Abstract
Acinetobacter calcoaceticus-baumannii complex isolates have been frequently associated with hospital and community infections, with A. baumannii being the most common. Other Acinetobacter spp. not belonging to this complex also cause infections in hospital settings, and the incidence has increased over the past few years. Some species of the Acinetobacter genus possess a great diversity of antibiotic resistance mechanisms, such as efflux pumps, porins, and resistance genes that can be acquired and disseminated by mobilizable genetic elements. By means of whole-genome sequencing, we describe in the clinical Acinetobacter haemolyticus strain AN54 different mechanisms of resistance that involve blaOXA-265, blaNDM-1, aphA6, aac(6’)-Ig, and a resistance-nodulation-cell division-type efflux pump. This strain carries six plasmids, of which the plasmid pAhaeAN54e contains blaNDM-1 in a Tn125-like transposon that is truncated at the 3′ end. This strain also has an insertion sequence IS91 and seven genes encoding hypothetical proteins. The pAhaeAN54e plasmid is nontypable and different from other plasmids carrying blaNDM-1 that have been reported in Mexico and other countries. The presence of these kinds of plasmids in an opportunistic pathogen such as A. haemolyticus highlights the role that these plasmids play in the dissemination of antibiotic resistance genes, especially against carbapenems, in Mexican hospitals.
Keywords: A. haemolyticus, plasmid, NDM-1, antibiotic resistance
Introduction
The genus Acinetobacter includes a group of bacteria that can be isolated from a wide variety of environmental sources, including soil and water. However, some of them have become important nosocomial pathogens, such as those included within the Acinetobacter calcoaceticus-baumannii complex. Members of this genus have been associated with severe nosocomial and community infections with high mortality rates. Nevertheless, isolates of other non-baumannii spp. have gained medical relevance because of their increased frequency in recent years, for example, Acinetobacter haemolyticus, Acinetobacter lwoffii, Acinetobacter ursingii, Acinetobacter parvus, and Acinetobacter junii.1–3
Acinetobacter spp. have developed resistance to multiple classes of antimicrobial agents, including broad-spectrum cephalosporins, carbapenems, fluoroquinolones, and aminoglycosides. This resistance is due to multiple mechanisms, such as resistance-nodulation-cell division (RND)-type efflux pumps, CarO porin, and resistance genes. In addition, the ability of Acinetobacter spp. to acquire mobilizable elements that carry antibiotic resistance genes increases the resistance dissemination.4–6 In particular, blaNDM-1, which encodes the New Delhi Metallo-β-lactamase-1 (NDM-1), hydrolyzes a broad spectrum of β-lactam antibiotics, including carbapenems, and is among the most worrisome resistance determinants that have spread around the world, severely complicating the treatment of nosocomial infections.7–10 In Latin America, its presence has been reported in Enterobacteriaceae isolates such as Klebsiella pneumoniae, Escherichia coli, and Providencia rettgeri,11–13 and in nonfermentative bacilli, such as A. baumannii, Acinetobacter pittii, Acinetobacter bereziniae, and A. haemolyticus.14–17 In this work, we report the presence of diverse antibiotic resistance genes in a strain of A. haemolyticus obtained from a Mexican pediatric patient and the characterization of the complete plasmid carrying blaNDM-1 isolated from this strain.
Materials and Methods
Bacterial isolation
Acinetobacter haemolyticus AN54 was recovered from peritoneal dialysis fluid culture from a 12-year-old male patient who had been admitted to the hospital for end-stage renal disease in February 2016. The patient was previously treated with ceftriaxone (CRO) and trimethoprim-sulfamethoxazole (SXT). The isolate was identified with the VITEK 2 system (bioMérieux) and molecular typing by sequencing of the rpoB gene.18,19
Antimicrobial susceptibility testing
An antimicrobial susceptibility test was performed by using the agar disk diffusion method according to the Clinical and Laboratory Standards Institute guidelines.20 The following antimicrobials were tested: piperacillin (PIP), ticarcillin (TIC), ampicillin/sulbactam, piperacillin/tazobactam, ticarcillin-clavulanic acid (TIM), ceftazidime (CAZ), cefepime (FEP), cefotaxime (CTX), CRO, imipenem (IPM), meropenem (MEM), gentamicin, amikacin (AN), tetracycline, ciprofloxacin, levofloxacin, and SXT. The minimal inhibitory concentration (MIC) for CTX, CAZ, FEP, MEM, IPM, and AN was determined by the agar dilution method.20 Metallo-β-lactamase (MBL) detection was performed by IPM and MEM disks supplemented with 10 μL of 0.5 M ethylenediaminetetraacetic acid.21
Test of the activity of the efflux pump in antibiotic resistance
The activity of the efflux pump was evaluated by using phenylalanine-arginine β-naphthylamide (Sigma-Aldrich) as an efflux pump inhibitor (EPI). The test was performed as follows: MICs for AN, CTX, and MEM were determined by the agar dilution method in the presence and absence of EPI (25 mg/L). A twofold or greater decrease in MIC in the presence of EPI was considered indicative of a role of RND-type efflux pumps in the resistance to the antibiotics tested. Test veracity was checked by using the strain Acinetobacter haemolyticus HNP11 as a positive control. To evaluate the effect of EPI on bacterial growth, all bacteria were cultured in Mueller–Hinton broth with and without EPI (25 mg/L).22
Whole-genome sequencing and data analysis
A high-quality draft genome sequence from isolate AN54 was obtained by using an Illumina MiSeq platform (2 × 300 paired-end reads) (IBT-UNAM) and one SMRT cell of PacBio RS II system (Yale Center for Genome Analysis). With data obtained from both platforms, a hybrid assembly was performed with the Unicycler assembler version 0.4.1.23 and SPAdes version 3.11.1.24 ResFinder 2.125 was used to identify and determine the location of antibiotic resistance genes. MAUVE version 20150226,26 CLC Sequence Viewer 8.0 and BLAST were used to align and compare sequences. EASYFIG 2.2.2 was used to draw figures.27
Pulsed-field gel electrophoresis and Southern blot
The S1 nuclease-pulsed-field gel electrophoresis (PFGE) method was carried out to determine the plasmids number by using the Escherichia coli strain NCTC 50192 as a reference. To detect the resistance gene in the plasmid, the PFGE gel was transferred to a nylon membrane (Hybond-N; GE Healthcare Life Sciences), and hybridization was performed with the Dig-High Prime DNA Labeling and Detection Starter Kit II (Roche).
