Abstract
Background
Fibrin-associated diffuse large B-cell lymphoma (FA-DLBCL) is a rare Epstein-Barr virus (EBV) positive lymphoproliferative disorder included in the current World Health Organization (WHO) classification. It arises within fibrinous material in the context of hematomas, pseudocysts, cardiac myxoma or in relation with prosthetic devices. In these clinical settings the diagnosis requires an high index of suspicion, because it does not form a mass itself, being composed of small foci of neoplastic cells. Despite overlapping features with diffuse large B-cell lymphoma associated with chronic inflammation, it deserves a separate classification, being not mass-forming and often following an indolent course.
Case presentation
A 64-year-old immunocompetent woman required medical care for cerebral hemorrhage. Computed Tomography (CT) angiography identified an aneurysm in the left middle cerebral artery. A FA-DLBCL was incidentally identified within thrombotic material in the context of the arterial aneurysm. After surgical removal, it followed a benign course with no further treatment.
Conclusions
The current case represents the first report of FA-DLBCL identified in a cerebral artery aneurysm, expanding the clinicopathologic spectrum of this rare entity. A complete literature review is additionally made.
Keywords: Fibrin, B-cell, Lymphoma, Epstein-Barr virus
Background
In the current WHO classification, diffuse large B-cell lymphoma associated with chronic inflammation (DLBCL-CI) is defined as an EBV-driven neoplasm, occurring in longstanding chronic inflammation in restricted spaces [1]. The prototype is pyothorax-associated lymphoma (PAL) arising in patients with a long history of pyothorax, following artificial pneumothorax as treatment for tuberculosis [1]. Recently, another EBV-related entity has been included among DLBCL-CI, but renamed fibrin-associated diffuse large B-cell lymphoma (FA-DLBCL) because it develops within fibrinous material [1].
It has been reported in association with pseudocysts, cardiac myxoma, valve prosthesis, fibrin thrombus, synthetic tube graft, hydrocele, metallic implants, and chronic subdural hematoma [1–25]. Differently from PAL, it does not form masses, being composed of rare neoplastic cells and it represents often an incidental finding [1]. Whereas PAL follows an aggressive course, the majority of FA-DLBCL behave favorably and may not require therapies other than surgery. Rare cases with persistent or localized recurrent disease have been described [9]. Only one case with a poor outcome has been reported so far [24]. We present the first report of FA-DLBCL incidentally disclosed in a cerebral artery aneurysm, widening the clinicopathological spectrum of this rare entity.
Case presentation
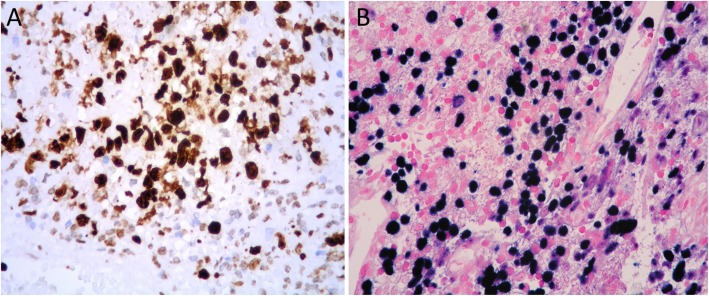
A 64-year-old immunocompetent woman was referred to hospital for cerebral hemorrhage in left temporal-parietal region. CT angiography detected an aneurysm in the distal segment of left middle cerebral artery. Tiny fragments of brain tissue together with partially organized thrombus were surgically removed. Histologically, it was identified an artery, with an interrupted wall, occluded by thrombotic material (Fig. 1). Small foci of large atypical lymphoid cells (Fig. 1, inset; Fig. 2) were disclosed within thrombus. The cells were positive for PAX5 (Fig. 2, inset left), CD30 and MUM1 (Fig. 2, inset right) with partial expression of CD79α and CD20. The proliferative index (Fig. 3 a) was high (Ki67 about 90%). The cells expressed LMP-1 and were diffusely positive for EBV by in situ hybridization for EBV-encoded RNA (EBER) (Fig. 3, b). Clonal immunoglobulin heavy chain (IGH) rearrangement was detected. A fibrin-associated diffuse large B-cell lymphoma was diagnosed. Staging procedures (CT scan and bone marrow biopsy) were negative. Three months later, CT scan showed an almost complete hemorrhage resorption. No further treatment was given. The patient is alive, free of disease at 8 months from diagnosis.
Fig. 1.
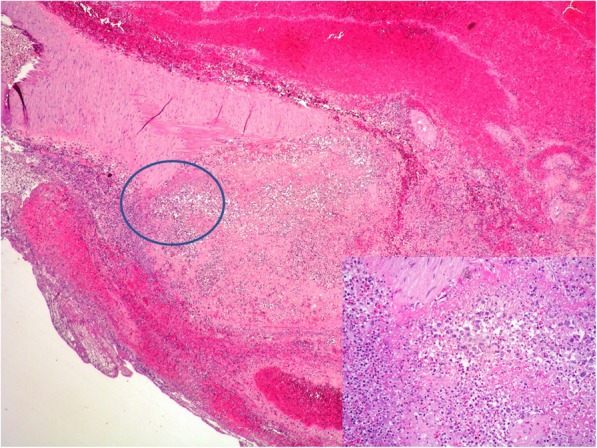
Low power view of artery with interrupted wall and containing thrombotic material (HE 4x); inset Rare atypical lymphoid cells lying within the thrombus are recognizable at high power view (HE 20x)
Fig. 2.
