Abstract
Patients with febrile neutropenia (FN) often are subject to antibiotic and diagnostic test overuse. We sought to improve appropriate use of antimicrobials and diagnostic tests for patients with FN. We used a blended quality approach with Lean Six Sigma tools and iterative improvement of a clinical decision aid to guide providers through empirical antimicrobial selection and diagnostic evaluation of patients with FN during a yearlong period. We evaluated the incidence of nonadherence to best practice before, during, and after rollout of a clinical decision aid in conjunction with an educational initiative. At baseline, 71% of patients with FN had at least one critical deviation from best practice. During the project, the percentage decreased to 27.3%; 4 months after the project was completed, the percentage was 33.3% (P = .04). A clinical decision aid can improve adherence to best practices for the empirical management of FN.
INTRODUCTION
Neutropenia induced by antineoplastic chemotherapy is a common complication of cancer treatment. Patients with hematologic malignancies are at increased risk for infectious complications during neutropenia. More than 80% of neutropenic episodes in this population may be complicated by fever.1 Febrile neutropenia (FN) in high-risk patients with hematologic malignancies is considered a medical emergency (average mortality, 14.3%).2
Several national and international societies have developed evidence-based guidelines for clinicians treating patients with FN.1,3-5 The guidelines outline management strategies that focus on a time-sensitive, patient-centered evaluation with judicious and effective antimicrobial selection.
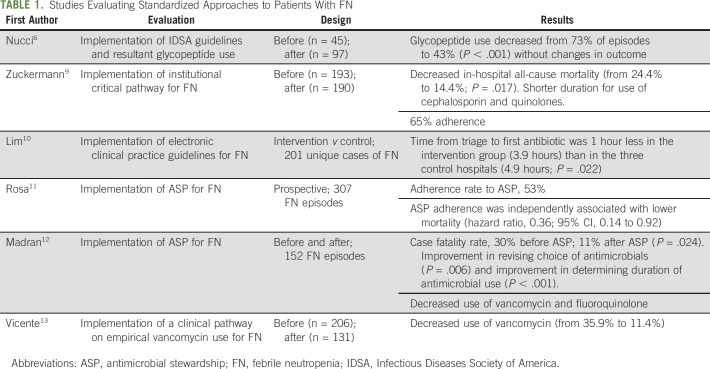
Compliance with FN guidelines improves the effective use of antimicrobials,6 but the suboptimal rate of adherence to guidelines at many institutions results in poor antimicrobial stewardship practices, larger pharmaceutical expenses, and negative patient care outcomes.7,8 In many studies, implementation of institutional evidence-based care models for patients with FN has improved outcomes (Table 1).
TABLE 1.
Studies Evaluating Standardized Approaches to Patients With FN
Implementation of antimicrobial stewardship programs for patients with FN is often challenging, because they are vulnerable hosts for various microbial infections. These patients may need more aggressive regimens because of their ongoing exposure to health care environments, the potential for colonization with nosocomial pathogens, and their ongoing immunosuppression. However, data suggest that implementing antimicrobial stewardship programs for patients with FN improves mortality.11,12,14-17 Thus, we sought to systematically evaluate and improve our institutional guidance for management of FN.
