Abstract
Binding of angiopoietin-1 (Ang-1) to its receptor Tie2 on endothelial cells (ECs) promotes vessel barrier integrity and angiogenesis. Here, we identify PAK2 and paxillin as critical targets of Ang-1 responsible for EC migration, polarization, and sprouting. We found that Ang-1 increases PAK2-dependent paxillin phosphorylation and remodeling of focal adhesions and that PAK2 and paxillin are required for EC polarization, migration, and angiogenic sprouting in response to Ang-1. Our findings show that Ang-1 triggers Cdc42 activation at the leading edges of migrating ECs, which is dependent on PAK2 and paxillin expression. We also established that the polarity protein Par3 interacts with Cdc42 in response to Ang-1 in a PAK2- and paxillin-dependent manner. Par3 is recruited at the leading edges of migrating cells and in focal adhesion, where it forms a signaling complex with PAK2 and paxillin in response to Ang-1. These results show that Ang-1 triggers EC polarization and angiogenic sprouting through PAK2-dependent paxillin activation and remodeling of focal adhesions, which are necessary for local activation of Cdc42 and the associated polarity complex. We have shown that PAK2 controls a signaling pathway important for angiogenic sprouting that links focal adhesions to polarity signaling in ECs.
INTRODUCTION
Angiogenesis, the formation of new blood vessels from preexisting ones, is a multistep process that requires accurate regulation of proliferation, migration, invasion, and differentiation of endothelial cells (ECs). Once formed, new blood vessels must stabilize and mature in order to sustain blood perfusion (Jain, 2003). Among the angiogenic factors involved in the maturation of blood vessels, angiopoietin-1 (Ang-1) has been shown to promote angiogenic sprouting and blood vessel stabilization (Thomas and Augustin, 2009). Multiple intracellular signaling pathways in ECs have been shown to be involved in the tightening of cell junctions between ECs and in blood vessel stabilization by Ang-1 and its tyrosine kinase receptor, Tie2. Ang-1–induced activation of Tie2 stabilizes cell–cell junctions through activation of the phosphatase receptor VE-PTP, which prevents VE-cadherin phosphorylation and internalization (Saharinen et al., 2008; Winderlich et al., 2009). Furthermore, Ang-1 binding to Tie2 prevents endothelial barrier permeability from being increased by VEGF by mediating PKCζ-dependent phosphorylation of eNOS, which inhibits NO release from ECs (Oubaha and Gratton, 2009). Last, Ang-1 prevents VEGF-induced Src activation leading to VE-cadherin phosphorylation and internalization through RhoA/mDia (Gavard et al., 2008). In addition to its roles at cell–cell junctions, Ang-1 has been shown to accumulate in the extracellular matrix, where it activates Tie2, which can interact with α5β1-integrin, leading to PI3K-dependent regulation of focal adhesion kinase (FAK) involved in EC migration and in the angiogenic response (Kim et al., 2000; Cascone et al., 2005; Fukuhara et al., 2008; Saharinen et al., 2008).
We previously showed that Ang-1 promotes collective and oriented migration of ECs through the formation of a β-catenin and PKCζ complex leading to Rac1 activation (Oubaha et al., 2012). This study linked Tie2 signaling at adherens junctions to the establishment of EC polarity and collective cell migration. Many effectors of Rac1 have been characterized so far; these include PAKs (p21-activated kinases), which are involved in lamellipodial protrusion and cell migration. The six PAK members are divided into two groups, I and II, that share similar characteristics and functions. PAK1 enzymatic activity has been extensively studied in vitro, and its expression is mostly restricted to the brain, spleen, and skeletal muscles. PAK1-deficient mice are viable and fertile, with minor immune and glucose homeostasis defects (McDaniel et al., 2008). In contrast, PAK2 is ubiquitously expressed and its global deletion is lethal at E8.0 due to hemorrhages (Kelly and Chernoff, 2012; Radu et al., 2015). ECs express mostly PAK2, and its conditional deletion in the endothelium of adult mice triggers an increase in vascular permeability, demonstrating its critical role in the maintenance of endothelial barrier integrity (Radu et al., 2015). The role of PAKs in the control of cell migration is well documented (Galan Moya et al., 2009). One of the mechanisms involved is the regulation of paxillin by phosphorylation (Nayal et al., 2006). Paxillin is a structural component of the focal adhesions, which interacts with many “integrin adhesome” network partners, including focal adhesion kinase (FAK), Src, β-PIX, and GIT1 (Brown and Turner, 2004; Zaidel-Bar et al., 2007a,b). Integrins’ engagement with the extracellular matrix triggers the formation of focal adhesions, which transmit mechanical forces to the cell cytoskeleton, leading to cell adhesion and lamellipodial protrusions and promoting cell migration (Nayal et al., 2006). The PAK–paxillin signaling pathway is well established as a regulator of cell protrusion and migration, but its role in cell polarization, a critical step of directed cell migration and sprouting, remains undefined.
We previously performed a phosphoproteome profiling of ECs stimulated with Ang-1 and identified PAK2 as being phosphorylated on Ser141 following Ang-1 stimulation and as part of interaction networks of phosphoproteins associated with cytoskeletal organization and cell motility (Chidiac et al., 2016). This work prompted us to investigate the role of PAK2 in the angiogenic response induced by Ang-1 on ECs. We characterize a novel mechanism where PAK2 promotes paxillin reorganization in focal adhesions in response to Ang-1 leading to Cdc42 activation and EC polarization. We show that PAK2 activation by Ang-1 links focal adhesions to polarity signaling in ECs, which leads to angiogenic sprouting.
RESULTS
PAK2 is required for Ang-1–induced oriented migration and polarization of Ecs
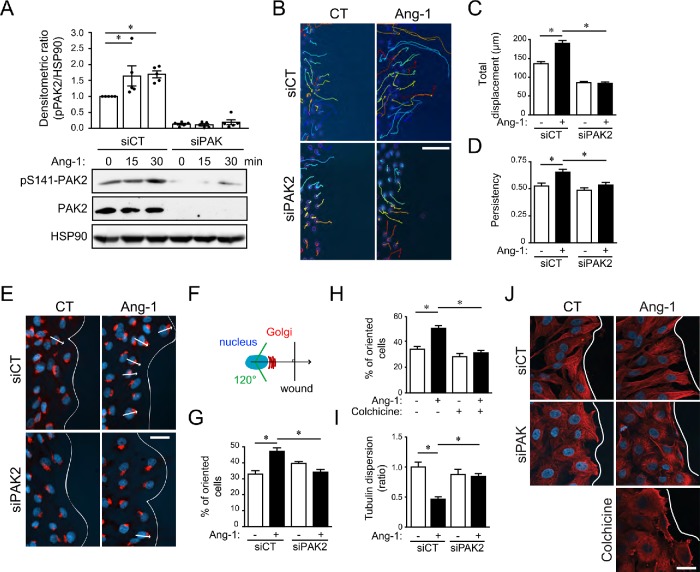
We previously found by phosphoproteomics analyses that PAK2 is phosphorylated on Ser141 following treatment of bovine aortic endothelial cells (BAECs) with Ang-1 (Chidiac et al., 2016). This study also revealed that PAK2 phosphorylation in response to Ang-1 stimulation of ECs was associated with subnetworks of phosphoproteins involved in cytoskeleton organization and cell motility (Chidiac et al., 2016). Building on these findings, we confirmed the phosphorylation of PAK2 on Ser141 following Ang-1 stimulation of BAECs by Western blot analyses (Figure 1A). To explore the role of PAK2 in Ang-1 signaling in ECs, we designed and validated small interfering RNA (siRNA) duplexes targeting PAK2 (Figure 1A and Supplemental Figure S1A). First, we determined the role of PAK2 in Ang-1–induced EC migration, using a scratch assay and tracking ECs during migration into the wounded area. PAK2 depletion by siRNA strongly prevented the induction of cell migration by Ang-1 (Figure 1, B and C). Interestingly, we observed that the persistence of the residual migration of PAK2-depleted cells in response to Ang-1 was also affected (Figure 1D). This suggests that PAK2 might be involved in cell polarization stimulated by Ang-1. Thus, we examined the ability of ECs to orient their Golgi apparatus to the migration front as a marker of cell polarization (Etienne-Manneville and Hall, 2001). We observed that 50% of ECs displayed proper orientation of the Golgi toward the wound following 60 min of Ang-1 stimulation, whereas the Golgi complexes of untreated cells were still randomly oriented (30%, Figure 1, E–G). PAK2 depletion by siRNA completely inhibited this Ang-1–induced orientation of the Golgi. Together, these results suggest that PAK2 is required for polarization and migration of ECs stimulated by Ang-1.