Conjugation assays
Conjugation assays from AN54 to recipient strains Escherichia coli C600 (rifampicin resistant) and Escherichia coli DH5α (nalidixic acid resistant) were performed. Mueller–Hinton agar plates (BD Bioxon) supplemented with rifampicin (100 μg/mL) or nalidixic acid (32 μg/mL) containing AN (32 μg/mL) and MEM (8 μg/mL) were used for the selection of transconjugant strains.
Nucleotide sequence accession numbers
Draft genome and plasmid sequences of the AN54 strain were deposited in the GenBank database, with accession numbers CP041224.1 to CP041229.1.
Results
In this work, we report the identification of an Acinetobacter haemolyticus (AN54) strain resistant to carbapenems, which was isolated from peritoneal dialysis fluid. This strain was initially identified as Acinetobacter spp. by a VITEK 2 System and subsequently reclassified as A. haemolyticus by analysis of the rpoB gene. The AN54 strain exhibited resistance to AN, and to the broad-spectrum β-lactams antibiotics, except for TIM. The blaNDM-1 and blaOXA-265 were previously detected by PCR and sequencing. A twofold decrease in the MIC for AN and no change in the MICs for CTX and MEM in the presence of EPI were observed. In addition, the phenotypic test to detect MBL production was positive (Table 1).
Table 1.
Antimicrobial Susceptibilities Test and Genotype of Acinetobacter haemolyticus AN54
| MIC (μg/mL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Strain | Anatomical site | Resistance phenotype | CAZ | FEP | IPM | CTX (CTX + EPI) | MEM (MEM + EPI) | AN (AN + EPI) | Genotypea | Efflux pumps genesa |
| AN54 | Peritoneal dialysis fluid | PIP, TIC, SAM, TZP, CAZ, FEP, CTX, CRO, IPM, MEM, AN | >128 | >128 | >128 | >128 (>128) | >128 (>128) | 64 (32) | blaNDM-1, blaOXA-265, aphA6, aac-(6’)-Ig | adeA, adeB, adeC, adeI, adeK, adeS,badeR,bmacA, macB |
Control strain Acinetobacter haemolyticus HNP11 MIC values: CTX: 16 μg/mL, CTX + EPI: 8 μg/mL; AN: 64 μg/mL, AN + EPI: 32 μg/mL.
Obtained by PCR and whole-genome sequencing analysis.
Regulators of AdeABC efflux pump.
AN, amikacin; CAZ, ceftazidime; CRO, ceftriaxone; CTX, cefotaxime; EPI, efflux pumps inhibitor (phenylalanine-arginine-β-naphthylamide) (PAβN) 25 mg/L; FEP, cefepime; IPM, imipenem; MEM, meropenem; MIC, minimal inhibitory concentration; PIP, piperacillin; SAM, ampicillin/sulbactam; TIC, ticarcillin; TZP, piperacillin/tazobactam.
