Abstract
Novel drug delivery systems have ameliorated drugs’ pharmacokinetics and declined undesired ramifications while led to a better patient compliance by extending the time of release. In fact, although there has been a multitude of encouraging achievements in controlled drug release, the application of micro- and nano-carriers is confronted with some challenges such as rapid clearance and inefficient targeting. In addition, since cell systems can be an appropriate alternative to micro- and nano-particles, they have been used as biological carriers. In general, features such as stable release into blood, slow clearance, efficient targeting, and high biocompatibility are the main properties of cells applied as drug carriers. Furthermore, some cells such as erythrocytes, leukocytes, stem cells, and platelets have been used as release systems. Hence, most common cells that were used as aforementioned release systems are going to be presented in this review article.
Keywords: Drug delivery, erythrocytes, macrophages, platelets, stem cells
Introduction
Drug delivery systems have been widely investigated as powerful tools to achieve an effective therapy with proper pharmacokinetics and pharmacodynamics[1] in the last decades. In fact, various polymers, ceramics, and metals are used to fabricate drug-releasing systems for loading and transferring drugs and biological factors.[2,3,4] Almost 30 releasing systems are commercially available nowadays.
Unquestionably, the main problem concerning systemic injection of drugs is their inappropriate distribution in the body as a percentage of drug degrades in natural body tissues; it should be injected in high amounts to reach the desired therapeutic dosage.[5] It can be mentioned that a perfect drug system must ideally implement its pharmaceutical activity in the targeting site. In this way, the lowest dosage can be prescribed.[6]
As a matter of fact, novel drug delivery systems are spreading broadly as alternatives to conventional drug delivery systems. For instance, lipid nanoparticles such as micelles and liposomes are being investigated for drug delivery to targeted tissues. Yet, the problem of such delivery systems is the reduction of their half-time by phagocytic absorption and hepatic filtration as well as targeted release and nanoparticles’ toxicity.[7] To overcome the challenges, cells are proposed to be employed as pharmaceutical carriers.[8] Cellular carriers have a complex mechanism to evade the immune system,[9] the potential of implantation in specific tissues as well as passing impermeable barriers such as the blood–brain barrier (BBB) in comparison with drug delivery systems in nanoscale. In addition, other cell features including long circulation time, abundant surface ligands, flexible morphology, cellular signaling, and metabolism of drugs can be used.[1] Drug diffusion from carrier cells results in dosage control, accessibility, and delivery of drugs to the desired site of patient's body. Drug release is conducted through inactive transport, exocytosis, or efflux pumps that can be set by biological signals.[10] Further, different cells such as erythrocytes, platelets, leukocytes, stem cells (SCs), fibroblasts, and cancerous cells have been investigated as novel drug delivery systems.[1]
Not only do cells but also their membranes can be used in releasing systems, and there are two ways in which cell-based systems can be used.[11] In the first one, the DNA which contains the genes for therapeutic factors, mostly specific proteins, is imported into the cell through particular vectors, and the expression of these genes leads to the formation of therapeutic factors.[12] In the second way, a synthetic drug or a set of nanoparticles containing it are loaded in a cell or on its surface through various methods.[6] To illustrate, we can load therapeutic factors in macrophages through phagocytosis[13] or a drug on the surface of erythrocytes through avidin–biotin bridge.[14] Table 1 shows some cell or cell membrane, their potential targets, and the kind of substance that may be incorporated. In this article, the most common cells that are used as drug delivery systems will be reviewed, and the methods of drug loading on them are going to be discussed.
Table 1.
Some of biological carrier
| Cell or cell membrane carrier | Cell or tissue target | Therapeutic substance | Remark | Reference |
|---|---|---|---|---|
| RBCs | Reticuloendothelial systems and macrophages | Drugs, enzymes, and peptides | Biocompatibility, biodegradation, long blood circulation, sustained drug release | [15] |
| RBCs membrane | Reticuloendothelial systems | Drugs, enzymes, and peptides | Improvement of pharmacokinetics and pharmacodynamics in combination chemotherapy | [16] |
| Platelets | Targeting to the injury sites or the sites with higher density of proliferating cells, metastatic cells | Drugs | Critical roles in hemostasis, tumor tropism property, high storage, and trafficking capacities | [8] |
| Stem cells | Tumor tissues | Drugs, genetic material, tumor imaging agents | High loading capacity | [17] |
| Neutrophils | Tumor tissues, lungs | Drugs, photodynamic therapy agents | Increased drug concentration due to aggregation of neutrophils at the inflammation site | [18] |
| Macrophages | Tumor tissue, inflammation location | Drugs, photodynamic therapy agents, tumor imaging agents | Tumor tropism property, ability to pass the blood-brain barrier | [19] |
| Bacteria ghosts | Dendritic cells, macrophages, tumor cells, and endothelial cells | Drugs, antigens | Easy and safe production, high bioavailability, and a long shelf-life | [20] |
RBCs – Red blood cells
The methods of drug loading inside of or on the surface of cells
Various methods are employed to load drugs into or on the surface of cells including hypotonic dialysis, electroporation, incubation, and encapsulation of therapeutic factors through biological pathways and conjugation of them on the surface of cells. In sum, all the mentioned methods have been experimented to load drugs into or on the surface of erythrocytes, and for other cells, one of the methods should be used according to and based on the properties of the cells.
