Abstract
Traumatic brain injury (TBI) is a well-known consequence of participation in activities such as military combat or collision sports. But the wide variability in eliciting circumstances and injury severities makes the study of TBI as a uniform disease state impossible. Military Service members are under additional, unique threats such as exposure to explosive blast and its unique effects on the body. This review is aimed toward TBI researchers, as it covers important concepts and considerations for studying blast-induced head trauma. These include the comparability of blast-induced head trauma to other mechanisms of TBI, whether blast overpressure induces measureable biomarkers, and whether a biodosimeter can link blast exposure to health outcomes, using acute radiation exposure as a corollary. This examination is contextualized by the understanding of concussive events and their psychological effects throughout the past century’s wars, as well as the variables that predict sustaining a TBI and those that precipitate or exacerbate psychological conditions.
Disclaimer: The views expressed in this article are solely the views of the authors and not those of the Department of Defense Blast Injury Research Coordinating Office, US Army Medical Research and Development Command, US Army Futures Command, US Army, or the Department of Defense.
Keywords: Blast, traumatic brain injury, concussion, biomarkers, radiation, biodosimetry
Introduction
Traumatic brain injury (TBI) is a brain dysfunction caused by an external force that may have short- and long-term effects on Service members and their units, families, and caregivers. Per Defense and Veterans Brain Injury Center1 (DVBIC) statistics, 383 947 individuals within the Department of Defense (DoD) sustained a TBI from 2001 to 2018, more than one-third of whom were exposed to a blast event.2,3 Management of TBI in the acute and chronic phases have shifted over time and will continue to change as operational constraints and medical advances evolve. Researchers should have a basic understanding of the blast mechanisms of injury as well as the first- through fifth-order effects that are possible with any blast (Figure 1). The physiologic effects of primary blast are most understood for the pulmonary and auditory systems, but the effects of blast on the central nervous system are less established. Explosion energy outside the body is transformed into biokinetic energy that causes damage to the brain and structures of the cranium from the overpressure. The damage this mechanical energy causes is mechanistically similar to blunt causes of TBI. It is well understood that blunt head trauma—from subconcussive events to the most severe TBI—represents a very heterogeneous population of patients; blast injury adds an additional level of complexity. A more useful working paradigm or classification system for physiologic changes, to thresholds, through degrees of injury still needs to be developed, especially to advance research. Although military TBI and psychological health issues are not novel to this century of warfare, there are aspects of these disorders unique to the military, as opposed to the general public. The purpose of this article is to briefly introduce the factors that need to be considered when entering into the research of military blast (and therefore blunt) brain trauma. This article will focus on TBI, especially mild traumatic brain injury (mTBI) and blast-induced TBI with and without psychological health issues.
Figure 1.
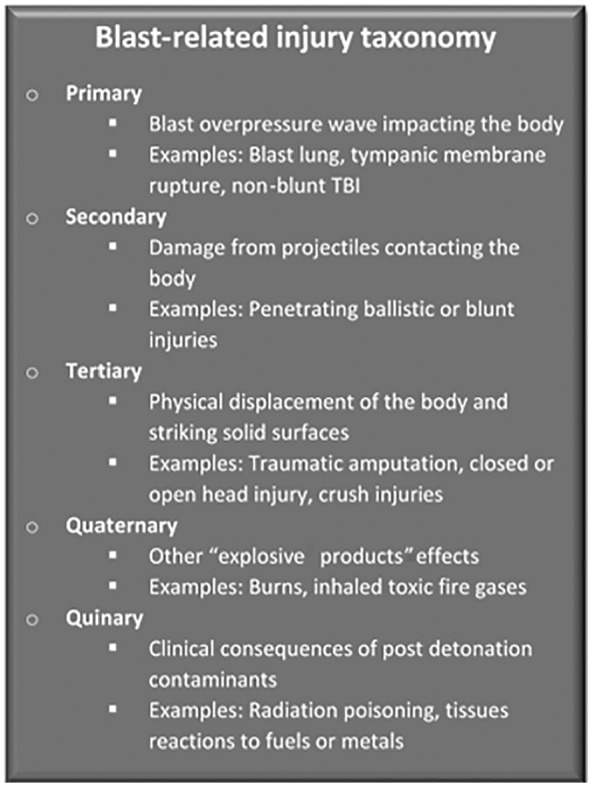
Blast-related injury taxonomy as outlined in Department of Defense4 directive 6025.21E. TBI indicates traumatic brain injury.
Historical Aspects
Descriptions of head injuries and the manners in which they were medically treated have been documented throughout history.5 Casualties with penetrating or severe TBI most often present to medical professionals with obvious injuries and symptoms, but mTBI is more difficult to define pathophysiologically. That is, the anatomical or functional disruption, clinical description, and mechanism(s) of injury are often challenging to ascertain.5 It was the advent of large, high-explosive high-projectile artillery developed in the late 1900s and made infamous during World War I that illuminated the effects of blast on the brain and spine, and the cause of “shell shock” (an early term for posttraumatic stress disorder [PTSD]). Descriptions by Elliott6 (function vs organic transient paraplegia), Myers,7-10 and Mott11,12 (organic cause of the neurological and psychiatric symptoms, especially in cases of the soldiers that died acutely after blast exposure) suggest that in many cases polytrauma—including neurotrauma associated with the effects of overpressure and blunt trauma—existed with other symptoms that could not be explained by anatomical or neurological lesions. By the end of the war and in the deliberations that continued after, the concensus was that shell shock was related to neurasthenia, dubbed a “war neurosis,” but required further investigation for an organic cause.13
One must consider cultural aspects within society, the military, and the medical profession that existed prior to, during, and after World War I to truly appreciate how shell shock, neurasthenia, and malingering were appreciated. A commission, which comprised a number of leading politicians and medical personnel, provided some insight in the years following the Armistice.14 These included intolerance for desertion regardless of nervous or mental status, abolition of the term “shell shock” in favor of “war neurosis,” sufficiency of the “simplest forms of psychotherapy” to treat war neurosis, and encouragement of short and frequent tours of duty.
As the war progressed, clinicians began to recognize that it was not only the “concussive effect of artillery and blast” that was causing these unexplained somatic and neurologic complaints among soldiers, but a recognition of the harsh conditions and the horrors of a new kind of warfare on a scale unknown in history. Without technological advances and diagnostic testing, medical professionals of this time were unable to differentiate the effects of blast exposure from those of harsh conditions, or the extent to which physical injury might contribute to a behavioral/psychological issue.
Neuropsychological effects of blast and blunt trauma continued to be issues in subsequent conflicts, but TBI, with and without psychological health issues, have been the major medical concerns of the last 18 years of armed combat with the use of improvised explosive devices (IEDs) against military and civilian targets. More recently, a growing concern is that the effects of repeated low- to medium-level blasts from training or combat on the brain are not well understood. As the research community evaluates blast-related physiologic and pathophysiologic changes, it must also consider pre-existing conditions (physiologic or pathologic), pre-existing medical/psychological conditions, the possibility that anatomic damage may cause psychological disorders and that psychological disorders may coexist with anatomic damage, and the effects of subsequent life factors.
Mechanisms of Head Trauma
Inconsistent classifications of TBI are due to its heterogeneity as a disease state. Particularly in the military setting, the variables that dictate clinically meaningful acute, subacute, and chronic effects are countless. These features include the severity of head trauma, extent of comorbid injuries, proximity to medical care, and first- through fifth-order blast effects. Despite the diversity of possibilities, an inciting event (ballistic, blunt, blast overpressure, or a combination of mechanisms) must occur to prompt downstream negative effects. The following sections briefly introduce the main mechanisms of military blast head trauma and discuss their distinguishing features and similarities.
