Abstract
Honey bees can be found all around the world and fulfill key pollination roles within their natural ecosystems, as well as in agriculture. Most species are typically docile, and most interactions between humans and bees are unproblematic, despite their ability to inject a complex venom into their victims as a defensive mechanism. Nevertheless, incidences of bee stings have been on the rise since the accidental release of Africanized bees to Brazil in 1956 and their subsequent spread across the Americas. These bee hybrids are more aggressive and are prone to attack, presenting a significant healthcare burden to the countries they have colonized. To date, treatment of such stings typically focuses on controlling potential allergic reactions, as no specific antivenoms against bee venom currently exist. Researchers have investigated the possibility of developing bee antivenoms, but this has been complicated by the very low immunogenicity of the key bee toxins, which fail to induce a strong antibody response in the immunized animals. However, with current cutting-edge technologies, such as phage display, alongside the rise of monoclonal antibody therapeutics, the development of a recombinant bee antivenom is achievable, and promising results towards this goal have been reported in recent years. Here, current knowledge on the venom biology of Africanized bees and current treatment options against bee envenoming are reviewed. Additionally, recent developments within next-generation bee antivenoms are presented and discussed.
Keywords: bee antivenom, bee allergy, bee envenoming, bee therapy, bee toxins, bee venom
Introduction
Bees are economically beneficial insects whose existence dates back to the Cretaceous period during the Mesozoic era (1). Bees have provided several products to humans, such as honey, beeswax, pollen, royal jelly, and propolis (2). They also pollinate a wide variety of agricultural crops (3). Although bees are extremely beneficial to crops and humans, they do present a danger due to their ability to inflict painful and toxic stings (4). Fortunately, most honey bees are not aggressive towards humans and only attack when they feel threatened. However, due to the human introduction of the Africanized bee, a hybrid with highly aggressive behavior, massive bee sting attacks have markedly increased and are now endemic in most of the Americas (excluding Chile and Canada) (5). Standardized medical approaches exist for handling cases, where victims allergic to venom components are stung by bees, or where milder envenomings are caused by only a few bee stings. Yet, no antivenom exists for treating severe bee envenomings. The underlying reason for this derives from the low immunogenicity of bee venom proteins (e.g., melittin), which hinders successful immunization of production animals to yield high antibody titers in their plasma and, consequently, complicates the development of a bee antivenom significantly (6). To develop a treatment against severe bee envenoming, the design of an effective antivenom is a necessity. Here, current knowledge on bee biology, spreading of Africanized bee hybrids in the Americas, and the bee venom apparatus and toxins are reviewed, and a discussion on current and next-generation treatments of bee envenomings is provided.
Bee Species, Behavior, and Epidemiology
Honey bees (Apis species) are social insects that live in well-organized communities and are very important to a significant proportion of the world economy due to the key role they fulfill as pollinators in agriculture (7). However, over the past decade, they have received increasing attention due to another physiological feature: their ability to deliver a venomous sting (8). The bee species predominantly responsible for human envenomings are Apis mellifera mellifera (A. m. mellifera) and A. m. ligustica in Europe, and A. m. scutellata in Africa (8).
Bee stings are not a novel phenomenon. In fact, significant exposure of humans to bee stings dates back over 7,000 years, when humans started to manage bee populations by providing them with artificial hives to enable an efficient harvest of their honey and wax, or for pollination purposes (9, 10). Despite significant breeding efforts, honey bees remain to be successfully domesticated, and a reduction in additive genetic variance, fixation of alleles associated with traits of economic importance, increased tameness, and the development of breed-specific characteristics amongst other properties have not been reported (11). In fact, targeted breeding appears to have increased rather than decreased genetic diversity (12).
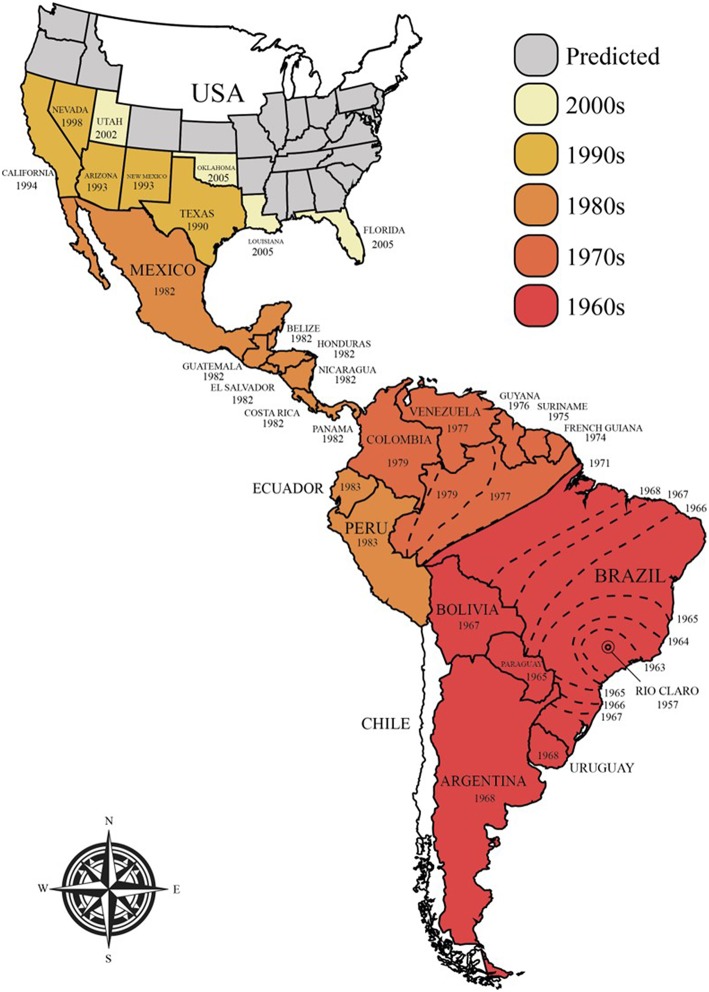
The majority of commercial honey bee populations are derived from Europe, although they from an evolutionary perspective originated from Africa and were introduced to Europe through two independent migration events (13). In the 1620s, European honey bees (A. m. mellifera) were successfully introduced to North America for pollination and honey production. Later, in 1822, they were introduced to Australia (14). Attempts to replicate the original successes from North America and Australia failed in 1839 in Brazil and in other tropical regions (15, 16). This failure was believed to stem from the very different climates on both continents. New attempts were made in 1955 involving the African honey bee (A. m. scutellata), which was crossbred with honey bees of European descent to create a hybrid species that would better thrive in tropical environments and would produce large quantities of high quality honey (15–18). In the subsequent year, however, 26 queens and their swarms of Africanized (hybrid) honey bees escaped the laboratory and invaded large parts of the Americas, expanding 300–500 km per year (Figure 1) (15–17). The bees reached Mexico in 1986, the USA in 1990 (Texas), and have since spread into many states, including California, Arizona, Utah, New Mexico, Oklahoma, Louisiana, Arkansas, Alabama, and Florida (16, 19–22). Although climate limitations, particularly cold winters, have significantly slowed down the spread of these hybrid bees and currently restrict the range of their habitat, they are still believed to be able to colonize North America, where the harsh winter will be their only natural barrier. This range is likely to expand with global increases in temperatures (16, 23).
Figure 1.
Current and predicted future spread of Africanized honey bees in the Americas.
The success of Africanized honey bees in the Americas has been attributed to a combination of ecological and genetic factors that have provided them with increased fitness compared to the resident pollinators (15, 16). Examples include higher reproductive rates, a shorter developmental cycle (i.e., the worker bees take 19–20 days, instead of 21, and queens take 14 days, instead of 16 to mature), higher drone production/abundance, higher absconding rates (i.e., forced colony relocation in case of food scarcity) and higher swarming rates (natural colony expansion and reproduction; 6–12 times per year in case of food abundance), lower honey-storing needs, disease resistance, and decreased selectivity when choosing nest sites (15, 24–26). Furthermore, Africanized bees are significantly more defensive than other bees. This is manifested in their propensity to attack with little stimulation, increased numbers of bees that co-attack at a greater distance to the hive than usual, their pronounced insistence to chase intruders for a longer period of time, and their release of putatively larger volumes of venom (15, 16). These characteristics have led to them being commonly known as “killer bees”.
The increased aggression of these bee hybrids is thought to cause significant ongoing livestock losses and human health issues, yet there is a scarcity of reliable information on the frequency of massive stinging events and severe envenomings (8, 27, 28). This lack of data is likely because the majority of bee stings are of minor medical importance and the stung individuals do not seek medical care (8, 27). Furthermore, few governmental agencies collect data on sting frequencies (8), and often group all animal bites and stings together, in the medical records from the emergency departments (29). In the US, for instance, the annual report of the American Association of Poison Control Centers stated that 41,850 animal bites/stings and four deaths occurred in 2017, yet the lack of specificity of the data makes it impossible to attribute a certain number of cases to bee envenomings (29). Indeed, although the recent report from the Centers for Disease Control and Prevention (CDC) shows that accidents and deaths by stinging insects increased over the last 5 years (annual average of 62 deaths), the report combines accidents within hornets, wasps, and bees together (30). However, though one exemplary report for envenomings by terrestrial animals in Brazil exists, where data was collected over the course of 12 years (8, 27). The study found that a total of 1,192,667 envenomings were recorded between 2001 and 2012, of which 66,283 (5.6%) could be attributed to bees. Notably, bee stings had the second highest case fatality rate (0.33%; 216 deaths), with only snakebites exceeding them (0.43%; 3,394 deaths) (27). The study showed that case fatality rates did not appear to undergo any significant fluctuations between 2001 and 2012 (27). Due to the significant incidence of bee stings in Brazil, it is likely that similar public health issues exist in other countries in the Americas with large numbers of Africanized honey bee colonies (27, 28). In fact, since their arrival in the USA, there have been several reports of deaths after Africanized bee swarm attacks (31). Taken together, the significant number of bee stings and the relatively high fatality rates of these stings suggest that there is a growing medical need for innovative treatment options, such as specific bee antivenoms to address severe envenomings. However, the financial prospects of developing antivenom products for the market are currently unknown and difficult to predict.
Bee Sting and Venom
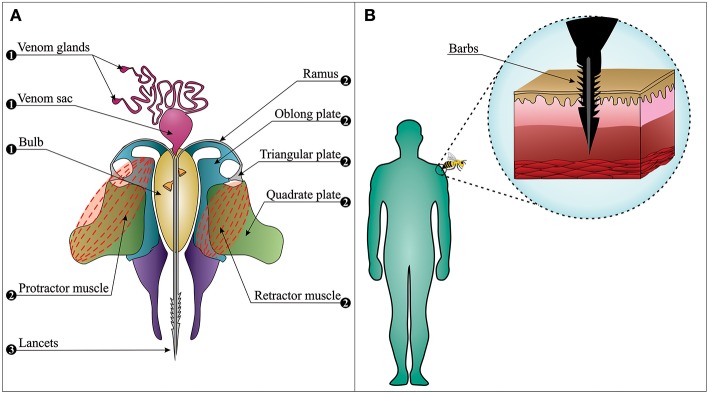
The bee sting apparatus exhibits three functionally distinct parts; the motor part, the piercing part, and the venom-related part (Figure 2A) (32–34). In the piercing part, stylet, and lancets have important roles. They are covered by tetrahedron-shaped barbs, which are distributed in a spiral right-handed manner. This specific type of distribution plays a fundamental role in the helically clockwise rotation of the sting during the penetration of the stinger into the wound and reduces the penetration force (35). These barbs make it almost impossible for the bee to retract its stinger from the elastic flesh of mammals when escaping (Figure 2B). This situation may easily lead to sting autotomy, where the sting apparatus and its associated muscles are separated from the rest of the abdomen upon the bee's escape from the victim (36).
Figure 2.
The bee sting apparatus. (A) The venom apparatus consists of three functionally distinct parts: (1) The venom-related part is composed of a venom sac, two venom glands, and a bulb. (2) The motor part is composed of muscles, plates, and ramus on each side. (3) The piercing part is composed of two lancets and a stylet (note: the stylet cannot be observed in this figure since the longitudinal section has passed from the middle of the venom canal that leaves the stylet on the upper section). (B) Barbs anchor the stinger into the skin, from where the stinger cannot be retracted when the bee escapes (i.e., sting autotomy).
