Abstract
Background
Evidence and treatment guidelines support the use of statins in patients with established atherosclerotic cardiovascular disease (ASCVD) for secondary prevention of subsequent cardiovascular (CV) event. However, treatment adherence and persistence are still a concern.
Methods
We constructed a retrospective population-based cohort of patients, who initiated statin treatment within 90 days after discharge from hospital for ASCVD using the claims database of Taiwan National Health Insurance. Proportion of days covered (PDC) was used to measure statin adherence, and PDC ≥80% was defined as good adherence. The study outcomes were subsequent rehospitalisation or in-hospital death due to composite ASCVD, myocardial infarction or ischaemic stroke. Their associations with statin prescription adherence or persistence were analysed using time-dependent Cox proportional hazards model.
Results
The study cohort included 185 252 postdischarge statin initiators. There were 50 015 subsequent ASCVD rehospitalisations including 2858 in-hospital death during 7 years of study period. Good adherence was significantly associated with lower risk of ASCVD rehospitalisation (adjusted HR (aHR) 0.90; 95% CI 0.87 to 0.92) and significantly lower risk of in-hospital death (aHR 0.59; 95% CI 0.53 to 0.65). Compared with constant use of statin, patients in the three less persistent states (recent stop, non-persistence and intermittent use) were associated with higher risk of subsequent ASCVD rehospitalisation, aHRs were 1.16, 1.13 and 1.26, respectively (all p<0.05). The increased risks were consistent with specific outcome of acute myocardial infarction and ischaemic stroke. Also, patients in the recent stop period had significantly higher risk for fatal CV event.
Conclusions
Good adherence and persistence to statin therapy are significantly associated with lower risk of secondary ASCVD rehospitalisation and in-hospital death.
Keywords: atherosclerosis, adherence, persistence, secondary prevention, statin
Key messages.
What is already known about this subject?
Despite the solid clinical evidence and strong recommendations for the long-term use of statins in secondary prevention of ASCVD, adherence and persistence are still issues that impact the treatment effect in real-world clinical practice.
What does this study add?
As most of the studies for statin adherence were done in western countries, this study add information on Asian population.
How might this impact on clinical practice?
Good adherence and persistence to statin therapy are significantly associated with lower risk of secondary ASCVD rehospitalisation and in-hospital death; optimising patient adherence and persistence behavior will improve treatment outcomes.
Introduction
Atherosclerotic cardiovascular disease (ASCVD) comprises several disorders that are of atherosclerotic origin,1 which leads to significant health and economic burdens.2 Among modifiable risk factors, hyperlipidaemia is one of the foremost risk factors for ASCVD.3 4 Landmark randomised controlled clinical trials (RCT) have established that patients on high-intensity statins have a lower incidence of secondary ASCVD.5–7 A systematic review of RCTs further confirmed the efficacy of statin therapy in preventing ASCVD.8 In recent years, several international lipid treatment guidelines9 10 have consistently recommended intensive statin therapy for patients with ASCVD to prevent subsequent cardiovascular (CV) events.
Despite the solid clinical evidence and strong recommendations for the long-term use of statins in secondary prevention of ASCVD, adherence and persistence are still issues that impact the treatment effect in real-world clinical practice. Poor adherence or non-persistence to statins increases the risk for CV events and mortality in patients with ASCVD history.11 12 A systematic review of 28 real-world studies published in 2014 showed that non-adherence and non-persistence to statin treatment significantly increased the relative risk of secondary CV events and mortality ranging from 1.22 to 5.26.13 However, most of the studies reviewed were conducted in western countries. The information of statin treatment adherence and persistence in patients with ASCVD for secondary prevention of CV disease is limited in Asian population. Thus, the aim of this study was to assess the effect of statin treatment adherence and persistence on the prevention of subsequent CV events in patients discharged from hospital for a new-onset ASCVD in a real-world setting in Taiwan.
Methods
Study design
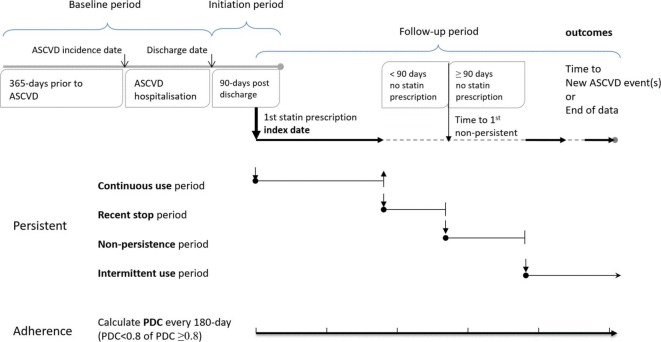
We constructed a retrospective population-based cohort using the claims database of National Health Insurance (NHI) in Taiwan.14 Figure 1 shows the study design schematic and definitions of statin adherence and persistence measurements.