Ballistic head trauma
Damage to the head from ballistic materials is dependent on the projectile velocity, mass, geometry, and region of impact. The unique aspects of ballistic head trauma are due to the propensity for penetration into the head. High-velocity projectiles such as shrapnel or bullets cause head trauma via laceration and cutting of the wound tract, stretching and shearing of adjacent regions from accompanying shock waves, and heavy blood loss.15 Of additional concern are the shock waves that cause the momentary dilation, then constriction, of a wound cavity producing diffuse neural damage, widespread edema, and brain tissue herniation. Fortunately, military helmets are designed to reduce penetrating injuries to the head.
Blunt head trauma
Head trauma sustained from impact with a blunt object often occurs in combat, vehicle crashes, or inward indentation of the helmet from a defeated ballistic threat—otherwise termed “backface deformation.” Traumatic skull deformations cause focal damage to the brain tissue and neurovasculature local to the site of impact resulting in coup lesions/contusions and epidural hematoma. With sufficient force, the brain can translate linearly and contact the skull distal to the site of impact, resulting in contrecoup lesions. When blunt trauma causes the cranium to rotate, angular acceleration can induce strain on, and shearing of, tissues throughout the brain, ie, the incompletely described “diffuse axonal injury.” It should be noted that translational and rotational acceleration of the brain need not be elicted by blunt trauma; inertial acceleration—most often via minor vehicular accident—can occur without external contact with the skull.
Primary blast-induced head trauma
The primary blast wave—propagation of a supersonic pressure gradient—is elicited from the detonation of an explosive device. In the millisecond range, a chemical reaction releases a high positive pressure wave that expands radially from the blast site, followed by a longer negative pressure phase. This idealized pressure pattern is known as the Friedlander16 curve which can be segregated into measurable components including peak overpressure, duration, and impulse, describing propagation through time and space. These components are useful because they vary by the distance from the explosive and can predict stand-off distance safety.17 However, although the Friedlander curve is a useful conceptual reference, it does not faithfully match the complex overpressure patterns observed in controlled or weaponized detonations; variables including reflections (eg, from the ground, buildings, or vehicles) or the structure/shape of the explosive can dramatically change the pressure profile.18,19 Furthermore, an individual may be subject to repeated blast exposures from the same incident as reflections of the blast wave bounce off solid objects, akin to an acoustic echo. More sophisticated methods are used to characterize complex overpressure patterns seen with reflection surfaces such as inside buildings, vehicles, or other enclosures.
Head trauma due to blast overpressure is incompletely understood and requires the continuum of multidisciplinary research efforts—in vitro work, animal testing, computational modeling, clinical research, neuropathology, and epidemiology—to elucidate how blast overpressure leads to the observed injury pathology. To date, there is insufficient evidence to support a single injury mechanism for all cases; however, many mechanisms have been substantiated through prior work. Although research can isolate or model the proposed mechanisms individually, they should not be considered mutually exclusive in military blast scenarios. Many notable reviews cover the following information in further depth.15,18,20-24
Acceleration mechanisms
The blast overpressure wave induced by an explosive can cause macroscopic translational and rotational acceleration of the brain, resulting in compression and shearing of the brain tissue. In addition, the brain in motion within the cranium can directly impact the skull causing contusions, lacerations, or subdural hematoma, and angular motion can induce diffuse micro-hemorrhaging and axonal injury. This mechanism of primary blast injury is particularly plausible as head acceleration thresholds for injury due to blunt force have been validated in the literature.25
Direct cranial transmission
When the pressure transient interacts with the human body, some of the shock wave is reflected but most of it is absorbed. Interfaces between parenchyma, blood vessels, and air- or fluid-filled compartments such as the lung, bowel, and brain are particularly susceptible to injury. The brain’s complex perfusing vasculature, deep sulci, and circulating cerebrospinal fluid within the vesicular and arachnoid spaces can facilitate damage through several processes.
Direct skull deformation can efficiently transmit compression waves via cerebrospinal fluid media that reverberate within the skull. Spalling can occur at tissue interfaces of different densities such that tissue fragments are displaced from the denser medium to the less dense medium. Inertial or shearing effects occur when the lightest tissue travels faster than the heavier tissue causing stress and strain, and possibly rupture of tissue. Implosion effects cause compression of liquid media which can lead to the dissolved gases therein to bubble and expand; the kinetic energy released from the bursting of these bubbles is called cavitation.
Vascular surge
A more controversial mechanism by which primary blast can damage the brain is through transmission of the pressure wave through the thorax. It is hypothesized that distortion pressure to the thorax causes volumetric blood surge which increases blood pressure within the cerebral vasculature to the extent that the blood-brain barrier is breached, capillaries rupture, and hemorrhage becomes probable. This hypothesis is supported by animal research whereby pigs,26 rats,27-29 or rabbits30 are exposed to experimental whole-body or thorax-only blast showing structural and biochemical changes in the brain, measureable changes in vascular pressure, and behavioral deficits.
Comparability between head trauma mechanisms
The 3 most common methods in which Service members sustain head trauma (ie, ballistic, blunt, or blast injury) have largely overlapping mechanisms of TBI. For instance, stretching and shearing forces can be initiated via a ballistic projectile, a blunt impact, or via primary blast overpressure. Likewise, accelerative translational and/or rotational forces can cause focal coup lesions, distal contrecoup lesions, and diffuse damage to microstructures throughout the brain. In addition, linear and depressed skull fractures can occur in penetrating head trauma, backface deformation, and blunt trauma.31,32 Pathophysiological differences between ballistic, blunt, and primary blast head trauma are still being explored and ongoing work seeks to determine loading thresholds for structural changes to the brain regardless of mechanism; one example is work from Army Research Laboratory that is pioneering the use of X-ray fiber diffraction to determine nanoscopic damage to myelin after trauma.33 It should be emphasized that recommended clinical management of head trauma casualties does not differ by the mechanism of injury34,35 and a systematic review comparing Service members and Veterans with blast- or non-blast-related TBI found minimal differences in clinical health outcomes.36 However, understanding the mechanisms of injury caused by blast overpressure is essential to finding in vivo markers of damage unique to blast. Further research in the area of modeling, scaling, and head-only or combined body injury should be strategically and collaboratively performed.
Biomarkers in Blast-Induced TBI
One of the many goals in TBI research, and especially in concussion/mTBI research, is to find biomarkers that can reliably indicate subtle changes caused by brain injury to help with identification, diagnosis, treatment, and prognosis of injury and sequelae. One of the most useful environments for head injury biomarkers is the combat casualty care scenario; polytrauma and mass casualty incidents are common, resources can be scarce, and operational demands may require delayed care. Therefore, any analysis of medical information that could aid in clinical decision-making (eg, evacuation, surgery) is warranted. Such biomarkers can be measured via neuroimaging or bodily fluid assay.
Imaging
Computed tomography (CT) imaging is particularly useful for identifying foreign bodies, skull fractures, and hemorrhage. When available in the military environment (Role of care 3), CT imaging is the standard of care for suspected TBI. By the current DoD definition, a CT abnormality that is consistent with a TBI, in an otherwise clinically mTBI patient, is reclassified as a moderate TBI. Most of the military-related TBI casualties present as mTBI. Therefore, the utility of CT imaging is for ruling out more severe injury rather than finding a radiographic diagnostic biomarker specific to mild TBI.
Other imaging modalities are less commonly used in theater (or in acute settings of civilian practice) and therefore acute injury data from the military population are sparse. These modalities include magnetic resonance imaging (MRI), functional MRI, diffusion tensor imaging, positron emission tomography, magnetoencephalography, and electroencephalography. Multiple reviews have stated the difficulty in finding an imaging biomarker with sufficient sensitivity and specificity to diagnose mTBI—or CT-negative blast-induced TBI—or prognosticate outcomes.37-39 Barriers to this goal are the countless variables present in the military blast scenario, the clinical heterogeneity of TBI itself, the lack of quality acute and longitudinal data, and the confounding comorbidities in the military population. This is in addition to the developing connections between blast-induced TBI neuroimaging findings and neurobehavioral consequences.
