Abstract
PURPOSE:
Fear of cancer recurrence is highly prevalent among adult survivors of cancer. The role of fear of recurrence in the emotional distress of survivors of cancer, as well as health behaviors that may directly affect their health, remains unclear. To advance oncology practice, this study sought to examine the extent to which fear of recurrence stemming from physical symptoms accounts for emotional distress in a large sample of adult survivors of cancer and to extend the model to explain postdiagnosis self-reported health behavior change.
METHODS:
In 2016, 258 survivors of cancer at an academic hospital completed a survey of psychosocial needs. Items assessed physical symptoms (checklist), fear of cancer recurrence (Assessment of Survivor Concerns), emotional distress (anxiety and depressed mood), and health behaviors (current alcohol use, physical activity, diet, and sunscreen use, as well as changes after cancer diagnosis) informed by National Comprehensive Cancer Network survivorship guidelines. Indirect effects regression models accounting for relevant covariates (age and treatment history) used 5,000-iteration bootstrapping.
RESULTS:
Higher fear of cancer recurrence was associated with greater number of physical symptoms (P < .001), greater emotional distress (P < .05), lower moderate or vigorous physical activity (P < .05), higher sunscreen use (P < .05), and postdiagnosis increases in alcohol use (P < .01) and reductions in physical activity (P < .01). Fear of cancer recurrence models accounted for almost half of the variance in distress of survivors of cancer (R2 = 0.44, P < .001) and, to a lesser yet significant extent, changes in alcohol consumption (R2 = 0.09, P < .001) and physical activity (R2 = 0.06, P = .003).
CONCLUSION:
Fear of cancer recurrence plays a central role in the emotional distress and key health behaviors of survivors of cancer. These findings support fear of cancer recurrence as a potential target for emotional health and health behavior change interventions.
INTRODUCTION
Cancer survivorship has increased over the past 50 years, with 19 million survivors projected to be living in the United States by 2024. After completion of active treatment, survivors of cancer are faced with both physical and emotional challenges. A prominent and common difficulty is fear of cancer recurrence,1 with moderate to high levels of fear present in 30% to 70% of survivors of cancer.2,3 Survivors’ worries about disease recurrence may continue for years after treatment ends4-7 and can persist at levels equal to that experienced at the time of diagnosis.8
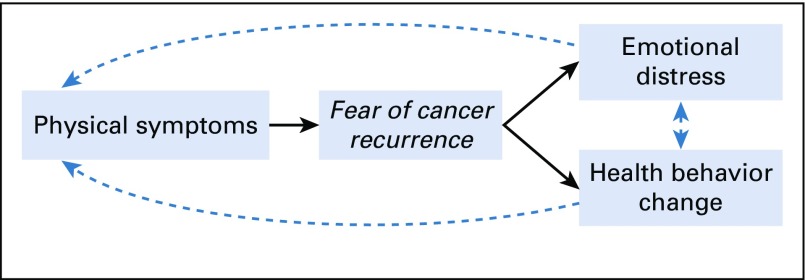
As cancer survivorship continues to increase, it is critical to develop a comprehensive understanding of fear of cancer recurrence, its precipitants, and its consequences on the emotional and physical health of survivors of cancer. Conceptual models of fear of cancer recurrence have integrated transdisciplinary theories of uncertainty in illness, health decision making, and health behavior change9,10 and suggest that physical symptoms are potent triggers of fear of cancer recurrence, whereby survivors of cancer interpret bodily sensations (eg, pain, fatigue) as potential indicators of cancer recurrence.4,7,11-13 If elevated, fear of cancer recurrence may lead to emotional distress; in extreme cases, fear of cancer recurrence may lead to hopelessness, demoralization, and even suicidal ideation.14 Recently, we found support for this pathway among a small sample of survivors of cancer, whereby fear of cancer recurrence stemming from physical symptoms explained 35% of the variability in distress levels13 (Fig 1). These results yield promising yet preliminary evidence requiring replication in a larger sample.
Fig 1.
Fear of cancer recurrence conceptual model.
To manage fear of cancer recurrence and its associated distress, survivors of cancer may develop coping behaviors that can affect their emotional and physical health. To date, most research describing behavioral consequences of fear of cancer recurrence has focused on patterns of seeking control (eg, seeking reassurance from medical care providers) or engaging in avoidance (eg, nonadherence to follow-up screening).6,8,15-26 However, few studies have examined links between fear of cancer recurrence and health behaviors that may more directly affect the clinical outcomes of survivors of cancer, such as minimizing alcohol intake, engaging in regular physical activity, maintaining a healthy diet, and applying sunscreen regularly.27 Recent findings suggest that as few as 7.6% of adult survivors of cancer adhere to lifestyle recommendations outlined by the National Comprehensive Cancer Network (NCCN) health behavior guidelines for survivors of cancer.27,28 Given what is known about behavioral consequences of fear of cancer recurrence, it is plausible that survivors of cancer with fear of cancer recurrence may either seek to gain control of their health by improving health behaviors or, alternatively, engaging in avoidance, thereby worsening health behaviors. To the best of our knowledge, links between fear of cancer recurrence and other specific health behaviors have either been mixed (ie, physical activity)29-33 or remain unexamined (ie, alcohol use, diet, and sunscreen use). In the current study, we aimed to examine the degree to which fear of cancer recurrence accounts for the association between physical symptoms and emotional distress (aim 1), as well as health behavior change after cancer diagnosis (aim 2), in a hospital outpatient sample of survivors of diverse cancers.