Figure 1.
Study design schematic. ASCVD, atherosclerotic cardiovascular disease; PDC, proportion of days covered.
Data source and study cohort
The Taiwan NHI programme was implemented on 1 March 1995. This is a compulsory universal programme. In 2014, over 99% of all the 23 million population in Taiwan were enrolled. The programme provides coverage including ambulatory care, emergency visits, hospitalisation, surgical procedures, laboratory tests, diagnostic examinations, dental services, traditional Chinese medicines and prescription medicines. The reimbursement claims data are captured through an electronic system and updated on a daily basis. This database provides a great resource for population-based research and has been widely used for various clinical and epidemiological studies.
The date of the first statin prescription within 90 days of patients discharged from hospital for new-onset ASCVD was defined as the index date. We identified cases with index dates between 1 January 2006 and 31 December 2012 from the NHI claims database. All data in the 1-year period prior to the ASCVD hospitalisation were examined to confirm it was a new-onset episode. ASCVD events were identified by hospital discharge diagnosis and in-hospital revascularisation procedure performed. The discharge diagnosis was coded based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). We included acute myocardial infarction (AMI) (ICD-9-CM code 410.xx), coronary heart disease (CHD) (ICD-9-CM code 414.01) and ischaemic stroke (ICD-9-CM codes 433.xx and 434.xx). From among the NHI procedure codes, we included percutaneous transluminal coronary angioplasty (PTCA) and coronary artery bypass graft (CABG). The validity of the records in NHI claims by using these diagnosis and procedure codes has been studied and published, the results of which have shown high positive predictive values (PPV): 0.96–1.00 for ischaemic stroke, 0.88 for AMI, 0.83 for CHD, 0.92 for CABG and 0.98 for PTCA. More details were described in the Outcome Variables section.15 16
To comply with personal confidentiality regulations, patient identity data were encrypted and analysed anonymously. All results were reported in aggregated manner and no individual data shall be revealed.
Baseline and covariate variables
Patients’ data 1 year prior to and during the hospitalisation were used as baseline variables for multivariate analysis. The baseline variables included age at admission, gender, discharge diagnosis, history of hyperlipidaemia before hospitalisation, Charlson Comorbidity Index, medication history, lipid testing before and during hospitalisation, accreditation level of healthcare organisation, index statin prescribing physicians’ specialty and dosage of statin. In addition to the baseline variables, a time-varying covariate of whether lipid examination ever performed 1 month before statin prescription change was also included as an indicator to adjust for potential healthy adherer effect.17
Treatment adherence and persistence variables
We calculated separate measurements for statin adherence and persistence. All statin prescription records were retrieved from the index date, when the first statin was prescribed within 90 days of hospital discharge to one of the following: (1) the last record available of each patient, (2) subsequent ASCVD event that led to hospitalisation, or (3) end of database (on 31 December 2012), whichever occurred the earliest. The changing on adherence and persistence to statin therapy were captured along follow-up. We measured statin adherence using proportion of days covered (PDC)18 every 180 days. PDC was the number of days covered by statin prescription divided by the number of calendar days in each 180-day period (or number of days in the last period), which was a proportion ranged from 0 to 1. A period with PDC ≥0.8 was defined as good adherence, while PDC <0.8 as suboptimal adherence. The level at 0.8 or greater was selected as it was found to be the most consistently associated with clinical benefits in a previous review.19 Adherence was analysed as a binary variable and allowed for variation across multiple exclusive periods along follow-up of one patient.
Statin persistence was classified into four states based on the daily status of a patient with or without statin prescription covered along follow-up.20 All patients included in the cohort commenced as ‘constant use’ as statin initiated. A patient entered the ‘non-persistence’ state on the day when the patient failed to refill statin prescription that reached the 90th day after the end of the latest prescription. Within the 90-day window without statin prescription covered, the period was defined as ‘recent stop’. If a statin prescription was refilled before the 90-day gap was reached, the patient returned to ‘constant use’ from ‘recent stop’. During the ‘non-persistence’ state, if statin prescription resumed, the patient was moved into ‘intermittent use’ state and stayed for the rest of the follow-up. Figure 1 illustrates the diagram of persistence and adherence measurements.
