Abstract
Context:
An umbrella term, acquired dermal macular hyperpigmentation (ADMH), has been proposed to denote conditions including ashy dermatosis, erythema dyschromicum perstans, lichen planus pigmentosus, and idiopathic macular eruptive pigmentation.
Aims:
To classify the patients manifesting ADMH on the basis of histology.
Settings and Design:
In this retrospective, cross-sectional study, histology specimens of patients of ADMH, who underwent skin biopsy in our institution from 1.1 2015 to 31.12.2017, were included after obtaining ethical clearance.
Materials and Methods:
The histology specimens of patients of ADMH were reviewed by the pathologist and classified. Clinical features of individual patient were collected from previous records and the data analyzed.
Statistical Analysis Used:
Pearson's Chi-square test was used to determine significance of association between age of onset and duration of pigmentation with histology type.
Results:
Three patterns of histology were identified in the study group (17 males and 13 females). Type 1: Basal cell degeneration and moderate to dense inflammation (12 patients, 40%), type 2: Significant pigment incontinence and sparse inflammation without basal cell degeneration, (12 patients, 40%), and type 3: sparse inflammation without basal cell degeneration or significant pigment incontinence (six patients, 20%). Statistically significant association was noted between age of onset of pigmentation and histology type (P value, 0.02).
Limitations:
Main limitation was the small sample size.
Conclusions:
Prospective studies evaluating the clinical progression and dermoscopy features and analyzing serial biopsies of ADMH patients may confirm whether the histology patterns observed represent different stages of same disease process or are different entities.
Keywords: Acquired dermal macular hyperpigmentation, ashy dermatosis, idiopathic eruptive macular pigmentation, lichen planus pigmentosus
Introduction
Terms ashy dermatosis, erythema dyschromicum perstans, and lichen planus pigmentosus (LPP) have been used by various authors in various regions while describing acquired small or large sized macules or diffuse hyperpigmentation of unknown etiology.[1,2,3] Consensus regarding whether all these represent different stages of same disease process or different entities is lacking.[4] Degos et al. in 1978 described idiopathic macular eruptive pigmentation (IMEP) characterized by pigmented macules over neck, trunk, and limbs in children and adolescents that resolved spontaneously.[5]
Zaynoun et al. in 2008 proposed a classification where they included most of the acquired hyperpigmentation disorders under the broad category of ashy dermatoses.[6] Pigmentary disorders society of India in 2017 proposed the umbrella term acquired dermal macular hyperpigmentation (ADMH) to denote LPP, Reihl's melanosis, ashy dermatosis, and IMEP owing to the difficulty encountered in differentiating these conditions clinically as well as histologically.[7] Global Consensus Forum in 2018 suggested using the terminology macular pigmentation of uncertain etiology for acquired macular pigmentation till a definite etiology/a definite name like LPP/ashy dermatosis/erythema dyschromicum perstans/Riehl's melanosis/IMEP could be attributed.[8]
Joshi and Rohatgi opined that presence of interface dermatitis and significant melanin deposition in dermis as negative criterion for IMEP which is considered as an epidermal melanosis.[9,10] Since some melanophages are expected in dermis, in pigmented skin, a method was proposed to determine whether their presence was significant or not. This was based on the magnification required to visualize them.[10] Vinay et al. have evaluated density of melanin incontinence by counting the average number of melanophages observed under magnification of 200× after examining five such fields.[11]
In this study, we have attempted to classify patients who attended our center with ADMH based on histology findings.
Study design
Retrospective cross-sectional study.
Study Subjects
Inclusion criteria: We undertook a cross-sectional study of archived biopsy samples of ADMH at the Dermatology department of a tertiary care center from 1.1.2015 to 31.12.2017.
Exclusion criteria: Patients who had a recorded pruritus, inflammation, and exposure to drugs known to induce hyperpigmentation were excluded. Histology specimens of poor quality (despite preparing fresh slides from paraffin embedded specimens) were also excluded from the study.
Materials and Methods
Methods: Patient characteristics and clinical details were collected using a preset questionnaire from the previous case records. Blind review of histology specimens was carried out by pathologist with special attention to basal cell hyperpigmentation, basal cell degeneration, type of inflammatory cells, pattern of inflammation, and extent of pigment incontinence. Toluidine blue staining was done in three cases where mastocytosis was a clinical differential and the two positive cases were excluded from the study.