METHODS
Sample and Procedures
In 2016, 636 patients attending outpatient oncology clinics in a large northeast academic cancer center completed a paper survey assessing psychosocial needs and care preferences. Surveys were distributed to a convenience sample of patients presenting to the Massachusetts General Hospital Cancer Center and distributed by clinic staff across multiple disease-specific clinics. For the purposes of this study, survey responses were analyzed from early-stage (stage 0 to III) survivors of cancer who had completed (ie, not currently receiving) primary treatment with curative intent presenting for routine follow-up (n = 258).
Measures
Sociodemographic and medical characteristics.
Survivors reported their age, sex, race, education level, partner status, insurance status, cancer type, date of diagnosis, and treatment history (yes or no for surgery, radiation therapy, intravenous chemotherapy, oral chemotherapy, hormonal or endocrine therapy, and complementary or alternative therapy).
Fear of cancer recurrence.
Fear of cancer recurrence was measured by the Assessment of Survivor Concerns.34 The Assessment of Survivor Concerns asks respondents to rate the frequency of worries over the past month related to future diagnostic tests, another type of cancer, the survivor’s cancer coming back, dying, and the survivor’s health. Respondents were given the following prompt: “The following set of questions highlight some of the concerns people commonly experience as a result of cancer and its treatment. For each, please circle the response that best describes how you have been feeling in the past month.” Response options were provided using a 4-point Likert scale, ranging from 1 (not at all) to 4 (very much). Items were averaged to create a total score. Internal consistency in the present sample was good (α = .87).
Physical symptoms.
Number of physical symptoms was measured by asking respondents to indicate whether the following 17 symptoms related to managing adverse effects of cancer and cancer treatment were problematic (yes or no): pain; fatigue; hot flashes; lymphedema or swelling; tingling or numbness in hands or feet; nausea or vomiting; trouble swallowing; dental or mouth problems; diarrhea or constipation; bowel or bladder changes; memory problems; concentration difficulties; body changes; hair or skin care changes; balance, walking, or mobility; weight gain; and weight loss. Items were summed to create a total score. Internal consistency was excellent (α = .91).
Emotional distress.
Survivors were asked to rate the extent to which they have felt “nervous or worried” and “sad or depressed” over the past month. Response options were provided using a 5-point Likert scale, ranging from 1 (never) to 5 (always). The two items were summed to create a total score. Internal consistency was good (α = .83).
Health behaviors (current and change after cancer diagnosis).
Current health behaviors were assessed following NCCN survivorship guidelines for diet, physical activity, sunscreen use, and alcohol use27 and by the Alcohol Use Disorders Identification Test–Alcohol Consumption measure.35 In addition, respondents were asked how each health behavior had changed compared with before their diagnosis of cancer. Responses were coded in the direction of positive changes, as follows: decreased or worsened (−1), no change (0), or increased or improved (1). In addition, each health behavior was assessed by adapting screening questions from the 2016 NCCN health behavior guidelines.27
Statistical Analyses
Survey data were entered by study staff into a secure online database using Research Electronic Data Capture (REDCap) software (https://www.project-redcap.org/). All data were analyzed using SPSS version 20.0 (SPSS, Chicago, IL). Data were examined for normality of distributions. Descriptive statistics assessed patients’ sociodemographic and cancer characteristics, fear of cancer recurrence, physical symptoms, perceived stress, health behaviors, and health behavior change. Interrelatedness among main study variables was calculated using Pearson’s r and Spearman’s ρ. Indirect effects were modeled using the Hayes PROCESS method36 with 5,000-iteration bootstrapping. This statistical approach produces the percentage of variance in the dependent variable accounted for by the model (R2) and also 95% CIs for each indirect effect. For each model, if the CI for the indirect effect did not contain zero, the effect was significantly different from zero, implying partial mediation. The magnitudes of these effects are presented as unstandardized regression coefficients. A series of regression models were constructed with physical symptom severity as the independent variable, fear of cancer recurrence as the mediating variable, and the dependent variable being either emotional distress (aim 1) or a health behavior change variable (aim 2). For each model, age and treatment history variables were explored as potential covariates and were included if significantly correlated with the dependent variable. Regression models were run with and without covariates. For all analyses, P values at the two-sided α = .05 were considered statistically significant.
RESULTS
Sample Characteristics
Sociodemographic characteristics are listed in Table 1. Most survivors of cancer were age 60 years or older (54%), female (64%), white (92%), and married or partnered (72%). Approximately half were employed full or part time (49%). One third had less than a 4-year college education (36%). One quarter lived in a household earning less than $60,000 annually (24%) and were insured through Medicare or Medicaid (23%).
TABLE 1.