Contrary to popular belief, worker bees stay alive for 18–114 h after the sting autotomization and continue playing their role as defenders (37). When the bee escapes, its autotomized sting continues to embed itself into the wound over a period of ~30 s (38), and venom can still be delivered. It is noteworthy that at least 90% of the venom sac content is delivered within the first 20 s after the stinging event (38), and removal of the stinger (see section Bee Envenomings: Clinical Manifestations) 1 min after this event is unlikely to reduce venom-induced toxicity. On average, 140–150 μg of venom is delivered in a stinging event, and the median lethal dose (LD50) of bee venom varies between 2.8 and 3.5 mg of venom per kg of human body weight (38–41). It can thus be speculated that a non-allergic person weighing 60–70 kg has a 50% chance of death upon being stung by 1,000–1,500 bees, although deaths caused by only 200–500 stings have also been reported (38, 42). Indeed, the severity of the envenoming is determined by victim age, body weight, number of stings, and individual characteristics of the victim (immune status, comorbidities, and previous sensitization) (43).
Bee venom is a complex mixture of compounds, which include proteins, peptides, amino acids, phospholipids, sugars, biogenic amines, volatile compounds, pheromones, and a high quantity of water (>80%) (44–46). The composition of bee venom has already been elucidated by omics techniques (47–49) and by fractionation of the venom (50–53). In this review, only components with important clinical and therapeutic effects, and with enough literature support, will be detailed, while other bee venom compounds are only listed in Table 1. It is important to emphasize that bees are insects from the Hymenoptera order, which includes wasps (80). Therefore, bee venoms contain some of the same compounds as wasp venoms, such as adrenaline, dopamine, histamine, hyaluronidase, noradrenaline, phospholipases A2 (PLA2s), phospholipases B (PLBs), and serotonin (81), while only bee venoms contain apamin (82), melittin (50), and mast cell-degranulating peptide (MCD) (83, 84).
Table 1.
Bee venom compounds.
| Name (others) | Mass (Da) | Access (uniprot) | % of dryed venom# | References |
|---|---|---|---|---|
| α-Glucosidase | 65,565 | Q17058 | 0.6 | (54, 55) |
| Acid phosphatase (Api m 3) | 45,389 | Q5BLY5 | 1 | (56) |
| Adolapin | 11,500 11,092 |
– | 0.1–0.8 | (57) |
| Apamin | 5,223 | P01500 | 1–3 | (58) |
| Api m 6.01 | 7,190 | P83563* | – | (59) |
| Api m 6.02 | 7,400 | |||
| Api m 6.03 | 7,598 | |||
| Api m 6.04 | 7,808 | |||
| Cardiopep | 1,940 | – | 0.7 | (60) |
| Dipeptidylpeptidase IV (Api m 5) | 87,937 | B2D0J4 | – | (61) |
| Hyaluronidase (Api m 2) | 44,260 | Q08169 | 1–3 | (62) |
| Icarapin (Api m 10) | 24,819 | Q5EF78 | – | (63, 64) |
| MRJP (1–5) | 49,000 | O18330 | – | (65) |
| 87,000 | ||||
| O77061 | ||||
| Q17060 | ||||
| Q17061 | ||||
| O97432 | ||||
| MRJP9 (Api m 11.0101) | 48,518 | Q4ZJX1 | – | (66) |
| MCD (Peptide 401) | 5,781 | P01499 | 1–3 | (67) |
| Melittin | 2,846 | P01501* | 50–60 | (68–70) |
| Melittin-S | 2,830 | 1–2 | ||
| Synthetic melittin | – | – | ||
| Melittin-F | 2,208 | – | 0.01 | (71) |
| Minimine | 6,000 | – | 2–3 | (72) |
| PLA2 (Api m 1) | 19,058 | P00630 | 10–12 | (73, 74) |
| PLB (Lysophospholipase) | – | – | – | (75) |
| Procamine | <1,000 | – | 1.4 | (44) |
| Secapin | 8,664 | P02852 | 1–2 | (71) |
| Secapin-1 | 2,822 | – | 1 | (76) |
| Secapin-2 | 2,872 | – | – | (77) |
| Serine proteases (Api m 7) | 39,000 | – | – | (78) |
| Tertiapin | 2,459 | P56587 | 0.1 | (79) |
MRJPs, Major Royal Jelly Proteins; MCD, Mast Cell-Degranulating peptide; PLA2, phospholipase A2; PLB, phospholipase B.
Isoforms are represented by the same entry in the UniprotKB due to the small differences in their amino acid sequence.
Dried venom excludes volatile compounds.
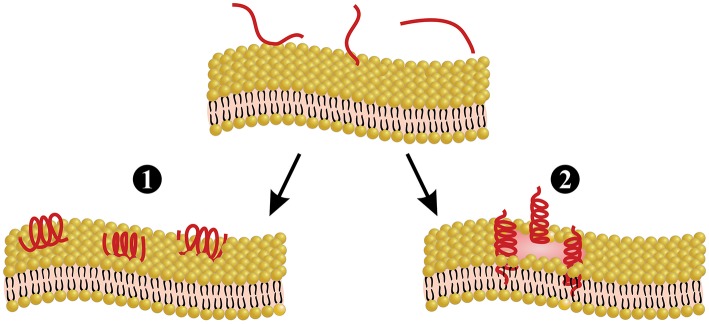
Melittin is the main and most toxic compound in bee venom, constituting 50–60% of the whole venom (85). Melittin only induces minor allergic reactions (86), but causes the majority of the pain associated with bee stings (4), which is induced through direct and indirect actions on primary nociceptor cells. The direct action is caused by melittin activation of thermal nociceptor transient receptor potential vanilloid 1 (TRPV1) via the PLA2 cascade pathway, resulting in sensitization of the primary nociceptors (87–89). The indirect action is based on the pore-forming actions of melittin (Figure 3), which allows for the release of pain-inducing substances such as H+, adenosine triphosphate (ATP), and 5-hydroxytryptamine (5-HT) from mast cells, as well as melittin causes tissue damage, resulting in activation of the pain receptors. Pore formation induced by melittin can also release mediators, such as histamine, bradykinin, and ATP, which activate G-protein-coupled receptors (GPCRs), resulting in the phosphorylation of phospholipase C (PLC). PLC cleaves phosphatidylinositol 4,5-bisphosphateintodiacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). DAG is an endogenous activator of transient receptor potential canonical (TRPC) channels, resulting in the indirect excitation of primary nociceptive neurons (i.e., pain). A more detailed overview of the pain-inducing mechanism of melittin can be found elsewhere (4). Melittin is classified as a lytic peptide that is able to destroy membrane phospholipids, which include erythrocytes, leading to hemolysis (85, 91). This action may derive from conformational modification, where melittin molecules have been proposed to bind perpendicularly to membranes forming a pore (90) (Figure 3). Furthermore, melittin can increase the activity of PLA2s (92). As an example, PLA2 activity was tested with and without melittin on lecithin liposomes, and it was observed that PLA2 activity was 5-fold higher in the presence of melittin (92). Different isoforms of melittin can be found in bee venom, such as melittin-S and melittin-F. However, these exist only in low abundance (68, 71). Melittin has been demonstrated to have antimicrobial activity in vitro and in vivo (91), anti-inflammatory effects in vitro (93), antiviral activity in vitro (94), and anti-cancer effects in vitro and in vivo (95). Finally, it is worth mentioning that melittin can be chemically synthesized to obtain high amounts of the peptide (69, 96, 97).
Figure 3.
Melittin-induced pore formation model. Melittin can bind to the membrane either in a parallel orientation (1) or a perpendicular orientation (2). The perpendicular orientation induces pore formation, whereas the parallel orientation is inactive. Parallel orientation has also been hypothesized to protect the membrane, since this prevents other melittin molecules from forming pores. Figure adapted from van den Bogaart et al. (90).
Phospholipase A2 (PLA2) is the second most abundant compound (10–12%) and the most allergenic and immunogenic protein in bee venom (83). Alone, PLA2 is a non-toxic protein (44, 83), but when PLA2 forms a complex with melittin, called bee hemolytic factor, it cleaves cellular membrane phospholipids (98). In vitro, bee PLA2 possesses several activities, such as trypanocidal and antibacterial effects (99, 100), neuronal protection caused by prion proteins (101), and anti-tumor properties (102). Moreover, bee PLA2 was able to decrease hepatotoxicity caused by acetaminophen in mice (103). Phospholipase B (PLB) has also been reported to be present in bee venom (75). PLB exhibits both PLA1 and PLA2 activity, being responsible for cleaving phospholipids on sn-1 and sn-2 position of acyl chains (104), which enhances PLA2 activity (46). Notably, PLBs are also important components of snake venoms (46, 75, 105, 106).
Apamin is another important peptide in bee venom, which comprises 1–3% of crude venom and is able to allosterically and selectively inhibit Ca2+-dependent K+ channels (SK channels), found in the central nervous system (CNS) (81, 107, 108). Only SK2 and SK3 channels are known to be sensitive to apamin, and when blocked, there is a decrease of the delayed hyperpolarization of cells, which results in increased continuous firing of neurons in the mesencephalon and cerebellum, elevating cell sensitivity to excitatory inputs (107, 109). Moreover, apamin is able to activate inhibitory muscarinic receptors of motor nerve terminals (i.e., reducing neuromuscular transmission), which has been experimentally explored as a potential treatment against diseases presenting high muscle excitability (110), such as Parkinson's disease (111), learning deficit disorder (112), and other disabilities (81, 113).
Hyaluronidase is an enzyme found in bee venom (1–3%), as well as many other animal venoms (114–117). Hyaluronidase is responsible for fast distribution of toxins, also known as the “spreading factor,” as this enzyme cleaves hyaluronic acid from the extracellular matrix (ECM) (83, 118, 119), leading to a faster and systemic envenoming by disrupting tissues (120). In addition, hyaluronidase is considered a potent allergen in bee venom (121).
Mast cell-degranulating (MCD) peptide is also considered an important component in bee venom based on its capability to induce histamine release from mast cells, which exhibit a central role on inflammation and allergy (122). In high quantities, however, MCD presents an opposite action, where it inhibits mast cell degranulation (i.e., by inhibiting histamine release). Thus, MCD can also act as an anti-allergic molecule (123). Indeed, studies have demonstrated that MCD peptide presents anti-inflammatory activity in vitro and in vivo (124, 125).
Beside the above mentioned components, bee venom also contains amines, such as histamine and catecholamines (81). Histamine is able to increase capillary permeability, contributing to the inflammatory response, while catecholamines (i.e., noradrenaline and dopamine) enhance bee venom distribution, since they, among other functions, increase cardiac output (122).
As for other venoms (105, 126, 127), bee venom is very susceptible to variability, depending on bee age, species, social condition, geographic localization, amongst other factors (44). For instance, young worker bees (foragers/guards/nurses) have higher levels of apamin and lower levels of melittin compared to old workers (foragers/guards). In contrast, queen bees present lower levels of melittin and apamin (128) and higher levels of histamine (129). Furthermore, young bees have low levels of histamine, while, at 35-days-old, they present high levels of this molecule. Melittin reaches maximum concentration when the bee is 4-weeks-old, and then decreases during bee aging; while promelittin is most prevalent when the bees are 8–10-days-old (130). Hyaluronidase levels also vary in bee venoms. Although hyaluronidase can be detected immediately after the pupae emerge from the eggs as adult bees (i.e., eclosion), the enzyme levels increase with bee aging (131). Whilst low concentrations of PLA2 are found during bee eclosion, they increase gradually and reach the highest levels when the bees are 7–10-days-old (51).
Regarding venom variations among different bee species, African bees release a low amount of venom when stinging, with lower quantities of melittin and hyaluronidase, and increased amounts of PLA2, which can be explained by the fact that these bees possess smaller venom glands than the European bees (132–135). Additionally, seasonal changes may have an impact on bee venom content, since the seasons affect flowers and fruits, and therefore also bee feeding (104). Melittin production changes during the summer (136), while melittin-S production increases during winter, allowing mellitin-S to reach an abundance of 10% of whole venom (68).