METHODS
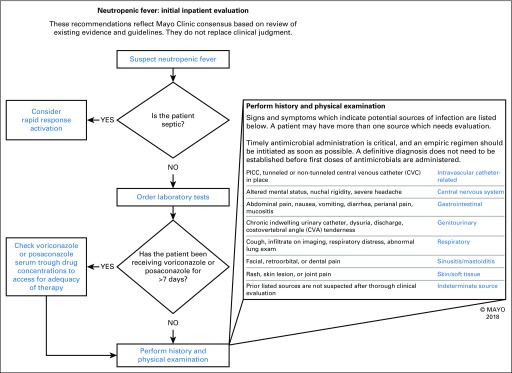
Our scope was the empirical treatment of fever (ie, treatment in the first 24 hours when a confirmed microbiologic or other cause was not available) in adult patients who had neutropenia according to results of laboratory testing. We first attempted to define the optimal FN care model within our institution. With an evidence-based review and the infectious disease physician stakeholders identified as subject matter experts, we used a modified Delphi approach to construct a care model on the basis of published guidelines and local practice. In the modified Delphi model, subject matter experts were asked to separately submit what they considered the most critical elements and pathways to optimal care of a patient with FN. Then, they were asked to vote anonymously on a ranking of these elements in sequential rounds of online voting. The separate submission and anonymous voting process were intended to facilitate experts’ convergence on an answer to a problem without concerns of disagreements leading to repercussions or rhetoric swaying opinions more than re-evaluation of evidence. When convergence on critical elements was consistent with the guidelines, we constructed a model identifying the critical elements for an evidence-based algorithm for local FN empirical care (Fig 1).
Fig 1.
Screenshot of the AskMayoExpert interface for the febrile neutropenia algorithm. PICC, peripherally inserted central catheter. (Used with permission of Mayo Foundation for Medical Education and Research.)
Measuring Compliance With Best Practice
The algorithm was used to evaluate our baseline compliance with best practice. Errors were categorized as critical (ie, having more immediate clinical impact; pertaining to treatment or antibiotic choices or to major diagnostic studies, such as radiologic studies or invasive studies) or noncritical. This division between critical and noncritical processes was based on discussion with subject matter experts and considered those that affect care immediately (eg, invasive procedures and antibiotic choices) or have a high cost (invasive or radiologic studies) as critical, whereas deviation from laboratory testing was designated as noncritical.
Using Mayo Clinic’s Advanced Cohort Explorer, an electronic health record search tool, we identified all potential patients with FN admitted in a 2-month baseline period (January 1, 2016 through March 1, 2016). Patients with potential FN were identified based on a combination of text searching for terms pertaining to febrile neutropenia in clinical notes as well as admission vital signs and absolute neutrophil counts. Manual review was used to determine if these patients truly met the criteria of febrile neutropenia. We subsequently assessed whether antimicrobials, radiographs, and laboratory studies were each matched with recommendations from the algorithm. This same methodology was used for project-end and post-project measurements. Project-end measurements were performed when the content of our algorithm was in its final form; post-project measurements were conducted 3 months after broader dissemination of the algorithm.
Interventions
From our baseline assessment, we aimed to decrease critical deviations from optimal FN management by 50%. Through several focus group meetings, we identified several factors contributing to nonstandard care. The focus groups identified knowledge gaps in best practice for restrictive use of antimicrobials in FN and showed that the present ordering systems did not facilitate appropriate antimicrobial selection for FN. Because our institution was preparing for a change in the electronic health record system and a moratorium prevented modifying the order set modification, restructuring the ordering system was not feasible. Therefore, we focused on creating a tool to assist providers in identifying and using appropriate diagnostics and antimicrobial therapy for management of FN in the first 24 hours.
We used AskMayoExpert (AME), a Mayo Clinic application for medical knowledge, to publish a care process model (CPM) for standardization of FN management. AME content is written and vetted by Mayo Clinic experts and is a culmination of best-practice advice on hundreds of medical conditions.
To create and disseminate the CPM, we used a plan-do-study-act cycle. We composed an algorithm for best-practice evaluation and antimicrobial prescribing on the basis of the suspected patient syndrome causing fever. We solicited additional input from the stakeholders in the Divisions of Hospital Infectious Diseases, Hematology, and Hospital Internal Medicine to make a poster and an electronic PDF file reference. This was disseminated through the Hospital Internal Medicine, Hematology, and Oncology disease-oriented groups (DOGs). From the multidisciplinary DOG members, we obtained endorsement for these processes to establish management support for the model as it stood. We sought monthly input from the DOG members to ensure that the algorithm was clear, addressed the situations being seen clinically, and did not contain errors or cause adverse outcomes. We then refined and updated the tool on the Hematology Website every other month, with serial improvements on the basis of the input we received.