Sociodemographic and Medical Characteristics of Survivors of Cancer
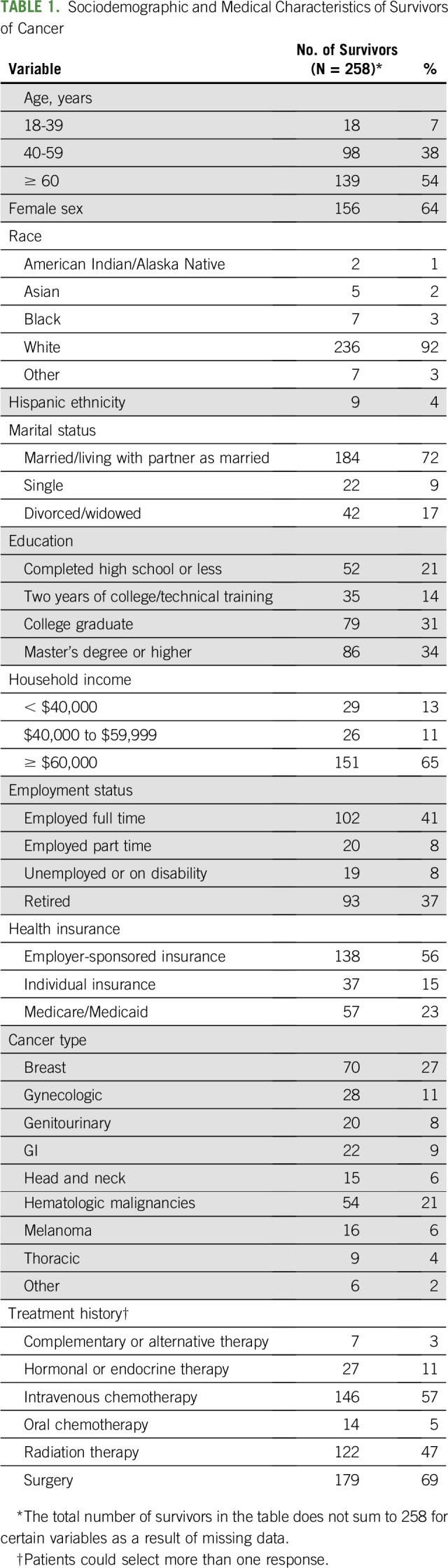
Survivors had been treated for a variety of cancers, most commonly breast (27%), hematologic (21%), gynecologic (11%), GI (9%), and genitourinary (8%). Treatment history included surgery (69%), intravenous chemotherapy (57%), radiation therapy (47%), hormone or endocrine therapy (11%), oral chemotherapy (5%), and/or complementary or alternative therapy (3%).
Aim 1: Replication of Fear of Cancer Recurrence Model
In support of the fear of cancer recurrence model (Fig 1), having more physical symptoms was positively correlated with greater fear of cancer recurrence (r = 0.26, P < .001). In turn, greater fear of cancer recurrence was positively correlated with emotional distress (r = 0.66, P < .001). Physical symptoms were also correlated with higher emotional distress (r = 0.16, P = .01). When analyzed separately, items assessing anxiety and depressed mood yielded similar correlations with fear of cancer recurrence (r = 0.67, P < .001 and r = 0.55, P < .001, respectively) and physical symptoms (r = 0.14, P < .05 and r = 0.17, P < .01, respectively).
Next, fear of cancer recurrence was examined as an intermediary linking physical symptoms with emotional distress (depicted in Fig 1). Controlling for age, the overall regression model of physical symptoms predicting distress via fear of cancer recurrence was supported, explaining almost half of the variance in distress levels (R2 = 0.44, F3,217 = 56.75, P < .001). Furthermore, fear of cancer recurrence was observed to drive this indirect effect (β = .17; bootstrapped SE, .05; 95% CI, .08 to .27). The pattern of results did not change when rerun without age as a covariate. Exploratory analyses yielded similar results when separately modeling anxiety (model R2 = .45; F3,218 = 58.50; P < .001; indirect effect β = .18; bootstrapped SE, .05; 95% CI, .08 to .28) and depressed mood (model R2 = .30; F3,218 = 30.79; P < .001; indirect effect β = .14; bootstrapped SE, .04; 95% CI, .06 to .22).
Aim 2: Extension to Health Behaviors
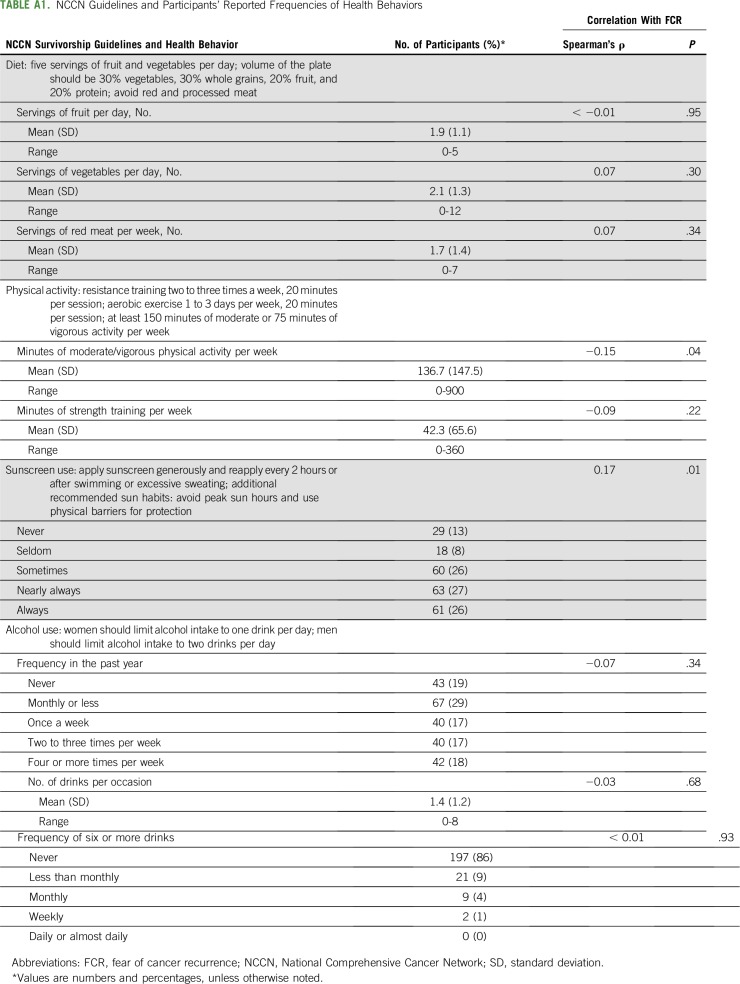
Current diet, physical activity, sunscreen use, and alcohol consumption, are listed in Appendix Table A1 (online only). When examined with respect to NCCN guidelines, 35% of the sample endorsed eating five or more servings of fruits and vegetables per day. In terms of physical activity, NCCN guidelines suggest 75 minutes of vigorous exercise or 150 minutes of moderate exercise per week. Participants reported on their minutes of moderate to vigorous activity as a single item; 56% of responses were 75 minutes or greater, and 37% were 150 minutes or greater. Thus, we estimate that approximately 37% to 56% of the sample met NCCN physical activity guidelines. As done in previous research,28 we collapsed responses for nearly always (27%) and always (26%) applying sunscreen before going outdoors on a sunny day, resulting in 53% of the sample meeting NCCN guidelines for sunscreen use. Because of the categorical nature of the Alcohol Use Disorders Identification Test–Alcohol Consumption measure, we were unable to compute drinks per day. Conservatively, we collapsed the categories of never having an alcoholic drink through having a drink two to three times a week to estimate that at least 82% of participants met NCCN guidelines.