Severity of inflammation was categorized into mild (when inflammatory infiltrate occupied less than 10% of upper dermis), moderate (when inflammatory infiltrate occupied 10%–25% of upper dermis), and severe (when inflammatory infiltrate occupied >25% of upper dermis).
Pigment incontinence was graded based on the average number of melanophages per high power field (400×) after examining five fields, which was a modification of one previously described method.[11]
Grade 3: Average number of melanophages per high power field is >20.
Grade 2: Average number of melanophages per high power field is 10–20.
Grade 1: Average number of melanophages per high power field is <10.
Grade 1 pigment incontinence was considered as insignificant.
The study group was classified histologically as following:
Type 1: Basal cell degeneration present.
Type 2: No basal cell degeneration; but significant pigment incontinence present.
Type 3: No basal cell degeneration; pigment incontinence absent or insignificant.
The data was entered in Microsoft excel and analyzed with SPSS version 16.
Data was analyzed with respect to the histology findings and clinical features. Pearson's Chi-square test was used to evaluate association between histology type and age of the patient at the onset of ADMH and between histology type and duration of disease.
P value below 0.05 was considered significant.
Results
A total of 30 patients who underwent skin biopsy for ADMH were identified. Males predominated in the study group (17 males and 13 females, male: female–1.3:1). Age of patients ranged from 4 to 64 years at the time of skin biopsy. Duration of cutaneous hyperpigmentation varied from 2 months to 14 years. None had recorded history of active erythematous border.
ADMH was observed on face (21 patients, 70%), upper limbs (18 patients, 60%), upper trunk (10 patients, 33.3%), lower limbs (8 patients, 26.7%), neck (7 patients, 23.3%), and abdomen (3 patients, 10%).
Color of pigmentation varied from violaceous (eight, 26.7%) to grayish black/gray (20 patients, 66.7%), and brown (two patients, 6.7%). No one manifested hyperpigmentation with an active erythematous border. A total of 16 patients manifested macular pattern (53.3%). Diffuse (13 patients, 43.3%) and reticular (1 patient, 0.3%) patterns were also recorded.
Three patients had lichen planus lesions elsewhere on the body. Hyperpigmentation spared palms, soles, and nails in all.
Histology findings
Histology findings observed included mild hyperkeratosis (28 cases, 93.3%), atrophic epidermis (5, 16.7%), basal cell degeneration (12, 40%) [Figure 1a], apoptotic keratinocytes (2, 6.7%), and pigment deposits in dermis (29, 96.7%). All specimens showed lymphohistiocytic inflammatory infiltrate. Moderate to dense inflammation was noted in 12 patients (40%) and 18 (60%) manifested mild inflammation. Majority (20, 66.7%) had perivascular infiltrate. Seven patients (23.3%) had perivascular as well as periappendageal distribution of inflammatory infiltrate [Figure 1b]. A lichenoid pattern [Figure 2] was observed in three patients (10%).
Figure 1.
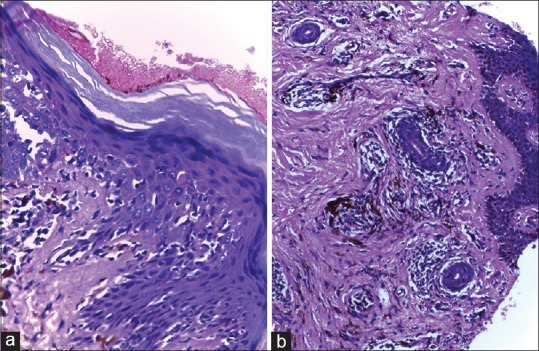
(a) Skin biopsy specimen manifesting basal cell degeneration and significant pigment incontinence in superficial dermis (H and E, 400x); 1 (b): perivascular and periappendageal inflammatory infiltrate (H and E, 200x)
Figure 2.
