Abstract
Syk/Zap70 family kinases are essential for signaling via multichain immune-recognition receptors such as tetrameric (αβγ2) FcεRI. Syk activation is generally attributed to cis binding of its tandem SH2 domains to dual phosphotyrosines within FcεRIγ-ITAMs (immunoreceptor tyrosine-based activation motifs). However, the mechanistic details of Syk docking on γ homodimers are unresolved. Here, we estimate that multivalent interactions for WT Syk improve cis-oriented binding by three orders of magnitude. We applied molecular dynamics (MD), hybrid MD/worm-like chain polymer modeling, and live cell imaging to evaluate relative binding and signaling output for all possible cis and trans Syk–FcεRIγ configurations. Syk binding is likely modulated during signaling by autophosphorylation on Y130 in interdomain A, since a Y130E phosphomimetic form of Syk is predicted to lead to reduced helicity of interdomain A and alter Syk’s bias for cis binding. Experiments in reconstituted γ-KO cells, whose γ subunits are linked by disulfide bonds, as well as in cells expressing monomeric ITAM or hemITAM γ-chimeras, support model predictions that short distances between γ ITAM pairs are required for trans docking. We propose that the full range of docking configurations improves signaling efficiency by expanding the combinatorial possibilities for Syk recruitment, particularly under conditions of incomplete ITAM phosphorylation.
INTRODUCTION
Spleen tyrosine kinase (Syk) is essential for signaling by B-cell receptors (BCR), Fc receptors for immunoglobulin G (FcγR), and Fc receptors for immunoglobulin E (FcεRI), all of which are members of the multichain immunorecognition receptor (MIRRs) family (Johnson et al., 1995; Tamir and Cambier, 1998). Syk activation by MIRRs switches on multiple pathways in mast cells and B cells (Geahlen, 2009; Mocsai et al., 2010), including protein kinase C (PKC) signaling (Kawakami et al., 2000), the PI3K-mediated Akt pathway (Jiang et al., 2003), and transcriptional regulation via the MAPK cascade (Wan et al., 1996). The coupling of Syk to MIRRs relies on a tandem pair of Src homology 2 (SH2) domains in the N-terminal region (Geahlen and Burg, 1994). Both SH2 domains adopt the canonical SH2 fold, comprising a β-sheet flanked by two α-helices (Kuriyan and Cowburn, 1993), and bind to short peptide motifs carrying phosphotyrosine (pTyr) residues (Pawson, 1995). The two SH2 domains are connected by a 45-residue linker referred to as interdomain A, which consists of three α-helices in a coiled-coil conformation leading to a Y-shaped structure for the N-terminal region (Fütterer et al., 1998). The only other member of the Syk family of nonreceptor protein tyrosine kinases is Zap70, which is expressed in T-cells and natural killer cells (Chan et al., 1992; Chu et al., 1998; Wang et al., 2010) and whose N-terminal region also adopts a Y-shaped structure (Hatada et al., 1995). In addition to binding pTyr motifs via its tandem SH2 domains (Kihara and Siraganian, 1994), the N-terminal region of Syk/Zap70 is also involved in the autoinhibition of its kinase domain (KD) through residue–residue interactions between interdomain A and the C lobe of the KD (Deindl et al., 2007; Yan et al., 2013). Syk and Zap70 can be functionally homologous for TCR and BCR signaling (Kong et al., 1995; Cheng et al., 1997), although Zap70 is more dependent on Src-family kinases (e.g., Lck) for its catalytic activation than Syk (Iwashima et al., 1994; Fasbender et al., 2017).
A distinguishing feature of the MIRRs, which lack intrinsic kinase activity, is the presence of pTyr-containing immunoreceptor tyrosine-based activation motifs (ITAMs) within the cytoplasmic tails of signaling subunits (Reth, 1989; Cambier, 1995; Sigalov, 2005; Harwood and Batista, 2008; Rivera et al., 2008; Smith-Garvin et al., 2009). MIRRs typically incorporate multiple ITAMS, often in disulfide-linked pairs such as the γ homodimers (common to FcγRIII, FcγRI, FcεRI), ζ homodimers (TCR), and Igα,Igβ heterodimers (BCR) (Reth, 2001). We focus here on high-affinity FcεRI, which is activated when multivalent antigens cross-link IgE–FcεRI complexes for increased defense against pathogens, wound healing, and allergic responses (Siraganian, 2003; Molfetta et al., 2007; MacGlashan, 2008; Mukai et al., 2018). FcεRI is an αβγ2 oligomeric complex with 3 ITAMs: one in the β subunit and one each in the disulfide-linked γ chains (Blank et al., 1989; Ra et al., 1989). In most models of ITAM-based signaling, phosphorylation of both tyrosines in the same ITAM is assumed to be required for docking of Syk’s tandem SH2 domains (Kihara and Siraganian, 1994; Shiue et al., 1995; Faeder et al., 2003). This cis-binding orientation is supported by crystal structures of Syk and Zap70 bound to a single dually tyrosine-phosphorylated peptide (Hatada et al., 1995; Fütterer et al., 1998). Mass spectrometry–based analysis of FcεRIγ found that phosphorylation of the N-terminal Tyr is more abundant than of the C-terminal Tyr (Yamashita et al., 2008), suggesting that ITAM phosphorylation is often incomplete and could limit availability for cis-oriented binding. The common presence of disulfide-linked ITAM-bearing pairs in the MIRRs, as mentioned above, raises the important question: do Syk (and Zap-70) bypass the requirement for full ITAM phosphorylation by docking in trans to pTyr pairs on adjacent signaling subunits? If so, is this alternative docking orientation equally efficient for signal propagation? We address these questions here through both modeling and experimental approaches.
Given that Syk has two distinct SH2 domains, we consider the evidence that individual SH2 domains show selectivity when interacting with phosphopeptides from their biologically relevant protein-binding partners. Prior studies have shown that SH2 domains exhibit 50- to 1000-fold higher affinity to phosphopeptides derived from binding partners than to randomized phosphopeptide sequences (Panayotou et al., 1993; Songyang et al., 1993; Ladbury et al., 1995). Binding is also enhanced by multivalent interactions (Ottinger et al., 1998), which is relevant for Syk–FcεRI, since the Syk tandem SH2 domains are bivalent and there are four available binding sites in a fully phosphorylated FcεRIγ chain homodimer. Other examples of signaling proteins incorporating tandem SH2 domains include the protein tyrosine phosphatases PTPN6 (or SHP-1; Pei et al., 1996) and PTPN11 (or SHP-2; Pluskey et al., 1995), as well as phospholipase C-γ1 (Ji et al., 1999). Interestingly, the recruitment of proteins bearing only a single SH2 domain, such as the phosphatase INPP5D (SHIP-1), is thought to be favored by monophosphorylation of BCR ITAMs (O’Neill et al., 2011; Getahun et al., 2016). In the case of Syk, experimentally measured binding affinities indicate micromolar affinity of individual SH2 domains to FcεRIγ, while engagement of the tandem SH2 domains results in stronger binding by around three orders of magnitude (Chen et al., 1996). However, the relationship between multivalent binding on Syk–FcεRIγ stoichiometry and signal initiation remains of keen interest.
The ability of tandem SH2 domains to enhance Syk-ITAM occupancy can be impacted by posttranslational modifications in interdomain A. We specially focus on Syk recruitment after phosphorylation at Y130 in interdomain A (Keshvara et al., 1998), since Y130E substitution in Syk resulted in a phosphomimetic recombinant protein with reduced binding of its tandem SH2 domains to phosphorylated ITAMs in coimmunoprecipitation studies (Keshvara et al., 1997; Zhang et al., 2008). This was initially attributed to the structural destabilization of interdomain A and the resulting partial decoupling of both SH2 domains by the phosphomimetic mutation (Zhang et al., 2008; Feng and Post, 2016; Roy et al., 2016). However, high-resolution imaging studies in Syk-deficient cells reconstituted with Syk Y130E recombinant protein revealed two interesting observations: 1) Syk recruitment to FcεRI aggregates is retained even for Y130E phosphomimetic, and 2) impaired downstream signaling correlates with an increased off-rate for FcεRI–Syk(Y130E) interactions (Schwartz et al., 2017). These results motivate the current studies to understand how interdomain A phosphorylation influences the conformation, binding orientation, and residency time of Syk’s dual SH2 domains on ITAMs.
In this work, we examine Syk–FcεRI binding modes using a combination of conventional and enhanced-sampling molecular dynamics (MD) simulations, as well as hybrid MD/polymer theory (Sethi et al., 2011). We first developed an analytical model based on simple structural arguments to evaluate how multivalent interactions cause a three–orders of magnitude increase in binding compared with that in individual SH2 domains. Next, conventional MD simulations show that Y130E substitution leads to increased flexibility in the relative orientations of the two SH2 domains in Syk. Replica-exchange MD (REMD) simulations of interdomain A further show that the amount of helical structure in this linker region is reduced by Y130E, thereby impacting the distance between the tandem SH2 domains. We then show using a hybrid approach of MD simulations and worm-like chain (WLC) polymer models that the combination of higher inter-SH2 distance and increased flexibility in the Syk Y130E mutant reduces its binding affinity to FcεRIγ in a cis orientation. Interestingly, wild type (WT) and Y130E mutant Syk show comparable affinities to FcεRIγ in WLC models of trans binding.
We also present structural models and binding energy estimates for the multiple trans binding modes that are possible for binding of two WT Syk molecules (one pair of tandem SH2 domains each) to a dimer of phosphorylated FcεRIγ chains (one pair of pTyr motifs each). Simulations predict that Syk tandem SH2 domains can bind phosphorylated γ-dimers effectively in both cis and trans orientations, with the caveat that the ITAM pairs must be in close proximity to accommodate trans binding. Given that the extent of ITAM phosphorylation is expected to be highly variable based upon antigen dose, valency, and occupancy of receptors by Ag-specific IgE, we propose that the availability of multiple trans and cis modes enhances the signaling capacity of FcεRI aggregates. Predictions of the computational modeling were supported by experiments in γ-KO rat basophilic leukemia (RBL-γKO) cells reconstituted with disulfide-linked γ subunits bearing either WT or mutant ITAM sequences. Experiments in cells expressing chimeric receptor monomers incorporating the γ cytoplasmic tail provide novel insights into the classes of immunoreceptors that bear a single ITAM (e.g., FcγRIIA; Nimmerjahn and Ravetch, 2008) or a hemITAM (e.g., CLEC-2 or Dectin-1; Bauer and Steinle, 2017).
