Abstract
Background:
Recent data suggests association of lichen planus (LP) with various systemic disorders. Relationship between LP and metabolic syndrome (MS) is not yet taken into account. MS has been associated with increased risk of cardiovascular diseases. Hence, earlier detection and treatment could potentially decrease mortality and improve the quality of life in these patients.
Objectives:
To find out the association of LP with MS.
Materials and Methods:
About 100 LP patients and 50 healthy adults were investigated for fasting blood glucose (FBS) and lipid profile. MS was diagnosed as per National Cholesterol Education Program's Adult Treatment Panel III guidelines.
Results:
Serum cholesterol, triglycerides, low density lipoprotein (LDL-C), and very low density lipoprotein (VLDL-C) values were significantly increased in cases as compared to controls (P < 0.05 in all). About 42% of patients showed raised FBS level as compared to 10% controls (P = 0.0003). MS was more prevalent in cases than in controls (43% versus 26% respectively, P = 0.045). Odds ratio was highest in FBS and waist circumference.
Limitations:
As the cases and controls are included from a local area, the result may differ from other parts of the world.
Conclusion:
Diabetes mellitus, dyslipidemia, and MS are seen more commonly in LP patients.
Keywords: Correlation, lichen planus, metabolic syndrome
Introduction
Lichen planus (LP) is a chronic inflammatory disease that involves the skin and oral mucosa affecting 0.22 to 4% of the general population.[1,2] Recent data throws some light on its association with various systemic and metabolic disorders and shows that LP is not as superficial as it was considered. This is of particular interest as they represent typical problems encountered by dermatologists in their daily ambulatory practices.[3]
There is a growing evidence that chronic dermatological conditions with autoimmune etiology like psoriasis and vitiligo affect not only the skin but may also be associated with metabolic abnormalities[4,5] As LP is also proved to be an immunological disorder, chance of patients suffering from LP to be having systemic diseases is high.
Patients with LP are more likely to have a number of systemic comorbidities than those without LP. There have been few studies in the past, which show correlation of LP with obesity, hypertension (HTN), hyperlipidemia, hepatic disorders, and diabetes mellitus (DM).[6,7,8] Metabolic syndrome (MS) and DM have been associated with an increased risk of cardiovascular disease (CVD).[9,10] There are reports of increased risk of CVD among patients with LP.[9]
These conditions, if detected and treated early could potentially decrease the mortality and improve the quality of life in these patients.
Materials and Methods
This is a cross-sectional study comprised of 100 LP patients and 50 age- and sex-matched controls. This hospital-based descriptive study was conducted from October 2012 to June 2014 at a tertiary care hospital inMysore. The study population included LP patients above 18 years attending Out Patient Department of Dermatology, Venereology and Leprosy. Pregnant women, patients on oral steroids, retinoids, or lipid lowering drugs were excluded from the study. An informed consent was taken from all subjects. A detailed history including the occupation, duration of the disease, treatment taken, family history, drug intake, and personal history was taken before clinical examination. Study was approved by the institutional ethics committee.
Blood pressure (BP) and waist circumference were recorded for all patients. BP was recorded after subjects have been lying in supine position for 5 minutes. Waist circumference was recorded by locating the upper iliac crest and placing a measuring tape at the level of its uppermost part around the abdomen (ensuring that the tape was horizontal). Measurement was made at the end of a normal expiration. Patients were investigated for blood sugar level and lipid profile after fasting overnight (minimum 8 hours).
Comparisons between patients and controls were performed by chi square analysis for qualitative variables. Odds ratios (ORs) and confidence intervals (CIs) were calculated with exact conditional logistic regression. A value of P ≤ 0.05 was considered statistically significant.
Results
In 100 LP patients, the age ranged from 18 to 81 years, with mean age of 42.02 ± 13.82 years and 35% were in age group of 41 to 50 years. In control group, the mean age was 40.72 ± 10.83 years with range of 20 to 64 years. There were 60 males and 40 females with sex ratio of 1.5:1, comparable to controls (χ2 = 0.0559, P = 0.813). About 66% of patients had LP for less than 6 months of duration. The mean duration of LP was 10.45 months. Oral mucosa was involved in 34% patients [Figure 1]. Lower limbs (85%) were the most commonly affected area followed by upper limbs (60%) and trunk (54%) [Figures 2-4].