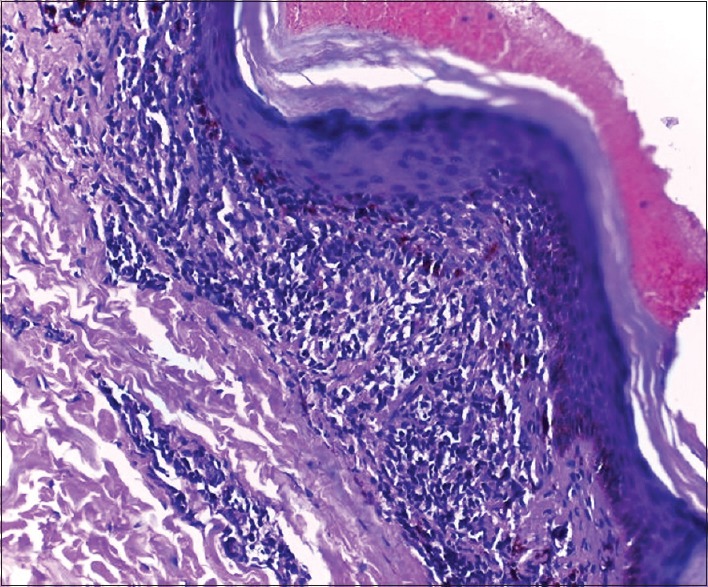
Skin biopsy from a patient with acquired idiopathic cutaneous hyperpigmentation showing basal cell degeneration, lichenoid inflammatory infiltrate, and significant pigment incontinence in superficial dermis (H and E, 200x)
In the study group, eight patients (26.7%) had grade 1, six (20%) had grade 2, and the remaining 16 patients (53.3%) had grade 3 pigment incontinence [Table 1]. One of the eight cases with grade 1 pigment incontinence did not manifest any melanophages in dermis.
Table 1.
Density of pigment incontinence in various histology types
| Histology types | Range | Mean with standard deviation |
|---|---|---|
| Type 1 | 0.6-46.4 | 32.27±16.99 |
| Type 2 | 12-51 | 25.78±11.11 |
| Type 3 | 0-8.8 | 3.33±3.32 |
Three patterns of histology were identified in the study group.
Type 1 (12 patients, 40%): Basal cell degeneration was present in all. Ten patients (83.3%) had moderate to dense inflammation [Figures 1 and 2]. A total of 9 out of the 12 patients (75%) had grade 3 [Figures 3a], one had grade 2 (8.3%) [Figures 3b], and two others had grade 1 (16.7%) pigment incontinence [Figure 3c].
Figure 3.
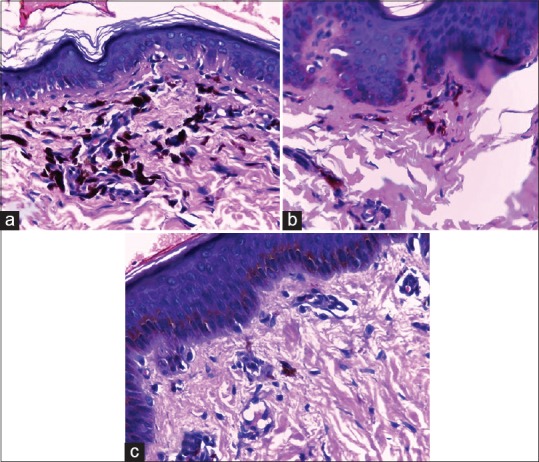
(a) Grade 3 pigment incontinence - more than 20 melanophages in upper dermis in average high power field (H and E, 400x); (b): Grade 2 pigment incontinence – 10–20 melanophages in upper dermis in average high power field (H and E, 400x); (c): Grade 1 pigment incontinence – Less than ten melanophages per average high power field (H and E, 400x)
Type 2 (12 patients, 40%): Significant pigment incontinence was documented in upper dermis in all cases-- 7 of the 12 (58.3%) cases had grade 3 and 5 (41.7%) had grade 2 pigment incontinence. Sparse perivascular inflammation was observed in ten patients (83.3%). Basal cell degeneration was absent [Figure 4a].
Figure 4.
