Abstract
Objective
Staphylococcus aureus with the ability of biofilm formation and the drug resistance acquisition is one of the most frequently isolated pathogens from chronic rhinosinusitis patients. Ultrasound as an alternative therapy is effectively able to kill the bacteria by cavitation in or on the bacterial cells and peroxide generation and hence improving antibiotic treatment efficacy.
Results
Staphylococcus aureus was detected in 4 and 6 out of 14 patients by phenotypic and qPCR assays, respectively. Four patients were completely resolved after pulsed ultrasound treatment. However, presence of the S. aureus was confirmed in three healthy controls by bacterial cultivation. Pulsed ultrasound have been quantitatively decreased the S. aureus population in chronic rhinosinusitis patients (p < 0.05). Further studies need to be investigated the effectiveness of pulsed ultrasound as an alternative course of CRS patient’s treatment.
Keywords: Pulsed ultrasound, Chronic rhinosinusitis, Staphylococcus aureus, Treatment, Real-time PCR
Introduction
Bacterial communities within a polymeric matrix which called biofilms are difficult for cultivation and highly persistent during antibiotic treatment [1]. Approximately, 65% of human bacterial infections are accompanied with biofilms including sinusitis [2]. Chronic rhinosinusitis (CRS) is a prevalent disease and have been documented with bacterial biofilms complicated to control. Staphylococcus aureus with the ability of biofilm formation and the drug resistance acquisition is one of the most frequently isolated pathogens from CRS patients [3]. Because of the complex and not well-known nature of the rhinosinusitis and drug resistance development of involved pathogens, treatment of the disease is sophisticated [4]. Medical management of the CRS is difficult and needs a prolonged antibiotic therapy. In addition to the complications of long-term medical treatment, persistence promotion and emerging the drug resistant bacterial populations made investigators to explore alternative therapies [4, 5].
Therapeutic ultrasound (US) has been advocated as a treatment option for CRS patients in recent years [1, 4]. The high-frequency sound waves are used in ultrasound treatment and can be implemented as continuous or pulsed type. Ultrasound is effectively able to kill the bacteria by cavitation in or on the bacterial cells and peroxide generation and hence improving antibiotic treatment efficacy [1]. It has been demonstrated that ultrasound could completely treat CRS patients [6]. Therefore, the aim of this study was to survey the impact of pulsed ultrasound on the S. aureus population of chronic rhinosinusitis patients.
Main text
Materials and methods
Study setting
This study was a double blind, randomized clinical trial performed from April to September 2018. The included individuals were CRS infected adult patients confirmed by clinical diagnostic criteria (more than or equal to two major symptoms or one major symptom (nasal obstruction, facial pain/pressure, postnasal drip and hyposmia) and two minor symptoms (headache, halitosis, fatigue, dental pain and ear pain)) and also the CT scan results based on the Lund-Mackay staging system [7]. Exclusion criteria were having any head and neck malignancy. Totally, fourteen CRS patients were referred by an Ear, Nose, and Throat (ENT) surgeon. Additionally, ten voluntarily healthy people were enrolled in this study. The informed consent form was filled before entering the study.
Specimen collection
Specimen collection from all participants were carried out in two times within a 21 days interval (before and after of US exposure to CRS patients, first and second time without any therapy for healthy control group). Briefly, Dacron swabs was used to collect specimens from three sites, including nasopharynx, the right and left meatus and kept in sterile tubes, containing 1 ml saline solution. Following sampling, all specimens were transferred to clinical laboratory for further analysis. Also, CT scan was done for all precipitants in two times, as described above and SNOT-20 questionnaire (Additional file 1) was filled out.
Therapeutic ultrasound
Patients were subjected to 10 times pulsed US therapy with 4 cm/s of the probe velocity movement. Ultrasound frequency was 1 MHz. The intensity for maxillary and frontal sinuses were reported 1 W/cm2 and 0.5 W/cm2, respectively. Duty cycle for ultrasound was 10%. Specimen collection, CT scans and SNOT20 questionnaire was repeated following the last exposure of pulsed US therapy. If recovery was not seen by CT scan findings or patient didn’t report recovery, routinely medical therapy started for patients by ENT specialist.