Figure 1.
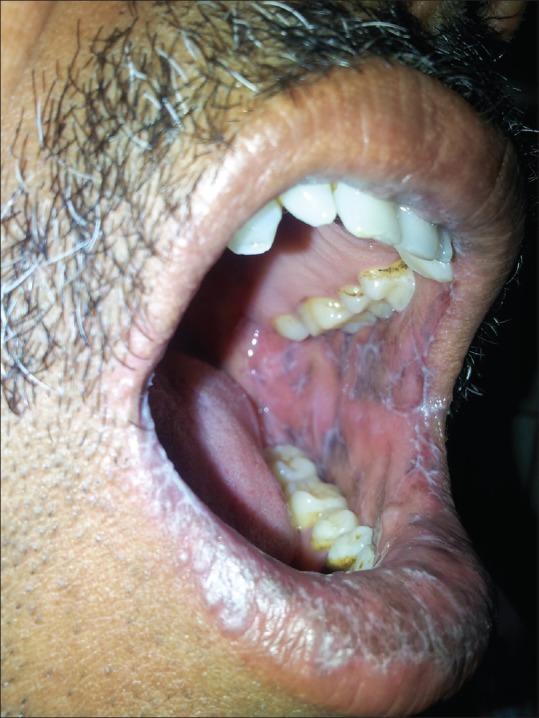
Reticular pattern in oral lichen planus
Figure 2.
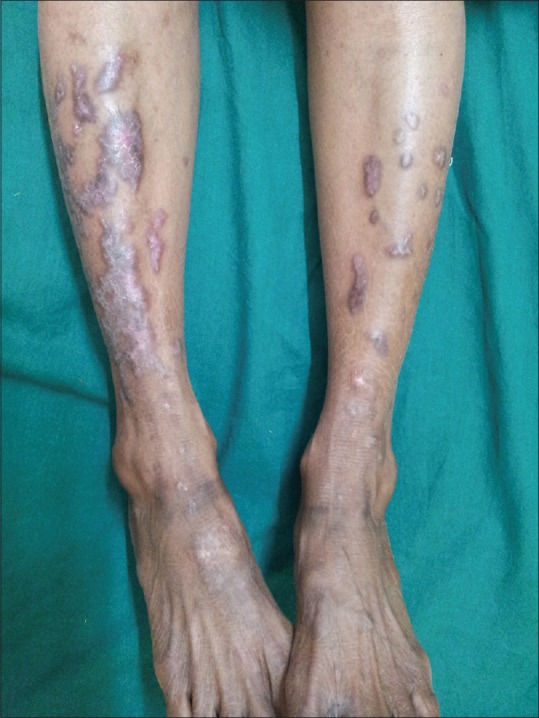
Lichenified plaques on legs
Figure 4.
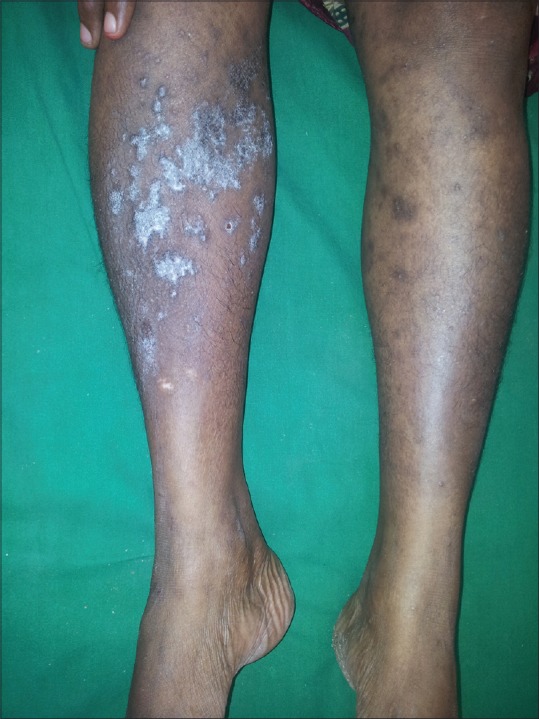
Scaly plaques over legs
Figure 3.
