pathophysiology
neEGID has been hypothesized to involve IgE and non-IgE-mediated immune mechanisms. Patients are suspected to have an abnormal response to food or environmental allergens, which may differ based on demographics, geographic location, and/or season [4]. Eotaxin, a selective chemokine regulating eosinophil migration, binds to the eosinophilic chemokine receptor CCR3, which leads to further upregulation of cytokines, including interleukins (IL) IL-3, IL-5, and IL-13 [5]. Mice exposed to oral antigens develop eosinophilic inflammation of the esophagus, stomach, and small intestine through eotaxin-mediated signaling (6).
Torrente, et al. [6], found that patients with EC had a higher density of CD3+ T cells, eotaxin-2+ intraepithelial lymphocytes, and IgE+ cells in the lamina propria than patients with inflammatory bowel disease (IBD). There were a higher number of degranulating eosinophils and mast cells along with elevated tryptase and IgE concentrations near mucosal enteric nerves in patients with EC, but not those with IBD or healthy controls. It is thought that these changes lead to mucosal permeability, dysmotility, and visceral hyperalgesia in EC.
STAT6-mediated immune mechanisms are involved in food-induced intestinal inflammation in mice [7]. STAT6 signaling leads to the production of IL-4 and IL-13 by Th-2 cells, and unlike control mice, STAT−/− mice do not develop diarrhea, exhibit intestinal eosinophilia, or produce Th-2 cytokines or IgE antibodies when stimulated with oral ovalbumin.
The role of mast cell release of leukotrienes has been debated in EoE and requires further investigation in neEGIDs too [8]. Allergens bind to the IgE receptor on mast cells and basophils leading to cell activation and release of inflammatory mediators, including IL-13 which is involved with eosinophil recruitment [9, 10]. Mast cells may also increase intestinal permeability; patients with EGE and protein-losing enteropathy had higher numbers of mucosal mast cells than those without [11]. Future investigations will determine if these molecular markers can be therapeutic targets.
Clinical Presentation
Patients with neEGID generally demonstrate symptoms based on the location and the depth of tissue eosinophilia (Table 1). EG may present with epigastric pain, vomiting, or weight loss [2]. Patients with EGE may have upper and lower GI symptoms, including abdominal pain, vomiting, hematemesis, or diarrhea. EC may manifest as abdominal pain, diarrhea and/or constipation, or lower GI bleeding. Mucosal eosinophilia may cause protein-losing enteropathy leading to hypoalbuminemia, whereas eosinophilic inflammation of the muscularis mucosa may lead to impaired motility or anatomical obstruction. Eosinophilic infiltration of the serosa may result in non-specific bloating or more worrisome ascites (Table 1) [4, 12, 13]. Approximately 60–70% of patients with EGE have peripheral eosinophilia which differs from the wide range in EoE of 20–100% of children; some patients may have elevated serum IgE levels [5, 8, 14].
Endoscopic and Histologic Features
As with EoE, the diagnosis of neEGID requires endoscopic evaluation and histological examination of the GI tract. Endoscopy may be visually normal or demonstrate mucosal erythema, edema, ulcerations, nodularity, or polypoid lesions. Histologically, there is eosinophilia along with degranulated eosinophils, cryptitis, crypt abscesses, and/or chronic architectural changes. Infiltration of other inflammatory cells, such as neutrophils or plasma cells, may be encountered but is generally not proportional to the density of eosinophilic inflammation [2]. Eosinophils may be located in lamina propria, muscularis mucosa, and/or submucosa [4]. However, endoscopically-obtained tissue biopsies contain only mucosa/lamina propria, and the diagnosis might be missed if only deeper tissue levels contain eosinophilia.