Statistical Analysis
Trends toward improvement or worsening were measured with the Cochrane-Armitage trend test. P values less than .05 were considered statistically significant. All analyses were performed with JMP 13 software (SAS Institute, Cary, NC).
RESULTS
At baseline, we identified 28 potential patients with FN, of whom 14 had true FN. Of 26 project-end patients, 11 had true FN. Of 32 post-project patients, 15 had true FN.
Source Evaluation
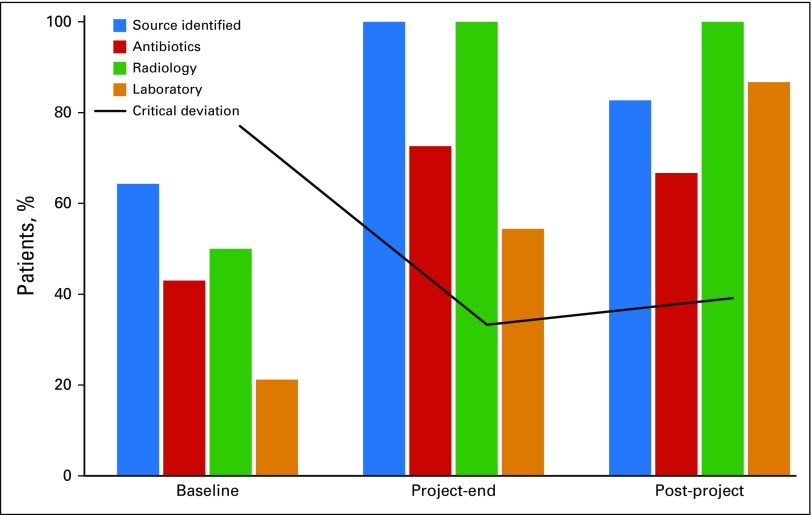
Evaluation for a source of infection was critical for determining the appropriate pathway. At baseline, 64.3% of patients had documentation of confirmed or suspected sources. For the project-end measurements, 100% of patients had documentation; for post-project measurements, 82.5% had documentation (Fig 2; P = .12).
Fig 2.
Adherence to best practice over time. Adherence is summarized for patients with febrile neutropenia at the baseline, project-end, and post-project periods. The bars summarize results for the following: documentation of the source of infection (P = .12), appropriate use of antibiotics (P = .19), appropriate use of radiologic studies (P < .01), appropriate use of laboratory studies (P < .01), and critical deviation from the algorithm (P = .04). Courtesy of the Mayo Clinic.
Antimicrobial Use
Antimicrobial use consistent with the best-practice algorithm was 42.9% at baseline, 72.7% at project end, and 66.6% at post project (Fig 2; P = .19). In every instance of antimicrobial overuse, vancomycin was used inappropriately, either alone or in combination with other antimicrobials. Other examples of overuse included inappropriate agent selection for double coverage for gram negatives, use of anaerobic coverage when not necessary, or use of carbapenems when not β-lactam or cephalosporin coverage would have been adequate.
Radiology and Laboratory Studies
Radiologic studies such as computed tomography, radiography, and magnetic resonance imaging were consistent with the algorithm for 50% of patients at baseline and for 100% at project end and at post project (Fig 2; P < .01). Laboratory studies were matched with the algorithm 21.4% of the time at baseline, 54.6% at project end, and 86.7% at post project (Fig 2; P < .01).
Critical Deviation
Our combined critical deviation end point, for patients who received nonstandard antimicrobials or underwent inappropriate use of major diagnostic studies, showed significant improvement (P = .04). The proportion of patients having a critical deviation was 71% at baseline, 27.3% at project end, and 33.3% at post project. Thus, we met our target of a 50% reduction in critical deviations (Fig 2).
Continued Use
Use of the CPM continued through 2017, with a monthly mean of 40 (standard deviation, 11) from April through December 2017. This use was fairly consistent with our use during the project, indicating ongoing adoption into the practice.