Venom milking methods can also affect bee venom composition. Bee venom can be collected by extraction of glandular venom or by electrical stimulation, and venoms collected by these methods present differences on chromatographic profiles. Volatile components such as histamine can disappear when bee venom is collected by electrical stimulation (44, 137). Moreover, through proteomic analysis, bee venom obtained by gland extraction may have contamination of proteins from the gland tissue, so that down to only 40% of the obtained material is actual bee venom proteins. However, generally when electrical stimulation is used, more than 80% of the obtained material is venom proteins (48).
Bee Envenomings: Clinical Manifestations
Bee envenomings can result in mild to severe clinical manifestations depending mainly on the number of stings that the victim has received. Patient age, weight, co-morbidities, and medical care can also influence the severity of an envenoming (28). Moreover, atopic individuals (e.g., individuals with asthma or allergic rhinitis) and a family history of bee sting allergy are associated with higher incidence of severe reactions (138). Typically, the clinical manifestations of bee envenoming can be divided into local inflammatory reactions (1), allergic manifestations (2), anaphylactic shock (3), and systemic toxic reactions (4) (43, 139). (1) Local inflammatory reactions are characterized by pain, swelling (edema and erythema), itching, and pruritus at the sting site. These reactions are experienced by most non-allergic individuals and are normally resolved within 24 h (39). (2) Bee sting allergic reactions are IgE-dependent and are classified as hypersensitivity type I reactions. These reactions occur about 10 min after the sting, and the symptoms can vary in severity. PLA2 is considered the main compound that induces IgE-sensitization of mast cells, although hyaluronidases and melittin are also considered allergens (140) (see section Bee Sting and Venom). Allergic patients can develop systemic urticaria, pruritus, angioedema, vomiting, and diarrhea (28). (3) In some cases, the allergic reactions can evolve to an anaphylactic reaction, resulting in bronchoconstriction and anaphylactic shock (39). Between 25% and 70% of patients with insect allergies exhibit systemic reactions when challenged with the allergen (i.e., bee venom) (140). Interestingly, some non-allergic individuals can also develop bee anaphylaxis due to systemic mastocytosis (141–143). Systemic mastocytosis is a heterogeneous disorder characterized by proliferation of mast cells and the extent of granulation, which is caused by mutations in the c-Kit gene (a growth factor for mast cells) (144, 145). (4) Systemic toxic reactions are characterized by direct toxic effects of the bee venom, independent of immune mechanisms, which are also known as venom volume-dependent reactions. Systemic toxic reactions are always considered severe and are caused by multiple stings (about 50 simultaneous stings). Patients suffering from systemic toxic reactions may present fatigue, dizziness, nausea, vomiting, and diarrhea, which can evolve into myocardial injury, hypertension, hepatic injury, rhabdomyolysis, hemolysis, comatose, and acute renal failure (43, 146, 147). Deaths are likely to occur when the victim has received about 500 stings, which are considered necessary to cause death by direct toxicity (42), although fewer stings (30–50) have proven fatal in children (148).
Other rare clinical manifestations have also been reported for bee stings, including peripheral neuritis (149), Fisher's syndrome (150), acute inflammatory polyradiculoneuropathy (Guillain-Barré syndrome) (151), optic neuropathy (152), septicemia (153), bilateral empyema (154), and even urticaria for a baby (12-day-old) being breastfed by its mother who had been stung by a bee (155).
Current Treatment
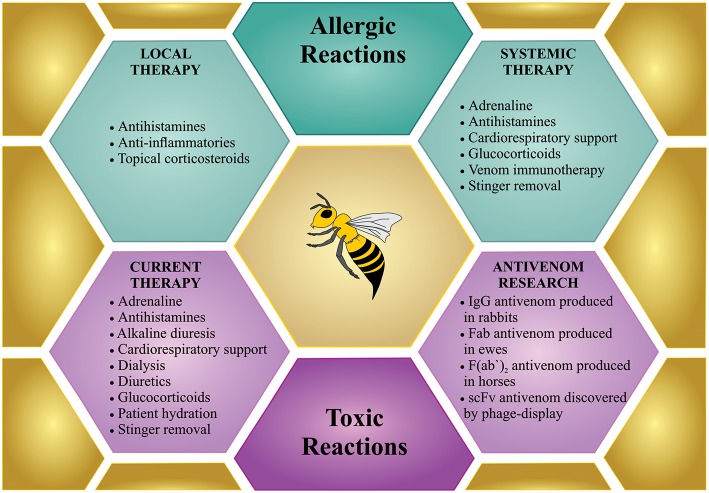
There are generally three different bee sting scenarios that require treatment. (1) Few stings on a non-sensitized person; (2) one or more stings on a hypersensitive person; and (3) massive bee envenoming by multiple stings (Figure 4).
Figure 4.
Treatment for bee sting(s). Bees incidents can involve few stings, which can cause local reactions or anaphylactic shock, which request a treatment similar to any allergic reactions (in green). However, mass stinging events can prove life-threatening via the toxic action of the venom when injected in large amounts, which demands intensive treatment (in purple). Although specific treatment is not available so far, only few antivenom researchers are working on developing new therapies against bee envenoming.
Treatment of a Few Stings on a Non-sensitized Person
Non-sensitized people present only a localized reaction to a bee sting. In the normal reaction to a bee sting, the skin manifests itself as an area of pain, redness, and swelling that is generally <10 cm in diameter and normally disappears within 24 h (156). Experiments conducted in guinea-pigs stung by bees, which had their tissues removed and subjected to histologic analysis, demonstrated only local marked inflammation (i.e., edema, high cellular infiltration, and necrosis) during 24 h after the sting (157). Medical assistance is unnecessary in these cases, although the use of topical corticosteroids is recommended, which produce anti-inflammatory, immunosuppressive, and anti-mitogenic effects (158). However, in the so-called large local reactions to an insect sting, the area of swelling and redness may be larger than 10 cm in diameter, and pain can persists for several days. Although this reaction is presumably of allergic origin, it is not necessarily mediated by IgE. In this situation, oral antihistamines can be prescribed (156).
Treatment of Hypersensitivity
If a person who is hypersensitive to bee venom (i.e., an allergic person) is stung by at least one bee, immediate medical attention is required, since anaphylaxis might occur. The majority of the deaths that occur in this case are due to allergic individuals not reaching medical care fast enough (159). The preferred first line of action against anaphylaxis differs from study to study. Some studies report as first line of action to scrape off the bee stingers carefully (by avoiding to pull or squeeze the stingers, which could lead to injection of more venom) (160), although this action is mainly relevant if performed within 60 s of the stinging event, in which period the stinger ejects all its venom (39). Other studies state that the first-line of action should be to administer intramuscular adrenaline (also known as epinephrine), and only then remove the stinger (161). While these studies might disagree on whether stinger removal should be the first action followed by adrenaline, or the opposite, they do agree that the first drug to be used is intramuscular adrenaline (39, 160–162). Even a few minutes delay in the administration of adrenaline can lead to hypoxia or death. Indeed, the lack of access to emergent adrenaline plays a critical role in the mortality and morbidity for allergic patients. Thus, there has been an increased awareness of the need for adrenaline auto-injectors in public locations including schools, parks, airports, and shopping malls (163, 164).
Adrenaline acts as an α and β-agonist. Through its α-1 agonistic effect, it works as a vasoconstrictor, which prevents and relieves airway edema, hypotension, and shock. The β-1 agonistic effects of adrenaline are chronotropic and inotropic and thus increase the rate and force of cardiac contractions, while the β-2 agonistic effects of adrenaline lead to bronchodilation (162). Furthermore, the β2-adrenergic agonistic effects of adrenaline also increase the intracellular levels of cyclic adenosine monophosphate in mast cells, which inhibits further release of inflammatory mediators, such as histamine, leukotrienes, and prostaglandin D2 (165). Following the administration of adrenaline, other first-line treatments include oxygen, intravenous fluid resuscitation, and inhaled short-acting β2 agonists (161, 166, 167). Second-line treatment usually consists of H1-antihistamines, H2-antihistamines, and glucocorticoids, which are given as adjuvant therapy and are considered optional. The antihistamines are only recommended for the relief of cutaneous symptoms, while glucocorticoids may be effective for treating airway edema and could prevent protracted anaphylaxis symptoms (161, 166).
A preventive treatment available to allergic individuals is venom immunotherapy (VIT). VIT consists of inoculating small increasing amounts of purified venom extracts in the allergic individual over a period of time. Venom extracts were introduced in the 1970s, and since then, VIT has become increasingly popular. Several different administration regimens have been developed to shorten the time required to reach the maintenance period and to minimize side effects (168). According to the European Academy of Allergy and Clinical Immunology (EAACI), VIT can be performed using different venom products (purified and non-purified, aqueous or depot) and different treatment protocols (conventional, cluster, rush, and ultra-rush), administered by the subcutaneous or sublingual routes (169).
Effective VIT restores immunotolerance to allergens by different mechanisms: (1) desensitization of mast cells and basophils; (2) suppression of innate lymphoid cells (ILC2); (3) activation of regulatory T cells (Tregs), which increase the levels of interleukin 10 (IL-10) and transforming growth factor-β (TGF-β); (4) and immunoglobulin cell-switch to IgG4 and IgA induced by Treg cytokines (170). The decision whether to start VIT depends on an accurate diagnosis, an assessment of the person's risk of having another allergic reaction, the degree to which the allergy affects their quality of life, the person's age and comorbid medical conditions, as well as whether the person suffers from concurrent mast cell disorder. Moreover, the allergen preparation (see EAACI guidelines) and the administered dose should be taken into account to avoid adverse effects as well as to ensure therapeutic success (169). In any event, VIT needs to be performed under medical supervision due to the risk of an allergic reaction. A study found that almost one in 10 people treated with VIT had an allergic reaction to the treatment (171). However, adverse events are normally mild and, although it is recommended to reduce the allergen dose in case of systemic adverse reactions, patients should not discontinue the therapy, since VIT is effective in reducing the risk of a subsequent systemic reaction to a bee sting in 77–84% of the treated patients (169).
Therapy Against Massive Bee Envenoming
The initial treatment against massive bee envenoming follows the same course as for a case of hypersensitivity. Allergic and systemic toxic reactions are difficult to differentiate, especially in the first minutes, and anaphylactic shock is the most immediate danger to the patient (172). However, once it is established that a hypersensitive event is not (or not only) occurring, specific treatment for massive bee envenoming is initiated. The ideal treatment against the severe toxic effects of bee venom would likely be antivenom. However, there are no specific antivenoms available, although major efforts are being made (see section Next-Generation Antivenom Therapy) (139). Patients who have more than 50 stings should be monitored, since the circulating venom toxins may persist in their body for hours or days and may have the potential to cause delayed reactions. Initially, the stung victim may be stable. Hours later though, the victim's conditions may deteriorate (28, 172, 173). Clinical monitoring should focus on levels of creatinine, serum urea nitrogen, electrolytes, and myoglobin to asses renal function and the risk of rhabdomyolysis (172, 174). Furthermore, to check for the development of acute respiratory distress syndrome and acidosis, blood pH, and oxygen levels should be monitored. If a patient shows signs of myoglobinuria, intravenous injection of sodium bicarbonate can be performed for alkalization of urine (i.e., to accelerate renal excretion). Alkaline diuresis can prevent the crystallization of myoglobin in kidney tubules, which may eventually lead to acute renal failure (172). Additionally, aggressive hydration and diuretics are often administered (139, 174). The patient can be started on either hemo or peritoneal dialysis, exchange transfusion, or plasmapheresis, to eliminate low molecular weight components of the venom, such as melittin or PLA2, or if acute renal failure develops (5, 172).
Next-Generation Antivenom Therapy
One of the obstacles for producing antibodies by immunization procedures for bee envenoming therapies is the lack of immunogenicity of several of the key bee venom toxins, such as melittin. As earlier mentioned (see section Bee Sting and Venom), melittin is a cell membrane lytic factor (85, 175) with a small molecular size (5, 176, 177), random conformation (178), and very hydrophobic regions (177), resulting in low immunogenicity (6), which highly complicates the production of effective bee antivenoms, as melittin fails to induce a strong antibody response in immunized animals.