As seen in Appendix Table A1, higher fear of cancer recurrence scores were correlated with fewer minutes per week of current moderate to vigorous physical activity (Spearman’s ρ = −0.15, P = .04). In contrast, fear of cancer recurrence was unrelated to minutes of strength training (Spearman’s ρ = −0.09, P = .22). In addition, higher fear of cancer recurrence was associated with higher frequency of sunscreen use (Spearman’s ρ = 0.17, P = .01). All other current health behaviors were unrelated to fear of cancer recurrence levels (P > .05).
Next, we examined changes in each health behavior after cancer diagnosis. Overall, 36% of survivors of cancer reported having an improved diet after cancer diagnosis (worsened diet, 8%; no change, 56%). In terms of physical activity, 39% of survivors reported decreases (increased activity, 18%; no change, 43%). Use of sunscreen increased among 29% of survivors of cancer (decreased use, 1%; no change, 69%). Notably, 21% of survivors reported increased alcohol intake after cancer diagnosis (decreased intake, 4%; no change, 75%).
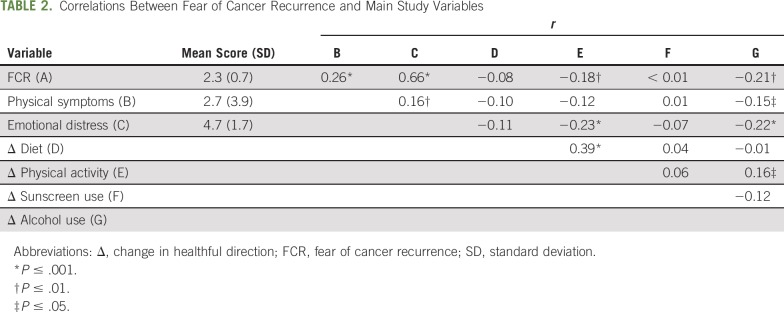
Correlations between fear of cancer recurrence and change in each health behavior after cancer diagnosis are listed in Table 2. Higher fear of cancer recurrence was associated with increased alcohol use (r = −0.21, P < .01) and reduced physical activity (r = −0.18, P < .01). Physical symptoms were associated with increased alcohol use and were borderline correlated with reduced physical activity (r = −0.15, P < .05 and r = −0.12, P = .06, respectively). Neither fear of cancer recurrence nor physical symptoms were associated with changes in diet and sunscreen use. Thus, subsequent analyses focused on explicating links between physical symptoms, fear of recurrence, and changes in alcohol use and physical activity (Fig 1).
TABLE 2.
Correlations Between Fear of Cancer Recurrence and Main Study Variables
The first overall indirect effects regression model explained a significant degree of variability in change in alcohol use, controlling for history of radiation therapy and intravenous chemotherapy (R2 = 0.09, F4,200 = 4.96, P < .001), which was driven by an indirect effect via fear of cancer recurrence (β = −.04; SE, .02; 95% CI, −.09 to −.003). The second overall indirect effects model explained change in physical activity, controlling for history of surgery (R2 = 0.06, F3,211 = 4.87, P = .003). This model was also driven by an indirect effect via fear of cancer recurrence (β = −.04; SE, .02; 95% CI, −.08 to −.002). For both regression models, the pattern of results did not change when rerun without covariates.