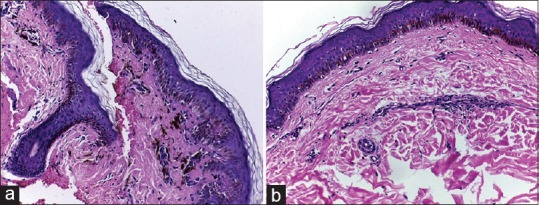
(a) Skin biopsy specimen showing significant pigment incontinence and sparse perivascular inflammation without basal cell degeneration (H and E, 100x). (b): Skin biopsy specimen showing sparse perivascular inflammation without basal cell degeneration or pigment incontinence (H and E, 200x)
Type 3 (six patients, 20%): Sparse perivascular (with or without periappendageal) inflammation was observed in all cases. All patients manifested grade 1 pigment incontinence. Basal cell degeneration was absent [Figure 4b].
Clinical profile of different histology types
Average age of the patients at the onset of pigmentation and average duration of disease process at the time of biopsy varied among the three histology groups [Table 2]. Association between the age of the patient at the onset of pigmentation and histology type was statistically significant (P value, 0.02). No association was noted between duration of pigmentation at the time of biopsy and histology types.
Table 2.
Age of disease onset and duration of pigmentation at the time of biopsy in study group manifesting different histology patterns
| Histology type | Age of the patients (in years) | Duration of pigmentation at the time of biopsy | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0-15 | 16-30 | 31-45 | 46-60 | 61-75 | 0-6 months | >6 months - 2 years | >2-5 years | >5 years | |
| Type 1 (12 patients) | 1 (8.3%) | 0 (0%) | 7 (58.3%) | 2 (16.7%) | 2 (16.7%) | 2 (16.7%) | 5 (41.7%) | 3 (25%) | 2 (16.7%) |
| Type 2 (12 patients) | 4 (33.3%) | 5 (41.7%) | 1 (8.3%) | 2 (16.7%) | 0 (0%) | 7 (58.3%) | 3 (25%) | 2 (16.7%) | 0 (0%) |
| Type 3 (6 patients) | 4 (66.7%) | 1 (16.7%) | 0 (0%) | 1 (16.7%) | 0 (0%) | 4 (66.7%) | 2 (33.3%) | 0 (0%) | 0 (0%) |
| Total (30 patients) | 9 (30%) | 6 (20%) | 8 (26.7%) | 5 (16.7%) | 2 (6.7%) | 13 (43.3%) | 10 (33.3%) | 5 (16.7%) | 2 (6.7%) |
Clinical profile, and age and sex profile of study subjects varied among the three histology types [Table 3].
Table 3.
Clinical profile of patients of various histology types
| Histology type | Mean age | Sex predilection | Mean duration of disease at the time of biopsy | Photopredilection | Most common pattern of pigmentation |
|---|---|---|---|---|---|
| Type 1 | 39.4 years | No | 41.3 months | Yes | Diffuse |
| Type 2 | 23.9 years | Males | 12.7 months | No | Macular |
| Type 3 | 15.3 years | Slight female predilection | 9.7 months | No | Macular |
Type 1 (12 patients): 11 patients (92%) were above 30 years at the onset of ADMH. No sex predilection was noted in this group (six males and six females). 10 patients (83.3%) had disease duration of more than 6 months at the time of biopsy. Nine patients (75%) manifested diffuse pigmentation. Reticular and macular patterns were documented in one and two cases, respectively. Color of pigmentation was violaceous in eight (66.7%) and grayish black in the rest (33.3%). 10/12 (77%) had lesions confined to photoexposed sites.
Type 2 (12 patients): Males predominated in this group (9/12, 75%). Nine patients (75%) were below 30 years. Disease duration was less than 6 months in more than 50% cases. Most commonly observed pigmentation pattern was macular (eight cases, 66.7%). Diffuse pigmentation was seen in four patients (33.3%). Color of pigmentation was grayish black in all. Nine patients (75%) had lesions on both photoexposed and photoprotected sites.
Type 3 (six patients): Females outnumbered males (four females, 66.7%) in this group. 5/6 patients (83.3%) were 20 years or below. Disease duration documented was less than 6 months in more than 60% cases. All (100%) had macular pigmentation. Color of pigmentation varied from brown to gray. Three patients had lesions confined to photoexposed sites; the other three had lesions on unexposed sites also.