Over the past decades, several attempts to develop an effective bee envenoming therapy have been reported. In 1996, Schumacher et al. reported the first attempt to produce heterologous antibody-based bee antivenom. Here, a polyclonal mixture of immunoglobulin G (IgG) antibodies was produced by successive immunizations of rabbits with purified PLA2, melittin, or crude bee venom, and neutralization capacities were further assessed in mice. It was observed that the specific anti-PLA2 antibodies clearly reduced PLA2-associated toxicity, when the toxin was administrated alone to mice. In contrast, it had no significant effect on lethality once crude venom was employed. Even when a combination of anti-PLA2 and anti-melittin antibodies was used, crude venom lethality did not decrease in mice, although authors identified melittin-binding antibodies in the rabbit serum (176).
In 1999, Jones et al. described a different approach based on Fab (fragment antigen binding) antibody fragments. In their study, Welsh ewes were successively immunized with bee venom for the production of IgGs, which were further digested with papain to obtain Fab fragments. Using an enzyme-linked immunosorbent assay (ELISA), researchers demonstrated that the Fab-based antivenom was able to recognize melittin. In addition, using standard efficacy (ED50) tests in vivo, the researchers determined that 20.5 mg of the ovine Fab-based antiserum was required to neutralize the toxic effects and to prevent lethality in mice when the antivenom was pre-incubated with 1 mg of bee venom (179). Later, a horse antibody fragment F(ab')2-based antivenom, described by Santos et al. (180), was demonstrated to be efficient in neutralizing the toxic activities of bee venom in vitro and in vivo. In vitro, the hemolytic activity of 1 mg of bee venom was neutralized by ~50 mg of the antivenom. In vivo, the horse antivenom was able to completely neutralize the myotoxic effects of bee venom with an effective dose (ED50) of 1.11 mg/mL (mg of bee venom/mL of antivenom) (180). Also, using horse immunization, Barraviera and co-authors recently developed a horse F(ab')2-based antivenom with an ED50 of 1.25 mg/mL (181). This study also developed a protocol for phase I/II clinical trials using the generated equine antivenom (181).
The current methods for producing antivenoms are based on successive immunizations of different animals, followed by low-cost purification of the animal plasma-derived IgGs. In spite of the historical clinical success achieved with animal plasma-derived antivenoms, these envenoming therapies have a propensity to cause adverse effects due to their heterologous nature (182, 183). It has been observed that 6–59% of the snakebite patients treated with plasma-derived antivenoms experienced early-onset adverse reactions after the administration of the antivenom, whereas 5–23% of patients experienced some delayed-onset (serum sickness) reactions, with symptoms such as fever, rash, and urticaria (182, 184). These antivenom-related adverse reactions are mainly a result of the composition and quality of the antivenom, the antibody format, and/or the total amount of protein administrated to the patient (185). Plasma-derived antivenoms are the only commercially available option for envenoming therapy. However, current progress made within the fields of biotechnology and monoclonal antibodies has positively contributed to the development of experimental antivenoms based on mixtures of specific recombinant monoclonal antibodies (186–188). Although these experimental antivenoms are yet to enter the clinical setting, envenoming therapies based on recombinant monoclonal antibodies and antibody fragments are predicted to one day be brought to the market and to be economically feasible to manufacture in the future (189).
In the field of recombinant bee antivenoms, Barbosa et al. were the first to report the discovery of fully human single-chain variable fragment (scFv)-based antibodies obtained via phage display technology against melittin and PLA2. These toxins act synergistically, and the combination of monoclonal antibodies against these toxins may therefore find its utility in treating severe bee envenomings. Specific monoclonal antibodies against melittin and PLA2 were selected from a phage display library and further selected for toxin specificity via ELISA. Two different scFv clones, named A7 and C12, against PLA2 and melittin, respectively, were discovered. Neutralization studies demonstrated that these two clones were able to neutralize the hemolytic activity of bee venom in vitro at a mass to mass ratio of 3:1 (scFv:bee venom). Moreover, the same monoclonal scFvs inhibited myotoxicity and delayed mortality in mice challenged with 1.5 LD50 of bee venom at the same ratio (190). Later, the same researchers selected two new monoclonal scFv-based antibodies against melittin and PLA2 using phage display technology, named Afribumab 1 and Afribumab 2, respectively. Afribumab 1 and 2 presented the capacity to inhibit bee venom hemolysis (0.5 μg) in vitro at a mass to mass ratio of 1:1:1 (bee venom:Afribumab 1:Afribumab 2). Using mice challenged with 2 LD50s of bee venom (corresponding to 9.484 μg/g of bee venom), the combination of Afribumabs 1 and 2 with the same ratio of 1:1:1 was demonstrated to reduce edema and prolong mouse survival for more than 400 min (compared to around 100 min when the mice were only challenged with 2 LD50s of bee venom) (191). Combined, these studies demonstrated that phage display technology can be an effective methodology for selecting antibodies with specificity against non-immunogenic components of bee venom (e.g., melittin). Such antibodies could not have easily been generated by more traditional antibody discovery approaches relaying on animal immunization. Potentially, such monoclonal antibodies against key toxins from bee venom could be formulated into a recombinant antivenom for treating severe bee envenoming. Although the precise timing for efficacious administration of such a bee antivenom cannot be predicted due to limited knowledge on the toxicokinetics of bee venom components in human subjects, it is likely that antivenom administration should occur within 24 h, since kinetic studies in mice have demonstrated that bee venom can be detected in different organs, such as the kidneys, during this period (192).
As effective therapeutic intervention is essential for the most severe cases of massive bee envenoming, and as scientific prior art demonstrates the applicability of different biotechnological techniques and antibody discovery methodologies in this field, it is likely that significant advances within the development of recombinant antivenoms against bee envenoming will occur in the next few decades. It seems eminent that antivenom design and development approaches from the neighboring field of snakebite envenoming may be adopted in the development of next-generation bee envenoming therapies. Particularly, the investigation of the utility of different monoclonal antibody formats (including nanobodies) and possibly non-antibody-based binding proteins (such as DARPins and other emerging scaffold proteins) is warranted, as this may enable the design of recombinant antivenom products with beneficial pharmacokinetic properties, such as rapid distribution and the ability to penetrate and target toxins deep within tissues (182, 193, 194). Such investigations may also prompt the exploration of low-cost manufacturing strategies for oligoclonal antibodies (189) or formulation strategies for improved stability and extended shelf-life. Also, the use of low-cost small molecule inhibitors may be an area relevant for further research. Finally, it is even possible that efforts within the development of improved bee envenoming therapeutics may encourage research and development in the field of bee envenoming diagnostics, which may aid stratification of patients and clinical decision making.
Final Remarks
Africanized bee attacks are considered a public health concern in Brazil, where they originated from. Other American countries have also noticed the effects of this serious threat, as these bee hybrids are currently spreading across the Americas. As a consequence, bee stings and envenomings will likely increase. The solution to this severe problem requires well-prepared medical emergency services and specific treatments against bee envenoming, such as antivenoms. To this date, only a few reports demonstrating positive results using animal immunization exist in the scientific literature, and no antivenom for treating severe bee envenomings is so far available to treating physicians. A possible explanation for the lack of commercial bee antivenoms is the difficulty of obtaining specific antibodies against key components of the bee venom, as these have low immunogenicity. Traditional methods based on successive animal immunizations therefore fail to generate high enough antibody titres for therapeutic utility. In contrast, phage display technology has proven to be a promising methodology for generating antibodies against key bee toxins with low immunogenicity. This technology may thus enable the development of effective recombinant bee antivenoms in the future (186–188). However, this technology is still a quite recent addition to the field of antivenom development, and many efforts are still needed before an effective antivenom for the treatment of severe bee envenomings will see the light of day.
Author Contributions
FC, IO, TJ, LA, CS, and SA wrote part of the review and provided critical feedback. FC and TJ prepared figures. MP and AL designed the review, wrote part of the manuscript, and provided revisions. JB gave his valuable and professional suggestions. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
JB (1949–2019) passed away during the final stages of the preparation of this article. This work is dedicated to his memory in gratitude for all his discoveries within bee antivenom and for his mentorship. We thank Cecilie Mullerup Kiel for proofreading the manuscript.
Footnotes
Funding. We thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, The National Council for Scientific and Technological Development, grant no. 307155/2017-0); Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, São Paulo Research Foundation, grant no. 2017/04724-4, scholarship to FC no. 2017/14035-1 and 2018/14158-9, and scholarship to ISO 2017/03580-9 and 2018/21233-7); the Villum Foundation (grant no. 00025302).
References
- 1.Engel MS. A new interpretation of the oldest fossil bee (Hymenoptera: Apidae). Novi. (2000) 2000:1–11. [DOI] [Google Scholar]
- 2.Mizrahi A, Lensky Y. Bee Products: Properties, Applications, and Apitherapy. Tel Aviv: Springer Science & Business Media; (2013). [Google Scholar]
- 3.Hoover SE, Ovinge LP. Pollen collection, honey production, and pollination services: managing honey bees in an agricultural setting. J Econ Entomol. (2018) 111:1509–16. 10.1093/jee/toy125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, Guan S-M, Sun W, Fu H. Melittin, the major pain-producing substance of bee venom. Neurosci Bull. (2016) 32:265–72. 10.1007/s12264-016-0024-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schumacher MJ, Egen NB. Significance of Africanized bees for public health: a review. Arch Intern Med. (1995) 155:2038–43. 10.1001/archinte.155.19.2038 [DOI] [PubMed] [Google Scholar]
- 6.King TP, Coscia MR, Kochoumian L. Structure-immunogenicity relationship of melittin and its N-terminal truncated analogs. Biochemistry. (1993) 32:3506–10. 10.1021/bi00064a039 [DOI] [PubMed] [Google Scholar]
- 7.Gallai N, Salles J-M, Settele J, Vaissière BE. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol Econ. (2009) 68:810–21. 10.1016/j.ecolecon.2008.06.014 [DOI] [Google Scholar]
- 8.Schmidt JO. Clinical consequences of toxic envenomations by Hymenoptera. Toxicon. (2018) 150:96–104. 10.1016/j.toxicon.2018.05.013 [DOI] [PubMed] [Google Scholar]
- 9.Crane E. The World History of Beekeeping and Honey Hunting. New York, NY: Routledge; (1999). [Google Scholar]
- 10.Bloch G, Francoy TM, Wachtel I, Panitz-Cohen N, Fuchs S, Mazar A. Industrial apiculture in the Jordan valley during Biblical times with Anatolian honeybees. Proc Natl Acad Sci USA. (2010) 107:11240–4. 10.1073/pnas.1003265107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oxley PR, Oldroyd BP. Chapter 3–the genetic architecture of honeybee breeding. Adv Insect Physiol. 39:83–118. 10.1016/B978-0-12-381387-9.00003-8 [DOI] [Google Scholar]
- 12.Harpur BA, Minaei S, Kent CF, Zayed A. Management increases genetic diversity of honey bees via admixture. Mol Ecol. (2012) 21:4414–21. 10.1111/j.1365-294X.2012.05614.x [DOI] [PubMed] [Google Scholar]
- 13.Whitfield CW, Behura SK, Berlocher SH, Clark AG, Johnston JS, Sheppard WS, et al. Thrice out of Africa: ancient and recent expansions of the honey bee, Apis mellifera. Science. (2006) 314:642–5. 10.1126/science.1132772 [DOI] [PubMed] [Google Scholar]
- 14.Crosby AW. Ecological Imperialism: The Biological Expansion of Europe, 900–1900. Cambridge: Cambridge University Press; (2004). [Google Scholar]
- 15.Scott Schneider S, DeGrandi-Hoffman G, Smith DR. The African honey bee: factors contributing to a successful biological invasion. Ann Rev Entomol. (2004) 49:351–76. 10.1146/annurev.ento.49.061802.123359 [DOI] [PubMed] [Google Scholar]
- 16.Ferreira RS, Jr, Almeida RAMB, Barraviera SRCS, Barraviera B. Historical perspective and human consequences of Africanized bee stings in the Americas. J Toxicol Environ Health B. (2012) 15:97–108. 10.1080/10937404.2012.645141 [DOI] [PubMed] [Google Scholar]
- 17.Kumschick S, Devenish A, Kenis M, Rabitsch W, Richardson DM, Wilson JRU. Intentionally introduced terrestrial invertebrates: patterns, risks, and options for management. Biol Invasions. (2016) 18:1077–88. 10.1007/s10530-016-1086-5 [DOI] [Google Scholar]
- 18.Kerr WE. The history of the introduction of African bees in Brazil. South African Bee J. (1967) 39:33–5. [Google Scholar]
- 19.Rinderer TE, Oldroyd BP, Sheppard WS. Africanized bees in the US. Sci Am. (1993) 269:84–90. 10.1038/scientificamerican1293-84 [DOI] [Google Scholar]
- 20.Lin W, McBroome J, Rehman M, Johnson BR. Africanized bees extend their distribution in California. PLoS ONE. (2018) 13:e0190604. 10.1371/journal.pone.0190604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rangel J, Giresi M, Pinto MA, Baum KA, Rubink WL, Coulson RN, et al. Africanization of a feral honey bee (Apis mellifera) population in South Texas: does a decade make a difference? Ecol Evol. (2016) 6:2158–69. 10.1002/ece3.1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Portman ZM, Tepedino VJ, Tripodi AD, Szalanski AL, Durham SL. Local extinction of a rare plant pollinator in Southern Utah (USA) associated with invasion by Africanized honey bees. Biol Invasions. (2018) 20:593–606. 10.1007/s10530-017-1559-1 [DOI] [Google Scholar]
- 23.Harrison JF, Fewell JH, Anderson KE, Loper GM. Environmental physiology of the invasion of the Americas by Africanized honeybees. Integr Comp Biol. (2006) 46:1110–22. 10.1093/icb/icl046 [DOI] [PubMed] [Google Scholar]
- 24.Caron DM. Africanized Honey Bees in the Americas. Medina: AI Root Co; (2001). [Google Scholar]
- 25.Schmidt JO, Hurley R. Selection of nest cavities by Africanized and European honey bees. Apidologie. (1995) 26:467–75. 10.1051/apido:19950603 [DOI] [Google Scholar]
- 26.Winston ML. The biology and management of Africanized honey bees. Ann Rev Entomol. (1992) 37:173–93. 10.1146/annurev.en.37.010192.001133 [DOI] [Google Scholar]
- 27.Chippaux J-P. Epidemiology of envenomations by terrestrial venomous animals in Brazil based on case reporting: from obvious facts to contingencies. J Venom Anim Toxins. (2015) 21:13. 10.1186/s40409-015-0011-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan HW, Kalil J. Massive bee envenomation. In: Brent J, Burkhart K, Dargan P, Hatten B, Megarbane B, Palmer R, White J, editors. Critical Care Toxicology: Diagnosis and Management of the Critically Poisoned Patient. Cham: Springer; (2017). p. 2627–36. [Google Scholar]
- 29.Gummin DD, Mowry JB, Spyker DA, Brooks DE, Osterthaler KM, Banner W. 2017 Annual report of the american association of poison control centers' national poison data system (NPDS): 35th annual report. Clin Toxicol. (2018) 56:1213–415. 10.1080/15563650.2018.1533727 [DOI] [PubMed] [Google Scholar]
- 30.CDC QuickStats: Number of deaths from hornet, wasp, and bee stings, among males and females—National vital statistics system, United States, 2000–2017. MMWR Morb Mortal Wkly Rep. (2019) 68:649 10.15585/mmwr.mm6829a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughes RL. A fatal case of acute renal failure from envenoming syndrome after massive bee attack: a case report and literature review. Am J Foren Med Pathol. (2019) 40:52–7. 10.1097/PAF.0000000000000451 [DOI] [PubMed] [Google Scholar]
- 32.Bücherl W, Buckley EE, Deulofeu V. Venomous Animals and Their Venoms. Academic Press (1968). Available online at: https://www.elsevier.com/books/venomous-animals-and-their-venoms/bucherl/978-1-4832-2949-2 (accessed April 8, 2019).
