Abstract
Objectives:
To determine whether burden of multiple chronic conditions (MCCs) influences the risk of receiving inappropriate vs. appropriate device therapies.
Design:
Retrospective cohort study.
Setting:
Seven U.S. healthcare delivery systems.
Participants:
Adults with left ventricular systolic dysfunction receiving an ICD for primary prevention.
Measurements:
Data on twenty-four comorbid conditions were captured from electronic health records and categorized into quartiles of comorbidity burden (0-3, 4-5, 6-7 and ≥8). Incidence of ICD therapies (shock and anti-tachycardia pacing therapies), including appropriateness, were collected for three years after implantation. Outcomes included time to first ICD therapy, total ICD therapy burden, and risk of inappropriate versus appropriate ICD therapy.
Results:
Among 2,235 patients (mean age 69±11 years, 75% men), the median number of comorbidities was 6 (interquartile range 4, 8), with 98% having at least two comorbidities. During a mean 2.2 years of follow-up, 18.3% of patients experienced at least one appropriate therapy and 9.9% experienced at least one inappropriate therapy. Higher comorbidity burden was associated with an increased risk of first inappropriate therapy (adjusted hazard ratio [HR] for 4-5 comorbidities 1.94 [95%CI:1.14-3.31]; HR 2.25 [95%CI:1.25-4.05] for 6-7 comorbidities; and HR 2.91 [95%CI:1.54-5.50] for ≥8 comorbidities. Participants with ≥8 comorbidities had a higher total burden of ICD therapy (adjusted relative risk [RR] 2.12 [95%CI:1.43-3.16]), higher burden of inappropriate therapy (RR 3.39 [95%CI:1.67-6.86]), and higher risk of receiving inappropriate versus appropriate therapy (RR 1.74 [95%CI:1.07-2.82]). Comorbidity burden was not significantly associated with receipt of appropriate ICD therapies. Patterns were similar when separately examining shock or anti-tachycardia pacing therapies.
Conclusions:
In primary prevention ICD recipients, MCC burden was independently associated with an increased risk of inappropriate but not appropriate device therapies. Comorbidity burden should be considered when engaging patients in shared decision-making about ICD implantation.
Keywords: Comorbidity, multimorbidity, chronic disease, implantable cardioverter defibrillator, patient-centered outcomes
INTRODUCTION
Use of implantable cardioverter defibrillators (ICDs) for the primary prevention of sudden cardiac death (SCD) has grown dramatically in recent decades, based on results from pivotal randomized clinical trials showing 23-60% relative reductions in SCD among selected patients with left ventricular systolic dysfunction1-4. National clinical practice guidelines have recommended ICD implantation for primary prevention of SCD in patients with left ventricular ejection fraction (LVEF) ≤35% and New York Heart Association class II or III heart failure who have an estimated life expectancy with good functional status of greater than one year5. Under these guidelines, more than half a million Medicare beneficiaries are now eligible for primary prevention ICD implantation6.
While ICDs are effective in reducing risk of SCD in selected patients with left ventricular systolic dysfunction, they are also associated with potential drawbacks. For example, receipt of shocks (appropriate or inappropriate) can be painful7 and are associated with increased mortality,7, 8 higher healthcare utilization9, psychological distress10, and reduced quality of life11. Evidence also suggests that ICD shocks or anti-tachycardia pacing may be pro-arrhythmic12 and are associated with myocardial damage 13—mechanisms that may explain, in part, the increased death rates observed among recipients who receive multiple shocks8, 14. Inappropriate shocks—those that result from non-lethal tachyarrhythmias or improper device sensing—account for up to one third of all ICD shocks12, offer no clinical benefit to patients, and are associated with adverse outcomes7, 15.
Clinical characteristics that predispose a patient to inappropriate versus appropriate therapy remain poorly understood, and an area of growing interest concerns how comorbidity burden may affect the risk of appropriate and inappropriate ICD therapies. Comorbid conditions such as hypertension16, ischemic heart disease17, 18, diabetes mellitus 17, 18, atrial fibrillation17, 18, chronic lung disease17, 18, anemia17, 18, and chronic kidney disease17, 18 are extremely common among ICD recipients, and the presence of multiple chronic conditions (MCCs) is common19-21. Certain conditions such as chronic lung disease22 and diabetes23 have been associated with higher risks of appropriate shocks, whereas others (e.g., atrial fibrillation15, 16, 24, hypertrophic cardiomyopathy24) have been associated with a greater incidence of inappropriate shocks. The impact of MCC burden on risks of appropriate and inappropriate ICD therapies has not been systematically evaluated but may be important when counseling patients about the net clinical benefit or harm of primary prevention ICD implantation.
Using data from the multicenter Cardiovascular Research Network Longitudinal Study of Implantable Cardioverter Defibrillators (CVRN LS-ICD), we characterized the prevalence of MCCs in a contemporary, community-based cohort receiving ICD therapies for primary prevention and examined the independent association of MCC burden on the frequency and appropriateness of ICD therapies.
METHODS
Study design and data sources
Data were derived from the CVRN LS-ICD, a retrospective cohort study of primary prevention ICD implant patients recruited from seven health care delivery system members of the CVRN25, affiliated with the Health Care Systems Research Network26. Details of this study have been previously described27. Data for the LS-ICD were aggregated from three entities: the National Cardiovascular Data Registry ICD Registry (), the CVRN Virtual Data Warehouse, and an ICD device therapy data repository developed specifically for the LS-ICD27. The registry provided data on ICD implantation eligibility and other clinical criteria, device and provider details, and adverse events during the index implant hospitalization. The Virtual Data Warehouse provided longitudinal data on health plan enrollment, insurance coverage details, demographics, comorbidities, health care utilization, pharmacy, and laboratory values. The ICD therapy data repository provided information on device therapies as described in more detail below.
We identified 2,787 patients with left ventricular systolic dysfunction who received a first-time ICD between January 1, 2006 and December 31, 2009 for primary prevention of SCD. Participants were recruited from one of 14 hospitals affiliated with the seven participating health care systems. All eligible patients received a primary prevention ICD, had no prior ICD implantation, had documented left ventricular systolic dysfunction, and were members of a participating health system. We excluded participants who had <1 year of continuous health plan membership before ICD implantation (n=545), those aged <21 years old (n=3), and those who died during the ICD implant procedure (n=7), leaving a total of 2,235 participants for analysis. Participants were followed through December 2011 (mean follow-up: 2.2 years, maximum: 3 years) to ascertain ICD therapy outcomes.