Phenotypic identification by conventional methods
All the specimens were vortexed carefully and worked out as soon as possible after collection. Ten microliters of each specimen were subjected to serial dilution from 101 to 10−6 concentration in PBS. By spreading plate technique, 10 microliters of each diluted suspensions were cultured separately in 2 sheep Blood agar, 2 chocolate agar and MacConkey agar plates and were incubated aerobically and anaerobically at 37 °C for 24 h. After the incubation period, the colony-forming units (CFUs)/ml of each specimen was analyzed using Miles and Misra Method [8]. Also, the Gram staining, standard routine biochemical and microbiological tests were used to identify all the bacterial colonies [9].
DNA extraction
Total Bacterial DNA was extracted from clinical specimens using Macherey–Nagel (MN) DNA purification kit according to the manufacturer’s instruction (Macherey–Nagel, Germany). Finally, extracted DNA was stored in − 20 °C for further analysis. S. aureus ATCC 29213 strain was used as standard strain.
Quantitative real time PCR
The primers and probe were used to detect fibronectin-binding protein A (FnbA) gene of S. aureus are as follows: forward primer 5-AGTGAGCGACCATACAACAG-3, reverse primer 5-CATAATTCCCGTGACCATTT-3 and probe 5-FAM-AAGCACAAGGACCAATCGAGG-BHQ-1-3 [10]. First, by using the Nanodrop 1000 (Thermo Scientific, USA), the amounts of DNA templates were checked. For calculating the DNA molecules of each template per gram, following formula was used; molecules of DNA = mass (in gram) Avogadro’s number/average molecular weight of a base × template length [11]. A standard curve was drawn using tenfold serially diluted DNA to gain the efficacy of the PCR reactions. The qPCR assay was performed by LinGene K Real Time PCR apparatus (Bioer, Hangzhou, China). The control strain was S. aureus ATCC 29213 and all the samples were run in duplicate.
Statistical analysis
All data were analyzed by SPSS software version 21. The presence of S. aureus among patients and control groups were analyzed by Chi Square test. S. aureus detected in samples before and after the therapy by conventional culture technique were analyzed by nonparametric McNemar’s test. For qPCR results between related groups before and after the treatment, Wilcoxon test was used. A P value of < 0.05 was considered as statistically significant.
Results
Bacterial isolates
According to conventional bacterial identification, S. aureus was detected in both CRS patients and healthy group before treatment. In this case, S. aureus was detected in 3 and 4 cases in healthy and CRS patients, respectively. Importantly, although S. aureus prevalence among CRS patients and healthy group was not significant (p > 0.05), but the presence of other pathogenic bacteria such as Streptococcus pneumoniae, Streptococcus pyogenes, Haemophilus influenza, Klebsiella pneumoniae and Escherichia coli was detected only in CRS patients.
Ultrasound treatment output
US therapy effectively resolved S. aureus in 3 cases and decreased bacterial CFU/ml in one case by phenotypic analysis. This decreased bacterial load was in concomitant with the site changing of S. aureus from the right meatus to the nasopharynx. Although this difference is considered to be not statistically significant (p > 0.05), but is clinically significant. In addition, S. pneumoniae, S. pyogenes, H. influenza, K. pneumoniae and E. coli were removed from different sites of patients except one case that, decreasing of the Streptococcus pneumoniae load was seen phenotypically. The CT score of all the patients was decreased considerably after the treatment. As well as, the patient’s scores from the SNOT-20 questionnaire were less after treatment compared to the initial stage. All these findings are in favor of patient’s remission due to pulsed ultrasound treatment (Table 1, Fig. 1).
Table 1.
The patient’s scores from CT and SNOT-20 questionnaire before and after the treatment
| Patient ID | CT scores | Questionnaire scores | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| 1 | 8 | 1 | 56 | 10 |
| 2 | 17 | 0 | 38 | 8 |
| 3 | 15 | 5 | 48 | 25 |
| 4 | 7 | 0 | 24 | 1 |
| 5 | 27 | 4 | 36 | 9 |
| 6 | 6 | 1 | 17 | 2 |
| 7 | 18 | 4 | 17 | 1 |
| 8 | 24 | 6 | 40 | 25 |
| 9 | 33 | 11 | 85 | 25 |
| 10 | 11 | 2 | 45 | 15 |
| 11 | 6 | 0 | 64 | 1 |
| 12 | 17 | 2 | 48 | 6 |
| 13 | 24 | 0 | 54 | 0 |
| 14 | 17 | 2 | 62 | 10 |
The CT score and questionnaire scores were decreased after treatment showing patient’s remission
Fig. 1.