- 33.Zhao Z-L, Zhao H-P, Ma G-J, Wu C-W, Yang K, Feng X-Q. Structures, properties, and functions of the stings of honey bees and paper wasps: a comparative study. Biol Open. (2015) 4:921–8. 10.1242/bio.012195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bridges AR, Owen MD. The morphology of the honey bee (Apis mellifera L.) venom gland and reservoir. J Morphol. (1984) 181:69–86. 10.1002/jmor.1051810107 [DOI] [PubMed] [Google Scholar]
- 35.Wu J, Yan S, Zhao J, Ye Y. Barbs Facilitate the Helical Penetration of Honeybee (Apis mellifera Ligustica) Stingers. PLoS ONE. (2014) 9:e103823. 10.1371/journal.pone.0103823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hermann HR, Blum MS. Defensive mechanisms in the social hymenoptera. Soc Insects. (1981) 2:77–197. 10.1016/B978-0-12-342202-6.50009-5 [DOI] [Google Scholar]
- 37.Nouvian M, Reinhard J, Giurfa M. The defensive response of the honeybee Apis mellifera. J Exp Biol. (2016) 219:3505–17. 10.1242/jeb.143016 [DOI] [PubMed] [Google Scholar]
- 38.Schumacher MJ, Tveten MS, Egen NB. Rate and quantity of delivery of venom from honeybee stings. J Allergy Clin Immunol. (1994) 93:831–5. 10.1016/0091-6749(94)90373-5 [DOI] [PubMed] [Google Scholar]
- 39.Fitzgerald KT, Flood AA. Hymenoptera Stings. Clin Tech Small Anim Pract. (2006) 21:194–204. 10.1053/j.ctsap.2006.10.002 [DOI] [PubMed] [Google Scholar]
- 40.Schumacher MJ, Schmidt JO, Egen NB. Lethality of “killer” bee stings. Nature. (1989) 337:413. 10.1038/337413a0 [DOI] [PubMed] [Google Scholar]
- 41.Ali MAA-SM. Studies on bee venom and its medical uses. Int J Adv Res Technol. (2012) 1:69–83. [Google Scholar]
- 42.França FO, Benvenuti LA, Fan HW, Dos Santos DR, Hain SH, Picchi-Martins FR, et al. Severe and fatal mass attacks by “killer” bees (Africanized honey bees–Apis mellifera scutellata) in Brazil: clinicopathological studies with measurement of serum venom concentrations. Q J Med. (1994) 87:269–82. [PubMed] [Google Scholar]
- 43.Toledo LFM de, Moore DCBC, Caixeta DM da L, Salú MDS, Farias CVB, Azevedo ZMA de. Multiple bee stings, multiple organs involved: a case report. Rev Soc Bras Med Trop. (2018) 51:560–2. 10.1590/0037-8682-0341-2017 [DOI] [PubMed] [Google Scholar]
- 44.Abd El-Wahed AA, Khalifa SAM, Sheikh BY, Farag MA, Saeed A, Larik FA, et al. Chapter 13–bee venom composition: from Chemistry to biological activity. In Atta-ur-Rahman editor. Studies in Natural Products Chemistry. Oxford: Elsevier; (2017). p. 459–84. [Google Scholar]
- 45.Szókán G, Horváth J, Almás M, Saftics G, Palócz A. Liquid chromatographic analysis and separation of polypeptide components from honey bee venoms. J Liq Chromatogr. (1994) 17:3333–49. 10.1080/10826079408013516 [DOI] [Google Scholar]
- 46.Hossen MS, Shapla UM, Gan SH, Khalil MI. Impact of bee venom enzymes on diseases and immune responses. Molecules. (2016) 22:1–16. 10.3390/molecules22010025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Danneels EL, Van Vaerenbergh M, Debyser G, Devreese B, de Graaf DC. Honeybee venom proteome profile of queens and winter bees as determined by a mass spectrometric approach. Toxins. (2015) 7:4468–83. 10.3390/toxins7114468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li R, Zhang L, Fang Y, Han B, Lu X, Zhou T, et al. Proteome and phosphoproteome analysis of honeybee (Apis mellifera) venom collected from electrical stimulation and manual extraction of the venom gland. BMC Genom. (2013) 14:766. 10.1186/1471-2164-14-766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Resende VMF, Vasilj A, Santos KS, Palma MS, Shevchenko A. Proteome and phosphoproteome of Africanized and European honeybee venoms. Proteomics. (2013) 13:2638–48. 10.1002/pmic.201300038 [DOI] [PubMed] [Google Scholar]
- 50.Teoh ACL, Ryu K-H, Lee EG. One-step purification of melittin derived from Apis mellifera bee venom. J Microbiol Biotechnol. (2017) 27:84–91. 10.4014/jmb.1608.08042 [DOI] [PubMed] [Google Scholar]
- 51.Owen MD, Pfaff LA, Reisman RE, Wypych J. Phospholipase A2 in venom extracts from honey bees (Apis mellifera L.) of different ages. Toxicon. (1990) 28:813–20. 10.1016/S0041-0101(09)80004-4 [DOI] [PubMed] [Google Scholar]
- 52.Dotimas EM, Hamid KR, Hider RC, Ragnarsson U. Isolation and structure analysis of bee venom mast cell degranulating peptide. Biochim Biophys Acta. (1987) 911:285–93. 10.1016/0167-4838(87)90069-0 [DOI] [PubMed] [Google Scholar]
- 53.Banks BE, Dempsey CE, Pearce FL, Vernon CA, Wholley TE. New methods of isolating been venom peptides. Anal Biochem. (1981) 116:48–52. 10.1016/0003-2697(81)90320-1 [DOI] [PubMed] [Google Scholar]
- 54.Ohashi K, Sawata M, Takeuchi H, Natori S, Kubo T. Molecular cloning of cDNA and analysis of expression of the gene for alpha-glucosidase from the hypopharyngeal gland of the honeybee Apis mellifera L. Biochem Biophys Res Commun. (1996) 221:380–5. 10.1006/bbrc.1996.0604 [DOI] [PubMed] [Google Scholar]
- 55.Kubo T, Sasaki M, Nakamura J, Sasagawa H, Ohashi K, Takeuchi H, et al. Change in the expression of hypopharyngeal-gland proteins of the worker honeybees (Apis mellifera L.) with age and/or role. J Biochem. (1996) 119:291–5. 10.1093/oxfordjournals.jbchem.a021237 [DOI] [PubMed] [Google Scholar]
- 56.Hoffman DR, Weimer ET, Sakell RH, Schmidt M. Sequence and characterization of honeybee venom acid phosphatase. J Allergy Clin Immunol. (2005) 115:S107 10.1016/j.jaci.2004.12.442 [DOI] [Google Scholar]
- 57.Shkenderov S, Koburova K. Adolapin–a newly isolated analgetic and anti-inflammatory polypeptide from bee venom. Toxicon. (1982) 20:317–21. 10.1016/0041-0101(82)90234-3 [DOI] [PubMed] [Google Scholar]
- 58.Haux P, Sawerthal H, Habermann E. Sequence analysis of bee venom neurotoxin (apamine) from its tryptic and chymotryptic cleavage products. Hoppe-Seyler's Z Physiol Chem. (1967) 348:737–8. [PubMed] [Google Scholar]
- 59.Kettner A, Hughes GJ, Frutiger S, Astori M, Roggero M, Spertini F, et al. Api m 6: a new bee venom allergen. J Allergy Clin Immunol. (2001) 107:914–20. 10.1067/mai.2001.113867 [DOI] [PubMed] [Google Scholar]
- 60.Vick JA, Shipman WH, Brooks R. Beta adrenergic and anti-arrhythmic effects of cardiopep, a newly isolated substance from whole bee venom. Toxicon. (1974) 12:139–44. 10.1016/0041-0101(74)90237-2 [DOI] [PubMed] [Google Scholar]
- 61.Blank S, Seismann H, Bockisch B, Braren I, Cifuentes L, McIntyre M, et al. Identification, recombinant expression, and characterization of the 100 kDa high molecular weight Hymenoptera venom allergens Api m 5 and Ves v 3. J Immunol. (2010) 184:5403–13. 10.4049/jimmunol.0803709 [DOI] [PubMed] [Google Scholar]
- 62.Gmachl M, Kreil G. Bee venom hyaluronidase is homologous to a membrane protein of mammalian sperm. Proc Natl Acad Sci USA. (1993) 90:3569. 10.1073/pnas.90.8.3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peiren N, de Graaf DC, Brunain M, Bridts CH, Ebo DG, Stevens WJ, et al. Molecular cloning and expression of icarapin, a novel IgE-binding bee venom protein. FEBS Lett. (2006) 580:4895–9. 10.1016/j.febslet.2006.08.005 [DOI] [PubMed] [Google Scholar]
- 64.Peiren N, Vanrobaeys F, de Graaf DC, Devreese B, Van Beeumen J, Jacobs FJ. The protein composition of honeybee venom reconsidered by a proteomic approach. Biochim Biophys Acta. (2005) 1752:1–5. 10.1016/j.bbapap.2005.07.017 [DOI] [PubMed] [Google Scholar]
- 65.Schmitzová J, Klaudiny J, Albert S, Schröder W, Schreckengost W, Hanes J, et al. A family of major royal jelly proteins of the honeybee Apis mellifera L. Cell Mol Life Sci. (1998) 54:1020–30. 10.1007/s000180050229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klaudiny J. MRJP9, an ancient protein of the honeybee MRJP family with non-nutritional function. J Apicult Res. (2007) 46:99–104. 10.3896/IBRA.1.46.2.06 [DOI] [Google Scholar]
- 67.Haux P. Amino acid sequence of MCD-peptide, a specific mast cell-degranulating peptide from bee venom. Hoppe-Seyler's Z Physiol Chem. (1969) 350:536–46. 10.1515/bchm2.1969.350.1.536 [DOI] [PubMed] [Google Scholar]
- 68.Sciani JM, Marques-Porto R, Lourenço Junior A, Orsi R de O, Ferreira Junior RS, Barraviera B, et al. Identification of a novel melittin isoform from Africanized Apis mellifera venom. Peptides. (2010) 31:1473–9. 10.1016/j.peptides.2010.05.001 [DOI] [PubMed] [Google Scholar]
- 69.Tosteson MT, Levy JJ, Caporale LH, Rosenblatt M, Tosteson DC. Solid-phase synthesis of melittin: purification and functional characterization. Biochemistry. (1987) 26:6627–31. 10.1021/bi00395a010 [DOI] [PubMed] [Google Scholar]
- 70.Schröder E, Lübke K, Lehmann M, Beetz I. Haemolytic activity and action on the surface tension of aqueous solutions of synthetic melittins and their derivatives. Experientia. (1971) 27:764–5. 10.1007/BF02136851 [DOI] [PubMed] [Google Scholar]
- 71.Gauldie J, Hanson JM, Shipolini RA, Vernon CA. The structures of some peptides from bee venom. Eur J Biochem. (1978) 83:405–10. 10.1111/j.1432-1033.1978.tb12106.x [DOI] [PubMed] [Google Scholar]
- 72.Lowy PH, Sarmiento L, Mitchell HK. Polypeptides minimine and melittin from bee venom: effects on Drosophila. Arch Biochem Biophys. (1971) 145:338–43. 10.1016/0003-9861(71)90044-0 [DOI] [PubMed] [Google Scholar]
- 73.Shipolini RA, Callewaert GL, Cottrell RC, Vernon CA. The amino-acid sequence and carbohydrate content of phospholipase A2 from bee venom. Eur J Biochem. (1974) 48:465–76. 10.1111/j.1432-1033.1974.tb03787.x [DOI] [PubMed] [Google Scholar]
- 74.Kuchler K, Gmachl M, Sippl MJ, Kreil G. Analysis of the cDNA for phospholipase A2 from honeybee venom glands. The deduced amino acid sequence reveals homology to the corresponding vertebrate enzymes. Eur J Biochem. (1989) 184:249–54. 10.1111/j.1432-1033.1989.tb15014.x [DOI] [PubMed] [Google Scholar]