Hypotonic dialysis
Any method which is used to encapsulate therapeutic factors in cells should make minimum alterations to cells. Hypotonic dialysis is among the desired methods as it maintains biochemical and physiological properties and provides the highest percentage of encapsulation.[6] In fact, in this method, cells are swelled in a hypotonic solution under osmotic pressure and some pores appear on the cell membrane; so, consequently, therapeutic molecules can pass through the membrane. Finally, pores are filled by submergence of the cell in an isotonic buffer.[14,21]
Electroporation
In fact, in this method, cells are in a strong electrical field that induces pores in their membranes and let pharmaceutical factors to be imported into cells.[22] Hence, for this purpose, the cell suspension is put in a conductive solution and subjected to an electrical field with high intensity. Furthermore, it should be noted that the used conductive solutions should contain sodium tetraborate 0.0125 mol/L and NaOH 0.00313 mol/L. In addition, an electrical field with the intensity of 20 Kv/cm for 20 μs is exposed for making pores in the membrane. Then, a perturbation in the bilayer phospholipid leads to the dysfunctionality of cell membrane that lets molecules to get encapsulated. Indeed, this method is employed for encapsulation of enzymes, nucleic acids, and anionic drugs such as diclofenac into erythrocytes.[1]
Incubation
As a matter of fact, some drugs such as hydrocortisone can be imported into cells through direct incubation or inactive transport mechanism. In addition, nanoparticles can attach to cells’ surfaces through electrostatic interaction and then be imported into it.[23] For example, in a study, immunoglobulin-G was conjugated with PEG-DSPE – a combination of a drug and polyethylene glycol, and it was concluded that the combination of PEG-DSPE-IgG can be imported into erythrocytes through lipid transfer.[16]
Encapsulation of pharmaceutical factors through biological pathways
A simple method for loading drugs into circulating cells is to use biological pathways. Various drugs can get encapsulated in cells through these pathways. In this way, both morphology and functionality of cells are retained. Cellular endocytosis is a basic mechanism in which eukaryotic cells aspire liquids, molecules, or cells. Most materials, in particular, big polar molecules, cannot pass the hydrophobic cell membrane directly.[24] Endocytosis is a pathway for materials to enter cells through alterations in the plasma membrane.[25] Some parameters including the size, shape, charge, and mechanical features of the external body affect endocytosis, and this fact provides an opportunity to regulate the encapsulation of pharmaceutical carriers. Pinocytosis, a type of endocytosis, imports liquids and prebroken molecules into cells. Eukaryotic cells conduct pinocytosis regularly with the preference to import small molecules.[26] The homeostasis of plasma membrane can be employed to encapsulate pure drugs in erythrocytes. Some drugs such as hydrocortisone, primaquine, vinblastine, and chlorpromazine have the ability to enter cells through pinocytosis. Pinocytosis is dependent on adenosine triphosphate. This method is based on simple absorption.[26] In contrast, phagocytosis occurs in specific cells such as macrophages.[19]
In fact, when immune responses excite phagocytic cells, these cells can import micro- and nano-particles through an actin-dependent mechanism while detection of some receptors such as FCs, complements, and mannose facilitates this mechanism.[27] The size of pharmaceutical carriers such as nanoparticles, micelles, and liposomes is usually in this range. Then, imported particles are combined with lysosomes and lysed by digestion enzymes. Indeed, as phagocytosis is an immune defense method against external invaders, it is tremendously effective for drug loadings in cells. Hence, phagocytic cells can be used as drug delivery systems instead of designing drug-containing particles to stand against the reticuloendothelial system. In addition, phagocytosis can be raised by opsonization; therefore, drug-containing particles are coated with either proteins or antibodies in order for their detection by mononuclear phagocyte system to rise. For instance, the surface of Fe2O3 particles was coated with immunoglobulin and albumin in a study to raise phagocytosis. In brief, a multitude of drug-containing nanomaterials such as inorganic, polymers, and liposomal ones were loaded in phagocytic cells through this method, and studies have revealed that the size, geometry, and charge of drug carriers play an essential role in phagocytosis.[28]
Conjugation of therapeutic factors on the surface of cells
There are two different approaches to attach therapeutic factors to the surface of cells. First one is the direct method in which drugs are bound to the receptors available on the surface of cells, while in the second one which is entitled indirect method, drugs are bound to a conjugated ligand like antibodies on the surface of cells.[7] Plenty of various functional groups have been discovered on the surface of cells that enable biological conjugation, and among these functional groups, type one amines and thiol groups are used, thanks to their working convenience and less toxicity. Conjugation through covalent bonds usually provides stronger attachments in comparison with ligand-receptor bounds; therefore, it prevents the detachment of therapeutic factors during cell migration.[1] Furthermore, nanoparticles can be attached to the surface of cells to raise the circulating time of drug-containing nanoparticles in blood. For example, studies have demonstrated that polystyrene nanoparticles can bind to the surface of red blood cells (RBCs) and detach from erythrocytes inactively due to shear forces and cell–cell interactions; thus, the concentration of novel systems was three times more than the primary system in blood.[29]
Conjugation of drugs or pharmaceutical carriers on the surface of cells can take place through avidin–biotin bridge. Biotin is a water-soluble vitamin that has a great tendency to attach to avidin and streptavidin. Hence, the aforementioned biological bridge can be formed on the surface of erythrocytes through attachment of amine groups by either n-hydroxysuccinimide ester or oxidation of aldehyde groups on the membranes of erythrocytes through hydrazide biotin. Accordingly, Magnani et al. showed that it can be possible to bind to thousands of biotin molecules on the surface of each erythrocyte.[30,31] Another applicable instance is to bind a thrombolytic agent to the surface of erythrocytes through avidin–biotin bridge. Besides, click chemistry is another method for attachment of drugs or pharmaceutical factors on the surface of cells. Click chemistry is a fast, selective, and biorthogonal reaction with high production percentage and the ability to stabilize materials on the surface of cells. Chiefly, there are different diverse click chemistry methods for stabilization of molecules on the surface of cells like azide-alkyne cycloaddition reaction catalyzed by copper and Diels–Alder reaction.[32] Other methods include molecules stabilization based on antibody-antigen attachment.[33]
Proposed Cells and Cells Membranes for Drug Delivery
Erythrocytes
RBCs are the most blood cells and responsible for oxygen transfer. Furthermore, these biconcave cells have the diameter of 7–8 μm and the mean volume of 90 fL, and in the mammalian, erythrocytes do not have nuclei and lose their organelles throughout maturation. In fact, they age from 100 to 120 days, and afterward, macrophages eliminate them selectively. In addition, although the area of an erythrocyte's surface is 136 μm2, it can swell and get to the shape of a sphere having the volume of roughly 150 fL.[34] The idea of using erythrocytes as pharmaceutical agents was first presented in 1970 since in comparison with artificial drug delivery systems, erythrocytes have outstanding features making them special carriers for drugs.[7] As an illustration, these cells are inherently biocompatible, especially when drugs are loaded on autologous cells. Furthermore, erythrocytes are biodegradable cells that do not produce toxic by-products and age longer than artificial carriers. For example, on the occasions when loading condition is optimum, loaded erythrocytes can age as much as healthy and normal erythrocytes. Finally, the range of desired size, size alteration, and the ability of being loaded with high capacity are among the other features of the drug delivery systems based on erythrocytes.[30] Being biconcave and not having nucleus provide erythrocytes with the capability of being loaded with a broad range of drugs.[1] There are different methods for loading drugs in erythrocytes.