Institutional review boards at all participating sites approved the study, and waivers of informed consent were obtained because of the nature of the study.
Assessment of Multiple Chronic Conditions
We selected 24 comorbidities for analysis based on their high prevalence or association with poor outcomes among patients with ICDs. We included 14 of the 15 comorbidities recommended by the U.S. Department of Health and Human Services’ Strategic Framework on Multiple Chronic Conditions28 that are collected by the Centers of Medicare and Medicaid Services, including hypertension, dyslipidemia, coronary heart disease, arthritis, diabetes, chronic kidney disease, depression, chronic obstructive pulmonary disease, dementia, atrial fibrillation/flutter, cancer, osteoporosis, asthma, and stroke. To these 14 conditions, we added pre-existing ventricular tachycardia, sinus node dysfunction, aortic valvular disease, previous valvular surgery, anemia, abnormal thyroid function, peripheral artery disease, chronic liver disease, mobility impairment, and history of gastrointestinal hemorrhage. All comorbid conditions were identified using previously described methods25, 27, 29 using data on inpatient, emergency, and ambulatory diagnoses and procedures, prescribed medications, and laboratory test results from the Virtual Data Warehouse and NCDR ICD Registry (definitions available on request). The lookback period to capture comorbidities was up to three years pre-ICD implantation for all participants; comorbidities diagnosed after ICD implantation were not evaluated. Each participant’s comorbidities were summed, and participants were categorized into count-based quartiles of one measure of morbidity burden for analysis: 0 to 3, 4 or 5, 6 or 7, and 8 or more comorbidities.
Assessment of ICD therapy
Participants were followed for up to three years after ICD placement for occurrence of ICD therapy (shock or anti-tachycardia pacing [ATP]). Participants were censored at the time of death, health plan disenrollment, receipt of 10 confirmed device therapies, or end of follow-up (December 31, 2011). ICD therapies were identified and confirmed via a standardized protocol that included medical record abstraction at the study site, central clinical review, and expert external adjudication of source documents as previously described27. Briefly, data on arrhythmic episodes resulting in ICD therapy were abstracted from device reports (including intracardiac electrograms) and clinical notes (including local provider interpretation). A central review panel, consisting of three electrophysiologists and a hospitalist with expertise in ICD device interpretation30, confirmed the occurrence of treated episodes1, determined the initial type of therapy (shock or ATP)2, determined whether the episode required multiple therapies, and adjudicated the appropriateness of therapy using standardized criteria3. As previously described,31 therapies were classified as appropriate (in response to a potentially malignant ventricular tachyarrhythmia), or inappropriate (due to other causes, including supraventricular arrhythmias, or problems with device sensing or function). Abstraction of ICD therapy was truncated after ten episodes for each participant, and a maximum of three episodes were collected per 24-hour period to limit the potential burden of data generated by “arrhythmic storms.”31 Selected records were double-adjudicated by an external panel of electrophysiologists. All judgments were based on definitions developed through extensive discussion of central and external panel members using literature-based guidance. Disagreement among reviewers was resolved via reviewer conference or arbitration with external experts.
We examined three separate outcomes related to receipt of ICD therapy:30 time to first therapy (any, appropriate, and inappropriate),1 total burden of therapy (counts of total therapy, appropriate, and inappropriate over the course of follow-up), and risk (i.e., balance) of inappropriate versus appropriate therapy2 among the subset of participants who received at least one appropriate or inappropriate ICD therapy (n=562). .
Covariates
Covariates were gathered from the NCDR ICD Registry and each site’s VDW, captured during the three-year period before ICD implantation. Demographic characteristics (age, gender, race/ethnicity), smoking status, body mass index and medication use (angiotensin-converting enzyme [ACE] inhibitors, angiotensin II receptor blockers, aspirin, beta blockers, warfarin, digoxin, statins and antiplatelet agents) were gathered from the VDW. Clinical characteristics (family history of SCD, heart failure, New York Heart Association class, blood pressure, estimated glomerular filtration rate, hemoglobin, serum potassium level, left ventricular ejection fraction and ICD type) at the time of ICD implantation were gathered from the NCDR ICD Registry.
Analytic Approach
All analyses were performed using SAS software, version 9.3 (Cary, NC). We performed descriptive statistics to examine baseline characteristics across quartiles of comorbidity counts (i.e., 0 to 3, 4 or 5, 6 or 7, and ≥8 comorbidities), using ANOVA for continuous variables and chi-square tests for categorical variables.
We used Cox proportional hazards regression to examine the association of comorbidity counts with time to first ICD therapy (any, appropriate, or inappropriate). We confirmed absence of the violation of the proportional hazards assumption via visual inspection of log-negative log survival curves. We examined the association of comorbidity counts with burden (i.e., total counts) of any, appropriate, and inappropriate ICD therapy using Poisson regression. Finally, we examined the association of comorbidity counts with the risk of receiving inappropriate vs. appropriate ICD therapy using relative risk regression. The referent group for all models were participants in the lowest quartile of comorbidity burden, i.e., the group with 0 to 3 comorbidities. For each outcome, we performed a series of nested models that introduced covariates in the following order: an unadjusted model with comorbidity counts, followed by sequential addition of demographic characteristics and study site; and then baseline medical history, NYHA classification, left ventricular ejection fraction, smoking status, vital signs and laboratory results, baseline medication use and ICD type. Due to prior evidence suggesting atrial fibrillation is a strong risk factor for inappropriate shocks, we performed sensitivity analyses after removing atrial fibrillation as an eligible comorbidity in the comorbidity counts (Supplemental Figure A). We performed additional sensitivity analyses separately evaluating shock therapy and ATP therapies to address potential differences in the association of MCC burden with different types of device therapies (Supplemental Figures B and C, respectively).