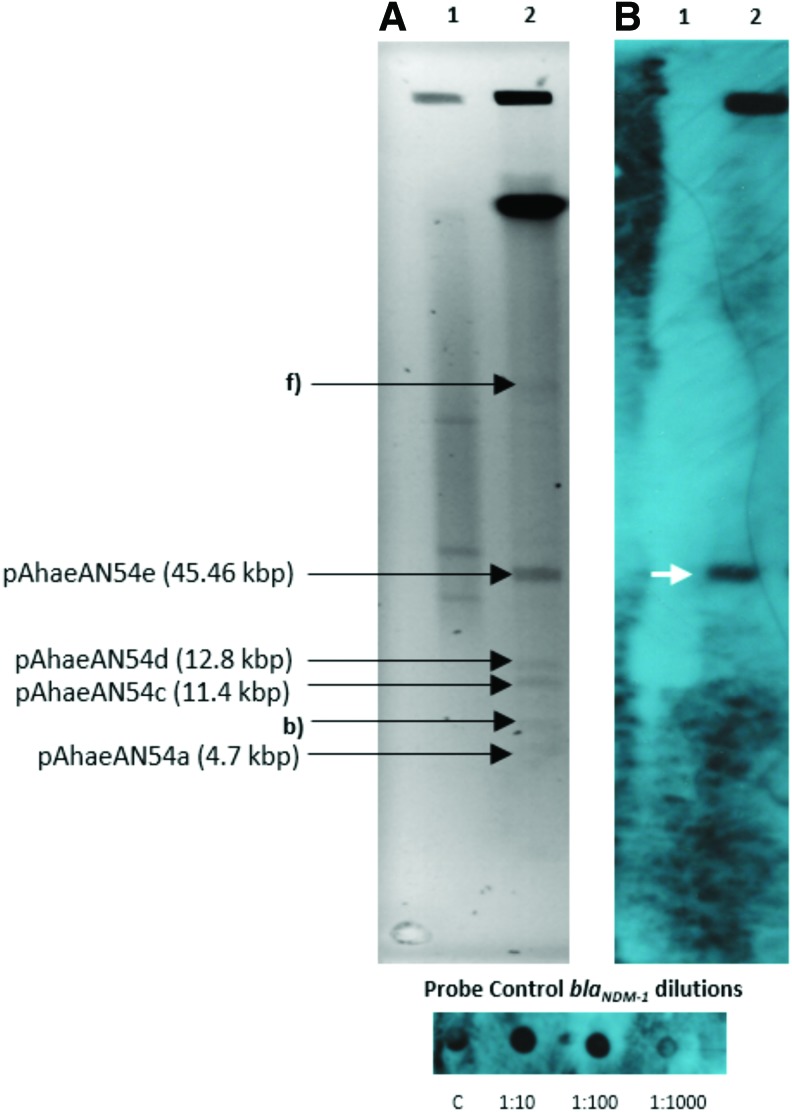
Plasmid DNA extraction revealed that the AN54 isolate carries six different bands. Southern blot hybridization indicated that blaNDM-1 was present in one of the plasmids (Fig. 1).
FIG. 1.
(A) PFGE-S1 gel showing the plasmids of Acinetobacter haemolyticus AN54 strain, Line 1: Control strain Escherichia coli NCTC 50192, Line 2: AN54. (B) Hybridization autoradiography with the blaNDM-1 probe (DIG-High Prime DNA Labeling and Detection Starter Kit II, Roche). The white arrow indicates that blaNDM-1 was detected in the plasmid pAhaeAN54e. The plasmid sizes were determined by WGS. PFGE, pulsed-field gel electrophoresis. Color images are available online.
Whole-genome sequencing analysis revealed that AN54 possess a chromosome with a size of ∼3.61 Mbp, and we obtained the complete sequence of four plasmids named pAhaeAN54a (4.7 kbp), pAhaeAN54c (11.4 kbp), and pAhaeAN54d (12.8 kbp) that do not carry antimicrobial resistance genes, and pAhaeAN54e (45.46 kbp) that carries blaNDM-1 and aphA6. In the chromosome, we detected the presence of blaOXA-265 and the aminoglycoside-modifying enzyme-encoding gene aac(6’)-Ig. In addition, genes for efflux pumps and their regulators that mediate multidrug resistance were found:, adeA, adeB, adeC, adeI, adeK, adeR, adeS, macA, and macB, as well as heavy metal resistance genes czcA and arsH.
The blaNDM-1 was located in the 45.46 kbp plasmid, which we named pAhaeAN54e. This plasmid has a 41% guanine-cytosine content and 53 open reading frames. Sixteen of these genes are related to plasmid maintenance and transfer functions (plasmid backbone) and include the plasmid-partitioning genes parA and parB, and transfer genes traA, traC, and traD, as well as some genes of the type IV secretion system (T4SS). Twelve genes of this plasmid are located within the composite transposon carrying blaNDM-1, and the remaining genes encode 25 hypothetical proteins. Conjugation assays were performed to test the transferability of this plasmid; however, no transconjugants were obtained under the tested conditions.