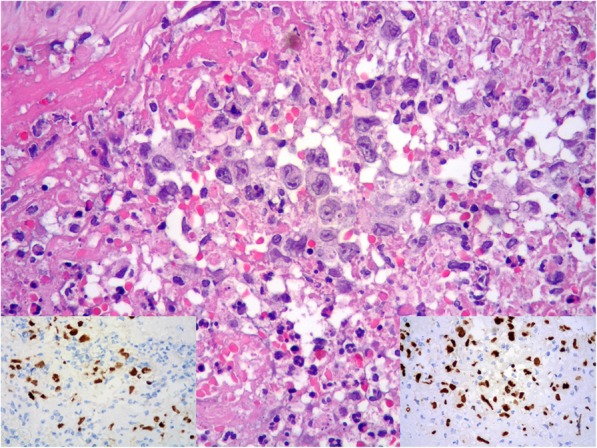
High power detail of large lymphoid cells (HE 40x); inset left PAX5 positivity of lymphoid cells; inset right MUM1 expression of lymphoid cells
Fig. 3.
High proliferative index (Ki67) (a); Epstein-Barr virus positivity in large-sized cells by in situ hybridization for EBV-encoded RNA (EBER) (b)
Discussion and conclusions
FA-DLBCL is a rare EBV-associated B-cell lymphoma included in the current WHO classification, in the chapter of DLBCL-CI [1]. Differently from DLBCL-CI, it is not mass-forming and therefore disclosed incidentally on histological evaluation of surgical specimens removed for other diseases [1]. Forty seven cases, including our, have been reported so far [1–25]. Clinicopathological data are summarized in Table 1. It shows male predominance with a wide age range. No ethnic differences have been apparently identified so far [9]. All cases, except 2 [9], occurred in immunocompetent individuals, presenting with different symptoms, depending on the underlying conditions in which FA-DLBCL occurred.
Table 1.
Demographic data, clinical data, and characteristics of reported cases of Fibrin-Associated Diffuse Large B-Cell Lymphoma
| SITE/REF. | AGE SEX | Immunosupp | CLINICAL FEATURES | HISTOLOGY | IIC/EBV/CLONALITY | STAGING THERAPY | FOLLOW-UP |
|---|---|---|---|---|---|---|---|
| Atrial myxoma Bagwan 2009 (ref [2]) | 81/M | negative | Multiple cerebral strokes | Foci of large lymphoid cells at myxoma surface | CD20+, CD79α+, CD10+, BCL6+, BCL2+, CD3-. Ki67:80% EBV: NV. Ig clonality NP. | NS Staging: neg; BM: neg. Surgery+ R-CHOP | NA |
| Atrial myxoma Dimitrova 2010 (ref [3]) | 51/M | negative | Acute obstructive left heart failure | Foci of large lymphoid cells at myxoma surface | CD20+, CD10+. Ki67 high EBV: NV. Ig clonality NP. | Imaging/BM Staging: neg. Surgery+ CHOP (VI) | NA |
| Atrial myxoma Loong 2010 (ref [4]) | 70/F | negative | Ischemic stroke | Foci of large lymphoid cells | CD20+, CD79α+, PAX5+, CD43+, MUM1+, CD10-, BCL6+, BCL2+, CD30+, CD138-, HHV8-, CD3-. Ki67 100%. LMP1+, EBNA2+, EBER+. Ig clonality +. | CT/BM Staging: neg. Surgery + R-CEOP (IV) | Died for CH complications (neutropenia+ pneumonia) at 5 mo. No autopsy |
| Atrial myxoma Svec 2012 (ref [5]) | 60/F | negative | Embolic brain stroke | Foci of large lymphoid cells | CD20+, CD79α+, PAX5+, CD10-MUM1+, CD23+, BCL2+, BCL6-, CD5-, CD3-, cyclin D1-, CD138-, CD38-; Ki67: 100%. LMP1+, EBER+, EBNA2+. FISH MYC, BCL2, BCL6 -. Ig clonality NP. | CT/PET/BM Staging: neg. Surgery+ R-CHOP (VI) | NED at 7 mo |
| Atrial myxoma Bartoloni 2013 (ref [6]) | 55/F | negative | Fatigue, fever | Foci of large lymphoid cells at myxoma surface | LCA+, CD20+, CD79α+, MUM1+, HHV8-, CD3-, CD5-. Ki67: 90%. LMP1+, EBNA2-, EBER+ Ig clonality NP. | CT/BM staging: neg. Surgery only | NED at 72 mo |
| Atrial myxoma Aguilar 2015 (ref [7]) | 52/M | negative | Dysarthria and hemiplegia | Foci of large lymphoid cells | CD20+, CD79α+, PAX5+, CD30+, MUM1+, ALK-1-, CD10-CD43-, cyclinD1-, CD3-, LMP1+, EBNA2+, EBER+. Ig clonality +. | CT/BM staging: neg. Surgery only | NED at 42 mo |
| Atrial myxoma Tapan 2015 (ref [8]) | 49/M | negative | Palpitations | Foci of large lymphoid cells | CD20+, CD79α+, CD30+, MUM1+, CD3-, CD5-, CD10-, CD138-, cyclin D1-, ALK1-, EMA-. Ki67 80%. EBNA2+, EBER+. Ig clonality NP. | NS Staging: neg; BM neg. Surgery + R-CHOP | NED at 12 mo |
| Atrial myxoma Boyer 2017 (ref [9]) | 54/F | negative | Syncope | Foci of large lymphoid cells | CD20+, PAX5+, CD79α+, BCL6+, CD30+, CD10-, CD138- CD3-, HHV8-, Ki67 80%. EBER+. Ig clonality NP. | NS Staging: neg. Surgery/ Other therapy: NA | NED at 130 mo |
| Atrial myxoma Boyer 2017 (ref [9]) | 55/F | negative | Syncope, cough, dyspnea | Foci of large lymphoid cells | CD20+, PAX5+, CD79α+, BCL6+, MUM1+, CD10-, CD45+, CD30+, HHV-8-, CD138-, CD3-. Ki67: > 95%. EBER+, LMP1+, EBNA2+. FISH for MYC-. Ig clonality NP. | NS Staging: neg. Surgery only | Died at 2 mo for cardiac cause. Autopsy: No lymphoma |
| Atrial myxoma Boyer 2017 (ref [9]) | 54/M | negative | Dyspnea, respiratory failure | Foci of large lymphoid cells | CD20+, PAX5+, BCL6+, MUM1+, CD10-, CD38+, CD45+, CD30+, CD3-. Ki67:90% EBER+, LMP1+, EBNA2+. FISH MYC, BCL6, BCL2 -. Ig clonality NP. | CT/BM Staging: neg. Surgery only | Recurrent FA-DLBCL at mitral valve after 25 mo. Died at 26 mo (embolic stroke). No autopsy. |