Outcome variables
We ascertained two groups of outcomes, including subsequent rehospitalisation and in-hospital death due to CV events. For each group, three types of diagnosis were identified: (1) ASCVD composite event, (2) AMI diagnosis, and (3) ischaemic stroke diagnosis. The composite ASCVD event included one of the following: AMI (ICD-9-CM code 410.xx); ischaemic stroke (ICD-9-CM codes 433.xx and 434.xx); or stable CHD (ICD-9-CM code 414.01) with revascularisation (PTCA or CABG NHI procedure code) but without AMI diagnosis. According to the published validation studies of this coding, the PPV for ischaemic stroke was from 0.96 to 1.00, for AMI 0.88, for revascularisation procedure of CABG 0.92 and for PTCA 0.98.15 16 The in-hospital death with AMI or stroke was validated as well with PPV of 0.79.21
Statistical analysis
We used Cox proportional hazards model to analyse the association of statin prescription adherence and persistence on the study outcomes in respective model. Patients’ data started at the index postdischarge statin prescription. Statin treatment adherence and persistence states were analysed as a time-dependent variable. We included baseline variables as time-fixed covariates and lipid test within 1 month prior to a statin prescription change as time-varying covariate to adjust for potential confounding on effect estimates. We presented adjusted HR (aHR) with associated 95% CI for each outcome. Two-sided p value <0.05 was considered statistically significant. We used Statistical Analysis System V.9.3 (SAS Institute) to carry out the analysis.
Results
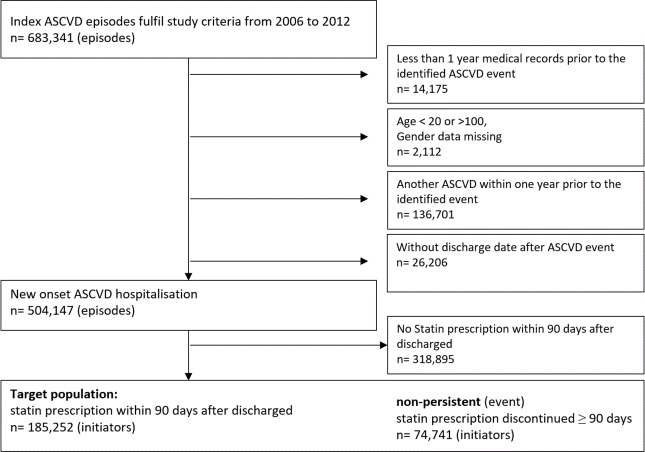
Figure 2 shows the patient selection flow for the study cohort. Within 90 days of hospital discharge for a new ASCVD, there were 185 252 cases initiated statin treatment, and were followed for a total of 412 752 patient-years, with a mean of 2.2 years, ranging from 1 to 7 years. Suboptimal adherence (PDC <0.8) accounted for 47% of the total follow-up duration. Distribution of follow-up duration by persistence states was: 50% in constant use, 13% in recent stop, 26% in non-persistence and 11% in intermittent use (table 2).
Figure 2.
Selection diagram of study cohort. ASCVD, atherosclerotic cardiovascular disease.
Table 2.
Study follow-up and outcomes
| Follow-up | Patient-years | |
| Total | 412 752 | 100% |
| By treatment persistence state | ||
| Constant use period | 208 225 | 50% |
| Recent stop period | 54 051 | 13% |
| Non-persistence period | 106 961 | 26% |
| Intermittent use period | 43 516 | 11% |
| By prescription adherence | ||
| PDC ≥0.8 (good adherence) | 218 412 | 53% |
| PDC <0.8 (suboptimal adherence) | 194 340 | 47% |
| Outcomes | Events (n) |
| Rehospitalisation | |
| All ASCVD hospitalisation | 50 015 |
| AMI hospitalisation | 7986 |
| Ischaemic stroke hospitalisation | 15 179 |
| In-hospital death | |
| Any ASCVD in-hospital death | 2858 |
| AMI in-hospital death | 1273 |
| Ischaemic stroke in-hospital death | 1072 |
AMI, acute myocardial infarction; ASCVD, atherosclerotic cardiovascular disease; PDC, proportion of days covered.
Baseline characteristics of patients and healthcare organisations were shown in table 1 and online supplementary file 1. At index statin prescription, patients’ mean age was 65.4 years, and 66% were male. During the 1 year prior to index hospitalisation, 44% had hyperlipidaemia, 65% had received lipid testing and 47% had ever prescribed statins. The most frequent comorbidity was hypertension (68%), followed by ischaemic heart disease (47%) and diabetes (41%). The mean Charlson comorbidity score was 1.79. The top three concomitant medications were antiplatelet drugs (65%), calcium channel blockers (57%) and β blockers (50%). The most common ASCVD diagnosis at hospital discharge was ischaemic stroke (40%), followed by CHD with revascularisation (37%), and AMI with or without revascularisation (18% and 5%, respectively). During hospitalisation, 82% of the patients had received statin treatment and 88% underwent lipid testing. After discharge, most of the statins were initially prescribed in medical centres (51%), followed by regional hospitals (31%) and district hospitals (9%). Most statins were prescribed by CV specialists (52% CV medicine plus 4% CV surgery), followed by neurologists (29%) (table 1).