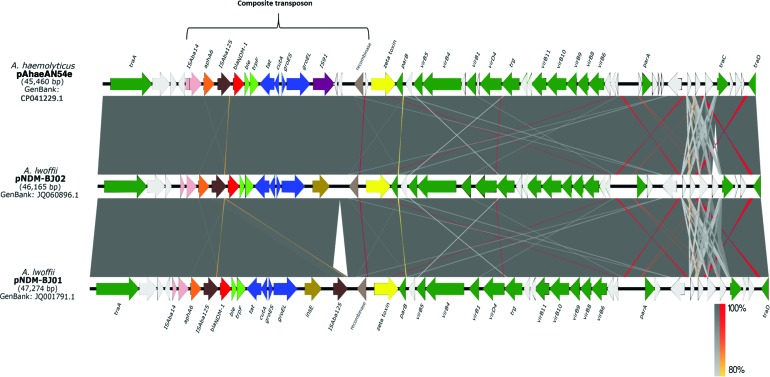
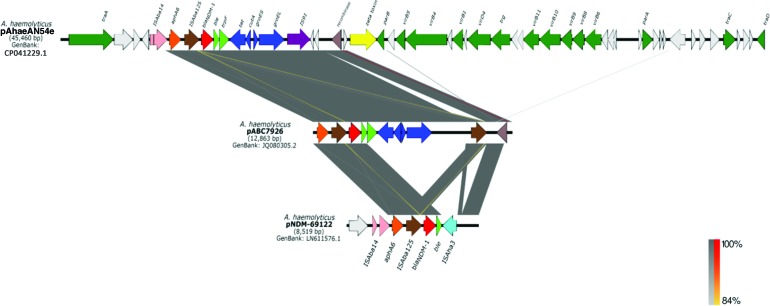
Plasmid pAhaeAN54e has 99% nucleotide identity, with 100% coverage, to plasmids pNDM-BJ02 (accession no. JQ060896.1) and pNDM-BJ01 (accession no. JQ001791.1) of A. lwoffii.28 Figure 2 shows the detected differences, especially the insertion of seven genes encoding hypothetical proteins along the T4SS gene cluster, near the parA and traA genes, and a deletion of a hypothetical protein upstream of traC. The pAhaeAN54e plasmid also has a truncated composite transposon that is similar to transposon Tn125. Both these transposons consist of the ISAba14 insertion sequence and the aphA6, blaNDM-1, ble, and trpF genes, but in contrast to Tn125, the truncated transposon of strain AN54 has only one copy of the insertion sequence ISAba125. The structure of the truncated transposon is similar to that found in plasmid pNDM-BJ02 and to the partially sequenced plasmids pABC7926 (accession no. JQ080305.2) and pNDM-69122 (accession no. LN611576.1) of A. haemolyticus (Fig. 3).29,30
FIG. 2.
The schematic representation and alignment of plasmid pAhaeAN54e of Acinetobacter haemolyticus and plasmids pNDM-BJ02 and pNDM-BJ01 of Acinetobacter lwoffii are divided into two sections: the composite transposon in which ISAba125, blaNDM-1, and IS91 are inserted and the putative conjugation machinery. The known genes are marked in green, and putative proteins are marked in white. The gray bars indicate 99% identity, and the inverted regions are indicated in red. Color images are available online.
FIG. 3.
Comparison between the plasmid pAhaeAN54e and the partially sequenced plasmids pABC7926 and pNDM69122 of Acinetobacter haemolyticus. Color images are available online.
Plasmid pAhaeAN54e contains an IS91 family transposase, which is 330 bp larger than the insE transposase gene carried on the pNDM-BJ02 and pNDM-BJ01 plasmids in A. lwoffii. In addition, pAhaeAN54e has an insertion of two genes encoding hypothetical proteins downstream of IS91 and one more that is adjacent to the recombinase gene.
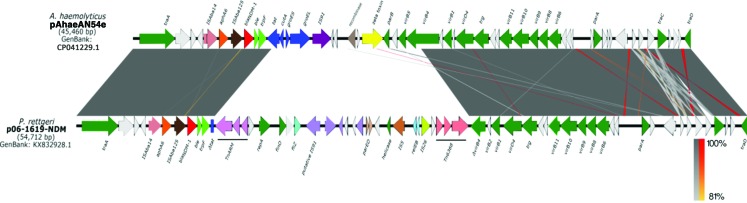
Interestingly, plasmid pAhaeAN54e has 99% identity with more than 73% of coverage with the P. rettgeri plasmid p06-1619-NDM (accession no. KX832928.1) reported in Mexico (Fig. 4).31 These plasmids share 33 genes, including traA, traC, traD, and genes from the type IV secretion system. Plasmid p06-1619-NDM also has a Tn125-like element.
FIG. 4.
Comparison between plasmids pAhaeAN54e of Acinetobacter haemolyticus and p06-1619-NDM of Providencia rettgeri, both of which are isolated from México. Color images are available online.
Discussion
A. baumannii is one of the principal etiological agents causing nosocomial infections in Mexico and the rest of the world.32–34 In Mexican hospitals, there are a few reports of carbapenem-resistant Acinetobacter isolates that are not included in the A. calcoaceticus-baumannii complex,17 possibly because the molecular typing of these species is not routinely implemented in Mexican hospitals. In this work, we studied the resistome of an A. haemolyticus strain resistant to carbapenems. The strain carries different resistance mechanisms, such as blaOXA-265, which is a member of the blaOXA-214-like family on the A. haemolyticus chromosome.35 The strain also harbors aminoglycoside-modifying enzyme genes, such as aac(6’)-Ig, which is responsible for AN resistance and belongs to the AAC(6’)-I aminoglycoside N-acetyltransferase family reported in Acinetobacter spp.,36,37 and aphA6, which confers resistance to AN and is associated with the presence of blaNDM-1 in a plasmid from this strain, as has been reported in another study.38
Another antibiotic resistance mechanism studied in A. baumannii is the use of efflux pumps, mainly the RND family of efflux pumps, such as the AdeABC and AdeIJK systems, which are located on the bacterial chromosome. This family exhibits a wide substrate range that includes dyes, biocides, detergents, and antiseptics; however, its presence has been little studied in non-baumannii spp.39 The overexpression of this system was shown to be responsible for decreasing susceptibility to a broad spectrum of antimicrobials, such as aminoglycosides, tetracyclines, erythromycin, chloramphenicol, trimethoprim, fluoroquinolones, some β-lactams, and ethidium bromide, and has recently also been associated with tigecycline.40 The genes that encode the AdeABC efflux pump are organized in an operon (adeABC). There are two regulatory genes, adeS and adeR, and their products are closely related to proteins of the two-component regulatory system. These genes regulate efflux pump expression in response to cellular environment stimuli (antibiotics). This type of expression is called inductive.41
AdeIJK encoded by the adeIJK operon is the second RND efflux system described in A. baumannii,42 which contributes to the resistance to β-lactams, such as TIC, cephalosporins, aztreonam, fluoroquinolones, tetracyclines, tigecycline, lincosamides, rifampicin, and chloramphenicol; however, aminoglycosides are not substrates for this pump.43 The Acinetobacter haemolyticus AN54 strain contains homologues of the regulator proteins AdeS and AdeR and the efflux pump components AdeA, AdeB and AdeC with the Acinetobacter baumannii AYE strain, these proteins share an amino acid identity of 72%, 85%, 86%, 92% and 77% respectively, to the corresponding proteins. In addition, homologues of AdeI and AdeK proteins with 82% and 89% identity with Acinetobacter baumannii AYE were also found. However, the AdeJ subunit in strain AN54 was not located.