Diagnosis
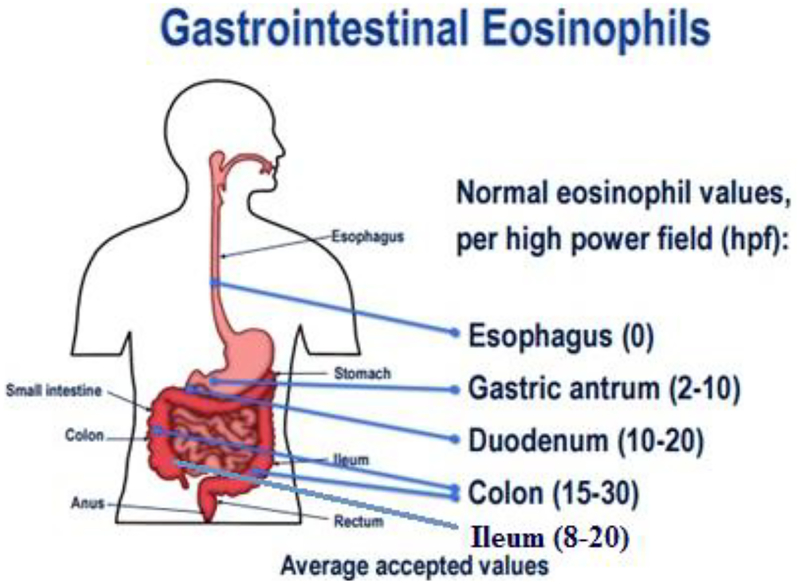
Diagnosing neEGIDs can be challenging as the diseases have a variable clinical presentation, may be segmental in location, and be outside the reach of endoscopically-obtained biopsies. Diagnostic criteria have not been conclusively established, and diagnosis is often based on eosinophil load on biopsies in the appropriate clinical setting. Physiological eosinophil load in different parts of the GI tract generally increases in a descending manner, save the colon where it is higher in the right colon compared to the distal colon (Figure 1) [4, 15, 16]. Additional studies have evaluated the number of tissue eosinophils based on age, sex, and history of atopic disease, but no significant correlations were found [15, 17].
Figure 1: Expected physiological numbers of eosinophils in the intestinal tract.
DeBrosse CW et.al. Pediatr Dev Pathol. 2006;9(3):210–8.
Figure adapted and modified from “Eosinophilic Esophagitis: Diagnosis and Management – Full Reference Set.” 2014. Permission granted by the NASPGHAN Foundation.
The histologic criteria for neEGIDs include a significant increase in intestinal eosinophil load; EG exhibits ≥30 eos/HPF in 5 HPFs or ≥70 eosinophils ≥3 HPFs [18, 19]. EGE demonstrates ≥52 eos/HPF in the duodenum and ≥56 eos/HPF in the ileum. EC is considered if there are greater than twice the normal number of eosinophils, with ≥100 eos/HPF in the right colon, ≥84 eos/HPF in the transverse and descending colon, and ≥64 eos/HPF in the rectosigmoid colon. Biopsies should be evaluated for cellular degranulation, crypt architectural distortion, and eosinophilic cryptitis [20]. IBD may be the most difficult to differentiate from EC as there is eosinophilia in the lamina propria, but IBD also has acute and chronic inflammatory cells [21]. EC typically does not demonstrate acute inflammation, and its diagnosis is often made after excluding other etiologies. Thus, the diagnosis of neEGIDs, as for IBD, should take into consideration clinical symptoms, endoscopic findings, and pathology.
Management
The low prevalence of neEGIDs, the limited number of placebo-controlled clinical trials, and gaps in our understanding of the natural history lead to empiric therapeutic and management decisions based on experience rather than evidenced-based protocols. As with EoE, it is unclear if treatment end-points in neEGIDs should be solely based on histological criteria, i.e. return of eosinophil load to physiological numbers or a composite of clinical, endoscopic, and histologic criteria [22].
Dietary restriction or corticosteroids are common treatment methods, though other agents, such as biologics, are being investigated. Dietary therapy is often recommended due to the lower risk of adverse effects than corticosteroids and a high rate of efficacy [19, 22]. In a systemic review by Lucendo, et al. [22], 86 patients with EG and EC were given an elemental or hydrolyzed protein formula or advised to follow the 6- or 7-food elimination diet. 68 (79%) patients demonstrated clinical improvement, and 16/20 (80%) patients who had follow-up biopsies had histological improvement or remission. Remission was defined as <20 eos/HPF in the stomach, small intestine, or colon [23]. Ko, et al. [19] evaluated elemental formula or food elimination diet in 17 patients with EG. 14/17 (82%) patients had symptom resolution and 11/14 (79%) who had post-therapy biopsies had histological remission with <10 eos/HPF.