RESULTS
Sample characteristics
Among 2235 eligible patients who received a primary prevention ICD, mean follow-up time was 2.2 (SD= 0.9) years. Mean age was 69 ±11 years, 25% were women, 77% were white/European, and 14% were Hispanic (Table 1). The distribution of ICD type was 33% single chamber, 36% dual chamber and 32% biventricular. Among 24 possible comorbid conditions, the median number of comorbidities per patient was 6 (IQR: 4, 8), and 98% of the cohort had at least two comorbidities. Participants with higher comorbidity burden were more likely to be older, white/European, current or former smokers, have government-based insurance, receive dual-chamber or biventricular ICDs, have a recent admission for heart failure or more symptomatic heart failure, higher systolic blood pressure, lower estimated glomerular filtration rate, higher blood urea nitrogen, lower hemoglobin, longer QRS duration, have abnormal IV conduction, be less likely to receive ACE inhibitor therapy, and more likely to receive an antiplatelet agent, anticoagulant, digoxin or statin (Table 1).
Table 1.
Baseline characteristics of adults receiving implantable cardioverter defibrillator for primary prevention for sudden cardiac death, overall and stratified by quartile of comorbidity burden.
| Characteristic | Overall (N=2235) |
0 to 3 comorbidities (N=317) |
4 or 5 comorbidities (N=650) |
6 or 7 comorbidities (N=681) |
≥8 comorbidities (N=587) |
P-value |
|---|---|---|---|---|---|---|
| Age, years, Mean (SD) | 68.5 (10.9) | 59.7 (13.4) | 66.2 (10.5) | 70.9 (9.2) | 72.9 (7.9) | <0.001 |
| Women, n (%) | 567 (25.4) | 88 (27.8) | 147 (22.6) | 174 (25.6) | 158 (26.9) | 0.23 |
| Race, n (%) | 0.25 | |||||
| White/European | 1711 (76.6) | 226 (71.3) | 494 (76.0) | 531 (78.0) | 460 (78.4) | |
| Hispanic, n (%) | 313 (14.0) | 38 (12.0) | 88 (13.5) | 109 (16.0) | 78 (13.3) | 0.41 |
| Current or former tobacco use, n (%) | 1275 (57.0) | 153 (48.3) | 352 (54.2) | 386 (56.7) | 384 (65.4) | <0.001 |
| Insurance type, n (%) | <0.001 | |||||
| Government | 1358 (60.8) | 106 (33.4) | 330 (50.8) | 477 (70.0) | 445 (75.8) | |
| Commercial | 30 (1.3) | 9 (2.8) | 12 (1.8) | 4 (0.6) | 5 (0.9) | |
| HMO | 842 (37.7) | 202 (63.7) | 305 (46.9) | 199 (29.2) | 136 (23.2) | |
| None / self pay | 5 (0.2) | 0 (0.0) | 3 (0.5) | 1 (0.1) | 1 (0.2) | |
| Follow-up, mean (SD), yr | 2.2 (0.9) | 2.3 (0.9) | 2.3 (0.8) | 2.2 (0.9) | 2.0 (1.0) | <0.001 |
| ICD device type, n (%) | <0.001 | |||||
| Single chamber | 728 (32.6) | 132 (41.6) | 257 (39.5) | 187 (27.5) | 152 (25.9) | |
| Dual chamber | 797 (35.7) | 84 (26.5) | 220 (33.8) | 274 (40.2) | 219 (37.3) | |
| Biventricular | 710 (31.8) | 101 (31.9) | 173 (26.6) | 220 (32.3) | 216 (36.8) | |
| Pre-implantation left ventricular ejection fraction, %, mean (SD) | 25.2 (6.6) | 23.6 (6.5) | 24.8 (6.5) | 25.5 (6.6) | 26.0 (6.7) | <0.001 |
| Comorbidities, per person | ||||||
| Mean (SD) | 6.0 (2.4) | 2.3 (0.9) | 4.5 (0.5) | 6.5 (0.5) | 9.1 (1.3) | <0.001 |
| Median (interquartile) | 6.0 (4.0-8.0) | 3.0 (2.0-3.0) | 5.0 (4.0-5.0) | 6.0 (6.0-7.0) | 9.0 (8.0-10.0) | <0.001 |
| Range | 0.0-16.0 | 0.0-3.0 | 4.0-5.0 | 6.0-7.0 | 8.0-16.0 | |
|
Comorbidities, n (%) 3 years prior to or on index date |
||||||
| Atrial fibrillation or flutter | 729 (32.6) | 33 (10.4) | 129 (19.8) | 232 (34.1) | 335 (57.1) | <0.001 |
| Aortic valvular disease | 878 (39.3) | 54 (17.0) | 173 (26.6) | 272 (39.9) | 379 (64.6) | <0.001 |
| Cerebrovascular disease | 321 (14.4) | 7 (2.2) | 47 (7.2) | 98 (14.4) | 169 (28.8) | <0.001 |
| Coronary artery disease | 1477 (66.1) | 97 (30.6) | 406 (62.5) | 488 (71.7) | 486 (82.8) | <0.001 |
| Ventricular tachycardia | <0.001 | |||||
| Non-sustained VT | 334 (14.9) | 15 (4.7) | 67 (10.3) | 105 (15.4) | 147 (25.0) | |
| Monomorphic sustained VT | 34 (1.5) | 1 (0.3) | 9 (1.4) | 9 (1.3) | 15 (2.6) | |