Pharmacokinetics of erythrocytes and targeted drug release through them
Autologous plasma or an isosmotic buffer in temperature of 37°C or dialysis sac is used for ex vivo investigation of loaded drugs in erythrocytes. Studies have shown that both zero and first order release are possible to be approached[35] and that the release of drugs from erythrocytes is of the sustained and slow ones. Furthermore, as it was mentioned in advance, the lifetime of loaded erythrocytes can be as long as that of normal ones provided that drug loading is conducted under optimum conditions. In addition, another outstanding merit of erythrocyte-based release systems is a tangible decline in the fluctuations of drug concentration in comparison with ordinary drug prescription methods.[29]
Aged erythrocytes are identified by the reticuloendothelial system and eliminated; therefore, erythrocytes can be utilized to release drugs in phagocytic cells like macrophages and in fact, in the tissues in which macrophages are available. Accordingly, liver and spleen can be considered as two examples of these tissues. Thus, macrophages of different tissues can be targeted.[36]
Some conducted research on erythrocytes as drug carriers
Most research on cell-based drug delivery systems has been carried out on erythrocytes. In these studies, erythrocytes have been employed to carry some drugs such as antiparasitic, antiretroviral agents, cardiovascular drugs, and enzymes.[37]
In some studies, an impermeable predrug was encapsulated in erythrocytes and then transformed into an active drug through enzymes of the erythrocyte and released into the bloodstream. The release is usually based on phosphorylation. In fact, dephosphorylation makes the impermeable predrug an active impermeable molecule. Therefore, all drugs are not appropriate in this case since dephosphorylated part must be excreted through the membrane through either inactive transfer mechanisms with intermediaries. Some of these drugs are predrugs, anti-inflammatory, antiviral, and anticancer drugs.[38,39] Magnani et al. showed that dexamethasone 21-phosphate can be loaded in erythrocytes in a wide concentration range so that it is released into the bloodstream through inactive transport after dephosphorylation. This professional drug delivery and release system can be employed to release drugs for chronic diseases such as intestinal inflammation and ulcerative colitis. This system provides dexamethasone to be released in 28 days.[40]
In addition to the above method that loaded drugs in erythrocytes, active drugs can also be loaded in erythrocytes to elongate the release time.[41] This approach not only provides longer release time but also prevents protein-based drugs from clearance, antibody attachments, and as a result, detection by the immune system and lysis through circulating proteases.[39] Loading active drugs in erythrocytes is a suitable approach to release enzymatic drugs as liver eliminates enzymes quickly. The best instance for enzyme loading in erythrocytes is the loading of L-asparaginase. This loading was successfully used to treat acute lymphoblastic leukemia in children and adults.[42]
As was mentioned before, macrophages of the reticuloendothelial system such as peritoneal, hepatic Kupffer, and pulmonary alveolar macrophages as well as vascular endothelial cells are the effective place for degradation of aged or abnormal erythrocytes. It should be noted that aging of erythrocytes and a group of factors and tensions make them detectable for macrophages.[43] Almost all the erythrocytes that undergo structural changes are captured by the reticuloendothelial system, in particular, that of spleen and liver. This takes a short time; the drug is thus transferred to the reticuloendothelial system. Therefore, encapsulation of drugs in erythrocytes results in fast drug delivery to reticuloendothelial system. In this method, either macrophages or the tissue containing them such as spleen and liver are targets.[29]
Specific drug release into the macrophage-monocyte system can be a benefit as macrophages are able to function as containers for viruses. In the second case, spleen and liver can be targeted for some disease. Lots of metabolic diseases result from dysfunctionality or insufficiency of enzymes; thus, they can be cured by these enzymes. Short half-life, allergic reactions, and toxigenicity are the main problems of enzyme therapy. If enzymes be loaded in erythrocytes, these problems are resolved. Used enzymes consist of beta-glucosidase, beta-glucuronidase, and beta-galactosidase. Gaucher's disease leads glucocerebroside to accumulate in spleen and liver; hence, loading glucocerebrosidase enzyme in erythrocytes cures this disease.[29] In Table 2, some enzymes that were loaded in erythrocyte are shown. Moreover, loaded deferoxamine in erythrocytes can be used for the treatment of extra accumulated iron for thalassemia patients who receive blood. Delivering such a drug to RES is advantageous as it makes the aged and transformed erythrocytes degrade in the reticuloendothelial system. Thanks to iron accumulation in spleen and liver; these tissues can be targeted through this method and prevented dysfunction. Furthermore, there are a group of methods for the evolution of reticuloendothelial system targeting. For instance, if antibody-G is loaded on the surface of erythrocytes, targeting liver is preferred whereas spleen is targeted when erythrocytes are coated with immunoglobulin-M.[52] Figure 1 demonstrates targeted drug delivery to the liver and spleen through opsonization by IgG and IgM.