Topical or systemic corticosteroids have been used to induce remission, but patients may develop resistance to corticosteroids or relapse upon discontinuation as reported in 42% of a cohort of EGE patients [5]. Budesonide can be given for EG and EGE, and the controlled ileal-release capsule can be used in EC. Swallowed fluticasone may benefit patients with concomitant EoE and EG, but the delivery may not treat EGE [23]. Patients may require low-dose maintenance therapy, particularly if they relapse upon discontinuation of corticosteroids [12].
Various other medications have been studied, including montelukast, a leukotriene receptor antagonist, and PPIs. Case reports demonstrate inconsistency of montelukast in maintaining remission in EGE, even after induction therapy with corticosteroids [5, 24, 25]. PPIs have exhibited variable and limited results in EG and EGE, and more studies are needed. In case reports, patients who were unresponsive to a PPI required treatment with corticosteroids or dietary elimination [26–28].
Biologic agents have produced mixed results with EoE, thereby tempering the enthusiasm for neEGIDs [29–31]. Reslizumab and mepolizumab, monoclonal antibodies to IL-5, demonstrated sub-optimal attainment of remission in EoE. QAX576, a monoclonal antibody to IL-13, has been studied in EoE but not neEGIDs. Omalizumab, a monoclonal antibody to IgE, has been investigated in EoE and EGE, but did not demonstrate consistent improvement in symptoms or endoscopic and histologic findings [9, 32, 33]. Biologic agents need further study in neEGIDs and may need to be used with other therapeutic modalities to target multiple pathways that result in intestinal eosinophilia.
Adherence to treatment is crucial in order for patients to maintain remission. In a study by Hommel, et al. [34], 30% of patients with EoE or EGE demonstrated non-adherence to dietary and medical therapy. Compliance must be emphasized in order to mitigate relapse and long-term complications.
Another unmet need is guidance on endoscopic and histological evaluation and monitoring of patients with neEGIDs. A high index of suspicion should be maintained not only for initial diagnosis, but during follow-up as eosinophilia may manifest in another segment(s) of the GI tract which was/were previously unaffected [35]. Prospective studies are needed to determine the appropriate timing of follow-up endoscopy and the location and number of biopsies to obtain.
The NIH-funded Consortium for Eosinophilic Gastrointestinal Disease Research (CEGIR) is conducting multicenter, longitudinal studies to evaluate clinical outcomes, histology, and molecular markers associated with EG, EGE, and EC. These studies will allow for the creation of standardized treatment guidelines. In addition, CEGIR has a portal where patients can register and input their information, https://www.rarediseasesnetwork.org/cms/cegir/Get-Involved/Contact-Registry [37].
Conclusions and Future Directions
It is important for gastroenterologists to consider neEGIDs in the appropriate clinical setting. Dietary modifications and corticosteroids are the most commonly used treatment options, though further studies in these and other therapies are needed. Improved understanding of the pathophysiology and natural history of neEGIDs will help drive the therapeutic field, and several drugs, including small molecules and biologics, are currently in development to target biological markers in eosinophilic diseases [36]. Long-term follow-up including timing of endoscopic evaluation, location and number of biopsies for histological evaluation, and duration of therapy are unmet needs. Ongoing individual efforts and multi-center consortia, such as CEGIR, provide platforms for us and patients to collectively move the field forward.
What is Known:
Eosinophilic gastrointestinal disorders can be difficult to diagnose due to variability in clinical presentation and a low prevalence, particularly in children.
Dietary elimination of food allergens and treatment with corticosteroids provide clinical and histological improvement.
What is New:
Mouse models have demonstrated key aspects to the pathophysiology of eosinophilic gastrointestinal disease.
Translational studies are needed to identify new therapeutic targets and clinical applicability in humans.
Large, longitudinal clinical investigations, including multi-center consortium, aim to define the natural history of these rare disorders and develop standardized diagnostic and management guidelines.
