Abstract
Helminths induce type 2 immune responses and establish an anti-inflammatory milieu in their hosts. This immunomodulation was previously shown to improve diet-induced insulin resistance which is linked to chronic inflammation. In the current study, we demonstrate that infection with the filarial nematode Litomosoides sigmodontis increased the eosinophil number and alternatively activated macrophage abundance within epididymal adipose tissue (EAT) and improved glucose tolerance in diet-induced obese mice in an eosinophil-dependent manner. L. sigmodontis antigen (LsAg) administration neither altered the body weight of animals nor adipose tissue mass or adipocyte size, but it triggered type 2 immune responses, eosinophils, alternatively activated macrophages, and type 2 innate lymphoid cells in EAT. Improvement in glucose tolerance by LsAg treatment remained even in the absence of Foxp3+ regulatory T cells. Furthermore, PCR array results revealed that LsAg treatment reduced inflammatory immune responses and increased the expression of genes related to insulin signaling (Glut4, Pde3b, Pik3r1, and Hk2) and fatty acid uptake (Fabp4 and Lpl). Our investigation demonstrates that L. sigmodontis infection and LsAg administration reduce diet-induced EAT inflammation and improve glucose tolerance. Helminth-derived products may, therefore, offer new options to improve insulin sensitivity, while loss of helminth infections in developing and developed countries may contribute to the recent increase in the prevalence of type 2 diabetes.
Key Words: Adipose tissue, Alternatively activated macrophages, Eosinophils, Glucose tolerance, Filariae, Helminths, Immunomodulation, Inflammation, Insulin resistance, Litomosoides sigmodontis, Regulatory T cells, Type 2 diabetes
Introduction
Diabetes imposes a tremendous burden on local health care systems and has a substantial impact on the quality of life of affected individuals. It is estimated that approximately 387 million people currently suffer worldwide from diabetes, and it is expected that the number of diabetic patients will increase up to almost 600 million by 2035 [1]. In most populations, 90% of all diabetes cases are type 2 diabetes (T2D) [1]. Obesity is a major risk factor to develop T2D and is associated with low-grade chronic inflammation. Excessive uptake of lipids in white adipose tissue causes (low-grade) inflammation and infiltration of classically activated macrophages (CAM), and changes in the cellular composition [2]. Those changes include the loss of eosinophils, alternatively activated macrophages (AAM), regulatory T cells, as well as innate lymphoid cells (ILC) [2, 3, 4], and the accumulation of CAM and B cells that further trigger the inflammatory response which eventually results in insulin resistance [5]. Several lines of evidence indicate that chronic activation of intracellular proinflammatory pathways within insulin target cells can lead to obesity-related insulin resistance. Proinflammatory cytokines such as IL-6, IL-1β, and TNFα can be released by both adipocytes and macrophages [6], and elevated levels of TNFα and IL-6 have been shown in individuals with insulin resistance and diabetes [7].
Accordingly, suppression of chronic inflammation in diet-induced obesity (DIO) may improve insulin sensitivity. In general, parasitic helminth infections induce a suppressive, regulatory immune response in their hosts including regulatory T cells, AAM, and anti-inflammatory cytokines such as IL-10 and TGFβ. In addition, helminths drive type 2 immune responses that counterbalance proinflammatory Th1 immune responses in the host [8]. The immunomodulation by helminths enables not only the long-term survival of the parasite within the host, but also affects responses to bystander antigens [9]. Several studies have shown that helminth infections prevent or ameliorate autoimmune diseases like type 1 diabetes (T1D), rheumatoid arthritis, chronic inflammatory bowel disease, and multiple sclerosis [10, 11], and clinical studies are currently testing the beneficial effect of Trichuris suis ova treatment on autoimmune diseases [12]. Moreover, in vivo infection studies with the rodent filarial nematode Litomosoides sigmodontis (L.s.) prevented the onset of T1D in nonobese diabetic mice [13]. This protection was independent of a type 2 immune shift but dependent on TGFβ signaling [14]. Although a protective role of helminth infections is well accepted for autoimmune diseases like T1D, its impact on the development of metabolic diseases like insulin-resistant T2D is less well studied.
Recent cross-sectional studies in India, Indonesia, rural China, and in aborigines in Australia revealed that the prevalence of helminth infections in T2D patients is significantly lower than in nondiabetic controls, independent of socioeconomic differences in income and nutrition, suggesting that helminth infections may indeed prevent or delay the onset of T2D [15, 16, 17, 18]. Possible helminth-mediated protective mechanisms include the downregulation of proinflammatory immune responses that are associated with T2D, the induction of type 2 immune responses, the promotion of fatty acid uptake, or a stronger expression of genes that are associated with insulin sensitivity. This is supported by the finding that administration of a recombinant receptor antagonist (anakinra) to block the signaling of the proinflammatory cytokine IL-1β ameliorated glycemia and insulin secretion from β-islet cells of T2D patients [19]. Similarly, anti-TNFα therapy improved glucose uptake in a murine model of T2D [20]. The beneficial effect of improved fatty acid uptake on the improvement in insulin sensitivity was further shown in several studies [21, 22].
In this study, we investigated whether L.s. infection or L.s. adult worm extract (LsAg) administration improves glucose tolerance in DIO mice and analyzed possible protective mechanisms.
Materials and Methods
Ethics Statement
Animal housing conditions and the procedures used in this work were performed according to the European Union animal welfare guidelines. All protocols were approved by the Landesamt für Natur, Umwelt und Verbraucherschutz, Cologne, Germany (87-51.04.2010.A355 and 84-02.04.2014.A131).
Animals and Animal Care
The experiments were performed using male wild-type (WT) and ΔdblGATA mice on a BALB/c background as well as C57BL/6J WT and C57BL/6 DEREG mice. BALB/c and C57BL/6J mice were purchased from Janvier Labs (Le Genest-St.-Isle, France). ΔdblGATA mice were originally obtained from The Jackson Laboratory (Bar Harbor, Maine, USA), and DEREG C57BL/6 mice were provided by Prof. Dr. Tim Sparwasser and Dr. Katharina Lahl (the TWINCORE Center for Experimental and Clinical Infection Research, Hannover, Germany). Mice were bred and housed at the animal facilities of the University Hospital of Bonn. Mice were maintained in individually ventilated cages with a 12-hour day/night cycle and food and water ad libitum. Starting at 6-8 weeks of age, subsets of mice were fed a high-fat (HF) diet which provides 60% of calories from fat (Research Diets, Inc., Brogaarden, Denmark).
L.s. Infection
L.s. infection was performed by natural infection as previously described [23]. If not stated otherwise, susceptible BALB/c mice were infected at 8-10 weeks of age, 1-2 weeks after the onset of HF diet. For natural infection, infective L3 larvae were transmitted with the blood meal of infected Ornithonyssus bacoti mites. The L3 larvae migrate to the thoracic cavity where they molt into adult worms (∼30 days after infection). At the time of necropsy, the infection status of mice was confirmed by screening for adult worms in the thoracic cavity.
LsAg Preparation and Administration
LsAg was prepared as described previously [24]. Briefly, L.s. adult worms were harvested from the thoracic cavity of infected cotton rats or gerbils and mechanically homogenized on ice in endotoxin-free PBS (PAA, Pasching, Austria) using a sterile glass potter. Following centrifugation at 3,200 g, the supernatant was collected and the protein concentration measured by the Bradford assay (Cytoskeleton, Denver, Colo., USA). Aliquots of LsAg were stored for later usage at −80°C.
Daily intraperitoneal injections of 2 µg LsAg per mouse for 2 weeks were given to male C57BL/6J DIO mice during weeks 8-10 or 12-14 of HF diet. Controls received equal amounts of sterile PBS (PAA). After the final LsAg injection, all groups of mice were subjected to a glucose tolerance test (GTT), and immunological studies were performed 1 week thereafter. In a further experiment employing DEREG C57BL/6 (depletion in regulatory T cells) mice [25, 26], groups of mice were placed on HF diet for a period of 14 weeks. Between weeks 8 and 10 and 12 and 14, groups received either daily intraperitoneal injections of LsAg (2 µg/mouse) or PBS. After the final LsAg administration, mice were monitored for glucose tolerance.
GTT and Insulin Tolerance Test
After 6 h of fasting, mice were injected i.p. with 2 g glucose solution per kilogram body weight. Blood glucose levels were measured from tail vein blood using a blood glucose meter (Accu-Check Advantage; Roche Diagnostics GmbH, Mannheim, Germany) immediately before and 15, 30, 60, 90, and 120 min after glucose injection. The area under the curve (AUC) was obtained by calculating the area between the x-axis and a given curve using GraphPad Prism software (version 5.03; GraphPad Software, San Diego, Calif., USA). As mentioned above, in a separate experiment using DEREG C57BL/6 mice, Foxp3+ T cells were depleted by two consecutive intraperitoneal injections of 1 µg diphtheria toxin (Merck KGaA, Darmstadt, Germany) 3 and 2 days before the GTT (using 1.5 g glucose/kg body weight).
For the insulin tolerance test, food was removed immediately before the injection of insulin. Insulin (1 U/kg body weight) was administered intraperitoneally, and blood glucose levels were taken immediately before and 15, 30, 60, 90, and 120 min after insulin injection.