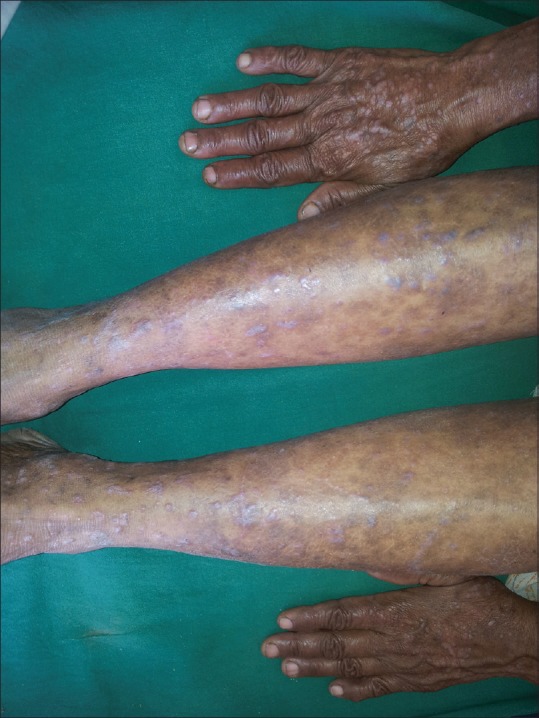
Hyperpigmented to violaceous papules and plaques over dorsum of hands and legs
About 9% of LP patients visiting OPD were already taking treatment for DM, whereas 8% for HTN.
When raised fasting blood glucose level (FBS) was considered as FBS >100 mg/dl,[11] it was seen in 42% (n = 42) of cases and 10% (n = 5) of controls. It was statistically significant in cases compared to controls (P = 0.0003). Mean values of FBS in cases and controls were 102.14 mg/dl and 85.68 mg/dl ranging from 66 to 236 mg/dl and 65 to 143 mg/dl respectively.
When lipid profile of the two groups was compared, we found that cases had higher lipid values. The total cholesterol (TC), low density lipoprotein (LDL-C), very low density lipoprotein (VLDL-C), and triglycerides (TG) values were significantly higher in cases than in controls (P < 0.05 in all) [Table 1].
Table 1.
Comparison of lipid profile between cases and controls
| Serum Lipids | Cases | Controls | t-test, P | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| TC | 203.96 | 49.633 | 175.3 | 40.217 | 0.000117 |
| HDL-C | 47.87 | 13.462 | 46.22 | 11.523 | 0.218337 |
| LDL-C | 125.01 | 50.087 | 104.54 | 38.256 | 0.003175 |
| VLDL-C | 31.08 | 15.784 | 24.54 | 9.742 | 0.001091 |
| TG | 157.5 | 78.950 | 124.84 | 48.361 | 0.001072 |
TC=Total cholesterol, HDL-C=High density lipoprotein, LDL-C=Low density lipoprotein, VLDL-C=Very low density lipoprotein, TG=Triglycerides, SD=Standard deviation
Total cholesterol was elevated in 50% cases, low levels of HDL-C in 29% cases, elevated VLDL-C, LDL-C, and TG in 43%, 38%, and 44% respectively [Table 2].
Table 2.
Analysis of lipid profile between cases and controls
| Lipid profile | Cases (in %) | Controls (in %) | P |
|---|---|---|---|
| TC (>200 mg/dl) | 50 | 24 | 0.003 |
| HDL-C (<40 mg/dl) | 29 | 34 | 0.531 |
| LDL-C (>130 mg/dl) | 38 | 16 | 0.007 |
| VLDL-C (>30 mg/dl) | 43 | 10 | 0.0002 |
| TG (>150 mg/dl) | 44 | 26 | 0.034 |
TC=Total cholesterol, HDL-C=High density lipoprotein, LDL-C=Low density lipoprotein, VLDL-C=Very low density lipoprotein, TG=Triglycerides
According to National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) guidelines, dyslipidemia is defined as presence of any one of the following parameters: TG >150 mg/dl, TC >200 mg/dl, and LDL-C >130 mg/dl.[12] In the present study, dyslipidemia was found in 65% of cases as compared to 38% of controls, which was statistically significant (P = 0.002).