DISCUSSION
Fear of cancer recurrence is widely recognized as a chief concern facing survivors of cancer after treatment ends. This study provides empirical evidence that fear of cancer recurrence can be a key contributor to the current emotional distress of survivors, as well as a contributor to adverse health behaviors, including increased alcohol use and decreased physical activity, after cancer diagnosis. Overall, these findings support theoretical conceptualizations of fear of cancer recurrence, which posit that worries about cancer recurrence have significant emotional and behavioral consequences,9,10 and highlight several clinical and research opportunities to improve patient outcomes.
The findings in our study, obtained among patients with diverse cancer histories, support other institutional reports and theoretical models of fear of cancer recurrence and physical symptoms.10,15-19 We found that almost half of the variability in survivors’ emotional distress was accounted for by our fear of cancer recurrence model. These findings are consistent with those reported from a smaller sample of survivors of cancer.13 Cumulatively, these data indicate that fear of cancer recurrence is a robust correlate of survivor distress and specifically helps to account for the emotional burden associated with lasting physical symptoms after treatment ends.
Overall, rates of health risk behaviors were similar to those reported in other samples of survivors of cancer.28,37-42 With regard to NCCN guidelines, adherence rates were an estimated 35% for diet, 37% to 56% for physical activity, 54% for sunscreen use, and 82% for alcohol consumption. Higher fear of cancer recurrence was associated with lower levels of aerobic exercise, yet higher levels of sunscreen use. Thus, fear of cancer recurrence may have a nuanced relationship with survivors’ current health behaviors, potentially serving as a risk factor for certain areas of health promotion (ie, less moderate to vigorous physical activity) while facilitating others (ie, increasing protection from sun exposure). In terms of physical activity, these findings are consistent with those from a large cross-sectional survey measuring fear of cancer recurrence and physical activity among survivors of colorectal cancer,30 as well as recent evidence that distress and physical activity are inversely correlated for survivors of various cancer types.28 Potentially, survivors experiencing fear of cancer recurrence may engage in less physical activity as a result of concerns about exacerbating persistent physical symptoms, such as fatigue, pain, or shortness of breath. Alternatively, sedentary behavior may be a maladaptive behavioral strategy for coping with fear of cancer recurrence; indeed, two recent meta-analyses have shown that physical activity may improve anxiety and overall well-being among survivors of cancer.43,44 Additional research is needed to better clarify these potentially bidirectional relationships. Higher fear of cancer recurrence was also associated with greater current sunscreen use. Some degree of fear of cancer recurrence may be adaptive, prompting survivors to protect themselves from controllable risk factors for future disease.9,10,18 It is plausible that sunscreen application may be perceived by survivors as salient, controllable, and directly related to cancer risk. Additional research is needed to identify potentially modifiable intermediaries of these relationships, such as locus of control, self-efficacy, or health education.
Notably, survivors’ self-reported health behavior change after cancer diagnosis was generally consistent with previous reports.28,45 Although most survivors endorsed not making any health behavior changes, those who reported a change described increased alcohol use, less physical activity, improved diet, and increased sunscreen use. Indeed, survivors of cancer may make positive changes in certain health behaviors to balance out maladaptive changes in others.46 Here, greater fear of cancer recurrence was associated with maladaptive health behavior change, specifically increased alcohol consumption and sedentary behavior. Furthermore, fear of cancer recurrence was a significant intermediary of the relationships between physical symptoms on the one hand and poorer health behavior change on the other. Recent studies suggesting the benefits of light alcohol consumption for adults with cancer have gained significant media attention, although overall empirical support is mixed.47,48 Thus, survivors of cancer with fear of cancer recurrence may increase alcohol use in an attempt to promote their physical health. In addition, fear of cancer recurrence had a similar role in accounting for reduced physical activity since diagnosis. Although the influence of fatigue, nausea, and pain on restricting physical activity among survivors of cancer is well known,49,50 our findings suggest that interpretations of physical symptoms as potential signs of recurrence may prompt sedentary behavior. Both physical inactivity and alcohol use may be maladaptive emotion regulation strategies for coping with fear of cancer recurrence and related worries51,52 and, critically, may increase risk for worse clinical outcomes.39,49,53
Our study was conducted at a single institution using a cross-sectional design, and responses are dependent on patient self-report. Patient-reported health behavior change may be subject to recall biases and less accurate than objective tracking or rating of changes. The patient population in the study was predominantly white and educated, with access to health care, and there is undoubtedly some degree of selection bias among those who elected to participate in the survey. However, none of these factors are intuitively likely to bias the association between fear of cancer recurrence and emotional distress or fear of cancer recurrence and negative behavior changes identified here. Future studies could use longitudinal measures to parse out the directionality of these relationships. To better account for variability in health behaviors, additional precipitating triggers of fear of cancer recurrence could be examined, including external events such as follow-up appointments and new diagnoses in family or friends.4,7,11 In addition, certain common concerns of survivors of cancer such as cardiac health, sleep, and sexual function could be included in future modeling of fear of recurrence. Finally, this study included survivors of more than eight different cancer types, enhancing the generalizability of these findings to a variety of cancer populations.9,13
Overall, these findings highlight the contribution of fear of cancer recurrence in accounting for survivors’ emotional distress, current health behaviors, and changes in health behaviors after diagnosis. Importantly, there are direct implications for guiding clinical oncology practice. Our findings suggest the need for enhanced routine screening for fear of cancer recurrence and targeted interventions to mitigate the impacts of fear of cancer recurrence. Screening for fear of cancer recurrence in oncology and primary care clinics is feasible and acceptable for clinicians, support staff, and patients.10,54,55 Furthermore, clinicians treating survivors of cancer are well positioned to assess and intervene on fear of cancer recurrence, distress, and health behaviors.56 Once survivors of cancer with fear of cancer recurrence are identified, clinicians can refer patients for psychosocial support10,54 or empirically supported, mind-body programs for managing fear of cancer recurrence.57 Collectively, our findings identify fear of cancer recurrence as a key factor underlying emotional and behavioral difficulties faced by survivors of cancer and suggest the need for targeting fear of cancer recurrence in future psychosocial and behavioral health interventions in this population.