Isolation of the Adipose Tissue Stromal Vascular Fraction
Isolation of the stromal vascular fraction (SVF) from adipose tissue was performed as previously described [3]. In brief, epididymal fat pads from male mice fed a normal chow or HF diet were excised and minced in DMEM/low-glucose media containing 1 g/l D-glucose, 4 mML-glutamine (PAA), 25 mM HEPES (Gibco, Life Technologies, Carlsbad, Calif., USA), 1% BSA (PAA), and 1% penicillin-streptomycin (PAA). Minced tissue was subsequently treated with medium containing 1.5 mg/ml collagenase-P (Roche) for 20 min at 37°C. Following centrifugation, floating adipocytes were discarded with the supernatant, and the SVF pellet was resuspended in 1× red blood cell lysis buffer (eBioscience, San Diego, Calif., USA). Following a washing step, cells were blocked for FACS analysis by incubation with anti-CD16/anti-CD32 (BD Biosciences, Heidelberg, Germany) in 1× Ca2+- and Mg2+-free Dulbecco's phosphate-buffered saline including 2 mM EDTA and 1% FCS (PAA) at a final concentration of 0.5-1 μg/106 cells for 1 h. The cell suspension was filtered afterwards through a 100-µm filter.
Flow Cytometry
Cell surface marker analysis was performed using flow cytometry. Briefly, cell surface markers were stained for 30 min at 4°C with rat anti-mouse F4/80 PerCP-Cy5.5, CD4 FITC, CD11c APC, CD19 PE, CD23 FITC, CD5 PE-Cy-7, Gr1 PerCp-Cy5.5 (all eBioscience), and Siglec-F PE (BD Bioscience). For intracellular staining, cells were fixed with fixation/permeabilization buffer (eBioscience) overnight, washed and blocked in PBS containing 1% BSA (PAA) and rat immunoglobulin (1 µg/ml; Sigma, St. Louis, Mo., USA). For RELMα staining, fixed cells were incubated in permeabilization buffer (eBioscience) and stained with rabbit anti-mouse RELMα (Peprotech, Rocky Hill, N.J., USA). After a washing step, cells were subsequently stained with a secondary antibody (goat anti-rabbit Alexa 488; Invitrogen, Carlsbad, Calif., USA). Regulatory T cells from non-DEREG mice were analyzed after overnight fixation and permeabilization and staining with CD4 FITC and Foxp3 PE (eBioscience). Foxp3+ T cells from DEREG mice were identified through their expression levels of egfp which is within the Foxp3 promoter [25].
To identify type 2 ILCs (ILC2s), isolated SVF cells were resuspended in FACS buffer, and Fc receptors were blocked using rat IgG before staining with monoclonal antibodies. ILC2s were characterized as Sca-1+KLRG-1+ and lineage negative. As lineage markers, the following antibodies were used: PerCP-conjugated CD4, CD8, CD5, and F4/80 (lineage 1) as well as APC-conjugated CD11c, FcεR1α, CD49b, Ly6C, and CD19 (lineage 2).
Online supplementary figures (for all online suppl. material, see www.karger.com/doi/10.1159/000448401) show the gating strategies used to identify AAM and CAM (online suppl. fig. 1), eosinophils and CD4+ T cells (online suppl. fig. 2), B cell subpopulations (online suppl. fig. 3), regulatory T cells (online suppl. fig. 4), as well as ILC2s (online suppl. fig. 5). Data were acquired using a BD FACS Canto and analyzed with BD FACS DIVA software (BD Bioscience).
Antibody Measurements
IgG2a levels were measured by ELISA according to the manufacturer's protocol. Wells (Nunc, Roskilde, Denmark) were coated with 2 µg/ml IgG2a primary antibody (BD Pharmingen, San Diego, USA) in coating buffer [0.1 M Na2HPO4 (Merck) in distilled water, pH 7.0] overnight at 4°C. For blocking, ELISA plates were incubated with PBS/1% BSA for a minimum of 2 h at room temperature and were subsequently washed with 1× PBS and 0.05% Tween 20 (Sigma-Aldrich); 50 μl of serum diluted 1:2,000 were added to the wells and incubated at 4°C overnight. After a washing step, the detection antibody was added for 2 h. Following another washing step, horseradish peroxidase-streptavidin (R&D Systems) was added for 30 min. After washing, TMB (tetramethylenebenzidine) (Carl Roth GmbH, Karlsruhe, Germany) was added and incubated for 20 min at room temperature. Subsequently, the color reaction was stopped with 1 M H2SO4 (Merck), optical density was measured at 450 nm using the SpectraMAX 340 microplate reader, and data were analyzed using the SoftMax Pro software (both Molecular Devices, Sunnyvale, Calif., USA).
Measurement of LsAg-specific antibodies was performed analogous to the measurement of total IgG2a levels, except that the plates were coated with 10 µg/ml LsAg overnight at 4°C, and secondary antibodies against mouse IgG2a/b (1:100 serum dilution) as well as IgG1 (1:10,000 serum dilution) (BD Biosciences) were used.
Adipose Tissue Histology Staining
Epididymal adipose tissue (EAT) was fixed in 4% paraformaldehyde (Otto Fischar GmbH, Saarbrücken, Germany) for at least 24 h. After fixation, tissue was dehydrated with ethanol and then embedded in paraffin for cutting. Hematoxylin-eosin staining was performed following standard procedures.
RNA Isolation and Quantitative Real-Time PCR
About 30 mg of epididymal fat were collected, snap frozen in liquid nitrogen and stored at −80°C until RNA isolation. Frozen adipose tissue was homogenized in innuSPEED Lysis Tubes W (Analytik-Jena, Jena, Germany) including TRIzol (Invitrogen) using a Precellys homogenizer (BERTIN Corp., Rockville, Md., USA) for 10 s (6,000 rpm). RNA was then extracted with the RNeasy mini kit (Qiagen, Hilden, Germany). RNA concentration was quantified by NanoVue (GE Healthcare Lifesciences, Chalfont St Giles, UK). Total RNA was reverse transcribed with an Omniscript RT Kit (Qiagen) according to the manufacturer's instructions with oligo-d(T) primers (Roche). Quantitative real-time PCR was performed using a Rotorgene cycler (Qiagen). For relative quantification of gene expression, the (ΔΔCt) method was applied. The primer sequences were as follows: arginase 1: forward - CCTATGTGTCATTTGGGTGGA, reverse - CAGGAGAAAGGACACAGGTTG; Foxp3: forward - TCTTGCCAAGCTGGAAGACT, reverse - GGGGTTCAAGGAAGAAGAGG; IL-10: forward - GGTTGCCAAGCCTTATCGGA, reverse - ACCTGCTCCACTGCCTTGCT; GATA3: forward - GTCATCCCTGAGCCACATCT, reverse - AGGGCTCTGCCTCTCTAACC, and β-actin: forward - AGAGGGAAATCGTGCGTGAC, reverse - CAATAGTGATGACCTGGCGGT.
PCR Array
RNA from EAT was digested with DNAse (Ambion, Carlsbad, Calif., USA). RNA integrity and RNA quality were analyzed using the Experion gel electrophoresis system and RNA StdSens chips (Bio-Rad, Hercules, Calif., USA). cDNA synthesis was performed with the RT2PreAMP cDNA Synthesis Kit (Qiagen). PCR amplification was performed according to the SABioscience protocols in 100-well disk formats of the mouse insulin resistance PCR array (PAMM-156Z) (Qiagen). Gene expression was normalized to housekeeping genes (B2m and Gapdh), and PCR array data were analyzed using the RT2Profiler PCR array data analysis (version 3.5) from SABioscience. A complete list of the genes included in the PCR array is provided in online supplementary table 1.
Statistics
Data were tested for statistical significance using GraphPad Prism software (version 5.03; GraphPad Software, San Diego, Calif., USA). The Mann-Whitney U test was applied to test differences between two unpaired groups with nonparametric distribution for statistical significance. Data that were normally distributed were tested for statistical significance using the unpaired t test for comparisons of two groups or the ANOVA test followed by the Newman-Keuls multiple-comparison test to compare more than two groups. Values of p < 0.05 were considered statistically significant. PCR array data analysis was performed using the online software provided by SABioscience (Frederick, Md., USA). p values were calculated based on a Student's t test of the replicate 2-ΔΔCt values for each gene in the control group and the treatment group, and values of p < 0.05 were considered significant.