A total of 14 of the 16 (87.5%), cases who manifested macular pigmentation had mild inflammation histologically while 10 of the 14 (71.4%) patients who developed diffuse pigmentation showed moderate to dense inflammation. This was found to be statistically significant (P value, <0.1).
Discussion
Several acquired hyperpigmentation disorders of uncertain etiology are described in literature. Lack of consensus exists regarding their classification and evaluation of individual patient.[1,2,3,4]
The male predominance for ADMH noted in our study was contrary to the findings of Vinay et al.[11] Three patients with ADMH manifesting lichen planus lesions elsewhere on body as noted by us was recorded earlier and this was one of the reasons that prompted Bhutani et al. to coin the term LPP.[3]
Different studies have documented different histology findings in ADMH. It was suggested that the histology patterns vary based on the duration of disease and many of the entities described under ADMH are different stages of same disease.[12,13] But the better prognosis and earlier age of onset observed for IMEP indicates it to be a separate entity.[9,10]
Biopsy findings (sparse inflammation and insignificant pigment incontinence) as well as the clinical profile (majority below the age of 20 years and all of them manifesting macular pigmentation of lighter shade) of the patients classified in our type 3 histology group match the IMEP category which manifests only epidermal hyperpigmentation and is rather observed as a disease of children and adolescents and presents with macular lesions.[9,10] We need to differentiate it from the conditions that produce dermal melanosis, since compared to the latter, IMEP undergoes spontaneous resolution and hence has a better prognosis, though cases manifesting persistent pigmentation have been rarely reported.[14]
Two patients in type 1 histology group manifested insignificant pigment incontinence; one was a 32-year-old female and the other was a 13-year-old boy. Both had well-defined bluish gray macules confined to photoexposed sites. Basal cell degeneration and moderate inflammation observed in both placed them under type 1 histology group as per the present study design. Follow up to assess prognosis may help to accurately categorize such patients.
Two distinct patterns of histology (types 1 and 2) were identified in the current study in the patients who manifested significant dermal melanosis. Absence of basal cell degeneration and lack of significant inflammation in group 2 patients distinguish them from group 1. Similar finding already reported in literature was attributed to the difference in the age of the lesions biopsied.[12] But the clinical differences including the statistically significant difference documented in age of onset of pigmentation in subjects manifesting different histology patterns suggest the need to explore the possibility of two separate disease processes.
In our study, most patients with Group 1 histology were above 30 years with diffuse pigmentation mainly affecting photoexposed sites manifesting basal cell degeneration and significant inflammation and pigment incontinence. Majority of Group 2 patients were younger individuals with macular pigmentation without any photo predilection showing significant pigment incontinence in upper dermis, sparse inflammation, and absence of basal cell degeneration. In Ramirez series of ashy dermatosis, most of the patients had disease onset in early adult life.[1] Others have described sparse inflammation and mild basal cell degeneration in erythema dyschromicum perstans.[4]
Vinay et al. in their study observed severity of pigmentation by dermoscopy was comparable to severity in histology.[11] Most of ADMH cases manifesting macular pigmentation showing mild inflammation, whereas most patients with diffuse pigmentation manifesting moderate to severe inflammation histologically, as noted by us suggests the comparability between clinical and histological findings. The lighter shade of pigmentation documented in those with insignificant pigment incontinence in the current study is also supportive of the comparability between clinical and histological findings.
The mean density of dermal melanophages documented by Vinay et al. ranged from 7.80 ± 1.79 to 15.00 ± 2.8, among various categories of patients based on dermoscopy findings, whereas in our study this ranged from 3.33 ± 3.32 to 32.27 ± 16.99 among different histology types. High upper level of mean density observed in our study could be explained on the basis of using higher magnification (400× in our study and 200× in the previous study) to count the melanophages. Despite using higher magnification to count the melanophages, the lower limit of mean density of pigment incontinence documented by us was less than that observed by Vinay et al. This could be attributed to the selection of treatment naïve patients as subjects in their study.[11]
Limitations
The major limitation of our study was the retrospective nature and small sample size. Another drawback of the study was the lack of information on age of the lesion biopsied which might have contributed to the lack of association noted between histology pattern and duration of disease.