Fluid biomarkers
Resultant injuries from blast can disrupt the structure of the body and brain within the milliseconds after explosion. These disruptions initiate subsequent short- and long-term cellular and molecular changes—so-called “secondary cascade”23,40—that change the concentrations of biomolecules in circulating bodily fluids which have potential value if their levels can be detected. There is further promise if a biomarker can be measured rapidly to diagnose TBI and/or repeatedly to track the progression of neurophysiological changes in response to low-level blast, particularly in the absence of more definitive diagnostic tests. Unfortunately, recent research seeking such biomarkers in military “breachers” exposed to repeated low-level blast yielded no measurable blood-based biomarker changes over a 5-year period.41 The state of the science is far from defining biomarkers with the characteristics necessary to provide useful blast TBI information to medical providers. Despite the ongoing concentrated efforts to identify definitive blood-based biomarkers over the last decade,42-45 the first panel was recently approved by the US Food and Drug Administration46 in February 2018.
Blast Dose Response
A central tenet of toxicology is the dose-response relationship. The blast overpressure dose should have a direct correlation with medical outcomes of interest after TBI. This ongoing research endeavor has revealed many factors that predict blast-related TBI severity such as the blast substrate (eg, air, water), energy of the blast, and distance from the blast origin; the position of the victim (eg, standing, laying); and the extent of reflected energy.47-49 Blast loads create very brief acceleration durations that may cause distinct neurophysiological outcomes.15 Although accelerative loading criteria have been validated in non-blast head injury scenarios,25 the primary blast wave poses a unique hazard that does not apply well to these established criteria.
Because controlled blast experiments cannot be conducted with humans, injury thresholds from animals from small to large have been extrapolated with the expectation that body mass scales well with blast injury thresholds.48 More nuanced scaling efforts have used brain mass and skull thickness,21,50,51 but geometric and physiological parameters make interpretation of the data difficult. Promising unpublished research from Applied Research Associates has compiled data from animal and observational human studies and augmented them with computational modeling to develop “sensor-based stand-down guidelines for Service members.”52 Results of similar experiments, if further validated, may provide objective exposure thresholds that can indicate when to return to duty, or when to withdraw from further blast-related training. Unfortunately, no blast dosimetry biosensor has yet overcome the many technological, logistical, and medical challenges that impede their ultimate integration into military use.
Recently, the North Atlantic Treaty Organization (NATO) Human Factors and Medicine (HFM)-234 (RTG), a NATO S&T panel working group consisting of prominent researchers and technical experts, framed blast exposures as an “environmental toxicology problem” and released a report guiding the future of such research.53 They noted that substantial future work is needed to standardize experimental blast induction parameters, validate blast biosensors, and link blast exposure to clinical outcome data.
Role of modeling
Research developing injury thresholds for Service members relies on the basic fundamental science that informs parameters for neurological deficits based on the established mechanisms of injury. The following sections broadly discuss the use of animal and computational models as a means to advance collective understanding of the science.
Animal models
Animal models are an invaluable tool in blast-induced TBI research. Because of its multivariate nature, elucidating the properties and mechanisms underlying individual blast-induced TBI components can be challenging. Animal models provide an avenue to study specific variables of blast injury with a level of control that is difficult to achieve in human studies. Common animal models include the rat, mouse, pig, and non-human primate. Rats and mice are commonly used due to their cost-effectiveness, tractability, and the number of standardized neurobehavioral outcome measures. Mice have the added benefit of genetically modified strains that can be used to study the role of specific genes in blast-related pathology. However, interspecies scaling issues and anatomical differences can be a barrier when translating animal research findings to clinical studies.54 Similar to the human, non-human primates and pigs have a large, gyrencephalic brain which responds to blast in a much different way than the lissencephalic brain of rodents. The anatomical and physiological similarities of pig and non-human primate brains (ie, head size and shape, and skull thickness) to human brains make these models particularly useful for increased fidelity, and many techniques used in the clinical setting for humans are also used for porcine models. However, the cost, ethical considerations, and extensive regulation surrounding the use of non-human primate models should also be considered.
Shock tubes
The most commonly used tool to replicate blast injury in the laboratory is the shock tube. The shock tube consists of 3 primary components: an expansion chamber, a frangible diaphragm, and a compression chamber. To simulate blast, the pressure within the compression chamber is raised to above the thickness of the diaphragm, creating a high-velocity pressure wave in the expansion chamber. Using diaphragms of various thicknesses, the peak overpressure can be modified and controlled. Enhancements to shock tubes in recent years have more faithfully reproduced free-field explosion profiles; helium ignited by an explosive charge may be used instead of pressurized air to better represent the conventional Friedlander curve and the addition of an “end wave eliminator” in the advanced blast simulator at Defence Research and Development Canada—Suffield Research Centre has successfully impeded reflective waves coming back toward the animal. Unfortunately, the experimental heterogeneity of shock tube experiments can make blast-induced TBI research difficult to evaluate in aggregate. Variability in pressure profiles, the positioning of the animal (eg, inside or outside the tube, head-on or side-on blast), restraints to minimize tertiary brain injury, and distance from the diaphragm can alter the injury type and/or severity significantly.37
Guidelines
To mitigate the obstacles and increase translatability of animal research findings, the NATO HFM-234 (RTG) published guidelines for animal model use in blast injury. The goals of these guidelines are to improve standardization of blast injury animal research and encourage development of well-designed, validated studies that properly replicate human features of blast injuries. The guidelines emphasize consideration of animal model choice based on the study purpose and physiological similarity to humans; clear description and justification of data collection and analysis strategies used; development of highly reproducible injury components; and use of injury-component-relevant outcomes.55
Computational models
Modern mathematical and computational techniques allow researchers to bypass the ethical, logistical, and financial burdens of generating a blast wave and studying its impact on biological tissue. Researchers have established computer-generated models of the blast wave and brain/body to predict how various blast wave parameters affect biological outcomes. Although extremely promising, this is not a trivial endeavor. Creation of a realistic computational model relies on a deep understanding of blast wave physics, anatomy, computational modeling techniques, relevant operational circumstances, and acute and long-term neurotrauma outcomes. Because each of these variables is inextricable and heavily interdependent, meaningful progress in this field is gradual. A comprehensive description of computational modeling principles and applications is outside the scope of this article, but relevant reviews are available.23,40,56,57
A common method of computational modeling is finite element modeling, which assumes the material properties of smaller comprising portions of larger organs and estimates their response to an external load; once the strength of the tissue or organ is exceeded, damage occurs. In a complex medium like the brain, however, tissue strength and its material properties are still being established by animal research, the downstream impacts of any given element breaking are incompletely understood, and the various properties of the blast wave have unpredictable effects on how each element interacts with others.
To inform computational models, non-biological surrogate models have been used to measure the interaction between blast loading materials of various properties. These so-called “biofidelic” models incorporate strategically placed sensors—typically on human surrogate chest and head structures—to measure biomechanical parameters such as displacement, velocity, and pressure in response to blast.58
Guidelines
Heterogeneity among current research efforts and their reporting standards make comparisons of computational models difficult. Guidance, therefore, is needed to create actionable consensuses on military-relevant computational modeling to reduce cost, minimize redundancy and waste, and benefit Service members. Cernak and Noble-Haeusslein59 developed particularly insightful criteria that they argue should be satisfied for any blast injury model. These include a thorough and quantifiable description of the blast wave and inflicted injury which mimic relevant human circumstances, established metrics of injury outcomes, and an analysis of how the mechanical properties of the blast scale with outcome severity.
What Can Be Learned From Acute Radiation Syndrome?
Radiobiological research has many similarities to the research done in TBI, particularly TBI secondary to blast. The first is the ethical limitations to exposing research participants to a controlled and predictable dose of radiation/overpressure. Much of the acquired knowledge is obtained from animal models, occupational exposure, or accidents and incidents. In the case of exposure to ionizing radiation, the health physics field has decades of such data and an understanding of acute and chronic effects of exposure based on dose. This knowledge is also the basis of radiation safety dosimetry programs to monitor radiation in workers. Sensing unsafe levels of blast overpressure or concussive impact, and their potential health effects, is a long-term goal of blast-related TBI research.