Table 1.
Patient and treatment characteristics at baseline and initiation of statin treatment
| Variable | n* | Proportion (%) |
| Total | 185 252 | 100 |
| Age (mean±SD), years | 65.4±12 | |
| Male | 122 881 | 66 |
| 1 year prior to the ASCVD onset | ||
| Prior use of statins | 87 191 | 47 |
| Prior lipid examination | 119 874 | 65 |
| Comorbidity | ||
| Charlson Comorbidity Index score (mean±SD) | 1.79±1.8 | |
| Hyperlipidaemia | 81 655 | 44 |
| Hypertension | 126 530 | 68 |
| Ischaemic heart disease | 87 031 | 47 |
| Diabetes | 76 390 | 41 |
| COPD | 18 112 | 10 |
| Prior use of other medications | ||
| Antiplatelet | 119 947 | 65 |
| CCB | 104 848 | 57 |
| β blockers | 92 994 | 50 |
| ARB | 72 550 | 39 |
| OAD | 68 073 | 37 |
| Baseline ASCVD hospitalisation | ||
| Type of ASCVD diagnosis | ||
| Ischaemic stroke | 74 753 | 40 |
| Stable CHD receiving revascularisation | 67 779 | 37 |
| AMI with PTCA/CABG | 33 021 | 18 |
| AMI without PTCA/CABG | 9699 | 5 |
| In-hospital statin use | 151 020 | 82 |
| In-hospital lipid examination | 163 329 | 88 |
| Index date statin prescription | ||
| Healthcare institute accreditation level | ||
| Medical centre | 95 326 | 51 |
| Regional hospital | 58 111 | 31 |
| District hospital | 17 129 | 9 |
| Other hospital | 10 440 | 6 |
| Clinic | 4246 | 2 |
| Physician specialty | ||
| Cardiovascular medicine | 96 018 | 52 |
| Cardiovascular surgery | 7540 | 4 |
| Neurology | 53 050 | 29 |
| Metabolism and endocrinology | 7899 | 4 |
| Internal medicine | 13 798 | 7 |
| Family medicine | 2971 | 2 |
| Others | 3976 | 2 |
*Except for the variables ‘age’ and ‘Charlson Comorbidity Index score’.
AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; ASCVD, atherosclerotic cardiovascular disease; CABG, coronary artery bypass graft; CCB, calcium channel blocker; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disorder; OAD, oral antidiabetic drug; PTCA, percutaneous transluminal coronary angioplasty.
heartasia-2018-011176supp001.pdf (80.5KB, pdf)
In terms of outcome, a total of 50 015 subsequent ASCVD rehospitalisations were found: among them 7986 were AMI and 15 179 were ischaemic stroke. In-hospital death occurred in 2858 events, out of which 1273 and 1072 fatal events were due to AMI and ischaemic stroke, respectively (table 2). Table 3A summarises the results of association between statin adherence and risk of subsequent ASCVD. Good adherence to treatment (PDC ≥0.8) was associated with statistically significant lower risk of subsequent hospitalisation. The aHRs (95% CI) were 0.90 (0.87 to 0.92) for composite ASCVD outcome, 0.86 (0.81 to 0.92) for AMI and 0.82 (0.78 to 0.86) for ischaemic stroke. Good adherence also associated with significantly lower risk of in-hospital death. The aHRs (95% CI) were 0.59 (0.53 to 0.65) for fatal ASCVD, 0.57 (0.49 to 0.66) for in-hospital death with AMI and 0.61 (0.52 to 0.73) for ischaemic stroke.
Table 3A.
Effect of statin prescription adherence on time to subsequent ASCVD events
| Rehospitalisation outcomes | All ASCVD | AMI | Ischaemic stroke | |||
| aHR* | (95% CI) | aHR* | (95% CI) | aHR* | (95% CI) | |
| Adherence | ||||||
| Suboptimal (PDC <0.8) | Ref | Ref | Ref | |||
| Adherent (PDC ≥0.8) | 0.90 | (0.87 to 0.92)† | 0.86 | (0.81 to 0.92)† | 0.82 | (0.78 to 0.86)† |
| In-hospital fatal outcomes | Fatal ASCVD | Fatal AMI | Fatal ischaemic stroke | |||
| aHR* | (95% CI) | aHR* | (95% CI) | aHR* | (95% CI) | |
| Adherence | ||||||
| Suboptimal (PDC <0.8) | Ref | Ref | Ref | |||
| Adherent (PDC ≥0.8) | 0.59 | (0.53 to 0.65)† | 0.57 | (0.49 to 0.66)† | 0.61 | (0.52 to 0.73)† |
Table 3B.