| Polymorphic sustained VT | 15 (0.7) | 3 (0.9) | 2 (0.3) | 4 (0.6) | 6 (1.0) | |
| Previous valvular surgery | 131 (5.9) | 2 (0.6) | 18 (2.8) | 36 (5.3) | 75 (12.8) | <0.001 |
| Abnormal sinus node function | 394 (17.6) | 13 (4.1) | 61 (9.4) | 136 (20.0) | 184 (31.3) | <0.001 |
| Peripheral artery disease | 61 (2.7) | 0 (0.0) | 4 (0.6) | 17 (2.5) | 40 (6.8) | <0.001 |
| Hypertension | 1651 (73.9) | 124 (39.1) | 426 (65.5) | 576 (84.6) | 525 (89.4) | <0.001 |
| Dyslipidemia | 1866 (83.5) | 160 (50.5) | 532 (81.8) | 610 (89.6) | 564 (96.1) | <0.001 |
| Anemia | 543 (24.3) | 11 (3.5) | 94 (14.5) | 176 (25.8) | 262 (44.6) | <0.001 |
| Diabetes mellitus | 952 (42.6) | 35 (11.0) | 223 (34.3) | 331 (48.6) | 363 (61.8) | <0.001 |
| Abnormal thyroid function | 260 (11.6) | 8 (2.5) | 43 (6.6) | 89 (13.1) | 120 (20.4) | <0.001 |
| Chronic liver disease | 73 (3.3) | 5 (1.6) | 12 (1.8) | 25 (3.7) | 31 (5.3) | <0.01 |
| Asthma | 312 (14.0) | 20 (6.3) | 65 (10.0) | 92 (13.5) | 135 (23.0) | <0.001 |
| Chronic obstructive pulmonary disease | 568 (25.4) | 31 (9.8) | 113 (17.4) | 182 (26.7) | 242 (41.2) | <0.001 |
| Chronic kidney disease | 1136 (50.8) | 46 (14.5) | 231 (35.5) | 405 (59.5) | 454 (77.3) | <0.001 |
| Chronic cancer | 222 (9.9) | 9 (2.8) | 34 (5.2) | 84 (12.3) | 95 (16.2) | <0.001 |
| Gastrointestinal hemorrhage | 54 (2.4) | 0 (0.0) | 3 (0.5) | 9 (1.3) | 42 (7.2) | <0.001 |
| Depression | 405 (18.1) | 27 (8.5) | 85 (13.1) | 112 (16.4) | 181 (30.8) | <0.001 |
| Dementia | 76 (3.4) | 1 (0.3) | 8 (1.2) | 20 (2.9) | 47 (8.0) | <0.001 |
| Arthritis | 716 (32.0) | 32 (10.1) | 134 (20.6) | 239 (35.1) | 311 (53.0) | <0.001 |
| Osteoporosis | 153 (6.8) | 8 (2.5) | 16 (2.5) | 53 (7.8) | 76 (12.9) | <0.001 |
| Mobility impairment | 63 (2.8) | 0 (0.0) | 7 (1.1) | 13 (1.9) | 43 (7.3) | <0.001 |
Frequency of ICD therapies
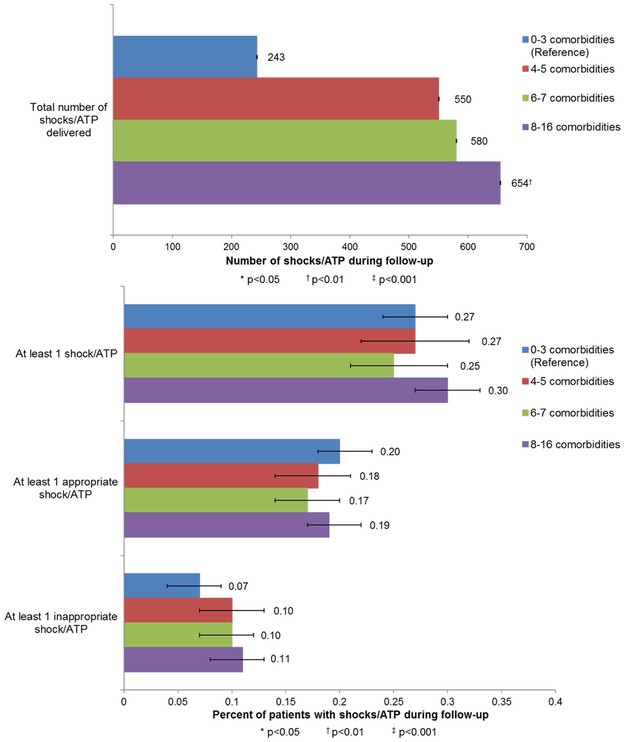
ICD therapy, including shock and ATP, occurred in 605 (27.1%) participants, with a total of 2027 therapies (647 shocks, 1349 ATP) delivered. At least one appropriate shock/ATP occurred in 410 participants (18.3%) and at least one inappropriate shock/ATP occurred in 221 participants (9.9%). Incidence of at least one shock/ATP (any, appropriate, or inappropriate) did not differ significantly according to comorbidity burden in bivariate analyses, but total burden of shock/ATP was significantly higher among participants with ≥8 comorbidities than among participants with lower comorbidity burden (P<0.01) (Figure 1).
Figure 1.
Frequencies of shock/ATP, stratified by quartiles of comorbidity count in adults with a primary prevention ICD.
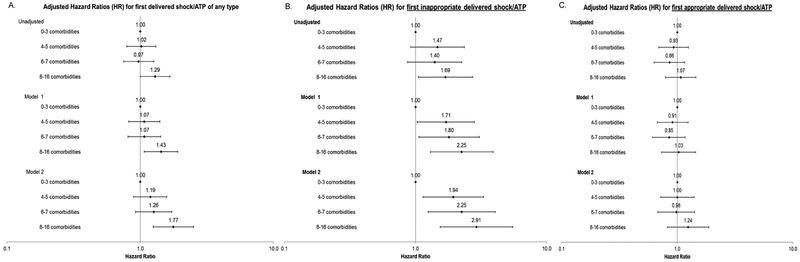
Comorbidity burden and time to first ICD therapy
Compared to participants with the lowest comorbidity burden (0 to 3 comorbidities), participants with higher comorbidity burden were at higher risk of receiving a first ICD therapy (any or inappropriate), but not appropriate therapy (Figure 2). For time to first ICD therapy of any kind, only participants with ≥8 comorbidities had a significantly higher adjusted rate of therapy (adjusted hazard ratio [HR] 1.77, 95% CI:1.25-2.51 in the fully adjusted model). The rate of receiving a first inappropriate therapy was greater among participants with 4 or 5 (HR 1.94, 95%CI:1.14-3.31), 6 or 7 (HR 2.25, 95%CI:1.25-4.05), and ≥8 (HR 2.91, 95% CI1.54-5.50) comorbidities (Figure 2). In contrast, comorbidity burden was not independently associated with time to first appropriate therapy in any models.
Figure 2. Association of baseline counts of comorbidities and time to first shock/ATP among 2235 participants who received a primary prevention ICD for cox proportional hazard regression models.
Panel A represents results for time to first delivered device therapy of any type; panel B represents time to first inappropriate device therapy, and panel C represents time to first appropriate device therapy.
