Abstract
The vaginal microbiota has great significance in maintaining vaginal health and protecting the host from disease. Recent advances in molecular techniques and informatics allow researchers to explore microbial composition in detail and to compare the structure of vaginal microbial communities with behavior and health outcomes, particularly acquisition and transmission of sexually transmitted diseases (STDs) and poor birth outcomes. Vaginal flora have been found to cluster into a limited number of communities, although community structure is dynamic. Certain community types are more associated with poor reproductive outcomes and STDs; communities dominated by Lactobacillus species, particularly Lactobacillus crispatus, are most associated with vaginal health. Modifiable and nonmodifiable factors are strongly associated with community composition, including behavior, race or ethnicity, and hygiene. In this review, we describe the state of the science on the vaginal microbiome and its relationship to behavior, sexual health, and STDs, including determinants of the microbiome that go beyond an individual level.
Human beings are amalgams of our own cells and the cells of our resident microbes. The relatively small number of human genetic protein-coding genes found by the Human Genome Project—approximately 20,000, similar to the number of genes of the flatworm Caenorhabditis elegans—does not account for the genomes of the mutualistic microbes that inhabit us and that are estimated to outnumber our own 10 to 1.1,2 Specific and complex microbial communities, termed the microbiota, and their collective genetic material, termed the micro biome, differ greatly between body sites as well as between individuals.3 There is a growing body of evidence demonstrating the enormous effect of the micro biome on host metabolism and susceptibility to disease, enabled by use of laboratory and statistical methods that use high-throughput DNA and RNA sequencing technology, rather than culture-dependent methods, to identify communities of microorganisms.1,4
The bacteria inhabiting the human vagina are thought to be the first line of defense against vaginal infection as a result of both the competitive exclusion5 and direct killing6–8 of other, pathogenic microbes. Disruptions of normal vaginal flora have long been linked to pelvic inflammatory disease,9 miscarriages,10 and prematurity.11 There has been enormous recent growth in the understanding of the vaginal ecosystem, although the interactions among host, the external environment, and bacterial communities are very complex.12 The objective of this review is to describe the current state of the science related to the vaginal microbiome, sexual health, and sexually transmitted diseases (STDs).
References for this review were identified through searches performed between January 2015 and October 2016 of all articles published in English in Google Scholar, EMBASE, and PubMed using search terms such as “vaginal microbiome,” “dysbiosis,” “bacterial vaginosis,” and “microbiome STI.” Pertinent original peer-reviewed articles and reviews were included. The publication dates were not limited to fully review the literature available regarding STDs, behavior, and the vaginal microbiome. Ancestry searches using the references from selected articles were also performed. For the purposes of this review, the term “bacterial vaginosis” or “BV” is used when discussing research into bacterial vaginosis as diagnosed by Amsel’s or Nugent’s criteria. “Vaginal dysbiosis” refers to any state in which the vaginal flora is disrupted, whether or not it is symptomatic or defined as bacterial vaginosis.“Community-state type” or “CST” is used when discussing molecular research that classifies vaginal organisms into these clustered microbiomes.
VAGINAL BACTERIAL COMMUNITIES CLUSTER INTO TYPES BUT ARE DYNAMIC
Vaginal ecology depends on the interactions of the vaginal environment and relatively limited types of flora, particularly Lactobacillus spp. Cultivation-independent methods have shown that vaginal bacterial communities cluster into anywhere from three to nine discrete groups, most of which are dominated by lactobacilli.12–14 A widely used method of classifying sequencing data was described by Ravel et al,15 who used next-generation molecular sequencing techniques to characterize the vaginal microbiota of 396 asymptomatic North American women from four ethnic groups. The authors found that the vaginal communities in these women clustered into five core vaginal microbiomes, which they termed community-state types. Four of these community-state types, found in 73% of the women tested, were dominated by different species of Lactobacillus (Lactobacillus crispatus, CST I; Lactobacillus gasseri, CST II; Lactobacillus iners, CST III; and Lactobacillus jensenii, CST V). The remaining 27% of communities (CST IV) were heterogeneous and typified by a higher proportion of obligate anaerobic bacteria, including Atopobium, Gardnerella, and Prevotella spp. and others.15 Community-state type IV has been further subdivided in some studies into subtypes IV-A and IV-B, both heterogeneous in composition but with CST IV-B containing fewer lactobacilli and more anaerobic bacterial taxa including Gardnerella, Atopobium, Leptotrichia, and Sneathia spp. and other bacterial vaginosis–associated organisms. Many studies have also confirmed the important finding that 20–30% of women at any given time have a Lactobacillus poor, diverse microbiome that has not historically been considered healthy.5,8,15,16
Further investigation into the vaginal microbiome using longitudinal study design has shown that vaginal communities are dynamic and capable of rapid shifts, although in many women, the microbiome is fairly stable.13,17–21 There is evidence that shifts of the vaginal microbiome from one community state to another might be preferential; that is, a given community-state type tends to transition only to certain others.11,16 Emerging evidence appears to show that CST I tends to be most stable and to promote vaginal community stability,8,11,13,16 whereas CST IV appears to frequently transition to multiple other states.11
Significant evidence now indicates that a micro biome dominated by Lactobacillus species other than Liners is optimal for vaginal health.13,22 Recent studies have shown that the presence of vaginal lactobacilli, particularly L crispatus, is strongly correlated with the absence of bacterial vaginosis.11,18,21,23 Lactic acid has been shown to inhibit the growth of pathogenic bacteria in the vagina17,22; additionally, lactobacilli important to vaginal health, elaborate the disinfectant H2O2, antimicrobial molecules, and bacteriocins. These bacteriocins can kill urogenital pathogens in vitro under various conditions,24 and lactic acid may act as an antimicrobial agent beyond maintaining highly acidic pH by disrupting bacterial cell membranes and stimulating host immunity in the presence of bacterial lipopolysaccharide.22 Interestingly, the healthy yet diverse vaginal microbial communities seen in a minority of women are dominated by taxa that also produce lactic acid25;the conservation of lactic acid production across all healthy vaginal communities may indicate that its presence is key to maintaining healthy vaginal function.8
The different isomers of lactic acid may also have unique roles in the human vagina: L-lactic acid, which is produced by both bacteria and vaginal epithelial cells, activates certain immune cells and can induce vaginal epithelial cells to release proinflammatory cytokines.26 The role of D-lactic acid (produced almost exclusively by bacteria) is less well-known; however, the ratio of L- to D-lactic acid may modulate the expression of host signaling molecules and affect the risk of infection-related preterm birth.26
DETERMINANTS OF THE VAGINAL MICROBIOME
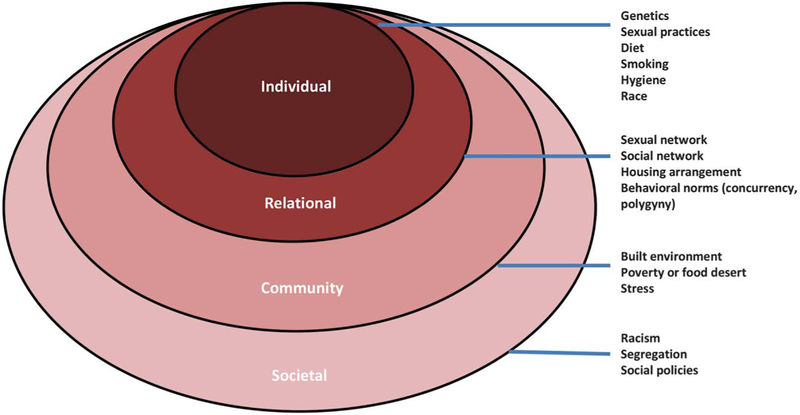
It has been known for more than a century that disrupting vaginal Lactobacillus species can result in bacterial vaginosis, an often symptomatic condition in which vaginal lactobacilli are lost and anaerobic bacteria are concomitantly increased.13 Differences in vaginal microbiota composition, including temporal shifts within a given individual, are almost certainly caused by a complicated interaction among host characteristics, environment, and behavior that is incompletely understood (Fig. 1). However, a number of modifiable and nonmodifiable factors have been shown to affect the vaginal microbiome.