FIGURE 1:
PAK2 is required for Ang-1–induced BAEC oriented migration, polarization, and microtubule organization. (A) BAECs were transfected with control siRNA (siCT) or targeting PAK2 (siPAK2). siCT- and siPAK2-transfected cells were starved and treated with Ang-1 (100 ng/ml) and cell lysates were extracted at the indicated times. Lysates were immunoblotted using an anti-phopho-Ser141-PAK2 antibody. Hsp90 was used as a loading control. Bar graph shows the average densitometric ratios of five experiments. (B) BAECs were transfected with siCT or siPAK2. After 48 h, confluent cell monolayers were scratched, and migration into the wound was monitored over 6 h in the presence or absence of Ang-1 (100 ng/ml). Representative pictures of individual cell tracking over 6 h are shown. (C, D) Quantifications of the total cell migration and persistence of displacements are shown. Persistence is calculated by the ratio of the net (distance between the start and the final position) and the total displacement for each track. The graphs are representative of three independent experiments yielding identical results (siCT: n = 80 cells; siCT+Ang-1: n = 120 cells; siPAK: n = 121 cells; siPAK+Ang-1: n = 107 cells). Bar: 100 µm. (E) Confluent monolayers of BAECs transfected with siCT or siPAK2 were scratched and treated for 30 min with Ang-1 (100 ng/ml) before fixation and staining for GM130 (Golgi marker, red) and nucleus (DAPI, blue). The arrows indicate the orientation of the cells considered as polarized toward the wound (white line). (F) Diagram representing the orientation of the Golgi and the nucleus according to the position of the wound. (G) Quantification showing the percentage of cells with the Golgi oriented toward the wound (120°). The graph is representative of three independent experiments yielding identical results (siCT: n = 36 cells; siCT+Ang-1: n = 36 cells; siPAK: n = 36 cells; siPAK+Ang-1: n = 35 cells). White lines show the migration front. Bar: 25 µm. (H) Effect of colchicine treatment (10 nM; 60 min) on Ang-1–induced (100 ng/ml) Golgi orientation toward the wound (120°). The graph is representative of three independent experiments yielding identical results. n = 30 cells per condition; experiment was repeated three times. (I, J) BAECs were transfected with control (siCT) or siPAK2. Scratches were performed on confluent monolayer and microtubule organization was observed by immunofluorescence for tubulin (red) and nucleus (DAPI). Quantification of tubulin dispersion using ImageJ is shown in I (see Materials and Methods). n = 20 cells per condition were quantified; experiment was repeated three times. White lines in J show the migration front. Bar: 20 µm. * p < 0.05.
We then confirmed that microtubule reorganization was important for Golgi orientation stimulated by Ang-1. Treatment of ECs with colchicine (10 μM; 60 min) inhibited microtubule polymerization, had minimal effect on the integrity of the Golgi apparatus, but abolished Ang-1–induced orientation of the Golgi toward the migration front (Figure 1H). Furthermore, Ang-1 stimulation of ECs induced the organization of microtubules, measured as the dispersion of the tubulin staining of cells. Indeed, Ang-1 stimulation resulted in a decrease in the dispersion of tubulin; lower dispersion implies a higher organization of microtubules. This microtubule reorganization induced by Ang-1 was inhibited in ECs where PAK2 was down-regulated (Figure 1, I and J).
PAK2-dependent activation of Cdc42 at the leading edge
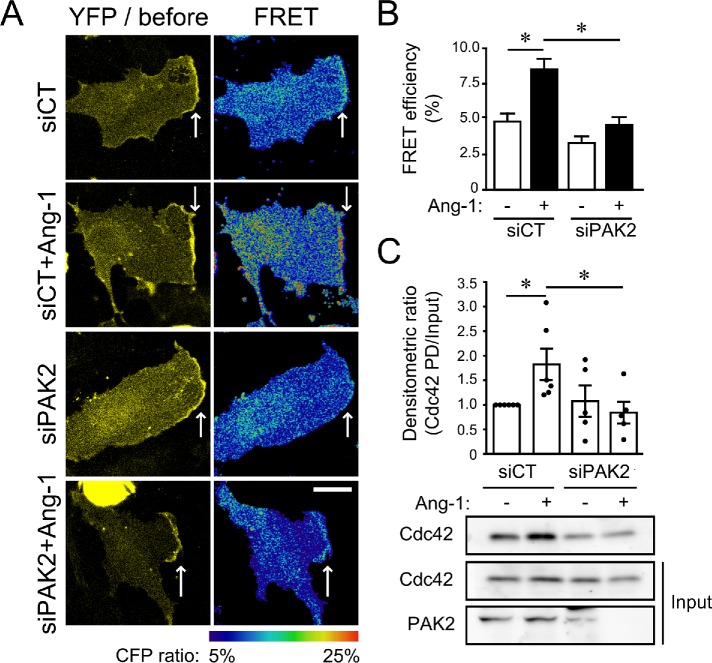
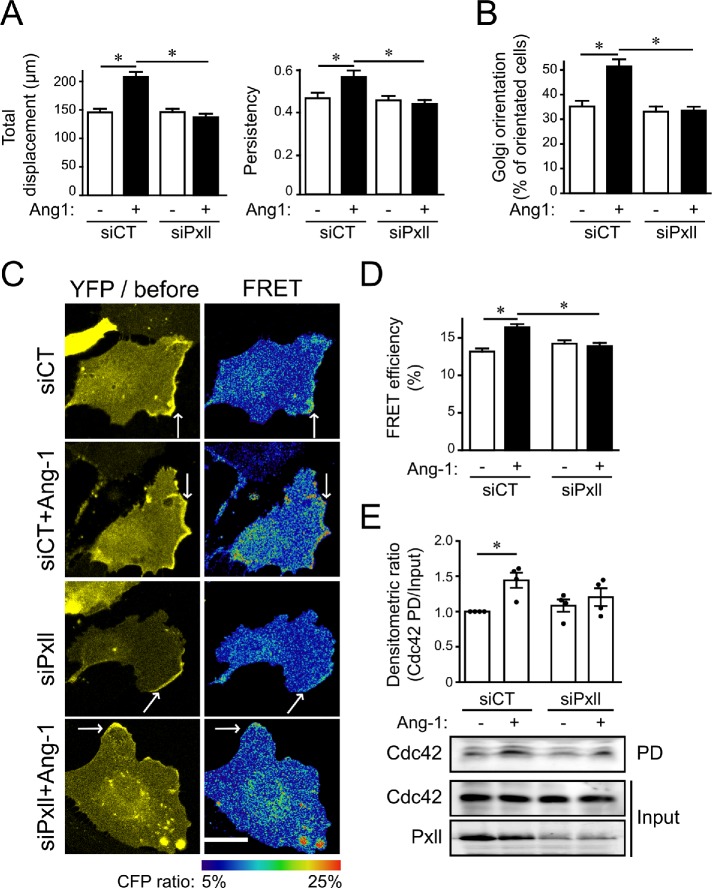
Cdc42 activation is considered as the early step of cell polarization during oriented cell migration (Etienne-Manneville and Hall, 2001; Cau and Hall, 2005). To understand how cell polarization is regulated by Ang-1, we determined the role of PAK2 in the activation of Cdc42. We used the Raichu-Cdc42 probe to perform Förster resonance energy transfer (FRET) by photobleaching in order to visualize the localization of activated Cdc42 (Itoh et al., 2002). We observed that Cdc42 (YFP signal) and activated Cdc42 (FRET signal) accumulated at the leading edges of migrating ECs following 30 min of stimulation with Ang-1 (Figure 2A). Some increases in active Cdc42 signal are visible in the perinuclear region, which may be at the Golgi complex (Farhan and Hsu, 2016). The depletion of PAK2 by siRNA prevented the recruitment and activation of Cdc42 at the leading edges of ECs in response to Ang-1 (Figure 2, A and B). Pull-down assays confirmed that depletion of PAK2 prevents Cdc42 activation (Figure 2C). In contrast, Rac1 activation following Ang-1 stimulation was not affected by PAK2 depletion ECs (Supplemental Figure S2). These results suggest that PAK2 is required for Cdc42 activation in response to Ang-1, which leads to ECs’ orientation.
FIGURE 2:
PAK2 is required for Ang-1–induced Cdc42 activation at the leading edge of migrating cells. (A) BAECs were transfected with plasmids coding for the Cdc42-Raichu probe and control siRNA (siCT) or targeting PAK2 (siPAK2). After 48 h, confluent BAEC monolayers were scratched and treated or not treated with Ang-1 (100 ng/ml; 30 min). After fixation, FRET by photobleaching was performed on cells at the migration front and the ratio of CFP was measured at the leading edge of cells where Cdc42-Raichu accumulates (white arrows). (B) Histogram representing the average of four independent experiments shown in A measuring the CFP ratio at the leading edge (siCT: n = 29 cells; siCT+Ang-1: n = 36 cells; siPAK2: n = 27 cells; siPAK2+Ang-1: n = 31 cells). * p < 0.05; bar: 20 µm. (C) Cdc42 activation was determined by pull-down (PD) assays using GST-PAK-CD. BAECs were transfected with siCT or siPAK2. After 48 h, cells were treated for 5 min with Ang-1 and PD assays were performed on protein extracts to reveal the amount of activated Cdc42 (Cdc42-GTP) in comparison with the total amount of Cdc42. Down-regulation of PAK2 was confirmed by immunoblotting and HSP90 was used as a loading control. Bar graph shows the mean ± SEM densitometric ratio of at least five experiments. * p < 0.05.