RESULTS
Impact of multivalency on the cis-oriented binding of wild type Syk tandem SH2 domains to a single FcεRIγ chain
We first constructed a structure-based analytical model that describes how local concentration effects lead to an enhancement of overall cis binding between the linked SH2 domains from Syk and the paired pTyrs in a FcεRIγ chain ITAM. In Figure 1A, the initial binding steps of either N-SH2:C-ITAM or C-SH2:N-ITAM are shown with equilibrium association constants of KN and KC, respectively. The subsequent binding steps involve either 1) binding of C-SH2:N-ITAM after N-SH2:C-ITAM with an effective equilibrium association constant of  , or 2) binding of N-SH2:C-ITAM after C-SH2:N-ITAM with an effective equilibrium association constant of
, or 2) binding of N-SH2:C-ITAM after C-SH2:N-ITAM with an effective equilibrium association constant of  . Both
. Both  and
and  are given by multiplying KN and KC by a factor Ceff that represents the effective concentration of unbound ITAM that unbound SH2 experiences upon binding of the other SH2:ITAM pair. This effective concentration factor is the same in both pathways described above (either binding of N-SH2:C-ITAM first or binding of C-SH2:N-ITAM first). This model is similar to the one we used previously to investigate the Grb2–Sos1 multivalent complex (Sethi et al., 2011), where the effective equilibrium association constant for binding of both motifs, Koverall (red arrows in Figure 1A), is given by
are given by multiplying KN and KC by a factor Ceff that represents the effective concentration of unbound ITAM that unbound SH2 experiences upon binding of the other SH2:ITAM pair. This effective concentration factor is the same in both pathways described above (either binding of N-SH2:C-ITAM first or binding of C-SH2:N-ITAM first). This model is similar to the one we used previously to investigate the Grb2–Sos1 multivalent complex (Sethi et al., 2011), where the effective equilibrium association constant for binding of both motifs, Koverall (red arrows in Figure 1A), is given by
FIGURE 1:

Structure-based analytical model for multivalent binding of Syk tandem SH2 domains to a single FcεRIγ chain. (A) Reaction network from unbound (left) to dual-bound (right) Syk. N-SH2, C-SH2, and interdomain A of Syk are shown in green, blue, and orange, respectively. The unstructured cytoplasmic region of the γ chain is shown as a wavy gray line, with pY64 and pY75 as pink and yellow symbols, respectively. (B) Schematic showing the accessible spherical shell where unbound SH2 can search for unbound ITAM and the orientation factor representing the flexibility of the γ chain, given prior binding of the other SH2:pTyr pair. Syk and the γ chain are colored as in A. (C) The six complexes in the asymmetric unit of the crystal structure of Syk tandem SH2 domains bound to CD3ε (PDB 1A81; Fütterer et al., 1998) were structurally aligned at the N-terminal SH2 (green cartoons). The translational (2 Å) and orientational (18°) variability range for C-terminal SH2 (magenta to blue cartoons) among these six aligned complexes are shown with red arrows.
| 1 |
or the product of both monomeric association constants with the effective concentration.
To derive a relation for the effective concentration, we modeled the second binding step using the top path in Figure 1A, although the other direction will give the same results. The binding of the first SH2 domain restricts the region in space that the second SH2 domain can access. The effective concentration of sites the second domain can bind to is just the number of unbound pTyr in the accessible region that are oriented so that binding can occur, divided by the volume of the accessible region Vacc. We model Vacc as a spherical shell (colored gray in Figure 1B) with the bound SH2:ITAM pair at the center of the sphere. Based on the crystal structure of Syk tandem SH2 domains bound to CD3ε (PDB 1A81; Fütterer et al., 1998) that contained six complex structures in the asymmetric unit cell, the spherical shell is located between 3.2 and 3.4 nm from the sphere center (C-SH2 translational variability of 2 Å in Figure 1C). The CD3ε chain also has an extended backbone in the crystal structure such that the inter-pTyr orientation can be assumed to be uniformly distributed between 0° and 360°; however, only a fraction of these orientations lead to successful complexes with Syk (C-SH2 orientational variability of 18° in Figure 1C). This can be expressed as an orientation factor (for) of 18°/360° or 0.05, which is the probability of the extended chain adopting an inter-ITAM orientation that allows multivalent binding with Syk. The effective concentration is thus given by
 |
2 |
where NA is the Avogadro constant. We note that the binding orientation found in the crystal structure, where N-SH2 interacts with C-ITAM and C-SH2 with N-ITAM, is the only cis mode considered here. This is because a physical atomistic model cannot be built for the other cis mode, with interactions between N-SH2:N-ITAM and C-SH2:C-ITAM, since the linker sequence between the two ITAMs on a single γ chain is not long enough to accommodate these interactions (Supplemental Figure 1).
For the system shown in Figure 1C, the estimated Ceff is around 3.03 mM. The experimentally measured equilibrium dissociation constants for binding of doubly phosphorylated FcεRIγ tail to N-SH2 ( ) and C-SH2 (
) and C-SH2 ( ) are >2.3 and 1.3 µM (Chen et al., 1996), respectively, whose reciprocals are KN and KC. Plugging these values into Eq. 1 and calculating the reciprocal of Koverall gives an estimated value for the effective equilibrium dissociation constant
) are >2.3 and 1.3 µM (Chen et al., 1996), respectively, whose reciprocals are KN and KC. Plugging these values into Eq. 1 and calculating the reciprocal of Koverall gives an estimated value for the effective equilibrium dissociation constant  of around 1 nM, which provides only a lower bound, since the value used for
of around 1 nM, which provides only a lower bound, since the value used for  is also only a lower bound. The experimentally measured value is 1.4 nM (Chen et al., 1996). Thus, this simple analytical model captures the three–orders of magnitude multivalent effect observed in going from monovalent binding (with µM affinity) to bivalent binding (with nM affinity) between WT Syk tandem SH2 domains and the two pTyrs on a single FcεRIγ chain.
is also only a lower bound. The experimentally measured value is 1.4 nM (Chen et al., 1996). Thus, this simple analytical model captures the three–orders of magnitude multivalent effect observed in going from monovalent binding (with µM affinity) to bivalent binding (with nM affinity) between WT Syk tandem SH2 domains and the two pTyrs on a single FcεRIγ chain.
Effect of the Y130E phosphomimetic mutation on Syk inter-SH2 distance
Our next goal was to use unbiased MD simulations to investigate the impact of interdomain A phosphorylation on the cis binding of Syk’s tandem SH2 domains, using the Y130E phosphomimetic as a model system. The challenge of applying the earlier analytical model for the Syk Y130E mutant is that there is no experimentally resolved structure for this mutant. We thus turned to MD simulations to investigate how the Y130E mutation affects the structure of the tandem SH2 domains. We applied two order parameters to quantify the flexibility between SH2 domains in our simulations (Figure 2A). The first coordinate was the distance between Cα’s of R21 on N-SH2 and R174 on C-SH2, which are both part of the pTyr-binding sites on their respective SH2 domains, and the second coordinate was the dihedral angle involving the Cα’s of R21 and L28 on N-SH2 with R174 and V181 on C-SH2. These coordinates provided measures of inter-SH2 distance and orientation, respectively. For both constructs, seven simulation replicates were performed for 2 µs simulation time per replicate.
FIGURE 2:

Unbiased MD simulations show higher inter-SH2 flexibility in the Y130E phosphomimetic form of Syk. (A) Distance (red dashed line) and dihedral (across red arrows) reaction coordinates for describing the inter-SH2 positions and orientations. (B) Free energy surface map for the WT Syk simulations based on the two reaction coordinates. (C) Corresponding free energy surface map for the Y130E phosphomimetic Syk. The white X in B and C gives the position of the starting conformation for the MD simulations. Data were collected from seven replicates per construct, with 2 µs simulation time per replicate.
These runs showed that the Y130E phosphomimetic has higher translational and orientational flexibility than WT Syk (Figure 2, B and C). We then chose two replicates per construct and extended the simulation time to 6 µs per replicate, and found the same higher flexibility trends for the mutant as compared with the WT (Supplemental Figure 2). The higher flexibility seen in the simulations of the WT construct (Figure 2B) compared with the crystal structure (Figure 1C) is likely because the latter had a bound dually phosphorylated ITAM tail that constrained the inter-SH2 flexibility. Closer inspection of these simulations showed that, in contrast with experimental findings, there was no destabilization of the helical structure of interdomain A in the Y130E phosphomimetic (Supplemental Figure 3). We instead observed changes in the number of domain–domain contacts for Syk due to Y130E, particularly a decreased number of contacts between N-SH2/C-SH2 and C-SH2/interdomain A, as well as an increased number of contacts between N-SH2/interdomain A (Supplemental Figure 4). Overall there was a net loss of domain–domain contacts in these simulations, which may account for the increased flexibility of the Y130E mutant. We note that verification of the structural destabilization of interdomain A is not accessible within the time scales of unbiased MD simulations considered here.
To address this point, we next used an enhanced sampling simulation technique called replica-exchange MD (REMD) that allows more efficient sampling of the conformational ensemble for the system under study (Sugita and Okamoto, 1999; Garcia and Sanbonmatsu, 2001). The REMD simulations were limited here to residues K115 to T159 that comprise interdomain A, since we were interested here on Y130E-induced conformational changes in this linker and not in the SH2 tandem domains. This simplification by removal of both SH2 domains also allows for a smaller system size to further speed up conformational sampling of the linker. For the WT and Y130E constructs, we ran 63 replicas each within the temperature range 275–475 K, with 1.2 µs of simulation time per replica for a cumulative simulation time of 75.6 µs. Analysis was performed for each construct cumulatively on the 15 replicas with temperatures less than 310 K.