Fig. 1.
Socioecologic framework for determinants of the vaginal micro-biome. Individual and relational determinants associated with differences in the microbiome have been well-studied, and emerging research may show that community-level factors may shape the composition of the microbiome as well. Societal factors that are posited to influence prevalence of sexually transmitted disease (STD) such as segregation, racism, and other societal-level policies may also be determinants of the microbiome. Research that addresses the role of higher level spheres of influence on the microbiome may identify modifiable risk factors that can be addressed. Modified from Scribner R, Theall KP, Simonsen N, Robinson W; National Institute on Alcohol Abuse and Alcoholism. HIV risk and the alcohol environment. Available at: https://pubs.niaaa.nih.gov/publications/arh333/179-183.htm. Retrieved January 4, 2017.
Race–Ethnicity
Bacterial vaginosis prevalence varies by ethnic group in essentially all of the populations studied to date.27 Acquisition of bacterial vaginosis in the United States has long been associated with black race27–30; this association has been shown to persist even after adjustment for sexual practices and other confounders.28–31 Bacterial vaginosis prevalence in the United Kingdom and Canada was higher in Afro-Caribbeans and aboriginal populations, respectively, whereas in Spain and China, Gypsy and Tibetan ethnicity had higher prevalence, respectively.27 Interestingly, all of these groups represent a minority population within the country studied.
More recent studies of the vaginal microbiome of U.S.-born black and white women show a significant difference in microbiota between the two groups, with black women having more microbial diversity and less likelihood of colonization with lactobacilli than white women.15,31 Multiple studies performed in several sub-Saharan African countries have shown a far lower proportion of women with vaginal communities dominated by L crispatus when compared with women of European or Asian ancestry.15,31,32 Rather, the communities of the African women were dominated by Liners and a variable mix of facultative anaerobic bacteria.32–34 Microbiome composition was significantly associated with ethnic origin in a Dutch study with women of African descent having the highest prevalence of clusters dominated by Gardnerella vaginalis or dysbiosis (Borgdorff H. The vaginal microbiome of women residing in Amsterdam: association with ethnicity. World HIV/STI Congress, Brisbane, Australia, 2015). Differences by race or ethnicity persisted after adjustment in some of these studies as well.28,31
There is evidence that host genetic variation, which may at times correlate with race or ethnicity, can affect microbiome composition: one large study using Human Microbiome Project metagenomic data has found multiple associations between key host genes related to immunity and the abundance of specific microbial taxa across four different body sites, although the vagina was not included.35
Sex Hormones and Hormonal Contraception
The effects of sex hormones on the vaginal microbiota are not entirely known; however, estrogen seems to play an important role in promoting the growth of lactobacilli by stimulating the accumulation of glycogen in the vaginal mucosa.36,37 High levels of estrogen are thought to contribute to the increased Lactobacillus spp. predominance and stability of the microbiota that is seen in healthy pregnant women.20 Conversely, postmenopausal women not on hormonal therapy have been found to have significantly lower free glycogen levels and lower levels and diversity of Lactobacillus spp. compared with those who use hormone therapy.38 Menstruation can be associated with significant disruption of the microbiota, although this may depend on community type.16,21
Importantly, certain types of hormonal contraceptives can alter the vaginal microbiota. There is a consistent association between oral contraceptive use and a decrease in prevalent bacterial vaginosis.29,39,40 Some studies have shown a decrease in prevalent bacterial vaginosis in women using depot medroxyprogesterone acetate injection or implant40;however, depot medroxyprogesterone acetate has also been found to decrease vaginal lactobacilli39,41 and is associated in some studies with an increased risk of human immunodeficiency virus (HIV) acquisition and transmission, possibly in part mediated by the effects of the microbiota on cervicovaginal inflammation.42 However, a recent systematic review and meta-analysis demonstrated a robust negative association between any hormonal contraception regardless of type (excluding intrauterine devices) and prevalent, incident, or recurrent bacterial vaginosis.43
Sexual Behavior
There has been debate about whether bacterial vaginosis can be classified as an STD as opposed to a sexually associated condition; however, the preponderance of evidence demonstrates that bacterial vaginosis can be sexually transmitted from women to male and female partners.44,45 Epidemiologic studies have consistently associated bacterial vaginosis with risk factors associated with STD.44,46 More frequent vaginal intercourse is associated with increased risk of bacterial vaginosis.30 Multiple, new, or increased number of male partners are strongly associated with bacterial vaginosis in multiple studies.34,44 Recent unprotected sex as evidenced by the presence of prostate-specific antigen in vaginal fluid has been associated with a more than twofold increased risk of bacterial vaginosis47 and recurrent bacterial vaginosis48 and is negatively associated with the presence and concentration of healthy Lactobacillus species.34 Additionally, there is a significant association between bacterial vaginosis and female sex partners,29 because women who have sex with women appear to be at increased risk when compared with women who have sex with men only.29,46 There is a strong inverse association between bacterial vaginosis and condom use.44 In one study, bacterial vaginosis–associated bacteria were detected more commonly in the urine and coronal sulcus of men with asymptomatic STDs than in healthy men.49 Finally, both bacterial vaginosis by Nugent scoring50 and detection of bacterial vaginosis–associated anaerobes29 are far less frequent in sexually inexperienced than in sexually experienced women.
Data on the influence of specific sexual practices on bacterial vaginosis are relatively few: in one study, vaginal intercourse immediately after receptive anal intercourse was associated with bacterial vaginosis30;other studies demonstrated an association between receptive oral sex and bacterial vaginosis.46 The increased detection and gene copies of G vaginalis in the oral cavity among women who have sex with women with bacterial vaginosis adds some biological plausibility to this association51; however, several other studies have not demonstrated an association with receptive oral sex.46 One study has found an association between bacterial vaginosis and receptive oral or anal sex, whereas several others did not46;receptive digital sex (either vaginal or anal) does not seem to be associated with bacterial vaginosis.46 One well-designed longitudinal study found that recurrent bacterial vaginosis was nearly twice as likely in women who had the same sex partner before and after treatment, regardless of coital frequency.52
Male circumcision may play a role in male-to-female transmission of bacterial vaginosis as it does in other STDs.44 Circumcision has been shown to significantly decrease the load of anaerobic bacteria (including bacterial vaginosis–associated species) on the coronal sulcus,53 and other studies have correlated circumcision with a decrease in bacterial vaginosis among female partners.30,54 A recent study showed that uncircumcised men with a higher prevalence of bacterial vaginosis–associated anaerobes in their penile microbiota were significantly more likely to have a partner with a high Nugent score; moreover, this type of microbiota was significantly associated with having two or more extramarital partners.55
Intravaginal Practices
Vaginal douching has long been associated with the acquisition of bacterial vaginosis and longitudinal data suggest that those who douche are at increased risk of incident bacterial vaginosis.56 The effects of other intravaginal practices are not well-studied, although some have been shown to kill vaginal bacteria and may be more associated with bacterial vaginosis than others.57,58
Studies of the effect of intravaginal practices are likely to be confounded by ethnicity and may be of limited statistical power as a result of heterogeneity of the practices.31,32,34 Because use of intravaginal products and practices is widespread in many cultures, more investigation is needed.