| Atrial myxoma Yan 2017 (ref [9]) | 54/M | negative | Congestive heart failure | Foci of large lymphoid cells within fibrin | CD20+, CD79α+, MUM1+, CD10-, BCL6+, CD30+. ALK-, BCL2+, CD3-, CD5-, Ki67 90% LMP1+, EBNA-2+, EBER+, FISH for MYC, Bcl6, BCL2-. Ig clonality NP. | CT/BM Staging: neg. Surgery only | NED at 7 MO |
| Atrial myxoma Yan 2017 (ref [10]) | 61/F | negative | Congestive heart failure | Foci of large lymphoid cells within fibrin | CD20+, CD79α+, MUM1+, CD10+, BCL6+, CD30+. ALK-, BCL2+, CD3-, CD5-, Ki67 95% LMP1+, EBNA-2+, EBER+, FISH for MYC, Bcl6, BCL2-. Ig clonality NP. | CT/BM Staging: neg. Surgery only | NED at 84 mo |
| Atrial myxoma Yan 2017 (ref [10]) | 46/F | negative | Congestive heart failure | Foci of large lymphoid cells within fibrin | CD20+, CD79α+, MUM1+, CD10-, BCL6+, CD30+. ALK-, BCL2+, CD3-, CD5-, Ki67 90% LMP1+, EBNA-2+, EBER+, FISH for MYC, Bcl6, BCL2-. Ig clonality NP. | CT/BM Staging: neg. Surgery only | NED at 3 mo |
| Atrial myxoma Yan 2017 (ref [10]) | 46/F | negative | Congestive heart failure | Foci of large lymphoid cells within fibrin | CD20+, CD79α+, MUM1+, CD10-, BCL6-, CD30+. ALK-, BCL2-, CD3-, CD5-, Ki67 85% LMP1+, EBNA-2+, EBER+, FISH for MYC, Bcl6, BCL2-. Ig clonality NP. | CT/BM Staging: neg. Surgery only | NED at 120 mo |
| Atrial thrombus Qigley 2003 (ref [11]) | 29/M | negative | Cerebral embolic stroke | Foci of large lymphoid cells at clot’s surface | CD45+, CD20+, CD79α+, CD43+, CD30+, CD3, LMP -, HHV8-, EBER+. Clonality:κ rearrangement +. IGH -, TCR-. | Imaging/BM Staging: neg. Surgery+ R-CHOP (VI) | NED at 24 mo |
| Atrial thrombus Gruver 2012 (ref [12]) | 56/M | negative | Short breath | Foci of large lymphoid cells within fibrin thrombus | CD20+, CD79α+, PAX5+, CD30+, CD43-, CD45+, BCL6+, MUM1+, BCL2+, CD10-, CD3-, CD5-, HHV8-, MYC + 30%; KI67 > 90% LMP1+, EBNA2 + .EBER+. Ig clonality +. | NS Staging: neg. Surgery+ R-CHOP (VI) | NED at 8 mo |
| Myxomatous mitral valve Gruver 2012 (ref [12]) | 75/M | negative | Dyspnea, aortic insufficiency, mitral valve regurgitation | Foci of large lymphoid cells within fibrin on mitral valve | CD20+, CD79α+, PAX5+, CD30-, CD43-, CD45+, BCL6-, MUM1+, BCL2+, CD10-, CD3-, CD5-, HHV8-, MYC -. KI67100%. LMP1-, EBNA2-. EBER-. Ig clonality +. | NS Staging: neg. Surgery+R-CVP (I) + R-CHOP (VI) | NED at 39 mo |
| Prosthesis (knee) Cheuk 2005 (ref [13]) | 78/M | negative | Pain at knee prosthesis (implanted 22 yrs. before) | Foci of large lymphoid cells within fibrin and necrosis | CD20+, CD79α+, CD138+/−. CD2-, CD3-, CD5-, CD10-, BCL6-, HHV8-. Ki67:70%. LMP1+, EBER+. Ig clonality +. | NS Staging: neg. Surgery+RT | NED at 24 mo |
| Prosthesis (aortic valve) Bagwan 2009 (ref [2]) | 50/M | negative | Symptoms of aortic regurgitation. Aortic valve prosthesis (16 yrs. before) | Foci of large lymphoid cells within aortic valve leaflets | CD45+, CD20+, CD79α+, CD10+, BC6+/−, BCL2+/−, Ki67:80% LMP1-. Ig clonality: NP. | NS Staging: neg; BM: neg. Surgery+ R-CHOP | Died after 6mo for prosthesis rupture. Autopsy: no lymphoma |
| Prosthesis (aortic valve) Berrio 2010 (ref [14]) | 60/M | negative | Acute left heart failure. History of aortic valve prosthesis for stenosis | Foci of large lymphoid cells within valve vegetations | CD20+, CD43+, CD3-. Ki67:80–90% EBV: NV. Ig clonality: NP. | NS Staging: neg. Surgery only | Died for tricuspidal endocarditis, pneumonia 2 yrs. later. No autopsy. |
| Prosthesis (aortic graft) Miller 2010 (ref [15]) | 48/M | negative | Ischemic attack. Marfan sy. Asc.a. aneurysm graft+ aortic valve prosthesis (24 yrs. before) | Foci of large lymphoid cells within fibrin | CD20+, MUM1+, CD10-, BCL6- BCL2+, CD3-, HHV8-. EBER+. Ig clonality +. | CT/PET/BM Staging: neg. Surgery only | NED at 6 mo |
| Prosthesis (aortic valve) Miller 2010 (ref [15]) | 80/F | negative | Heart failure. Aortic valve prosthesis (8 yrs. before) | Foci of large lymphoid cells within fibrin | CD20+, MUM1+, CD10-, BCL6-BCL2-, CD3-, HHV8-, EBER+. Ig clonality +. | CT/PET/BM Staging: neg. Surgery only | Died (for breast cancer 18 mo after aortic valve surgery). No autopsy. |
| Prosthesis (aortic graft) Miller 2010 (ref [15]) | 79/F | negative | Short breath, thoracic pulsing sensation. Tube graft for asc. a. dissection (5 yrs. before) | Foci of large lymphoid cells within fibrin | CD20+, MUM1+, CD10-, BCL6+, BCL2+, CD3-, HHV8-, EBER+, Ig clonality +. | CT/PET/BM Staging: neg. Surgery only | Died for surgical complications. No autopsy |