In this work, EPI was used to evaluate the role of the efflux pump in antibiotic resistance. The results obtained indicate that the efflux pump only participates in conferring resistance to AN, as shown by a twofold decrease in the MIC (from 64 to 32 μg/mL). The strain did not show a decrease in the MICs of CTX and MEM, possibly due to the presence of blaNDM-1.6
The presence of blaNDM-1 in a non-baumannii spp. of environmental origin such as A. haemolyticus is medically and epidemiologically relevant. This is especially true considering the lack of successful treatment for patients with some underlying diseases and the ease of blaNDM-1 dissemination through complex recombination events mediated by insertion sequences, transposons, and plasmids, as reported in previous works.31,44,45
The Acinetobacter haemolyticus AN54 strain harbors blaNDM-1 within a Tn125-like transposon in a plasmid highly similar to pNDM-BJ02 of A. lwoffii, which has been previously reported in China.28 The backbone of the plasmids carrying blaNDM-1 between Acinetobacter spp. is relatively conserved; however, one difference found in our plasmid is the presence of IS91. This gene is designated as ISCR, and one of its functions is the mobilization of additional sequences upstream of the transposase gene.46,47 The second difference is the insertion of seven genes encoding hypothetical proteins in the putative conjugation region; the function of these proteins is yet unknown. Currently, there are no methodologies to characterize this kind of replicon, due to the absence of a typical Rep protein, such as the one presented by Enterobacteriaceae plasmids, indicating that these plasmids possess an uncharacterized replication system; therefore, additional studies are needed. No transconjugants were obtained under the tested conditions. However, we cannot exclude other alternative mechanisms of plasmid transference, as reported in other studies of Acinetobacter spp. carrying blaNDM-1.14,15,38,48
Recently, Duran-Bedolla et al.17 partially reported the genetic context of blaNDM-1 in strains of Acinetobacter spp., including the Acinetobacter haemolyticus 10256 strain isolated in a different geographical area of Mexico. The plasmid reported in our work showed specific differences in comparison with the plasmid of Acinetobacter haemolyticus 10256 (55 kbp plasmid); these included size (45.4 kbp), absence of ISAba125 sequence in the 3′ end, presence of genes encoding hypothetical proteins along the structure of the plasmid, and IS91 instead of ISCR27. These results suggest that the genetic context of blaNDM-1 in A. haemolyticus is partially conserved.17,29,30
Our study shows that plasmid pAhaeAN54e has a high similarity with plasmid p06-1619-NDM of P. rettgeri and, as noted by Marquez-Ortiz et al.,31 the similarities among pNDM-BJ01-like plasmids and plasmids of P. rettgeri may be the result of recombination events that lead to a chimeric plasmid that can be transmitted and replicated among Enterobacteriaceae and Acinetobacter isolates.49
Concluding Remarks
In this study, we report a multidrug-resistant clinical A. haemolyticus strain isolated from a pediatric patient carrying blaNDM-1 in a novel variant of pNDM-BJ01-like plasmids. This plasmid could constitute a dissemination mechanism of antimicrobial resistance genes among diverse bacterial species. The findings reported in this work contribute to providing information about the diverse mechanisms of resistance that can coexist in A. haemolyticus strains.
Acknowledgments
The authors thank Liliana López-Pliego, PhD and Ángeles Pérez-Oseguera, MS for technical support in the laboratory. This work was funded by Benemérita Universidad Autonóma de Puebla, VIEP grant numbers: LOZP-NAT17-I and VIEP/2497/16 and partially supported by “Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica PAPIIT” (IN200318). Bello-López and Castro-Jaimes received Doctoral fellowships from CONACyT México with numbers: 273320 and 606188, respectively.
Ethical Statement
Acinetobacter haemolyticus AN54 isolate was collected during routine sampling, and patient data were anonymized. The protocol for this study was approved by the Ethical Committee of Hospital number: HNP/ENS/177/2016.
Disclosure Statement
No competing financial interests exist.