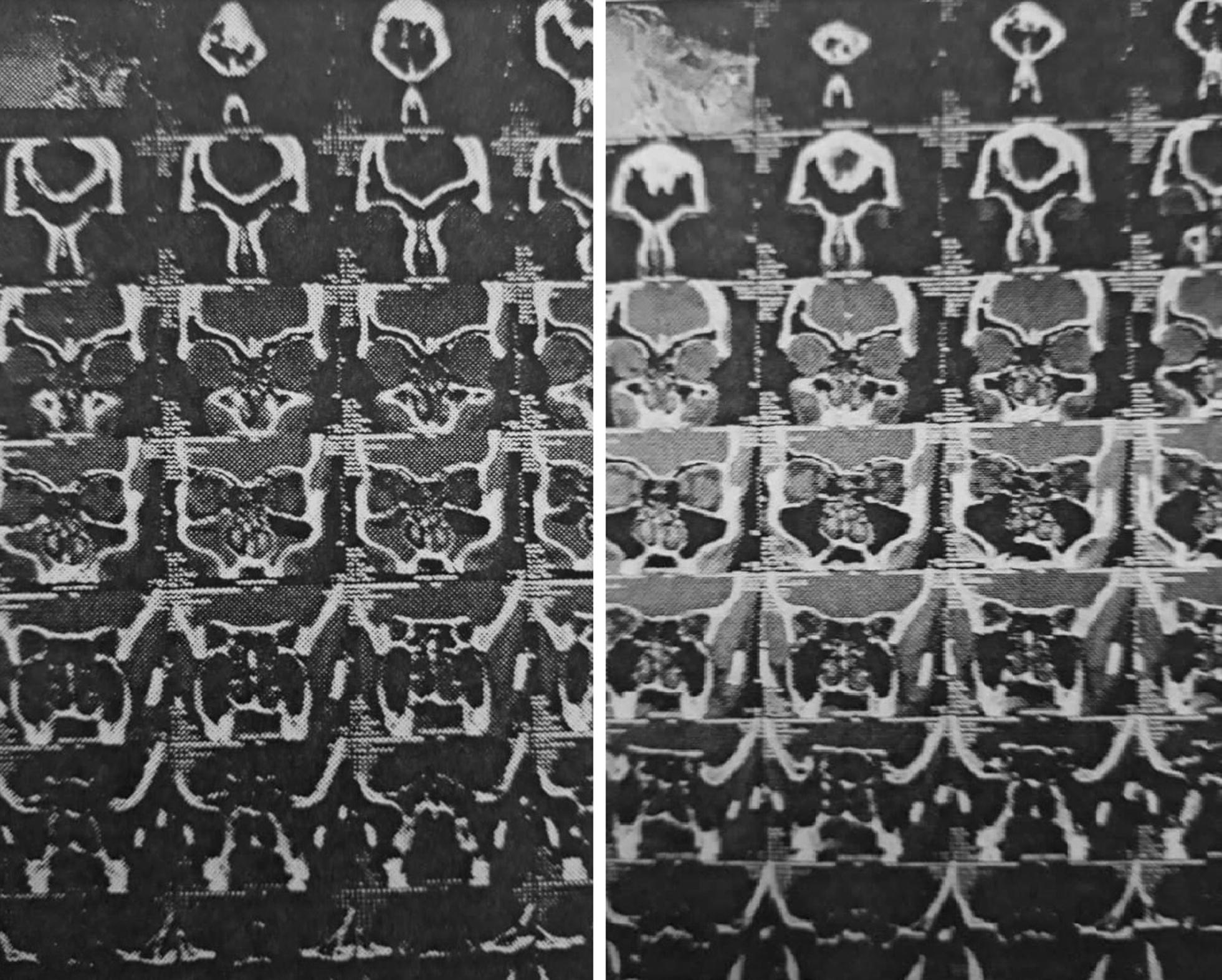
Pre-treatment (left) and post-treatment (right) findings by CT scan
Quantitative PCR findings
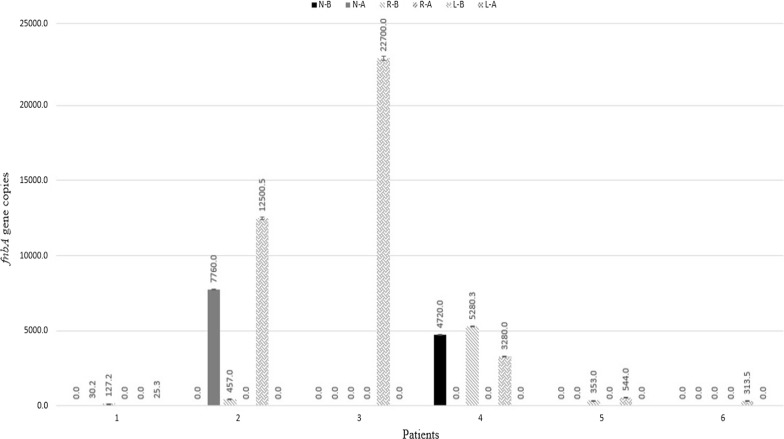
Six patients were quantitatively demonstrated to have S. aureus. From these, 4 patients were completely recovered after treatment and the bacterial load was significantly reduced (p = 0.02). However, two patients (No. 1, 2) that were negative at the beginning showed S. aureus after treatment. This may be because of bacterial transferring during sampling or subsequent colonization of bacteria. The qPCR results are detailed in Fig. 2.
Fig. 2.
qPCR results of fnbA gene in positive patients. (N-B nasopharynx-before treatment, N-A nasopharynx-after treatment, R-B right meatus-before treatment, R-A right meatus-after treatment, L-B left meatus-before treatment, L-A left meatus-after treatment)
Discussion
Treatment of CRS patients is encountering to the problem and is challengeable. Mucosal surfaces of the sinuses of these patients is source of multi species of bacterial population within the extracellular matrix which called biofilm [10]. This matrix is elaborated by bacterial population and it causes tolerance and resistance against harsh conditions such as the presence of antimicrobial agents [12]. Already, S. aureus is considered as an important infectious agent which can produce the thick layer of biofilm [13, 14].
Despite classical antibiotic therapy in CRS patients commonly is led to failure in therapy, but antibiotics are used as an empiric choice for CRS patients [15, 16] which resulting development of resistant bacteria and thus treatment failure [16]. It has also been shown, exposure of S. aureus living in the sinuses to antibiotics led emerging and distributing of methicillin resistant S. aureus (MRSA) in CRS patients [16]. Complication of CRS patients by the noticed challenges