- 75.Doery HM, Pearson JE. Phospholipase B in snake venoms and bee venom. Biochem J. (1964) 92:599–602. 10.1042/bj0920599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meng Y, Yang XX, Sheng YX, Zhang JL, Yu DQ. A novel peptide from Apis mellifera and solid-phase synthesis of its analogue. Chin Chem Lett. (2012) 23:1161–4. 10.1016/j.cclet.2012.09.003 [DOI] [Google Scholar]
- 77.Mourelle D, Brigatte P, Bringanti LDB, De Souza BM, Arcuri HA, Gomes PC, et al. Hyperalgesic and edematogenic effects of Secapin-2, a peptide isolated from Africanized honeybee (Apis mellifera) venom. Peptides. (2014) 59:42–52. 10.1016/j.peptides.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 78.Georgieva D, Greunke K, Betzel C. Three-dimensional model of the honeybee venom allergen Api m 7: structural and functional insights. Mol BioSyst. (2010) 6:1056. 10.1039/b923127g [DOI] [PubMed] [Google Scholar]
- 79.Ovchinnikov YA, Miroshnikov AI, Kudelin AB, Kostina MB, Boikov VA, Magazanik LG, et al. Structure and presynaptic activity of tertiapin, a neurotoxin from honeybee venom. Bioorganicheskaya Khimiya. (1980) 6:359–65. [Google Scholar]
- 80.Vetter RS, Visscher PK. Bites and stings of medically important venomous arthropods. Int J Dermatol. (1998) 37:481–96. 10.1046/j.1365-4362.1998.00455.x [DOI] [PubMed] [Google Scholar]
- 81.Moreno M, Giralt E. Three valuable peptides from bee and wasp venoms for therapeutic and biotechnological use: melittin, apamin and mastoparan. Toxins. (2015) 7:1126–50. 10.3390/toxins7041126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ramalingam K, Snyder GH. Selective disulfide formation in truncated apamin and sarafotoxin. Biochemistry. (1993) 32:11155–61. 10.1021/bi00092a027 [DOI] [PubMed] [Google Scholar]
- 83.Elieh Ali Komi D, Shafaghat F, Zwiener RD. Immunology of bee venom. Clin Rev Allergy Immunol. (2018) 54:386–96. 10.1007/s12016-017-8597-4 [DOI] [PubMed] [Google Scholar]
- 84.Buku A. Mast cell degranulating (MCD) peptide: a prototypic peptide in allergy and inflammation. Peptides. (1999) 20:415–20. 10.1016/S0196-9781(98)00167-3 [DOI] [PubMed] [Google Scholar]
- 85.Raghuraman H, Chattopadhyay A. Melittin: a membrane-active peptide with diverse functions. Biosci Rep. (2007) 27:189–223. 10.1007/s10540-006-9030-z [DOI] [PubMed] [Google Scholar]
- 86.Paull BR, Yunginger JW, Gleich GJ. Melittin: an allergen of honeybee venom. J Allergy Clin Immunol. (1977) 59:334–8. 10.1016/0091-6749(77)90056-2 [DOI] [PubMed] [Google Scholar]
- 87.Ding J, Xiao Y, Lu D, Du Y-R, Cui X-Y, Chen J. Effects of SKF-96365, a TRPC inhibitor, on melittin-induced inward current and intracellular Ca2+ rise in primary sensory cells. Neurosci Bull. (2011) 27:135–42. 10.1007/s12264-011-1018-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hao J, Liu M-G, Yu Y-Q, Cao F-L, Li Z, Lu Z-M, et al. Roles of peripheral mitogen-activated protein kinases in melittin-induced nociception and hyperalgesia. Neuroscience. (2008) 152:1067–75. 10.1016/j.neuroscience.2007.12.038 [DOI] [PubMed] [Google Scholar]
- 89.Ding J, Zhang J-R, Wang Y, Li C-L, Lu D, Guan S-M, et al. Effects of a non-selective TRPC channel blocker, SKF-96365, on melittininduced spontaneous persistent nociception and inflammatory pain hypersensitivity. Neurosci Bull. (2012) 28:173–81. 10.1007/s12264-012-1213-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van den Bogaart G, Guzmán JV, Mika JT, Poolman B. On the mechanism of pore formation by melittin. J Biol Chem. (2008) 283:33854–7. 10.1074/jbc.M805171200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Memariani H, Memariani M, Shahidi-Dadras M, Nasiri S, Akhavan MM, Moravvej H. Melittin: from honeybees to superbugs. Appl Microbiol Biotechnol. (2019) 103:3265–76. 10.1007/s00253-019-09698-y [DOI] [PubMed] [Google Scholar]
- 92.Mollay C, Kreil G. Enhancement of bee venom phospholipase A2 activity by melittin, direct lytic factor from cobra venom and polymyxin B. FEBS Lett. (1974) 46:141–4. 10.1016/0014-5793(74)80354-6 [DOI] [PubMed] [Google Scholar]
- 93.Moon D-O, Park S-Y, Lee K-J, Heo M-S, Kim K-C, Kim M-O, et al. Bee venom and melittin reduce proinflammatory mediators in lipopolysaccharide-stimulated BV2 microglia. Int Immunopharmacol. (2007) 7:1092–101. 10.1016/j.intimp.2007.04.005 [DOI] [PubMed] [Google Scholar]
- 94.Baghian A, Jaynes J, Enright F, Kousoulas KG. An amphipathic alpha-helical synthetic peptide analogue of melittin inhibits herpes simplex virus-1. (HSV-1)-induced cell fusion and virus spread. Peptides. (1997) 18:177–83. 10.1016/S0196-9781(96)00290-2 [DOI] [PubMed] [Google Scholar]
- 95.Oršolić N. Bee venom in cancer therapy. Cancer Metastasis Rev. (2012) 31:173–94. 10.1007/s10555-011-9339-3 [DOI] [PubMed] [Google Scholar]
- 96.Juvvadi P, Vunnam S, Merrifield RB. Synthetic melittin, its enantio, retro, and retroenantio isomers, and selected chimeric analogs: their antibacterial, hemolytic, and lipid bilayer action. J Am Chem Soc. (1996) 118:8989–97. 10.1021/ja9542911 [DOI] [Google Scholar]
- 97.Sun X, Chen S, Li S, Yan H, Fan Y, Mi H. Deletion of two C-terminal Gln residues of 12-26-residue fragment of melittin improves its antimicrobial activity. Peptides. (2005) 26:369–75. 10.1016/j.peptides.2004.10.004 [DOI] [PubMed] [Google Scholar]
- 98.Mingarro I, Pérez-Payá E, Pinilla C, Appel JR, Houghten RA, Blondelle SE. Activation of bee venom phospholipase A2 through a peptide-enzyme complex. FEBS Lett. (1995) 372:131–4. 10.1016/0014-5793(95)00964-B [DOI] [PubMed] [Google Scholar]
- 99.Boutrin M-CF, Foster HA, Pentreath VW. The effects of bee (Apis mellifera) venom phospholipase A2 on Trypanosoma brucei brucei and enterobacteria. Exp Parasitol. (2008) 119:246–51. 10.1016/j.exppara.2008.02.002 [DOI] [PubMed] [Google Scholar]
- 100.Leandro LF, Mendes CA, Casemiro LA, Vinholis AHC, Cunha WR, de Almeida R, et al. Antimicrobial activity of apitoxin, melittin and phospholipase A2 of honey bee (Apis mellifera) venom against oral pathogens. An Acad Bras Cienc. (2015) 87:147–55. 10.1590/0001-3765201520130511 [DOI] [PubMed] [Google Scholar]
- 101.Jeong J-K, Moon M-H, Bae B-C, Lee Y-J, Seol J-W, Park S-Y. Bee venom phospholipase A2 prevents prion peptide induced-cell death in neuronal cells. Int J Mol Med. (2011) 28:867–73. 10.3892/ijmm.2011.730 [DOI] [PubMed] [Google Scholar]
- 102.Son DJ, Lee JW, Lee YH, Song HS, Lee CK, Hong JT. Therapeutic application of anti-arthritis, pain-releasing, and anti-cancer effects of bee venom and its constituent compounds. Pharmacol Ther. (2007) 115:246–70. 10.1016/j.pharmthera.2007.04.004 [DOI] [PubMed] [Google Scholar]
- 103.Kim H, Keum DJ, Kwak J won, Chung H-S, Bae H. Bee venom phospholipase A2 protects against acetaminophen-induced acute liver injury by modulating regulatory T cells and IL-10 in mice. PLoS ONE. (2014) 9:e114726. 10.1371/journal.pone.0114726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abusabbah M, Lau WH, Mahmoud ME, Salih AM, Omar D. Prospects of using carbohydrates as supplemented-diets and protein rich mixture as alternative-diet to improve the quality of venom produced by Apis cerana L. J Entomol Zool Stud. (2016) 4:23–6. [Google Scholar]
- 105.Oliveira IS de, Cardoso IA, Bordon K de CF, Carone SEI, Boldrini-França J, Pucca MB, et al. Global proteomic and functional analysis of Crotalus durissus collilineatus individual venom variation and its impact on envenoming. J Proteomics. (2019) 191:153–65. 10.1016/j.jprot.2018.02.020 [DOI] [PubMed] [Google Scholar]
- 106.Wiezel GA, Shibao PYT, Cologna CT, Morandi Filho R, Ueira-Vieira C, De Pauw E, et al. In-depth venome of the Brazilian rattlesnake Crotalus durissus terrificus: an integrative approach combining its venom gland transcriptome and venom proteome. J Proteome Res. (2018) 17:3941–58. 10.1021/acs.jproteome.8b00610 [DOI] [PubMed] [Google Scholar]
- 107.Lamy C, Goodchild SJ, Weatherall KL, Jane DE, Liégeois J-F, Seutin V, et al. Allosteric block of KCa2 channels by apamin. J Biol Chem. (2010) 285:27067–77. 10.1074/jbc.M110.110072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Adelman JP, Maylie J, Sah P. Small-conductance Ca2+-activated K+ channels: form and function. Annu Rev Physiol. (2012) 74:245–69. 10.1146/annurev-physiol-020911-153336 [DOI] [PubMed] [Google Scholar]
- 109.Liegeois J, Mercier F, Graulich A, Graulich-Lorge F, Scuvee-Moreau J, Seutin V. Modulation of small conductance calcium-activated potassium (SK) channels: a new challenge in medicinal chemistry. Curr Med Chem. (2003) 10:625–47. 10.2174/0929867033457908 [DOI] [PubMed] [Google Scholar]
- 110.de Matos Silva LFC, de Paula Ramos ER, Ambiel CR, Correia-de-SáP, Alves-Do-Prado W. Apamin reduces neuromuscular transmission by activating inhibitory muscarinic M(2) receptors on motor nerve terminals. Eur J Pharmacol. (2010) 626:239–43. 10.1016/j.ejphar.2009.09.064 [DOI] [PubMed] [Google Scholar]
- 111.Alvarez-Fischer D, Noelker C, Vulinović F, Grünewald A, Chevarin C, Klein C, et al. Bee venom and its component apamin as neuroprotective agents in a Parkinson disease mouse model. PLoS ONE. (2013) 8:e61700. 10.1371/journal.pone.0061700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Messier C, Mourre C, Bontempi B, Sif J, Lazdunski M, Destrade C. Effect of apamin, a toxin that inhibits Ca(2+)-dependent K+ channels, on learning and memory processes. Brain Res. (1991) 551:322–6. 10.1016/0006-8993(91)90950-Z [DOI] [PubMed] [Google Scholar]
- 113.Chase TN, Oh-Lee JD. Composition for Treating Parkinson's Disease (2013). Available online at: https://patents.google.com/patent/WO2013083574A1/en?oq=Thomas%2c+N.C.;+Justin%2c+D.O.L.+Composition+for+Treatins+Parkinsin%E2%80%99s+Disease.+WO2013083574+A1%2c+13+June+2013+ (accessed April 14, 2019).