| Prosthesis (aortic graft) Gruver 2012 (ref [12]) | 55/M | negative | Stroke. Aortic graft for aneurysm (4 yrs. before) | Foci of large lymphoid cells within thrombus | CD20+, CD79α+, PAX5+, CD30+, CD43+, CD45+, BCL6+, MUM1+, BCL2-, CD10-, CD3-, CD5-, HHV8-, MYC-; KI67 100%. LMP1+, EBNA2 + .EBER+. Ig clonality +. | NS Staging: neg. Surgery + R-CEOP (VIII) | NED at 16 mo |
| Prosthesis (vascular graft) Boyer 2017 (ref [9]) | 56/M | negative | IR aorta+ CIA aneurysms. TAA aneurysm graft + thrombectomy (1 yr. before). Asc a. dissection graft (9 yrs. before). | Foci of large lymphoid cells within thrombus of IR aorta and CIA aneurysms. In retrospect foci within thrombus of TAA aneurysm | CD20+, PAX5+, BCL6-, MUM1+, CD10-, CD138-, HHV8-, CD30+, KI67: 95%. EBER+, LMP1+, EBNA2+. FISH for MYC -. Ig clonality +. | CT/PET/BM Staging: neg. Surgery+ R-CHOP (VI) + IT MTX | AWSD at 24 mo. Surgical revision of aortic graft: persistent foci of EBV+ large B cell. |
| Prosthesis (vascular graft) Boyer 2017 (ref [9]) | 68/M | negative | Lower limbs ischemia. AA aneurysm repair with IR graft (7 yrs. before). | Foci of large lymphoid cells within thrombus | CD20+, PAX5+, BCL6+, CD10-MUM1+, CD30+, HHV8-, KI67 90%, EBER+, LMP1+, EBNA2+. FISH for MYC -. Ig clonality NP. | CT/PET Staging neg. 3 mo after: PET/CT/biopsy: foci of EBV+ cells near adrenal gland. R-COEP (II) | Died at 10 mo for embolic stroke. No progressive lymphoma. No autopsy |
| Prosthesis (vascular) Boyer 2017 (ref [9]) | 71/M | MG for THY treated with surgery+ steroids+ AZA | AF graft (6 yrs. before). | Foci of large lymphoid cells within thrombus associated with graft | CD20+, CD79α+, PAX5+, CD10-BCL6+, MUM1+, CD30+, CD45+, CD138-, HHV8-, KI67 > 95%, EBER+, LMP1+. Ig clonality +. | NS Staging: neg. Surgery only | NED at 10 mo |
| Pseudocyst (kidney) Lee 2009 (ref [16]) | 61/M | negative | Renal cyst (for 20 yrs) | Foci of large lymphoid cells within necrosis | CD22+, CD45+, CD79α+, MUM1+, PAX5+, CD3-, CD10-, CD20-, CD138-, BCL6-, ALK1-, HHV8-, κ-, λ-, EBER+. Ig clonality NP. | Staging NA. Surgery+ CHOP (VI) | NA |
| Pseudocyst (spleen) Loong 2010 (ref [4]) | 29/M | negative | Abdominal pain | Foci of large lymphoid cells within necrosis | CD20+, CD79α+, PAX5+, CD43+, MUM1+, CD10-, BCL6-CD138-, BCL2+, CD30-, HHV8-, CD3-. Ki67 90%. LMP1+, EBNA2+, EBER+. Ig clonality +. | PET/BM Staging: neg. Surgery (splenectomy) + R (IV) | NED at 6 mo |
| Pseudocyst (kidney) Valli 2011 (ref [17]) | 46/M | negative | Left-sided flank pain | Foci of large lymphoid cells within necrosis | CD20+, MUM1+, CD10-, BCL6-BCL2+, CD30-, HHV8-;Ki67:90%. EBER+. Ig clonality NP. | CT/PET/BM Staging: neg. Surgery+ R-CHOP (VI) | NED at 1 mo |
| Pseudocyst (adrenal gland) Boroumand 2012 (ref [18]) | 63/F | negative | Right abdominal pain | Foci of large lymphoid cells within fibrin | CD20+, CD79α+, PAX5+, MUM1+, BCL2+, CD3-, CD10-, CD30-, BCL6-, HHV8-. Ki67 > 90%. LMP1-; EBER+. Ig clonality NP. | NS Staging: neg. Surgery + R-CHOP (VI) + RT | NED at 40 mo |
| Pseudocyst (testis) Boroumand 2012 (ref [18]) | 27/M | negative | R. scrotal swelling. Herniorraphy followed by l. scrotal hematoma (removed 3 yrs. before) | Foci of large lymphoid cells within fibrin | CD20+, CD79α+, CD30+, MUM1+, BCL2+, CD3-, CD10- BCL6-, HHV8-. Ki67 > 90%. LMP1+, EBER+. Ig clonality NP. | NS Staging: neg. Surgery only | NED at 9 mo |
| Pseudocyst (spleen) Boyer 2017 (ref [9]) | 37/F | negative | Splenic mass (9 cm), incidentally found | Foci of large lymphoid cells within fibrin | CD20+, PAX5+, MUM1+, CD10-BCL6-, CD30-, CD45+, KI67 > 90% EBER+. Ig clonality NP. | CT/PET/BM Staging: neg. Surgery + R-CHOP (III) | NED at 32 mo |
| Pseudocyst (retrop.) Boyer 2017 (ref [9]) | 73/M | negative | Femoral a. aneurysm repair | Foci of large lymphoid cells within fibrin | CD20+, PAX5+, CD79α+, BCL6-, CD10-, MUM1+, CD30+, CD45+, HHV8-, KI67 > 95%, EBER+. Ig clonality NP. | CT/BM Staging: neg. Surgery+ R-CHOP (VI) | NED at 43 mo |
| Pseudocyst (adrenal gland) Boyer 2017 (ref [9]) | 70/M | negative | Adrenal mass (7 cm) causing bladder obstruction | Foci of large lymphoid cells within fibrin | CD20-, PAX5+, CD79α+, BCL6-, CD10-, MUM1+, CD45+, CD30+, CD138-, HHV8-, KI67 > 90%, LMP1-, EBNA2+, EBER+. FISH for MYC -. Ig clonality NP. | CT/PET Staging: neg. Surgery only | NED at 14 mo |
| Pseudocyst (retrop.) Boyer 2017 (ref [9]) | 44/M | negative | Right flank pain | Foci of large lymphoid cells within fibrin | CD20+, PAX5+, CD10-, BCL6-, MUM1+, CD45+, CD30-, KI67 40%, LMP1+, EBNA2+, EBER+, FISH for MYC -. Ig clonality +. | BM/imaging Staging: neg. 5-CHOP | NED at 84 mo |