Results
L.s. Infection Improves Glucose Tolerance in DIO Mice
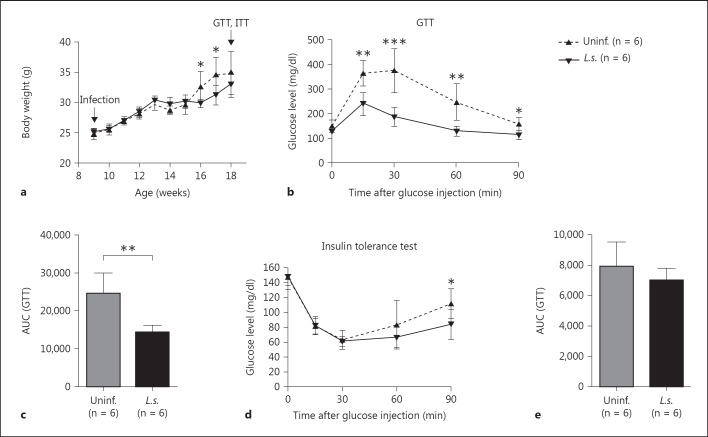
To induce obesity and glucose intolerance, male BALB/c mice were maintained on a HF diet for 10 weeks starting 6 weeks from birth. This diet provided 60% of calories from fat, and a subset of these animals were infected with L.s. 2 weeks after HF diet initiation. As expected, both L.s.-infected and control mice developed body weights exceeding 30 g after 10 weeks on HF diet. The development of body weight did not differ between both groups until 6 weeks after infection. At 7-8 weeks after infection, a time point when microfilaremia starts, L.s.-infected mice had a lower body weight than uninfected mice. This difference in body weight was no longer observed 9 weeks after infection (18 weeks of age) (fig. 1a) and was not present in repeat experiments. The adipose tissue weight between L.s.-infected and uninfected DIO mice did not show any significant difference [EAT: uninfected: 1.17 g (range 0.38-1.85 g), L.s. infected: 1.33 g (0.69-2.44 g), p = 0.866, and subcutaneous adipose tissue: uninfected: 0.287 g (0.11-0.46 g) and L.s. infected: 0.290 g (0.1-0.69 g), p = 0.771].
Fig. 1.
L.s. infection improves glucose tolerance in DIO mice. a Body weight of L.s.-infected and control mice maintained on a 60% HF diet. b Blood glucose levels over time following intraperitoneal glucose challenge (GTT) in L.s.-infected BALB/c mice and uninfected controls after a 10-week HF diet. c AUC obtained from the GTT. d Blood glucose levels over time following intraperitoneal insulin challenge (insulin tolerance test) in L.s.-infected BALB/c mice and uninfected controls that received a HF diet for 10 weeks. e AUC obtained from the insulin tolerance test. a-e n = 6 animals/group; representative experiment of 1 in 3 independent infection studies. Means ± SD. * p < 0.05, ** p < 0.01, *** p < 0.001 (unpaired t test).
In L.s.-infected DIO mice, blood glucose levels returned earlier to baseline levels during the GTT than in uninfected DIO mice suggesting an improved glucose tolerance in L.s.-infected DIO mice (fig. 1b). Accordingly, the AUC of the GTT, calculated as the area between the x-axis and the respective glucose tolerance curves, was significantly lower in L.s.-infected DIO mice than uninfected DIO mice (fig. 1c). Although L.s.-infected DIO mice showed an improvement in glucose tolerance, insulin tolerance was not improved by L.s. infection as the drop in blood glucose levels following insulin injection and AUC were similar in both groups (fig. 1d, e).
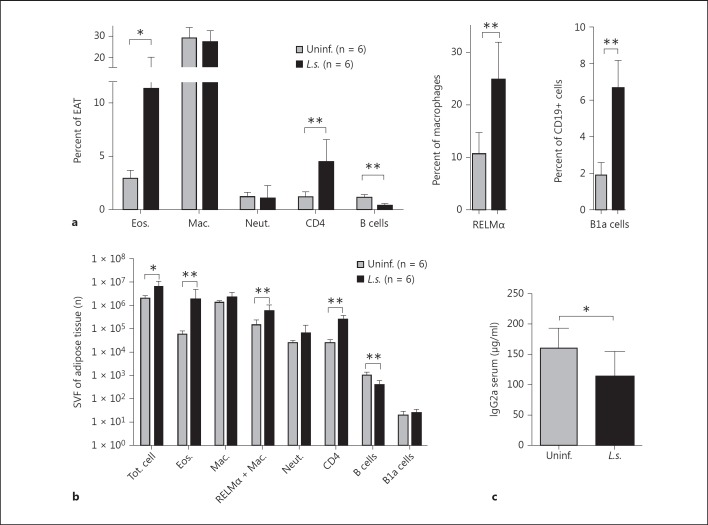
L.s. Infection Increases the Frequency of RELMα+ Macrophages and Eosinophils within EAT of DIO Mice
During obesity, proinflammatory CD11c+ macrophages (CAM) and B cells infiltrate EAT [5, 27, 28], which is accompanied by a loss of AAM and eosinophils [3, 5]. This leads to low chronic inflammation and eventually results in a diabetic phenotype with insulin resistance. We therefore investigated whether L.s. infection alters the cellular composition of EAT of DIO mice. FACS analysis of the SVF showed that L.s.-infected DIO mice had significantly increased frequencies of eosinophils (L.s.: 11.3%; control: 2.9%), RELMα+ macrophages (L.s.: 24.8%; control: 10.6%), and CD4+ T cells (L.s.: 4.5%; control: 1.2%) compared to uninfected DIO controls, while the frequency of macrophages (L.s.: 27.1%; control: 29.2%) and neutrophils (L.s.: 1.0%; control: 1.3%) did not significantly differ (fig. 2a). Similarly, L.s.-infected DIO mice had significantly increased numbers of eosinophils (L.s.: 1.97 × 106; control: 5.82 × 104), RELMα+ macrophages (L.s.: 6.31 × 105; control: 1.53 × 105), and CD4+ T cells (L.s.: 2.67 × 105; control: 2.77 × 104), while no differences were observed in the number of macrophages (L.s.: 2.38 × 106; control: 1.37 × 106) or neutrophils (L.s.: 6.94 × 104; control: 2.78 × 104) (fig. 2b).
Fig. 2.
Increased frequencies and total numbers of eosinophils, AAMs, and CD4+ T cells and a reduction in B cells in EAT of L.s.-infected DIO mice. a Frequencies of eosinophils, macrophages, neutrophils, B cells, and CD4+ T cells within the SVF of EAT, B1a cell frequencies of B cells, and RELMα+ macrophages with the macrophage population of L.s.-infected and uninfected mice that received a HF diet for 10 weeks. b Absolute number (n) of total cells, eosinophils, macrophages, RELMα+ macrophages, neutrophils, B cells, B1a cells, and CD4+ T cells within the SVF of EAT of L.s.-infected and uninfected mice that received a HF diet for 10 weeks. c Total IgG2a serum antibody levels. a-c Representative data of 1 of 3 independent experiments with at least n = 5 animals/group. Means ± SD. * p < 0.05, ** p < 0.01 (Mann-Whitney U test).
As B cells also promote insulin resistance in DIO mice by producing pathogenic IgG2 antibodies [5], we further analyzed B cell subsets in EAT of L.s.-infected DIO mice. L.s.-infected DIO mice had significantly lower frequencies (L.s.: 0.4%; control: 1.2%) and total numbers of CD19+ B cells (L.s.: 436 cells; control: 1,160 cells) compared to uninfected DIO controls (fig. 2a, b). However, the frequency of B cells that represents the B1a cell subset (CD19+CD5+CD23-) was higher in L.s.-infected DIO mice than DIO controls (L.s.: 6.6%; control: 1.8%; fig. 2a), whereas the absolute numbers of B1a cells were not significantly different (L.s.: 27.3; control: 21.0; fig. 2b). Consistent with the reduction in total B cells, the level of potentially pathogenic total IgG2a antibodies was significantly lower in EAT of L.s.-infected than uninfected DIO controls (fig. 2c). L.s.-specific antibodies were only induced in L.s.-infected mice with significantly increased IgG2a/b and especially IgG1 levels, indicating a type 2 antibody response in L.s.-infected DIO mice (online suppl. fig. 6).
These results suggest that L.s. infection maintains a cellular composition within EAT that is characterized by increased AAM and eosinophil numbers, while B cells are restricted, which may reduce inflammation and improve glucose tolerance.
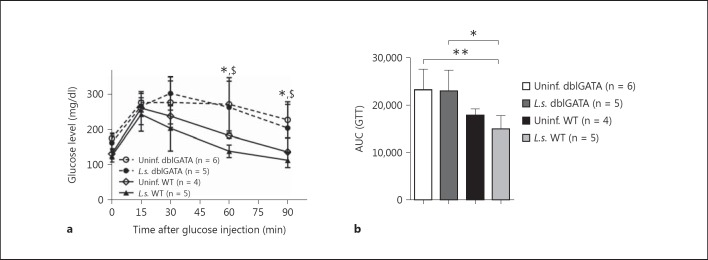
Improvement in Glucose Tolerance by L.s. Infection Is Dependent on Eosinophils
Previously, it has been suggested that eosinophils play a major role in the improvement in insulin sensitivity in DIO mice by inducing AAM in adipose tissue via IL-4 [3]. Since we found an increased frequency and absolute number of eosinophils in L.s.-infected DIO mice (fig. 2a, b), we used eosinophil-deficient ΔdblGATA mice to investigate whether the improvement in glucose tolerance in L.s.-infected DIO mice was dependent on eosinophils. Confirming previous findings that eosinophils are essential for insulin sensitivity in DIO mice [3], uninfected eosinophil-deficient DIO mice maintained increased blood glucose levels over an extended period of time following glucose challenge and had an increased AUC of the GTT compared to uninfected WT DIO controls (fig. 3a, b). As expected, L.s. infection improved glucose tolerance in WT BALB/c mice (fig. 3a). However, glucose tolerance curves remained comparable between L.s.-infected and uninfected ΔdblGATA mice (fig. 3a). Furthermore, the AUCs of both L.s.-infected (AUC mean = 23,003) and uninfected ΔdblGATA mice (AUC = 23,288; fig. 3b) were not different, suggesting that the improvement in glucose tolerance by L.s. infection is dependent on eosinophils.