About 32% of cases and 12% of controls were found to be hypertensive (BP ≥140/90 mm Hg[13]). It was statistically significant (P = 0.01).
MS was diagnosed as per NCEP ATP III guidelines.[14] It was found to be more prevalent in cases than in controls (43% versus 26% respectively, P = 0.045) with an OR of 2.15. A significant difference was seen between waist circumference, FBS, and TG, which were higher in cases (P = 0.0082, 0.0003, and 0.0343 respectively). Although, more number of cases had elevated BP as compared to controls, the difference was not statistically significant (P = 0.167). OR was highest in FBS and waist circumference being 6.517 and 4.472 respectively, making them most important parameters of MS [Table 3].
Table 3.
Comparison of MS and its individual components in cases and controls
| Variable | Cases (%) | Controls (%) | P | OR (95%/CI) | Z statistics |
|---|---|---|---|---|---|
| Waist circumference ≥102 cm (M) or ≥88 cm (F) | 28 (28) | 4 (8) | 0.0082 | 4.4722 (1.4723-13.5848) | 2.642 |
| Triglyceride ≥150 mg/dl, n (%) | 44 (44) | 13 (26) | 0.0343 | 2.2363 (1.0615-4.7113) | 2.117 |
| HDL-C <40 mg/dl (M) or <50 mg/dl (F), n (%) | 41 (41) | 26 (52) | 0.2027 | 0.6415 (0.3240-1.2701) | 1.274 |
| BP ≥130/85 mm Hg, n (%) | 52 (52) | 20 (40) | 0.1669 | 1.6250 (0.8163-3.2350) | 1.382 |
| FBS ≥100 mg/dl, n (%) | 42 (42) | 5 (10) | 0.0003 | 6.5172 (2.3840-17.8166) | 3.653 |
| MS, n (%) | 43 (43) | 13 (26) | 0.0446 | 2.1471 (1.0186-4.5259) | 2.008 |
HDL-C=High density lipoprotein, BP=Blood pressure, FBS=Fasting blood sugar, MS=Metabolic syndrome, M=Male, F=Female, OR=Odds ratio, CI=Confidence interval
Dyslipidemia, HTN, and MS were diagnosed in 65%, 32%, and 43% cases respectively and were found to be statistically significant when compared to controls. 36% cases and 18% controls showed both MS and dyslipidemia (P < 0.05). 25 cases had both MS and HTN among which 23 had dyslipidemia also (P = 0.009).
Discussion
LP is a chronic mucocutaneous disease and has been linked to HTN, DM, and dyslipidemia.[9] The association between LP and DM has been studied by various authors but it has still remained controversial.[15,16]
A slightly high male preponderance seen in our study correlates with other published studies.[17]
The age group ranged from 18 to 81 years, mean age being 42.02 years. Omal PM et al.[2] found that prevalence of LP was highest in 40 to 60 years age group.
When FBS > or equal to 126 mg/dl was considered as DM, it was diagnosed in 10% cases and 2% controls. Denli YG et al.[15] found DM in 15.7% cases and 7.1% controls but few other studies have found a higher incidence.[16,18]
About 65% of cases had dyslipidemia as compared to 38% of controls, which was statistically significant (P = 0.002) [Table 4]. In other studies, TL, TG, VLDL-C, and LDL-C were significantly increased, whereas HDL-C was lower in cases as compared to controls.[9,19] Similar findings were observed in the present study.
Table 4.
Dyslipidemia in various studies
| Studies | Cases (%) | Controls (%) | P | OR, CI 95% |
|---|---|---|---|---|
| Dreither J et al.[21] | 42.5 | 37.8 | 0.003 | 1.21, 1.06-1.38 |
| Lopez Jornet P et al.[22] | 58 | 50 | - | - |
| Arias Santiago S et al.[19] | 61 | 33 | 0.001 | 3.17, 1.77-5.66 |
| Present study | 65 | 38 | 0.002 | 3.03, 1.499-6.123 |
OR=Odds ratio, CI=Confidence interval
HTN was observed in 32% cases and 12% controls. These results were found to be statistically significant (P = 0.011). However, Chattopadhyay[20] did not find significant association between the two. The present study supports the hypothesis of relationship between LP and HTN.