convinced researchers to study and elaborate alternative strategy for treatment of CRS patients [17]. In this regard, US therapy is a suggested strategy for treatment of CRS patients by breaking down the biofilm structure of the bacterial population. It has been reported that, US can decrease bacterial load through biofilm destruction [18]. In pulsed US strategy, it reduces thermal activities by allowing time for heat to dissipate from the coupling medium during treatment [19]. Although, biofilm formation of bacterial strains and its disruption by therapy was not evaluated in our study, but according to previous studies and published paper {see above), it has been demonstrated that, S. aureus is considered as an important CRS agent which can produce the thick layer of biofilm. In another hand, according to literature, US therapy was using as biofilm disruption option [13–18].
It has also been shown, Pulsed US therapy combined with gentamicin decrease bacterial viability in E. coli model [1, 20]. A study conducted on pulsed US therapy against P. aeruginosa, E. coli in implants [21]. These researchers reported that, pulsed US therapy only was effective against E. coli. It shows pulsed US therapy is more effective when combined with the antibiotics, which can decrease bacterial viability dramatically [22]. Ansari et al. [23] treated 57 CRS cases using low-intensity pulsed US successfully. According to their reports, most major and minor symptoms indicated significant changes after pulsed US therapy. Young et al. [24] treated 22 CRS cases with six treatments of low-intensity pulsed US which who were being considered for endoscopic sinus surgery. In accordance to these studies, our results derived from bacterial culture and quantitative molecular tests showed a significant reduction in S. aureus load in majority of patients after pulsed US treatment.