Fig. 3.
L.s. infection does not improve glucose tolerance in eosinophil-deficient ΔdblGATA mice. Kinetics of blood glucose levels after intraperitoneal glucose challenge (a) and AUC (b) from the GTT in L.s.-infected and uninfected WT BALB/c as well as ΔdblGATA mice at 20 weeks of age and after 14 weeks of HF diet. * p < 0.05, $ p < 0.05, between uninfected and L.s.-infected dblGATA mice in comparison to uninfected WT controls, respectively (ANOVA followed by Newman-Keuls multiple-comparison test). Means ± SD.
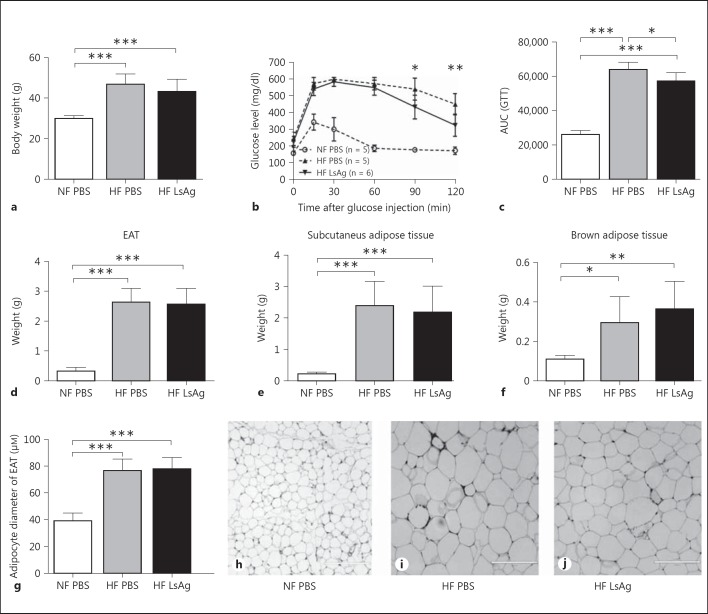
Daily LsAg Administration for 2 Weeks Improves Glucose Tolerance in DIO Mice
In order to test whether filarial antigen treatment is able to improve glucose tolerance in insulin-resistant DIO mice, C57BL/6 mice received daily intraperitoneal injections of LsAg during the last 2 weeks of a 14-week HF diet. As expected, mice on HF diet had significantly higher body weights than mice on normal chow diet (normal fat; NF) (fig. 4a). No differences in body weight were observed between DIO mice that received PBS and LsAg treatment after 14 weeks of HF diet (fig. 4a). A GTT was performed 4 days after the last LsAg injection. Blood glucose levels of L.s.-infected DIO mice were significantly reduced compared to PBS-treated DIO controls starting 90 min after glucose injection (fig. 4b), resulting in a significantly decreased AUC of the GTT (reduction by 10.6%; fig. 4c). A second experiment with 2 weeks of LsAg administration also significantly improved glucose tolerance in LsAg-treated DIO mice compared to PBS-treated DIO controls (online suppl. fig. 7A, B). Those results demonstrate that glucose tolerance was improved by LsAg treatment in DIO mice. However, fasting insulin levels were not altered between LsAg-treated [mean: 2.87 ng/ml (range 0.09-4.37 ng/ml)] and PBS-treated mice [mean: 3.22 ng/ml (range 0.78-13.6 ng/ml)], and there was no improvement in the insulin tolerance test (online suppl. fig. 7C, D). Body weights were also not altered in DIO mice treated with LsAg in the second experiment (online suppl. fig. 7E).
Fig. 4.
Two weeks of daily LsAg injections improve glucose tolerance but do not affect adipose tissue weight in DIO mice. a Comparison of body weight of LsAg-treated DIO mice and PBS-treated DIO controls that received a HF diet for 14 weeks with PBS-treated mice given a standard chow diet (NF). b Blood glucose levels over time during a GTT 14 weeks after HF diet initiation. c AUC obtained from the GTT. Comparison of adipose tissue composition in PBS- and LsAg-treated animals that received a HF diet and PBS-treated controls on a chow diet: EAT weight (d), subcutaneous adipose tissue weight (e), brown adipose tissue weight (f), as well as epididymal adipocyte size (g). Representative pictures of EAT of control mice on chow diet (h), PBS-treated DIO mice (i), and LsAg-treated DIO mice (j). Bars = 200 µm. b Mann-Whitney U test. a-g Representative data of 1 of 2 independent experiments with at least n = 5 animals/group. a, c-g ANOVA followed by the Newman-Keuls multiple-comparison test. Means ± SD. * p < 0.05, ** p < 0.01, *** p < 0.001.
To investigate whether LsAg administration inhibits adipogenesis in vivo, body weight and fat tissue composition were analyzed. As shown in figure 4, there were no significant differences in body weight as well as epididymal, subcutaneous, and brown adipose tissue weight between PBS- and LsAg-treated DIO groups (fig. 4a, d-f, respectively). As adipose inflammation is correlated with adipocyte hypertrophy, adipocyte size in EAT was analyzed in LsAg- and PBS-treated DIO mice as well as uninfected controls on chow diet. Despite the improvement in glucose tolerance by LsAg administration, epididymal adipocytes did not differ in diameter size between DIO mice treated with LsAg and PBS (fig. 4g, h, i, j), indicating that 2 weeks of LsAg administration did not suppress adipogenesis and fatty-acid storage in adipocytes in vivo when administered after the development of glucose intolerance.
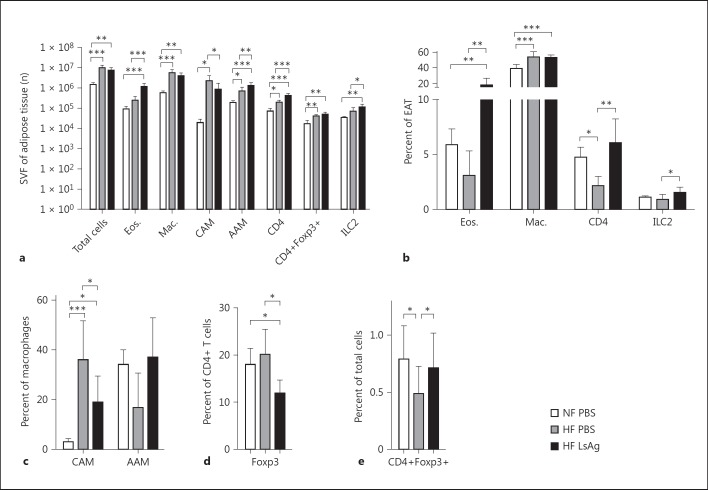
Interestingly, although body weight (fig. 4a), EAT mass (fig. 4d, e, f) as well as epididymal adipocyte size (fig. 4g), and the total cell number within the SVF of EAT (fig. 5a) did not differ between LsAg- and PBS-treated DIO mice, the cellular composition of the SVF of EAT was altered. Consistent with our findings in L.s.-infected DIO mice, analysis of the cellular composition of EAT by flow cytometry revealed a significantly higher number of eosinophils, AAM, and CD4+ T cells. In line with previous studies that have used the combination of F4/80 and CD11c to identify CAMs in adipose tissue [27, 28], we analyzed the frequency of these cells in our treated groups and detected a lower number of CD11c+ CAM in LsAg-treated DIO mice compared to PBS-treated DIO controls (fig. 5a). Similarly, flow-cytometric analysis of the cellular composition of EAT showed significantly higher frequencies of eosinophils, AAM (p > 0.05), and CD4+ T cells, as well as significantly lower frequencies of CAM in LsAg-treated DIO mice compared to PBS-treated DIO controls (fig. 5b, c). Frequencies of macrophages in EAT of DIO mice were significantly increased compared to mice on normal chow diet (fig. 5b). Those results suggest that LsAg administration improves glucose tolerance by increasing eosinophil and AAM numbers in adipose tissue. Unexpectedly, frequencies of CD4+ T cells expressing Foxp3+ were significantly higher in PBS-treated DIO controls when compared to LsAg-treated mice (LsAg: 11.9%; PBS: 20.1%; fig. 5d). However, frequencies of CD4+Foxp3+ regulatory T cells within LsAg DIO mouse EAT were comparable to those of the PBS-treated chow diet group and were significantly higher than in the PBS-treated DIO group (fig. 5e).
Fig. 5.
LsAg administration increases the frequency of eosinophils and AAM within EAT. a Total cell number (n) within the SVF of EAT and absolute number of eosinophils, macrophages, RELMα+ macrophages (AAM), macrophages expressing CD11c (CAM), CD4+ T cells, CD4+Foxp3+ T cells, and ILC2s within the SVF of EAT. b Frequency of eosinophils, macrophages, CD4+ T cells, and ILC2s within the SVF of EAT. c Frequencies of macrophages expressing CD11c (CAM) or RELMα (AAM) within the SVF of EAT. d Frequencies of T cells expressing Foxp3. e Frequency of CD4+Foxp3+ T cells of total cells in EAT. a-e Representative data of 1 of 2 independent experiments with at least n = 5 animals/group (single experiment for ILC2s). Means + SD. * p < 0.05, ** p < 0.01; *** p < 0.001, between the HF diet groups (Mann-Whitney U test).