References
- 1. Peleg A.Y., de Breij A., Adams M.D, Cerqueira G.M, Mocali S., Galardini M., Nibbering P.H, Earl A.M, Ward D.V, Paterson D.L, Seifert H., and Dijkshoorn L. 2012. The success of Acinetobacter species; genetic, metabolic and virulence attributes. PLoS One. 7:e46984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Touchon M., Cury J., Yoon E.-J, Krizova L., Cerqueira G.C, Murphy C., Feldgarden M., Wortman J., Clermont D., Lambert T., Grillot-Courvalin C., Nemec A., Courvalin P., and Rocha E.P.C. 2014. The genomic diversification of the whole Acinetobacter genus: origins, mechanisms, and consequences. Genome Biol. Evol. 6:2866–2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wong D., Nielsen T.B, Bonomo R.A, Pantapalangkoor P., Luna B., and Spellberg B. 2017. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin. Microbiol. Rev. 30:409–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Esterly J., Richardson C.L, Eltoukhy N.S, Qi C., and Scheetz M.H. 2011. Genetic mechanisms of antimicrobial resistance of Acinetobacter baumannii. Ann. Pharmacother. 45:218–228 [DOI] [PubMed] [Google Scholar]
- 5. Traglia G.M., Almuzara M., Vilacoba E., Tuduri A., Neumann G., Pallone E., Centrón D., and Ramírez M.S. 2014. Bacteremia caused by an Acinetobacter junii strain harboring class 1 integron and diverse DNA mobile elements. J. Infect. Dev. Ctries. 8:666–669 [DOI] [PubMed] [Google Scholar]
- 6. Rumbo C., Gato E., López M., Ruiz de Alegría C., Fernández-Cuenca F., Martínez-Martínez L., Vila J., Pachón J., Cisneros J.M, Rodríguez-Baño J., Pascual A., Bou G., and Tomás M. 2013. Contribution of efflux pumps, porins, and β-lactamases to multidrug resistance in clinical isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 57:5247–5257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berrazeg M., Diene S.M, Medjahed L., Parola P., Drissi M., Raoult D., and Rolain J.M. 2014. New Delhi Metallo-beta-lactamase around the world: An eReview using Google Maps. Euro. Surveill. 19:pii: [DOI] [PubMed] [Google Scholar]
- 8. Shahid M. 2011. Environmental dissemination of NDM-1: time to act sensibly. Lancet Infect. Dis. 11:334–335 [DOI] [PubMed] [Google Scholar]
- 9. Roca I., Espinal P., Vila-Farrés X., and Vila J. 2012. The Acinetobacter baumannii oxymoron: commensal hospital Dweller turned pan-drug-resistant menace. Front. Microbiol. 3:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Robledo I.E., Aquino E.E, Santé M.I, Santana J.L, Otero D.M, León C.F, and Vázquez G.J. 2010. Detection of in KPC Acinetobacter spp. in Puerto Rico. Antimicrob. Agents Chemother. 54:1354–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pasteran F., Albornoz E., Faccone D., Gomez S., Valenzuela C., Morales M., Estrada P., Valenzuela L., Matheu J., Guerriero L., Arbizu E., Calderon Y., Ramon-Pardo P., and Corso A. 2012. Emergence of NDM-1-producing Klebsiella pneumoniae in Guatemala. J. Antimicrob. Chemother. 67:1795–1797 [DOI] [PubMed] [Google Scholar]
- 12. Torres-González P., Bobadilla-Del Valle M., Tovar-Calderón E., Leal-Vega F., Hernández-Cruz A., Martínez-Gamboa A., Niembro-Ortega M.D, Sifuentes-Osornio J., and Ponce-de-León A. 2015. Outbreak caused by Enterobacteriaceae harboring NDM-1 metallo-β-lactamase carried in an IncFII plasmid in a tertiary care hospital in Mexico City. Antimicrob. Agents Chemother. 59:7080–7083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carvalho-Assef A.P.D., Pereira P.S, Albano R.M, Beriao G.C, Chagas T.P.G, Timm L.N, Da Silva R.C.F, Falci D.R, and Asensi M.D. 2013. Isolation of NDM-producing Providencia rettgeri in Brazil. J. Antimicrob. Chemother. 68:2956–2957 [DOI] [PubMed] [Google Scholar]
- 14. Pasteran F., Mora M.M, Albornoz E., Faccone D., Franco R., Ortellado J., Melgarejo N., Gomez S., Riquelme I., Matheu J., Ramon-Pardo P., and Corso A. 2014. Emergence of genetically unrelated NDM-1-producing Acinetobacter pittii strains in Paraguay. J. Antimicrob. Chemother. 69:2575–2578 [DOI] [PubMed] [Google Scholar]
- 15. Brovedan M., Marchiaro P.M, Morán-Barrio J., Cameranesi M., Cera G., Rinaudo M., Viale A.M, and Limansky A.S. 2015. Complete sequence of a bla(NDM-1)-harboring plasmid in an Acinetobacter bereziniae clinical strain isolated in Argentina. Antimicrob. Agents Chemother. 59:6667–6669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pillonetto M., Arend L., Vespero E.C, Pelisson M., Chagas T.P.G, Carvalho-Assef A.P.D, and Asensi M.D. 2014. First report of NDM-1-producing Acinetobacter baumannii sequence type 25 in Brazil. Antimicrob. Agents Chemother. 58:7592–7594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duran-Bedolla J., Bocanegra-Ibarias P., Silva-Sánchez J., Garza-González E., Morfín-Otero R., Hernández-Castro R., Lozano L., Garza-Ramos U., and Barrios-Camacho H. 2018. Genetic characterization of multiple NDM-1-producing clinical isolates in Mexico. Diagn. Microbiol. Infect. Dis. 94:195–198 [DOI] [PubMed] [Google Scholar]