PAK2 is required for EC sprouting by Ang-1
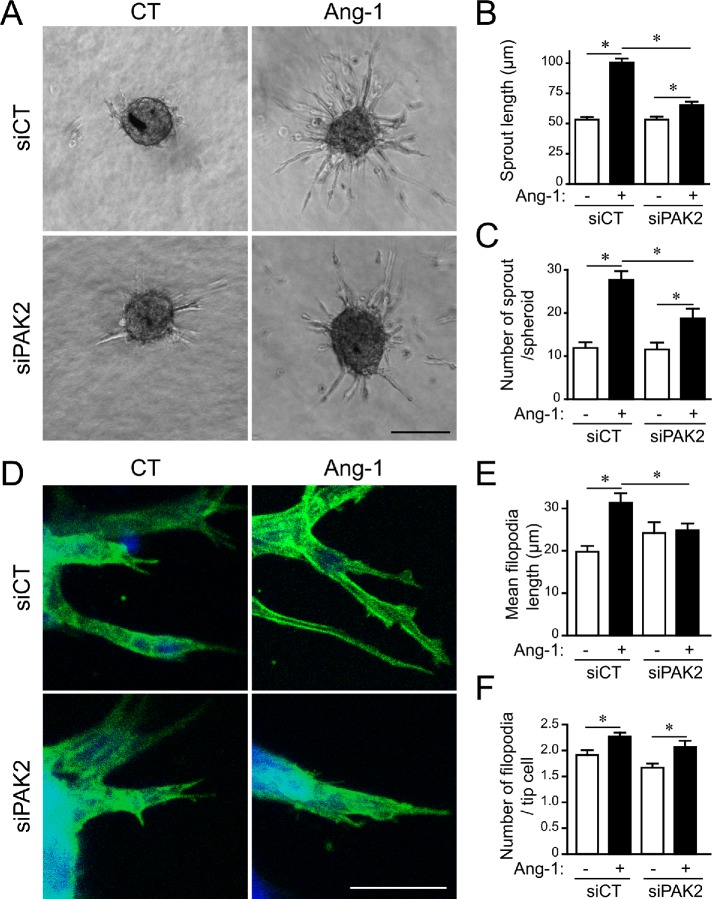
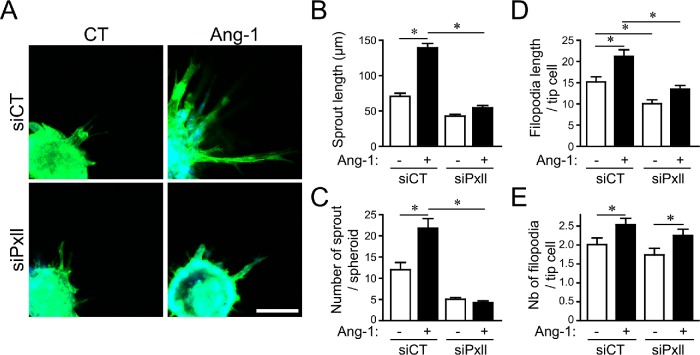
We previously showed that Ang-1 promotes directional migration and sprouting of ECs from spheroids cultured in collagen (Oubaha et al., 2012). We examined the role of PAK2 in the formation of sprouts from EC spheroids in response to Ang-1. We found that spheroids of BAECs transfected with siPAK2 had fewer sprouts and a shorter sprout length in the presence of Ang-1 than those that were transfected with control siRNA (Figure 3, A–C). We also found that PAK2-depleted cells at sprout tips had shorter filopodial extensions in response to Ang-1 than control cells (Figure 3, D and E). However, the number of filopodia per tip cell was not affected by PAK2 depletion (Figure 3, D and F). This shows that PAK2 is required for the growth of angiogenic EC sprouts induced by Ang-1.
FIGURE 3:
PAK2 is required for Ang-1–induced sprouting of BAECs. (A) Representative images from spheroid-based angiogenesis assays generated from BAECs transfected with control siRNA (siCT) or targeting PAK2 (siPAK2) and exposed to Ang-1 (100 ng/ml) or left untreated (CT). Pictures were taken 24 h after embedding in a collagen matrix. Scale bar represents 100 μm. (B) Quantification of capillary-like sprouting from spheroids was measured under every condition as indicated. Data are shown as means of sprout length. (C) Number of sprouts per spheroid. Results are displayed as mean values ± SEM and show one of three experiments with eight spheroids per condition. (D) Spheroids were generated and treated with Ang-1 as in A and were stained for actin (phalloidin in green) and nucleus (DAPI in blue) to reveal filopodial extensions from the tip cells in response to Ang-1. Bar: 40 µm. (E) The mean filopodium length and (F) the number of filopodia per tip cell were measured. Results are displayed as mean values ± SEM and show the result of one over three experiments. n = 15–30 tip cells from five different spheroids per condition. * p < 0.05.
Ang-1 promotes PAK2-dependent reorganization of paxillin in focal adhesions
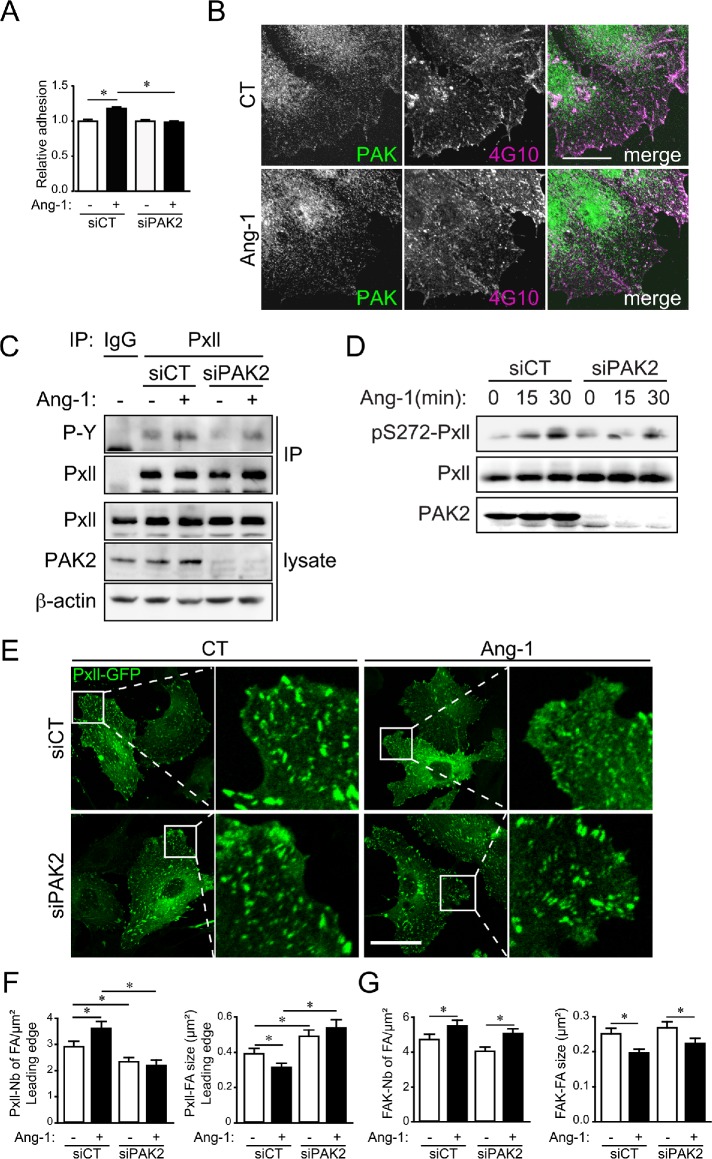
PAK2 participates in the process of adhesion to extracellular matrix and in focal adhesion remodeling in many cell types. First, we show that Ang-1 treatment of ECs increased their adhesion to a gelatin substrate, and this effect was abolished in siPAK2-transfected ECs (Figure 4A). In addition, Ang-1 stimulation of ECs induced an increase and a relocalization of total phosphotyrosine staining (4G10 antibody), which is generally considered a marker of focal adhesions (Figure 4B). Compared with untreated cells, phosphotyrosine staining in Ang-1–treated ECs was located in smaller and more abundant puncta throughout the cell. PAK2 staining colocalized with phosphotyrosine in control and Ang-1–treated cells. The focal adhesion protein paxillin is known to be regulated by PAKs and phosphorylated on multiple serine, threonine, and tyrosine residues (Ostergaard et al., 1998; Nayal et al., 2006). We show that Ang-1 stimulation increased the phosphorylation of paxillin on tyrosine residues and on the putative PAK phosphorylation site Ser 272 of paxillin. Both these Ang-1–dependent phosphorylation events were reduced in PAK2-depleted cells (Figure 4, C and D). To study in more detail the role of PAK2 in paxillin and focal adhesion regulation, we transfected paxillin-GFP or FAK-GFP in BAECs in combination with siRNA targeting PAK2. We found that in paxillin-GFP (Figure 4, E and F)- and FAK-GFP (Figure 4G)-expressing cells, Ang-1 treatment induced a decrease in the size of focal adhesions and an increase in their number, in agreement with a motile phenotype. In sparse PAK2-depleted BAECs, Ang-1 could not provoke a remodeling of paxillin-GFP-stained focal adhesions (Figure 4E). In addition, dynamics of focal adhesion remodeling was determined by fluorescence recovery after photobleaching (FRAP). Ang-1 stimulation increased the recovery and half-life of paxillin-GFP fluorescence in focal adhesions after photobleaching and these increases where inhibited in PAK2 depleted BAECs (Supplemental Figure S3). To study the regulation of focal adhesions during Ang-1–stimulated BAEC migration, we performed a scratch assay on confluent monolayers of BAECs transfected with paxillin-GFP and monitored the quantity and size of paxillin-GFP-stained focal adhesions at the leading edges of Ang-1–stimulated BAECs (Figure 4F). As in sparse cells, Ang-1 stimulation increased the numbers and decreased the size of paxillin-GFP-positive focal adhesions located at the leading edges of migrating cells, and PAK2 depletion by siRNA abrogated the effects of Ang-1 (Figure 4F). In contrast, FAK-GFP-stained focal adhesions in sparse PAK2-depleted ECs were still being remodeled by Ang-1 treatment (Figure 4G). These results suggest that PAK2-dependent signaling controls Ang-1–induced remodeling of paxillin-containing focal adhesions during EC migration. However, remodeling of FAK-containing focal adhesions by Ang-1 is not affected by the down-regulation of PAK2.