Smoking
Cigarette smoking has been strongly associated with increased prevalence of bacterial vaginosis in multiple epidemiologic studies, sometimes in a dose-dependent manner.30,59 Several compounds from cigarette smoking are detectable in the cervical mucus of smokers, one of which has been associated with the induction of bacteriophages in lactobacilli.59 Recent data using sequence analysis have shown a correlation between smoking and dysbiosis even after adjusting for other factors.31 A 2014 study found that the vaginal microbiota of smokers was significantly more likely to be in a low-lactobacillus state and that there was a significant trend in increasing amounts of smoking metabolites with a high Nugent score.59
Diet
Research into the gut microbiome has consistently demonstrated the striking effect of diet on bacterial community composition and function, which seems to have a profound influence on human health, including obesity and metabolic disorders, inflammatory bowel disease, and cancer.60 The proinflammatory effects of disrupted gut microbiota on distal body systems is increasingly recognized60; moreover, the gut may serve as an extravaginal reservoir for both lactobacilli and bacterial vaginosis–associated bacteria.51 Subclinical deficiencies of iron and vitamin D in pregnancy have been associated with increased risk of bacterial vaginosis,61,62 although a large longitudinal study found no association between vitamin D and bacterial vaginosis using the proxy variable of season.63 Other analyses performed on subsets of women from this study demonstrated an association among increased dietary fat, higher glycemic load, and lower nutritional density64,65 with bacterial vaginosis and an inverse association between bacterial vaginosis and an increased intake of folate, vitamin E, and calcium.64 Additionally, glycemic load was significantly associated with bacterial vaginosis progression and persistence.65 Bacterial vaginosis has also been epidemiologically associated with obesity.29
Network-Level Risk Factors: Built Environment, Poverty, and Likelihood of Partnerships Based on Ethnicity
Limited research suggests that social determinants often associated with STD such as sexual and social milieu may be associated with the composition of the microbiome. Families, particularly sexual partners in a household, have been demonstrated to have shared microbiota in the fecal and oral compartments.66 Animal studies have demonstrated that social group membership and frequent physical contact and social interaction among individuals correlate with shared gut microbiome.67 Emerging research shows that the influence of the built environment has an effect on the composition of human microbiota,68 as might the influence of stress.69,70 A combination of unhealthy neighborhood, diet, social conditions, stress, and other factors such as is seen in poverty may contribute to a less healthy vaginal microbiome in multiple ways; the association of neighborhood with STD71 and low birth weight72 is intriguing when viewed in this light.
Population-level parameters affect the prevalence of STD,73 and the structure of social and sexual networks may be important in explaining the difference in prevalence of bacterial vaginosis among different ethnic groups.27,74 Although research is limited, there is a strong ecologic-level association between the prevalence of concurrency among men and bacterial vaginosis prevalence.74 Bacterial vaginosis prevalence is higher in minority populations of different ethnicities in multiple different countries; additionally, the populations with more bacterial vaginosis also had markers of higher risk sexual behaviors than those of majority ethnicity.27,75 Another study performed with historical data from Uganda, the United States, and Thailand demonstrated that HIV prevalence differentials aligned perfectly with differences in prevalence, duration, and coital exposure of concurrent partnerships among males.76
CONSEQUENCES OF DYSBIOSIS
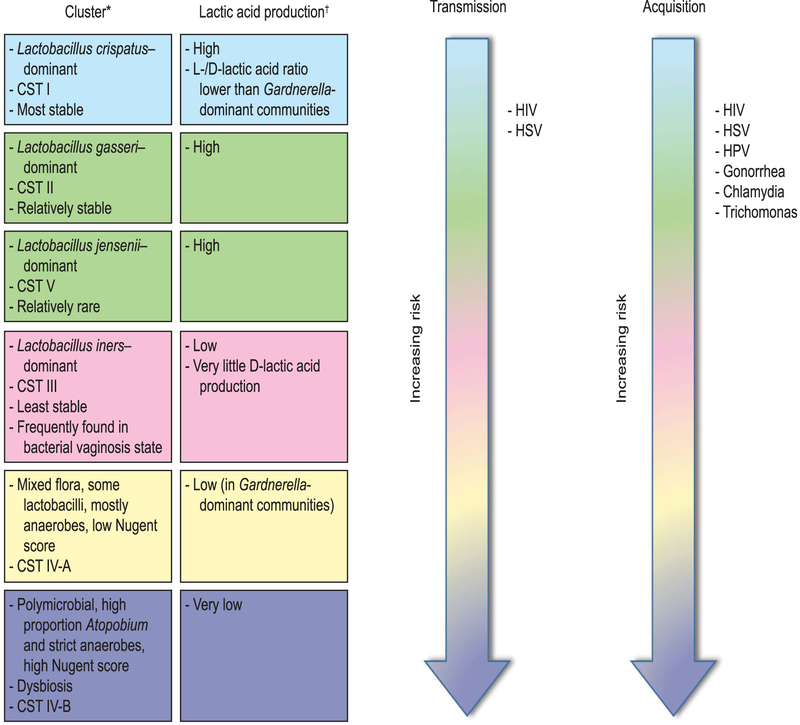
An unhealthy vaginal microbiome, in addition to its significant psychosocial effect on symptomatic women,77 is an important risk factor for acquisition of STDs and adverse reproductive and obstetric sequelae.78 Increasingly diverse vaginal microbiota seem to demonstrate increasingly less resilience to disturbance and more susceptibility to disease8,11,33(Fig. 2).
Fig. 2.
Vaginal communities and risk of sexually transmitted diseases (STDs). Risk of STD acquisition and transmission increases with increasing diversity of vaginal flora and is lowest with Lactobacillus crispatus–dominant communities. Higher levels of lactic acid have been strongly associated with vaginal health, and production of lactic acid is conserved across healthy vaginal communities. L- and D-lactic acid isomers may have different functions within the vaginal microenvironment, and their ratio may influence expression of host genes and immune response. CST, community-state types; HIV, human immunodeficiency virus; HSV, herpes simplex virus; HPV, human papillomavirus. *Data from references 10 and 19. †Data from reference 10 and the following: Witkin SS, Mendes-Soares H, Linhares IM, Jayaram A, Ledger WJ, Forney LJ. Influence of vaginal bacteria and D- and L-lactic acid isomers on vaginal extracellular matrix metalloproteinase inducer: implications for protection against upper genital tract infections. MBio 2013 Aug 6;4. pii: e00460–13. DOI: 10.1128/mBio.00460-13.