| Pseudocyst (adrenal gland) Zanelli 2019 (ref [19]) | 71/F | negative | Lower limbs edema+ abdominal distension | Foci of large lymphoid cells within fibrin | CD20+, PAX5+, CD30+, MUM1+, CD10-, BCL6-, EBER+, Ki67 90%. Ig clonality NP. | CT Staging: neg. Surgery only | NED at 6 mo |
| Teratoma (ovary) Valli 2014 (ref [20]) | 56/F | negative | Abdominal pain+ swelling | Foci of large lymphoid cells | CD20+, MUM1+, CD45+, PAX5+, CD30-, BCL6-, CD10-, CD3-, CD2-, HHV8-, CD138-. Ki67: 80%. EBER+. Ig clonality +. | CT/PET Staging: neg. Surgery+ R-CHOP (VI) | NED at 8 mo |
| Hydrocele (testis) Loong 2010 (ref [4]) | 88/M | negative | Fever, scrotal pain, swelling | Foci of large lymphoid cells within necrosis | CD20+, CD79α+, PAX5+, MUM1+, CD10-, BCL6-, CD138-, BCL2+, CD30-, HHV8-, CD3+, CD2-, CD5-, CD7-. Ki67 70% LMP1+, EBNA2+, EBER+. Ig clonality -. | Staging NA. Surgery only (Orchidectomy) | NA |
| Hematoma (testis) Boyer 2017 (ref [9]) | 79/M | negative | Testicular trauma (5 yrs. before) | Foci of large lymphoid cells within hematoma | CD20+, PAX5+, CD79α+, CD10-CD138-, BCL6-, MUM1+, CD45+, CD30+, HHV8-, KI67 > 90%, EBER+, LMP1+, EBNA2+. Ig clonality +. | NS Staging: neg. Surgery only | NED. Died at 17 mo |
| Hematoma (thigh) Hayes 2014 (ref [21]) | 91/M | negative | Thigh hematoma. (6 yrs. before leg amputation for popl. a. aneurysm rupture at prior artery bypass graft site) | Foci of large lymphoid cells | CD45+, CD20+, MUM1+, CD30+, CD43+, BCL2+/−, MYC+, p53+/−, HHV8-, CD3-, CD5-, CD10-, BCL1-, BCL6-. Ki67: 90%. LMP1-, EBER+. Ig clonality NP. | NS Staging: neg. Surgery only | NED at 18 mo |
| Subdural hematoma Reyes 1990 (ref [22]) | 56/M | negative | Headaches, dizziness, unsteady gait | Foci of large lymphoid cells within fibrin, clots, necrosis | B-cell phenotype. EBV NV. Ig clonality NP. | CT/BM Staging: neg. Surgery only | NA |
| Subdural hematoma Sugita 2012 (ref [23]) | 77/M | negative | Dementia due to head trauma (20 yrs. before) | Foci of large lymphoid cells | CD20+, CD79α+, MUM1+, CD3-, BCL6-, CD10-. Ki67 high. EBNA2+, LMP1-, EBER+. Ig clonality - (rare neoplastic foci). | Imaging Staging: neg. Surgery only | NA |
| Subdural hematoma Kameda 2015 (ref [24]) | 96/M | negative | Gait disturbs+ anorexia. Trauma+ subdural hematoma (7 mo before). | Brain mass: DLBCL EBV+. Subdural hematoma: FA-DLBCL. No continuity among 2 lesions | CD20+, CD79α+, CD3-, CD4-, CD7-, CD8-, LMP1+, EBNA2+, EBER+. Ig clonality NP. | CT Staging: neg at presentation. Brain mass + subdural hematoma resection. IT MTX + cytarabine+ glucocorticoids | Died after 3 mo for lymphoma dissemination. No autopsy |
| Subdural hematoma Boyer 2017 (ref [9]) | 25/M | negative | SD hematoma since child. Hydrocephalus+ SD catheter. Steroid tp for pituitary overactivity | Foci of large lymphoid cells within hematoma | CD20+, PAX5+, MUM1+, CD10-BCL6-, CD30+, HHV8-, KI67 > 90%; EBER+, LMP1+. Ig clonality NP. | CT/PET/BM Staging: neg. Surgery only | NED at 7 mo |
| Arachnoid cyst Kirshenbaum 2017 (ref [25]) | 81/M | negative | Tremor, gait ataxia, memory disturbs | Foci of large lymphoid cells within fibrin | CD20+ CD30+, BCL2+, MUM1+, BCL6+/−, CD10-, TdT-, CD5-, cMYC+ (50%) Ki67: > 80%. EBER+. FISH MYC -. Ig clonality +. | CT/PET staging: neg. Surgery (cyst excision) + R-lenalidomide | NA |
| Cerebral artery aneurysm Present case | 64/F | negative | Cerebral hemorrhage. Left middle cerebral artery aneurysm | Foci of large lymphoid cells within fibrin | CD20+/−, PAX5+, CD79α+, CD30+, MUM1+, CD10-, BCL6-, EBER+, Ki67 90%. Ig clonality NP. | CT/BM Staging: neg. Surgery only | NED at 5 mo |
Literature review of fibrin-associated diffuse large B-Cell Lymphoma
A artery, AA abdominal aorta, AF, aortofemoral, Asc. A ascending aorta, AWSD alive with stable disease, AZA azathioprine, BM bone marrow, CEOP cyclophosphamide, etoposide, oncovin, prednisone, CHOP cyclophosphamide, doxorubicin, vincristine, prednisone, retro retroperitoneum, CIA common iliac arteries, CH chemotherapy, CVP cyclophosphamide, vincristine, prednisone, CT Computerized tomography, DEXA dexamethasone, F female, IT intrathecal, IR infrarenal, Ig immunoglobulin, IGH immunoglobulin heavy chain, mo months, M male, MTX methotrexate, MG myasthenia gravis, NA not available, NED not evidence of disease, Neg negative, NP not performed, NS not specified, PBL plasmablastic lymphoma, popl. A popliteal artery, R rituximab, Retrop retroperitoneum, RT radiotherapy, SD subdural, sy syndrome, TAA thoracoabdominal aorta, TCR T cell receptor, THY Thymoma, Tp therapy, yrs. years