A significant association between MS and LP was seen (P = 0.044). Very few studies done on LP have mentioned MS in literature. Arias-Santiago S et al.[9] found higher prevalence of MS in patients with LP as compared to controls (27% vs. 20%), although it was not statistically significant (P = 0.31). In his study, most frequently recorded MS criteria in LP patients was hypertriglyceridemia. The most common abnormal metabolic feature in cases in our study was elevated BP. Although the BP values recorded in cases were higher than controls, no statistical significance was seen in comparison with other components of MS (P = 0.167).
Next most common abnormal feature of MS in cases was triglycerides. Arias Santiago et al.[19] in his study found similar results.
Most important parameter of MS was FBS with highest OR of 6.51. When raised FBS was considered as a part of MS, it was found in 42% of cases as compared to 10% controls, which was similar to other studies.[16,18,19]
Other component of MS which showed significant association with LP was waist circumference. LP patients had a higher waist circumference as compared to controls and this difference was highly significant (P = 0.0082). Though there is paucity of case–control studies, many authors have remarked regarding the association between obesity and LP.[9]
In the present study, 9% patients were found to have both MS and DM. MS and dyslipidemia were both diagnosed in 36% cases and 18% controls. 5% of the cases showed presence of all DM, MS, dyslipidemia, and hypertension. These observations suggest multiple autoimmune disorders association.
Our study revealed a significant association between LP and dyslipidemia, DM, and MS. The presence of all three parameters was significant in cases as compared to healthy individuals. MS represents a cluster of risk factors including central obesity, atherogenic dyslipidemia, HTN, and glucose intolerance. The diagnosis of MS puts the patient for future risk of DM and CVS morbidity. Thus, clinicians should be aware and attentive to the symptoms of DM, dyslipidemia, and MS in these patients. Timely screening of LP patients is essential. These findings of LP with DM, dyslipidemia, and MS may help us to formulate guidelines for investigation and proper management of LP.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
This research was supported by Department of Dermatology, Venereology and Leprosy, JSS Medical College. We would like to give our thanks to the subjects for their participation in this project. We are also grateful to all the staff members and my colleagues in our department for their concern and support our work.
References
- 1.Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53–60. [PubMed] [Google Scholar]
- 2.Omal PM, Jacob V, Prathap A, Thomas NG. Prevalence of oral, skin, and oral and skin lesions of lichen planus in patients visiting a dental school in Southern India. Indian J Dermatol. 2012;57:107–9. doi: 10.4103/0019-5154.94276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sampogna F, Picardi A. Association between poorer quality of life and psychiatric morbidity in patients with different Dermatological conditions. Psychosom Med. 2004;66:620–4. doi: 10.1097/01.psy.0000132869.96872.b2. [DOI] [PubMed] [Google Scholar]
- 4.Cohen AD, Sherf M, Vidavsky L, Vardy DA, Shapiro J, Meyerovitch J. Association between psoriasis and the metabolic syndrome. A cross-sectional study. Dermatology. 2008;216:152–5. doi: 10.1159/000111512. [DOI] [PubMed] [Google Scholar]
- 5.Akay BN, Bozkir M, Anadolu Y, Gullu S. Epidemiology of vitiligo, associated autoimmune diseases and audiological abnormalities: Ankara study of 80 patients in Turkey. J Eur Acad Dermatol Venereol. 2010;24:1144–50. doi: 10.1111/j.1468-3083.2010.03605.x. [DOI] [PubMed] [Google Scholar]