Bacterial Vaginosis and Herpes Simplex Virus
Bacterial vaginosis and herpes simplex virus (HSV) have been epidemiologically linked in multiple cross sectional and prospective studies. On a population level, Nugent scores of 4 or higher were significantly associated with a 32% increase in concurrent HSV-2 and an 8% increase in HSV-1.79 In a meta-analysis of 16 cross-sectional studies, the authors found that the pooled odds of prevalent bacterial vaginosis were 60% greater among HSV-2-positive women when compared with HSV-2-negative women.80 Cherpes et al81 followed 670 women for 1 year and found that a diagnosis of bacterial vaginosis was associated with a twofold risk of HSV-2 seroconversion. This association may be bidirectional: HSV-2 infection was associated with an increased risk of bacterial vaginosis episodes in female sex workers in Burkina Faso82 and this meta-analysis also demonstrated a relative risk of 1.55 for incident bacterial vaginosis in HSV-2-infected women.80 A recent study found that antibiotic-induced vaginal dysbiosis in mice resulted in severe impairment of antiviral protection against HSV-2 infection.83
Bacterial Vaginosis and Human Papillomavirus
The literature exploring the relationship between bacterial vaginosis and human papillomavirus (HPV) is consistent. Longitudinal studies have shown an increased association of prevalent and incident HPV in women with both intermediate flora and bacterial vagionsis,84 a small but significant increase in risk for prevalent HPV, an increase in odds of incident HPV, and delayed clearance of HPV in women with Nugent scores 7 or greater.85 Two more recent molecular analyses found that women who were HPV-positive had a lower proportion of protective vaginal Lactobacillus spp. when compared with HPV-negative women86,87; furthermore, women with microbiota dominated by L gasseri seemed to have increased rates of HPV clearance.86 Other studies have demonstrated that severity of cervical intraepithelial dysplasia was significantly associated with increasing vaginal microbial diversity, regardless of HPV status (demonstrated that community-state type was significantly associated with prevalent HPV and CST IV-B was associated with HPV positivity [although not at a significant level], that severity of cervical intraepithelial dysplasia was significantly associated with increasing vaginal microbial diversity, regardless of HPV status,88 and that severity of cervical intraepithelial dysplasia was significantly associated with increasing vaginal microbial diversity, regardless of HPV status.88
Bacterial Vaginosis and Human Immunodeficiency Virus
There is considerable evidence associating vaginal dysbiosis with increased risk of acquisition and transmission of HIV-1. A meta-analysis of 23 studies showed that bacterial vaginosis was associated with a 60% increase in risk of acquiring HIV-1; this included four longitudinal studies that examined incident HIV-1 infection.89 A vaginal mucosal model demonstrated that lactobacilli, particularly L crispatus, suppressed HIV-1 replication.90 Cervicovaginal mucus with high levels of D-lactic acid and an L crispatus–dominated microbiome effectively trapped HIV-1 significantly better than did mucus dominated by other microbes,91 and lactic acid at concentrations found in the vagina can inactivate HIV far more potently in vitro than can other acids.92 Importantly, a recent study among Rwandan sex workers showed that those with L crispatus–dominant microbiota had the lowest prevalence of both HIV and sexually transmitted infections, and that dysbiosis increased the risk of acquiring HIV and STD in a dose–response relationship; moreover, significantly fewer of the HIV-positive women with Lactobacillus spp.–dominant microbiota had detectable cervicovaginal levels of HIV-1.33
Bacterial Vaginosis and Bacterial Sexually Transmitted Disease
Epidemiologic studies have associated bacterial vaginosis with increased risk of both gonorrhea and chlamydia infection.78 Vaginal lactobacilli in vitro inhibit growth of Neisseria gonorrhoeae93,94 as well as other bacterial pathogens.95 One cross-sectional study found that Nugent scores higher than 3 were associated with a fourfold increase in risk for gonorrhea and a threefold increase in risk for chlamydia infection.96 Well-designed longitudinal studies have also demonstrated the association with the largest study showing an increased risk for incident chlamydia and gonorrhea in women with Nugent scores higher than 3.97 A randomized trial showed that the treatment of asymptomatic bacterial vaginosis with intravaginal metronidazole was significantly associated with a more than a threefold decrease in incident chlamydia98; however, more recent data from a prospective randomized trial showed that home screening and treatment for bacterial vaginosis did not decrease incidence of either chlamydia or gonorrhea.99
Bacterial Vaginosis and Trichomonas
Trichomonas vaginalis infection has been strongly associated with bacterial vaginosis.97 In the 2001–2004 National Health and Examination Survey, cooccurrence occurred in approximately half of women infected with T vaginalis.100 T vaginalis alters vaginal pH, has been associated with lower levels of healthy vaginal lactobacilli, and has been positively associated with increased Nugent score.101 In vitro evidence indicates thatT vaginalis presence reduces epithelial-associated lactobacilli but not bacterial vaginosis–associated species.102 Recent longitudinal analyses have demonstrated that a Nugent score higher than 3 was associated with a significantly increased risk of acquiring T vaginalis.103 Studies of T vaginalis and the microbiome using sequencing techniques are few; however, one study found that CST-IV was significantly associated with T vaginalis detection.104 Furthermore, T vaginalis and bacterial vaginosis are independently associated with increased vaginal shedding of HIV-1, and their cooccurrence has been associated with greatly increased odds of vaginal shedding.105
Bacterial Vaginosis and Pelvic Inflammatory Disease
There is some question whether bacterial vaginosis can cause pelvic inflammatory disease (PID) or whether the epidemiologic association between them is the result of the increased attributable risk of bacterial vaginosis to STD acquisition.9 Although it is typically associated with gonorrhea and chlamydia infection, PID has been shown to frequently occur in the absence of known STD and can be of multimicrobial etiology.106–108 The anaerobic organisms found in many cases of acute salpingitis and endometritis are often bacterial vaginosis–associated organisms.108 One large longitudinal cohort study found that vaginal carriage of bacterial vaginosis–associated organisms was associated with a twofold increase in incident PID risk.109 Another study did not demonstrate an association between incident PID and bacterial vaginosis carriage in the prior 6 months; however, dense growth of anaerobic, pigmented Gram-negative rods was significantly associated with PID.9 Detection of similar organisms was associated in another study with a more than fourfold increase in PID risk; other anaerobes or Nugent scores of 7–10 were also significantly associated with PID.108 A small molecular study of patients with and without found DNA of bacterial vaginosis–associated bacteria and un-characterized species in most viable case samples but none in control patients; moreover, only one case sample was positive for bacterial STD.