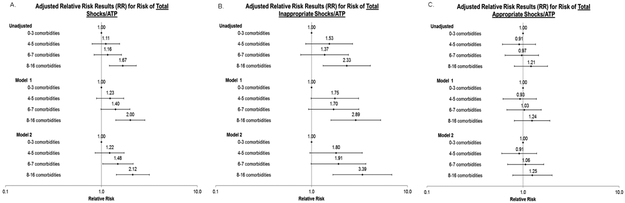
Comorbidity burden and total burden of ICD therapy
Compared to participants with 0 to 3 comorbidities, those with 6 or 7 comorbidities had a 48% significantly higher adjusted relative risk of any ICD therapy (RR 1.48, 95%CI:1.03-2.14) and those with ≥8 comorbidities had a more than twofold higher adjusted relative risk of any ICD therapy in fully-adjusted models (RR 2.12, 95%CI:1.43-3.16; Figure 3). Higher comorbidity burden was also associated with a greater burden of inappropriate therapy, with a more than threefold higher adjusted relative risk for ≥8 comorbidities compared with 0 to 3 comorbidities (RR 3.39, 95%CI:1.67-6.86, Figure 3). In contrast, comorbidity burden was not associated with greater burden of appropriate ICD therapy (Figure 3).
Figure 3. Association of baseline counts of comorbidities and burden of total delivered shocks/ATPs among 2235 participants who received a primary prevention ICD.
Panel A represents results for burden of device therapy of any type; panel B represents burden of inappropriate device therapy, and panel C represents burden of appropriate device therapy.
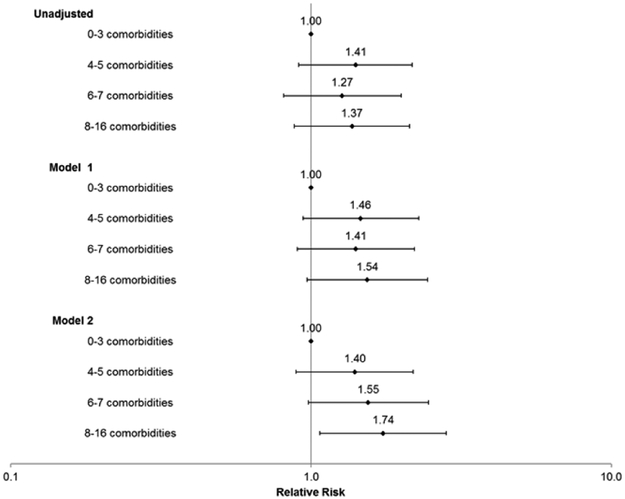
Comorbidity burden and risk of inappropriate vs. appropriate device therapy
Finally, for the outcome of the relative balance of receiving inappropriate vs. appropriate therapy, compared to patients who had 0 to 3 comorbidities, only patients with ≥8 comorbidities had a statistically significant higher risk of receiving inappropriate vs. appropriate therapy (RR 1.74, 95% CI:1.07-2.82) in the fully adjusted model (Figure 4).
Figure 4.
Association of baseline counts of comorbidities with risk of receiving an inappropriate shock/ATP vs. appropriate shock/ATP among 562 adults who received at least one inappropriate or appropriate shock from primary prevention ICD.
Sensitivity analyses
Findings from all sensitivity analyses that removed atrial fibrillation from the comorbidity count were in some cases attenuated but not differ materially from our main findings (see Supplementary Figure A). Patterns were also similar when individually examining shock vs. ATP therapies, although there was lower precision in the estimates (Supplemental Figures B and C).
DISCUSSION
Within a large, diverse, community-based cohort of primary prevention ICD recipients, we examined the association of comorbidity burden with receipt and appropriateness of ICD therapy. We found that higher comorbidity burden was consistently associated with shorter time to first ICD therapy and higher burden (i.e., counts) of device therapy over three years of follow-up. Furthermore, those with higher comorbidity burden were at greater risk for inappropriate, but not appropriate, therapies. The excess risk of inappropriate ICD therapy was greatest among participants with ≥8 comorbidities, with more attenuated risks observed among participants with 4 or 5 and 6 or 7 comorbidities compared to those with 0 to 3 comorbidities. Patterns were similar when separately examining shocks vs. ATP therapies.
To our knowledge, this is the first published study to report on the association between MCCs and device therapies among primarily older adults receiving a primary prevention ICD. Previous studies have found that selected individual medical conditions increase the probability of receipt of appropriate (e.g., diabetes23) and inappropriate (e.g., atrial fibrillation15) ICD device therapy. Taken together with existing studies, our results provide strong support for the importance of MCC burden influencing device-related outcomes in persons with a primary prevention ICD.
The reasons for the excess rate of inappropriate device therapies associated with greater comorbidity burden are unclear. Although patients with greater number of comorbidities had significantly higher percentages of atrial fibrillation and non-sustained ventricular tachycardia that can directly lead to increased inappropriate ICD therapy, this did not account for the observed increased risk of inappropriate device therapies. Greater comorbidity burden is also associated with higher levels of circulating inflammatory factors32 which, in turn, are associated with greater arrhythmogenicity33. It is also possible that both higher comorbidity burden and/or selected conditions may lead to other types of metabolic or physiologic changes that affect the sensitivity or accuracy of ICD sensing. Alternatively, device programming differences associated with comorbidity burden or targeted comorbid conditions during the study time period may have influenced the rate and appropriateness of device therapies, but systematic information on device settings was unavailable.
Our study had several notable strengths, including analysis of a large, multi-center cohort that included a wide range of information on comorbid conditions from complementary site-specific electronic medical records and national ICD registry data sources. Our cohort was demographically diverse and included patients from various practice settings and geographic locations throughout the U.S. Importantly, our study ascertained longitudinal information on ICD therapies that were subsequently adjudicated and classified as appropriate or inappropriate by clinical experts using standardized criteria. Our study also had certain limitations. Despite extensive review of available electronic and paper medical records and discussion, 15% of device therapies were unable to be given an appropriateness classification. Additional information on the specific causes of inappropriate device therapy were also unavailable. As noted, detailed information on ICD device settings were unavailable, but our results reflect the heterogeneity of clinical ICD care across the seven participating health systems during the time period of study. However, given our study time period, our study results may not be fully generalizable to current practice given the evoluation in ICD programming algorithms which reduce inappropriate and appropriate shocks. We also were unable to determine the potential impact of appropriate or inappropriate device therapies on subsequent mortality or other clinical outcomes. As a retrospective study, we also cannot rule out residual confounding of the observed association of MCCs with ICD therapies.