DISCUSSION
Our project successfully implemented a complex algorithm for standardizing and optimizing care for patients with FN. Since its implementation, the CPM has been continually used within Mayo Clinic.
We identified vancomycin overuse as the main agent in antimicrobial nonadherence, which was our most persistent issue and a good marker of overall algorithm nonadherence. The antimicrobial stewardship group monitors adherence to this algorithm and vancomycin use on hematology services as part of its ongoing stewardship efforts and the control phase of our intervention. Since quarter 4 of 2017, use of vancomycin on the day of admission for patients on hematology services has remained relatively flat, between 2.08% and 4.64%. Although this reflects all patients and does not take into account other contributing factors, such as reasons for admission changing and competing stewardship initiatives, this would support that there has not been a drift toward increasing use of vancomycin and deviation from the protocol.
AME has previously been shown to be an effective tool for rapid, point-of-care access to accurate clinical data.18 Our study, to our knowledge, is the first to show a clinical outcome related to this tool; deviations from the protocol for antimicrobials and diagnostic tests were decreased and a CPM was implemented. Our project showed the utility of AME as a quality-improvement tool in clinical practice.
We found high rates of nonadherence to the best-practice algorithms at baseline, which, despite improvements, persisted into the post-project period. Part of this persistence was likely the result of the highly variable and complex nature of patients with FN, and part was likely the result of our use of clinical decision support rather than to a more direct effect on ordering. Follow-up work will be performed to investigate the utility and advisability of order sets for patients with FN with this algorithm and the comparative effectiveness of this clinical decision support approach.
Overall, through our project, we successfully developed a consensus best-practice standard for evaluating and treating patients with FN. We showed that AME can be used to modify clinician practices to better align with best practices. Finally, we identified key metrics, such as vancomycin overuse, which can be used for ongoing monitoring of adherence to best practices. These tools and template algorithm can be used to inform similar efforts at other institutions for developing syndromic-based, locally acceptable guidelines for treatment of FN.
ACKNOWLEDGMENT
Supported by National Center for Research Resources/National Center for Advancing Translational Sciences Clinical and Translational Science Awards Grant No. UL1 TR002377. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
AUTHOR CONTRIBUTIONS
Conception and design: John C. O’Horo, Jasmine R. Marcelin, Omar M. Abu Saleh, Christina G. Rivera, Aaron J. Tande, John W. Wilson, Pritish K. Tosh, Douglas R. Osmon
Collection and assembly of data: John C. O’Horo, Jasmine R. Marcelin, Omar M. Abu Saleh, Amelia K. Barwise, Patricia M. Odean, Pritish K. Tosh
Data analysis and interpretation: John C. O’Horo, John W. Wilson
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Standardizing Febrile Neutropenia Management: Antimicrobial Stewardship in the Hematologic Malignancy Population
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jop/site/ifc/journal-policies.html.