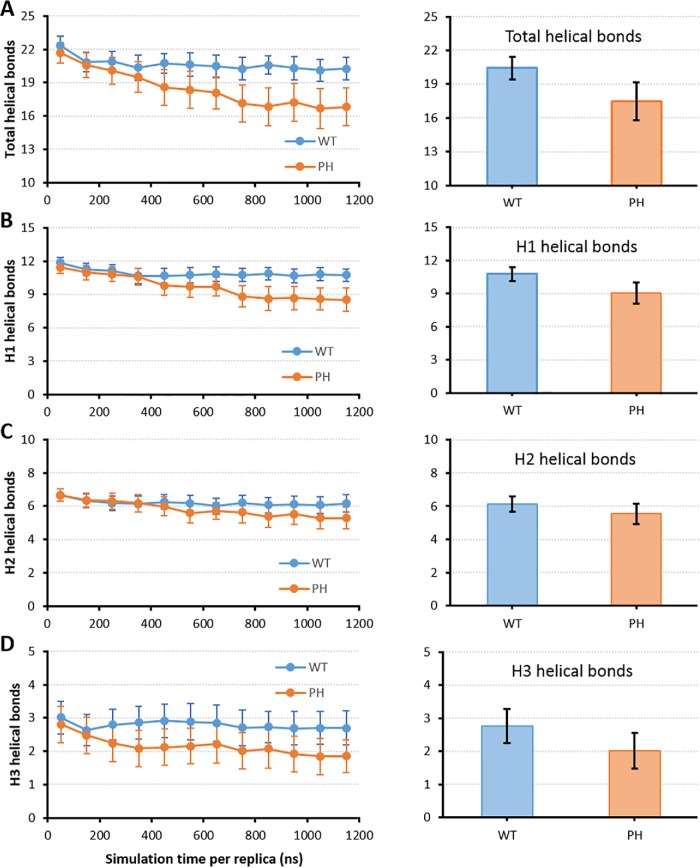
We again counted the number of helical bonds as a measure of the stability of the helical structure of interdomain A for both constructs in these simulations. Overall, we found that the Y130E mutant showed more helical unfolding than the WT construct, with around five helical bonds lost for Y130E compared with around two helical bonds for WT by the end of the simulations (Figure 3A). For the three individual helices that comprise the interdomain A structure, we found that more unfolding occurred in helix 1 that contains the Y130 phosphorylation site, with the mutant destabilizing around three helical bonds in contrast to <1 for WT (Figure 3B). In contrast, only around one helical bond was destabilized in helices 2 and 3 for the phosphomimetic mutant (Figure 3, C and D).
FIGURE 3:
REMD simulations show more helical unfolding of interdomain A in the Y130E phosphomimetic mutant. Time profiles over the entire trajectory (left) and cumulative bar plots of the last 500 ns (right) for the number of helical bonds from REMD simulations of WT (blue) and Y130E mutant (orange) interdomain A. Helical bond counts were done for (A) total helices, (B) helix 1 (H1), (C) helix 2 (H2), or (D) helix 3 (H3). Error bars give SEM.
Given the helical unfolding observed here for Y130E, we were next interested to see how this might affect the distances between the SH2 domains. Thus, for the conformational ensembles obtained for the WT and Y130E interdomain A constructs in these REMD simulations, we reattached both SH2 domains in silico to every snapshot in order to assess the inter-SH2 distance distributions for both constructs. Given that the two ends of interdomain A were free to move in the REMD simulations, we filtered for only those conformations that did not show steric clashes between N-SH2, C-SH2, and interdomain A upon reattachment of both SH2 domains. About 5% of the total number of models in both constructs remained physically viable after this filtering.
We found that the distance distributions for both constructs from the REMD-based models were broader than those from the unbiased simulations (Figure 4, A and B; compare with x axes in Figure 2, B and C), but consistently showed that the Y130E mutant samples more inter-SH2 distance values than the WT. The mean inter-SH2 distance values for the WT construct were very similar, at around 32 Å, between the REMD-based models and the unbiased simulations. For the Y130E construct, however, the mean inter-SH2 distance was larger for the REMD-based models (around 46 Å) compared with the unbiased simulations (around 36 Å), which is consistent with the increased structural instability of interdomain A seen in the REMD simulations. Using these distance distributions, a hybrid MD/polymer approach can be employed to estimate the multivalent effect on binding for WT and Y130E mutant Syk to FcεRI.
FIGURE 4:
Distance distributions between tandem SH2 domains from REMD-based models and between ITAM pTyrs from WLC models. (A) Distribution between SH2 domains from REMD-based models of WT tandem SH2. (B) Distribution between SH2 domains from REMD-based models of Y130E mutant tandem SH2. (C) The WT Syk distribution from A is shown with the distribution between ITAM pTyrs from the WLC model of a single FcεRIγ chain for cis binding (gray curve). (D) The Y130E Syk distribution from B is shown with the distribution between ITAM pTyrs from the WLC model of a single FcεRIγ chain for cis binding (gray curve). (E) The WT Syk distribution from A is shown with the distribution between ITAM pTyrs from WLC model of two FcεRIγ chains for trans binding (gray curve). (F) The Y130E Syk distribution from B is shown with the distribution between ITAM pTyrs from WLC model of two FcεRIγ chains for trans binding (gray curve). In C–F, the product of both distance distributions is shown by the red curve, whose integrated area gives Ceff. The magnitudes of the red curves have been increased here 100× to facilitate their visualization and comparison.
Changes in multivalent cis-oriented binding of Syk to a single FcεRIγ chain due to the Y130E mutation
As mentioned earlier, multivalent binding for Syk–FcεRI depends on the effective concentration Ceff of unbound ITAM that unbound SH2 experiences when the other SH2:ITAM pair is bound. Given distance distributions between the Syk SH2 domains (pSH2-SH2) and between the ITAM pTyrs (pITAM-ITAM), it has been shown that Ceff can be calculated by determining the overlapping regions between the two distributions and integrating over the products of the probabilities in these regions for all possible distances (Van Valen et al., 2009; Sethi et al., 2011):
 |
3 |
Here, we use the distance distributions derived from the REMD-based models of WT and Y130E mutant Syk tandem SH2 domains as the corresponding pSH2-SH2 in Eq. 3. To derive pITAM-ITAM, we treat the FcεRIγ chains using a WLC polymer model. For binding of a Syk tandem SH2 to a single FcεRIγ chain (i.e., cis binding),
 |
4 |
where lp and lc are the persistence and contour lengths of the peptide between the two pTyrs in the single FcεRIγ chain. Both of these length values can be expressed as a function of the number of residues in the peptide. The distance distribution plot for the cis binding WLC model is shown overlapped with corresponding plots for the WT and Y130E tandem SH2 REMD-based models in Figure 4, C and D. The Ceff values (proportional to the integrated area under red curves in Figure 4, C and D) for binding of a single FcεRIγ chain to WT and Y130E mutant Syk are estimated at 7.9 mM and 1.1 mM, respectively. These correspond to  values of 0.38 and 2.8 nM, respectively, indicating that WT Syk has stronger affinity than the Y130E mutant for cis binding to a single FcεRIγ chain by around an order of magnitude. We note that the WT
values of 0.38 and 2.8 nM, respectively, indicating that WT Syk has stronger affinity than the Y130E mutant for cis binding to a single FcεRIγ chain by around an order of magnitude. We note that the WT  value calculated here using a hybrid MD/WLC model is close to that calculated earlier using an analytical model.
value calculated here using a hybrid MD/WLC model is close to that calculated earlier using an analytical model.
Trans-oriented multivalent binding modes increase the complexity of Syk recruitment toward FcεRI
The above hybrid MD/polymer model assumes that Syk only binds via a cis mode to an FcεRIγ monomer. However, an outstanding question is whether Syk can also span and bind to pTyr residues in both chains of an FcεRIγ dimer (i.e., trans binding). We first explored trans binding using a WLC polymer model to derive pITAM-ITAM for the separate chains. For binding of a Syk tandem SH2 to separate pTyrs on two FcεRIγ chains,
 |
5 |
where separate persistence and contour length values can be used for the two chains, based on the number of residues between the phosphotyrosine and the anchor point to the membrane surface (see Supplemental Appendix 1 for derivation). This distribution can then be used as input into Eq. 3, along with pSH2-SH2 for Syk, to calculate Ceff. We note that there are three possible ways that trans binding of Syk to two FcεRIγ chains can occur: 1) binding to N-ITAM on one chain and to C-ITAM on the other chain (i.e., NC binding), 2) binding to N-ITAMs on both chains (i.e., NN binding), and 3) binding to C-ITAMS on both chains (i.e., CC binding).
The distance distribution plot for trans binding via the NC mode is shown overlapped with plots for the WT and Y130E tandem SH2 REMD-based models in Figure 4, E and F. The Ceff values for binding of WT and Y130E mutant to pTyrs on separate construct chains are estimated at around 36.9 and 28.8 mM, respectively. These correspond to  values of 0.08 and 0.10 nM, respectively, indicating that Y130E mutant Syk has affinity comparable to that of WT for trans NC binding. The corresponding distance distribution plots for trans binding via the NN and CC modes are shown in Supplemental Figure 5, and also show comparable affinities for WT and Y130E mutants. For NN binding, the Ceff values for WT and Y130E mutants are 42.1 and 20.1 mM, respectively, leading to
values of 0.08 and 0.10 nM, respectively, indicating that Y130E mutant Syk has affinity comparable to that of WT for trans NC binding. The corresponding distance distribution plots for trans binding via the NN and CC modes are shown in Supplemental Figure 5, and also show comparable affinities for WT and Y130E mutants. For NN binding, the Ceff values for WT and Y130E mutants are 42.1 and 20.1 mM, respectively, leading to  values of 0.07 and 0.15 nM, while for CC binding, the Ceff values for WT and Y130E mutants are 23.9 and 27.4 mM, respectively, leading to
values of 0.07 and 0.15 nM, while for CC binding, the Ceff values for WT and Y130E mutants are 23.9 and 27.4 mM, respectively, leading to  values of 0.12 and 0.11 nM.
values of 0.12 and 0.11 nM.