- 114.Fox JW. A brief review of the scientific history of several lesser-known snake venom proteins: l-amino acid oxidases, hyaluronidases and phosphodiesterases. Toxicon. (2013) 62:75–82. 10.1016/j.toxicon.2012.09.009 [DOI] [PubMed] [Google Scholar]
- 115.Boldrini-França J, Cologna CT, Pucca MB, Bordon K de CF, Amorim FG, Anjolette FAP, et al. Minor snake venom proteins: Structure, function and potential applications. Biochim Biophys Acta Gen Subj. (2017) 1861:824–38. 10.1016/j.bbagen.2016.12.022 [DOI] [PubMed] [Google Scholar]
- 116.Bordon KCF, Perino MG, Giglio JR, Arantes EC. Isolation, enzymatic characterization and antiedematogenic activity of the first reported rattlesnake hyaluronidase from Crotalus durissus terrificus venom. Biochimie. (2012) 94:2740–8. 10.1016/j.biochi.2012.08.014 [DOI] [PubMed] [Google Scholar]
- 117.Horta CCR, Magalhães B de F, Oliveira-Mendes BBR, do Carmo AO, Duarte CG, Felicori LF, et al. Molecular, immunological, and biological characterization of Tityus serrulatus venom hyaluronidase: new insights into its role in envenomation. PLoS Negl Trop Dis. (2014) 8:e2693. 10.1371/journal.pntd.0002693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kreil G. Hyaluronidases–a group of neglected enzymes. Protein Sci. (1995) 4:1666–9. 10.1002/pro.5560040902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Marković-Housley Z, Miglierini G, Soldatova L, Rizkallah PJ, Müller U, Schirmer T. Crystal structure of hyaluronidase, a major allergen of bee venom. Structure. (2000) 8:1025–35. 10.1016/S0969-2126(00)00511-6 [DOI] [PubMed] [Google Scholar]
- 120.Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci. (2007) 80:1921–43. 10.1016/j.lfs.2007.02.037 [DOI] [PubMed] [Google Scholar]
- 121.Clinton PM, Kemeny DM, Youlten LJ, Lessof MH. Histamine release from peripheral blood leukocytes with purified bee venom allergens: effect of hyperimmune beekeeper plasma. Int Arch Allergy Appl Immunol. (1989) 89:43–8. 10.1159/000234921 [DOI] [PubMed] [Google Scholar]
- 122.Habermann E. Bee and wasp venoms. Science. (1972) 177:314–22. 10.1126/science.177.4046.314 [DOI] [PubMed] [Google Scholar]
- 123.Birr C, Wengert-Müller M. Molecular perspectives of synthetic mast cell-degranulating peptide. In: Eberle AN, Wieland T, Geiger R, editors. Perspectives in Peptide Chemistry. Basel: Karger; (1981). p. 372–80. [Google Scholar]
- 124.Banks BE, Dempsey CE, Vernon CA, Warner JA, Yamey J. Anti-inflammatory activity of bee venom peptide 401 (mast cell degranulating peptide) and compound 48/80 results from mast cell degranulation in vivo. Br J Pharmacol. (1990) 99:350–4. 10.1111/j.1476-5381.1990.tb14707.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ziai MR, Russek S, Wang H-C, Beer B, Blume AJ. Mast cell degranulating peptide: a multi-functional neurotoxin. J Pharm Pharmacol. (1990) 42:457–61. 10.1111/j.2042-7158.1990.tb06595.x [DOI] [PubMed] [Google Scholar]
- 126.Jin A-H, Dutertre S, Dutt M, Lavergne V, Jones A, Lewis RJ, et al. Transcriptomic-proteomic correlation in the predation-evoked venom of the cone snail, Conus imperialis. Mar Drugs. (2019) 17:177. 10.3390/md17030177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pucca MB, Amorim FG, Cerni FA, Bordon K de CF, Cardoso IA, Anjolette FAP, et al. Influence of post-starvation extraction time and prey-specific diet in Tityus serrulatus scorpion venom composition and hyaluronidase activity. Toxicon. (2014) 90:326–6. 10.1016/j.toxicon.2014.08.064 [DOI] [PubMed] [Google Scholar]
- 128.Baracchi D, Turillazzi S. Differences in venom and cuticular peptides in individuals of Apis mellifera (Hymenoptera: Apidae) determined by MALDI-TOF MS. J Insect Physiol. (2010) 56:366–75. 10.1016/j.jinsphys.2009.11.013 [DOI] [PubMed] [Google Scholar]
- 129.Owen MD, Braidwood JL, Bridges AR. Age dependent changes in histamine content of venom of queen and worker honey bees. J Insect Physiol. (1977) 23:1031–5. 10.1016/0022-1910(77)90131-7 [DOI] [Google Scholar]
- 130.Bachmayer H, Kreil G, Suchanek G. Synthesis of promelittin and melittin in the venom gland of queen and worker bees: patterns observed during maturation. J Insect Physiol. (1972) 18:1515–21. 10.1016/0022-1910(72)90230-2 [DOI] [Google Scholar]
- 131.Owen MD. Relationship between age and hyaluronidase activity in the venom of queen and worker honey bees (Apis mellifera L.). Toxicon. (1979) 17:94–8. 10.1016/0041-0101(79)90260-5 [DOI] [PubMed] [Google Scholar]
- 132.Schumacher MJ, Schmidt JO, Egen NB, Dillon KA. Biochemical variability of venoms from individual European and Africanized honeybees (Apis mellifera). J Allergy Clin Immunol. (1992) 90:59–65. 10.1016/S0091-6749(06)80011-4 [DOI] [PubMed] [Google Scholar]
- 133.Owen MD. The venom system and venom hyaluronidase of the African honeybee (Apis mellifera Adansonii). Toxicon. (1983) 21:171–4. 10.1016/0041-0101(83)90061-2 [DOI] [PubMed] [Google Scholar]
- 134.Funari SRC, Zeidler PR, Rocha HC, Sforcin JM. Venom production by Africanized honeybees (Apis mellifera) and Africanized-European hybrids. J Venom Anim Toxins. (2001) 7:190–8. 10.1590/S0104-79302001000200005 [DOI] [Google Scholar]
- 135.Hoffman DR, Jacobson RS. Allergens in hymenoptera venom XII: how much protein is in a sting? Ann Allergy. (1984) 52:276–8. [PubMed] [Google Scholar]
- 136.Owen MD, Pfaff LA. Melittin synthesis in the venom system of the honey bee (Apis mellifera L.). Toxicon. (1995) 33:1181–8. 10.1016/0041-0101(95)00054-P [DOI] [PubMed] [Google Scholar]
- 137.Ferreira Junior RS, Sciani JM, Marques-Porto R, Junior AL, Orsi R de O, Barraviera B, et al. Africanized honey bee (Apis mellifera) venom profiling: seasonal variation of melittin and phospholipase A(2) levels. Toxicon. (2010) 56:355–62. 10.1016/j.toxicon.2010.03.023 [DOI] [PubMed] [Google Scholar]
- 138.Ediger D, Terzioglu K, Ozturk RT. Venom allergy, risk factors for systemic reactions and the knowledge levels among Turkish beekeepers. Asia Pac. Allergy. (2018) 8:e15. 10.5415/apallergy.2018.8.e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Almeida RAM de B, Olivo TET, Mendes RP, Barraviera SRCS, Souza L do R, Martins JG, et al. Africanized honeybee stings: how to treat them. Rev Soc Bras Med Trop. (2011) 44:755–61. 10.1590/S0037-86822011000600020 [DOI] [PubMed] [Google Scholar]
- 140.Hymenoptera Allergy Committee of the SEAIC. Alfaya Arias T, Soriano Gómis V, Soto Mera T, Vega Castro A, Vega Gutiérrez J, et al. Key issues in hymenoptera venom allergy: an update. J Invest Allergol Clin Immunol. (2017) 27:19–31. 10.18176/jiaci.0123 [DOI] [PubMed] [Google Scholar]
- 141.Jimenez-Rodriguez TW, Garcia-Neuer M, Alenazy LA, Castells M. Anaphylaxis in the 21st century: phenotypes, endotypes, and biomarkers. J Asthma Allergy. (2018) 11:121–42. 10.2147/JAA.S159411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Castells MC, Hornick JL, Akin C. Anaphylaxis after hymenoptera sting: is it venom allergy, a clonal disorder, or both? J Allergy Clin Immunol Pract. (2015) 3:350–5. 10.1016/j.jaip.2015.03.015 [DOI] [PubMed] [Google Scholar]
- 143.Greenhawt M, Akin C. Mastocytosis and allergy. Curr Opin Allergy Clin Immunol. (2007) 7:387–92. 10.1097/ACI.0b013e3282a6443e [DOI] [PubMed] [Google Scholar]
- 144.Bonadonna P, Zanotti R, Müller U. Mastocytosis and insect venom allergy. Curr Opin Allergy Clin Immunol. (2010) 10:347–53. 10.1097/ACI.0b013e32833b280c [DOI] [PubMed] [Google Scholar]
- 145.Piao X, Bernstein A. A point mutation in the catalytic domain of c-kit induces growth factor independence, tumorigenicity, and differentiation of mast cells. Blood. (1996) 87:3117–23. [PubMed] [Google Scholar]
- 146.Dart RC. Medical Toxicology. Philadelphia, PA: Lippincott Williams and Wilkins; (2004). [Google Scholar]
- 147.Nisahan B, Selvaratnam G, Kumanan T. Myocardial injury following multiple bee stings. Trop Doct. (2014) 44:233–4. 10.1177/0049475514525606 [DOI] [PubMed] [Google Scholar]
- 148.James ES, Walker WG. ACTH in wasp stings. Med Assoc J. (1952) 67:50–1. [PMC free article] [PubMed] [Google Scholar]
- 149.Ross AT. Peripheral neuritis: allergy to honeybee stings. J Allergy. (1939) 10:382–4. 10.1016/S0021-8707(39)90154-1 [DOI] [Google Scholar]
- 150.Marks HG, Augustyn P, Allen RJ. Fisher's syndrome in children. Pediatrics. (1977) 60:726–9. [PubMed] [Google Scholar]
- 151.Bachman DS, Paulson GW, Mendell JR. Acute inflammatory polyradiculoneuropathy following hymenoptera stings. JAMA. (1982) 247:1443–5. 10.1001/jama.247.10.1443 [DOI] [PubMed] [Google Scholar]
- 152.Maltzman JS, Lee AG, Miller NR. Optic neuropathy occurring after bee and wasp sting. Ophthalmology. (2000) 107:193–5. 10.1016/S0161-6420(99)00020-2 [DOI] [PubMed] [Google Scholar]
- 153.Truskinovsky AM, Dick JD, Hutchins GM. Fatal infection after a bee sting. Clin Infect Dis. (2001) 32:E36–8. 10.1086/318451 [DOI] [PubMed] [Google Scholar]
- 154.Venkataramanappa SK, Gowda A, Raju S, Harihar V. An unusual case of bilateral empyema associated with bee sting. Case Rep Med. (2014) 2014:985720. 10.1155/2014/985720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kaya A, Okur M. Bee sting in mother and urticarial rash in her baby. Indian Pediatr. (2012) 49:499. [DOI] [PubMed] [Google Scholar]