References
- 1.Eluri S, Dellon ES Proton pump inhibitor-responsive oesophageal eosinophilia and eosinophilic oesophagitis: more similarities than differences. Curr Opin Gastroenterol 2015;31(4):309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen ET, Martin CF, Kappelman MD, et al. Prevalence of Eosinophilic Gastritis, Gastroenteritis, and Colitis: Estimates From a National Administrative Database. J Pediatr Gastroenterol Nutr 2016;62(1):36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dellon ES, Jensen ET, Martin CF, et al. Prevalence of eosinophilic esophagitis in the United States. Clin Gastroenterol Hepatol 2014;12(4):589–96.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins MH Histopathology associated with eosinophilic gastrointestinal diseases. Immunol Allergy Clin North Am 2009;29(1):109–17, x–xi. [DOI] [PubMed] [Google Scholar]
- 5.Choi JS, Choi SJ, Lee KJ, et al. Clinical Manifestations and Treatment Outcomes of Eosinophilic Gastroenteritis in Children. Pediatr Gastroenterol Hepatol Nutr 2015;18(4):253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torrente F, Barabino A, Bellini T, et al. Intraepithelial lymphocyte eotaxin-2 expression and perineural mast cell degranulation differentiate allergic/eosinophilic colitis from classic IBD. J Pediatr Gastroenterol Nutr 2014;59(3):300–7. [DOI] [PubMed] [Google Scholar]
- 7.Kweon MN, Yamamoto M, Kajiki M, et al. Systemically derived large intestinal CD4(+) Th2 cells play a central role in STAT6-mediated allergic diarrhea. J Clin Invest 2000;106(2):199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 2007;133(4):1342–63. [DOI] [PubMed] [Google Scholar]
- 9.Foster B, Foroughi S, Yin Y, et al. Effect of anti-IgE therapy on food allergen specific T cell responses in eosinophil associated gastrointestinal disorders. Clin Mol Allergy 2011;9(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mir SA, Schady D, Olive AP, et al. Mucosal mast cell counts in pediatric eosinophilic gastrointestinal disease. Pediatr Allergy Immunol 2014;25(1):94–5. [DOI] [PubMed] [Google Scholar]
- 11.Chehade M, Magid MS, Mofidi S, et al. Allergic eosinophilic gastroenteritis with protein-losing enteropathy: intestinal pathology, clinical course, and long-term follow-up. J Pediatr Gastroenterol Nutr 2006;42(5):516–21. [DOI] [PubMed] [Google Scholar]
- 12.Mori A, Enweluzo C, Grier D, et al. Eosinophilic gastroenteritis: review of a rare and treatable disease of the gastrointestinal tract. Case Rep Gastroenterol 2013;7(2):293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonsalves N, Furuta GT, Atkins D Eosinophilic Gastrointestinal Disorders Affect More Than Just the Esophagus. J Pediatr Gastroenterol Nutr 2016;62(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mikhail I, Sampson H. Difficult cases: eosinophilic gastrointestinal diseases (EGIDS) 2017. [DOI] [PubMed]
- 15.DeBrosse CW, Case JW, Putnam PE, et al. Quantity and distribution of eosinophils in the gastrointestinal tract of children. Pediatr Dev Pathol 2006;9(3):210–8. [DOI] [PubMed] [Google Scholar]
- 16.Lowichik A, Weinberg AG A quantitative evaluation of mucosal eosinophils in the pediatric gastrointestinal tract. Mod Pathol 1996;9(2):110–4. [PubMed] [Google Scholar]
- 17.Saad AG Normal quantity and distribution of mast cells and eosinophils in the pediatric colon. Pediatr Dev Pathol 2011;14(4):294–300. [DOI] [PubMed] [Google Scholar]
- 18.Lwin T, Melton SD, Genta RM Eosinophilic gastritis: histopathological characterization and quantification of the normal gastric eosinophil content. Mod Pathol 2011;24(4):556–63. [DOI] [PubMed] [Google Scholar]