- 18. Gundi V.A., Dijkshoorn L., Burignat S., Raoult D., and La Scola B. 2009. Validation of partial rpoB gene sequence analysis for the identification of clinically important and emerging Acinetobacter species. Microbiology. 155:2333–2341 [DOI] [PubMed] [Google Scholar]
- 19. La Scola B., Gundi V.A.K.B, Khamis A., and Raoult D. 2006. Sequencing of the rpoB gene and flanking spacers for molecular identification of Acinetobacter species. J. Clin. Microbiol. 44:827–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clinical Laboratory Standards Institute. 2017. Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 21. Bonnin R.A., Naas T., Poirel L., and Nordmann P. 2012. Phenotypic, biochemical, and molecular techniques for detection of metallo-β-lactamase NDM in Acinetobacter baumannii. J. Clin. Microbiol. 50:1419–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. López-García A., Rocha-Gracia R.C, Bello-López E., Juárez-Zelocualtecalt C., Sáenz Y., Castañeda-Lucio M., López-Pliego L., González-Vázquez M.C, Torres C., Ayala-Nuñez T., Jiménez-Flores G., de la Paz Arenas-Hernández M.M, and Lozano-Zarain P. 2018. Characterization of antimicrobial resistance mechanisms in carbapenem-resistant Pseudomonas aeruginosa carrying IMP variants recovered from a Mexican hospital. Infect. Drug Resist. 11:1523–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wick R.R., Judd L.M, Gorrie C.L, and Holt K.E. 2017. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 13:e1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bankevich A., Nurk S., Antipov D., Gurevich A.A, Dvorkin M., Kulikov A.S, Lesin V.M, Nikolenko S.I, Pham S., Prjibelski A.D, Pyshkin A.V, Sirotkin A.V, Vyahhi N., Tesler G., Alekseyev M.A, and Pevzner P.A. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 19:455–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zankari E., Hasman H., Cosentino S., Vestergaard M., Rasmussen S., Lund O., Aarestrup F.M, and Larsen M.V. 2012. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 67:2640–2644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Darling A.C.E., Mau B., Blattner F.R, and Perna N.T. 2004. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 14:1394–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sullivan M.J., Petty N.K, and Beatson S.A. 2011. Easyfig: a genome comparison visualizer. Bioinformatics. 27:1009–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hu H., Hu Y., Pan Y., Liang H., Wang H., Wang X., Hao Q., Yang X., Yang X., Xiao X., Luan C., Yang Y., Cui Y., Yang R., Gao G.F, Song Y., and Zhu B. 2012. Novel Plasmid and its variant haboring both a blaNDM-1 gene and type IV secretion System in clinical isolates of Acinetobacrer lwoffii. Antimicrob. Agents Chemother. 56:1698–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jones L.S., Carvalho M.J, Toleman M.A, White P.L, Connor T.R, Mushtaq A., Weeks J.L, Kumarasamy K.K, Raven K.E, Török M.E, Peacock S.J, Howe R.A, and Walsh T.R. 2015. Characterization of plasmids in extensively drug-resistant Acinetobacter strains isolated in India and Pakistan. Antimicrob. Agents Chemother. 59:923–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fu Y., Liu L., Li X., Chen Y., Jiang Y., Wang Y., Yu Y., and Xie X. 2015. Spread of a common blaNDM-1-carrying plasmid among diverse Acinetobacter species. Infect. Genet. Evol. 32:30–33 [DOI] [PubMed] [Google Scholar]
- 31. Marquez-Ortiz R.A., Haggerty L., Olarte N., Duarte C., Garza-Ramos U., Silva-Sanchez J., Castro B.E, Sim E.M, Beltran M., Moncada M.V, Valderrama A., Castellanos J.E, Charles I.G, Vanegas N., Escobar-Perez J., and Petty N.K. 2017. Genomic epidemiology of NDM-1-encoding plasmids in Latin American clinical isolates reveals insights into the evolution of multidrug resistance. Genome Biol. Evol. 9:1725–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bocanegra-Ibarias P., Peña-López C., Camacho-Ortiz A., Llaca-Díaz J., Silva-Sánchez J., Barrios H., Garza-Ramos U., Rodríguez-Flores A.M, and Garza-González E. 2015. Genetic characterisation of drug resistance and clonal dynamics of Acinetobacter baumannii in a hospital setting in Mexico. Int. J. Antimicrob. Agents. 45:309–313 [DOI] [PubMed] [Google Scholar]