Effect of statin prescription persistence on time to subsequent ASCVD events
| Rehospitalisation outcomes | All ASCVD | AMI | Ischaemic stroke | |||
| aHR* | (95% CI) | aHR* | (95% CI) | aHR* | (95% CI) | |
| Persistence | ||||||
| Constant use period | Ref | Ref | Ref | |||
| Recent stop period | 1.16 | (1.13 to 1.19)† | 1.51 | (1.42 to 1.62)† | 1.38 | (1.32 to 1.45)† |
| Non-persistence period | 1.13 | (1.10 to 1.16)† | 1.60 | (1.49 to 1.71)† | 1.47 | (1.40 to 1.54)† |
| Intermittent use period | 1.26 | (1.21 to 1.31)† | 1.21 | (1.09 to 1.35)† | 1.26 | (1.17 to 1.35)† |
| In-hospital fatal outcomes | Fatal ASCVD | Fatal AMI | Fatal ischaemic stroke | |||
| aHR* | (95% CI) | aHR* | (95% CI) | aHR* | (95% CI) | |
| Persistence | ||||||
| Constant use period | Ref | Ref | Ref | |||
| Recent stop period | 1.42 | (1.28 to 1.59)† | 1.47 | (1.25 to 1.71)† | 1.54 | (1.29 to 1.84)† |
| Non-persistence period | 0.91 | (0.82 to 1.01) | 0.86 | (0.73 to 1.01) | 1.10 | (0.92 to 1.30) |
| Intermittent use period | 1.17 | (0.98 to 1.39) | 1.16 | (0.88 to 1.52) | 1.04 | (0.77 to 1.39) |
*Using Cox proportional hazards model and adjusted for age, sex, types of baseline ASCVD diagnosis, history of hyperlipidaemia 1 year before hospitalisation, Charlson Comorbidity Index 1 year before hospitalisation, medications 1 year before the enrolment date (antiplatelet, statin), number of lipid examination 1 year before and during hospitalisation, level of healthcare organisation, physician specialty of index statin, dosage of index statin and time-varying covariate of lipid examination 1 month before statin dose change.
†P<0.05.
aHR, adjusted HR; AMI, acute myocardial infarction; ASCVD, atherosclerotic cardiovascular disease; PDC, proportion of days covered.
Table 3B summarises the results of statin persistence on subsequent ASCVD outcomes. Compared with constant use of statins, the other three lower persistence states (recent stop, non-persistence and intermittent use) were all associated with significantly higher risk of subsequent ASCVD rehospitalisation. The aHR for ASCVD composite outcome ranged from 1.13 to 1.26; for AMI from 1.21 to 1.60; and for ischaemic stroke from 1.26 to 1.47 (all p<0.05). For in-hospital death, the patient in the state of recent stop with statin treatment (failed to refill prescription for less than 90 days) was associated with significantly higher fatal risk compared with that of patients in the constant use state (p<0.05). The aHRs (90% CI) were 1.42 (1.28 to 1.59), 1.47 (1.25 to 1.71) and 1.54 (1.29 to 1.84) for fatal ASCVD, fatal AMI and fatal ischaemic stroke, respectively. The associations of non-persistence and intermittent use states with fatal events were not statically significant.
Discussion
Statin therapy is important for secondary prevention of both non-fatal and fatal subsequent ASCVD events. The evidence has shown that the effect of statins persists if they are taken for long term.22 The use of high-intensity statins is recommended by several international treatment guidelines published in recent years.1 9 10 In this long-term follow-up of ASCVD discharge cohort, we found good adherence (PDC ≥0.8) with statin treatment was associated with a 10% lower risk of subsequent ASCVD rehospitalisation. The strength of association was even stronger, specifically with AMI and ischaemic stroke (14% and 18% lower risk, respectively); the strongest association was with in-hospital death (40% lower risk). With respect to persistence, compared with the ‘constant use’ period, all the three lower persistence states (recent stop, non-persistence and intermittent use) were also associated with significantly higher risk of rehospitalisation outcomes across the composite ASCVD event as well as with AMI and ischaemic stroke events. These findings in the real-world practice for the effectiveness of use of statin for secondary prevention of ASCVD were consistent with the efficacy results of RCTs for statins compared with placebo on secondary prevention of CV events with the effect size of relative risk reduction in the range of 21%–30%.9 Effectiveness of statin treatment on secondary prevention of ASCVD should be as expected if the medications were well taken.