Finally, ILC2 cells were analyzed within LsAg- and PBS-treated DIO EAT, demonstrating that LsAg injection in DIO mice significantly increased ILC2 frequencies and total cells in EAT compared to DIO controls (fig. 5a, b).
LsAg Administration Reduces Inflammatory Immune Responses and Promotes Insulin Signaling
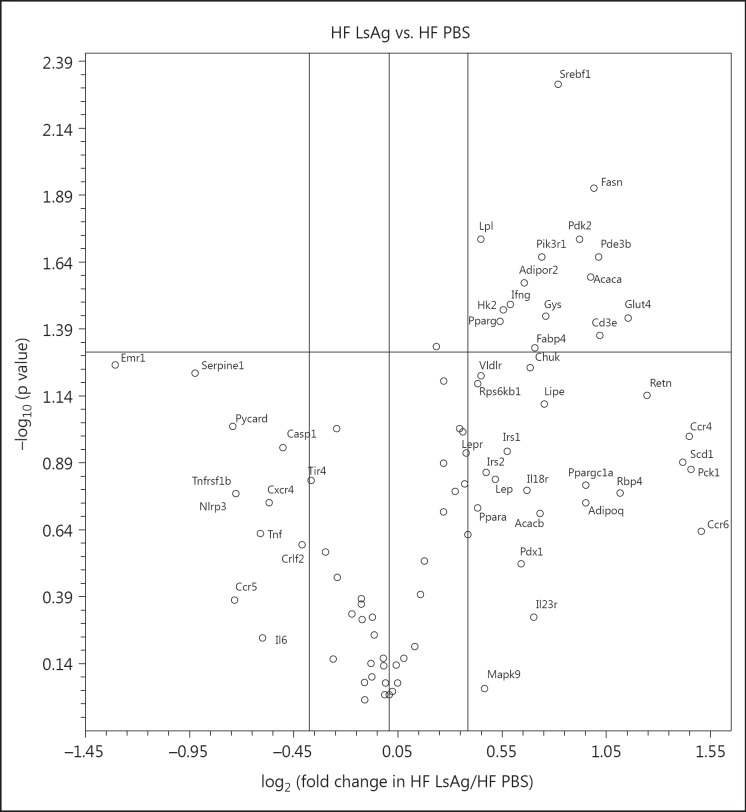
In order to identify the impact of LsAg administration on mRNA levels of genes related to insulin resistance in EAT, the expression of 84 genes was analyzed using PCR arrays (SABioscience) of individual EAT samples from LsAg-treated mice (n = 10) and PBS-treated controls (n = 8) on a HF diet. Genes that had a fold change >1.3 are presented in figure 6, and the complete list of genes including p values and fold changes are given in online supplementary table 1.
Fig. 6.
Volcano plot representing gene expression data from individual EAT samples of DIO mice which were treated with LsAg (n = 10) and compared to PBS-treated DIO controls (n = 8). The x-axis represents the fold change, whereas the y-axis represents the p value for statistical significance (as -log10). The mid horizontal line indicates p = 0.05 with listed genes above the line having p < 0.05. The circles on the upper-right quadrant indicate genes with a fold change >1.3, the circles on the lower-left quadrant indicate genes that have a fold change <-1.3.
DIO mice treated with LsAg injections had a significantly higher expression of genes related to insulin signaling, such as Slc2a4 (Glut4), Pde3b, Pik3r1, and Hk2, than DIO controls that received PBS (p < 0.05). Moreover, there were tendencies to higher expression of Irs1 (p = 0.11) and Irs2 (p = 0.14), indicating that LsAg administration may improve insulin sensitivity in EAT of DIO mice. Subsequently, a tendency to upregulate expression of adipoq (p = 0.18), an adiponectin-coding gene, and increased expression of its receptor (adipor2; p < 0.05) also support an improvement in insulin signaling in DIO mice treated with LsAg. Upregulation of Srebf1 (p < 0.01), Fasn (p < 0.05), Acaca (p < 0.05), and Pparg (p < 0.05) further indicates that the lipogenesis in adipocytes of LsAg-treated DIO mice may be increased. In parallel, PCR analysis revealed that expression of genes related to fatty acid uptake, including Fabp4 and Lpl (p < 0.05), as well as Vldr (p = 0.06), were increased in DIO mice that received LsAg treatment. Upregulation of Gys1 expression in LsAg-treated DIO mice (p < 0.05) may further indicate that the storage of excessive energy is not only generated as lipid formation but also as glycogen.
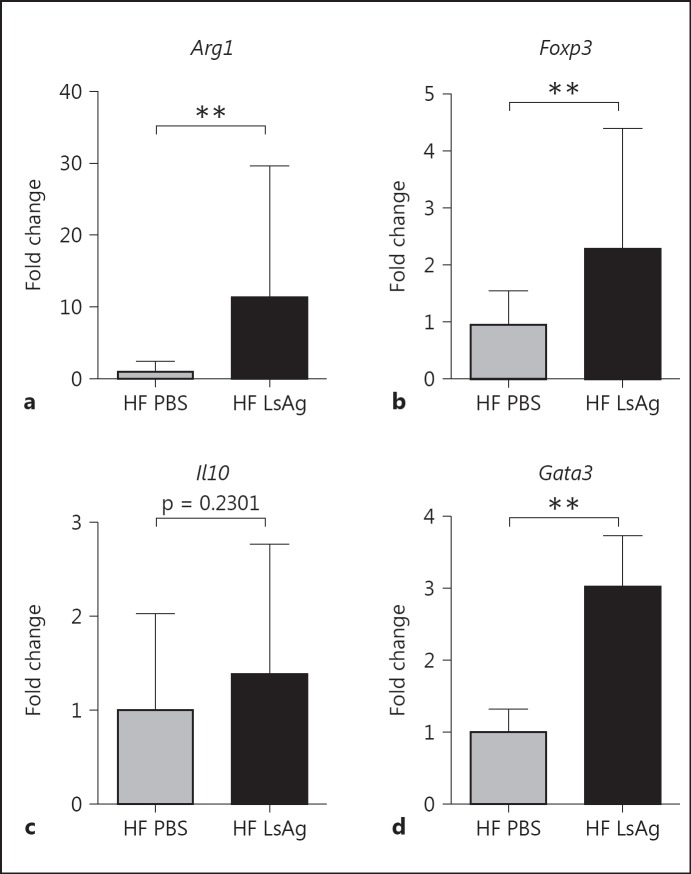
In addition to the increased expression of genes related to insulin signaling and fatty acid uptake, a downregulation of genes that are linked to inflammatory immune responses was observed in LsAg-treated mice. LsAg administration downregulated the expression of the macrophage marker Emr1 (F4/80) (p = 0.056), indicating a reduced macrophage infiltration in EAT of LsAg-treated mice. Similarly, genes related to proinflammatory immune responses such as Tnfrsf1b tended to be suppressed in LsAg-treated DIO mice (p = 0.17). Moreover, LsAg administration may suppress inflammasome activation, as indicated by the downregulation of Nlrp3 (p = 0.17), Pycard (p = 0.09), and Casp1 (p = 0.11). On the other hand, infiltration of CD4+ T cells was increased in LsAg-treated mice, as was shown by a stronger expression of Cd3e (p < 0.05), and confirmed our previous results on CD4+ T cells by flow cytometry. Furthermore, the trend towards a higher expression of Ccr4 (p = 0.10) in EAT of LsAg-treated DIO may suggest an increased Th2 polarization in EAT. Interestingly, LsAg administration in DIO mice downregulated the expression of Serpine1 (p = 0.06), which is related to atherothrombotic lesion formation [29], suggesting that LsAg administration may modulate cardiovascular diseases. Whereas several genes that are related to inflammatory immune responses were downregulated by LsAg administration, Ifng expression was increased in DIO mice that received LsAg (p < 0.05). In addition, array analysis also showed upregulation of Pdk2, which was previously associated with T2D [30]. Nevertheless, the results of the array analysis indicate that LsAg administration may suppress apoptosis-induced inflammasome activation and promote insulin signaling in EAT, thus boosting glucose as well as fatty acid uptake into white adipocytes. Additional gene expression analyses of EAT were performed to investigate protective mechanisms by which LsAg administration may improve glucose tolerance. According to the increased frequencies of F4/80+RELMα+ AAM shown by flow cytometry, LsAg administration also increased the expression of arginase-1 (p < 0.01) (fig. 7a), a gene expressed in AAM. Since our FACS analyses indicated an increase in regulatory CD4+ T cells, but Foxp3 as well as Gata3 were not included in the PCR array, Foxp3 and Gata3 gene expression analysis was also performed. As expected, Foxp3 gene expression was increased in EAT of LsAg-treated DIO mice when compared to PBS-treated DIO controls (p < 0.01; fig. 7b), suggesting that increased total numbers of regulatory T cells may contribute to the protective effect of LsAg treatment. However, LsAg administration did not significantly increase the expression of Il10 (p = 0.23; fig. 7c). In a further comparison and in line with the increased Ccr4 expression in the PCR array (fig. 6), the Th2 transcription factor Gata3, which is also important for regulatory T cells, was significantly upregulated by LsAg administration in DIO mice (p < 0.01; fig. 7d).