Cardiac myxoma represents one of the most frequent site of occurrence with 14 cases identified, whereas only occasional cases arose in atrial thrombi and within mixomatous valve degeneration. Some cases have been identified in association with prosthetic devices such as endovascular graft, cardiac valve prosthesis and metallic implant. Time from placement of devices to lymphoma diagnosis is extremely variable, ranging from 1 to more than 20 years. A rather frequent site of presentation is represented by pseudocysts, with a total of 10 cases, in different organs (adrenal gland, spleen, kidney, retroperitoneum, testis). Single descriptions at unusual sites as within testicular hydrocele, ovarian teratoma and testicular hematoma are also reported. The intracranial location appears to be rare, with only 4 cases within chronic subdural hematomas [9, 22–24] and 1 within an arachnoid cyst [25]. Our case represents the first report in a patient with a brain hemorrhage and incidentally identified within thrombotic material in a cerebral artery aneurysm. Notably in all cases evaluated (45/47) staging workup at diagnosis revealed no other sites of disease.
Histologically all cases were remarkably similar and found incidentally, being composed of microscopic foci of large lymphoid cells, embedded within fibrin and not invading adjacent tissue structures. Most cases had a non-germinal center B-cell phenotype and high proliferative index. A strong association with EBV infection is present; as 41/43 evaluated were positive for EBV by EBER-ISH. Notably a type III EBV latency profile, with positivity for LMP-1 and Epstein-Barr nuclear antigen-2 (EBNA-2) was found in most cases (18/22 tested). Type III latency of EBV infection is the hallmark of lymphoproliferative disorders arising in the setting of severe immunosuppression. EBV-infected cells expressing EBNA-2 do not survive in immunocompetent individuals, because destroyed by cytotoxic T-lymphocytes. As patients with FA-DLBCL are immunocompetent, it has been assumed that the restricted environment where FA-DLBCL occurs, allows the EBV-infected B-cells to escape T-cell surveillance [9]. Clonal immunoglobulin rearrangement was identified in most cases evaluated. None of the cases tested by fluorescence in situ hybridization (FISH) showed c-MYC, BCL6 and/or BCL2 rearrangements or amplifications: a rather striking difference from PAL, presenting MYC amplification in 80% of cases [9]. Clinical course of FA-DLBCL is commonly indolent. Remarkably of 36 cases with available follow-up, 30 pursued a benign course, with no evidence of disease from 1 to 130 months. Treatment is variable, although surgery alone often represents the treatment of choice. Sixteen/30 cases were treated with surgery alone, 11 with surgery plus chemotherapy, 1 with surgery plus radiotherapy, 1 with surgery plus immunotherapy, and 1 with surgery plus chemotherapy and radiotherapy. All cases arising within pseudocysts behaved favorably. Local recurrences or persistent disease were seen only in isolated cases in which the primary disease had arisen either within an atrial myxoma (1) or at sites of previous vascular graft (2) [9]. The recurrent or persistent disease presented close to the site initially involved. Two/3 patients died of thromboembolic disease and 1 is alive with stable and localized disease. It has been hypothesized that FA-DLBCL arising at cardiac or vascular sites can recur or persist more easily than cases occurring in sites more amenable to complete surgical removal [9]. Kameda et al reported the unique case with an aggressive course, occurring in an elderly patient within a chronic subdural hematoma observed conservatively [1, 24]. Seven months later, a de novo brain mass developed beneath the hematoma [24]. After surgical removal, the neoplasm within the subdural hematoma appeared consistent with FA-DLBCL and the brain mass was an EBV-positive DLBCL [24]. The authors hypothesized that the lymphoid process developed in the hematoma before infiltrating the brain parenchyma [24]. Once the lymphoma infiltrates outside the subdural hematoma, the prognosis becomes poor [1]. FA-DLBCL shares similarities with breast implant-associated anaplastic large B-cell lymphoma (BIA-ALCL), although the latter is a T/null lymphoma, not EBV-related [1]. Both entities portend a worse prognosis, when infiltrate the surrounding tissues outside the restricted space of origin.