Our results have several important implications for patients and providers. While ICDs reduce the risk of death in high-risk patients, some studies have observed reduced mortality benefit and higher hospitalization rates among ICD recipients with MCCs21, whereas others have not.34 ICD shocks are painful7 and may produce adverse psychological effects10, particularly among patients with multimorbidity35. Additionally, both appropriate and inappropriate shocks may damage myocardium and put patients at increased risk for death8, 14. In patients with primary prevention ICDs and multimorbidity, consideration of the type of ICD and settings as well as possible adjustment of adjuvant antiarrhythmic strategies36, 37 may help to reduce the frequency of inappropriate device therapies and associated negative impacts on patients. The willingness of older patients to accept a medical therapy for primary cardiovascular prevention has been reported to be relatively insensitive to its benefits, but highly sensitive to its adverse effects—emphasizing the need to fully incorporate information on both benefits and harms into decision-making38. Given the excess risk of inappropriate ICD therapy associated with greater comorbidity burden, our findings support informing patients with multimorbidity about the spectrum of potential risks and benefits as part of shared decision-making for primary prevention ICD implantation.
CONCLUSIONS
Among adults receiving ICD implantation for primary prevention of sudden cardiac death, greater comorbidity burden was independently associated with time to first ICD therapy and total burden of ICD therapy. These outcomes appear to be driven by excess risk of inappropriate therapies, with no significant difference in risk of appropriate therapies. Additional research is needed to delineate the mechanisms affecting this excess risk and the potential influence of specific comorbid conditions and associated therapies to inform the development of strategies to mitigate the risk of inappropriate device therapy and optimize the net benefit of ICD therapy in the growing population of multimorbid patients receiving primary prevention ICDs.
Supplementary Material
Figure SA1. Frequencies of shock/ATP, stratified by quartiles of comorbidity count in adults with a primary prevention ICD and no known atrial fibrillation.
Figure SA2. Association of baseline counts of comorbidities (excluding atrial fibrillation) and time to first shock/ATP among 2235 participants who received a primary prevention ICD for cox proportional hazard regression models. Panel A represents results for time to first delivered device therapy of any type; panel B represents time to first inappropriate device therapy, and panel C represents time to first appropriate device therapy.
Figure SA3. Association of baseline counts of comorbidities (excluding atrial fibrillation) and burden of total delivered shocks/ATPs among 2235 participants who received a primary prevention ICD. Panel A represents results for burden of device therapy of any type; panel B represents burden of inappropriate device therapy, and panel C represents burden of appropriate device therapy.
Figure SA4. Association of baseline counts of comorbidities (excluding atrial fibrillation) with risk of receiving an inappropriate shock/ATP vs. appropriate shock/ATP among 562 adults who received at least one inappropriate or appropriate shock from primary prevention ICD.
Figure SB1. Frequencies of shocks, stratified by quartiles of comorbidity count in adults with a primary prevention ICD and no known atrial fibrillation.
Figure SB2. Association of baseline counts of comorbidities (excluding atrial fibrillation) and time to first shock among 2235 participants who received a primary prevention ICD for cox proportional hazard regression models. Panel A represents results for time to first delivered device therapy of any type; panel B represents time to first inappropriate device therapy, and panel C represents time to first appropriate device therapy.
Figure SB3. Association of baseline counts of comorbidities (excluding atrial fibrillation) and burden of total delivered shocks among 2235 participants who received a primary prevention ICD. Panel A represents results for burden of device therapy of any type; panel B represents burden of inappropriate device therapy, and panel C represents burden of appropriate device therapy.
Figure SB4. Association of baseline counts of comorbidities (excluding atrial fibrillation) with risk of receiving an inappropriate shock vs. appropriate shock among 300 adults who received at least one inappropriate or appropriate shock from primary prevention ICD.
Figure SC1. Frequencies of ATPs, stratified by quartiles of comorbidity count in adults with a primary prevention ICD and no known atrial fibrillation.
Figure SC2. Association of baseline counts of comorbidities (excluding atrial fibrillation) and time to first ATP among 2235 participants who received a primary prevention ICD for cox proportional hazard regression models. Panel A represents results for time to first delivered device therapy of any type; panel B represents time to first inappropriate device therapy, and panel C represents time to first appropriate device therapy.
Figure SC3. Association of baseline counts of comorbidities (excluding atrial fibrillation) and burden of total delivered ATPs among 2235 participants who received a primary prevention ICD. Panel A represents results for burden of device therapy of any type; panel B represents burden of inappropriate device therapy, and panel C represents burden of appropriate device therapy.
Figure SC4. Association of baseline counts of comorbidities (excluding atrial fibrillation) with risk of receiving an inappropriate ATP vs. appropriate ATP among 367 adults who received at least one inappropriate or appropriate shock from primary prevention ICD.
Impact statement: We certify that this work is novel as it is the first to evaluate the impact of multiple chronic conditions on therapy outcomes among older adults with implantable cardioverter defibrillators for primary prevention of sudden death.
ACKNOWLEDGMENTS
Disclosures: Dr Masoudi has a contract with the American College of Cardiology for his role as the Senior Medical Officer of the National Cardiovascular Data Registries. Dr Gupta serves as local site PI on multi-center clinical trials sponsored by St. Jude Medical, Boston Scientific, and Medtronic. The other authors have no relevant disclosures to report.
Funding: National Heart, Lung, and Blood Institute through the Cardiovascular Research Network (CVRN) (U19HL091179), Agency for Healthcare Research and Quality, US Department of Health and Human Services Contract No. 290-05-0033 Developing Evidence to Inform Decisions about Effectiveness (DEcIDE) program, American Heart Association Mentored Clinical Research Awards (#17MCPRP33670631 & 17MCPRP33370062), American College of Cardiology Foundation, National Institute on Aging through the Advancing Geriatrics Infrastructure & Network Growth (AGING) Initiative (R24AG045050), National Institute on Aging T32AG019134.
ABBREVIATIONS
- ATP
anti-tachycardia pacing
- ICD
implantable cardioverter defibrillator
- LS-ICD
Longitudinal Study of Implantable Cardioverter Defibrillators
- MCC
multiple chronic conditions
- NCDR
National Cardiovascular Data Registry
- SCD
sudden cardiac death
- VDW
virtual data warehouse
Footnotes
Sponsor's Role: Sponsors played no role in the design, methods, subject recruitment, data collection, analysis, or preparation of this manuscript.