Fig. 7.
Daily LsAg administration for 2 weeks increases the expression of genes associated with type 2 immune responses in EAT of DIO mice. Gene expression of Arg1 (a), Foxp3 (b), Il10 (c), and Gata3 (d) given as fold changes after normalization to β-actin (n = 6/group). Means ± SD. ** p < 0.01 (Mann-Whitney U test).
Collectively, these gene expression analyses suggest that LsAg administration induced type 2 immune responses, increased AAM as well as regulatory T cells, and promoted insulin signaling in EAT, thus increasing glucose and free fatty acid uptake into white adipocytes.
LsAg-Mediated Glucose Tolerance Is Independent of Foxp3+ T Cells
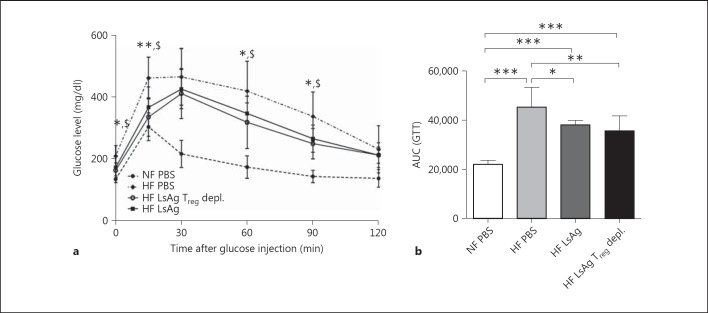
Since LsAg administration increased regulatory T cells within EAT and gene expression analysis revealed an increased Foxp3 expression in adipose tissue, we investigated whether LsAg-mediated improvement in glucose intolerance was dependent on regulatory T cells. Therefore, groups of DEREG C57BL/6 mice were placed on a HF diet for 14 weeks. LsAg was then administered between weeks 8 and 10 and 12 and 14 with a 2-week washout period. Three and 2 days before the GTT, Foxp3+ T cells were depleted in one group of mice, and then all groups were subjected to a GTT. Interestingly, despite the previous elevations in regulatory T cells in EAT, the depletion of Foxp3+ T cells did not abolish LsAg-mediated improvement in glucose tolerance in DEREG DIO mice. Figure 8a shows that both the ‘HF LsAg’ and ‘HF LsAg Treg-depleted' groups had significantly lower blood glucose levels than the ‘HF PBS’ group (fig. 8a). This was confirmed after calculating the AUC of the GTT (fig. 8b).
Fig. 8.
LsAg-mediated glucose tolerance is independent of Foxp3+ T cells. Groups of C57BL/6 DEREG mice were placed on a HF diet for 14 weeks. During weeks 8 and 10 and 12 and 14, groups of mice were given LsAg injections (2 µg/day) or PBS. Three days before the GTT, one group of LsAg-treated DEREG mice on a HF diet was depleted of Foxp3+ T cells through diphtheria toxin injection. a Blood glucose levels were measured over time during a GTT. Means ± SD in each group: NF PBS n = 4; HF PBS n = 8; HF LsAg Treg depleted n = 9 and HF LsAg n = 6. *, $ Statistically significant differences between LsAg-treated WT and DEREG DIO mice in comparison to PBS WT DIO controls, respectively. * p < 0.05, ** p < 0.01 (ANOVA). b AUC obtained from the GTT. Means ± SD. ANOVA followed by Newman-Keuls multiple-comparison test. * p < 0.05, ** p < 0.01, *** p < 0.001.
Discussion
The objective of this study was to investigate whether L.s. infection and filarial antigen administration improve insulin sensitivity in DIO mice and to elucidate the underlying mechanisms. For this purpose, male BALB/c mice were used to study the impact of L.s. infection, as this mouse strain is susceptible to L.s. infection and develops chronic infections. On the other hand, C57BL/6 mice are semisusceptible to L.s. infection [31] but develop increased body weights on HF diet with aggravated insulin resistance compared to BALB/c mice, and therefore they were used to test the protective effects of LsAg. Such differences in body weight and grade of insulin resistance of both mouse strains explain the observed variations in the GTTs in our study. Several studies previously reported that chronic inflammation in obesity-induced insulin resistance is associated with increased numbers of CAM and a reduction in AAM in visceral adipose tissue [6, 32]. Since the presence of AAM in adipose tissue correlates with improved insulin sensitivity, numerous investigations were conducted to maintain AAM in adipose tissue to counter the obesity-induced chronic inflammation [33, 34, 35]. Locksley's group demonstrated that the presence of AAM in visceral adipose tissue of DIO mice is maintained by eosinophils which are the major source of IL-4 in adipose tissue, indicating that eosinophils play a key role to maintain insulin sensitivity in DIO [3]. Accordingly, Ricardo-Gonzalez et al. [36] demonstrated that IL-4 signaling via STAT6 reduces adipose tissue inflammation thus ameliorating insulin sensitivity by regulating nutrient metabolism.
In addition to the well-known function of filariae to induce systemic type 2 immune responses, findings from our study demonstrate that EAT in L.s.-infected DIO mice contained increased numbers of eosinophils and AAM and improved glucose tolerance. These findings are therefore in accordance with the results of Wu et al. [3] who showed an improvement in glucose tolerance in Nippostrongylus brasiliensis-infected mice that was accompanied by increased numbers of eosinophils and AAM in perigonadal adipose tissue and suggested that tissue-dwelling filariae as well as intestinal nematodes affect immune responses of the adipose tissue. Consistent with the findings of Wu et al. [3], our study demonstrates that lack of eosinophils worsens glucose tolerance in DIO mice. Furthermore, absence of eosinophils in L.s.-infected ΔdblGATA mice prevented the improvement in glucose tolerance, suggesting that eosinophils are required for helminth-mediated improvement in glucose tolerance and no redundant eosinophil-independent mechanisms are in place. In line with this, 2 weeks of daily LsAg administration increased eosinophil and AAM numbers in EAT and led to improved glucose tolerance in DIO mice. Collectively, these findings suggest that both L.s. infection and LsAg administration protect against diet-induced glucose intolerance by increasing eosinophil and AAM frequencies in EAT.
The improvement in glucose tolerance in our experiments was further accompanied by lower numbers of B cells in EAT of L.s.-infected DIO mice. Winer et al. [5] reported that the presence of B cells in visceral adipose tissue is associated with increased levels of proinflammatory IgG2c serum levels, thus promoting insulin resistance in DIO mice. The observed restrictions of B cells in EAT and lower levels of total IgG2a (which corresponds to IgG2c in C57BL/6 mice) of L.s.-infected DIO BALB/c mice may therefore help to limit HF diet-induced activation of proinflammatory macrophages and T cells, therefore reducing adipose tissue inflammation. Interestingly, despite the reduced B cell population in L.s.-infected DIO mice, the percentage of B1 cells was significantly higher in L.s.-infected than uninfected DIO control mice. Expansion of the B1 cell subset may contribute to a reduced adipose tissue inflammation, as B1 cells have been identified as a source of IL-10 [37].
Despite the increased numbers of potentially IL-10- producing cell types like B1 cells, regulatory T cells and AAM, our gene expression data showed no significant differences for Il10 expression after both L.s. infection and LsAg administration, suggesting that IL-10 may not essentially be involved in the improvement in glucose tolerance in this context. According to the increased frequencies of AAM and eosinophils in EAT of LsAg-treated mice, the classical marker for type 2 immune responses Gata3 was significantly upregulated and Ccr4, which is expressed on Th2 cells, tended to be increased after 2 weeks of LsAg administration. LsAg-induced type 2 immune responses may improve glucose tolerance in DIO mice, as IL-5 has a key role in maintaining eosinophils, and IL-4 promotes AAM activation in visceral adipose tissue [3]. Interestingly, Th2-associated cytokines are also produced by ILC2s, which also express Gata3[38]. ILC2 cells were increased in EAT of LsAg-treated DIO mice, and it was previously shown that ILC2s improve glucose homeostasis in adipose tissue by maintaining eosinophils and AAM in EAT [4]. Moreover, the increased frequency of ILC2s may drive browning of adipose tissue, as a recent study showed that IL-33 activated ILC2s promote browning of adipose tissue and restrict obesity [39, 40]. However, whether L.s. infection and LsAg administration improve glucose tolerance via browning of adipose tissue needs further investigation.
Increased expression of Gata3, as was observed in LsAg-treated DIO mice, is also linked to regulatory T cell stability [41, 42, 43]. Accordingly, flow-cytometric and quantitative RT-PCR analysis indicated that CD4+Foxp3+ regulatory T cells were more abundant in EAT of LsAg-treated DIO mice compared to DIO controls. In order to investigate whether regulatory T cells are implemented in the LsAg-mediated improvement in glucose tolerance in DIO mice, experiments using regulatory T cell-depleted DEREG mice were conducted. However, depletion of regulatory T cells following LsAg therapy did not impair the beneficial impact on glucose tolerance, suggesting that LsAg treatment improves glucose tolerance independent of regulatory T cells.