The discovery of ionizing radiation caused an increase in scientific and consumer use. The use in both fields was so rapid; it outstretched the understanding of the dangers this new discovery posed. Both researchers and consumers suffered acute and chronic effects of radiation exposures during the early days of radiation use. In fact, it is the understanding of these radiation accidents and incidents that underlies the current knowledge of radiation exposure.
Subsequently, during the race to discover atomic weapons and to develop atomic energy, additional research in the field of radiation exposure and the effects on biological systems from ionizing radiation was performed. Over the next 6 decades, these in-depth studies provided a comprehensive dose-response model, to which blunt and blast TBI aspires. Acute radiation syndrome (ARS) is an archetype which describes a threshold of whole-body or significant partial-body radiation exposure (approximately 1 Gy) and defines a series of subsyndromes with predictable deterministic effects based on increasing dose.60 Simply put, based on the radiation dose, one is able to discern the amount and type of tissue damage; physical dosimetry and biomarkers provide initial and definitive exposure dosage (Figure 2).
Figure 2.
Severity of pathology increases with increasing dose once a threshold has been reached. Below this level, reversible physiological changes occur. ARS indicates acute radiation syndrome.
Source: Adapted from Waselenko et al,60 Medical Effects of Ionizing Radiation (MEIR) Course,61 and Walker et al.62
Biomarkers used to determine biodosimetry in the field of radiobiology such as time to emesis, lymphocyte depletion, amylase, C-reactive protein, and cultured lymphocytes with dicentric chromosome analysis (the latter is considered the “gold standard”) point to certain levels of radiation exposure that clinicians can use to help triage, direct medical support, and provide prognosis. It has been well accepted within the field of radiobiology that no one biodosimetric tool is perfect to answer all the questions needed for research or for clinical care; multiple biodosimetry measurements are needed to more accurately evaluate, diagnose, and prognosticate in cases of radiation exposure. The Armed Forces Radiobiology Research Institute has even created tools which incorporate different biodosimetric readings to more accurately determine an exposed individual’s radiation dose.63-67
The current concept of ARS considers the physiologic changes that occur with subthreshold radiation exposure. There are measurable changes in lab parameters with exposure to ionizing radiation that are reversible. In addition, one’s status (age, medical condition, full- or partial-body radiation exposure) may change the threshold of 1 Gy that predicts acute effects. The coexisting exacerbating and/or mitigating factors that precede, happen concurrently, or develop independently of the radiation exposure impact the clinical course of an ARS patient. It is well known that a concomitant conventional injury like a laceration or burn in association with ARS level radiation exposure increases mortality and morbidity. Traumatic brain injury experts agree that there are factors which, in a similar way, impact a TBI patient’s clinical course. Therefore, a precision medicine approach and not a “one size fits all” approach may provide better outcomes.
The “Life Effect”
The paucity of evidence regarding the specific long-term effects of blast-induced TBI on military populations is unsurprising as the long-term outcomes of TBI as a whole are not fully understood. As the mechanisms by which the brain incurs damage and the studied long-term outcomes between non-blast- and blast-related TBI are largely similar, it stands to reason that influencing external factors should also be similar.
Most individuals who sustain a TBI experience physical, cognitive, behavioral, and psychological deficits that subside within weeks or months of injury. Yet, a significant subset experience persistent symptoms, known collectively as postconcussion syndrome (PCS) that can last for years after the original injury. In addition, comorbid psychiatric and somatic symptoms may accompany other TBI-related symptoms. It is essential that investigators bear in mind that TBIs does not occur in a vacuum but influence, and are influenced by, multiple life factors occurring prior to, during, and after injury that can have a significant impact on long-term health. The current section explores factors outside of the original injury or “life effects” that may impact TBI—and likely blast-induced TBI—outcomes in military populations.
Prior to injury
Genetic polymorphisms
There is increasing evidence of a genetic component for certain TBI outcomes. Genetic polymorphisms in a number of genes have been associated with variance in TBI outcomes including APOE,68,69 BDNF,70 and COMT.71 An in-depth review of genetic polymorphism susceptibility for long-term TBI outcomes is available.72
Apolipoprotein E (APOE), a gene that encodes glycoproteins involved in lipid transportation and cell membrane formation for neurons, has 3 allelic variants (ε2-ε4) that encode 3 protein isoforms (E2-E4). Several studies have shown that the presence of at least 1 ε4 allele is associated with a poorer outcome after TBI.73-75 However, a handful of others studies have found that there is no association between the presence of the allele and outcome.76-78 These conflicting results suggest that the predictive power of the ε4 allele may be population specific.72
Catechol-O-methyltransferase (COMT) encodes a catalyst that metabolizes catecholamines. The presence or absence of polymorphism Val158Met determines the catalyst’s activity level and may contribute to variance in cognition. The Val158Met genotype has been linked to better cognitive and psychological outcomes after TBI.79-81
The brain-derived neurotrophic factor (BDNF) is involved in several cellular processes including survival and plasticity. The polymorphism Val66Met has been linked with poor neurocognitive function after TBI as well as in healthy adults.82
Premorbid conditions
Similar to the genetic predisposition that polymorphisms provide, pre-existing conditions and experiences can impact the trajectory of TBI outcome and recovery. Psychiatric histories prior to injury are associated with variations in cognitive functioning and life satisfaction. A longitudinal, prospective study of psychological and demographic factors associated with TBI outcomes found that pre-injury psychiatric history and education are associated with life satisfaction.83 Seagly et al84 observed that long-term post-TBI functional outcomes were predicted by cognitive and physical independence more than age at injury and Glasgow Coma Scale (GCS) score. Bertisch et al85 found that, whereas individuals with TBI either with or without psychiatric histories did not show a difference in treatment, return to work delay, or social activity, TBI patients with psychiatric history did display slower processing speed and less satisfaction with their work. This suggests that premorbid conditions may account for long-term outcomes after TBI. Despite the potential impact of these psychiatric history and other conditions pre-injury, few studies have investigated the mechanism and role of pre-existing conditions on long-term TBI outcomes. Further investigation into the role of premorbidities on TBI outcomes could elucidate the trajectory of recovery in certain TBI populations.
Previous injuries
It has been well established that a significant risk factor of military TBI and predictor of post-TBI sequelae is previous brain injury.86,87 In addition, repetitive TBI and subconcussive injuries/impacts have a cumulative detrimental effect on neurological functioning and resiliency.89,90 Chronic traumatic encephalopathy (CTE)—a tauopathy associated with an array of behavioral, cognitive, and physical deficits—is associated with repeated trauma to the head.91 Previous injuries are likely underreported in both civilian and military populations primarily due to the inability to recognize or perceive symptoms of particularly mild injuries and the asymptomatic nature of subconcussive events.92
During injury
Combat and military training activities present unique conditions that influence TBI outcomes.93 The increased use of IEDs in recent warfare has made injuries related to one, or often multiple, blast(s) commonplace in theater. It is possible that unique characteristics of primary blast may be associated with a distinct pathology that could alter long-term TBI outcomes, but as detailed above blast- and non-blast-related TBI share similar mechanisms of injury and the current literature has reported few differences in blast and non-blast outcomes.36 However, inconsistencies in classification of injuries among the studies and a lack of individuals with purely primary blast exposure make it difficult to confidently isolate outcomes of blast injury from non-blast injury. Additional controlled studies could help elucidate the unique effects of blast on TBI.