REFERENCES
- [1].Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346: 877–883. [DOI] [PubMed] [Google Scholar]
- [2].Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352: 225–237. [DOI] [PubMed] [Google Scholar]
- [3].Tung P, Albert CM. Causes and prevention of sudden cardiac death in the elderly. Nature reviews Cardiology. 2013;10: 135–142. [DOI] [PubMed] [Google Scholar]
- [4].Peck KY, Lim YZ, Hopper I, Krum H. Medical therapy versus implantable cardioverter -defibrillator in preventing sudden cardiac death in patients with left ventricular systolic dysfunction and heart failure: a meta-analysis of > 35,000 patients. International journal of cardiology. 2014;173: 197–203. [DOI] [PubMed] [Google Scholar]
- [5].Epstein AE, DiMarco JP, Ellenbogen KA, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2013;127: e283–352. [DOI] [PubMed] [Google Scholar]
- [6].McClellan MB, Tunis SR. Medicare coverage of ICDs. The New England journal of medicine. 2005;352: 222–224. [DOI] [PubMed] [Google Scholar]
- [7].Borne RT, Varosy PD, Masoudi FA. Implantable cardioverter-defibrillator shocks: epidemiology, outcomes, and therapeutic approaches. JAMA internal medicine. 2013;173: 859–865. [DOI] [PubMed] [Google Scholar]
- [8].Larsen GK, Evans J, Lambert WE, Chen Y, Raitt MH. Shocks burden and increased mortality in implantable cardioverter-defibrillator patients. Heart rhythm : the official journal of the Heart Rhythm Society. 2011;8: 1881–1886. [DOI] [PubMed] [Google Scholar]
- [9].Bhavnani SP, Giedrimiene D, Coleman CI, Guertin D, Azeem M, Kluger J. The healthcare utilization and cost of treating patients experiencing inappropriate implantable cardioverter defibrillator shocks: a propensity score study. Pacing and clinical electrophysiology : PACE. 2014;37: 1315–1323. [DOI] [PubMed] [Google Scholar]
- [10].Morken IM, Isaksen K, Karlsen B, Norekval TM, Bru E, Larsen AI. Shock anxiety among implantable cardioverter defibrillator recipients with recent tachyarrhythmia. Pacing and clinical electrophysiology : PACE. 2012;35: 1369–1376. [DOI] [PubMed] [Google Scholar]
- [11].Mark DB, Anstrom KJ, Sun JL, et al. Quality of life with defibrillator therapy or amiodarone in heart failure. The New England journal of medicine. 2008;359: 999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Germano JJ, Reynolds M, Essebag V, Josephson ME. Frequency and causes of implantable cardioverter-defibrillator therapies: is device therapy proarrhythmic? The American journal of cardiology. 2006;97: 1255–1261. [DOI] [PubMed] [Google Scholar]
- [13].Semmler V, Biermann J, Haller B, et al. ICD Shock, Not Ventricular Fibrillation, Causes Elevation of High Sensitive Troponin T after Defibrillation Threshold Testing--The Prospective, Randomized, Multicentre TropShock-Trial. PloS one. 2015;10: e0131570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. The New England journal of medicine. 2008;359: 1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].van Rees JB, Borleffs CJ, de Bie MK, et al. Inappropriate implantable cardioverter-defibrillator shocks: incidence, predictors, and impact on mortality. Journal of the American College of Cardiology. 2011;57: 556–562. [DOI] [PubMed] [Google Scholar]
- [16].Desai H, Aronow WS, Ahn C, et al. Risk factors for appropriate cardioverter-defibrillator shocks, inappropriate cardioverter-defibrillator shocks, and time to mortality in 549 patients with heart failure. The American journal of cardiology. 2010;105: 1336–1338. [DOI] [PubMed] [Google Scholar]
- [17].Borne RT, Peterson PN, Greenlee R, et al. Temporal trends in patient characteristics and outcomes among Medicare beneficiaries undergoing primary prevention implantable cardioverter-defibrillator placement in the United States, 2006-2010. Results from the National Cardiovascular Data Registry’s Implantable Cardioverter-Defibrillator Registry. Circulation. 2014;130: 845–853. [DOI] [PubMed] [Google Scholar]
- [18].Bruch C, Sindermann J, Breithardt G, Gradaus R. Prevalence and prognostic impact of comorbidities in heart failure patients with implantable cardioverter defibrillator. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2007;9: 681–686. [DOI] [PubMed] [Google Scholar]
- [19].Clarke B, Howlett J, Sapp J, Andreou P, Parkash R. The effect of comorbidity on the competing risk of sudden and nonsudden death in an ambulatory heart failure population. The Canadian journal of cardiology. 2011;27: 254–261. [DOI] [PubMed] [Google Scholar]
- [20].Bhavnani SP, Coleman CI, Guertin D, Yarlagadda RK, Clyne CA, Kluger J. Evaluation of the Charlson comorbidity index to predict early mortality in implantable cardioverter defibrillator patients. Annals of noninvasive electrocardiology : the official journal of the International Society for Holter and Noninvasive Electrocardiology, Inc. 2013;18: 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Steinberg BA, Al-Khatib SM, Edwards R, et al. Outcomes of implantable cardioverter-defibrillator use in patients with comorbidities: results from a combined analysis of 4 randomized clinical trials. JACC Heart failure. 2014;2: 623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Naksuk N, Kunisaki KM, Benditt DG, Tholakanahalli V, Adabag S. Implantable cardioverter-defibrillators in patients with COPD. Chest. 2013;144: 778–783. [DOI] [PubMed] [Google Scholar]
- [23].Ruwald MH, Zareba W, Jons C, et al. Influence of diabetes mellitus on inappropriate and appropriate implantable cardioverter-defibrillator therapy and mortality in the Multicenter Automatic Defibrillator Implantation Trial-Reduce Inappropriate Therapy (MADIT-RIT) Trial. Circulation. 2013;128: 694–701. [DOI] [PubMed] [Google Scholar]
- [24].Olde Nordkamp LR, Brouwer TF, Barr C, et al. Inappropriate shocks in the subcutaneous ICD: Incidence, predictors and management. International journal of cardiology. 2015;195: 126–133. [DOI] [PubMed] [Google Scholar]
- [25].Go AS, Magid DJ, Wells B, et al. The Cardiovascular Research Network: a new paradigm for cardiovascular quality and outcomes research. Circ Cardiovasc Qual Outcomes. 2008;1: 138–147. [DOI] [PubMed] [Google Scholar]