FIGURE 4:
PAK2 is required for Ang-1–induced paxillin remodeling in focal adhesions. (A) Quantification of EC adhesion. BAECs were transfected with control siRNA (siCT) or targeting PAK2 (siPAK2). Transfected cells were left to adhere on 1% gelatin for 1 h in the absence or presence of Ang-1 (100 ng/ml). The graph is representative of three independent experiments performed in quadruplicate yielding identical results. (B) Scratches were performed on monolayers of BAECs treated or not with Ang-1 (100 ng/ml; 30 min). After fixation, cells were stained by immunofluorescence for PAK2 (green) and total phosphotyrosine, known to massively stain focal adhesions (4G10, purple), and observed by confocal microscopy. Colocalization of PAK2 with phosphotyrosine in focal adhesions appears as white in the right panels. Bar: 15 µm. (C) Levels of paxillin (Pxll) tyrosine phosphorylation revealed by immunoprecipitation followed by immunoblotting using phosphotyrosine antibody (P-Y; 4G10). BAECs were transfected with control siRNA (siCT) or targeting PAK2 (siPAK2) and treated with Ang-1 (100 ng/ml; 10 min) as indicated. Nonimmune immunoglobulin G (IgG) is used as negative control for immunoprecipitation. Cell lysates were immunoblotted against paxillin, PAK2, and actin to monitor for input levels and down-regulation of PAK2. (D) Immunoblots showing the phosphorylation of paxillin on Ser272 in response to Ang-1 stimulation (100 ng/ml) for 15 and 30 min in BAECs transfected with siCT or siPAK2. (E) Representative confocal micrograph of paxillin-GFP (Pxll-GFP)-labeled focal adhesions in BAECs cultured under sparse conditions. BAECs were transfected with Pxll-GFP and siCT or siPAK2. After 48 h, cells were treated or not with Ang-1 (100 ng/ml) and fixed after 30 min. Bar: 25 µm. (F) Histograms showing the quantification of the number (left panel) and size (right panel) of paxillin-GFP–labeled focal adhesions at the leading edges of migrating BAECs. Scratches were performed on confluent cell monolayers and Pxll-GFP–labeled focal adhesions were quantified within 1 μm of the leading edge of the cells (siCT: n = 22 cells; siCT+Ang-1: n = 22 cells; siPAK: n = 25 cells; siPAK2+Ang-1: n = 19 cells). (G) Histograms showing the quantification of the number (left panel) and size (right panel) of FAK-GFP–labeled focal adhesions in BAECs cultured under sparse conditions (siCT: n = 25 cells; siCT+Ang-1: n = 20 cells; siPAK2: n = 17 cells; siPAK2+Ang-1: n = 24 cells). BAECs were transfected with FAK-GFP and siCT or siPAK2. After 48 H, cells were treated or not with Ang-1 (100 ng/ml) and fixed after 30 min. The experiments were repeated three times with identical results. * p < 0.05.
Paxillin is required for Ang-1–induced oriented migration and polarization of BAECs
Because paxillin is a known target of PAKs, we explored its role in Ang-1–mediated effects in ECs. To do so, siRNAs targeted against bovine paxillin were characterized in BAECs (Supplemental Figure S1B). We observed that, similarly to PAK2, depletion of paxillin prevents Ang-1–induced cell migration (Figure 5A). More interestingly, we found that paxillin depletion affected the persistency of cell migration as observed for PAK2-depleted BAECs. We also observed that siRNAs targeting paxillin prevented Ang-1–induced orientation of the Golgi to the leading edge of migrating cells (Figure 5B), suggesting that paxillin is required for Ang-1–induced cell polarization. Using the Cdc42-Raichu probe, we found that, similarly to PAK2 deletion, paxillin depletion prevented activation of Cdc42 at the leading edge in response to Ang-1, without affecting its overall localization (Figure 5, C and D). These effects were confirmed by pull downs of activated Cdc42 showing that paxillin depletion decreased Ang-1–induced Cdc42 activation (Figure 5E). Finally, we found that paxillin is required for Ang-1–induced EC sprouting from 3D spheroids (Figure 6A). Indeed, paxillin depletion affected the length of sprouts and their number, as well as the length of filopodia, but not their number, at the tips of sprouts in response to Ang-1 stimulation (Figure 6, B–E). Altogether, these results suggest that, similarly to PAK2, paxillin is required for Cdc42 activation in response to Ang-1, leading to EC polarization, oriented migration, and sprouting.
FIGURE 5:
Paxillin is required for Ang-1–induced EC migration, polarization, and Cdc42 activation. (A) BAECs were transfected with control siRNA (siCT) or targeting paxillin (siPxll). After 48 h, confluent cell monolayers were scratched, and wound healing was monitored over 6 h in the presence or absence of Ang-1 (100 ng/ml). Quantifications of the total cell migration (left panel) and persistence (right panel) of displacements are shown. Persistence is calculated by the ratio of the net (distance between the start and the final position) and the total displacement for each track. The graphs are representative of three independent experiments each yielding identical results. n = 80–120 cells per condition. Bar: 100 µm. (B) Confluent monolayers of BAECs transfected with siCT or siPxll were scratched and treated for 30 min with Ang-1 (100 ng/ml) before fixation and staining for GM130 (Golgi marker) and nucleus (DAPI). Quantifications represent the percentages of cells with the Golgi oriented toward the wound (120°). The graph is representative of three independent experiments yielding identical results. n = 30–50 cells per condition. (C) BAECs were transfected with plasmids coding for the Cdc42-Raichu probe and siCT or siPxll. After 48 h, confluent BAEC monolayers were scratched and treated or not with Ang-1 (100 ng/ml; 30 min). After fixation, FRET by photobleaching was performed on cells at the migration front and the ratio of CFP was measured at the leading edges of cells where Cdc42-Raichu accumulates (white arrows). (D) Bar graph representing the average of four independent experiments shown in A measuring the CFP ratio at the leading edge (siCT: n = 29 cells; siCT+Ang-1: n = 21 cells; siPxll: n = 20 cells; siPxll+Ang-1, n = 19 cells). Bar: 20 µm. (E) Cdc42 activation was determined by pull-down assays using GST-PAK-CD. BAECs were transfected with siCT or siPxll. After 48 h, cells were treated for 5 min with Ang-1 and PD assays were performed on protein extracts to reveal the amount of activated Cdc42 (Cdc42-GTP) in comparison with the total amount of Cdc42. Down-regulation of paxillin (Pxll) was confirmed by immunoblotting. Bar graph shows the average densitometric ratio of four experiments. * p < 0.05.
FIGURE 6:
Paxillin is required for EC sprouting angiogenesis. (A) Representative images from spheroid-based angiogenesis assays generated from BAECs transfected with control siRNA (siCT) or targeting paxillin (siPxll) and exposed to Ang-1 (100 ng/ml) or left untreated (CT). At 24 h after embedding in collagen matrices, spheroids were fixed and stained for actin (phalloidin, green) and nucleus (DAPI, blue). Bar: 100 µm. Quantification of (B) capillary-like sprouting from spheroids measured as mean of sprout length, (C) number of sprouts per spheroid, (D) mean filopodium length, and (E) the number of filopodia per tip cell. Results are displayed as mean values ± SEM and show one of three experiments with at least 10 spheroids per condition. * p < 0.05.