Table 2.
Example of therapeutic enzymes encapsulated in carrier red blood cells
| Enzyme | Size | Function | Condition to treat | Reference |
|---|---|---|---|---|
| Galactosidase | 464 kDa | Degrades galactosides | Lysosomal storage diseases | [44] |
| Glucuronidase | 80 kDa | Degrades heparin and mucopolysaccharides | Lysosomal storage diseases | [45] |
| Carbonic anhydrase | 30 kDa | Converts CO2 into bicarbonate | Detoxification of CO2 | [46] |
| Uricase | 33 kDa | Oxidizes uric acid | Uric acid detoxification | [47] |
| Thiosulfate-cyanide sulfurtransferase | 37 kDa | Converts cyanides into thiocyanate | Cyanide detoxification | [48] |
| Phosphodiesterase | 15-35 kDa | Thioester hydrolysis | Detoxification of organophosphorus compounds | [49] |
| Alcohol oxidase | 140-600 kDa | Converts alcohols into aldehydes | Detoxification of alcohol | [50] |
| Adenosine deaminase | 47 kDa | Degrades adenosine | Elimination of deoxyadenosine that inhibits immune cells in patients with reduced ADA human studies | [51] |
ADA – Adenosine deaminase
Figure 1.
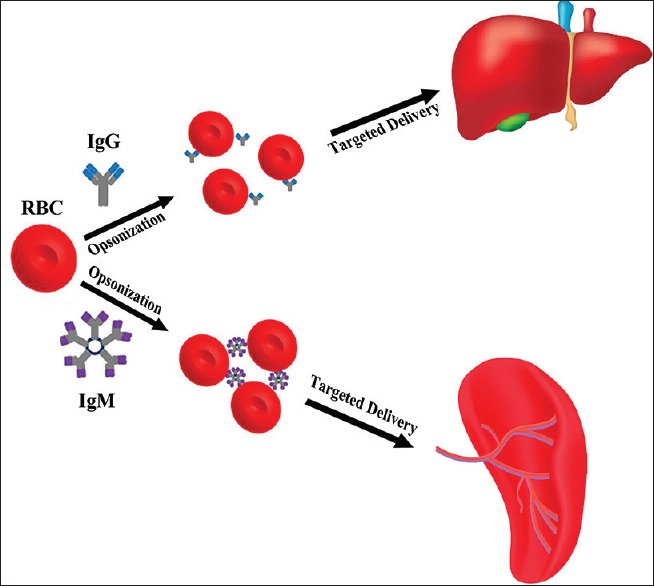
Targeted delivery strategies for liver and spleen tissues
Another example of loading drugs on the surface of erythrocytes is the attachment of the tissue plasminogen-activating agent to the surface of erythrocytes to treat and prevent thrombosis formation. This claim has been shown to have encouraging results.[53] Plenty of studies have been carried out to develop the release of erythrocytes-based therapeutic factors albeit various challenges such as fast leakage of drugs, drug-induced physiological alterations of erythrocytes, and contamination of blood sources. Furthermore, successful clinical trials of erythrocyte-based carriers have revealed that these cells have the capacity to be employed as novel drug delivery carriers.[30]
Platelets
Megakaryocytes produce platelets. Small size and not having a nucleus are the features of platelets. They play an essential role in homeostasis.[54] Although platelets age from 7 to 10 days that is lower compared to other blood cells, some features thereof such as storing capacity and high payload make them interesting drug carriers.[8] Platelets can be targeted perfectly and migrate to destroyed parts of the body or the places where cell proliferation rate is higher. Furthermore, these cells have a great importance in inflammation; thus, they are available in the tumor site. Drugs can be loaded in platelets through either electroporation or incubation. Studies have shown that loading doxorubicin in platelets has better consequences in comparison with the drug only. The results of this study were encouraging both in vivo and in vitro.[55]
Other researches have shown that metastatic cells have a great tendency to adhere to platelets through surface proteins. Therefore, anticancer drugs can be loaded in platelets to sabotage and eliminate metastatic cells.[56,57] Figure 2 illustrates specialized targeting to metastasized tumor cells through platelets.
Figure 2.