- 19.Ko HM, Morotti RA, Yershov O, et al. Eosinophilic gastritis in children: clinicopathological correlation, disease course, and response to therapy. Am J Gastroenterol 2014;109(8):1277–85. [DOI] [PubMed] [Google Scholar]
- 20.Collins MH Histopathologic features of eosinophilic esophagitis and eosinophilic gastrointestinal diseases. Gastroenterol Clin North Am 2014;43(2):257–68. [DOI] [PubMed] [Google Scholar]
- 21.Pensabene L, Brundler MA, Bank JM, et al. Evaluation of mucosal eosinophils in the pediatric colon. Dig Dis Sci 2005;50(2):221–9. [DOI] [PubMed] [Google Scholar]
- 22.Lucendo AJ, Serrano-Montalban B, Arias A, et al. Efficacy of Dietary Treatment for Inducing Disease Remission in Eosinophilic Gastroenteritis. J Pediatr Gastroenterol Nutr 2015;61(1):56–64. [DOI] [PubMed] [Google Scholar]
- 23.Prussin C Eosinophilic gastroenteritis and related eosinophilic disorders. Gastroenterol Clin North Am 2014;43(2):317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neustrom MR, Friesen C Treatment of eosinophilic gastroenteritis with montelukast. J Allergy Clin Immunol 1999;104(2 Pt 1):506. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz DA, Pardi DS, Murray JA Use of montelukast as steroid-sparing agent for recurrent eosinophilic gastroenteritis. Dig Dis Sci 2001;46(8):1787–90. [DOI] [PubMed] [Google Scholar]
- 26.Wong GW, Lim KH, Wan WK, et al. Eosinophilic gastroenteritis: Clinical profiles and treatment outcomes, a retrospective study of 18 adult patients in a Singapore Tertiary Hospital. Med J Malaysia 2015;70(4):232–7. [PubMed] [Google Scholar]
- 27.Yamada Y, Toki F, Yamamoto H, et al. Proton pump inhibitor treatment decreased duodenal and esophageal eosinophilia in a case of eosinophilic gastroenteritis. Allergol Int 2015;64 Suppl(S83–5. [DOI] [PubMed] [Google Scholar]
- 28.Tomizawa T, Kawamura O, Kusano M A Case of Proton Pump Inhibitor-Resistant Multiple Gastric Ulcers Caused by Eosinophilic Gastroenteritis. Clin Gastroenterol Hepatol 2015;13(13):A23–4. [DOI] [PubMed] [Google Scholar]
- 29.Sawas T, Dhalla S, Sayyar M, et al. Systematic review with meta-analysis: pharmacological interventions for eosinophilic oesophagitis. Aliment Pharmacol Ther 2015;41(9):797–806. [DOI] [PubMed] [Google Scholar]
- 30.Spergel JM, Rothenberg ME, Collins MH, et al. Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol 2012;129(2):456–63, 63.e1–3. [DOI] [PubMed] [Google Scholar]
- 31.Assa’ad AH, Gupta SK, Collins MH, et al. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology 2011;141(5):1593–604. [DOI] [PubMed] [Google Scholar]
- 32.Foroughi S, Foster B, Kim N, et al. Anti-IgE treatment of eosinophil-associated gastrointestinal disorders. J Allergy Clin Immunol 2007;120(3):594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clayton F, Fang JC, Gleich GJ, et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology 2014;147(3):602–9. [DOI] [PubMed] [Google Scholar]
- 34.Hommel KA, Franciosi JP, Hente EA, et al. Treatment adherence in pediatric eosinophilic gastrointestinal disorders. J Pediatr Psychol 2012;37(5):533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaur S, Rosen JM, Kriegermeier AA, et al. Utility of Gastric and Duodenal Biopsies During Follow-up Endoscopy in Children With Eosinophilic Esophagitis. J Pediatr Gastroenterol Nutr 2017;65(4):399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Legrand F, Klion AD Biologic therapies targeting eosinophils: current status and future prospects. J Allergy Clin Immunol Pract 2015;3(2):167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.“Join the RDCRN CEGIR Contact Registry.” https://www.rarediseasesnetwork.org/cms/cegir/Get-Involved/Contact-Registry. Rare Diseases Clinical Research Network; Accessed 14 July 2017. [Google Scholar]