Although insulin tolerance tests did not show an improved insulin sensitivity in LsAg treated mice, PCR array analyses of EAT indicated that LsAg treatment alters the expression of genes that are linked to insulin sensitivity in DIO mice. Accordingly, significantly higher expression of Pik3r1, Pde3b, and Slac2a4 (Glut4) was observed. Pik3r1 encodes the p50α, p55α, and p85α regulatory subunits of class IA phosphatidylinositol 3 kinases and has a key role in insulin signaling by activating AKT [44]. Activated AKT in turn phosphorylates PDE3B, an enzyme that promotes GLUT-4 translocation, thereby enhancing the expression of Glut4, which increases glucose uptake into the cells [45]. In addition, the adiponectin receptor coding gene (Adipor2) tended to be increased in EAT of LsAg-treated DIO mice, which may further support insulin signaling.
Under normal conditions, insulin induces an anabolic metabolism which stores excessive energy in the form of glycogen and lipids. In contrast, insulin resistance leads to a catabolism which results in increased blood glucose and fatty acid levels [46]. Genes that are associated with lipogenesis and glycogenesis were stronger expressed in LsAg- than PBS-treated DIO mice, as was shown for Srebf1, Fasn, and Acaca, which may contribute to fatty acid synthesis, as well as Gys1, which is involved in glycogenesis. In addition to the possibility of an enhanced glucose uptake, gene expression data may further indicate an increased cellular uptake of fatty acids, as Fabp4,Lpl, as well as Vldlr expression was increased by LsAg treatment. This may indicate that LsAg treatment does not suppress lipogenesis and explain the lack of differences in body weight and adipocyte size of LsAg-treated DIO mice compared to PBS-treated DIO controls. The lack of changes in body weight and adipose tissue mass in L.s.-infected and LsAg-treated DIO mice in comparison to previous studies using N. brasiliensis[3] and Schistosoma mansoni[47] may also be linked to differences in parasite locations in the host and the compositions of antigens that were used. Adult L.s. worms live in the thoracic cavity, whereas N. brasiliensis is a gastrointestinal parasite which may affect absorption in the intestine. S. mansoni worms on the other hand reside in the portal vein and cause liver pathology, which could modulate glucose metabolism and food intake. Furthermore, we used a crude adult worm extract which may also include proinflammatory components. L. sigmodontis worms contain endosymbiotic Wolbachia bacteria that are known to trigger TLR responses [48]. Accordingly, our PCR array results indicated for example the induction of IFNγ with LsAg treatment. Thus, identification of an active component within LsAg may further improve the protective effect on diet-induced glucose intolerance which may resemble the protective effect observed with S. mansoni soluble egg antigens [47], which is known to be a strong inducer of type 2 immune responses.
Increased Pparg expression in EAT of LsAg-treated animals may also contribute to an improved glucose tolerance without inhibiting adipogenesis. Such a paradoxical effect of Pparg by promoting adipogenesis and improving insulin sensitivity was previously reported [49]. Mice with increased Pparg activity are protected from obesity-associated insulin resistance [50], whereas mice lacking Pparg expression in fat, muscle, or liver are predisposed to develop insulin resistance [49]. Accordingly, thiazolidinedione is a high-affinity agonist for Pparg and effectively reduces systemic insulin resistance in peripheral tissues which can be used to treat T2D in humans [51]. In addition, Pparg possesses anti-inflammatory effects by inhibition of NFκB and IFNγ [52]. Furthermore, Pparg has also been identified as a critical orchestrator of the regulatory T cell phenotype, function, and accumulation in EAT of mice which was associated with an improvement in insulin sensitivity [53]. Taken together, although Pparg promotes adipocyte differentiation and adipogenesis, Pparg enhances insulin sensitivity and suppresses inflammation, thereby improving glucose tolerance in DIO mice. Thus, upregulation of Pparg in LsAg-treated DIO mice may contribute to the improved glucose tolerance.
Free fatty acid-induced lipotoxicity may activate the inflammasome, thus resulting in adipocyte apoptosis and enhanced inflammatory responses [54]. Several genes that are associated with inflammasome activation (Nlrp3, Pycard, and Casp1) were downregulated by LsAg administration, which may indicate an increased uptake of free fatty acids by adipocytes upon LsAg administration and a restricted inflammasome activation. In this context, reduced numbers of CAM and a reduced expression of Tnfrsf1b as well as Emr1(F4/80) were also observed in LsAg-treated DIO mice, suggesting that filarial antigen treatment reduced adipose tissue inflammation. However, Ifng expression was significantly increased in adipose tissue of LsAg-treated mice. This increased Ifng expression could be due to endosymbiotic Wolbachia bacteria that are found in most human pathogenic filariae as well as L.s. [55].
Epidemiological studies suggest that beneficial effects of helminth infections on T2D are also present in humans. People in a rural area in China who have a history of Schistosoma infections compared to endemic controls who never suffered from schistosomiasis had a lower body mass index and fasting blood glucose levels, and reduced prevalence of T2D and metabolic syndrome [16]. Accordingly, prevalence of lymphatic filariasis was significantly lower in diabetic subjects than nondiabetic subjects in Chennai, India [15]. Moreover, a recent study in Flores Island, Indonesia, revealed that people with soil-transmitted helminth infections have an improved insulin sensitivity compared to uninfected individuals [17]. A cross-sectional study in Australian aborigines further suggested that a history of Strongyloides stercoralis infection reduces the risk to develop T2D [18]. These findings should be confirmed by additional human epidemiological follow-up studies investigating helminth infections and their impact on the metabolic syndrome and should include further analysis about the underlying protective mechanism.
In conclusion, our study demonstrates that both L.s. infection and LsAg treatment of DIO mice improve glucose tolerance. This improvement is associated with type 2 immune responses and increased numbers of eosinophils, AAM, and regulatory T cells. Eosinophils were required to mediate the protective effect in L.s.-infected DIO mice, while regulatory T cells were dispensable to improve glucose tolerance by LsAg administration. LsAg administration further increased expression of genes that are linked to an increasing fatty acid uptake and restricted inflammasome activation, which may improve insulin sensitivity. These findings indicate that filariae induce several protective mechanisms that should be pursued in order to develop new strategies to ameliorate insulin resistance in human T2D.
Disclosure Statement
There are no competing financial interests.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Acknowledgments
We thank Benedikt C. Buerfent, Wiebke Stamminger, and Khaldoun Aslan for their technical support. This work was funded by the German Research Foundation (HU 2144/1-1); intramural funding by the University Hospital of Bonn (BONFOR, 2010-1-16 and 2011-1-10), and the People Program (Marie Curie Actions) of the European Union's Seventh Framework Program FP7/2007-2013 under Research Executive Agency Grant GA 276704. A.B. was supported by the German Academic Exchange Service (DAAD). J.S. received a fellowship by the Alexander von Humboldt Foundation. A.H. is a member of the German Center for Infection Research (DZIF) and of the Excellence Cluster Immunosensation (DFG, EXC 1023). L.S.H. was supported by BONFOR.