In conclusion, the clinical improvements of patients showed beneficial recovery and significantly reduced bacterial load were seen in target patients. However, the effectiveness of pulsed ultrasound is thought to be worthy of investigation in further studies.
Limitations
The low number of volunteers to participate in the study and difficulty to access and following up of all members are limitation of this study.
Supplementary information
Additional file 1. SINO-NASAL OUTCOME TEST (SNOT-20).
Acknowledgements
We are thankful to all Members of the Department of Microbiology, School of Medicine, Tehran University of Medical Sciences.
Abbreviations
- CRS
chronic rhinosinusitis
- US
ultrasound
- CT scan
computed tomography scan
- ENT surgeon
nose, and throat surgeon
- SNOT
Sino-Nasal Outcome test
- CFUs
colony-forming units
- FnbA
fibronectin-binding protein A
- qPCR
quantitative real time PCR
Authors’ contributions
JS and MMF: conceived, designed and supervised the study; MF revised the manuscript; SHS, JKY: specimen collection; NF: US treatment; HK: Microbiological analysis; NF, BVG, MD: collected and analyzed the data; NF: drafted the manuscript. All authors read and approved the final manuscript.
Funding
This project was supported by Iran University of Medical sciences (project No. IRCT20140810018754N8). The funding body and its agents didn’t have any role in the designing of the study, collection, analysis and interpretation of data as well as in writing the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are presented within the manuscript, supplementary file and also additional information such as patients’ history are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study protocol and ethical issue were approved by the Ethics Committee of Iran University of Medical sciences (IRCT20140810018754N8).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Javad Sarrafzadeh, Email: j.sarrafzadeh@gmail.com.
Mohammad Mehdi Feizabadi, Email: mfeizabadi@tums.ac.ir.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13104-019-4579-3.
References
- 1.Bartley J, Young D. Ultrasound as a treatment for chronic rhinosinusitis. Med Hypotheses. 2009;73(1):15–17. doi: 10.1016/j.mehy.2008.12.049. [DOI] [PubMed] [Google Scholar]
- 2.Costerton JW, Stewart PS. Biofilms and device-related infections. Persistent bacterial infections. Washington, D.C.: American Society of Microbiology; 2000. pp. 423–439. [Google Scholar]
- 3.Dworniczek E, Frączek M, Seniuk A, Kassner J, Sobieszczańska B, Adamski J, et al. Bacterial biofilms in patients with chronic rhinosinusitis. Folia Microbiol. 2009;54(6):559–562. doi: 10.1007/s12223-009-0082-x. [DOI] [PubMed] [Google Scholar]
- 4.Nakhostin Ansari N, Naghdi S, Farhadi M. Physiotherapy for chronic rhinosinusitis: the use of continuous ultrasound. Int J Ther Rehabil. 2007;14(7):306–310. doi: 10.12968/ijtr.2007.14.7.23837. [DOI] [Google Scholar]
- 5.Ansari NN, Fathali M, Naghdi S, Hasson S. Effect of pulsed ultrasound on chronic rhinosinusitis: a case report. Physiother Theory Pract. 2010;26(8):558–563. doi: 10.3109/09593981003628153. [DOI] [PubMed] [Google Scholar]
- 6.Ansari NN, Naghdi S, Farhadi M. Therapeutic ultrasound as a treatment for chronic sinusitis. Physiother Res Int. 2004;9(3):144–146. doi: 10.1002/pri.315. [DOI] [PubMed] [Google Scholar]
- 7.Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology. 1993;31(4):183–184. [PubMed] [Google Scholar]
- 8.Miles AA, Misra S, Irwin J. The estimation of the bactericidal power of the blood. Epidemiol Infect. 1938;38(6):732–749. doi: 10.1017/S002217240001158X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahon CR, Lehman DC, Manuselis G. Textbook of diagnostic microbiology. Amsterdam: Elsevier Health Sciences; 2014. [Google Scholar]
- 10.Ghodousi A, Nomanpour B, Davoudi S, Maleknejad P, Omrani M, Kashef N, et al. Application of fnbA gene as new target for the species-specific and quantitative detection of Staphylococcus aureus directly from lower respiratory tract specimens by real time PCR. Indian J Pathol Microbiol. 2012;55(4):490. doi: 10.4103/0377-4929.107787. [DOI] [PubMed] [Google Scholar]