- [26].Greenlee RT, Coleman LA, Nelson AF, Selby JV. Partnerships in translation: advancing research and clinical care. The 14th Annual HMO Research Network Conference, April 13-16, 2008, Minneapolis, Minnesota Clinical medicine & research; 2008;6: 109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Masoudi FA, Go AS, Magid DJ, et al. Longitudinal study of implantable cardioverter-defibrillators: methods and clinical characteristics of patients receiving implantable cardioverter-defibrillators for primary prevention in contemporary practice. Circulation Cardiovascular quality and outcomes. 2012;5: e78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Goodman RA, Posner SF, Huang ES, Parekh AK, Koh HK. Defining and measuring chronic conditions: imperatives for research, policy, program, and practice. Preventing chronic disease. 2013;10: E66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Saczynski JS, Go AS, Magid DJ, et al. Patterns of Comorbidity in Older Adults with Heart Failure: The Cardiovascular Research Network PRESERVE Study. J Am Geriatr Soc. 2013;61: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009--a World Society of Arrhythmia’s project. Pacing and clinical electrophysiology : PACE. 2011;34: 1013–1027. [DOI] [PubMed] [Google Scholar]
- [31].Greenlee RT, Go AS, Peterson PN, et al. Device Therapies Among Patients Receiving Primary Prevention Implantable Cardioverter-Defibrillators in the Cardiovascular Research Network. J Am Heart Assoc. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fabbri E, An Y, Zoli M, et al. Aging and the burden of multimorbidity: associations with inflammatory and anabolic hormonal biomarkers. The journals of gerontology Series A, Biological sciences and medical sciences. 2015;70: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lewek J, Kaczmarek K, Cygankiewicz I, Wranicz JK, Ptaszynski P. Inflammation and arrhythmias: potential mechanisms and clinical implications. Expert review of cardiovascular therapy. 2014;12: 1077–1085. [DOI] [PubMed] [Google Scholar]
- [34].Khazanie P, Hellkamp AS, Fonarow GC, et al. Association Between Comorbidities and Outcomes in Heart Failure Patients With and Without an Implantable Cardioverter-Defibrillator for Primary Prevention. J Am Heart Assoc. 2015;4: e002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cho EN, von Kanel R, Marten-Mittag B, et al. Determinants and trajectory of phobic anxiety in patients living with an implantable cardioverter defibrillator. Heart. 2012;98: 806–812. [DOI] [PubMed] [Google Scholar]
- [36].Ruwald MH, Abu-Zeitone A, Jons C, et al. Impact of carvedilol and metoprolol on inappropriate implantable cardioverter-defibrillator therapy: the MADIT-CRT trial (Multicenter Automatic Defibrillator Implantation With Cardiac Resynchronization Therapy). Journal of the American College of Cardiology. 2013;62: 1343–1350. [DOI] [PubMed] [Google Scholar]
- [37].Patel D, Hasselblad V, Jackson KP, Pokorney SD, Daubert JP, Al-Khatib SM. Catheter ablation for ventricular tachycardia (VT) in patients with ischemic heart disease: a systematic review and a meta-analysis of randomized controlled trials. Journal of interventional cardiac electrophysiology : an international journal of arrhythmias and pacing. 2016;45: 111–117. [DOI] [PubMed] [Google Scholar]
- [38].Fried TR, Tinetti ME, Towle V, O’Leary JR, Iannone L. Effects of benefits and harms on older persons’ willingness to take medication for primary cardiovascular prevention. Archives of internal medicine. 2011;171: 923–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure SA1. Frequencies of shock/ATP, stratified by quartiles of comorbidity count in adults with a primary prevention ICD and no known atrial fibrillation.
Figure SA2. Association of baseline counts of comorbidities (excluding atrial fibrillation) and time to first shock/ATP among 2235 participants who received a primary prevention ICD for cox proportional hazard regression models. Panel A represents results for time to first delivered device therapy of any type; panel B represents time to first inappropriate device therapy, and panel C represents time to first appropriate device therapy.
Figure SA3. Association of baseline counts of comorbidities (excluding atrial fibrillation) and burden of total delivered shocks/ATPs among 2235 participants who received a primary prevention ICD. Panel A represents results for burden of device therapy of any type; panel B represents burden of inappropriate device therapy, and panel C represents burden of appropriate device therapy.
Figure SA4. Association of baseline counts of comorbidities (excluding atrial fibrillation) with risk of receiving an inappropriate shock/ATP vs. appropriate shock/ATP among 562 adults who received at least one inappropriate or appropriate shock from primary prevention ICD.
Figure SB1. Frequencies of shocks, stratified by quartiles of comorbidity count in adults with a primary prevention ICD and no known atrial fibrillation.
Figure SB2. Association of baseline counts of comorbidities (excluding atrial fibrillation) and time to first shock among 2235 participants who received a primary prevention ICD for cox proportional hazard regression models. Panel A represents results for time to first delivered device therapy of any type; panel B represents time to first inappropriate device therapy, and panel C represents time to first appropriate device therapy.
Figure SB3. Association of baseline counts of comorbidities (excluding atrial fibrillation) and burden of total delivered shocks among 2235 participants who received a primary prevention ICD. Panel A represents results for burden of device therapy of any type; panel B represents burden of inappropriate device therapy, and panel C represents burden of appropriate device therapy.
Figure SB4. Association of baseline counts of comorbidities (excluding atrial fibrillation) with risk of receiving an inappropriate shock vs. appropriate shock among 300 adults who received at least one inappropriate or appropriate shock from primary prevention ICD.
Figure SC1. Frequencies of ATPs, stratified by quartiles of comorbidity count in adults with a primary prevention ICD and no known atrial fibrillation.
Figure SC2. Association of baseline counts of comorbidities (excluding atrial fibrillation) and time to first ATP among 2235 participants who received a primary prevention ICD for cox proportional hazard regression models. Panel A represents results for time to first delivered device therapy of any type; panel B represents time to first inappropriate device therapy, and panel C represents time to first appropriate device therapy.
Figure SC3. Association of baseline counts of comorbidities (excluding atrial fibrillation) and burden of total delivered ATPs among 2235 participants who received a primary prevention ICD. Panel A represents results for burden of device therapy of any type; panel B represents burden of inappropriate device therapy, and panel C represents burden of appropriate device therapy.
Figure SC4. Association of baseline counts of comorbidities (excluding atrial fibrillation) with risk of receiving an inappropriate ATP vs. appropriate ATP among 367 adults who received at least one inappropriate or appropriate shock from primary prevention ICD.