References
- 1.International Diabetes Federation IDF Diabetes Atlas, ed 6, 2014. Brussels, International Diabetes Federation. 2014 http://www.idf.org/sites/default/files/Atlas-poster-2014_EN.pdf. [Google Scholar]
- 2.Cipolletta D, Kolodin D, Benoist C, Mathis D. Tissular T(regs): a unique population of adipose-tissue-resident Foxp3+CD4+ T cells that impacts organismal metabolism. Semin Immunol. 2011;23:431–437. doi: 10.1016/j.smim.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Wu D, Molofsky AB, Liang H-E, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molofsky AB, Nussbaum JC, Liang H, Van Dyken SJ, Cheng LE, Mohapatra A, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 7.Spranger J, Kroke A, Möhlig M, Hoffmann K, Bergmann MM, Ristow M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 8.McSorley HJ, Hewitson JP, Maizels RM. Immunomodulation by helminth parasites: defining mechanisms and mediators. Int J Parasitol. 2013;43:301–310. doi: 10.1016/j.ijpara.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Kreider T, Anthony RM, Urban JF, Gause WC. Alternatively activated macrophages in helminth infections. Curr Opin Immunol. 2007;19:448–453. doi: 10.1016/j.coi.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wammes LJ, Mpairwe H, Elliott AM, Yazdanbakhsh M, Hoeppli R, Bundy D, et al. Helminth therapy or elimination: epidemiological, immunological, and clinical considerations. Lancet Infect Dis. 2014;14:1150–1162. doi: 10.1016/S1473-3099(14)70771-6. [DOI] [PubMed] [Google Scholar]
- 11.Berbudi A, Ajendra J, Wardani APF, Hoerauf A, Hübner MP. Parasitic helminths and their beneficial impact on type 1 and type 2 diabetes. Diabetes Metab Res Rev. 2016;32:238–250. doi: 10.1002/dmrr.2673. [DOI] [PubMed] [Google Scholar]
- 12.Maizels RM, Maizels RM, Yazdanbakhsh M, Asher MI, Montefort S, Bjorksten B, et al. Parasitic helminth infections and the control of human allergic and autoimmune disorders. Clin Microbiol Infect. 2016;22:481–486. doi: 10.1016/j.cmi.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 13.Hübner MP, Stocker JT, Mitre E. Inhibition of type 1 diabetes in filaria-infected non-obese diabetic mice is associated with a T helper type 2 shift and induction of FoxP3+ regulatory T cells. Immunology. 2009;127:512–522. doi: 10.1111/j.1365-2567.2008.02958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hübner MP, Shi Y, Torrero MN, Mueller E, Larson D, Soloviova K, et al. Helminth protection against autoimmune diabetes in nonobese diabetic mice is independent of a type 2 immune shift and requires TGF-β. J Immunol. 2012;188:559–568. doi: 10.4049/jimmunol.1100335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aravindhan V, Mohan V, Surendar J, Muralidhara Rao M, Pavankumar N, Deepa M, et al. Decreased prevalence of lymphatic filariasis among diabetic subjects associated with a diminished pro-inflammatory cytokine response (CURES 83) PLoS Negl Trop Dis. 2010;4:e707. doi: 10.1371/journal.pntd.0000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Lu J, Huang Y, Wang T, Xu Y, Xu M, et al. Association of previous schistosome infection with diabetes and metabolic syndrome: a cross-sectional study in rural China. J Clin Endocrinol Metab. 2013;98:E283–E287. doi: 10.1210/jc.2012-2517. [DOI] [PubMed] [Google Scholar]
- 17.Wiria AE, Hamid F, Wammes LJ, Prasetyani MA, Dekkers OM, May L, et al. Infection with soil-transmitted helminths is associated with increased insulin sensitivity. PLoS One. 2015;10:e0127746. doi: 10.1371/journal.pone.0127746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hays R, Esterman A, Giacomin P, Loukas A, McDermott R, Krolewiecki AJ, et al. Does Strongyloides stercoralis infection protect against type 2 diabetes in humans? Evidence from Australian aboriginal adults. Diabetes Res Clin Pract. 2015;107:355–361. doi: 10.1016/j.diabres.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Malozowski S, Sahlroot JT. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;357:302–303. doi: 10.1056/NEJMc071324. [DOI] [PubMed] [Google Scholar]
- 20.Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2000;11:212–217. doi: 10.1016/s1043-2760(00)00272-1. [DOI] [PubMed] [Google Scholar]
- 21.Santomauro AT, Boden G, Silva ME, Rocha DM, Santos RF, Ursich MJ, et al. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes. 1999;48:1836–1841. doi: 10.2337/diabetes.48.9.1836. [DOI] [PubMed] [Google Scholar]
- 22.Frayn KN. Adipose tissue as a buffer for daily lipid flux. Diabetologia. 2002;45:1201–1210. doi: 10.1007/s00125-002-0873-y. [DOI] [PubMed] [Google Scholar]
- 23.Volkmann L, Bain O, Saeftel M, Specht S, Fischer K, Brombacher F, et al. Murine filariasis: interleukin 4 and interleukin 5 lead to containment of different worm developmental stages. Med Microbiol Immunol. 2003;192:23–31. doi: 10.1007/s00430-002-0155-9. [DOI] [PubMed] [Google Scholar]
- 24.Ajendra J, Specht S, Neumann A-L, Gondorf F, Schmidt D, Gentil K, et al. ST2 deficiency does not impair type 2 immune responses during chronic filarial infection but leads to an increased microfilaremia due to an impaired splenic microfilarial clearance. PLoS One. 2014;9:e93072. doi: 10.1371/journal.pone.0093072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, et al. Selective depletion of Foxp3 + regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lahl K, Sparwasser T. In vivo depletion of FoxP3+ Tregs using the DEREG mouse model. Methods Mol Biol. 2011;707:157–172. doi: 10.1007/978-1-61737-979-6_10. [DOI] [PubMed] [Google Scholar]
- 27.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 28.Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 2009;58:2574–2582. doi: 10.2337/db08-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaughan DE. PAI-1 and atherothrombosis. J Thromb Haemost. 2005;3:1879–1883. doi: 10.1111/j.1538-7836.2005.01420.x. [DOI] [PubMed] [Google Scholar]
- 30.Jeoung NH, Harris RA. Knocking out PDK2 and PDK4 lowers fasting blood glucose levels, increases insulin sensitivity, and greatly improves glucose tolerance. FASEB J. 2007;21:LB35–LB36. [Google Scholar]
- 31.Layland LE, Ajendra J, Ritter M, Wiszniewsky A, Hoerauf A, Hübner MP. Development of patent Litomosoides sigmodontis infections in semi-susceptible C57BL/6 mice in the absence of adaptive immune responses. Parasit Vectors. 2015;8:396. doi: 10.1186/s13071-015-1011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, et al. Alternative M2 activation of Kupffer cells by PPARδ ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhargava P, Li C, Stanya KJ, Jacobi D, Dai L, Liu S, et al. Immunomodulatory glycan LNFPIII alleviates hepatosteatosis and insulin resistance through direct and indirect control of metabolic pathways. Nat Med. 2012;28:1–9. doi: 10.1038/nm.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ricardo-Gonzalez RR, Red Eagle A, Odegaard JI, Jouihan H, Morel CR, Heredia JE, et al. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proc Natl Acad Sci USA. 2010;107:22617–22622. doi: 10.1073/pnas.1009152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Garra A, Chang R, Go N, Hastings R, Haughton G, Howard M. Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. Eur J Immunol. 1992;22:711–717. doi: 10.1002/eji.1830220314. [DOI] [PubMed] [Google Scholar]
- 38.Zhou L. Striking similarity: GATA-3 regulates ILC2 and Th2 cells. Immunity. 2012;37:589–591. doi: 10.1016/j.immuni.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee M-W, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, et al. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160:74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242–246. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wohlfert EA, Grainger JR, Bouladoux N, Konkel JE, Oldenhove G, Ribeiro CH, et al. GATA3 controls Foxp3+ regulatory T cell fate during inflammation in mice. J Clin Invest. 2011;121:4503–4515. doi: 10.1172/JCI57456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Su MA, Wan YY. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity. 2011;35:337–48. doi: 10.1016/j.immuni.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barbi J, Pardoll D, Pan F. Treg functional stability and its responsiveness to the microenvironment. Immunol Rev. 2014;259:115–139. doi: 10.1111/imr.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thauvin-Robinet C, Auclair M, Duplomb L, Caron-Debarle M, Avila M, St-Onge J, et al. PIK3R1 mutations cause syndromic insulin resistance with lipoatrophy. Am J Hum Genet. 2013;93:141–149. doi: 10.1016/j.ajhg.2013.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zmuda-Trzebiatowska E, Oknianska A, Manganiello V, Degerman E. Role of PDE3B in insulin-induced glucose uptake, GLUT-4 translocation and lipogenesis in primary rat adipocytes. Cell Signal. 2006;18:382–390. doi: 10.1016/j.cellsig.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Aronoff SL, Berkowitz K, Shreiner B. Glucose metabolism and regulation: beyond insulin and glucagon. Diabetes Spectr. 2004;17:183–190. [Google Scholar]
- 47.Hussaarts L, Garcia-Tardon N, van Beek L, Heemskerk MM, Haeberlein S, van der Zon GC, et al. Chronic helminth infection and helminth-derived egg antigens promote adipose tissue M2 macrophages and improve insulin sensitivity in obese mice. FASEB J. 2015;7:3027–3039. doi: 10.1096/fj.14-266239. [DOI] [PubMed] [Google Scholar]
- 48.Hise AG, Daehnel K, Gillette-Ferguson I, Cho E, McGarry HF, Taylor MJ, et al. Innate immune responses to endosymbiotic Wolbachia bacteria in Brugia malayi and Onchocerca volvulus are dependent on TLR2, TLR6, MyD88, and Mal, but not TLR4, TRIF, or TRAM. J Immunol. 2007;178:1068–1076. doi: 10.4049/jimmunol.178.2.1068. [DOI] [PubMed] [Google Scholar]
- 49.Lehrke M, Lazar MA. The many faces of PPARγ. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 50.Rangwala SM, Rhoades B, Shapiro JS, Rich AS, Kim JK, Shulman GI, et al. Genetic modulation of PPARγ phosphorylation regulates insulin sensitivity. Dev Cell. 2003;5:657–663. doi: 10.1016/s1534-5807(03)00274-0. [DOI] [PubMed] [Google Scholar]
- 51.Nolan JJ, Ludvik B, Beerdsen P, Joyce M, Olefsky J. Improvement in glucose tolerance and insulin resistance in obese subjects treated with troglitazone. N Engl J Med. 1994;331:1188–1193. doi: 10.1056/NEJM199411033311803. [DOI] [PubMed] [Google Scholar]
- 52.Welch JS, Ricote M, Akiyama TE, Gonzalez FJ, Glass CK. PPARγ and PPARδ negatively regulate specific subsets of lipopolysaccharide and IFN-γ target genes in macrophages. Proc Natl Acad Sci USA. 2003;100:6712–6717. doi: 10.1073/pnas.1031789100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, et al. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT-H, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoerauf A, Satoguina J, Saeftel M, Specht S. Immunomodulation by filarial nematodes. Parasite Immunol. 2005;27:417–429. doi: 10.1111/j.1365-3024.2005.00792.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data