- 33. Garza-González E., Llaca-Díaz J.M, Bosques-Padilla F.J, and González G.M. 2010. Prevalence of multidrug-resistant bacteria at a tertiary-care teaching hospital in Mexico: Special focus on Acinetobacter baumannii. Chemotherapy. 56:275–279 [DOI] [PubMed] [Google Scholar]
- 34. Morfín-Otero R., Alcántar-Curiel M.D, Rocha M.J, Alpuche-Aranda C.M, Santos-Preciado J.I, Gayosso-Vázquez C., Araiza-Navarro J.R, Flores-Vaca M., Esparza-Ahumada S., González-Díaz E., Pérez-Gómez H.R, and Rodríguez-Noriega E. 2013. Acinetobacter baumannii infections in a tertiary care hospital in mexico over the past 13 years. Chemotherapy. 59:57–65 [DOI] [PubMed] [Google Scholar]
- 35. Figueiredo S., Bonnin R.A, Poirel L., Duranteau J., and Nordmann P. 2012. Identification of the naturally occurring genes encoding carbapenem-hydrolysing oxacillinases from Acinetobacter haemolyticus, Acinetobacter johnsonii, and Acinetobacter calcoaceticus. Clin. Microbiol. Infect. 18:907–913 [DOI] [PubMed] [Google Scholar]
- 36. Doi Y., Wachino J.-I, Yamane K., Shibata N., Yagi T., Shibayama K., Kato H., and Arakawa Y. 2004. Spread of novel aminoglycoside resistance gene aac(6’)-Iad among Acinetobacter clinical isolates in Japan. Antimicrob. Agents Chemother. 48:2075–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lambert T., Gerbaud G., Galimand M., and Courvalin P. 1993. Characterization of Acinetobacter haemolyticus aac(6’)-Ig gene encoding an aminoglycoside 6’-N-acetyltransferase which modifies amikacin. Antimicrob. Agents Chemother. 37:2093–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fu Y., Du X., Ji J., Chen Y., Jiang Y., and Yu Y. 2012. Epidemiological characteristics and genetic structure of blaNDM-1 in non-baumannii Acinetobacter spp. in China. J. Antimicrob. Chemother. 67:2114–2122 [DOI] [PubMed] [Google Scholar]
- 39. Chu Y.W., Chau S.L, and Houang E.T.S. 2006. Presence of active efflux systems AdeABC, AdeDE and AdeXYZ in different Acinetobacter genomic DNA groups. J. Med. Microbiol. 55:477–478 [DOI] [PubMed] [Google Scholar]
- 40. Gholami M., Hashemi A., Hakemi-Vala M., Goudarzi H., and Hallajzadeh M. 2015. Efflux pump inhibitor phenylalanine-arginine B-naphthylamide effect on the minimum inhibitory concentration of imipenem in Acinetobacter baumannii strains isolated from hospitalized patients in Shahid Motahari Burn Hospital, Tehran, Iran. Jundishapur. J. Microbiol. 8:e19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wieczorek P., Sacha P., Hauschild T., Zórawski M., Krawczyk M., and Tryniszewska E. 2008. Multidrug resistant Acinetobacter baumannii—the role of AdeABC (RND family) efflux pump in resistance to antibiotics. Folia Histochem. Cytobiol. 46:257–267 [DOI] [PubMed] [Google Scholar]
- 42. Damier-Piolle L., Magnet S., Brémont S., Lambert T., and Courvalin P. 2008. AdeIJK, a resistance-nodulation-cell division pump effluxing multiple antibiotics in Acinetobacter baumannii. Antimicrob. Agents Chemother. 52:557–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Coyne S., Guigon G., Courvalin P., and Périchon B. 2010. Screening and quantification of the expression of antibiotic resistance genes in Acinetobacter baumannii with a microarray. Antimicrob. Agents Chemother. 54:333–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Al Atrouni A., Joly-Guillou M.-L, Hamze M., and Kempf M. 2016. Reservoirs of Non-baumannii Acinetobacter Species. Front. Microbiol. 7:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nordmann P., Poirel L., Walsh T.R, and Livermore D.M. 2011. The emerging NDM carbapenemases. Trends Microbiol. 19:588–595 [DOI] [PubMed] [Google Scholar]
- 46. Toleman M.A., Bennett P.M, and Walsh T.R. 2006. ISCR elements: novel gene-capturing systems of the 21st century? Microbiol. Mol. Biol. Rev. 70:296–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Toleman M.A., and Walsh T.R. 2010. ISCR elements are key players in IncA/C plasmid evolution. Antimicrob. Agents Chemother. 54:3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Montaña S., Cittadini R., Del Castillo M., Uong S., Lazzaro T., Almuzara M., Barberis C., Vay C., and Ramírez M.S. 2016. Presence of New Delhi metallo-β-lactamase gene (NDM-1) in a clinical isolate of Acinetobacter junii in Argentina. New Microbes New Infect. 11:43–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Barrios H., Garza-Ramos U., Reyna-Flores F., Sanchez-Perez A., Rojas-Moreno T., Garza-Gonzalez E., Llaca-Diaz J.M, Camacho-Ortiz A., Guzman-Lopez S., and Silva-Sanchez J. 2013. Isolation of carbapenem-resistant NDM-1-positive Providencia rettgeri in Mexico. J. Antimicrob. Chemother. 68:1934–1936 [DOI] [PubMed] [Google Scholar]