We next applied computational modeling to explore how Syk molecules can bind to the ITAMs in the FcεRIγ dimer, using a maximum stoichiometry of two Syk molecules (a total of four SH2 domains) to one FcεRIγ dimer (a total of four pTyrs). We assume here that all ITAM tyrosines have been phosphorylated. From a total of 24 possible permutations, we found that only six lead to viable atomistic models after structurally redundant or physically unrealistic models are filtered out. One of these six models is the cis binding mode, while the other five models are different trans binding modes (Figure 5A). Three of the five trans binding modes contain variations of trans NC binding (Trans1, Trans2, and Trans3 in Figure 5A), while the remaining two (Trans4 and Trans5) combine trans NN and trans CC binding.
FIGURE 5:
WT tandem SH2 domains show higher affinity for cis binding in complex models of Syk–FcεRI with 2:2 binding stoichiometry. (A) Diagrams and corresponding atomistic structures of one cis and five trans binding modes that are physically viable and consistent with a 2:2 binding stoichiometry. Syk is depicted here using cartoons for N-SH2 (green), C-SH2 (blue), and interdomain A (orange). In each model, the two γ chains are colored differently (black and light pink), and how pY64 (pink) and pY75 (yellow) are bound is shown. Portions of the linker that connect the N-terminal end of the ITAM to the γ-chain transmembrane helices are shown for each model using artificial extended conformations, to give a sense of how each model may be oriented relative to the surface of the cytoplasmic leaflet of the cell membrane. (B) Unbiased MD simulations of these models in solution were then performed, with the artificial linker regions that connect the ITAMs to the transmembrane helices removed. The overall binding free energies were then calculated using the linear interaction energy (LIE) method (Aqvist et al., 1994) and are plotted here relative to the cis binding free energy. Error bars give SEM.
Unbiased MD simulations for each of these 2:2 models of Syk–FcεRIγ were then performed using the soluble portions of the complexes, with three replicates per model at 1 µs simulation time per replicate. To evaluate the relative stability of each of these models, we used the linear interaction energy (LIE) method (Aqvist et al., 1994) to compute the total free energies of the binding of ITAMs to their corresponding SH2 domains in each model. Results show that the cis orientation results in the highest binding free energy for WT Syk at a 2:2 stoichiometry between Syk–FcεRIγ (Figure 5B). Among the trans modes, Trans1 binding is strongest (80% of cis), followed by Trans4 (∼70% of cis). Trans 2, 3, and 5 binding modes were feasible, but with interaction strengths approximately half that of cis binding.
We then assessed whether these differences in stability arise from interactions of individual Syk SH2 domains with the individual γ chain pTyrs: the N-terminal pTyr site (pY64) or the C-terminal pTyr site (pY75). As shown in Supplemental Figure 6A, we computed interaction energies for each ITAM:SH2 pair and found similar results regardless of the binding mode. Two exceptions occur for pY75:N-SH2 in the Trans2 and pY64:N-SH2 binding in the Trans5 orientation; however these are due to unbinding of the pairs, which took place in only one out of three simulation replicates. Overall, the various binding modes do not appear to influence the affinity of individual Syk SH2 domains for particular ITAM pTyrs strongly.
It is noteworthy that the interaction energies for binding of Syk’s C-SH2 domain to pY64 in all orientations are predicted to be slightly higher than for the binding of the same C-SH2 domain to pY75 (Supplemental Figure 6B). Conversely, the binding of Syk’s N-SH2 domain to pY75 is predicted to be slightly stronger than its binding to pY64. Supplemental Figure 6B also shows that, on average, Syk C-SH2 interactions with either pTyr site are stronger than the corresponding N-SH2 interactions. For most binding orientations, the simulations suggest that the interactions by Syk’s N-SH2 domain are comparable. These results are qualitatively similar to experimental measurements of binding constants between individual Syk SH2 domains and CD3ε ITAMs, in the order pY64:C-SH2 > pY75:C-SH2 > pY75:N-SH2 > pY75:N-SH2 (Feng and Post, 2016).
We next considered the possibility that the engagement of tandem SH2 domains with both pTyrs of the ITAM induces additional contact sites. As shown in Figure 6A, we made the novel observation that the interaction stability of a Syk tandem SH2 domain bound to a dually phosphorylated ITAM is improved by additional residue–residue interactions (red circles) of the C-SH2 domain with pY75 (which is bound to N-SH2). These interactions likely contribute to the stronger binding observed in Cis and Trans1 orientations, as well as for Trans4 (Figure 6B). Note that these unexpected contacts are asymmetric, as pY64 (which is bound to C-SH2) is not capable of additional interactions with N-SH2.
FIGURE 6:
Additional contacts contribute to further stabilization of the cis binding mode. (A) Illustration showing that in the binding of the Syk N-SH2 domain to pY75 and the C-SH2 domain to pY64, asymmetrical contacts between pY75 and C-SH2 are formed (red encircled region). This is not the case for pY64 and N-SH2. Key: N-SH2 (green), C-SH2 (blue), interdomain A (orange), γ chain (magenta). (B) Average interaction energies of these asymmetrical contacts when engaged in each of the different 2:2 binding modes. (C) Illustration of additional contacts between the pTyrs bound to the Syk molecule on the right with the “bystander” Syk molecule on the left (red circles) for the cis orientation of the 2:2 Syk–FcεRIγ complex. Proteins are colored as in A. (D) Average interaction energies of these additional contacts, illustrating that these are significant only in the cis orientation. Error bars give SEM.
Figure 6C indicates that additional asymmetric contacts further contribute to the stability of Syk docking when engaged in 2:2 Syk–FcεRIγ complexes. The structural model shows that the pTyrs and nearby residues of the γ chain bound to one Syk molecule are actually engaging in additional residue–residue contacts with the other Syk molecule (red circles). Because these interactions likely are unique to the cis orientation (Figure 6D), these data provide mechanistic support for the cis binding mode as the most stable mode of multivalent binding between Syk and FcεRIγ (Figure 5B). We do note, however, that while individual trans modes are each weaker than the cis mode, the availability of multiple trans modes should allow a stronger gain for trans binding than for the single cis mode (see Figure 4, C and D for cis vs. E and F for trans).
Trans-oriented multivalent binding facilitates signaling during partial ITAM phosphorylation
We next evaluated experimentally the above computational predictions that formation of Syk–FcεRI complexes can involve both cis- and trans-oriented binding modes. Experimental measures of calcium mobilization provide a sensitive indicator of FcεRI and Syk activation in cultured mast cells (Smith et al., 2001). We used two complementary approaches to study the FcεRIγ ITAM–Syk axis in rat basophilic leukemia (RBL-2H3) cells. In the first approach, we took advantage of CRISPR-Cas 9 gene editing methods (Richardson et al., 2016; Cleyrat et al., 2017) to create RBL cells homozygous for the deletion of the ITAM-bearing γ subunit of FcεRI. Because complete assembly of the αβγ2 heterotetrameric complex is a prerequisite for trafficking of IgE receptors to the cell surface (Fiebiger et al., 2005), we used cell sorting to select γ knockout cells based on a complete loss of binding to fluorescent IgE (unpublished data). This cell population was expanded in cell culture and then subjected to subcloning and Western blotting analysis to produce RBL cells completely lacking FcεRIγ expression (FcεRI γ-KO cells). This new cell line then served as the basis for FcεRI reconstitution with γ constructs for expression of wild type gamma (γWT) or mutants with substitutions of alanine for one or both tyrosine residues in the γ ITAM (γY64A; γY75A; γY64A\Y75A).
Results of this reconstitution model system to study Syk-dependent signaling are reported in Figure 7, based upon ratio imaging of cells loaded with the calcium reporter Fura-2AM (Smith et al., 2001). Cells were primed overnight with Alexa647-IgE to screen for cells with comparable FcεRI surface expression following transient transfection. As expected, antigen-mediated cross-linking of FcεRIγ-KO cells reconstituted with γWT led to marked elevation of cytoplasmic calcium in 100% of cells (Figure 7, A and B; Supplemental Figure 7, A and B). Prior work has shown that antigen stimulation of bone marrow–derived mast cells induces multiple species of FcεRIγ phosphorylation (Yamashita et al., 2008). If one assumes no bias for phosphorylation of the four tyrosines in the paired ITAMs, the resulting FcεRI aggregates could represent up to 10 chemical species (four phosphorylation states per γ chain leading to 16 total states for two chains, although six of these states are redundant). This includes the full range from incompletely phosphorylated γ pairs (zero to three phosphotyrosines) to fully phosphorylated (four phosphotyrosines) that can recruit Syk in both cis and trans configurations, thereby increasing the combinatorial complexity of this system (Faeder et al., 2005).
FIGURE 7:
Calcium imaging of transiently FcεRIγ-reconstituted RBL-2H3 FcεRIγ-KO cells after antigen cross-linking supports the concept of trans docking of Syk onto pairs of monophosphorylated ITAMs, but with a greatly diminished response compared with that for cis docking. (A) RBL-2H3 FcεRIγ-KO were antigen cross-linked with DNP25-BSA (0.1 μg/ml) calcium imaged after FcεRIγ reconstitution with (top to bottom) WT, Y64A, Y75A, a combination of Y64A and Y75A single mutants, or the double mutant Y64A\Y75A. (B) The respective percentages of response after antigen cross-linking. Error bars indicate the 95% CI. (C) The time between antigen cross-linking and calcium release. (D) The relative increase in the Fura-2 ratio per cell after antigen cross-linking. SEM and mean are reported in C and D by error bars and crosses, respectively. ** P < 0.001, * P < 0.01 by the Fisher test and the two-sample Kolmogorov–Smirnov test. Experiments were conducted over a period of 5 d.
Cells reconstituted with γY64A or γY75A express FcεRI complexes where each of the γ ITAMs can only be monophosphorylated. Thus, if Syk engages with two γ ITAMs through both of the SH2 domains in these cells, it can only be in one of the trans configurations. We observed the most consistent antigen-stimulated calcium responses in cells expressing γ subunits bearing only the ITAM Tyr75 residue (FcεRIγ–Y64A), where 73% of cells had a measurable change in cytosolic calcium (Figure 7, A and B; Supplemental Figure 7B). In comparison, calcium responses were triggered in only 54% of cells expressing γ subunits solely bearing ITAM Tyr64 (FcεRIγ–Y75A). Cells transiently transfected with both mutant γ constructs, which have the potential to express FcεRI composed of a mixture of γY75A and γY64A and could present a combination of all trans binding modes (see Figure 5A), showed calcium responses similar to those of cells expressing γY64A alone. Finally, cells reconstituted with the γY64A\γY75A double mutant remain capable of triggering a weak calcium response, which may be attributed to the presence of an intact FcεRI β-ITAM.