ACKNOWLEDGMENT
E.R.P. and J.M.P. contributed equally to this work. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. This work was supported by internal funds from Massachusetts General Hospital. D.L.H. and G.Y.Y. were supported by the National Center for Complementary and Integrative Health at the National Institutes of Health (Grant No. T32AT000051 to D.L.H. and Grant No. K24AT009465 to G.Y.Y.), and E.R.P. was supported by the National Cancer Institute at the National Institutes of Health (Grant No. K24CA197382). Presented, in part, at the 14th Annual International Conference of the Society for Integrative Oncology, Chicago, IL, November 12-14, 2017.
APPENDIX
TABLE A1.
NCCN Guidelines and Participants’ Reported Frequencies of Health Behaviors
AUTHOR CONTRIBUTIONS
Conception and design: Daniel L. Hall, Rachel B. Jimenez, Giselle K. Perez, Elyse R. Park, Jeffrey M. Peppercorn
Administrative support: Daniel L. Hall, Julia Rabin
Provision of study material or patients: Daniel L. Hall, Julia Rabin, Jeffrey M. Peppercorn
Collection and assembly of data: Daniel L. Hall, Rachel B. Jimenez, Giselle K. Perez, Julia Rabin, Katharine Quain, Elyse R. Park, Jeffrey M. Peppercorn
Data analysis and interpretation: Daniel L. Hall, Giselle K. Perez, Gloria Y. Yeh, Elyse R. Park, Jeffrey M. Peppercorn
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Fear of Cancer Recurrence: A Model Examination of Physical Symptoms, Emotional Distress, and Health Behavior Change
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jop/site/ifc/journal-policies.html.
Rachel B. Jimenez
Employment: Biogen (I)
Research Funding: Focal Therapeutics
Elyse R. Park
Honoraria: UpToDate
Research Funding: Pfizer
Jeffrey M. Peppercorn
Employment: GlaxoSmithKline (I)
Stock and Other Ownership Interests: GlaxoSmithKline (I)
Consulting or Advisory Role: Athenex
Research Funding: Pfizer
No other potential conflicts of interest were reported.
REFERENCES
- 1.Crist JV, Grunfeld EA. Factors reported to influence fear of recurrence in cancer patients: A systematic review. Psychooncology. 2013;22:978–986. doi: 10.1002/pon.3114. [DOI] [PubMed] [Google Scholar]
- 2.Savard J, Ivers H. The evolution of fear of cancer recurrence during the cancer care trajectory and its relationship with cancer characteristics. J Psychosom Res. 2013;74:354–360. doi: 10.1016/j.jpsychores.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Thewes B, Butow P, Zachariae R, et al. Fear of cancer recurrence: A systematic literature review of self-report measures. Psychooncology. 2012;21:571–587. doi: 10.1002/pon.2070. [DOI] [PubMed] [Google Scholar]
- 4.Gil KM, Mishel M, Belyea M, et al. Triggers of uncertainty about recurrence and long-term treatment side effects in older African American and Caucasian breast cancer survivors. Oncol Nurs Forum. 2004;31:633–639. doi: 10.1188/04.onf.633-639. [DOI] [PubMed] [Google Scholar]
- 5.Hilton BA. The phenomenon of uncertainty in women with breast cancer. Issues Ment Health Nurs. 1988;9:217–238. doi: 10.3109/01612848809140926. [DOI] [PubMed] [Google Scholar]
- 6.McKinley ED. Under Toad days: Surviving the uncertainty of cancer recurrence. Ann Intern Med. 2000;133:479–480. doi: 10.7326/0003-4819-133-6-200009190-00019. [DOI] [PubMed] [Google Scholar]
- 7.Koch L, Jansen L, Brenner H, et al. Fear of recurrence and disease progression in long-term (≥ 5 years) cancer survivors: A systematic review of quantitative studies. Psychooncology. 2013;22:1–11. doi: 10.1002/pon.3022. [DOI] [PubMed] [Google Scholar]
- 8.Nelson JP. Struggling to gain meaning: Living with the uncertainty of breast cancer. ANS Adv Nurs Sci. 1996;18:59–76. doi: 10.1097/00012272-199603000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Simonelli LE, Siegel SD, Duffy NM. Fear of cancer recurrence: A theoretical review and its relevance for clinical presentation and management. Psychooncology. 2017;26:1444–1454. doi: 10.1002/pon.4168. [DOI] [PubMed] [Google Scholar]
- 10.Butow P, Sharpe L, Thewes B, et al. Fear of cancer recurrence: A practical guide for clinicians. Oncology (Williston Park) 2018;32:32–38. [PubMed] [Google Scholar]
- 11.McGinty HL, Small BJ, Laronga C, et al. Predictors and patterns of fear of cancer recurrence in breast cancer survivors. Health Psychol. 2016;35:1–9. doi: 10.1037/hea0000238. [DOI] [PubMed] [Google Scholar]
- 12.Hall DL, Mishel MH, Germino BB. Living with cancer-related uncertainty: Associations with fatigue, insomnia, and affect in younger breast cancer survivors. Support Care Cancer. 2014;22:2489–2495. doi: 10.1007/s00520-014-2243-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall DL, Lennes IT, Pirl WF, et al. Fear of recurrence or progression as a link between somatic symptoms and perceived stress among cancer survivors. Support Care Cancer. 2017;25:1401–1407. doi: 10.1007/s00520-016-3533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vehling S, Kissane DW, Lo C, et al. The association of demoralization with mental disorders and suicidal ideation in patients with cancer. Cancer. 2017;123:3394–3401. doi: 10.1002/cncr.30749. [DOI] [PubMed] [Google Scholar]