In addition to exposure to blast, the physical and mental states of the Warfighter at the time of injury may influence the outcome. Service members are often sleep deprived and under tremendous mental and emotional stress that civilians may not experience at the time of injury. Moreover, Service members often combat sleep deprivation with stimulants such as energy drinks, coffee, or caffeinated gum which are recommended by the DoD as safe and effective for maintaining solider performance when sufficient sleep is not attainable.94 However, the potential medical risks of caffeine overconsumption and the interacting effects of environmental factors in the deployed setting may have further detrimental or beneficial effects on short-, intermediate-, and long-term outcomes of TBI.95
Although much of the blast-related TBI research in military populations is focused on injuries incurred during combat, Service members are also at risk of exposure to multiple low-level blasts during operational and training activities, such as breacher training. Exposure to repetitive low-level blasts could result in subconcussive injury and subsequent cumulative neurological effects. For more information on the effects of repetitive low-level blast exposure on military operations in garrison, see the meeting proceedings from the Seventh DoD International State-of-the-Science Meeting: The Neurological Effects of Repeated Exposure to Military Occupational Blast: Implications for Prevention and Health.52
Comorbidities
In many cases, particularly in military populations, TBI is accompanied by substance abuse and psychiatric comorbidities.96 The substantial overlap in symptomology between TBI and psychological disorders, namely, PTSD, can make it difficult to parse out symptom cause and their role in long-term outcomes (Figure 3). Several studies have shown that TBI with comorbid conditions is associated with a greater likelihood of experiencing PCS, suggesting that TBI alone may not account for the symptoms or severity of outcome.98,99 There is a growing body of evidence that some of the outcomes attributed to TBI are actually a result of the comorbidities alone. To delve into the relationship of TBI and comorbidities with outcome, consider these well-written reviews.36,100
Figure 3.
Overlap of TBI- and PTSD-related symptoms. TBI indicates traumatic brain injury; PTSD, posttraumatic stress disorder.
Source: Defense and Veterans Brain Injury Center.97
Military demographics
The demographics of the military population can have a significant impact on propensity for sustaining a TBI and the acute and chronic outcomes. Most of the Service-related TBI cases are diagnosed in the non-deployed setting101,102; they therefore tend to be the same mechanisms by which civilians sustain a TBI (ie, sports, motor vehicle accidents). Given the demographic overlap between Service members and collegiate athletes—athletic men and women in their early 20s—the National Collegiate Athletic Association (NCAA) and DoD partnered to create the NCAA/DoD Concussion Assessment, Research and Education (CARE) Consortium,103 a massive undertaking that has surveyed more than 10 000 cadets and student athletes to study the neurobiology and natural history of TBI in this population.
Service members may be especially vulnerable due to their neurodevelopmental stage as myelination of the adolescent brain continues well into the early 20s.104 Given the white matter shearing that occurs in response to accelerations during blast-related injury, this raises questions about additional trauma that may occur in this population. Damage to these areas prior to complete myelination could potentially impact plasticity and neurological development and have long-term consequences.
After injury
Military culture impacts treatment seeking and injury reporting
Military culture may also impact TBI outcomes by altering how Service members and Veterans approach reporting of their injuries. Key values such as selflessness, not accepting failure, and support of the needs of the group over personal needs are impressed on Service members throughout their career. Even after service, military culture and values tend to remain with Veterans decades later. The focus on self-sacrifice can lead Service members and Veterans to envision seeking treatment as accepting failure,102 which skews population-based estimates of injuries or symptoms. Furthermore, although attempts have been made to destigmatize reporting of mental health conditions, many Service members may be apprehensive about seeking treatment for psychiatric comorbidities due to fear that it may limit their career. This fear can also lead to underreporting of TBI that occur outside of a military setting such as injuries incurred while playing a recreational sport or through misconduct.
Domestic violence
Another factor that should be considered when investigating TBI outcomes in a military population is the role of violence outside of the military setting. A large subset of Veterans that sustained a TBI in theater also experience intimate partner violence (IPV). In a study by Iverson et al,105 a subset of women from a reintegration study reported experiencing lifetime IPV. Those that experienced IPV reported greater neurobehavioral symptoms and were more likely to be clinically diagnosed with back pain or substance abuse. IPV itself can also result in TBI. Iverson and Pogoda106 reported that nearly 20% of the Veterans screened met the criteria for IPV-related TBI history. Female Veterans that experienced an IPV-related TBI reported higher levels of depression and PTSD and perceived poorer heath compared with those that experienced IPV-related head injury without TBI.
Aging with TBI
It is recognized that TBI may influence aging in many ways, such as increasing risk for early onset of neurodegenerative diseases and microglial activation.107,108 However, aging can also influence TBI outcome through multiple pathways. One of the greatest risks that aging provides to TBI outcome is an increased risk of additional injuries. As an individual ages, they are also more likely to experience a fall, the leading cause of TBI in the elderly.109 TBI experienced later in life is often associated with more severe outcomes due to age-related changes in the dura and veins and brain atrophy.110 Older populations are more likely to have a chronic condition and to take medications that could impact recovery and outcome as well. Many neuropsychiatric conditions occur later in life, which can make it difficult to distinguish neuropsychiatric symptoms resulting from a new injury or due to age or other conditions.
Conclusions
To better prevent, protect, identify, treat, and rehabilitate Service members and Veterans from the effects of blast injury, the research performed must adequately consider the population for which it is addressing. Physicists and engineers are currently breaking through the nuances of blast injury through computational and animal models. A thorough understanding of the mechanisms behind blast, and perhaps even other directed energy sources (adding yet another layer of complexity), is paramount to modeling the human condition. These models need to also consider the aspects of the human condition that may dramatically alter critical assumptions, on which the models are based.
The large amounts of research data, from all sources such as large consortia to smaller pilot studies, need to be evaluated so that these data can be properly compared. This is especially important in the era of big data and machine learning where it is crucial to ensure comparability of data being put into algorithms. The purpose of this article is to make those researchers who are currently undertaking blast-related research endeavors, or those who plan to enter the field, aware of some of the basic tenants of blast-related research as it affects the central nervous system with particular emphasis on the brain. The challenges inherent in this area of research are numerous, but these obstacles further emphasize the need for substantial and cooperative work to be done for the benefit of Service members, Veterans, and their families.
Acknowledgments
The authors would like to thank Mr Mike Leggieri, Dr Raj Gupta, Ms Katrina Caravelli, and Mr Charles Woodruff for their support in this article.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Conceptualization: SRH; Supervision: SRH; Visualization: SRH, DWB, JIT; Writing: SRH, DWB, JIT.
ORCID iD: Sidney R Hinds II  https://orcid.org/0000-0003-2231-6770
https://orcid.org/0000-0003-2231-6770
References
- 1. Defense and Veterans Brain Injury Center (DVBIC). DoD worldwide numbers for TBI. https://dvbic.dcoe.mil/system/files/tbi-numbers/worldwide-totals-2000-2018Q1-total_jun-21-2018_v1.0_2018-07-26_0.pdf. Updated 2019. Accessed April 15, 2019.
- 2. Regasa LE, Agimi Y, Stout KC. Traumatic brain injury following military deployment: evaluation of diagnosis and cause of injury. J Head Trauma Rehabil. 2019;34:21-29. [DOI] [PubMed] [Google Scholar]
- 3. External causes of traumatic brain injury, 2000-2011. MSMR. 2013;20:9-14. [PubMed] [Google Scholar]
- 4. Department of Defense. Medical research for prevention, mitigation, and treatment of blast injuries (Directive Number 6025.21E). https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodd/602521p.pdf?ver=2018-10-24-112151-983. Accessed April 16, 2019.