Importantly, the profiles of calcium responses in cells that allow only two phosphorylation sites and a limited subset of trans binding modes were markedly less robust than in cells constituted with γWT, where both cis and trans modes are possible in combination. Differences from γWT include a slower onset for the initial calcium response (increase in response time, Figure 7C) as well as a lower overall magnitude of change (decrease in rise height, Figure 7D). They were also frequently highly oscillatory, which we classify as a weak response in Supplemental Figure 7C. The signaling output is weaker than in FcεRI aggregates reconstituted with γWT, which also permit the full range of Syk binding configurations, including the canonical cis orientation as well as multiple trans orientations, depending on the extent of receptor phosphorylation.
Membrane anchoring distance is a critical factor in Syk recruitment in trans-oriented binding
We also explored the importance of proximity between ITAM pairs for recruitment of Syk in the trans binding mode, combining the power of experimental and modeling approaches. As an experimental strategy, we transiently transfected parental RBL-2H3 cells with plasmids encoding a chimeric Tac-Tac-γ (TT-γ) receptor monomer derived from the coding sequences of extracellular and transmembrane domains of the Tac antigen (IL2α subunit) fused in frame with the γ subunit cytoplasmic tail sequence (Letourneur and Klausner, 1991; Wilson et al., 1995). We prepared vectors for expression of either WT chimeric receptors (TT-γ WT) or mutant versions with tyrosine-to-alanine substitutions in each of the two ITAM tyrosines (TT-γ Y64A, TT-γ Y75A and TT-γ Y64A\Y75A). The chimeric receptors are first converted to a quasidimer state by pretreating cells with Alexa647-tagged, murine monoclonal anti-Tac antibodies. Because this pretreatment does not initiate measurable mast cell signaling (Wilson et al., 1995), it provides a good model system to explore the role of distance in trans binding for Syk. We again used Alexa647 labeling as a thresholding method to select transiently transfected cells with comparable surface expression of chimeric receptors.
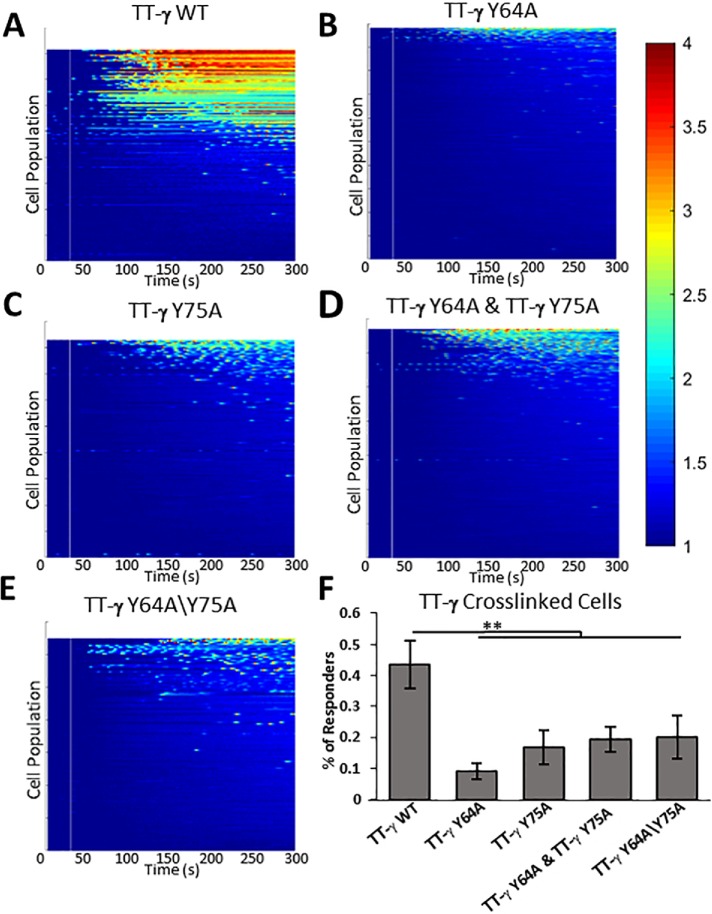
Results in Figure 8 show that aggregation of TT-γ WT chimeric receptors with anti-mouse secondary antibodies led to robust calcium responses in 41% of the cells examined, with weak and oscillatory calcium bursts in an additional 2% of cells (Figure 8, A and F; Supplemental Figure 7D). These data confirm that aggregation of receptor monomers with a single γ ITAM is generally less efficient at signaling than stimulation via cross-linking of the intact receptor (see Figure 7). Weaker responses were observed after aggregation of either of the TT-γ mutant forms, where only monophosphorylation is possible (TT-γ Y64A and TT-γ Y75A; Figure 8, B and C). As summarized in Figure 8F and Supplemental Figure 7D, these responses were limited to a small fraction of cells examined and are not significantly better than for cells expressing TT-γ chimeras that lack both tyrosines (TT-γ Y64A\Y75A; Figure 8, E and F). Cotransfection of TT-γ Y64A and TT-γ Y75A into RBL-2H3 cells led to the possibility of mixed TT-γ aggregates containing both monophosphorylated forms of the γ ITAM but did not improve the consistency of responses after cross-linking (Figure 8, D and F). The inability of TT-γ single-tyrosine mutants to signal is consistent with the separation between cross-linked TT-γ outside the limits of the binding distance calculated for SH2 domains (Figure 4; Supplemental Figure 5).
FIGURE 8:
Calcium imaging after cross-linking of chimeric TTγ receptors demonstrates that Syk can bind in cis to a single ITAM but requires sufficiently close ITAMs for trans binding. Data are reported for RBL-2H3 cells transiently expressing (A) TT-FcεRIγ(WT), (B) TT-FcεRIγ(Y64A), (C) TT-FcεRIγ(Y75A), (D) the combination of TT-FcεRIγ(Y64A) and TT-FcεRIγ(Y75A), or (E) TT-FcεRIγ(Y64A\Y75A). Cells were preincubated for 10 min with Alexa647-labeled anti-Tac murine monoclonal antibodies and then cross-linked with anti-mouse secondary antibodies, as described in Materials and Methods. Heat maps indicate Ca2+ mobilization, with red indicating a strong response and blue indicating no release. (F) The respective percentages of response after TT cross-linking. Error bars indicate the 95% CI. ** P < 0.001 by the Fisher test. Experiments were conducted over a period of 5 d.
DISCUSSION
Efficient immune-cell signaling relies on protein–protein interactions, which are often facilitated by reversible phosphorylation events (Hunter, 1995; Pawson and Scott, 2005; Basson, 2012; Ardito et al., 2017; Gelens et al., 2018). The recruitment of the Syk/Zap-70 family of cytosolic tyrosine kinases to ITAM motifs is an essential step in signaling by the MIRR family of immunoreceptors (Geahlen, 2009; Mocsai et al., 2010). In this work, we have applied multiple computational modeling approaches to characterize the structural features that control interactions between Syk and the FcεRI γ-ITAM. We report contributions of multiple factors to the specificity and strength of the Syk–FcεRI complex, including how the combination of multivalency and cis versus trans binding orientations increases signaling output in situations where phosphorylation of γ-ITAMs is incomplete. We unveil novel residue–residue interactions made possible only in specific orientations. We also provide mechanistic insight into the impaired binding of the Syk-Y130E that mimics structural changes induced by phosphorylation in interdomain A.
The influence of multivalency was explored through development of a structure-based analytical model (Figure 1). We show that local concentration effects arise due to the bivalent nature of Syk’s tandem SH2 domains together with the paired pTyrs in the FcεRI γ-ITAM. We applied an effective equilibrium association constant for binding of the linked motifs, previously derived (Sethi et al., 2011), that comprises the product of the two monomeric association constants and a quantity called Ceff as shown in Eq. 1. Ceff represents the effective local concentration of pTyr available for SH2 binding, given that one SH2:pTyr is already engaged. We used the translational variability between the Syk tandem SH2 domains observed in the six structures from PDB 1A81 (Fütterer et al., 1998) to define the volume of the spherical shell where unbound SH2 can search for unbound pTyr. The orientational variability between the tandem SH2 domains in these six structures was also used to obtain a factor for the fraction of orientations within this search shell that allow for successful binding events. Combining these values with the experimentally measured monomeric binding constants (Chen et al., 1996), we arrived at a calculated effective binding constant of ∼1 nM for the system, which is consistent with the experimentally measured affinity for Syk SH2–SH2 bound to a dually phosphorylated ITAM (Chen et al., 1996).
Binding characteristics of the Syk Y130E mutant are particularly of interest, since expression of this phosphomimetic has impaired signaling capability (Schwartz et al., 2017). Because there is no resolved structure for the phosphomimetic, we used a hybrid MD/polymer model to generate two distance distributions (inter-SH2 for Syk and inter-pTyr for FcεRIγ). First, the inter-SH2 distance distribution was computed from unbiased MD simulations of the Syk tandem SH2 domains connected by the interdomain A linker using WT and Y130E mutant constructs. These simulations showed that the mutant sampling conformations cover a wider distance distribution than the WT (x-axes in Figure 2, B and C). The mutant also shows higher orientational variability in the conformations it sampled in these simulations (y-axes in Figure 2, B and C). These conformational differences were attributed to changes in the number of domain–domain contacts in the mutant relative to the WT construct (Supplemental Figure 4). In particular, there is a decrease in the number of contacts between the N-SH2 and C-SH2 domains in the presence of the mutation, which is consistent with the experimentally observed partial decoupling of both SH2 domains in the mutant (Zhang et al., 2008; Feng and Post, 2016; Roy et al., 2016).