Bacterial colonization of the upper cervix and uterus may be physiological110; however, one study demonstrated that at least one bacterial species was found 95% of the time in the upper cervix and uterus of women without endometritis undergoing hysterectomy for benign conditions, and bacterial composition varied significantly by race.111 Whether this reflects vaginal contamination or true upper tract commensal organisms is not yet known.111
Bacterial Vaginosis and Preterm Birth
Bacterial vaginosis has long been associated with adverse birth outcomes, although the mechanism by which dysbiosis affects pregnancy remains unclear112 and certain organisms may affect pregnancy outcomes differently at different gestational ages.10 Molecular studies have consistently shown pregnancy to be associated with decreased microbial diversity, Lactobacillus spp. dominance, and more stability of vaginal communities.11,20,113 Preterm labor has been associated with diverse vaginal communities in other studies11,114; moreover, no women with term deliveries had CST IV-B in one longitudinal study.20 In a large cohort of pregnant women with intermediate vaginal flora, the absence of lactobacilli was significantly associated with preterm delivery.115
GAPS AND RESEARCH PRIORITIES
Vaginal microbial communities are instrumental in vaginal health. Progress in this field is extremely rapid; however, important research gaps remain. One of the more important may be the influence of network and community-level risk factors on the vaginal microbiome. Given the centrality of the structure of sexual networks to transmission and prevalence of STDs,116 it is likely these factors are equally important to vaginal microbiota composition and transmission and prevalence of bacterial vaginosis. In particular, more detailed longitudinal studies on the effect of overlap and duration of concurrent partnerships on vaginal microbiota would be important. Given different patterns of formation and maintenance of sexual partnerships in different populations and cultures,27,117 such studies might go a long way in explaining the racial differences consistently seen in the vaginal microbiota. Additionally, further investigation into the effect of the order of sexual acts and coital frequency on the composition of the microbiota could provide practical risk reduction advice for women.
More effective treatments for bacterial vaginosis are necessary, because current cure rates range from 50–80% after metronidazole treatment and recurrence is very common.118 The role of biofilm disruption119 and probiotic administration120 in achieving a better cure and preventing recurrent infection should be further explored, although the efficacy of different combinations or strains of probiotic species on restoring the vaginal flora is an area of active research.22 Treating the sex partners of women with recurrent bacterial vaginosis has not decreased recurrence in several randomized controlled trials; however, this may be the result of study design limitations and ineffective treatment.55 More research into the efficacy of treating sex partners is needed.22
Given that cesarean deliveries have been shown to significantly affect the composition of the gut microbiome,121 investigation on the effect of mode of birth on establishment and maintenance of a healthy vaginal microbiome may be important. If female neonates delivered by cesarean are at risk for unhealthy sexual or reproductive outcomes as a result of unhealthy or inadequate colonization of the neonatal vagina, strategies could be devised to replenish necessary vaginal flora to improve health outcomes. This has recently been explored in a study of the neonatal gut microbiome of neonates delivered by cesarean.122
Finally, although the microbes that inhabit the vagina have been fairly well-characterized, it is important to better understand their metabolic interactions. Several studies have begun to elucidate the functionality of the microbiome123; further assessment of protein transcription of both microbes and host will help address gaps in knowledge about the pathogenesis of dysbiosis, microbial, and host interactions that lead to adverse clinical outcomes and the evaluation of interventions that aim to maintain or restore a healthy vaginal milieu.
Footnotes
The conclusions, findings, and opinions expressed by authors do not necessarily reflect the official position of the U.S. Department of Health and Human Services or the Centers for Disease Control and Prevention.
Continuing medical education for this article is available at http://links.lww.com/AOG/A933.
Each author has indicated that he or she has met the journal’s requirements for authorship.
Financial Disclosure
The authors did not report any potential conflicts of interest.
REFERENCES
- 1.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett C, Knight R, Gordon JI. The human microbiome project: exploring the microbial part of ourselves in a changing world. Nature 2007; 449:804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NIH Human Microbiome Project. Bethesda (MD): National Institutes of Health; 2015. [Google Scholar]
- 3.Human Microbiome Project Consortium. Structure, function and diversity of the health human microbiome. Nature 2012; 486:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Relman DA. The human microbiome and the future of medicine. JAMA 2015;314:1127–8. [DOI] [PubMed] [Google Scholar]
- 5.Bing M, Forney LJ, Ravel J. Vaginal microbiome: rethinking health and diseases. Annu Rev Microbiol 2012;66:371–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaewsrichan J, Peeyananjarassri K, Kongpraserkit J. Selection and identification of anaerobic lactobacilli producing inhibitory compounds against vaginal pathogens. FEMS Immunol Med Microbiol 2006;48:75–83. [DOI] [PubMed] [Google Scholar]
- 7.Ghartey JP, Smith BC, Chen Z, Buckley N, Lo Y, Ratner AJ, et al. Lactobacillus crispatus dominant vaginal microbiome is associated with inhibitory activity of female genital tract secretions against Escherichia coli. PLoS One 2014;9:e96659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aldunate M, Srbinovski D, Hearps AC, Latham CF, Ramsland PA, Gugasyan R, et al. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front Physiol 2015;6:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ness RB, Hillier SL, Kip KE, Soper DE, Stamm CA, McGregor JA, et al. Bacterial vaginosis and risk of pelvic inflammatory disease. Obstet Gynecol 2004;104:761–9. [DOI] [PubMed] [Google Scholar]
- 10.Nelson DB, Hanlon AL, Wu G, Liu C, Fredricks DN. First trimester levels of BV-associated bacteria and risk of miscarriage among women early in pregnancy. Matern Child Health J 2015;19:2682–7. [DOI] [PubMed] [Google Scholar]
- 11.DiGuilio DB, Callahan BJ, McMurdie PJ, Costello EK, Lyell DJ, Robaczewska A, et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci U S A 2015;112:11060–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou X, Brown CJ, Abdo Z, Davis CC, Hansmann MA, Joyce P, et al. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J 2007;1:121–33. [DOI] [PubMed] [Google Scholar]
- 13.van de Wijgert JH, Borgdorff H, Verhelst R, Crucitti T, Francis S, Verstraelen H, et al. The vaginal microbiota: what have we learned after a decade of molecular characterization? PLoS One 2014:9:e105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med 2005;353:1899–911. [DOI] [PubMed] [Google Scholar]