In the analysis of statin persistence, we found that patients in ‘recent stop’ period were associated with significantly increased risk of both fatal and non-fatal events. Recent stop was defined as ‘failed to refill statin within 90 days of end of last prescription,’ this observation characterised the early emergence of recurrent ASCVD facing treatment discontinuation. It may relate to the theory of low-density lipoprotein (LDL) variability suggested by Bangalore et al,23 who found that patients with LDL fluctuation were subjected to higher risk of coronary and other CV events in a post hoc analysis of the Treat to New Targets trial. The discontinuation of statin treatment that defined the ‘recent stop’ period would likely be associated with LDL level rebounded in majority of patients.24 Our observation was in consistent with the review of 13 studies of statin non-adherence or discontinuation on CV outcomes,25 which found that patients with highly variable statin adherence were at particular risk for secondary ASCVD events. Moreover, within 1 month following the discontinuation of statin, the CV risk in diabetes and rheumatoid arthritis populations was significantly increased.25 This phenomenon may also be complementary with the findings of early reduction of CV risk within few weeks of statin initiation in RCTs of patients with post-AMI or those who underwent revascularisation procedures.6 7 26 27 To summarise, the result of ‘recent stop’ revealed the importance of the short-term acute effect of failure to maintain optimal treatment in patients with established ASCVD and emphasised the importance of sustained statin treatment. We noted that the ‘non-persistence’ and ‘intermittent use’ states had little effect on the fatal ASCVD outcomes, which suggested that once a patient survived the ‘recent stop’ period, the risk of fatal ASCVD outcomes diminished and became similar to that of the constant use state. It is worthy for further studies on this phenomenon in the future.
Several issues in the analysis of treatment effect in real world were considered in the design of this study. Reduction or changing of treatment compliance over time is common in real-world practice, especially for the long-term prevention of recurrent disease. In order to assess the treatment pattern more accurately, we used time-dependent Cox model to analyse adherence and persistence as time-varying variables. This method captured real-life drug use patterns allowed for variation in adherence and persistence over the 7 years of study follow-up. This is in contrast to other studies that used time-fixed measurement by pooling prescriptions across the whole follow-up period28 or taking a single time point observation29 that may subject to higher measurement error and result in biased estimate of effect. Furthermore, we analysed adherence and persistence in separate models to account for the two different constructs.20 PDC is commonly used to analyse treatment adherence and more consistently defined in previous studies; thus the result supported the existing published literature.13 19 20 However, to calculate PDC, data need to be pooled across a period of time, the fluctuation within the calculation period will be smoothed out and the effect related to the change in prescription could not be captured. This drawback was recovered in our analysis of persistence and found recent stop associated with increased recurrent CV risk.
We used the new user design with all the subjects included in the analysis-initiated statin within the same 90 days’ period of hospital discharge to minimise the potential selection by treatment indication. To control for potential confounders, we included extended variables covering factors of all the five dimensions related to medication adherence, described by the WHO.30 We included the age and sex for ‘patient factors’; diagnosis of index ASCVD event, comorbidities and use of other medications for ‘condition factors’; prior use of statin, history of lipid examination for ‘therapy factors’; healthcare institute accreditation level and physician specialty for ‘healthcare system factors’; and geographic location and year of index prescription for ‘socioeconomic factors’. Most previous studies were not able to be as comprehensive with covering all the dimensions for adherence.13 In addition to the five dimensions of baseline factors, we included lipid testing prior to changing of statin prescription as another time-dependent variable to adjust for potential healthy adherer effect17 in the consideration of regular lipid testing, a potential indicator of better healthy-seeker behaviour.
Financial burden has been recognised as an important factor of statin adherence.31 One additional advantage of this study is that the claims data are taken from the NHI, which provides comprehensive coverage of medical services, including all the statins approved in Taiwan. This has minimised the potential impact of copayment affordability to adherence in this study. Also, the universal coverage of the NHI minimised the bias due to selection of patients or lost to follow-up due to change between health plans. The method used to define the study population and identify outcomes has been validated with high PPV in the NHI claims database.15 16
There were also limitations in this study. First, this study was based on the prescription records. We could not evaluate to what extent the prescribed medication was actually consumed by patients; thus, there could be potential exposure measure error, which may bias the estimates towards the null and result in conservative effect. Second, laboratory examination result of serum lipid level was not available in the database, which is an important parameter for the prescription of statins and predictor for CV outcomes. But this information might be important to the initiation of statin treatment; in the patients with established ASCVD and initiated treatment, their treatment should be maintained. Third, we could not capture lifestyle variables and socioeconomic factors, which may introduce confounding on the effect measurement. These could be factors that warrant further study to enhance treatment compliance and improve patient outcomes. Finally, we were not able to collect data on statin intolerance and side effects, although statins are generally well tolerated, the impact to the study result might be limited.