- 156.Przybilla B, Ruëff F. Insect Stings. Minich: Deutsches Aerzteblatt; (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gordon RM. Reactions produced by arthropods. BMJ. (1950) 2:316–8. 10.1136/bmj.2.4674.316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kragballe K. Topical corticosteroids: mechanisms of action. Acta Derm Venereol Suppl. (1989) 151:7–10. [PubMed] [Google Scholar]
- 159.Sherman RA. What physicians should know about Africanized honeybees. West J Med. (1995) 163:541–6. [PMC free article] [PubMed] [Google Scholar]
- 160.da Silva GB, Vasconcelos AG, Rocha AMT, de Vasconcelos VR, de Barros J, Fujishima JS, et al. Acute kidney injury complicating bee stings–a review. Rev Inst Med Trop São Paulo. (2017) 59:e25 10.1590/s1678-9946201759025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Muraro A, Roberts G, Worm M, Bilò MB, Brockow K, Rivas MF, et al. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. (2014) 69:1026–45. 10.1111/all.12437 [DOI] [PubMed] [Google Scholar]
- 162.Simons FER, Ebisawa M, Sanchez-Borges M, Thong BY, Worm M, Tanno LK, et al. 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Organ J. (2015) 8:32. 10.1186/s40413-015-0080-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dudley LS, Mansour MI, Merlin MA. Epinephrine for anaphylaxis: underutilized and unavailable. West J Emerg Med. (2015) 16:385–7. 10.5811/westjem.2015.3.25337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Campbell RL, Manivannan V, Hartz MF, Sadosty AT. Epinephrine auto-injector pandemic. Pediatr Emerg Care. (2012) 28:938–42. 10.1097/PEC.0b013e318267f689 [DOI] [PubMed] [Google Scholar]
- 165.Brown AF. Therapeutic controversies in the management of acute anaphylaxis. Emerg Med J. (1998) 15:89–95. 10.1136/emj.15.2.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lieberman P, Nicklas RA, Randolph C, Oppenheimer J, Bernstein D, Bernstein J, et al. Anaphylaxis—a practice parameter update 2015. Annal Allergy Asthma Immunol. (2015) 115:341–84. 10.1016/j.anai.2015.07.019 [DOI] [PubMed] [Google Scholar]
- 167.Simons FER, Ardusso LRF, Bilò MB, El-Gamal YM, Ledford DK, Ring J, et al. World Allergy Organization anaphylaxis guidelines: summary. J Allergy Clin Immunol. (2011) 127:587–93.e22. 10.1016/j.jaci.2011.01.038 [DOI] [PubMed] [Google Scholar]
- 168.Incorvaia C, Frati F, Dell'Albani I, Robino A, Cattaneo E, Mauro M, et al. Safety of hymenoptera venom immunotherapy: a systematic review. Expert Opin Pharmacother. (2011) 12:2527–32. 10.1517/14656566.2011.616494 [DOI] [PubMed] [Google Scholar]
- 169.Sturm GJ, Varga E-M, Roberts G, Mosbech H, Bilò MB, Akdis CA, et al. EAACI guidelines on allergen immunotherapy: hymenoptera venom allergy. Allergy. (2018) 73:744–64. 10.1111/all.13262 [DOI] [PubMed] [Google Scholar]
- 170.Głobińska A, Boonpiyathad T, Satitsuksanoa P, Kleuskens M, van de Veen W, Sokolowska M, et al. Mechanisms of allergen-specific immunotherapy: diverse mechanisms of immune tolerance to allergens. Ann Allergy Asthma Immunol. (2018) 121:306–12. 10.1016/j.anai.2018.06.026 [DOI] [PubMed] [Google Scholar]
- 171.Boyle RJ, Elremeli M, Hockenhull J, Cherry MG, Bulsara MK, Daniels M, et al. Venom immunotherapy for preventing allergic reactions to insect stings. Coch Datab Syst Rev. (2012) 10:CD008838. 10.1002/14651858.CD008838.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mitchell A. Africanized killer bees A case study. Crit Care Nurse. (2006) 26:23–31. [PubMed] [Google Scholar]
- 173.Tunget CL, Clark RF. Invasion of the “killer” bees. Separating fact from fiction. Postgrad Med. (1993) 94:92–4. 10.1080/00325481.1993.11945694 [DOI] [PubMed] [Google Scholar]
- 174.Bresolin NL, Carvalho FC, Goes JC, Fernandes V, Barotto AM. Acute renal failure following massive attack by Africanized bee stings. Pediatr Nephrol. (2002) 17:625–7. 10.1007/s00467-002-0888-0 [DOI] [PubMed] [Google Scholar]
- 175.Abbas AK, Lichtman AH, Pillai S. Basic Immunology: Functions and Disorders of the Immune System. 5th ed. St. Louis, MO: Elsevier; (2016). [Google Scholar]
- 176.Schumacher MJ, Egen NB, Tanner D. Neutralization of bee venom lethality by immune serum antibodies. Am J Trop Med Hyg. (1996) 55:197–201. 10.4269/ajtmh.1996.55.197 [DOI] [PubMed] [Google Scholar]
- 177.King TP, Sobotka AK, Kochoumian L, Lichtenstein LM. Allergens of honey bee venom. Arch Biochem Biophys. (1976) 172:661–71. 10.1016/0003-9861(76)90121-1 [DOI] [PubMed] [Google Scholar]
- 178.Terra RMS. Análise Conformacional da Melitina por Dinâmica Molecular e Caracterização dos Efeitos do Peptídeo na Função Plaquetária. Universidade do Rio Grande do Sul, Porto Alegre, Brazil (2006). Available online at: https://lume.ufrgs.br/handle/10183/10943
- 179.Jones RG, Bhogal G, Corteling RL, Landon J. A novel Fab-based antivenom for the treatment of mass bee attacks. Am J Trop Med Hyg. (1999) 61:361–6. 10.4269/ajtmh.1999.61.361 [DOI] [PubMed] [Google Scholar]
- 180.Santos KS, Stephano MA, Marcelino JR, Ferreira VMR, Rocha T, Caricati C, et al. Production of the first effective hyperimmune equine serum antivenom against Africanized bees. PLoS ONE. (2013) 8:e79971. 10.1371/journal.pone.0079971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Barbosa AN, Boyer L, Chippaux J-P, Medolago NB, Caramori CA, Paixão AG, et al. A clinical trial protocol to treat massive Africanized honeybee (Apis mellifera) attack with a new apilic antivenom. J Venom Anim Toxins Incl Trop Dis. (2017) 23:14. 10.1186/s40409-017-0106-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Laustsen AH, Gutiérrez JM, Knudsen C, Johansen KH, Bermúdez-Méndez E, Cerni FA, et al. Pros and cons of different therapeutic antibody formats for recombinant antivenom development. Toxicon. (2018) 146:151–75. 10.1016/j.toxicon.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 183.Pucca MB, Cerni FA, Janke R, Bermúdez-Méndez E, Ledsgaard L, Barbosa JE, et al. History of envenoming therapy and current perspectives. Front Immunol. (2019) 10:1598. 10.3389/fimmu.2019.01598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.LoVecchio F, Klemens J, Roundy EB, Klemens A. Serum sickness following administration of Antivenin (Crotalidae) Polyvalent in 181 cases of presumed rattlesnake envenomation. Wilderness Environ Med. (2003) 14:220–1. 10.1580/1080-6032(2003)14[220:SSFAOA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 185.León G, Herrera M, Segura Á, Villalta M, Vargas M, Gutiérrez JM. Pathogenic mechanisms underlying adverse reactions induced by intravenous administration of snake antivenoms. Toxicon. (2013) 76:63–76. 10.1016/j.toxicon.2013.09.010 [DOI] [PubMed] [Google Scholar]
- 186.Laustsen AH, Karatt-Vellatt A, Masters EW, Arias AS, Pus U, Knudsen C, et al. In vivo neutralization of dendrotoxin-mediated neurotoxicity of black mamba venom by oligoclonal human IgG antibodies. Nat Commun. (2018) 9:3928 10.1038/s41467-018-06086-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pucca MB, Cerni FA, Peigneur S, Arantes EC, Tytgat J, Barbosa JE. Serrumab: a novel human single chain-fragment antibody with multiple scorpion toxin-neutralizing capacities. J Immunotoxicol. (2014) 11:133–40. 10.3109/1547691X.2013.809175 [DOI] [PubMed] [Google Scholar]
- 188.Silva LC, Pucca MB, Pessenda G, Campos LB, Martinez EZ, Cerni FA, et al. Discovery of human scFvs that cross-neutralize the toxic effects of B. jararacussu and C. d. terrificus venoms. Acta Tropica. (2018) 177:66–73. 10.1016/j.actatropica.2017.09.001 [DOI] [PubMed] [Google Scholar]
- 189.Laustsen AH, Johansen KH, Engmark M, Andersen MR. Recombinant snakebite antivenoms: a cost-competitive solution to a neglected tropical disease? PLoS Negl Trop Dis. (2017) 11:e0005361. 10.1371/journal.pntd.0005361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Funayama JC, Pucca MB, Roncolato EC, Bertolini TB, Campos LB, Barbosa JE. Production of human antibody fragments binding to melittin and phospholipase A2 in Africanised bee venom: minimising venom toxicity. Basic Clin Pharmacol Toxicol. (2012) 110:290–7. 10.1111/j.1742-7843.2011.00821.x [DOI] [PubMed] [Google Scholar]
- 191.Pessenda G, Silva LC, Campos LB, Pacello EM, Pucca MB, Martinez EZ, et al. Human scFv antibodies (Afribumabs) against Africanized bee venom: advances in melittin recognition. Toxicon. (2016) 112:59–67. 10.1016/j.toxicon.2016.01.062 [DOI] [PubMed] [Google Scholar]
- 192.Yonamine CM, Costa H, Silva J a. A,, Muramoto E, Rogero JR, Troncone LRP. Biodistribution studies of bee venom and spider toxin using radiotracers. J Venom Anim Toxins Incl Trop Dis. (2005) 11:39–50. 10.1590/S1678-91992005000100006 [DOI] [Google Scholar]
- 193.Jenkins T, Fryer T, Dehli R, Jürgensen J, Fuglsang-Madsen A, Føns S, et al. Toxin neutralization using alternative binding proteins. Toxins. (2019) 11:53. 10.3390/toxins11010053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Laustsen AH. Guiding recombinant antivenom development by omics technologies. N Biotechnol. (2018) 45:19–27. 10.1016/j.nbt.2017.05.005 [DOI] [PubMed] [Google Scholar]