- 15.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 2011;108(suppl 1):4680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gajer P, Brotman RM, Bai G, Sakamoto J, Schütte UM, Zhong X, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med 2012;4:132ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hickey RJ, Zhou X, Pierson JD, Ravel J, Forney LJ. Understanding vaginal microbiome complexity from an ecological perspective. Transl Res 2012;160:267–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jespers V, Menten J, Smet H, Poradosú S, Abdellati S, Verhelst R, et al. Quantification of bacterial species of the vaginal microbiome in different groups of women, using nucleic acid amplification tests. BMC Microbiol 2012;12:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravel J, Brotman RM, Gajer P, Ma B, Nandy M, Fadrosh DW, et al. Daily temporal dynamics of vaginal microbiota before, during and after episodes of bacterial vaginosis. Microbiome 2013;1:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Nikita L, et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2014;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srinivasan S, Liu C, Mitchell CM, Fiedler TL, Thomas KK, Agnew KJ, et al. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS One 2010;5:e10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradshaw CS, Brotman RM. Making inroads into improving treatment of bacterial vaginosis—striving for long-term cure. BMC Infect Dis 2015;15:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srinivasan S, Hoffman NG, Morgan MT, Matsen FA, Fiedler TL, Hall RW, et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One 2012;7:e37818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruiz FO, Pascual L, Giordano W, Barberis L. Bacteriocins and other bioactive substances of probiotic lactobacilli as biological weapons against Neisseria gonorrhoeae. Pathog Dis 2015. February 11 [epub]. [DOI] [PubMed] [Google Scholar]
- 25.Petrova MI, van den Broek M, Balzarini J, Vanderleyden J, Lebeer S. Vaginal microbiota and its role in HIV transmission and infection. FEMS Microbiol Rev 2013;37:762–92. [DOI] [PubMed] [Google Scholar]
- 26.Beghini J, Linhares IM, Giraldo PC, Ledger WJ, Witkin SS. Differential expression of lactic acid isomers, extracellular matrix metalloproteinase inducer, and matrix metalloproteinase-8 in vaginal fluid from women with vaginal disorders. BJOG 2015; 122:1580–5. [DOI] [PubMed] [Google Scholar]
- 27.Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol 2013;209:505–23. [DOI] [PubMed] [Google Scholar]
- 28.Peipart JF, Lapane KL, Allsworth JE, Redding CA, Blume JD, Stein MD. Bacterial vaginosis, race, and sexually transmitted infections: does race modify the association? Sex Transm Dis 2008;35:363–7. [DOI] [PubMed] [Google Scholar]
- 29.Koumans EH, Sternberg M, Bruce C, McQuillan G, Kendrick J, Sutton M, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis 2007;34: 864–9. [DOI] [PubMed] [Google Scholar]
- 30.Cherpes TL, Hillier SL, Meyn LA, Busch JL, Krohn MA. A delicate balance: risk factors for acquisition of bacterial vaginosis include sexual activity, absence of hydrogen peroxide-producing lactobacilli, black race, and positive herpes simplex virus type 2 serology. Sex Transm Dis 2008;35:78–83. [DOI] [PubMed] [Google Scholar]
- 31.Fettweis JM, Brooks JP, Serrano MG, Sheth NU, Girerd PH, Edwards DJ, et al. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology 2014;160:2272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gautam R, Borgdorff H, Jespers V, Francis SC, Verhelst R, Mwaura M, et al. Correlates of the molecular vaginal micro-biota composition of African women. BMC Infect Dis 2015; 15:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borgdorff H, Tsivtsivadze E, Verhelst R, Marzorati M, Jurriaans S, Ndayisaba GF, et al. Lactobacillus-dominated cervicovaginal microbiota associated with reduced HIV/STI prevalence and genital HIV viral load in African women. ISME J 2014;8:1781–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jespers C, van de Wijgert J, Cools P, Verhelst R, Verstraelen H, Delany-Moretlwe S, et al. The significance of Lactobacillus crispatus and L. vaginalis for vaginal health and the negative effect of recent sex: a cross-sectional descriptive study across groups of African women. BMC Infect Dis 2015;15:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blekhman R, Goodrich JK, Huang K, Sun Q, Bukowski R, Bell JT, et al. Host genetic variation impacts microbiome composition across human body sites. Genome Biol 2015;16:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brotman RM, Ravel J, Bavoil PM, Gravitt PE, Ghanem KG. Microbiome, sex hormones, and immune responses in the reproductive tract: challenges for vaccine development against sexually transmitted infections. Vaccine 2014;32:1543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mirmonsef P, Hotton AL, Gilbert D, Burgad D, Landay A, Weber KM, et al. Free glycogen in vaginal fluids is associated with lactobacillus colonization and low vaginal pH. PLoS One 2014;9:e102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muhleisen AL, Herbst-Kralovetz MM. Menopause and the vaginal microbiome. Maturitas 2016;91:42–50. [DOI] [PubMed] [Google Scholar]
- 39.Achilles SL, Hillier SL. The complexity of contraceptives: understanding their impact on genital immune cells and vaginal microbiota. AIDS 2013;27(suppl 1):S5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riggs M, Klebanoff M, Nansel T, Zhang J, Schwebke J, Andrews W. Longitudinal association between hormonal contraceptives and bacterial vaginosis in women of reproductive age. Sex Transm Dis 2007;34:954–9. [PubMed] [Google Scholar]
- 41.Mitchell CM, McLemore L, Westerberg K, Astronomo R, Smythe K, Gardella C, et al. Long-term effect of depot medroxyprogesterone acetate on vaginal microbiota, epithelial thickness and HIV target cells. J Infect Dis 2014;210:651–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fichorova RN, Chen PL, Morrison CS, Doncel GF, Mendonca K, Kwok C, et al. The contribution of cervicovaginal infections to the immunomodulatory effects of hormonal contraception. MBio 2015;6:e00221–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vodstrcil LA, Hocking JS, Law M, Walker S, Tabrizi SN, Fairley CK, et al. Hormonal contraception is associated with a reduced risk of bacterial vaginosis: a systematic review and meta-analysis. PLoS One 2013;8:e73055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fethers KA, Fairley CK, Hocking JS, Gurrin LC, Bradshaw CS. Sexual risk factors and bacterial vaginosis: a systematic review and meta-analysis. Clin Infect Dis 2008;47:1426–35. [DOI] [PubMed] [Google Scholar]
- 45.Vodstrcil LA, Walker SM, Hocking JS, Law M, Forcey DS, Fehler G, et al. Incident bacterial vaginosis (BV) in women who have sex with women is associated with behaviors that suggest sexual transmission of BV. Clin Infect Dis 2015;60:1042–53. [DOI] [PubMed] [Google Scholar]
- 46.Forcey DS, Vodstrcil LA, Hocking JS, Fairley CK, Law M, McNair RP, et al. Factors associated with bacterial vaginosis among women who have sex with women: a systematic review. PLoS One 2015;10:e0141905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jespers V, Crucitti T, Menten J, Verhelst R, Mwaura M, Mandaliya K, et al. Prevalence and correlates of bacterial vaginosis in different sub-populations of women in sub-Saharan Africa: a cross-sectional study. PLoS One 2014;9:e109670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Norris Turner A, Carr Reese P, Snead MC, Fields K, Ervin M, Kourtis AP, et al. Recent biomarker-confirmed unprotected vaginal sex, but not self-reported unprotected sex, is associated with recurrent bacterial vaginosis. Sex Transm Dis 2016;43:172–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nelson DE, Van Der Pol B, Dong Q, Revanna KV, Fan B, Easwaran S, et al. Characteristic male urine microbiomes associate with asymptomatic sexually transmitted infection. PLoS One 2010;5:e14116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fethers K, Twin J, Fairley CK, Fowkes FJ, Garland SM, Fehler G, et al. Bacterial vaginosis (BV) candidate bacteria: associations with BV and behavioural practices in sexually-experienced and inexperienced women. PLoS One 2012;7:e30633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marazzo JM, Fiedler TL, Srinivasan S, Thomas KK, Liu C, Ko D, et al. Extravaginal reservoirs of vaginal bacteria as risk factors for incident bacterial vaginosis. J Infect Dis 2012;205:1580–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bradshaw CS, Vodstrcil LA, Hocking JS, Law M, Pirotta M, Garland SM, et al. Recurrence of bacterial vaginosis is significantly associated wit posttreatment sexual activities and hormonal contraceptive use. Clin Infect Dis 2013;56:777–86. [DOI] [PubMed] [Google Scholar]