- 5. McCrory PR, Berkovic SF. Concussion: the history of clinical and pathophysiological concepts and misconceptions. Neurology. 2001;57:2283-2289. [DOI] [PubMed] [Google Scholar]
- 6. Elliott TR. Transient paraplegia from shell explosions. Br Med J. 1914;2:1005-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Myers CS. Contributions to the study of shell shock: being an account of certain disorders of cutaneous sensibility. Lancet. 1916;187:608-613. [Google Scholar]
- 8. Myers CS. Contributions to the study of shell shock: being an account of certain cases treated by hypnosis. Lancet. 1916;187:65-69. [Google Scholar]
- 9. Myers CS. Contributions to the study of shell shock: being an account of certain disorders of speech, with special reference to their causation and their relation to malingering. Lancet. 1916;188:461-468. [Google Scholar]
- 10. Myers CS. A contribution to the study of shell shock: being an account of three cases of loss of memory, vision, smell, and taste, admitted into the Duchess of Westminster’s War Hospital, Le Touquet. Lancet. 1915;185:316-320. [Google Scholar]
- 11. Mott FW. The microscopic examination of the brains of two men dead of commotio cerebri (shell shock) without visible external injury. Br Med J. 1917;2:612-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mott FW. The Chadwick lecture on mental hygiene and shell shock during and after the war. Br Med J. 1917;2:39-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Myers CS. A final contribution: the study of shell shock: being a consideration of unsettled points needing investigation. Lancet. 1919;193:51-54. [Google Scholar]
- 14. Bogacz T. War neurosis and cultural change in England, 1914-22: the work of the War Office Committee of Enquiry into “shell-shock.” J Contemp Hist. 1989;24:227-256. [Google Scholar]
- 15. Young L, Rule GT, Bocchieri RT, Walilko TJ, Burns JM, Ling G. When physics meets biology: low and high-velocity penetration, blunt impact, and blast injuries to the brain. Front Neurol. 2015;6:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Friedlander FG. Propagation of a Pulse in an Inhomogeneous Medium. New York: Institute of Mathematical Sciences, New York University; 1955. [Google Scholar]
- 17. Champion HR, Holcomb JB, Young LA. Injuries from explosions: physics, biophysics, pathology, and required research focus. J Trauma. 2009;66:1468-1477; discussion 1477. [DOI] [PubMed] [Google Scholar]
- 18. Bandak FA, Ling G, Bandak A, De Lanerolle NC. Injury biomechanics, neuropathology, and simplified physics of explosive blast and impact mild traumatic brain injury. Handb Clin Neurol. 2015;127:89-104. [DOI] [PubMed] [Google Scholar]
- 19. Mayorga MA. The pathology of primary blast overpressure injury. Toxicology. 1997;121:17-28. [DOI] [PubMed] [Google Scholar]
- 20. Cernak I. Understanding blast-induced neurotrauma: how far have we come? Concussion. 2017;2:CNC42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Courtney A, Courtney M. The complexity of biomechanics causing primary blast-induced traumatic brain injury: a review of potential mechanisms. Front Neurol. 2015;6:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elder GA, Mitsis EM, Ahlers ST, Cristian A. Blast-induced mild traumatic brain injury. Psychiatr Clin North Am. 2010;33:757-781. [DOI] [PubMed] [Google Scholar]
- 23. Gupta RK, Przekwas A. Mathematical models of blast-induced TBI: current status, challenges, and prospects. Front Neurol. 2013;4:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Courtney A, Courtney M. A thoracic mechanism of mild traumatic brain injury due to blast pressure waves. Med Hypotheses. 2009;72:76-83. [DOI] [PubMed] [Google Scholar]
- 25. Lissner H, Lebow M, Evans F. Experimental studies on the relation between acceleration and intracranial pressure changes in man. Surg Gynecol Obstet. 1960;111:329-338. [PubMed] [Google Scholar]
- 26. Bauman RA, Ling G, Tong L, et al. An introductory characterization of a combat-casualty-care relevant swine model of closed head injury resulting from exposure to explosive blast. J Neurotrauma. 2009;26:841-860. [DOI] [PubMed] [Google Scholar]
- 27. Cernak I, Wang Z, Jiang J, Bian X, Savic J. Ultrastructural and functional characteristics of blast injury-induced neurotrauma. J Trauma. 2001;50:695-706. [DOI] [PubMed] [Google Scholar]
- 28. Chavko M, Watanabe T, Adeeb S, Lankasky J, Ahlers ST, McCarron RM. Relationship between orientation to a blast and pressure wave propagation inside the rat brain. J Neurosci Methods. 2011;195:61-66. [DOI] [PubMed] [Google Scholar]
- 29. Simard JM, Pampori A, Keledjian K, et al. Exposure of the thorax to a sublethal blast wave causes a hydrodynamic pulse that leads to perivenular inflammation in the brain. J Neurotrauma. 2014;31:1292-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cernak I, Savic J, Malicevic Z, et al. Involvement of the central nervous system in the general response to pulmonary blast injury. J Trauma. 1996;40: S100-S104. [DOI] [PubMed] [Google Scholar]
- 31. Rafaels KA, Cutcliffe HC, Salzar RS, et al. Injuries of the head from backface deformation of ballistic protective helmets under ballistic impact. J Forensic Sci. 2015;60:219-225. [DOI] [PubMed] [Google Scholar]
- 32. Weisenbach CA, Logsdon K, Salzar RS, Chancey VC, Brozoski F. Preliminary investigation of skull fracture patterns using an impactor representative of helmet back-face deformation. Mil Med. 2018;183:287-293. [DOI] [PubMed] [Google Scholar]
- 33. Orgel J, Madhurapantula RS, Eidsmore A, et al. X-ray diffraction reveals blunt-force loading threshold for nanoscopic structural change in ex vivo neuronal tissues. J Synchrotron Radiat. 2019;26:89-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamamoto S, DeWitt DS, Prough DS. Impact & blast traumatic brain injury: implications for therapy [published online ahead of print January 26, 2019]. Molecules. doi:10.3390/molecules23020245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Silver JM, McAllister TW, Arciniegas DB. Textbook of Traumatic Brain Injury. Arlington, VA: American Psychiatric Publishing; 2019. [Google Scholar]
- 36. Greer N, Sayer N, Koeller E, Velasquez T, Wilt TJ. Outcomes associated with blast versus nonblast-related traumatic brain injury in US military service members and veterans: a systematic review. J Head Trauma Rehabil. 2018;33:E16-E29. [DOI] [PubMed] [Google Scholar]
- 37. Agoston DV, Kamnaksh A. Modeling the neurobehavioral consequences of blast-induced traumatic brain injury spectrum disorder and identifying related biomarkers. In: Kobeissy FH, ed. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton, FL: CRC Press and Taylor & Francis; 2015:307-326. [PubMed] [Google Scholar]
- 38. Mu W, Catenaccio E, Lipton ML. Neuroimaging in blast-related mild traumatic brain injury. J Head Trauma Rehabil. 2017;32:55-69. [DOI] [PubMed] [Google Scholar]
- 39. Salat DH, Robinson ME, Miller DR, Clark DC, McGlinchey RE. Neuroimaging of deployment-associated traumatic brain injury (TBI) with a focus on mild TBI (mTBI) since 2009. Brain Inj. 2017;31:1204-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Przekwas A, Somayaji MR, Gupta RK. Synaptic mechanisms of blast-induced brain injury. Front Neurol. 2016;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kamimori GH, LaValle CR, Eonta SE, Carr W, Tate C, Wang KKW. Longitudinal investigation of neurotrauma serum biomarkers, behavioral characterization, and brain imaging in soldiers following repeated low-level blast exposure (New Zealand Breacher Study). Mil Med. 2018;183:28-33. [DOI] [PubMed] [Google Scholar]
- 42. Agoston DV, Shutes-David A, Peskind ER. Biofluid biomarkers of traumatic brain injury. Brain Inj. 2017;31:1195-1203. [DOI] [PubMed] [Google Scholar]
- 43. Azar S, Hasan A, Younes R, et al. Biofluid proteomics and biomarkers in traumatic brain injury. Methods Mol Biol. 2017;1598:45-63. [DOI] [PubMed] [Google Scholar]
- 44. Lorente L. Biomarkers associated with the outcome of traumatic brain injury patients [published online ahead of print October 27, 2017]. Brain Sci. doi:10.3390/brainsci7110142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Strathmann FG, Schulte S, Goerl K, Petron DJ. Blood-based biomarkers for traumatic brain injury: evaluation of research approaches, available methods and potential utility from the clinician and clinical laboratory perspectives. Clin Biochem. 2014;47:876-888. [DOI] [PubMed] [Google Scholar]
- 46. US Food & Drug Administration. FDA authorizes marketing of first blood test to aid in the evaluation of concussion in adults. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm596531.htm. Updated 2018. Accessed April 16, 2019.