Conclusions
Good statin prescription adherence and persistence are associated with significant reduction in risk for ASCVD rehospitalisation in the context of secondary prevention. There is a need for further investigation into the factors associated with improving statin adherence and persistence and in developing effective strategies. As suggested by the WHO, medication adherence and persistence are related to multidimensional factors. To treat lipid disorders and reduce CV risk, we need to rely on more than the pharmaceutical effect. Most clinical guidelines recommend that the management of lipid disorders must incorporate lifestyle modifications and patient compliance behaviour. Our study suggests that optimising patient adherence and persistence behaviours is a core driver to improving patient outcomes.
Footnotes
Contributors: JZL and SF have made substantial contributions to the conception and design of the study, interpretation of data and drafting of the article. WYS, STH, CLL, STC and MSL have made substantial contributions to the conception and design of the study, acquisition of data, analysis and interpretation of data and drafting the article. VCT has made substantial contributions to the interpretation of data and drafting of the article. All authors were involved in revising the study critically for important intellectual content and gave final approval of the version to be submitted.
Funding: This study was sponsored by Pfizer which is the manufacturer of Lipitor, the original brand of atorvastatin (Study ID A2581195). Editorial assistance was provided by APCER Life Sciences and funded by Pfizer.
Disclaimer: Pfizer management was not involved in the review and interpretation of the results; the interpretation of the results was only from the authors. All analyses were done by the Formosan Medical Association; in fact, Pfizer did not have access to the data thus would not be able to, and did not, analyse the data. Because of this, the analyses were not repeated by another independent group. Pfizer follows the ICMJE standards, and this was clearly specified in the contract with the academic contractor Formosan Medical Association for this study: 'For all Publications, Investigator will comply with recognized ethical standards concerning publications and authorship, including Section II–'Ethical Considerations in the Conduct and Reporting of Research' of the Uniform Requirements for Manuscripts Submitted to Biomedical Journals, http://www.icmje.org/index.html%23authorship, established by the International Committee of Medical Journal Editors.'
Competing interests: Among the authors of this study, WYS, JZL and SF are employees of Pfizer. STH, STC and MSL received research grant from Pfizer. VCT, who is an employee of APCER, received funding from Pfizer. CLL received consultancy fee from Pfizer.
Ethics approval: The study protocol was reviewed by the Research Ethics Committee of the National Taiwan University, and was granted waiving of ethics approval and individual consent in accordance with local regulations.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data are available upon reasonable request.
References
- 1. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American heart association Task force on practice guidelines. J Am Coll Cardiol 2014;63:2889–934. 10.1016/j.jacc.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 2. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke Statistics-2016 update: a report from the American heart association. Circulation 2016;133:e38–60. 10.1161/CIR.0000000000000350 [DOI] [PubMed] [Google Scholar]
- 3. Dawber TR, Moore FE, Mann GV. Coronary heart disease in the Framingham study. Am J Public Health Nations Health 1957;47:4–24. 10.2105/AJPH.47.4_Pt_2.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham study. Am J Cardiol 1976;38:46–51. 10.1016/0002-9149(76)90061-8 [DOI] [PubMed] [Google Scholar]
- 5. Amarenco P, Bogousslavsky J, Callahan A, et al. High-Dose atorvastatin after stroke or transient ischemic attack. J Vasc Surg 2006;44:1374–59. 10.1016/j.jvs.2006.10.008 [DOI] [PubMed] [Google Scholar]
- 6. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495–504. 10.1056/NEJMoa040583 [DOI] [PubMed] [Google Scholar]
- 7. Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001;285:1711–8. 10.1001/jama.285.13.1711 [DOI] [PubMed] [Google Scholar]
- 8. Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366:1267–78. 10.1016/S0140-6736(05)67394-1 [DOI] [PubMed] [Google Scholar]
- 9. NICE clinical guideline CG181 Lipid modification: cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease. clinical guideline. methods, evidence and recommendations. NICE clinical guideline CG181 lipid modification: cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease clinical guideline methods, evidence and recommendations. London; 2014. [Google Scholar]
- 10. Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the European Assocciation for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis 2016;253:281–344. 10.1016/j.atherosclerosis.2016.08.018 [DOI] [PubMed] [Google Scholar]
- 11. Hippisley-Cox J, Coupland C. Effect of statins on the mortality of patients with ischaemic heart disease: population based cohort study with nested case-control analysis. Heart 2006;92:752–8. 10.1136/hrt.2005.061523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kumbhani DJ, Steg PG, Cannon CP, et al. Adherence to secondary prevention medications and four-year outcomes in outpatients with atherosclerosis. Am J Med 2013;126:693–700. 10.1016/j.amjmed.2013.01.033 [DOI] [PubMed] [Google Scholar]
- 13. De Vera MA, Bhole V, Burns LC, et al. Impact of statin adherence on cardiovascular disease and mortality outcomes: a systematic review. Br J Clin Pharmacol 2014;78:684–98. 10.1111/bcp.12339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. National Health Insurance Administration, Ministry of Health and Welfare, Taiwan, R.O.C National health insurance annual report 2014–2015; 2014.