- 6.Lodi G, Pellicano R, Carrozzo M. Hepatitis C virus infection and lichen planus: A systematic review with meta-analysis. Oral Dis. 2010;16:601–12. doi: 10.1111/j.1601-0825.2010.01670.x. [DOI] [PubMed] [Google Scholar]
- 7.Atefi N, Majedi M, Peyghambari S, Ghourchian S. Prevalence of diabetes mellitus and impaired fasting blood glucose in patients with Lichen Planus. Med J Islam Repub Iran. 2012;26:22–6. [PMC free article] [PubMed] [Google Scholar]
- 8.Naldi L, Sena P. Lichen planus and liver diseases: A multicentre case-control study. Gruppo Italiano Studi Epidemiologici in Dermatologia (GISED) Br Med J. 1990;300:22730. doi: 10.1136/bmj.300.6719.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arias-Santiago S, Buendía-Eisman A, Aneiros-Fernández J, Girón-Prieto MS, Gutiérrez-Salmerón MT, Mellado VG. Cardiovascular risk factors in patients with lichen planus. Am J Med. 2011;124:543–8. doi: 10.1016/j.amjmed.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 10.Parapid B, Ostojic MC, Lalic NM, Micic D, Damjanovic S, Bubanja D, et al. Risk factors clustering within the metabolic syndrome: A pattern or by chance? Hellenic J Cardiol. 2014;55:92–100. [PubMed] [Google Scholar]
- 11.Soliman A, DeSanctis V, Yassin M, Elalaily R, Eldarsy NE. Continuous glucose monitoring system and new era of early diagnosis of diabetes in high risk groups. Indian J Endocrinol Metab. 2014;18:274–82. doi: 10.4103/2230-8210.131130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Talbert RL. Role of the national cholesterol education program adult treatment panel III guidelines in managing dyslipidemia. Am J Health Syst Pharm. 2003;60:3–8. doi: 10.1093/ajhp/60.suppl_2.S3. [DOI] [PubMed] [Google Scholar]
- 13.Crim MT, Yoon SS, Ortiz E, Wall HK, Schober S, Gillespie C, et al. National surveillance definitions for hypertension prevalence and control among adults. Circ Cardiovasc Qual Outcomes. 2012;5:343–51. doi: 10.1161/CIRCOUTCOMES.111.963439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA. Grundy diagnosis and management of the metabolic syndrome: An American heart association/national heart, lung, and blood institute scientific statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 15.Denli YG, Durdu M, Karakas M. Diabetes and hepatitis frequency in 140 lichen planus cases in Cukurova region. J Dermatol. 2004;31:293–8. doi: 10.1111/j.1346-8138.2004.tb00675.x. [DOI] [PubMed] [Google Scholar]
- 16.Bagan JV, Donat JS, Penarrocha M, Milian MA, Sanchis JM. Oral lichen planus and diabetes mellitus. A clinico-pathological study. Bull Group Int Rech Sci Stomatol Odontol. 1993;36:3–6. [PubMed] [Google Scholar]
- 17.Kanwar AJ, De D. Lichen Planus in childhood: Report of 100 cases. Clin Exp Dermatol. 2010;35:257–62. doi: 10.1111/j.1365-2230.2009.03613.x. [DOI] [PubMed] [Google Scholar]
- 18.Romero MA, Seoane J, Vareola-Centelles P, Diz-Dios P, Garcia Pola MJ. Prevalence of diabetes mellitus amongst oral lichen planus patients. Clinical and pathological characteristics. Medicinia Oral. 2002;7:121–9. [PubMed] [Google Scholar]
- 19.Arias-Santiago S, Buendía-Eisman A, Aneiros-Fernández J, Girón-Prieto MS, Gutiérrez-Salmerón MT, García-Mellado V, et al. Lipid levels in patients with lichen planus: A case-control study. J Eur Acad Dermatol Venereol. 2011;25:1398–401. doi: 10.1111/j.1468-3083.2011.03983.x. [DOI] [PubMed] [Google Scholar]
- 20.Chattopadhyay A. Arterial blood pressure and blood glucose levels in oral lichen planus patients in Calcutta (India) Indian J Dent Res. 1992;3:84–9. [PubMed] [Google Scholar]
- 21.Dreiher J, Shapiro J, Cohen AD. Lichen Planus and Dyslipidaemia: A Case-Control Study. Br J Dermatol. 2009;161(3):626–9. doi: 10.1111/j.1365-2133.2009.09235.x. [DOI] [PubMed] [Google Scholar]
- 22.López-Jornet P, Camacho-Alonso F, Rodríguez-Martínes MA. Alterations in serum lipid profile patterns in oral lichen planus: A cross-sectional study. Am J Clin Dermatol. 2012;13(6):399–404. doi: 10.2165/11633600-000000000-00000. [DOI] [PubMed] [Google Scholar]