- 11.Adams PS. Data analysis and reporting. Real-time PCR. Abingdon: Taylor & Francis; 2007. pp. 65–88. [Google Scholar]
- 12.Kazemian H, Bourbour S, Beheshti M, Bahador A. Oral colonization by nosocomial pathogens during hospitalization in intensive care unit and prevention strategies. Recent Pat Anti-Infect Drug Discov. 2017;12(1):8–20. doi: 10.2174/1574891X12666170215152854. [DOI] [PubMed] [Google Scholar]
- 13.Mahdiyoun SM, Kazemian H, Ahanjan M, Houri H, Goudarzi M. Frequency of aminoglycoside-resistance genes in methicillin-resistant staphylococcus aureus (MRSA) isolates from hospitalized patients. Jundishapur J Microbiol. 2016;9(8):e35052. doi: 10.5812/jjm.35052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghafourian S, Mohebi R, Rezaei M, Raftari M, Sekawi Z, Kazemian H, et al. Comparative analysis of biofilm development among MRSA and MSSA strains. Roum Arch Microbiol Immunol. 2012;71(4):175–182. [PubMed] [Google Scholar]
- 15.Smith SS, Evans CT, Tan BK, Chandra RK, Smith SB, Kern RC. National burden of antibiotic use for adult rhinosinusitis. J Allergy Clin Immunol. 2013;132(5):1230–1232. doi: 10.1016/j.jaci.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhattacharyya N, Kepnes LJ. Assessment of trends in antimicrobial resistance in chronic rhinosinusitis. Ann Otol Rhinol Laryngol. 2008;117(6):448–452. doi: 10.1177/000348940811700608. [DOI] [PubMed] [Google Scholar]
- 17.Bordin A, Sidjabat HE, Cottrell K, Cervin A. Chronic rhinosinusitis: a microbiome in dysbiosis and the search for alternative treatment options. Microbiol Austr. 2016;37(3):149–152. [Google Scholar]
- 18.Bartley J, Ansari NN, Naghdi S. Therapeutic ultrasound as a treatment modality for chronic rhinosinusitis. Curr Infect Dis Rep. 2014;16(3):398. doi: 10.1007/s11908-014-0398-9. [DOI] [PubMed] [Google Scholar]
- 19.Polat BE, Hart D, Langer R, Blankschtein D. Ultrasound-mediated transdermal drug delivery: mechanisms, scope, and emerging trends. J Control Release. 2011;152(3):330–348. doi: 10.1016/j.jconrel.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rediske AM, Roeder BL, Nelson JL, Robison RL, Schaalje GB, Robison RA, et al. Pulsed ultrasound enhances the killing of Escherichia coli biofilms by aminoglycoside antibiotics in vivo. Antimicrob Agents Chemother. 2000;44(3):771–772. doi: 10.1128/AAC.44.3.771-772.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carmen JC, Roeder BL, Nelson JL, Ogilvie RLR, Robison RA, Schaalje GB, et al. Treatment of biofilm infections on implants with low-frequency ultrasound and antibiotics. Am J Infect Control. 2005;33(2):78–82. doi: 10.1016/j.ajic.2004.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ensing G, Roeder B, Nelson JLE, Van Horn J, Van der Mei H, Busscher H, et al. Effect of pulsed ultrasound in combination with gentamicin on bacterial viability in biofilms on bone cements in vivo. J Appl Microbiol. 2005;99(3):443–448. doi: 10.1111/j.1365-2672.2005.02643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ansari NN, Naghdi S, Farhadi M, Jalaie S. A preliminary study into the effect of low-intensity pulsed ultrasound on chronic maxillary and frontal sinusitis. Physiother Theory Pract. 2007;23(4):211–218. doi: 10.1080/09593980701209360. [DOI] [PubMed] [Google Scholar]
- 24.Young D, Morton R, Bartley J. Therapeutic ultrasound as treatment for chronic rhinosinusitis: preliminary observations. J Laryngol Otol. 2010;124(5):495–499. doi: 10.1017/S0022215109992519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. SINO-NASAL OUTCOME TEST (SNOT-20).
Data Availability Statement
The datasets used and/or analyzed during the current study are presented within the manuscript, supplementary file and also additional information such as patients’ history are available from the corresponding author on reasonable request.