Our case arose in a previously unreported setting, being identified in a cerebral artery aneurysm of a patient with a brain hemorrhage. The disease was totally confined within thrombotic material occluding the artery. After surgical removal, it pursued a benign course with no additional treatment.
In conclusion, FA-DLBCL is a rare EBV-related lymphoproliferative disorder, arising within fibrinous material in different clinical settings. Intracranial location is very rare. This represents the first report within a cerebral artery aneurysm. Diagnosis can be tricky, being FA-DLBCL not mass-forming and composed of tiny neoplastic foci. Clinical behavior is mostly indolent. The limited number of FA-DLBCL reported so far makes difficult to draw definitive conclusion regarding the best treatment. Further cases with longer follow-up would help to adopt the most appropriate therapeutic options for each individual patient.
Acknowledgements
None.
Abbreviations
- BIA-ALCL
Breast implant-associated anaplastic large B-cell lymphoma
- CT
Computed Tomography
- DLBCL-CI
Diffuse large B-cell lymphoma associated with chronic inflammation
- EBER
EBV-encoded RNA
- EBV
Epstein-Barr virus
- FA-DLBCL
Fibrin-associated diffuse large B-cell lymphoma
- PAL
Pyothorax-associated lymphoma
- WHO
World Health Organization
Authors’ contributions
ZaM wrote the manuscript and performed literature review; AS performed histopathological examination and designed the study; MM studied the patient; GV, MG, DL, FOG, MMP performed literature review; ZiM was involved in review, editing and validation of the manuscript. All authors have read and approved the manuscript.
Funding
The authors have no financial ties to disclose.
Availability of data and materials
All the original data supporting our research are described in the Case presentation section and in the figures’ legends.
Ethics approval and consent to participate
Local ethics committee (Comitato Etico dell’Area Vasta Emilia Nord, Italy) ruled that no formal ethics approval was required in this particular case.
Patient gave consent to participate.
Consent for publication
Written informed consent was obtained from patient for publication of this Case Report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Magda Zanelli, Email: Magda.Zanelli@ausl.re.it.
Maurizio Zizzo, Phone: +39-0522-296372, Email: zizzomaurizio@gmail.com.
Marco Montanaro, Email: mmontanaro51@gmail.com.
Vito Gomes, Email: semog@libero.it.
Giovanni Martino, Email: giovanni.martino@unipg.it.
Loredana De Marco, Email: Loredana.DeMarco@ausl.re.it.
Giulio Fraternali Orcioni, Email: fraternali.g@ospedale.cuneo.it.
Maria Paola Martelli, Email: maria.martelli@unipg.it.
Stefano Ascani, Email: s.ascani@aospterni.it.
References
- 1.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Arber DA, Hasserjian RP, Le Beau MM, Orazi A, Siebert R, editors. WHO classification of tumours of hematopoietic and lymphoid tissues. Lyon: IARC; 2017. [Google Scholar]
- 2.Bagwan IN, Desai S, Wotherspoon A, Sheppard MN. Usual presentation of primary cardiac lymphoma. Interact Cardiovasc Thorac Surg. 2009;9:127–129. doi: 10.1510/icvts.2009.204628. [DOI] [PubMed] [Google Scholar]
- 3.Dimitrova KR, Hoffman DM, Geller CM, Thiagarjah P, Master J, Berger M, Tranbaugh RF. Malignant B-cell lymphoma arising in a large, left atrial mass. Ann Thorac Surg. 2010;89:626–629. doi: 10.1016/j.athoracsur.2009.06.096. [DOI] [PubMed] [Google Scholar]
- 4.Loong F, Chan ACL, Ho BCS, Chau YP, Lee HY, Cheuk W, Yuen WK, Ng WS, Cheung HL, Chan JKC. Diffuse large B-cell lymphoma associated with chronic inflammation as an incidental finding and new clinical scenarios. Mod Pathol. 2010;23:493–501. doi: 10.1038/modpathol.2009.168. [DOI] [PubMed] [Google Scholar]
- 5.Svec A, Rangaiah M, Giles M, Jaksa R, Mc Aulay KA. EBV+ diffuse large B-cell lymphoma arising within atrial myxoma. An example of a distinct primary EBV+ DLBCL of immunocompetent patients. Pathol Res Pract. 2012;208:172–176. doi: 10.1016/j.prp.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Bartoloni G, Pucci A, Giorlandino A, Berretta M, Mignosa C, Italia F, Carbone A, Canzonieri V. Incidental Epstein-Barr virus associated atypical lymphoid proliferation arising in a left atrial myxoma: a case of long survival without any postsurgical treatment and review of the literature. Cardiovasc Pathol. 2013;22:5–10. doi: 10.1016/j.carpath.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Aguilar C, Beltran B, Quinones P, Carbajal T, Vilcapaza J, Yabar A, Segura P, Quintanilla-Martinez L, Miranda RN, Castillo JJ. Large B-cell lymphoma arising in cardiac myxoma or intracardiac fibrinous mass. A localized lymphoma usually associated with Epstein-Varr virus? Cardiovasc Pathol. 2015;24:60–64. doi: 10.1016/j.carpath.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Tapan U, Pestana JB, Lee JC, Lerner A. Epstein-Barr virus-associated diffuse large B-cell lymphoma arising in atrial myxoma: a proposal for a modified therapeutic approach. Leuk Lymphoma. 2015;56(2):505–507. doi: 10.3109/10428194.2014.919632. [DOI] [PubMed] [Google Scholar]