MD simulations provided insufficient information on the influence of the Y130E substitution on helical stability, due to limited sampling even at microsecond time scales. Motivated by experimental observations that Y130E does disrupt helical stability (Zhang et al., 2008; Feng and Post, 2016; Roy et al., 2016), we computed inter-SH2 distance distribution from REMD simulations of the interdomain A linker. Only the linker was included in these simulations in order to enhance the sampling of adopted conformations and to evaluate the impact of the Y130E mutation. The two SH2 domains were then reattached to each conformation in the WT and Y130E interdomain A ensembles, from which the inter-SH2 distance distribution was obtained after filtering of models that showed large steric clashes when both SH2 domains were added back. The models based on the REMD simulations of Y130E linker showed more unfolding of helical turns than the WT model (Figure 3). The inter-SH2 distance distribution for Y130E in these simulations was broader and had a higher average value than WT (Figure 4, A and B).
A major uncertainty in these calculations is that the FcεRIγ cytoplasmic tail is structurally disordered; adequate conformational sampling of these unstructured regions is challenging for MD simulations (Lopez et al., 2015) even using enhanced sampling techniques. To evaluate the inter-pTyr distance distribution within an ITAM, we used a WLC polymer model. When applied to protein chains, WLC modeling derives this distance distribution (gray curves in Figure 4, C and D) based on Eq. 4 as a function of the persistence and contour lengths. These length values provide the minimum length where the polymer can be modeled as a random walk and the maximum possible length of the polymer, respectively (Zhou, 2001; Ohashi et al., 2007; Rawat and Biswas, 2009). The product of the two distributions at each distance value is shown as a red curve in Figure 4, C and D, and integrating the area under this curve gives an estimate of Ceff as given in Eq. 3. The effective binding constant for the Y130E Syk mutant was found to be ∼10-fold lower than that for the WT. Taken together, these data support the experimental observation that the Y130E phosphomimetic form of Syk can be recruited to FcεRI complexes. If the 10-fold lower binding is mainly attributable to an increased koff (i.e., higher dissociation rate of formed complexes), then this would be consistent with the experimentally observed shorter lifetimes of bound Y130E mutant Syk (Schwartz et al., 2017).
Many MIRRs incorporate paired ITAM-bearing subunits, including the FcεRIγ2 subunit (shared with Fcγ receptors), as well as TCRζ2 and the BCR Igα–Igβ complex. These paired homodimers and heterodimers are stable by virtue of a disulfide linkage (Turner and Kinet, 1999; Call et al., 2006). This markedly expands the complexity and number of variations for possible multivalent interactions, in comparison to the simplest case considered in Figure 1 for Syk tandem SH2 domains bound to paired pTyrs in the cis orientation. We used WLC polymer models along with molecular modeling and simulations to explore the possibility that Syk can also bind stably to γ homodimers via several trans orientations. WLC polymer models for native FcεRIγ chains showed that trans binding can be comparable to or stronger than cis binding (see Figure 4, C and D for cis vs. E and F for trans). Even in the cases where individual trans modes are less efficient than the cis configuration for recruiting Syk (Figures 5 and 6), the availability of multiple trans modes vs. a single cis mode should allow enhanced opportunities to recruit Syk, given the evidence that multiple phosphorylation states are generated following receptor activation (Yamashita et al., 2008).
We tested predictions of the model by performing experiments in FcεRIγ-KO cells, where the intact receptor is reconstituted with either WT or Tyr-mutant versions of the γ cytoplasmic tail (Y64A and Y75A). Signaling is maximal in cells reconstituted with the WT form, which offer a full range of cis and trans docking modes in response to multivalent antigen (Figure 7, A and B). Receptors bearing mutant γ constructs are composed of disulfide-bonded γ homodimers with two, rather than four, ITAM tyrosines. Importantly, aggregation of these homodimers can generate measurable calcium responses when only trans binding modes are available for docking a single dual SH2 domain of Syk, but with markedly less robust responses (Figure 7, B–D). Our results are consistent with previous studies where γ-ITAM tyrosine mutants were expressed in γ-knockout bone marrow–derived mast cells (Yamashita et al., 2008), which focused on potential dephosphorylation kinetics rather than Syk orientation as a mechanism for controlling signal propagation. In addition, we point out that the β-ITAM is intact in the FcεRIγ reconstituted receptor complexes, and therefore we cannot rule out it contributing in part to Syk recruitment in the intact receptor. Indeed, this is supported by the observation that weak calcium signaling was observed on antigen stimulation of FcεRIγ KO cells after reconstitution with γ constructs deficient in both Tyr75 and Tyr64 (γY64A\Y75A).
We also compared signaling capabilities in cells expressing chimeric receptor monomers, where the transmembrane and extracellular domains of the Tac antigen (IL2α subunit) is fused to the γ cytoplasmic tail. We confirmed that extensive cross-linking of the WT version of the TT-γ receptor leads to a robust calcium response (Figure 8), although the response rate is less consistent than for cells with WT FcεRIγ reconstituted receptor complexes. These results are consistent with the distances of antibody–cross linked WT TT-γ constructs, which likely favors a cis orientation for Syk binding. We speculate that this behavior is relevant to signal initiation by monomeric receptors containing a single functional ITAM, such as FcγRIIA (Van den Herik-Oudijk et al., 1995).
We also generated chimeric TT-γ receptors with alanine substitutions at Y64 or Y75 that are similar to the naturally occurring class of “hemITAM” receptors, which include CLEC-2 and Dectin-1 (Bauer and Steinle, 2017). Because each monomer has a single tyrosine phosphorylation site, they can recruit Syk only via trans binding modes. Distance is again a contributing factor. We hypothesize that the very weak signaling observed for these mutant chimeric constructs is due in part to the limits of the experimental system, where dimerization with anti-Tac antibodies separates the two chains by a wide distance. We note that based upon a Fab separation distance of ∼120 Å in a typical IgG antibody (Harris et al., 1998), we expect that trans binding is essentially precluded, because the WT and Y130E mutant inter-SH2 distributions cover shorter distances (<80 Å; see Figure 4, A and B), leading to almost no overlap between the distributions and thus very small values for Ceff.
On the surface, the relative signaling capabilities of cis and trans configurations for Syk docking are distinguishing features that strikingly separate several distinct classes of immunoreceptors bearing either ITAM or hemITAM domains. Modeling predictions support the notion that multisubunit receptors that take advantage of multiple orientations for Syk docking should be the most efficient for signal transduction. We expect that this principle also applies to receptors that couple to other tandem SH2 domain–containing proteins, such as Zap-70, PTPN6, PTPN11, and PLCγ1 (Pluskey et al., 1995; Pei et al., 1996; Ji et al., 1999). However, there are still lessons to be learned about the recruitment of Syk to ITAM receptors under conditions of suboptimal cross-linking, where monophosphorylation of ITAMs is expected to predominate (O’Neill et al., 2011; Mahajan et al., 2014). In the case of hemITAM receptors, which have only one tyrosine phosphorylation site per monomer, these are limited to a trans binding mode for Syk recruitment. We speculate that the membrane anchor distance between hemITAMs after aggregation of this entire class of C-type lectin-like receptors will be the key determinant of signaling output, where trans docking must be the key event after dimerization (Hughes et al., 2010). In particular, a geometrical approach for quantifying multivalency when both chains are separated by a distance d between their membrane anchor points (see Supplemental Appendix 2 for derivation) indicates that Ceff, and thus the multivalent binding affinity of Syk, is inversely proportional to the square of distance d. This suggests a possible mechanism where the cell can modulate the binding of Syk to hemITAMs via changes in gene expression that can regulate the membrane density, and thus membrane spacing, between hemITAMs. A relatively weak signaling capability for Dectin-1 may also explain the need for a high density of surface-exposed β-1,3-Glucan on pathogens to trigger phagocytic responses to yeast (Wester et al., 2017; Graus et al., 2018).
MATERIALS AND METHODS
Analytical model of multivalent interactions in WT Syk–FcεRIγ complex
The crystal structure of the Syk tandem SH2 domains bound to the ITAMs from a single CD3ε tail (PDB 1A81; Fütterer et al., 1998) was used here to quantify the possible changes in Syk upon its binding to an immunoreceptor. In particular, the six complexes in the asymmetric unit cell of this crystal structure were compared after structural alignment of their N-SH2 in PyMOL (Schrödinger), which showed that the C-SH2 position varied within a range of 2.0 nm (i.e., translational variability) and the C-SH2 rotation varied within a range of 18° (i.e., orientational variability). The translational and orientational variabilities were used to compute the Vacc (volume of accessible spherical shell) and for (orientation factor) terms in Eq. 2, which gives the effective concentration Ceff of unbound ITAM that unbound SH2 experiences upon binding of the other SH2:ITAM pair. This effective concentration can then be used in Eq. 1 to estimate the effective equilibrium association constant Koverall for multivalent Syk–FcεRIγ, whose reciprocal is the effective equilibrium dissociation constant  , which can be compared with experimental measurements.
, which can be compared with experimental measurements.
System setup and unbiased MD simulations of Syk tandem SH2 and interdomain A
Chain A from PDB 1A81 was used as the initial conformation for performing unbiased MD simulations of Syk tandem SH2 and interdomain A. This chain contains the WT human sequence, and in silico mutagenesis was done using PyMOL to generate the WT murine sequence comprising residues 8–261. The initial conformation for the Syk Y130E mutant used this WT construct with the single phosphomimetic mutation done in silico using PyMOL. Acetyl and N-methyl neutral caps were added to the N- and C-termini of both constructs using PyMOL. All-atom topologies and parameters for both constructs were generated using the CHARMM36 force field (Klauda et al., 2010; Best et al., 2012). Solvent molecules were added using the TIP3P water model (Jorgensen et al., 1983) to fill a rhombic dodecahedral box around each construct, with a minimum distance of 1.2 nm from any protein atom to any edge of the simulation unit cell. Monovalent K+ and Cl– ions were added to neutralize the system charge and to reach a physiological ionic strength of 150 mM.