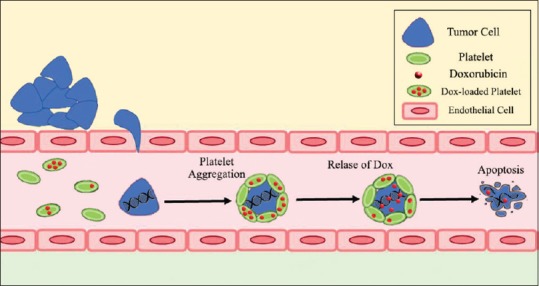
Targeted delivery to metastatic cell
Macrophages
Being a derivative of monocytes, macrophages are the first-level defenders in human body's immune system, and phagocytosis of microorganisms and external factors are their important characteristics. Moreover, monocytes can migrate to tumor sites and pathologic places suffering from infection or inflammation. When monocytes reach the desired site, they differentiate into macrophages. The first application of macrophages as drug carriers is carrying anti-infection drugs as they naturally migrate to infection sites. To illustrate, antiretroviral therapy is a suitable example because in this treatment, antivirus drugs are delivered to the sites where viruses are proliferating, and in case of AIDS virus, this method of drug delivery has shown to be effective for 2 weeks in the human body.[58]
Since the hypoxic condition is an outstanding feature of solid tumors, targeted drug delivery to tumors with limited toxicity and high efficiency is an important aim in the pharmacokinetics of cancer therapy. Tumor hypoxic cells secrete chemical absorbents or adherent agents through vascular system resulting in the migration of monocytes to the tumor site. Hence, macrophages’ tendency to migrate to tumor site which is called tumor-tropic feature can be used for releasing therapeutic agents or imaging contrast dyes into the tumor site. Furthermore, another interesting feature of macrophages is that these cells can pass the BBB, and for that reason, macrophages can be loaded with anticancer drugs ex vivo and then injected into the body to cure brain tumors. In addition, anticancer drugs should be loaded in liposomes to stay secured by the time they reach tumors. Furthermore, as it was mentioned in advance, incubation and phagocytosis can load drugs in macrophages.[13,58]
Stem cells
Studies based on SCs are expanding, and SCs are employed in tissue engineering and regenerative medicine. For example, mesenchymal and nervous SCs have been used as drug carriers.[12] These SCs are tumor-tropic and consequently can be used in treating and imaging tumors. More important, such a feature can be utilized in two ways. The first way is the encapsulation of therapeutic agents and anticancer drugs in stem cells and the second way is to import the genes that are related to therapeutic agents into the stem cells so that the genes can be expressed in the tumor site and demonstrate its anticancerous impact. Indeed, genetic engineering or gene therapy is used in the second case. As an example, tumor necrotic factor was released in the tumor site and activated caspase cascade of tumor cells leading to apoptosis.[7]
Numerous studies have been conducted pertinent to loading drugs or nanoparticles that contain drugs or contrast agents in SCs. In 2010, Roger et al. loaded coumarin-6 anticancer drug in polylactic acid nanoparticles and lipid nanocapsules and then the fabricated carriers in mesenchymal SCs. The results disclosed that drugs did not have any impact on cell viability or tumor-tropic feature.[59]
In addition to encapsulation of anticancer drugs in SCs, nanoparticles have been loaded in SCs. Most importantly, these nanoparticles are contrast agents in magnetic resonance imaging. Fe2O3-loaded nanoparticles were employed for tumor diagnosis in the lungs of mice that showed encouraging results. In this study, nanoparticles were imported into cells through unspecific absorption or pinocytosis.[60] Furthermore, coating with nanoparticles or liposomes that contain drugs with hyaluronic acid has been shown to cause attachment to CD44 receptors on the surface of mesenchymal SCs followed by phagocytosis.[61]
Application of the membranes of erythrocytes as drug delivery carriers
As was presented in previous sections, cell membranes or cell ghosts can be used in drug delivery systems. When the diameter of drug carriers is in nanoscale, for example, nanoliposomes and nanoparticles, cell absorption rises. Therefore, cell membranes can be engineered to form nanoerythrosomes. Nanoerythrosomes are erythrocytes’ membranes with the diameter of below 200 nm that increases cell absorption. Hence, for producing such nanoerythrosomes, hemoglobin should be eliminated at first through hypotonic lysis. Then, erythrosomes should pass through a polycarbonate membrane to achieve the particles, and drugs are loaded in nanoerythrosomes through coextrusion.[62] Furthermore, drugs can be loaded on the surface of erythrosomes which has proven to lead to suitable pharmacokinetic and pharmacodynamical conditions no matter loading in erythrosomes or their membranes. In addition, macrophages can be targeted through this method.[61]
Application of bacterial membranes as drug delivery carriers
Cytoplasm-free bacterial membranes can be used as drug delivery carriers. To illustrate, in the cases of negative-Gram bacteria, expression of a specific gene makes a hole in the membrane of bacteria resulting in the internal contents of the cell to leave. More importantly, the produced membranes retain their morphology, natural level, antigenic structure, and bioadherence. Hence, because they retain their natural level, they can stimulate humoral and cellular immune responses and consequently be used in vaccination. Furthermore, bacterial membranes can be used as antigens and nucleic acids’ predrugs while drugs can be attached to the surface of these membranes as well. Reported release from bacterial membranes has been of stable ones. In addition, high bioaccessibility, low cost, and simple production methods are the other features of such systems.[19]
The targeted release can be achieved with the application of bacterial membranes since these membranes can target dendritic cells, macrophages, tumor, and endothelial cells. For instance, the membrane of bacterium Mannheimia haemolytica has shown an appropriate adhesion to colorectal cancer cells. Therefore, with loading anticancer drugs in such membranes, cancer can be cured. Ex vivo studies on the treatment of colorectal cancer by loading doxorubicin in the membrane of M. haemolytica have led to encouraging results.[63]
Conclusion
In the recent decade, various cell types including erythrocytes, platelets, SCs, white blood cells, and also their membranes such as erythrocytes have been used as drug carriers. The most important benefits of cell-based drug carriers are enabling targeted drug delivery, improving pharmacokinetics and pharmacodynamics and greater biocompatibility. Therapeutic agents such as drugs, enzymes, hyperthermia therapeutic agents, and imaging agents have been loaded in aforementioned cells. Despite the challenges in cell-based drug delivery, this method can revolutionize therapeutic and diagnostic technologies. Among mentioned cell carriers, erythrocytes have been very noticed, thanks to their outstanding features. L-asparaginase-loaded erythrocytes have been used in clinical investigations for the treatment of pancreatic cancer and lymphoblastic leukemia. It should be considered that any development in the field of biomaterials indubitably extends cell-based drug delivery systems. Thus, combination of cells and nanobiomaterials is a novel drug delivery method that theoretically is the most creative and encouraging drug delivery method and demanding great efforts and attempts to achieve the optimum drug delivery patterns.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
BIOGRAPHIES

Seyed Mohammad Zargar received the B.S. degree in biomechanical engineering from University of Isfahan, Isfahan, Iran, in 2018. Now he is an M.S candidate of biomaterial engineering in University of Tehran, Tehran. His research interests are biomaterials and cell-based drug delivery systems.
Email: s.mohammad.zargar@gmail.com

Darioush Khodabakhshi Hafshejani received the B.S. degree in biomaterial engineering from Amirkabir University of Technology, Tehran, Iran, in 2016 and the M.S degree in biomaterial engineering from Isfahan University of Medical Sciences, Isfahan, Iran, in 2019. His research interests are advanced drug delivery system, cell based drug delivery system and transdermal drug delivery system.