- 15. Cheng C-L, Kao Y-HY, Lin S-J, et al. Validation of the National health insurance research database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf 2011;20:236–42. 10.1002/pds.2087 [DOI] [PubMed] [Google Scholar]
- 16. Cheng C-L, Lee C-H, Chen P-S, et al. Validation of acute myocardial infarction cases in the National health insurance research database in Taiwan. J Epidemiol 2014;24:500–7. 10.2188/jea.JE20140076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shrank WH, Patrick AR, Brookhart MA. Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med 2011;26:546–50. 10.1007/s11606-010-1609-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choudhry NK, Shrank WH, Levin RL, et al. Measuring concurrent adherence to multiple related medications. Am J Manag Care 2009;15:457–64. [PMC free article] [PubMed] [Google Scholar]
- 19. Simpson RJ, Mendys P. The effects of adherence and persistence on clinical outcomes in patients treated with statins: a systematic review. J Clin Lipidol 2010;4:462–71. 10.1016/j.jacl.2010.08.026 [DOI] [PubMed] [Google Scholar]
- 20. Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health 2008;11:44–7. 10.1111/j.1524-4733.2007.00213.x [DOI] [PubMed] [Google Scholar]
- 21. Cheng C-L, Chien H-C, Lee C-H, et al. Validity of in-hospital mortality data among patients with acute myocardial infarction or stroke in national health insurance research database in Taiwan. Int J Cardiol 2015;201:96–101. 10.1016/j.ijcard.2015.07.075 [DOI] [PubMed] [Google Scholar]
- 22. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016;388:2532–61. 10.1016/S0140-6736(16)31357-5 [DOI] [PubMed] [Google Scholar]
- 23. Bangalore S, Fayyad R, Laskey R, et al. Lipid lowering in patients with treatment-resistant hypertension: an analysis from the treating to new targets (TnT) trial. Eur Heart J 2014;35:1801–8. 10.1093/eurheartj/eht315 [DOI] [PubMed] [Google Scholar]
- 24. Mann DM, Glazer NL, Winter M, et al. A pilot study identifying statin nonadherence with visit-to-visit variability of low-density lipoprotein cholesterol. Am J Cardiol 2013;111:1437–42. 10.1016/j.amjcard.2013.01.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Phan K, Gomez Y-H, Elbaz L, et al. Statin treatment non-adherence and discontinuation: clinical implications and potential solutions. Curr Pharm Des 2014;20:6314–24. 10.2174/1381612820666140620162629 [DOI] [PubMed] [Google Scholar]
- 26. Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003;361:1149–58. 10.1016/S0140-6736(03)12948-0 [DOI] [PubMed] [Google Scholar]
- 27. Patti G, Pasceri V, Colonna G, et al. Atorvastatin pretreatment improves outcomes in patients with acute coronary syndromes undergoing early percutaneous coronary intervention: results of the ARMYDA-ACS randomized trial. J Am Coll Cardiol 2007;49:1272–8. 10.1016/j.jacc.2007.02.025 [DOI] [PubMed] [Google Scholar]
- 28. Li Y-C, Huang W-L. Effects of adherence to statin therapy on health care outcomes and utilizations in Taiwan: a population-based study. Biomed Res Int 2015;2015:149573 10.1155/2015/149573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ho PM, Spertus JA, Masoudi FA, et al. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med 2006;166:1842–7. 10.1001/archinte.166.17.1842 [DOI] [PubMed] [Google Scholar]
- 30. WHO Adherence to long-term therapies. Evidence for action, 2003. Available: http://apps.who.int/iris/bitstream/10665/42682/1/9241545992.pdf
- 31. Ellis JJ, Erickson SR, Stevenson JG, et al. Suboptimal statin adherence and discontinuation in primary and secondary prevention populations. J Gen Intern Med 2004;19:638–45. 10.1111/j.1525-1497.2004.30516.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartasia-2018-011176supp001.pdf (80.5KB, pdf)