The AMBER MD engine (version 16), which has been GPU-optimized for simulating explicit solvent systems (Salomon-Ferrer et al., 2012; Case et al., 2017) was used for running unbiased MD simulations of the Syk WT and Y130E systems. Particle mesh Ewald (PME) electrostatics (Darden et al., 1993) was used along with Coulomb and Lennard Jones cutoffs of 1.2 nm and potential switching at 1.0 nm. A constant temperature was maintained at 310 K via Langevin dynamics (Pastor et al., 1988) with a collision frequency of 1.0 ps–1. Semiisotropic pressure coupling was set for each system at 1 bar using a Monte Carlo barostat (Åqvist et al., 2004) with a relaxation time of 4.0 ps. Bonds containing hydrogen atoms were constrained using the SHAKE algorithm (Ryckaert et al., 1977). A hydrogen mass–repartitioning approach (Feenstra et al., 1999) allowed the use of a 4-fs time step. Both systems were energy-minimized using up to 10,000 steps of steepest descent. Seven replicates of each system were then equilibrated, with each replicate having different initial velocities. Position restraints were applied to all protein heavy atoms during equilibration, which took 100 ps in the NVT ensemble and 10 ns in the NPT ensemble. Production runs (without position restraints) of 2 µs in the NPT ensemble were then performed for each replicate with configurations and energies saved every 200 ps. Two out of seven replicates were randomly selected for each system, and the simulations extended to 6 µs. Analyses were performed on the last two-thirds of each simulation. The distance reaction coordinate was measured between the Cα atoms of residues R21 and R174, which are part of the corresponding pTyr-binding sites on N-SH2 and C-SH2, respectively. The dihedral reaction coordinate was measured using the Cα atoms of R21 and L28 from N-SH2 with R174 and V181 from C-SH2, which belong to stable α-helices near the pTyr-binding site of each SH2 domain.
System setup and REMD simulations of Syk interdomain A
The initial conformation for performing REMD simulations of Syk interdomain A was taken from the WT murine structure and comprised residues 115–159. As for the larger Syk construct (residues 8–261), the single phosphomimetic mutation Y130E was introduced in silico using PyMOL along with acetyl and N-methyl neutral caps on the N- and C-termini. Generation of all-atom topologies/parameters and the addition of solvent molecules and monovalent ions were performed as described earlier. The GROMACS MD engine (version 5.1.2; Abraham et al., 2015) was used for running all simulations described in this section. Simulation parameters were similar to those described earlier, with a few exceptions. Pressure coupling was done using a Parrinello–Rahman barostat (Parrinello and Rahman, 1981), and bonds containing hydrogen atoms were constrained using the LINCS algorithm for the protein (Hess, 2008) and the SETTLE algorithm for water (Miyamoto and Kollman, 1992). A 2-fs time step was used, as hydrogen mass repartitioning was not performed for these systems.
Pilot unbiased MD runs were first run for the WT and Y130E mutant systems to determine the optimal temperature distribution. After energy minimization, a copy of each system was equilibrated at nine different temperatures equally spaced within the range 275–475 K. Energy minimization and equilibration were performed as described earlier for the unbiased simulations. Production runs (without position restraints) of 100 ns in the NVT ensemble were then performed with configurations and energies saved every 10 ps. The average energy and its SD were then computed for each of the 10 simulations for a particular system and used as input for a REMD temperature scheduler (Garcia et al., 2006). This scheduler provided a temperature distribution comprising 63 temperatures within the range 275–475 K that would ensure a 20% exchange rate between all adjacent replicas in the REMD simulations. The same temperature distributions were given by this scheduler for the WT and Y130E mutant interdomain A systems.
These 63 temperatures were then used to run the REMD simulations. From the energy-minimized structures, a copy of each system was equilibrated at one of the 63 temperatures. All equilibration runs were performed as described earlier for the unbiased simulations. Production REMD runs (without position restraints) of 1.2 µs in the NVT ensemble were then performed for each replica with configurations and energies saved every 10 ps. Alternating exchanges between adjacent replicas were attempted every 2 ps, with swap acceptance or rejection determined by the Metropolis criterion. All analyses were done using the 15 replicas with temperatures less than 310 K (excluding the 275-K replica, which acted as a sink replica). Helicity for each of the 15 replicas was monitored over the entire trajectory, while subsequent processing and analyses used the last 500 ns of these trajectories.
Hybrid MD/polymer model of multivalent interactions in WT and Y130E Syk–FcεRIγ complexes
The conformational ensembles for WT and Y130E mutant Syk interdomain A were each taken from the 15 replicas described earlier for the REMD simulations. To generate the corresponding ensembles of WT and Y130E mutant Syk tandem SH2 and interdomain A, the N-SH2 (residues 8–116) and C-SH2 (residues 158–261) from the WT murine model of Syk were reattached in silico to every snapshot in the interdomain A ensembles using PyMOL. In particular, the atoms in the peptide bond between residues 115 and 116 were structurally aligned between N-SH2 and each interdomain A snapshot, and similarly for the atoms in the peptide bond between residues 158 and 159 to attach C-SH2. After filtering out of snapshots containing steric clashes between N-SH2 with interdomain A, C-SH2 with interdomain A, and N-SH2 with C-SH2, around 5% of the WT snapshots remained as physically viable models of Syk tandem SH2 and interdomain A, and similarly 5% of the Y130E mutant snapshots remained. The distance distribution between the two SH2 domains for each system was obtained using the distance reaction coordinate described earlier.
These inter-SH2 distance distributions were used as the MD component in the hybrid MD/polymer model to estimate the effective concentration Ceff in Eq. 3. The inter–ITAM-pTyr distance distribution comprised the polymer model component of this hybrid approach, and was generated using Eq. 4, which assumes that each Syk molecule binds to a single immunoreceptor tail in a cis mode. This equation requires a value for the peptide contour length, which represents the maximum/extended end-to-end length of the peptide as a function of the number of residues (N) (Zhou, 2001),
| 6 |
and a value for the peptide persistence length that gives the end-to-end length of the peptide beyond which a description of its behavior transitions from a flexible rod to a random walk (Rawat and Biswas, 2009),
| 7 |
A value of 12 was used for N in Eqs. 6 and 7, as this gives the length between the two pTyr residues in the FcεRIγ sequence (counting inclusive of both pTyr). Ceff is then calculated in Eq. 3 by integrating over the products of the probabilities in the overlapping regions between both distributions for all possible distances (Van Valen et al., 2009; Sethi et al., 2011). Ceff can then be used for estimating the equilibrium dissociation constant  via Eq. 1 and getting the reciprocal.
via Eq. 1 and getting the reciprocal.
System setup and unbiased MD simulations of WT Syk–FcεRIγ complexes with 2:2 stoichiometry
Although there are 24 possible permutations for combining four SH2 domains from two Syk molecules with four pTyrs from an FcεRIγ dimer, we found that only one cis and five trans modes can be built as physically viable structural models. The structure for the cis mode complex was obtained by first taking one of the Syk-CD3ε structures (chains A and B) from PDB 1A81 and performing in silico mutagenesis with PyMOL to get the WT murine Syk and FcεRIγ sequences, and then placing two of these structures adjacent to each other. Each of the five trans structures was then obtained from the cis structure via a combination of manual translation and rotation operations in PyMOL. Backbone breaks/reseals and rotations were performed on the N-Cα and Cα-C bonds of the FcεRIγ chain to keep the WT structure of Syk intact and to prevent accidental formation of peptide bonds with incorrect dihedral angles (i.e., not 180°). Acetyl and N-methyl neutral caps were added to the N- and C-termini of all chains using PyMOL. Generation of all-atom topologies/parameters, addition of solvent molecules and monovalent ions, and unbiased MD simulations using GPU-optimized AMBER were performed as described earlier for the unbiased simulations of Syk tandem SH2 and interdomain A.
Reagents and antibodies
MEM was purchased from Life Technologies (Grand Island, NY). Alexa647-labeled anti-Tac IgG was purchased from BioLegend (San Diego, CA) and anti-mouse IgG was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Amaxa Nucleofector and Solution L were purchased from Lonza (Basel, Switzerland). Fura-2AM was from Molecular Probes (Eugene, OR). DNP25-BSA was from ThermoFisher Scientific (Waltham, MA; catalogue #A23018) and anti-DNP-IgE was affinity-purified from ascites (Covance, Denver, PA) according to the methods of Liu et al. (1980). Alexa647-labeled IgE was prepared using Alexa647 NHS Ester (succinimidyl ester; ThermoFisher Scientific).
Cell culture and transfection
Parental RBL-2H3 cells (Metzger et al., 1986; Wilson et al., 2000) and their derivatives were cultured in MEM supplemented with 10% heat-inactivated fetal bovine serum, 5 U/ml penicillin, 0.05 mg/ml streptomycin, and 2 mM l-glutamine. Transient transfections were conducted using the Amaxa system (Program T-20) and cells were imaged the following day. Constructs for expression of chimeric TT-γ receptors have been described previously (Letourneur and Klausner, 1991). Constructs for reconstitution of FcεRIγ WT and mutant γ in FcεRIγ-KO cells were prepared in the pcDNA3.1 vector (Invitrogen). For all microscopy experiments, cells were plated overnight in eight-well Lab-Tek (Nunc) chambers (ThermoFisher Scientific) at a density of 50,000 cell/well.
Genome editing
The FcεRIγ-KO cell line was generated in RBL-2H3 cells by CRISPR-Cas9–mediated gene editing, resulting in insertion of a premature stop codon. RNA (5′-GCAAGAACAAGATCACCGCT-3′) targeting the first exon of rat FcεRIγ was designed using the http://crispr.mit.edu/ portal and then subcloned into PX458 vector (Addgene plasmid #48138) for simultaneous expression of gRNA, WT Cas9, and a GFP reporter. For gRNA subcloning, two partially complementary oligonucleotides (Integrated DNA Technologies) were assembled by PCR. Gel-purified PCR products were cloned into BbsI-digested PX458 using Gibson assembly (NEB) following the manufacturer’s specifications. GFP-positive cells were selected on an iCyt cell sorter 24 h after transfection using the Amaxa system and then dispensed at single-cell density into 96-well plates. Subclones were screened for lack of fluorescent IgE binding by flow cytometry, followed by Western blotting to confirm selected clones completely lack FcεRIγ expression.