- 15.Mishel MH. Uncertainty in illness. Image J Nurs Sch. 1988;20:225–232. doi: 10.1111/j.1547-5069.1988.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 16.Mishel MH. Reconceptualization of the uncertainty in illness theory. Image J Nurs Sch. 1990;22:256–262. doi: 10.1111/j.1547-5069.1990.tb00225.x. [DOI] [PubMed] [Google Scholar]
- 17.Butow P, Kelly S, Thewes B, et al. Attentional bias and metacognitions in cancer survivors with high fear of cancer recurrence. Psychooncology. 2015;24:416–423. doi: 10.1002/pon.3659. [DOI] [PubMed] [Google Scholar]
- 18.Curran L, Sharpe L, Butow P. Anxiety in the context of cancer: A systematic review and development of an integrated model. Clin Psychol Rev. 2017;56:40–54. doi: 10.1016/j.cpr.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Lee-Jones C, Humphris G, Dixon R, et al. Fear of cancer recurrence: A literature review and proposed cognitive formulation to explain exacerbation of recurrence fears. Psychooncology. 1997;6:95–105. doi: 10.1002/(SICI)1099-1611(199706)6:2<95::AID-PON250>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 20.Armenian SH, Lacchetti C, Barac A, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2017;35:893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 21.Earle CC. Failing to plan is planning to fail: Improving the quality of care with survivorship care plans. J Clin Oncol. 2006;24:5112–5116. doi: 10.1200/JCO.2006.06.5284. [DOI] [PubMed] [Google Scholar]
- 22.Nathan PC, Greenberg ML, Ness KK, et al. Medical care in long-term survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2008;26:4401–4409. doi: 10.1200/JCO.2008.16.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shelby R, Keefe F, Red S, et al. Symptom experiences and nonadherent medication-taking behaviors of breast cancer patients taking adjuvant hormone therapy. J Clin Oncol 29, 2011 (suppl 15; abstr 524) [Google Scholar]
- 24.Bestvina CM, Zullig LL, Rushing C, et al. Patient-oncologist cost communication, financial distress, and medication adherence. J Oncol Pract. 2014;10:162–167. doi: 10.1200/JOP.2014.001406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dieperink KB, Wagner L, Hansen S, et al. Embracing life after prostate cancer: A male perspective on treatment and rehabilitation. Eur J Cancer Care (Engl) 2013;22:549–558. doi: 10.1111/ecc.12061. [DOI] [PubMed] [Google Scholar]
- 26.Welch HG. Cancer screening, overdiagnosis, and regulatory capture. JAMA Intern Med. 2017;177:915–916. doi: 10.1001/jamainternmed.2017.1198. [DOI] [PubMed] [Google Scholar]
- 27.Denlinger CS, Sanft T, Baker KS, et al. Survivorship, version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:1140–1163. doi: 10.6004/jnccn.2017.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hyland KA, Jacobs JM, Lennes IT, et al. Are cancer survivors following the National Comprehensive Cancer Network health behavior guidelines? An assessment of patients attending a cancer survivorship clinic. J Psychosoc Oncol. 2018;36:64–81. doi: 10.1080/07347332.2017.1399193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skaali T, Fosså SD, Bremnes R, et al. Fear of recurrence in long-term testicular cancer survivors. Psychooncology. 2009;18:580–588. doi: 10.1002/pon.1437. [DOI] [PubMed] [Google Scholar]
- 30.Fisher A, Beeken RJ, Heinrich M, et al. Health behaviours and fear of cancer recurrence in 10 969 colorectal cancer (CRC) patients. Psychooncology. 2016;25:1434–1440. doi: 10.1002/pon.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hawkins NA, Smith T, Zhao L, et al. Health-related behavior change after cancer: Results of the American Cancer Society’s Studies of Cancer Survivors (SCS) J Cancer Surviv. 2010;4:20–32. doi: 10.1007/s11764-009-0104-3. [DOI] [PubMed] [Google Scholar]
- 32.Humphris GM, Rogers SN. The association of cigarette smoking and anxiety, depression and fears of recurrence in patients following treatment of oral and oropharyngeal malignancy. Eur J Cancer Care (Engl) 2004;13:328–335. doi: 10.1111/j.1365-2354.2004.00479.x. [DOI] [PubMed] [Google Scholar]
- 33.Simmons VN, Litvin EB, Jacobsen PB, et al. Predictors of smoking relapse in patients with thoracic cancer or head and neck cancer. Cancer. 2013;119:1420–1427. doi: 10.1002/cncr.27880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gotay CC, Pagano IS. Assessment of Survivor Concerns (ASC): A newly proposed brief questionnaire. Health Qual Life Outcomes. 2007;5:15. doi: 10.1186/1477-7525-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): An effective brief screening test for problem drinking—Ambulatory Care Quality Improvement Project (ACQUIP), Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 36.Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York, NY: Guilford Press; 2013. [Google Scholar]