Regulation of the Par polarity complex by PAK2 and paxillin
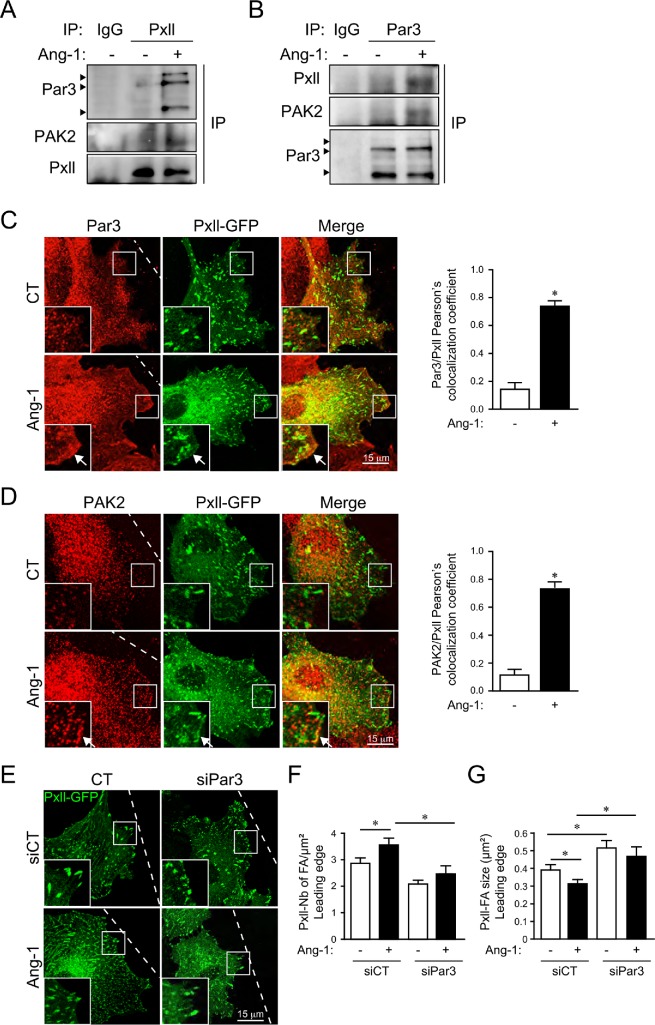
To understand how PAK2 and paxillin could affect EC polarization, we investigated their roles in the regulation of the Par polarity complex. In agreement with activation of the Par polarity complex following Ang-1 stimulation, we found an increased association between Cdc42 and Par3 in response to Ang-1, determined by Par3 immunoprecipitation (Figure 7, A and B). PAK2 or paxillin depletion prevented this increased interaction between Cdc42 and Par3 in response to Ang-1 (Figure 7, A and B). This suggests that PAK2 and paxillin are required for the formation of the Par polarity complex. Thus, we investigated the contribution of Par3 to Ang-1–stimulated Cdc42 activation and EC polarization. First, we examined the effects of down-regulation of Par3 on Cdc42 activation by Ang-1 using the Cdc42-Raichu probe. Down-regulation of Par3 by siRNA (siPar3; Supplemental Figure S1C) in BAECs prevented the Ang-1–stimulated activation of Cdc42. Interestingly, basal Cdc42 activity was increased in siPar3-transfected cells in the absence of Ang-1 stimulation; however, no further increase was observed following Ang-1 stimulation (Figure 7, C and D). Second, down-regulation of Par3 inhibited Ang-1–induced orientation of the Golgi to the leading edges of migrating cells (Figure 7E), confirming that Par3 is involved in Ang-1–induced EC polarization.
FIGURE 7:
PAK2, paxillin, and Par3 associate at the leading edge in response to Ang-1. Par3 immunoprecipitates from BAECs transfected with control siRNA (siCT) (A) targeting PAK2 (siPAK2) or (B) targeting paxillin (siPxll) and treated with Ang-1 (100 ng/ml; 5 min), as indicated. The presence of Cdc42 in the Par3 immunoprecipitate was detected by immunoblotting. Immunoprecipitation levels were controlled by Par3 immunoblotting (arrowheads point to bands at 100, 150, and 180 kDa) and down-regulation of PAK2 and paxillin (Pxll) was confirmed. Extracts from untreated cells were subjected to immunoprecipitation with neutral antibodies (IgG) as control. (C) BAECs were transfected with plasmids coding for the Cdc42-Raichu probe and siCT or targeting Par3 (siPar3). After 48 h, confluent BAEC monolayers were scratched and treated or not with Ang-1 (100 ng/ml; 30 min). After fixation, FRET by photobleaching was performed on cells at the migration front and the ratio of CFP was measured at the leading edges of cells where Cdc42-Raichu accumulates (arrows). (D) Histogram representing the average of four independent experiments shown in C measuring the CFP ratio at the leading edge. n = 20–40 cells per condition (siCT: n = 21 cells; siCT+Ang-1: n = 32 cells; siPar3: n = 25 cells; siPar3+Ang-1: n = 34 cells). * p < 0.05. Bar: 20 µm. (E) Cell polarization of confluent monolayers of BAECs transfected with siCT or siPar3 were scratched and treated for 30 min with Ang-1 (100 ng/ml) before fixation and staining for GM130. Bar graph represents the orientation of the Golgi and the nucleus according to the position of the wound (120°). The graph is representative of three independent experiments yielding identical results (siCT: n = 25 cells; siCT+Ang-1: n = 24 cells; siPar3: n = 20 cells; siPar3+Ang-1: n = 22 cells). * p < 0.05.
To investigate the relationship between PAK2, paxillin, and Par3, we performed immunoprecipitations of paxillin and Par3 in ECs stimulated or not with Ang-1 and determined the presence of PAK2, paxillin, and Par3 in the immunocomplexes (Figure 8, A and B). We found that stimulation of ECs with Ang-1 induced an association of PAK2 and Par3 with the paxillin immunoprecipitates (Figure 8A). Interestingly, Par3 immunoprecipitates also revealed increased association of PAK2 and paxillin with the Par3 immunocomplex in response to Ang-1 (Figure 8B). We also observed that Par3 accumulates in lamellipodia in response to Ang-1 in migrating ECs (Figure 8C). Interestingly, PAK2 and paxillin-GFP also accumulated at the leading edges of migrating BAECs in response to Ang-1 and partially colocalized with Par3 (Figure 8, C and D). This suggests cross-talk between PAK2, paxillin, and the Par polarity complex at the leading edge to promote EC polarization and migration in response to Ang-1.
FIGURE 8:
PAK2, paxillin, and Par3 associate at the leading edge in response to Ang-1. BAECs were treated with Ang-1 for 20 min before lysis and immunoprecipitation against (A) paxillin (Pxll) or (B) Par3. The presence of Par3 (arrowheads point to bands at 100, 150, and 180 kDa), paxillin, and PAK2 in the immunoprecipitates was detected by immunoblotting. Extracts from untreated cells were subjected to immunoprecipitation with neutral antibodies (IgG) as control. (C, D) Scratches were performed on monolayer of BAECs transfected with plasmids coding for paxillin-GFP (Pxll-GFP; green) and treated or not with Ang-1 (100 ng/ml; 20 min). After fixation, cells were stained for Par3 (C, red) or PAK2 (D, red). Zooms show the tips of the lamellipodia where paxillin, PAK2, and Par3 accumulate and colocalize with Pxll-GFP (arrow). Bar: 20 µm. Bar graphs (right panels) show Pearson’s colocalization coefficient between Pxll-GFP and Par3 (C) or Pxll-GFP and PAK2 (D). Pearson’s coefficient was determined in 6–8 areas per cell with 8–10 cells per condition. (E) Scratches were performed on confluent cell monolayers and paxillin-GFP–labeled focal adhesions were quantified within 1 μm of the leading edges of the cells. Bar graphs showing the quantification of the density (F) or size (G) of paxillin-GFP labeled focal adhesion in transfected BAECs (siCT: n = 22 cells; siCT+Ang-1: n = 21; siPar3: n = 23 cells; siPar3+Ang-1: n = 16 cells). The experiments were repeated three times with identical results. * p < 0.05.
Finally, we studied the influence of Par3 on regulation of focal adhesions by Ang-1. Focal adhesion remodeling was studied in paxillin-GFP–expressing BAECs subjected to Ang-1–stimulated migration (Figure 8E). Size and number of paxillin-GFP positive focal adhesions were determined at the leading edge after 30 min of migration (Figure 8, F and G). Similarly to PAK2, down-regulation of Par3 resulted in inhibition of Ang-1–stimulated increase in the number of paxillin-GFP positive focal adhesions at the leading edge of BAECs and prevented the reduction of their size (Figure 8, F and G).
DISCUSSION
Here, we show that Ang-1–induced PAK2 activation increases paxillin phosphorylation, leading to reorganization of focal adhesions, cell polarization, and increased migration of ECs. To induce these effects, this pathway triggers cell polarization by increasing Cdc42 activation and Par3 localization at the leading edges of migrating cells, which promotes oriented EC migration and sprouting in response to Ang-1. We also show that Par3 is recruited to focal adhesions at leading edge and interacts with PAK2 and paxillin in response to Ang-1. We propose a new mechanism in response to Ang-1 where PAK2, paxillin, and Par3 form signaling complexes at the leading edges of migrating ECs, regulating the activity of Cdc42 and triggering their polarization, which results in angiogenic sprouting.