Amelia K. Barwise
Stock and Other Ownership Interests: Ambient Clinical Analytics (I)
Patents, Royalties, Other Intellectual Property: My husband has intellectual property and is on the board of Ambient Clinical Analytics, a company that commercializes licensed software. However, none of this was relevant to the publication under review for this journal (I)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:e56–e93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 2.Kuderer NM, Dale DC, Crawford J, et al. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106:2258–2266. doi: 10.1002/cncr.21847. [DOI] [PubMed] [Google Scholar]
- 3.Rosa RG, Goldani LZ. Cohort study of the impact of time to antibiotic administration on mortality in patients with febrile neutropenia. Antimicrob Agents Chemother. 2014;58:3799–3803. doi: 10.1128/AAC.02561-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Averbuch D, Orasch C, Cordonnier C, et al. European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: Summary of the 2011 4th European Conference on Infections in Leukemia. Haematologica. 2013;98:1826–1835. doi: 10.3324/haematol.2013.091025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klastersky J, de Naurois J, Rolston K, et al. Management of febrile neutropaenia: ESMO Clinical Practice Guidelines. Ann Oncol. 2016;27(suppl 5):v111–v118. doi: 10.1093/annonc/mdw325. [DOI] [PubMed] [Google Scholar]
- 6.Nucci M, Landau M, Silveira F, et al. Application of the IDSA guidelines for the use of antimicrobial agents in neutropenic patients: Impact on reducing the use of glycopeptides. Infect Control Hosp Epidemiol. 2001;22:651–653. doi: 10.1086/501839. [DOI] [PubMed] [Google Scholar]
- 7.Wright JD, Neugut AI, Ananth CV, et al. Deviations from guideline-based therapy for febrile neutropenia in cancer patients and their effect on outcomes. JAMA Intern Med. 2013;173:559–568. doi: 10.1001/jamainternmed.2013.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baugh CW, Wang TJ, Caterino JM, et al. Emergency department management of patients with febrile neutropenia: Guideline concordant or overly aggressive? Acad Emerg Med. 2017;24:83–91. doi: 10.1111/acem.13079. [DOI] [PubMed] [Google Scholar]
- 9.Zuckermann J, Moreira LB, Stoll P, et al. Compliance with a critical pathway for the management of febrile neutropenia and impact on clinical outcomes. Ann Hematol. 2008;87:139–145. doi: 10.1007/s00277-007-0390-7. [DOI] [PubMed] [Google Scholar]
- 10. Lim C, Bawden J, Wing A, et al: Febrile neutropenia in EDs: The role of an electronic clinical practice guideline. Am J Emerg Med 30:5-11, 11.e1-5, 2012. [DOI] [PubMed]
- 11.Rosa RG, Goldani LZ, dos Santos RP. Association between adherence to an antimicrobial stewardship program and mortality among hospitalised cancer patients with febrile neutropaenia: A prospective cohort study. BMC Infect Dis. 2014;14:286. doi: 10.1186/1471-2334-14-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madran B, Keske Ş, Tokça G, et al. Implementation of an antimicrobial stewardship program for patients with febrile neutropenia. Am J Infect Control. 2018;46:420–424. doi: 10.1016/j.ajic.2017.09.030. [DOI] [PubMed] [Google Scholar]
- 13.Vicente M, Al-Nahedh M, Parsad S, et al. Impact of a clinical pathway on appropriate empiric vancomycin use in cancer patients with febrile neutropenia. J Oncol Pharm Pract. 2017;23:575–581. doi: 10.1177/1078155216668672. [DOI] [PubMed] [Google Scholar]
- 14.Aguilar-Guisado M, Espigado I, Martín-Peña A, et al. Optimisation of empirical antimicrobial therapy in patients with haematological malignancies and febrile neutropenia (How Long study): An open-label, randomised, controlled phase 4 trial. Lancet Haematol. 2017;4:e573–e583. doi: 10.1016/S2352-3026(17)30211-9. [DOI] [PubMed] [Google Scholar]
- 15.Le Clech L, Talarmin JP, Couturier MA, et al. Early discontinuation of empirical antibacterial therapy in febrile neutropenia: The ANTIBIOSTOP study. Infect Dis (Lond) 2018;50:539–549. doi: 10.1080/23744235.2018.1438649. [DOI] [PubMed] [Google Scholar]
- 16.Haddad HE, Chaftari AM, Hachem R, et al. Procalcitonin guiding antimicrobial therapy duration in febrile cancer patients with documented infection or neutropenia. Sci Rep. 2018;8:1099. doi: 10.1038/s41598-018-19616-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.United States Government National action plan for combating antibiotic-resistant bacteria. 2015 https://obamawhitehouse.archives.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf
- 18.Cook DA, Enders F, Linderbaum JA, et al. Speed and accuracy of a point of care web-based knowledge resource for clinicians: A controlled crossover trial. Interact J Med Res. 2014;3:e7. doi: 10.2196/ijmr.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]