- 53.Liu CM, Hungate BA, Tobian AA, Serwadda D, Ravel J, Lester R, et al. Male circumcision significantly reduces prevalence and load of genital anaerobic bacteria. MBio 2013;4: e00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gray RH, Kigozi G, Serwadda D, Makumbi F, Nalugoda F, Watya S, et al. The effects of male circumcision on female partners’ genital tract symptoms and vaginal infections in a randomized trial in Rakai, Uganda. Am J Obstet Gynecol 2009; 200:42.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu CM, Hungate BA, Tobian AA, Ravel J, Prodger JL, Serwadda D, et al. Penile microbiota and female partner bacterial vaginosis in Rakai, Uganda. MBio 2014;6:e00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brotman RM, Klebanoff MA, Nansel TR, Andrews WW, Schwebke JR, Zhang J, et al. A longitudinal study of vaginal douching and bacterial vaginosis—a marginal structural modeling analysis. Am J Epidemiol 2008;168:188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Low N, Chersich MF, Schmidlin K, Egger M, Francis SC, van de Wijgert JH, et al. Intravaginal practices, bacterial vaginosis, and HIV infection in women: individual participant data meta-analysis. PLoS Med 2011;8:e1000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fashemi B, Delaney ML, Onderdonk AB, Fichorova RN. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis 2013. February 25 [eCollection]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brotman RM, He X, Gajer P, Fadrosh D, Sharma E, Mongodin EF, et al. Association between cigarette smoking and the vaginal microbiota: a pilot study. BMC Infect Dis 2014;14:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, et al. The gut microbiota and host health: a new clinical frontier. Gut 2016;65:330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verstraelen H, Delanghe J, Roelens K, Blot S, Claeys G, Temmerman M. Subclinical iron deficiency is a strong predictor of bacterial vaginosis in early pregnancy. BMC Infect Dis 2005;5:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bodnar LM, Krohn M, Simhan H. Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. J Nutr 2009;139:1157–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klebanoff MA, Turner AN. Bacterial vaginosis and season, a proxy for vitamin D status. Sex Transm Dis 2014;41:295–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Neggers YH, Nansel TR, Andrews WW, Schwebke JR, Yu KF, Goldenberg RL, et al. Dietary intake of selected nutrients affects bacterial vaginosis in women. J Nutr 2007;137:2128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thoma ME, Klebanoff MA, Rovner AJ, Nansel TR, Neggers Y, Andrews WW, et al. Bacterial vaginosis is associated with variation in dietary indices. J Nutr 2011;141:1698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Song SJ, Lauber C, Costello EK, Lozupone CA, Humphrey G, Berg-Lyons D, et al. Cohabiting family members share microbiota with one another and with their dogs. eLife 2013; 2:e00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moeller AH, Foerster S, Wilson ML, Pusey AE, Hahn BH, Ochman H. Social behavior shapes the chimpanzee pan-microbiome. Sci Adv 2016;2:e1500997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kembel SW, Jones E, Kline J, Northcutt D, Stenson J, Womack AM, et al. Architectural design influences the diversity and structure of the built environment microbiome. ISME J 2012;6:1469–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nansel TR, Riggs MA, Yu KF, Andrews WW, Schwebke JR, Klebanoff MA. The association of psychosocial stress and bacterial vaginosis in a longitudinal cohort. Am J Obstet Gynecol 2006;194:381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jašarević E, Howerton CL, Howard CD, Bale TL. Alterations in the vaginal microbiome by maternal stress are associated with metabolic reprogramming of the offspring gut and brain. Endocrinology 2015;156:3265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holtgrave DR, Crosby RA. Social capital, poverty, and income inequality as predictors of gonorrhoea, syphilis, chlamydia and AIDS case rates in the United States. Sex Transm Infect 2003;79:62–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pearl M, Braveman P, Abrams B. The relationship of neighborhood socioeconomic characteristics to birthweight among 5 ethnic groups in California. Am J Public Health 2001;91:1808–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aral SO, Lipshutz J, Blanchard J. Drivers of STD/HIV epidemiology and the timing and targets of STD/HIV prevention. Sex Transm Infect 2007;83(suppl 1):i1–4. [DOI] [PubMed] [Google Scholar]
- 74.Kenyon CR, Colebunders R. Strong association between the prevalence of bacterial vaginosis and male point-concurrency. Eur J Obstet Gynecol Reprod Biol 2013;172:93–6. [DOI] [PubMed] [Google Scholar]
- 75.Kenyon C, Osbak K. Sexual networks, HIV, race and bacterial vaginosis. AIDS 2015;29:641–2. [DOI] [PubMed] [Google Scholar]
- 76.Morris M, Epstein H, Wawer M. Timing is everything: international variations in historical sexual partnership concurrency and HIV prevalence. PLoS One 2010;5:e14092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bilardi JE, Walker S, Temple-Smith M, McNair R, Mooney-Somers J, Bellhouse C, et al. The burden of bacterial vaginosis: women’s experience of the physical, emotional, sexual and social impact of living with recurrent bacterial vaginosis. PLoS One 2013;8:e74378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brotman RM. Vaginal microbiome and sexually transmitted infections: an epidemiologic perspective. J Clin Invest 2011; 121:4610–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Allsworth JE, Lewis VA, Peipert JF. Viral sexually transmitted infections and bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data. Sex Transm Dis 2008;35:791–6. [DOI] [PubMed] [Google Scholar]
- 80.Esber A, Vicetti Miguel RD, Cherpes TL, Klebanoff MA, Gallo MF, Turner AN. Risk of bacterial vaginosis among women with herpes simplex virus type 2 infection: a systematic review and meta-analysis. J Infect Dis 2015;212:8–17. [DOI] [PubMed] [Google Scholar]
- 81.Cherpes TL, Meyn LA, Krohn MA, Lurie JG, Hillier SL. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis 2003;37: 319–25. [DOI] [PubMed] [Google Scholar]
- 82.Nagot N, Ouedraogo A, Defer MC, Vallo R, Mayaud P, Van de Perre P. Association between bacterial vaginosis and herpes simplex virus type-2 infection: implications for HIV acquisition studies. Sex Transm Infect 2007;83:365–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oh JE, Kim BC, Chang DH, Kwon M, Lee SY, Kang D, et al. Dysbiosis-induced IL-33 contributes to impaired antiviral immunity in the genital mucosa. Proc Natl Acad Sci U S A 2016;116:E762–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Watts DH, Fazzari M, Minkoff H, Hillier SL, Sha B, Glesby M, et al. Effects of bacterial vaginosis and other genital infections on the natural history of human papillomavirus infection in HIV-1-infected and high-risk HIV-1-uninfected women. J Infect Dis 2005;191:1129–39. [DOI] [PubMed] [Google Scholar]