The role of PAKs in VEGF-mediated effects on ECs has been investigated previously. Activation of PAK by VEGF results in phosphorylation and regulation of MLC and MLCK (myosin light-chain kinase), leading to the activation of myosin and stress fibers formation. This increase of cell tension triggers cell retraction and increases transendothelial permeability (Zeng et al., 2000; Stockton et al., 2004, 2007). The critical role of PAKs in the development of the cardiovascular system has been well studied and it is now clear that PAK2 is the predominant isoform expressed in the endothelium (Sells and Chernoff, 1997; Knaus and Bokoch, 1998). Deletion of PAK2 causes embryonic lethality in mice due to multiple hemorrhages and deletion of Pak2a in zebrafish leads to cerebral hemorrhages, indicating that PAK2 is involved in maintenance of the endothelial barrier (Liu et al., 2007; Radu et al., 2015). In addition to its role in the integrity of the endothelium, PAK2 is also involved in EC migration and angiogenesis, which have been linked to activation of the Erk5 pathway (Nayal et al., 2006; Radu et al., 2015).
It has been shown previously that activated Tie2 associates with Dok-R and promotes the subsequent recruitment of Nck and PAK/PAK1, leading to PAK activation. Localization of this Dok-R-Nck-PAK complex to activated Tie2 promotes Ang-1 driven EC migration (Master et al., 2001). This study provided the first link between Tie2 and PAK1 and suggested that Ang-1 promotes EC migration through PAK signaling. We observed previously in a phosphoproteomic study of Ang-1 signaling in ECs that PAK2 is phosphorylated in response to Ang-1 and part of a subnetwork of phosphoproteins, including paxillin, β-Pix, and β-catenin, suggesting that PAK2 is a central regulator of cell adhesion in response to Ang-1 (Chidiac et al., 2016). Also, we previously showed that Ang-1 promotes oriented and collective migration of ECs through a PKCζ- and β-catenin–dependent localized Rac1 activation, and we observed that Par3 and Par6 accumulate at the leading edges of ECs in response to Ang-1 (Oubaha et al., 2012). Here, we show that PAK2 is necessary for the phosphorylation of paxillin, leading to localized Cdc42 activation, EC polarization, directional migration, and sprouting. This indicates that Tie2 uses different intracellular signaling pathways for the activation of Rac1 and Cdc42. Indeed, we now show that, in contrast to Cdc42 activation, PAK2 is not necessary for activation of Rac1 by Ang-1 (Supplemental Figure S2). To induce activation of Cdc42 at the leading edges of migrating ECs, Ang-1 promotes association between PAK2, paxillin, and Par3 (Figure 8). It is tempting to propose that activated Tie2 leads to PKCζ- and β-catenin–dependent activation of Rac1 that could result in PAK2 activation and paxillin phosphorylation at the leading edges of ECs. This would promote the recruitment of an activated Cdc42/Par3/Par6 polarity complex and the consequent oriented migration of ECs.
It is well established that the engagement of integrins with the extracellular matrix promotes cell spreading and orientation through the recruitment and activation of Cdc42 at the leading edges of migrating cells (Price et al., 1998; Etienne-Manneville and Hall, 2001; Cau and Hall, 2005). This results in the formation, in many cell types including ECs, of an active Cdc42/Par3/Par6 polarity complex and in the orientation of the microtubule network, resulting in cell polarization. Our results show that Ang-1 stimulation induces a PAK2-dependent reorganization of microtubules and that disruption of microtubules prevents Ang-1–induced polarization of ECs during migration. Intact microtubules have also been shown to be required for Tie2 trafficking to cell–matrix contacts and for modulation of cell–matrix interactions in ECs (Pietila et al., 2012). In addition, the Ang-1/Tie2 system induces intracellular signaling at cell-cell and cell-substratum contacts of ECs (Fukuhara et al., 2008; Saharinen et al., 2008). α5β1 integrin and Tie2 have been shown to interact in ECs plated on fibronectin. This interaction sensitizes Tie2 to Ang-1 stimulation and results in the recruitment of the p85 subunit of PI3K and of FAK to the α5β1/Tie2 complex (Cascone et al., 2005). Furthermore, Ang-1 stimulation induces interaction between Tie1 and Tie2 receptors at cell–cell junctions of ECs, which is responsible for α5β1 integrin-dependent EC adhesion, migration, and sprouting angiogenesis (Dalton et al., 2016). Interestingly, our study reveals that, in addition to its roles in cell adhesion, PAK2 is an essential molecular intermediate for cell polarization induced by Ang-1/Tie2 signaling in ECs.
PAK has been shown to promote Cdc42 activation and cell migration through its interaction with βPIX (Reddy et al., 2016). It has been proposed that integrin engagement promotes paxillin-dependent Cdc42 activation through the Arf-GAP protein paxillin kinase linker (PKL/GIT), which is known to promote the β-PIX–dependent activation of Cdc42, resulting in focal adhesion turnover and cell spreading (Brown et al., 2002, 2005). We now propose that in Ang-1–stimulated ECs, Cdc42 activation and polarization are controlled by the formation, in focal adhesions at leading edges, of a complex comprising PAK2, paxillin, and Par3 that results in activation of Cdc42 and the polarity complex, which promotes oriented and persistent EC migration and sprouting. Interestingly, PAK2 does not seem to be involved in the remodeling of FAK-positive focal adhesions by Ang-1, in contrast to paxillin-positive structures (Figure 4). While we cannot fully explain these results, it has been shown that FAK and paxillin have different dynamics at focal adhesions in protrusions of migrating ECs (Hu et al., 2014). It is reported that in migrating Ecs, FAK is assembled first at focal adhesions located at cell fronts, which is followed by paxillin recruitment to these focal adhesions. One could suggest that paxillin-positive focal adhesions are more involved in cell polarization and in localized activation of Cdc42, which happens later in the migration process. It would be interesting to determine if subsets of focal adhesions are involved in different aspects of EC migration controlled by Ang-1.
It has been shown in fibroblasts that Par3, aPKC, and the Rac1 GEF Tiam1 interact with PDGFRβ to mediate localized signaling of PDGF to Rac1 (Matsuzawa et al., 2016). This argues for the importance of the cellular localization of polarity complexes comprising Par3 for efficient signaling and cell migration. Our study unveils that PAK2 and paxillin interact and colocalize with Par3 in Ang-1–stimulated Ecs, suggesting that this molecular complex serves to localize Cdc42 activity and polarize ECs, which is important for directed cell migration and angiogenic sprouting. In addition, we show that down-regulation of Par3 leads to inhibition of Ang-1–induced remodeling of paxillin-positive focal adhesions. It has been reported previously that Par3 interacts with FAK and PI-3 kinase in focal adhesions, and Par3 has been involved in integrin endocytosis in focal adhesions at leading edge of migrating cells (Nishimura and Kaibuchi, 2007; Itoh et al., 2010). This underscores the importance of the cross-talk between focal adhesion and cell polarity. EC polarization is of particular interest during the angiogenic process, as endothelial tip cells at emerging vascular sprouts are polarized, extend filopodia, and migrate in a directional manner. In summary, our findings identify the PAK2/paxillin system as a central integrator of signaling in ECs, linking focal adhesions to cell polarization and angiogenic sprouting.
MATERIALS AND METHODS
Cell culture and transfection
Bovine aortic endothelial cells (BAECs), obtained from VEC Technologies, were cultured in DMEM supplemented with 10% fetal bovine serum (HyClone), 2.0 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were transfected using Lipofectamine 2000 in OptiMEM (Invitrogen) with RNA duplexes (Dharmacon; see Supplemental Table S1 for sequences) and with paxillin-GFP, FAK-GFP (from I.R. Nabi, UBC, Vancouver), or Cdc42-Raichu (Itoh et al., 2002). Recombinant human angiopoietin-1 was obtained from R&D Systems Colchicine (Sigma-Aldrich) and was dissolved in dimethylsulfoxide and used at a final concentration of 10 nM.
Antibodies
For immunofluorescence and immunoblots, Alexa-coupled antibodies from Life Technologies and horseradish peroxidase (HRP)-coupled antibodies from Jackson Laboratories were used, respectively. The primary antibodies used were rabbit anti-PAK2, rabbit anti-phospho-PAK2 (Ser141), and mouse anti-actin (Cell Signaling), mouse anti-paxillin, anti-GM130, anti-HSP90, and anti-Cdc42 (BD Biosciences), mouse anti-phosphotyrosine (4G10; Millipore), rabbit anti-Par3 (Millipore Sigma), rabbit anti-Cdc42 (Santa Cruz Biotechnology), rabbit anti-tubulin (Abcam), and rabbit anti-phospho-paxillin (S272; Life Technologies).