Email: darioush1373@gmail.com

Asghar Eskandarinia has received his MSc in Biomedical Engineering from Isfahan University of Medical Science, Isfahan, Iran. His research interests are Biomaterials, skin Tissue Engineering and Wound Healing.
Email: a.eskandarinia1@gmail.com

Mohamad Rafienia obtained his B.Sc and both M.Sc and Ph.D. from isfahan university of technology and Amir Kabir University of Technology, respectively. He is currently a professor at biosensor Research center of Isfahan University of medical Sciences. His research expertise is in biomaterials, tissue engineering, and advanced drug delivery.
Email: m_rafienia@med.mui.ac.ir

Anousheh Zargar Kharazi is an associated Professor of Biomaterials Science and Engineering in the school of Advanced Medical Technology in Isfahan university of medical sciences. She received her Bachelor's degree in Mechanical Engineering from Isfahan University of Technology and Master's degree in Biomechanics from the Iran University of Science and Technology. She completed her PhD in Biomaterials from the Isfahan University of Technology, Iran. Her research interests are tissue engineering and advanced drug delivery systems.
Email: anosh_zargar@yahoo.com
References
- 1.Su Y, Xie Z, Kim GB, Dong C, Yang J. Design strategies and applications of circulating cell-mediated drug delivery systems. ACS Biomater Sci Eng. 2015;1:201–17. doi: 10.1021/ab500179h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rafienia M, Orang F, Emami SH. Preparation and characterization of polyurethane microspheres containing theophylline. J Bioact Compat Polym. 2006;21:341–9. [Google Scholar]
- 3.Rafienia M, Mirzadeh H, Mirzadeh H, Mobedi H, Jamshidi A. In vitro evaluation of drug solubility and gamma irradiation on the release of betamethasone under simulated in vivo conditions. J Bioact Compat Polym. 2007;22:443–59. [Google Scholar]
- 4.Hassanzadeh-Tabrizi SA, Bigham A, Rafienia M. Surfactant-assisted sol-gel synthesis of forsterite nanoparticles as a novel drug delivery system. Mater Sci Eng C Mater Biol Appl. 2016;58:737–41. doi: 10.1016/j.msec.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Rezaei F, Rafienia M, Keshvari H, Sattary M, Naeimi M, Keyvani H. Fabrication of poly hydroxybutyrate-polyethylene glycol-folic acid nanoparticles loaded by paclitaxel. Curr Drug Deliv. 2016;13:57–64. doi: 10.2174/1567201812666150803153938. [DOI] [PubMed] [Google Scholar]
- 6.Fliervoet LAL, Mastrobattista E. Drug delivery with living cells. Adv Drug Deliv Rev. 2016;106:63–72. doi: 10.1016/j.addr.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 7.Pierigè F, Serafini S, Rossi L, Magnani M. Cell-based drug delivery. Adv Drug Deliv Rev. 2008;60:286–95. doi: 10.1016/j.addr.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 8.Sun Y, Su J, Liu G, Chen J, Zhang X, Zhang R, et al. Advances of blood cell-based drug delivery systems. Eur J Pharm Sci. 2017;96:115–28. doi: 10.1016/j.ejps.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Li T, Dong H, Zhang C, Mo R. Cell-based drug delivery systems for biomedical applications. Nano Res. 2018;25:1–18. [Google Scholar]
- 10.Shapira A, Livney YD, Broxterman HJ, Assaraf YG. Nanomedicine for targeted cancer therapy: Towards the overcoming of drug resistance. Drug Resist Updat. 2011;14:150–63. doi: 10.1016/j.drup.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Li R, He Y, Zhang S, Qin J, Wang J. Cell membrane-based nanoparticles: A new biomimetic platform for tumor diagnosis and treatment. Acta Pharm Sin B. 2018;8:14–22. doi: 10.1016/j.apsb.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krueger TEG, Thorek DLJ, Denmeade SR, Isaacs JT, Brennen WN. Concise review: Mesenchymal stem cell-based drug delivery: The good, the bad, the ugly, and the promise. Stem Cells Transl Med. 2018;7:651–63. doi: 10.1002/sctm.18-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi J, Kim HY, Ju EJ, Jung J, Park J, Chung HK, et al. Use of macrophages to deliver therapeutic and imaging contrast agents to tumors. Biomaterials. 2012;33:4195–203. doi: 10.1016/j.biomaterials.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 14.Jain S, Jain N. Engineered erythrocytes as a drug delivery system. Indian J Pharm Sci. 1997;59:275. [Google Scholar]
- 15.Villa CH, Cines DB, Siegel DL, Muzykantov V. Erythrocytes as carriers for drug delivery in blood transfusion and beyond. Transfus Med Rev. 2017;31:26–35. doi: 10.1016/j.tmrv.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan S, Wu T, Zhang D, Zhang Z. Cell or cell membrane-based drug delivery systems. Theranostics. 2015;5:863–81. doi: 10.7150/thno.11852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Labusca L, Herea DD, Mashayekhi K. Stem cells as delivery vehicles for regenerative medicine-challenges and perspectives. World J Stem Cells. 2018;10:43–56. doi: 10.4252/wjsc.v10.i5.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu D, Dong X, Shi X, Zhang C, Wang Z. Neutrophil-based drug delivery systems. Adv Mater. 2018;30:e1706245. doi: 10.1002/adma.201706245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pang L, Qin J, Han L, Zhao W, Liang J, Xie Z, et al. Exploiting macrophages as targeted carrier to guide nanoparticles into glioma. Oncotarget. 2016;7:37081–91. doi: 10.18632/oncotarget.9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutiérrez Millán C, Colino Gandarillas CI, Sayalero Marinero ML, Lanao JM. Cell-based drug-delivery platforms. Ther Deliv. 2012;3:25–41. doi: 10.4155/tde.11.141. [DOI] [PubMed] [Google Scholar]