- 47. Stuhmiller JH, Phillips Y, Richmond D. The physics and mechanisms of primary blast injury. In: Zajtchuk R, Jenkins DP, Bellamy RF, eds. Conventional Warfare: Ballistic, Blast, and Burn Injuries. Washington, DC: Department of the Army, Office of the Surgeon General; 1991:241-270. [Google Scholar]
- 48. Bowen IG, Fletcher ER, Richmond DR. Estimate of Man’s Tolerance to the Direct Effects of Air Blast. Albuquerque, NM: Lovelace Foundation for Medical Education and Research; 1968. [Google Scholar]
- 49. White CS. The scope of blast and shock biology and problem areas in relating physical and biological parameters. Ann N Y Acad Sci. 1968;152:89-102. [DOI] [PubMed] [Google Scholar]
- 50. Zhu F, Chou CC, Yang KH, King AI. Some considerations on the threshold and inter-species scaling law for primary blast-induced traumatic brain injury: a semi-analytical approach. J Mech Med Biol. 2013;13:1350065. [Google Scholar]
- 51. Panzer MB, Wood GW, Bass CR. Scaling in neurotrauma: how do we apply animal experiments to people? Exp Neurol. 2014;261:120-126. [DOI] [PubMed] [Google Scholar]
- 52. Engel CC, Hoch E, Simmons M. The neurological effects of repeated exposure to military occupational blast: implications for prevention and health. Paper presented at: Proceedings, Findings, and Expert Recommendations From the Seventh Department of Defense State-of-the-Science Meeting; 23–25 January 2018; Santa Monica, CA. [Google Scholar]
- 53. Leggieri MJ, Jr, Bieler D, Bjarnason S, et al. Environmental toxicology of blast exposures: injury metrics, modelling, methods and standards. J R Army Med Corps. 2019;165:7-9. [DOI] [PubMed] [Google Scholar]
- 54. Goldstein LE, McKee AC, Stanton PK. Considerations for animal models of blast-related traumatic brain injury and chronic traumatic encephalopathy. Alzheimers Res Ther. 2014;6:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Watts S, Kirkman E, Bieler D, et al. Guidelines for using animal models in blast injury research. J R Army Med Corps. 2019;165:38-40. [DOI] [PubMed] [Google Scholar]
- 56. Cernak I. Blast injuries and blast-induced neurotrauma: overview of pathophysiology and experimental knowledge models and findings. In: Kobeissy FH, ed. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton, FL: CRC Press and Taylor & Francis; 2015:629-642. [PubMed] [Google Scholar]
- 57. Stuhmiller JH, Santee WR, Friedl K. Blast Injury: Translating Research Into Operational Medicine. Washington, DC: Borden Institute; 2008. [Google Scholar]
- 58. Desmoulin GT, Dionne JP. Blast-induced neurotrauma: surrogate use, loading mechanisms, and cellular responses. J Trauma. 2009;67:1113-1122. [DOI] [PubMed] [Google Scholar]
- 59. Cernak I, Noble-Haeusslein LJ. Traumatic brain injury: an overview of pathobiology with emphasis on military populations. J Cereb Blood Flow Metab. 2010;30:255-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Waselenko JK, MacVittie TJ, Blakely WF, et al. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med. 2004;140:1037-1051. [DOI] [PubMed] [Google Scholar]
- 61. Medical Effects of Ionizing Radiation (MEIR) Course. Acute Radiation Syndrome and Combined Injury. 2013. Uniformed Services University of the Health Sciences: Armed Forces Radiobiology Research Institute. [Google Scholar]
- 62. Walker RI, Cerveny TJ, Alt LA; for Institute AFRR, Agency USDN. Medical Consequences of Nuclear Warfare. Falls Church, VA: TMM Publications, Office of the Surgeon General; 1989. [Google Scholar]
- 63. Armed Forces Radiobiology Research Institute (AFFRI). Assessment of radiation injury (biodosimetry). https://www.usuhs.edu/afrri/biodosimetry. Updated 2019. Accessed March 26, 2019.
- 64. Dainiak N, Albanese J, Kaushik M, et al. Concepts of operations for a US Dosimetry and Biodosimetry Network [published online ahead of print February 6, 2019]. Radiat Prot Dosimetry. doi:10.1093/rpd/ncy294. [DOI] [PubMed] [Google Scholar]
- 65. Blakely WF, Madrid JP, Sandgren DJ. Biodosimetry medical recording-use of the Biodosimetry Assessment Tool. Health Phys. 2010;99:S184-S191. [DOI] [PubMed] [Google Scholar]
- 66. Sandgren DJ, Salter CA, Levine IH, Ross JA, Lillis-Hearne PK, Blakely WF. Biodosimetry Assessment Tool (BAT) software-dose prediction algorithms. Health Phys. 2010;99:S171-S183. [DOI] [PubMed] [Google Scholar]
- 67. Goans RE. Clinical application of the AFRRI BAT computer program. Health Phys. 2010;99:S192-S196. [DOI] [PubMed] [Google Scholar]
- 68. Merritt VC, Clark AL, Sorg SF, et al. Apolipoprotein E (APOE) epsilon4 genotype is associated with reduced neuropsychological performance in military veterans with a history of mild traumatic brain injury. J Clin Exp Neuropsychol. 2018;40:1050-1061. [DOI] [PubMed] [Google Scholar]
- 69. Merritt VC, Lapira KM, Clark AL, et al. APOE-ε4 genotype is associated with elevated post-concussion symptoms in military Veterans with a remote history of mild traumatic brain injury [published online ahead of print December 6, 2018]. Arch Clin Neuropsychol. doi:10.1093/arclin/acy082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang YJ, Chen KY, Kuo LN, et al. The association between BDNF Val66Met polymorphism and emotional symptoms after mild traumatic brain injury. BMC Med Genet. 2018;19:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Willmott C, Withiel T, Ponsford J, Burke R. COMT Val158Met and cognitive and functional outcomes after traumatic brain injury. J Neurotrauma. 2014;31:1507-1514. [DOI] [PubMed] [Google Scholar]
- 72. Davidson J, Cusimano MD, Bendena WG. Post-traumatic brain injury: genetic susceptibility to outcome. Neuroscientist. 2015;21:424-441. [DOI] [PubMed] [Google Scholar]
- 73. McFadyen C, Zeiler FA, Newcombe V, et al. Apolipoprotein E4 polymorphism and outcomes from traumatic brain injury: a living systematic review and meta-analysis [published online ahead of print April 23, 2019]. J Neurotrauma. doi:10.1089/neu.2018.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhou W, Xu D, Peng X, Zhang Q, Jia J, Crutcher KA. Meta-analysis of APOE4 allele and outcome after traumatic brain injury. J Neurotrauma. 2008;25:279-290. [DOI] [PubMed] [Google Scholar]
- 75. Ponsford J, McLaren A, Schonberger M, et al. The association between apolipoprotein E and traumatic brain injury severity and functional outcome in a rehabilitation sample. J Neurotrauma. 2011;28:1683-1692. [DOI] [PubMed] [Google Scholar]
- 76. Millar K, Nicoll JA, Thornhill S, Murray GD, Teasdale GM. Long term neuropsychological outcome after head injury: relation to APOE genotype. J Neurol Neurosurg Psychiatry. 2003;74:1047-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Teasdale GM, Murray GD, Nicoll JA. The association between APOE epsilon4, age and outcome after head injury: a prospective cohort study. Brain. 2005;128:2556-2561. [DOI] [PubMed] [Google Scholar]
- 78. Chamelian L, Reis M, Feinstein A. Six-month recovery from mild to moderate traumatic brain injury: the role of APOE-epsilon4 allele. Brain. 2004;127:2621-2628. [DOI] [PubMed] [Google Scholar]