Immunofluorescence analyses
BAECs were cultured on coverslips coated with 1% gelatin for 48 h and fixed in 4% paraformaldehyde (PFA) before permeabilization in 0.1% Triton and blocking in 1% bovine serum albumin (BSA). Primary and secondary antibodies and phalloidin (Life Technologies) were incubated on fixed cells in 1% BSA-PBS (phosphate-buffered saline) solution for 1 h before being mounted in Fluoromount (Sigma-Aldrich). Acquisitions were performed on an LSM 800 confocal laser-scanning microscope (Carl Zeiss). To quantify cell orientation, BAECs were transfected with siRNA and cultured for 48 h until confluence. Scratches were performed with 10-μl tips. After 45 min, cells were fixed and nuclei (4′,6-diamidino-2-phenylindole [DAPI]; Sigma-Aldrich), Golgi (GM130), and F-actin (phalloidin; Life Technologies) were stained. Golgi orientation as a function of its position relative to the nucleus and perpendicular to the scratch was quantified manually on the first layer of cells behind the scratch. A total of 8–10 fields were quantified for each condition, representing 150–200 cells, and each experiment was repeated three times. In colchicine-treated cells (Figure 1H), only cells with intact Golgi integrity, seen with GM130 staining, were quantified. To quantify focal adhesion, BAECs were transfected with paxillin-GFP or FAK-GFP and fixed after 48 h. Only cells expressing moderate amounts of paxillin-GFP or FAK-GFP were acquired. Quantifications were performed on ImageJ (National Institutes of Health [NIH], Bethesda, MD) by applying a threshold on the GFP signal level and quantifying the number and size of GFP-positive spots. Quantification of intracellular tubulin organization was done using the Directionality plug-in in ImageJ. The directionality algorithm determines in an image the number of structures in a given direction and attributes numerical values of direction and angular dispersion. The higher the angular dispersion, the higher is the disorder of the structure. Numerical values per cells were exported to an Excel spreadsheet and plotted graphically.
Three-dimensional sprouting assay
Spheroids were prepared as previously described (Oubaha et al., 2012). Briefly, transfected BAECs were cultured in DMEM containing 25% methyl cellulose (Sigma-Aldrich), 10% FBS, 1% l-glutamine, and 1% penicillin/streptomycin in a U-shaped 96-well plate for 24 h to allow spheroid formation. Spheroids were transferred in complete medium containing 45% collagen, pH 7.4, and 20% methyl cellulose and cultured for 24 h before fixation in 4% PFA. Actin and nucleus were stained with phalloidin and DAPI, respectively. Image acquisitions were performed using an inverted Zeiss Axio-observer microscope with a 10× objective. Numbers of sprouts and filopodia lengths were determined manually using ZEN Blue 2.3 (Zeiss). Filopodia were determined as extensions from the tip cells of the sprout with <0.1 μm diameter and enriched in F-actin. For length quantification, only filopodia visible in one focus plane were manually counted by drawing lines from the bases of the cells to the tips of the filopodia using the line tool of Zen Blue.
Wound healing migration assay and time-lapse video microscopy
Assays were performed as previously (Oubaha et al., 2012). Briefly, transfected BAECs were cultured in 24-well tissue-culture plates and incubated with the fluorescent vital Hoechst dye (Life Technologies) for 10 min before scratches were performed with a 200-μl pipette tip on the confluent monolayer. Cell movements were recorded using an Axio-Observer Z1 microscope (Zeiss) equipped with an AxioCam MRm camera (Zeiss) and programmed to capture a frame every 10 min of the migration period (6 h). Temperature was maintained at 37°C and atmosphere within the chamber (PECON) was kept at 5% CO2/95% air throughout the experiment. Nuclei of the leading edge cells were tracked by time-lapse video microscopy with the Cell Tracker plug-in of ImageJ (NIH), and nucleus tracks were analyzed with the Track Manager plug-in of Icy—Open Source Image Processing Software. The total displacement, net displacement, and persistence were obtained for each track, and statistical analysis was performed.
FRET experiments and analyses
BAECs were cultured on 1% gelatin-coated coverslips and transfected with the Cdc42-Raichu probe (Itoh et al., 2002). Scratches were performed on confluent monolayers; cells were fixed in 4% PFA after 30 min of Ang-1 stimulation and mounted in Fluoromount medium. FRET after acceptor photobleaching was performed on fixed cells and used to quantify Cdc42 activation as described previously (Oubaha et al., 2012). Within a field, a region of interest, corresponding to leading edges of extruding cells in wounds, the acceptor (YFP) was photobleached (500 iterations of 514-nm laser at 100% power) using a Nikon A1R confocal laser system. Increase of donor (CFP) fluorescence intensity after acceptor photobleaching was measured as FRET. FRET efficiency was calculated using the “FRET calc” plug-in of ImageJ software (NIH) as the change in background with fluorescence intensity subtracted: 100 × {[(Donor post-bleach intensity) – (Donor pre-bleach intensity)]/(Donor post-bleach intensity)}.
Immunoprecipitation, Cdc42 pull down, and immunoblotting
For immunoprecipitations, cells were solubilized in lysis buffer containing 1% Triton X-100, 0.2% NP40, 50 mM Tris-HCl, pH 7.4, 140 mM NaCl, 1 mM EDTA, 1 mM ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), 20 mM sodium fluoride, 1 mM sodium pyrophosphate, 1 mM orthovanadate, and protease inhibitor cocktail (Roche Diagnostics). Soluble proteins were incubated with primary antibodies (2 μg) at 4°C overnight. Protein A-Sepharose (Sigma-Aldrich; 50 µl of a 50% slurry) was added and incubated for an additional hour. The immune complexes were precipitated, boiled in SDS sample buffer, and revealed by immunoblotting. Cdc42 activity was monitored by pull-down assay using a glutathione-S-transferase (GST)–PAK-CD (PAK1B-CRIB domain) fusion protein (Sander et al., 1998). BAECs were lysed in 1% Triton X-100, 0.2% NP-40, 50 mM Tris-HCl, pH 7.5, 110 mM NaCl, 1 mM EDTA, 1 mM EGTA, 20 mM sodium fluoride, 1 mM sodium pyrophosphate, 1 mM orthovanadate, and protease inhibitor cocktail. GST-PAK-CD (20 µg) bound to glutathione-coupled Sepharose 4B beads (Sigma-Aldrich) was used to isolate activated Cdc42 from cell extracts. The complexes were precipitated and boiled in SDS sample buffer, and bound Cdc42 was revealed by immunoblotting. Immunoblot analyses were performed as follows. Proteins were separated by SDS–PAGE, transferred onto a nitrocellulose membrane (Hybond-ECL; GE Healthcare), and Western blotted. Antibody detection was performed with HRP-coupled antibodies from Jackson Laboratories using the Image Quant LAS4000 chemiluminescence-based detection system (enhanced chemiluminescence; GE Healthcare).
Cell adhesion assay
BAEC adhesion was assayed in 96-well plates precoated overnight with 1% gelatin (Acevedo et al., 2004). At 48 h after transfection, 50,000 cells were plated and left to adhere at 37°C for 1 h. Adherent cells were stained with 0.2% crystal violet (Sigma Aldrich) and 20% methanol in PBS and adhesion was quantitated by measuring absorbance at 550 nm with a Wallac Victor 3V microplate reader (PerkinElmer). Each experimental point was measured in triplicate.
Statistical analyses
Data are represented as means ± SEM Two-tailed independent Student’s t tests were used in comparing two groups. Comparisons between multiple groups were made using one-way analysis of variance followed by post hoc Bonferroni multiple comparison tests among groups. p value < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Afnan Abu-Thuraia and Jean-François Côté (Montreal Clinical Research Institute, Canada) for critical reading of the manuscript. This work was supported by an operating grant from the Cancer Research Society and by the Université de Montréal—Merck Canada Chair in pharmacology to J.-P.G.
Abbreviations used:
- Ang-1
angiopoietin-1
- BAEC
bovine aortic endothelial cell
- BSA
bovine serum albumin
- CFP
cyan fluorescent protein
- CT
control
- DAPI
4′,6-diamidino-2-phenylindole
- EC
endothelial cell
- eNOS
endothelial nitric oxide synthase
- FAK
focal adhesion kinase
- FRET
Förster resonance energy transfer
- GFP
green fluorescent protein
- HRP
horseradish peroxidase
- NO
nitric oxide
- PAK
p21-activated kinase
- PBS
phosphate-buffered saline
- PFA
paraformaldehyde
- PKC
protein kinase C
- VEGF
vascular endothelial growth factor
- YFP
yellow fluorescent protein
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E18-08-0486) on May 29, 2019.