- 21.Gutiérrez Millán C, Zarzuelo Castañeda A, Sayalero Marinero ML, Lanao JM. Factors associated with the performance of carrier erythrocytes obtained by hypotonic dialysis. Blood Cells Mol Dis. 2004;33:132–40. doi: 10.1016/j.bcmd.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Tsong TY. Electroporation of cell membranes. Biophys J. 1991;60:297–306. doi: 10.1016/S0006-3495(91)82054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Millán CG, Marinero ML, Castañeda AZ, Lanao JM. Drug, enzyme and peptide delivery using erythrocytes as carriers. J Control Release. 2004;95:27–49. doi: 10.1016/j.jconrel.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 24.Batrakova EV, Li S, Reynolds AD, Mosley RL, Bronich TK, Kabanov AV, et al. A macrophage-nanozyme delivery system for Parkinson's disease. Bioconjug Chem. 2007;18:1498–506. doi: 10.1021/bc700184b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinman RM, Mellman IS, Muller WA, Cohn ZA. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983;96:1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canton I, Battaglia G. Endocytosis at the nanoscale. Chem Soc Rev. 2012;41:2718–39. doi: 10.1039/c2cs15309b. [DOI] [PubMed] [Google Scholar]
- 27.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annual review of immunology. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 28.Nowacek AS, Miller RL, McMillan J, Kanmogne G, Kanmogne M, Mosley RL, et al. NanoART synthesis, characterization, uptake, release and toxicology for human monocyte-macrophage drug delivery. Nanomedicine (Lond) 2009;4:903–17. doi: 10.2217/nnm.09.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chambers E, Mitragotri S. Long circulating nanoparticles via adhesion on red blood cells: Mechanism and extended circulation. Exp Biol Med (Maywood) 2007;232:958–66. [PubMed] [Google Scholar]
- 30.Hamidi M, Zarrin A, Foroozesh M, Mohammadi-Samani S. Applications of carrier erythrocytes in delivery of biopharmaceuticals. J Control Release. 2007;118:145–60. doi: 10.1016/j.jconrel.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 31.Magnani M, Chiarantini L, Mancini U. Preparation and characterization of biotinylated red blood cells. Biotechnol Appl Biochem. 1994;20:335–45. doi: 10.1111/j.1470-8744.1994.tb00321.x. [DOI] [PubMed] [Google Scholar]
- 32.Baskin JM, Bertozzi CR. Bioorthogonal click chemistry: Covalent labeling in living systems. QSAR Comb Sci. 2007;26:1211–9. [Google Scholar]
- 33.Li L, Guan Y, Liu H, Hao N, Liu T, Meng X, et al. Silica nanorattle-doxorubicin-anchored mesenchymal stem cells for tumor-tropic therapy. ACS Nano. 2011;5:7462–70. doi: 10.1021/nn202399w. [DOI] [PubMed] [Google Scholar]
- 34.Bratosin D, Mazurier J, Tissier JP, Estaquier J, Huart JJ, Ameisen JC, et al. Cellular and molecular mechanisms of senescent erythrocyte phagocytosis by macrophages. A review. Biochimie. 1998;80:173–95. doi: 10.1016/s0300-9084(98)80024-2. [DOI] [PubMed] [Google Scholar]
- 35.Hamidi M, Tajerzadeh H, Dehpour AR, Rouini MR, Ejtemaee-Mehr S. In vitro characterization of human intact erythrocytes loaded by enalaprilat. Drug Deliv. 2001;8:223–30. doi: 10.1080/107175401317245903. [DOI] [PubMed] [Google Scholar]
- 36.Hamidi M, Tajerzadeh H. Carrier erythrocytes: An overview. Drug Deliv. 2003;10:9–20. doi: 10.1080/713840329. [DOI] [PubMed] [Google Scholar]
- 37.Lotero LA, Olmos G, Diez JC. Delivery to macrophages and toxic action of etoposide carried in mouse red blood cells. Biochim Biophys Acta. 2003;1620:160–6. doi: 10.1016/s0304-4165(02)00536-6. [DOI] [PubMed] [Google Scholar]
- 38.Magnani M, Rossi L. Approaches to erythrocyte-mediated drug delivery. Expert Opin Drug Deliv. 2014;11:677–87. doi: 10.1517/17425247.2014.889679. [DOI] [PubMed] [Google Scholar]
- 39.Biagiotti S, Paoletti MF, Fraternale A, Rossi L, Magnani M. Drug delivery by red blood cells. IUBMB Life. 2011;63:621–31. doi: 10.1002/iub.478. [DOI] [PubMed] [Google Scholar]
- 40.Castro M, Rossi L, Papadatou B, Bracci F, Knafelz D, Ambrosini MI, et al. Long-term treatment with autologous red blood cells loaded with dexamethasone 21-phosphate in pediatric patients affected by steroid-dependent Crohn disease. J Pediatr Gastroenterol Nutr. 2007;44:423–6. doi: 10.1097/MPG.0b013e3180320667. [DOI] [PubMed] [Google Scholar]
- 41.Muzykantov VR. Drug delivery by red blood cells: Vascular carriers designed by mother nature. Expert Opin Drug Deliv. 2010;7:403–27. doi: 10.1517/17425241003610633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Domenech C, Thomas X, Chabaud S, Baruchel A, Gueyffier F, Mazingue F, et al. L-asparaginase loaded red blood cells in refractory or relapsing acute lymphoblastic leukaemia in children and adults: Results of the GRASPALL 2005-01 randomized trial. Br J Haematol. 2011;153:58–65. doi: 10.1111/j.1365-2141.2011.08588.x. [DOI] [PubMed] [Google Scholar]