- 79. Winkler EA, Yue JK, Ferguson AR, et al. COMT Val(158)Met polymorphism is associated with post-traumatic stress disorder and functional outcome following mild traumatic brain injury. J Clin Neurosci. 2017;35:109-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Winkler EA, Yue JK, McAllister TW, et al. COMT Val 158 Met polymorphism is associated with nonverbal cognition following mild traumatic brain injury. Neurogenetics. 2016;17:31-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lipsky RH, Sparling MB, Ryan LM, et al. Association of COMT Val158Met genotype with executive functioning following traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2005;17:465-471. [DOI] [PubMed] [Google Scholar]
- 82. McAllister TW, Tyler AL, Flashman LA, et al. Polymorphisms in the brain-derived neurotrophic factor gene influence memory and processing speed one month after brain injury. J Neurotrauma. 2012;29:1111-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Davis LC, Sherer M, Sander AM, et al. Preinjury predictors of life satisfaction at 1 year after traumatic brain injury. Arch Phys Med Rehabil. 2012;93:1324-1330. [DOI] [PubMed] [Google Scholar]
- 84. Seagly KS, O’Neil RL, Hanks RA. Pre-injury psychosocial and demographic predictors of long-term functional outcomes post-TBI. Brain Inj. 2018;32:78-83. [DOI] [PubMed] [Google Scholar]
- 85. Bertisch H, Satris G, Temkin N, Barber J, Manley GT. Rehabilitation trajectories and outcomes in individuals with mild traumatic brain injury and psychiatric histories: a TRACK-TBI Pilot study. J Head Trauma Rehabil. 2019;34:36-44. [DOI] [PubMed] [Google Scholar]
- 86. Ivins BJ, Schwab KA, Warden D, et al. Traumatic brain injury in U.S. Army paratroopers: prevalence and character. J Trauma. 2003;55:617-621. [DOI] [PubMed] [Google Scholar]
- 87. Lindquist LK, Love HC, Elbogen EB. Traumatic brain injury in Iraq and Afghanistan Veterans: new results from a national random sample study. J Neuropsychiatry Clin Neurosci. 2017;29:254-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yurgil KA, Barkauskas DA, Vasterling JJ, et al. Association between traumatic brain injury and risk of posttraumatic stress disorder in active-duty marines. JAMA Psychiatry. 2014;71:149-157. [DOI] [PubMed] [Google Scholar]
- 89. Bailes JE, Dashnaw ML, Petraglia AL, Turner RC. Cumulative effects of repetitive mild traumatic brain injury. Prog Neurol Surg. 2014;28:50-62. [DOI] [PubMed] [Google Scholar]
- 90. Bailes JE, Petraglia AL, Omalu BI, Nauman E, Talavage T. Role of subconcussion in repetitive mild traumatic brain injury. J Neurosurg. 2013;119:1235-1245. [DOI] [PubMed] [Google Scholar]
- 91. McKee AC, Stein TD, Kiernan PT, Alvarez VE. The neuropathology of chronic traumatic encephalopathy. Brain Pathol. 2015;25:350-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Nauman EA, Talavage TM. Subconcussive trauma. Handb Clin Neurol. 2018;158:245-255. [DOI] [PubMed] [Google Scholar]
- 93. Chapman JC, Diaz-Arrastia R. Military traumatic brain injury: a review. Alzheimers Dement. 2014;10:S97-S104. [DOI] [PubMed] [Google Scholar]
- 94. McLellan TM, Riviere LA, Williams KW, McGurk D, Lieberman HR. Caffeine and energy drink use by combat arms soldiers in Afghanistan as a countermeasure for sleep loss and high operational demands. Nutr Neurosci. 2018:1-10. [DOI] [PubMed] [Google Scholar]
- 95. Manchester J, Eshel I, Marion DW. The benefits and risks of energy drinks in young adults and military service members. Mil Med. 2017;182:e1726-e1733. [DOI] [PubMed] [Google Scholar]
- 96. Iverson KM, Hendricks AM, Kimerling R, et al. Psychiatric diagnoses and neurobehavioral symptom severity among OEF/OIF VA patients with deployment-related traumatic brain injury: a gender comparison. Womens Health Issues. 2011;21:S210-S217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Defense and Veterans Brain Injury Center. Concussion/mild traumatic brain injury and posttraumatic stress disorder. https://dvbic.dcoe.mil/system/files/resources/4628.1.1.3_ConcussionmTBI_and_PTSD%20FS_508.pdf. Updated February 2019.
- 98. Isokuortti H, Iverson GL, Kataja A, Brander A, Ohman J, Luoto TM. Who gets head trauma or recruited in mild traumatic brain injury research? J Neurotrauma. 2016;33:232-241. [DOI] [PubMed] [Google Scholar]
- 99. Broshek DK, De Marco AP, Freeman JR. A review of post-concussion syndrome and psychological factors associated with concussion. Brain Inj. 2015;29:228-237. [DOI] [PubMed] [Google Scholar]
- 100. Howe LL. Giving context to post-deployment post-concussive-like symptoms: blast-related potential mild traumatic brain injury and comorbidities. Clin Neuropsychol. 2009;23:1315-1337. [DOI] [PubMed] [Google Scholar]
- 101. Armed Forces Health Surveillance Center. Surveillance snaphshot: responses to the traumatic brain injury (TBI) screening questions on the 2012 version of the post-deployment health assessment (DD Form 2796). MSMR. 2015;22:12-13. [PubMed] [Google Scholar]
- 102. Armistead-Jehle P, Soble JR, Cooper DB, Belanger HG. Unique aspects of traumatic brain injury in military and Veteran populations. Phys Med Rehabil Clin N Am. 2017;28:323-337. [DOI] [PubMed] [Google Scholar]
- 103. Broglio SP, McCrea M, McAllister T, et al. A national study on the effects of concussion in collegiate athletes and US military service academy members: the NCAA-DoD Concussion Assessment, Research and Education (CARE) consortium structure and methods. Sports Med. 2017;47:1437-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77-85. [DOI] [PubMed] [Google Scholar]
- 105. Iverson KM, Sayer NA, Meterko M, et al. Intimate partner violence among female OEF/OIF/OND veterans who were evaluated for traumatic brain injury in the Veterans Health Administration: a preliminary investigation [published online ahead of print April 1, 2017]. J Interpers Violence. doi:10.1177/0886260517702491. [DOI] [PubMed] [Google Scholar]
- 106. Iverson KM, Pogoda TK. Traumatic brain injury among women veterans: an invisible wound of intimate partner violence. Med Care. 2015;53:S112-S119. [DOI] [PubMed] [Google Scholar]
- 107. Ziebell JM, Rowe RK, Muccigrosso MM, et al. Aging with a traumatic brain injury: could behavioral morbidities and endocrine symptoms be influenced by microglial priming. Brain Behav Immun. 2017;59:1-7. [DOI] [PubMed] [Google Scholar]
- 108. Johnson VE, Stewart W. Traumatic brain injury: age at injury influences dementia risk after TBI. Nat Rev Neurol. 2015;11:128-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kannus P, Palvanen M, Niemi S, et al. Increasing number and incidence of fall-induced severe head injuries in older adults: nationwide statistics in Finland in 1970-1995 and prediction for the future. Am J Epidemiol. 1999;149: 143-150. [DOI] [PubMed] [Google Scholar]
- 110. Karibe H, Hayashi T, Narisawa A, Kameyama M, Nakagawa A, Tominaga T. Clinical characteristics and outcome in elderly patients with traumatic brain injury: for establishment of management strategy. Neurol Med Chir. 2017;57:418-425. [DOI] [PMC free article] [PubMed] [Google Scholar]