REFERENCES
- Acevedo L, Yu J, Erdjument-Bromage H, Miao RQ, Kim JE, Fulton D, Tempst P, Strittmatter SM, Sessa WC. (2004). A new role for Nogo as a regulator of vascular remodeling. Nat Med , 382–388. [DOI] [PubMed] [Google Scholar]
- Brown MC, Cary LA, Jamieson JS, Cooper JA, Turner CE. (2005). Src and FAK kinases cooperate to phosphorylate paxillin kinase linker, stimulate its focal adhesion localization, and regulate cell spreading and protrusiveness. Mol Biol Cell , 4316–4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Turner CE. (2004). Paxillin: adapting to change. Physiol Rev , 1315–1339. [DOI] [PubMed] [Google Scholar]
- Brown MC, West KA, Turner CE. (2002). Paxillin-dependent paxillin kinase linker and p21-activated kinase localization to focal adhesions involves a multistep activation pathway. Mol Biol Cell , 1550–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascone I, Napione L, Maniero F, Serini G, Bussolino F. (2005). Stable interaction between alpha5beta1 integrin and Tie2 tyrosine kinase receptor regulates endothelial cell response to Ang-1. J Cell Biol , 993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cau J, Hall A. (2005). Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways. J Cell Sci , 2579–2587. [DOI] [PubMed] [Google Scholar]
- Chidiac R, Zhang Y, Tessier S, Faubert D, Delisle C, Gratton JP. (2016). Comparative phosphoproteomics analysis of VEGF and angiopoietin-1 signaling reveals ZO-1 as a critical regulator of endothelial cell proliferation. Mol Cell Proteomics , 1511–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton AC, Shlamkovitch T, Papo N, Barton WA. (2016). Constitutive association of Tie1 and Tie2 with endothelial integrins is functionally modulated by angiopoietin-1 and fibronectin. PLoS One , e0163732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. (2001). Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell , 489–498. [DOI] [PubMed] [Google Scholar]
- Farhan H, Hsu VW. (2016). Cdc42 and cellular polarity: emerging roles at the Golgi. Trends Cell Biol , 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara S, Sako K, Minami T, Noda K, Kim HZ, Kodama T, Shibuya M, Takakura N, Koh GY, Mochizuki N. (2008). Differential function of Tie2 at cell–cell contacts and cell–substratum contacts regulated by angiopoietin-1. Nat Cell Biol , 513–526. [DOI] [PubMed] [Google Scholar]
- Galan Moya EM, Le Guelte A, Gavard J. (2009). PAKing up to the endothelium. Cell Signal , 1727–1737. [DOI] [PubMed] [Google Scholar]
- Gavard J, Patel V, Gutkind JS. (2008). Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell , 25–36. [DOI] [PubMed] [Google Scholar]
- Hu YL, Lu S, Szeto KW, Sun J, Wang Y, Lasheras JC, Chien S. (2014). FAK and paxillin dynamics at focal adhesions in the protrusions of migrating cells. Sci Rep , 6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N, Nakayama M, Nishimura T, Fujisue S, Nishioka T, Watanabe T, Kaibuchi K. (2010). Identification of focal adhesion kinase (FAK) and phosphatidylinositol 3-kinase (PI3-kinase) as Par3 partners by proteomic analysis. Cytoskeleton (Hoboken) , 297–308. [DOI] [PubMed] [Google Scholar]
- Itoh RE, Kurokawa K, Ohba Y, Yoshizaki H, Mochizuki N, Matsuda M. (2002). Activation of rac and cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Mol Cell Biol , 6582–6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK. (2003). Molecular regulation of vessel maturation. Nat Med , 685–693. [DOI] [PubMed] [Google Scholar]
- Kelly ML, Chernoff J. (2012). Mouse models of PAK function. Cell Logist , 84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Kim HG, Moon SO, Chae SW, So JN, Koh KN, Ahn BC, Koh GY. (2000). Angiopoietin-1 induces endothelial cell sprouting through the activation of focal adhesion kinase and plasmin secretion. Circ Res , 952–959. [DOI] [PubMed] [Google Scholar]
- Knaus UG, Bokoch GM. (1998). The p21Rac/Cdc42-activated kinases (PAKs). Int J Biochem Cell Biol , 857–862. [DOI] [PubMed] [Google Scholar]
- Liu J, Fraser SD, Faloon PW, Rollins EL, Vom Berg J, Starovic-Subota O, Laliberte AL, Chen JN, Serluca FC, Childs SJ. (2007). A betaPix Pak2a signaling pathway regulates cerebral vascular stability in zebrafish. Proc Natl Acad Sci USA , 13990–13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Master Z, Jones N, Tran J, Jones J, Kerbel RS, Dumont DJ. (2001). Dok-R plays a pivotal role in angiopoietin-1-dependent cell migration through recruitment and activation of Pak. EMBO J , 5919–5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa K, Akita H, Watanabe T, Kakeno M, Matsui T, Wang S, Kaibuchi K. (2016). PAR3-aPKC regulates Tiam1 by modulating suppressive internal interactions. Mol Biol Cell , 1511–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel AS, Allen JD, Park SJ, Jaffer ZM, Michels EG, Burgin SJ, Chen S, Bessler WK, Hofmann C, Ingram DA, et al. (2008). Pak1 regulates multiple c-Kit mediated Ras-MAPK gain-in-function phenotypes in Nf1+/- mast cells. Blood , 4646–4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayal A, Webb DJ, Brown CM, Schaefer EM, Vicente-Manzanares M, Horwitz AR. (2006). Paxillin phosphorylation at Ser273 localizes a GIT1-PIX-PAK complex and regulates adhesion and protrusion dynamics. J Cell Biol , 587–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Kaibuchi K. (2007). Numb controls integrin endocytosis for directional cell migration with aPKC and PAR-3. Dev Cell , 15–28. [DOI] [PubMed] [Google Scholar]
- Ostergaard HL, Lou O, Arendt CW, Berg NN. (1998). Paxillin phosphorylation and association with Lck and Pyk2 in anti-CD3- or anti-CD45-stimulated T cells. J Biol Chem , 5692–5696. [DOI] [PubMed] [Google Scholar]
- Oubaha M, Gratton JP. (2009). Phosphorylation of endothelial nitric oxide synthase by atypical PKC zeta contributes to angiopoietin-1-dependent inhibition of VEGF-induced endothelial permeability in vitro. Blood , 3343–3351. [DOI] [PubMed] [Google Scholar]
- Oubaha M, Lin MI, Margaron Y, Filion D, Price EN, Zon LI, Cote JF, Gratton JP. (2012). Formation of a PKCzeta/beta-catenin complex in endothelial cells promotes angiopoietin-1-induced collective directional migration and angiogenic sprouting. Blood , 3371–3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietila R, Natynki M, Tammela T, Kangas J, Pulkki KH, Limaye N, Vikkula M, Koh GY, Saharinen P, Alitalo K, Eklund L. (2012). Ligand oligomerization state controls Tie2 receptor trafficking and angiopoietin-2-specific responses. J Cell Sci , 2212–2223. [DOI] [PubMed] [Google Scholar]
- Price LS, Leng J, Schwartz MA, Bokoch GM. (1998). Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol Biol Cell , 1863–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radu M, Lyle K, Hoeflich KP, Villamar-Cruz O, Koeppen H, Chernoff J. (2015). p21-activated kinase 2 regulates endothelial development and function through the Bmk1/Erk5 pathway. Mol Cell Biol , 3990–4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PN, Radu M, Xu K, Wood J, Harris CE, Chernoff J, Williams DA. (2016). p21-activated kinase 2 regulates HSPC cytoskeleton, migration, and homing via CDC42 activation and interaction with beta-Pix. Blood , 1967–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saharinen P, Eklund L, Miettinen J, Wirkkala R, Anisimov A, Winderlich M, Nottebaum A, Vestweber D, Deutsch U, Koh GY, et al. (2008). Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell–cell and cell–matrix contacts. Nat Cell Biol , 527–537. [DOI] [PubMed] [Google Scholar]
- Sander EE, van Delft S, ten Klooster JP, Reid T, van der Kammen RA, Michiels F, Collard JG. (1998). Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell–cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J Cell Biol , 1385–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sells MA, Chernoff J. (1997). Emerging from the Pak: the p21-activated protein kinase family. Trends Cell Biol , 162–167. [DOI] [PubMed] [Google Scholar]
- Stockton R, Reutershan J, Scott D, Sanders J, Ley K, Schwartz MA. (2007). Induction of vascular permeability: beta PIX and GIT1 scaffold the activation of extracellular signal-regulated kinase by PAK. Mol Biol Cell , 2346–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockton RA, Schaefer E, Schwartz MA. (2004). p21-activated kinase regulates endothelial permeability through modulation of contractility. J Biol Chem , 46621–46630. [DOI] [PubMed] [Google Scholar]
- Thomas M, Augustin HG. (2009). The role of the angiopoietins in vascular morphogenesis. Angiogenesis , 125–137. [DOI] [PubMed] [Google Scholar]
- Winderlich M, Keller L, Cagna G, Broermann A, Kamenyeva O, Kiefer F, Deutsch U, Nottebaum AF, Vestweber D. (2009). VE-PTP controls blood vessel development by balancing Tie-2 activity. J Cell Biol , 657–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. (2007a). Functional atlas of the integrin adhesome. Nat Cell Biol , 858–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel-Bar R, Milo R, Kam Z, Geiger B. (2007b). A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell–matrix adhesions. J Cell Sci , 137–148. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Lagunoff D, Masaracchia R, Goeckeler Z, Cote G, Wysolmerski R. (2000). Endothelial cell retraction is induced by PAK2 monophosphorylation of myosin II. J Cell Sci (Pt 3), 471–482. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.