- 43.Gottlieb Y, Topaz O, Cohen LA, Yakov LD, Haber T, Morgenstern A, et al. Physiologically aged red blood cells undergo erythrophagocytosis in vivo but not in vitro . Haematologica. 2012;97:994–1002. doi: 10.3324/haematol.2011.057620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ihler GM, Glew RH, Schnure FW. Enzyme loading of erythrocytes. Proc Natl Acad Sci U S A. 1973;70:2663–6. doi: 10.1073/pnas.70.9.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thorpe SR, Fiddler MB, Desnick RJ. Enzyme therapy. V. In vivo fate of erythrocyte-entrapped beta-glucuronidase in beta-glucuronidase-deficient mice. Pediatr Res. 1975;9:918–23. doi: 10.1203/00006450-197512000-00011. [DOI] [PubMed] [Google Scholar]
- 46.Alvarez FJ, Herráez A, Murciano JC, Jordán JA, Díez JC, Tejedor MC. In vivo survival and organ uptake of loaded carrier rat erythrocytes. J Biochem. 1996;120:286–91. doi: 10.1093/oxfordjournals.jbchem.a021411. [DOI] [PubMed] [Google Scholar]
- 47.Ihler G, Lantzy A, Purpura J, Glew RH. Enzymatic degradation of uric acid by uricase-loaded human erythrocytes. J Clin Invest. 1975;56:595–602. doi: 10.1172/JCI108129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Way J, Leung P, Ray L, Sander C. Vol. 51. Karger Publishers; 1985. Erythrocyte encapsulated thiosulfate sulfurtransferase. Red Blood Cells as Carriers for Drugs; pp. 75–81. [DOI] [PubMed] [Google Scholar]
- 49.Lizano C, Sanz S, Luque J, Pinilla M. In vitro study of alcohol dehydrogenase and acetaldehyde dehydrogenase encapsulated into human erythrocytes by an electroporation procedure. Biochim Biophys Acta. 1998;1425:328–36. doi: 10.1016/s0304-4165(98)00085-3. [DOI] [PubMed] [Google Scholar]
- 50.Bax BE, Fairbanks LD, Bain MD, Simmonds HA, Chalmers RA. The entrapment of polyethylene glycol-bound adenosine deaminase (Pegademase) in human carrier erythrocytes. Biochem Soc Trans. 1996;24:442S. doi: 10.1042/bst024442s. [DOI] [PubMed] [Google Scholar]
- 51.Bourgeaux V, Lanao JM, Bax BE, Godfrin Y. Drug-loaded erythrocytes: On the road toward marketing approval. Drug Des Devel Ther. 2016;10:665–76. doi: 10.2147/DDDT.S96470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Magnani M, DeLoach JR. The Use of Resealed Erythrocytes as Carriers and Bioreactors. Springer Science & Business Media. 2012;326:8. [Google Scholar]
- 53.Danielyan K, Ganguly K, Ding BS, Atochin D, Zaitsev S, Murciano JC, et al. Cerebrovascular thromboprophylaxis in mice by erythrocyte-coupled tissue-type plasminogen activator. Circulation. 2008;118:1442–9. doi: 10.1161/CIRCULATIONAHA.107.750257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saad J, Schoenberger L. Physiology, Platelet Activation. StatPearls. 2018 [PubMed] [Google Scholar]
- 55.Xu P, Zuo H, Chen B, Wang R, Ahmed A, Hu Y, et al. Doxorubicin-loaded platelets as a smart drug delivery system: An improved therapy for lymphoma. Sci Rep. 2017;7:42632. doi: 10.1038/srep42632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Modery-Pawlowski CL, Master AM, Pan V, Howard GP, Sen Gupta A. A platelet-mimetic paradigm for metastasis-targeted nanomedicine platforms. Biomacromolecules. 2013;14:910–9. doi: 10.1021/bm301996p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Läubli H, Borsig L, editors Selectins promote tumor metastasis. Seminars in Cancer Biology. Academic Press. 2010;20:3. doi: 10.1016/j.semcancer.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 58.Choi MR, Stanton-Maxey KJ, Stanley JK, Levin CS, Bardhan R, Akin D, et al. A cellular trojan horse for delivery of therapeutic nanoparticles into tumors. Nano Lett. 2007;7:3759–65. doi: 10.1021/nl072209h. [DOI] [PubMed] [Google Scholar]
- 59.Roger M, Clavreul A, Venier-Julienne MC, Passirani C, Sindji L, Schiller P, et al. Mesenchymal stem cells as cellular vehicles for delivery of nanoparticles to brain tumors. Biomaterials. 2010;31:8393–401. doi: 10.1016/j.biomaterials.2010.07.048. [DOI] [PubMed] [Google Scholar]
- 60.Loebinger MR, Kyrtatos PG, Turmaine M, Price AN, Pankhurst Q, Lythgoe MF, et al. Magnetic resonance imaging of mesenchymal stem cells homing to pulmonary metastases using biocompatible magnetic nanoparticles. Cancer Res. 2009;69:8862–7. doi: 10.1158/0008-5472.CAN-09-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Q, Cheng H, Peng H, Zhou H, Li PY, Langer R. Non-genetic engineering of cells for drug delivery and cell-based therapy. Adv Drug Deliv Rev. 2015;91:125–40. doi: 10.1016/j.addr.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 62.Lejeune A, Moorjani M, Gicquaud C, Lacroix J, Poyet P, Gaudreault R, et al. Nanoerythrosome, a new derivative of erythrocyte ghost: Preparation and antineoplastic potential as drug carrier for daunorubicin. Anticancer Res. 1994;14:915–9. [PubMed] [Google Scholar]
- 63.Jalava K, Eko FO, Riedmann E, Lubitz W. Bacterial ghosts as carrier and targeting systems for mucosal antigen delivery. Expert Rev Vaccines. 2003;2:45–51. doi: 10.1586/14760584.2.1.45. [DOI] [PubMed] [Google Scholar]