- 85.King CC, Jamieson DJ, Wiener J, Cu-Uvin S, Klein RS, Rom-palo AM, et al. Bacterial vaginosis and the natural history of human papillomavirus. Infect Dis Obstet Gynecol 2011;2011: 319460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brotman RM, Shardell MD, Gajer P, Tracy JK, Zenilman JM, Ravel J, et al. Interplay between the temporal dynamics of the vaginal microbiota and human papillomavirus detection. J Infect Dis 2014;210:1723–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee JE, Lee S, Lee H, Song YM, Lee K, Han MJ, et al. Association of the vaginal microbiota with human papillomavirus infection in a Korean twin cohort. PLoS One 2013;8:e63514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mitra A, MacIntyre DA, Lee YS, Smith A, Marchesi JR, Lehne B, et al. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci Rep 2015;5:16865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS 2008;22:1493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pyles RB, Vincent KL, Baum MM, Elsom B, Miller AL, Maxwell C, et al. Cultivated vaginal microbiomes alter HIV-1 infection and antiretroviral efficacy in colonized epithelial multilayer cultures. PLoS One 2014;9:e93419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nunn KL, Wang YY, Harit D, Humphrys MS, Ma B, Cone R, et al. Enhanced trapping of HIV-1 by human cervicovaginal mucus is associated with Lactobacillus crispatus-dominant microbiota. MBio 2015;6:e01084–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cone RA. Vaginal microbiota and sexually transmitted infections that may influence transmission of cell-associated HIV. J Infect Dis 2014;210(suppl 3):S616–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schwebke JR. Abnormal vaginal flora as a biological risk factor for acquisition of HIV infection and sexually transmitted diseases. J Infect Dis 2005;192:1315–7. [DOI] [PubMed] [Google Scholar]
- 94.St Amant DC, Valentin-Bon IE, Jerse AE. Inhibition of Neisseria gonorrhoeae by lactobacillus species that are commonly isolated from the female genital tract. Infect Immun 2002;70:7169–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kalyoussef S, Nieves E, Dinerman E, Carpenter C, Shankar V, Oh J, et al. Lactobacillus proteins are associated with the bactericidal activity against E. coli of female genital tract secretions. PLoS One 2012;7:e49506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wiesenfeld HC, Hillier SL, Krohn MA, Landers DV, Sweet RL. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis 2003;36:663–8. [DOI] [PubMed] [Google Scholar]
- 97.Brotman RM, Klebanoff MA, Nansel TR, Yu KF, Andrews WW, Zhang J, et al. Bacterial vaginosis assessed by gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. J Infect Dis 2010;202:1907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schwebke JR, Desmond R. A randomized trial of metronidazole in asymptomatic BV to prevent acquisition of STDs. Am J Obstet Gynecol 2007;196:517.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schwebke JR, Lee JY, Lensing S, Philip SS, Wiesenfeld HC, Seña AC, et al. Home screening for bacterial vaginosis to prevent sexually transmitted diseases. Clin Infect Dis 2016;62: 531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sutton M, Sternberg M, Koumans EH, McQuillan G, Berman S, Markowitz L. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001–2004. Clin Infect Dis 2007;45:1319–26. [DOI] [PubMed] [Google Scholar]
- 101.Mirmonsef P, Krass L, Landay A, Spear GT. The role of bacterial vaginosis and trichomonas in HIV transmission across the female genital tract. Curr HIV Res 2012;10: 202–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fichorova RN, Buck OR, Yamamoto HS, Fashemi T, Dawood HY, Fashemi B, et al. The villain team-up or how Trichomonas vaginalis and bacterial vaginosis alter innate immunity in concert. Sex Transm Infect 2013;89:460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Balkus JE, Richardson BA, Rabe LK, Taha TE, Mgodi N, Kasaro MP, et al. Bacterial vaginosis and the risk of trichomonas vaginalis acquisition among HIV-1 negative women. Sex Transm Dis 2014;41:123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brotman RM, Bradford LL, Conrad M, Gajer P, Ault K, Peralta L, et al. Association between Trichomonas vaginalis and vaginal bacterial community composition among reproductive-age women. Sex Transm Dis 2012;10:807–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fastring DR, Amedee A, Gatski M, Clark RA, Mena LA, Levison J, et al. Co-occurrence of Trichomonas vaginalis and bacterial vaginosis and vaginal shedding of HIV-1 RNA. Sex Transm Dis 2014;41:173–9. [DOI] [PubMed] [Google Scholar]
- 106.Soper DE, Brockwell NJ, Dalton HP, Johnson D. Observations concerning the microbial etiology of acute salpingitis. Am J Obstet Gynecol 1994;170:1014–14. [DOI] [PubMed] [Google Scholar]
- 107.Jossens MO, Schachter J, Sweet RL. Risk factors associated with pelvic inflammatory disease of differing microbial etiologies. Obstet Gynecol 1994;83:989–97. [DOI] [PubMed] [Google Scholar]
- 108.Haggerty CL, Hillier SL, Bass DC, Ness RB; PID Evaluation and Clinical Health study investigators. Bacterial vaginosis and anaerobic bacteria are associated with endometritis. Clin Infect Dis 2004;39:990–5. [DOI] [PubMed] [Google Scholar]
- 109.Ness RB, Kip KE, Hillier SL, Soper DE, Stamm CA, Sweet RL, et al. A cluster analysis of bacterial vaginosis-associated microflora and pelvic inflammatory disease. Am J Epidemiol 2005;162:585–90. [DOI] [PubMed] [Google Scholar]
- 110.Hebb JK, Cohen CR, Astete SG, Bukusi EA, Totten PA. Detection of novel organisms associated with salpingitis, by use of 16S rDNA polymerase chain reaction. J Infect Dis 2004;190:2109–20. [DOI] [PubMed] [Google Scholar]
- 111.Green KA, Zarek SM, Catherino WH. Gynecologic health and disease in relation to the microbiome of the female reproductive tract. Fertil Steril 2015;104:1351–7. [DOI] [PubMed] [Google Scholar]
- 112.Donati L, Di Vico A, Nucci M, Quagliozzi L, Spagnuolo T, Labianca A, et al. Vaginal microbial flora and outcome of pregnancy. Arch Gynecol Obstet 2010;281:589–600. [DOI] [PubMed] [Google Scholar]
- 113.MacIntyre DA, Chandiramani M, Lee YS, Kindinger L, Smith A, Angelopoulos N, et al. The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci Rep 2015;5:8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hyman RW, Fukushima M, Jiang H, Fung E, Rand L, Johnson B, et al. Diversity of the vaginal microbiome correlates with preterm birth. Reprod Sci 2014;21:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Farr A, Kiss H, Hagmann M, Machal S, Holzer I, Kueronya V, et al. Role of lactobacillus species in the intermediate vaginal flora in early pregnancy: a retrospective cohort study. PLoS One 2015;10:e0144181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hamilton DT, Morris M. The racial disparities in STI in the U.S.: concurrency, STI prevalence, and heterogeneity in partner selection. Epidemics 2015;11:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Adimora AA, Schoenbach VJ, Doherty IA. Concurrent sexual partnerships among men in the United States. Am J Public Health 2007;97:2230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eschenbach D Bacterial vaginosis: resistance, recurrence, and/or reinfection? Clin Infect Dis 2007;44:220–1. [DOI] [PubMed] [Google Scholar]
- 119.Hardy L, Jespers V, Dahchour N, Mwambarangwe L, Musengamana V, Vaneechoutte M, et al. Unravelling the bacterial vaginosis-associated biofilm: a multiplex Gardnerella vaginalis and Atopobium vaginae fluorescence in situ hybridization assay using peptide nucleic acid probes. PLoS One 2015;10:e0136658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mastromarino P, Vitali B, Mosca L. Bacterial vaginosis: a review on clinical trials with probiotics. New Microbiol 2013;36:229–38. [PubMed] [Google Scholar]
- 121.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A 2010; 107:11971–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dominguez-Bello MG, De Jesus-Laboy KM, Shen N, Cox LM, Amir A, Gonzalez A, et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat Med 2016;22:250–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Srinivasan S, Morgan MT, Fiedler TL, Djukovic D, Hoffman NG, Raftery D, et al. Metabolic signatures of bacterial vaginosis. MBio 2015;6 pii: e00204–15. [DOI] [PMC free article] [PubMed] [Google Scholar]