- 9.Boyer DF, McKelvie PA, de Leval L, Edlefsen KL, Ko YH, Aberman ZA, Kovach AE, Masih A, Nishino HT, Weiss LM, Meeker AK, Nardi V, Palisoc M, Shao L, Pittaluga S, Ferry JA, Lee Harris N, Sohani AR. Fibrin-associtaed EBV-positive large B-cell lymphoma. An indolent neoplasm with features distinct from diffuse large B-cell lymphoma associated with chronic inflammation. Am J Surg Pathol. 2017;41:299–312. doi: 10.1097/PAS.0000000000000775. [DOI] [PubMed] [Google Scholar]
- 10.Yan J, Luo D, Zhang F, He J, Yao S, Luo X, Hu F, Chen Y, Fu L, Xu J, Liu Y. Diffuse large B-cell lymphoma associated with chronic inflammation arising within atrial myxoma: aggressive histological but indolent clinical behaviour. Histopathology. 2017;71:951–959. doi: 10.1111/his.13336. [DOI] [PubMed] [Google Scholar]
- 11.Quiley MM, Schwartzman E, Boswell PD, Christensen RL, Gleason LA, Sharpe RW, d’Amato TA. A unique atrial primary cardiac lymphoma mimicking myxoma presenting with embolic stroke: a case report. Blood. 2003;101:4708–4710. doi: 10.1182/blood-2002-08-2550. [DOI] [PubMed] [Google Scholar]
- 12.Gruver AM, Huba MA, Dogan A, His ED. Fibrin-associated large B-cell lymphoma. Part of the spectrum of cardiac lymphomas. Am J Surg Pathol. 2012;36:1527–1537. doi: 10.1097/PAS.0b013e31825d53b5. [DOI] [PubMed] [Google Scholar]
- 13.Cheuk W, Chan ACL, Chan JKC, Lau GTC, Chan VNH, Yiu HHY. Metallic implant-associated lymphoma. A distinct subgroup of large B-cell lymphoma related to pyothorax-associated lymphoma? Am J Surg Pathol. 2005;29:832–836. doi: 10.1097/01.pas.0000157747.10967.f4. [DOI] [PubMed] [Google Scholar]
- 14.Berrio G, Suryadevara A, Singh NK, Wesly OH. Diffuse large B-cell lymphoma in an aortic valve allograft. Tex Heart Inst J. 2010;37(4):492–493. [PMC free article] [PubMed] [Google Scholar]
- 15.Miller DV, Firchau DJ, Mc Clure RF, Kurtin PJ, Feldman AL. Epstein-Barr virus-associated diffuse large B-cell lymphoma arising on cardiac prosthesis. Am J Surg Pathol. 2010;34:377–384. doi: 10.1097/PAS.0b013e3181ce9128. [DOI] [PubMed] [Google Scholar]
- 16.Lee MC, Aron M, His ED, Herts BR, Pohlman B, Gill IS. Age-related Epstein-Barr virus-associated lymphoproliferative disorder presenting as renal mass. Urology. 2009;74(3):505–508. doi: 10.1016/j.urology.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 17.Valli R, Piana S, Capodanno I, Cavazza A. Diffuse large B-cell lymphoma associated with chronic inflammation arising in a renal pseudocyst. Int J Surg Pathol. 2011;19(1):117–119. doi: 10.1177/1066896910391253. [DOI] [PubMed] [Google Scholar]
- 18.Boroumand N, Ly TL, Sonstein J, Medeiros LJ. Microscopic diffuse large B-cell lymphoma (DLBCL) occurring in pseudocysts. Do these tumors belong to the category of DLBCL associated with chronic inflammation?. Am J. Surg Pathol. 2012;36:1074–1080. doi: 10.1097/PAS.0b013e3182515fb5. [DOI] [PubMed] [Google Scholar]
- 19.Zanelli M, Zizzo M, De Marco L, Bisagni A, Ascani S. Fibrin-associated diffuse large B-cell lymphoma. Br J Haematol. 2019;185(3):397. doi: 10.1111/bjh.15786. [DOI] [PubMed] [Google Scholar]
- 20.Valli R, Froio E, Alvarez De Celis MI, Mandato VD, Piana S. Diffuse large B-cell lymphoma occurring in an ovarian cystica teratoma: expanding the spectrum of large B-cell lymphoma associated with chronic inflammation. Hum Pathol. 2014;45:2507–2511. doi: 10.1016/j.humpath.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Hayes C, Alkan S, Kitahara S. Indolent fibrin-associated EBV-positive large B-cell lymphoproliferative disorder in a lower extremity aneurysmal hematoma: a case report. J Hematop. 2014;7:139–143. doi: 10.1007/s12308-014-0213-4. [DOI] [Google Scholar]
- 22.Reyes MG, Homsi MF, Mangkornkanong M, Stone J, Glick RP. Malignant lymphoma presenting as a chronic subdural hematoma. Surg Neurol. 1990;33:35–36. doi: 10.1016/0090-3019(90)90222-B. [DOI] [PubMed] [Google Scholar]
- 23.Sugita Y, Ohta M, Ohshima K, Niino D, Nakamura Y, Okada Y, Nakashima S. Epstein-Barr virus-positive lymphoproliferative disorder associated with old organized chronic subdural hematoma. Pathol Int. 2012;62:412–417. doi: 10.1111/j.1440-1827.2012.02824.x. [DOI] [PubMed] [Google Scholar]
- 24.Kameda K, Shono T, Takagishi S, Kono S, Aoki T, Ito Y, Kamimura T, Sugita Y, Ohshima K. Epstein-Barr virus-positive diffuse large B-cell primary central nervous system lymphoma associated with organized chronic subdural hematoma: a case report and review of the literature. Pathol Int. 2015;65:138–143. doi: 10.1111/pin.12242. [DOI] [PubMed] [Google Scholar]
- 25.Kirschenbaum D, Prommel P, Vasella F, Haralambleva E, Maggio EM, Reisch R, Beer M, Camenisch U, Rushing EJ. Fibrin-associated diffuse large B-cell lymphoma in a hemorrhagic cranial arachnoid cyst. Acta Neuropatol Commun. 2017;5:60. doi: 10.1186/s40478-017-0463-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the original data supporting our research are described in the Case presentation section and in the figures’ legends.