- 37.Bellizzi KM, Rowland JH, Jeffery DD, et al. Health behaviors of cancer survivors: Examining opportunities for cancer control intervention. J Clin Oncol. 2005;23:8884–8893. doi: 10.1200/JCO.2005.02.2343. [DOI] [PubMed] [Google Scholar]
- 38.Blanchard CM, Courneya KS, Stein K, et al. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: Results from the American Cancer Society’s SCS-II. J Clin Oncol. 2008;26:2198–2204. doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- 39.Kwan ML, Kushi LH, Weltzien E, et al. Alcohol consumption and breast cancer recurrence and survival among women with early-stage breast cancer: The life after cancer epidemiology study. J Clin Oncol. 2010;28:4410–4416. doi: 10.1200/JCO.2010.29.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coups EJ, Ostroff JS. A population-based estimate of the prevalence of behavioral risk factors among adult cancer survivors and noncancer controls. Prev Med. 2005;40:702–711. doi: 10.1016/j.ypmed.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 41.Demark-Wahnefried W, Peterson B, McBride C, et al. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer. 2000;88:674–684. [PubMed] [Google Scholar]
- 42.Mowls DS, Brame LS, Martinez SA, et al. Lifestyle behaviors among US cancer survivors. J Cancer Surviv. 2016;10:692–698. doi: 10.1007/s11764-016-0515-x. [DOI] [PubMed] [Google Scholar]
- 43.Speck RM, Courneya KS, Mâsse LC, et al. An update of controlled physical activity trials in cancer survivors: A systematic review and meta-analysis. J Cancer Surviv. 2010;4:87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 44.Fong DY, Ho JW, Hui BP, et al. Physical activity for cancer survivors: Meta-analysis of randomised controlled trials. BMJ. 2012;344:e70. doi: 10.1136/bmj.e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blanchard CM, Denniston MM, Baker F, et al. Do adults change their lifestyle behaviors after a cancer diagnosis? Am J Health Behav. 2003;27:246–256. doi: 10.5993/ajhb.27.3.6. [DOI] [PubMed] [Google Scholar]
- 46.Park ER, Streck JM, Gareen IF, et al. A qualitative study of lung cancer risk perceptions and smoking beliefs among National Lung Screening Trial participants. Nicotine Tob Res. 2014;16:166–173. doi: 10.1093/ntr/ntt133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boffetta P, Hashibe M. Alcohol and cancer. Lancet Oncol. 2006;7:149–156. doi: 10.1016/S1470-2045(06)70577-0. [DOI] [PubMed] [Google Scholar]
- 48.LoConte NK, Brewster AM, Kaur JS, et al. Alcohol and cancer: A statement of the American Society of Clinical Oncology. J Clin Oncol. 2018;36:83–93. doi: 10.1200/JCO.2017.76.1155. [DOI] [PubMed] [Google Scholar]
- 49.Meyerhardt JA, Giovannucci EL, Holmes MD, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 50.Ormel HL, van der Schoot GGF, Sluiter WJ, et al. Predictors of adherence to exercise interventions during and after cancer treatment: A systematic review. Psychooncology. 2018;27:713–724. doi: 10.1002/pon.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greer JA, Solis JM, Temel JS, et al. Anxiety disorders in long-term survivors of adult cancers. Psychosomatics. 2011;52:417–423. doi: 10.1016/j.psym.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lown EA, Goldsby R, Mertens AC, et al. Alcohol consumption patterns and risk factors among childhood cancer survivors compared to siblings and general population peers. Addiction. 2008;103:1139–1148. doi: 10.1111/j.1360-0443.2008.02242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Playdon MC, Bracken MB, Sanft TB, et al. Weight gain after breast cancer diagnosis and all-cause mortality: Systematic review and meta-analysis. J Natl Cancer Inst. 2015;107:djv275. doi: 10.1093/jnci/djv275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thewes B, Husson O, Poort H, et al. Fear of cancer recurrence in an era of personalized medicine. J Clin Oncol. 2017;35:3275–3278. doi: 10.1200/JCO.2017.72.8212. [DOI] [PubMed] [Google Scholar]
- 55.Humphris GM, Watson E, Sharpe M, et al. Unidimensional scales for fears of cancer recurrence and their psychometric properties: The FCR4 and FCR7. Health Qual Life Outcomes. 2018;16:30. doi: 10.1186/s12955-018-0850-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Demark-Wahnefried W, Pinto BM, Gritz ER. Promoting health and physical function among cancer survivors: Potential for prevention and questions that remain. J Clin Oncol. 2006;24:5125–5131. doi: 10.1200/JCO.2006.06.6175. [DOI] [PubMed] [Google Scholar]
- 57.Hall DL, Luberto CM, Philpotts LL, et al. Mind-body interventions for fear of cancer recurrence: A systematic review and meta-analysis. Psychooncology. 2018;27:2546–2558. doi: 10.1002/pon.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]