Summary
We suggest that the possibility of different dermal melanosis inducing conditions manifesting as ADMH needs to be explored, in addition to disease processes that predominantly cause epidermal melanosis. Prospective studies incorporating clinical, histological, and dermoscopic evaluation may help to define the diagnosis and prognosis of conditions described as ADMH.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ramirez CO. Los Cenicrentas-Problem Clinico, Report 1st Central American Congress of Dermatology. San Salvador. 1957 [Google Scholar]
- 2.Convit J, Kerdel-Vegas F, Rodriguez G. Erythema dyschromicumperstans. A hitherto undescribed skin disease. J Invest Dermatol. 1961;36:457–62. [Google Scholar]
- 3.Bhutani LK, Bedi TR, Pandhi RK, Nayak NC. Lichen planuspigmentosus. Dermatologica. 1974;149:43–50. doi: 10.1159/000251470. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh A, Condoo A. Lichen planus pigmentosus: The controversial consensus. Indian J Dermatol. 2016;61:482–6. doi: 10.4103/0019-5154.190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Degos R, Civatte J, Belaïch S. Idiopathic eruptive macular pigmentation (author's transl) Ann Dermatol Venereol. 1978;105:177–82. [PubMed] [Google Scholar]
- 6.Zaynoun S, Rubeiz N, Kibbi AG. Ashy dermatoses – A criticalreview of the literature and a proposed simplified clinical classification. Int J Dermatol. 2008;47:542–4. doi: 10.1111/j.1365-4632.2008.03625.x. [DOI] [PubMed] [Google Scholar]
- 7.Podder I, Das A, Sarkar R. Pigmentarycon. Indore, India: 2017. Nov 10–12, Pigmentary disorders and their management–analyzing current evidence: Conference proceedings and learning points. Indian Dermatol Online J 2018;9:143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumarasinghe SPW, Pandya A, Chandran V, Rodrigues M, Dlova NC, Kang HY, et al. A global consensus statement on ashy dermatosis, erythema dyschromicumperstans, lichen planuspigmentosus, idiopathic eruptive macular pigmentation, and Riehl's melanosis. Int J Dermatol. 2018;58:263–72. doi: 10.1111/ijd.14189. [DOI] [PubMed] [Google Scholar]
- 9.Joshi RS, Rohatgi S. Idiopathic eruptive macular pigmentation: A critical review of published literature and suggestions for revision of criteria for diagnosis. Indian J Dermatol Venereol Leprol. 2015;81:576–80. doi: 10.4103/0378-6323.168323. [DOI] [PubMed] [Google Scholar]
- 10.Joshi R. Idiopathic eruptive macular pigmentation in an Indian male. Indian Dermatol Online J. 2018;9:64–5. doi: 10.4103/idoj.IDOJ_298_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vinay B, Bishnoi A, Parsad D, Saikia UN, Kumaran MS. Dermoscopic evaluation and histopathological correlation of acquired dermal macular hyperpigmentation. Int J Dermatol. 2017;56:1395–99. doi: 10.1111/ijd.13782. [DOI] [PubMed] [Google Scholar]
- 12.Kanwar AJ, Dogra S, Handa S, Parsad D, Radotra BD. A study of 124 Indian patients with lichen planuspigmentosus. Clin Exp Dermatol. 2003;28:481–5. doi: 10.1046/j.1365-2230.2003.01367.x. [DOI] [PubMed] [Google Scholar]
- 13.Vega ME, Waxtein L. Ashy dermatosis and lichen planuspigmentosus: A clinicopathologic study of 31 cases. Int J Dermatol. 1992;31:90–4. doi: 10.1111/j.1365-4362.1992.tb03244.x. [DOI] [PubMed] [Google Scholar]
- 14.Subhadarshani S, Singh A, Ramteke PP, Verma KK. Idiopathic eruptive macular pigmentation in an Indian male. Indian Dermatol Online J. 2018;8:367–70. doi: 10.4103/idoj.IDOJ_274_16. [DOI] [PMC free article] [PubMed] [Google Scholar]