Calcium measurements
Calcium measurements and data analysis were performed as previously described (Schwartz et al., 2015). Parental or FcεRIγ-KO derivatives were primed overnight with DNP-specific Alexa647-labled IgE (1 μg/ml). Fura-2AM loading (2 μM in Hanks buffer) was performed for 30 min at room temperature. Cells expressing chimeric TT-γ receptors were incubated with Alexa647-labled anti-Tac IgG (1 μg/ml) for the final 10 min. In all cases, cells were washed with buffer and ratio images were acquired at 35°C using an Olympus IX71 inverted microscope outfitted with a UPLANSAPO 60X/NA1.2 water emersion objective coupled to an objective heater (Bioptechs). Cells were activated by cross-linking with DNP25-BSA (0.1 μg/ml; ThermoFisher Scientific catalogue #A23018) or anti-mouse IgG (25 μg/ml), added at 30 s during a total of 5 min of imaging. Ratiometric changes in cytosolic calcium were determined by alternating between 350 and 380 nm at 1 Hz with a xenon arc lamp monochromator (Cairn Research OptoScan) and collecting the interleaved Fura-2 fluorescence emissions at 510 nm with an iXon 887 EMCCD camera using IQ3 imaging software (Andor Technology). Offline ratiometric analysis was performed over time with a custom MATLAB script for each cell (5–10 per field of view) to assess calcium release and subsequently normalized to a minimum threshold of Alexa647 emission (100 AU) based on surface expression of receptors in transiently transfected cells.
Supplementary Material
Acknowledgments
This work was supported by federal grants and the New Mexico Spatiotemporal Modeling Center (NIH P50GM085273 for B.S.W.; the Department of Energy through contract DE-AC5206NA25396 for S.G. and T.T.; NIH R35GM126934 for D.S.L.). T.T. was also supported by the Center for Nonlinear Studies (CNLS) at the Los Alamos National Laboratory (LANL). E.J. was supported by K12 GM088021. Computing resources were made available through Extreme Science and Engineering Discovery Environment (XSEDE) Allocation MCB170148, which is supported by National Science Foundation (NSF) Grant ACI-1548562, and through LANL Institutional Computing. We thank Shayna Lucero for assistance with cell culture and gratefully acknowledge use of the University of New Mexico Cancer Center fluorescence microscopy and flow cytometry facilities, as well as NIH-NCI support via P30CA118100 for this core.
Abbreviations used:
- BCR
B-cell receptor
- IgE
immunoglobulin E
- ITAM
immunoreceptor tyrosine-based activation motif
- KD
kinase domain
- KO
knockout
- MD
molecular dynamics
- MIRR
multichain immunorecognition receptor
- RBL
rat basophilic leukemia
- REMD
replica-exchange molecular dynamics
- SH2
Src homology 2
- TCR
T-cell receptor
- TT
Tac-Tac
- WLC
worm-like chain
- WT
wild type.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E18-11-0722) on June 19, 2019.
REFERENCES
- Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E. (2015). GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX , 19–25. [Google Scholar]
- Aqvist J, Medina C, Samuelsson JE. (1994). A new method for predicting binding affinity in computer-aided drug design. Protein Eng , 385–391. [DOI] [PubMed] [Google Scholar]
- Åqvist J, Wennerström P, Nervall M, Bjelic S, Brandsdal BO. (2004). Molecular dynamics simulations of water and biomolecules with a Monte Carlo constant pressure algorithm. Chem Phys Lett , 288–294. [Google Scholar]
- Ardito F, Giuliani M, Perrone D, Troiano G, Lo Muzio L. (2017). The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy (review). Int J Mol Med , 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basson MA. (2012). Signaling in cell differentiation and morphogenesis. Cold Spring Harb Perspect Biol , a008151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer B, Steinle A. (2017). HemITAM: a single tyrosine motif that packs a punch. Sci Signal , eaan3676. [DOI] [PubMed] [Google Scholar]
- Best RB, Zhu X, Shim J, Lopes PE, Mittal J, Feig M, Mackerell AD., Jr (2012). Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone phi, psi and side-chain chi(1) and chi(2) dihedral angles. J Chem Theory Comput , 3257–3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank U, Ra C, Miller L, White K, Metzger H, Kinet JP. (1989). Complete structure and expression in transfected cells of high affinity IgE receptor. Nature , 187–189. [DOI] [PubMed] [Google Scholar]
- Call ME, Schnell JR, Xu C, Lutz RA, Chou JJ, Wucherpfennig KW. (2006). The structure of the ζζ transmembrane dimer reveals features essential for its assembly with the T cell receptor. Cell , 355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier JC. (1995). Antigen and Fc receptor signaling. The awesome power of the immunoreceptor tyrosine-based activation motif (ITAM). J Immunol , 3281–3285. [PubMed] [Google Scholar]
- Case DA, Ben-Shalom IY, Brozell SR, Cerutti DS, Cheatham TEI, Cruzeiro VWD, Darden TA, Duke RE, Ghoreishi D, Gilson MK, et al. (2017). AMBER 2017, University of California, San Francisco. [Google Scholar]
- Chan AC, Iwashima M, Turck CW, Weiss A. (1992). ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR ζ chain. Cell , 649–662. [DOI] [PubMed] [Google Scholar]
- Chen T, Repetto B, Chizzonite R, Pullar C, Burghardt C, Dharm E, Zhao Z, Carroll R, Nunes P, Basu M, et al. (1996). Interaction of phosphorylated FcεRIγ immunoglobulin receptor tyrosine activation motif-based peptides with dual and single SH2 domains of p72syk. assessment of binding parameters and real time binding kinetics. J Biol Chem , 25308–25315. [DOI] [PubMed] [Google Scholar]
- Cheng AM, Negishi I, Anderson SJ, Chan AC, Bolen J, Loh DY, Pawson T. (1997). The Syk and ZAP-70 SH2-containing tyrosine kinases are implicated in pre-T cell receptor signaling. Proc Natl Acad Sci USA , 9797–9801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DH, Morita CT, Weiss A. (1998). The Syk family of protein tyrosine kinases in T-cell activation and development. Immunol Rev , 167–180. [DOI] [PubMed] [Google Scholar]
- Cleyrat C, Girard R, Choi EH, Jeziorski É, Lavabre-Bertrand T, Hermouet S, Carillo S, Wilson BS. (2017). Gene editing rescue of a novel MPL mutant associated with congenital amegakaryocytic thrombocytopenia. Blood Adv , 1815–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darden T, York D, Pedersen L. (1993). Particle mesh Ewald: an N⋅log(N) method for Ewald sums in large systems. J Chem Phys , 10089–10092. [Google Scholar]
- Deindl S, Kadlecek TA, Brdicka T, Cao X, Weiss A, Kuriyan J. (2007). Structural basis for the inhibition of tyrosine kinase activity of ZAP-70. Cell , 735–746. [DOI] [PubMed] [Google Scholar]
- Faeder JR, Blinov ML, Goldstein B, Hlavacek WS. (2005). Combinatorial complexity and dynamical restriction of network flows in signal transduction. Syst Biol , 5–15. [DOI] [PubMed] [Google Scholar]
- Faeder JR, Hlavacek WS, Reischl I, Blinov ML, Metzger H, Redondo A, Wofsy C, Goldstein B. (2003). Investigation of early events in FcεRI-mediated signaling using a detailed mathematical model. J Immunol , 3769–3781. [DOI] [PubMed] [Google Scholar]
- Fasbender F, Claus M, Wingert S, Sandusky M, Watzl C. (2017). Differential requirements for Src-family kinases in SYK or ZAP70-mediated SLP-76 phosphorylation in lymphocytes. Front Immunol , 789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feenstra KA, Hess B, Berendsen HJC. (1999). Improving efficiency of large time-scale molecular dynamics simulations of hydrogen-rich systems. J Comput Chem , 786–798. [DOI] [PubMed] [Google Scholar]
- Feng C, Post CB. (2016). Insights into the allosteric regulation of Syk association with receptor ITAM, a multi-state equilibrium. Phys Chem Chem Phys , 5807–5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebiger E, Tortorella D, Jouvin M-H, Kinet J-P, Ploegh HL. (2005). Cotranslational endoplasmic reticulum assembly of FcεRI controls the formation of functional IgE-binding receptors. J Exp Med , 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fütterer K, Wong J, Grucza RA, Chan AC, Waksman G. (1998). Structural basis for Syk tyrosine kinase ubiquity in signal transduction pathways revealed by the crystal structure of its regulatory SH2 domains bound to a dually phosphorylated ITAM peptide. J Mol Biol , 523–537. [DOI] [PubMed] [Google Scholar]
- Garcia AE, Herce H, Paschek D. (2006). Simulations of temperature and pressure unfolding of peptides and proteins with replica exchange molecular dynamics. Annu Rep Comput Chem , 83–95. [Google Scholar]
- Garcia AE, Sanbonmatsu KY. (2001). Exploring the energy landscape of a beta hairpin in explicit solvent. Proteins , 345–354. [DOI] [PubMed] [Google Scholar]
- Geahlen RL. (2009). Syk and pTyr’d: signaling through the B cell antigen receptor. Biochim Biophys Acta , 1115–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geahlen RL, Burg DL. (1994). The role of Syk in cell signaling. Adv Exp Med Biol , 103–109. [DOI] [PubMed] [Google Scholar]
- Gelens L, Qian J, Bollen M, Saurin AT. (2018). The importance of kinase-phosphatase integration: lessons from mitosis. Trends Cell Biol , 6–21. [DOI] [PubMed] [Google Scholar]
- Getahun A, Beavers NA, Larson SR, Shlomchik MJ, Cambier JC. (2016). Continuous inhibitory signaling by both SHP-1 and SHIP-1 pathways is required to maintain unresponsiveness of anergic B cells. J Exp Med , 751–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus MS, Wester MJ, Lowman DW, Williams DL, Kruppa MD, Martinez CM, Young JM, Pappas HC, Lidke KA, Neumann AK. (2018). Mannan molecular substructures control nanoscale glucan exposure in Candida. Cell Rep , 2432–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris LJ, Skaletsky E, McPherson A. (1998). Crystallographic structure of an intact IgG1 monoclonal antibody. J Mol Biol , 861–872. [DOI] [PubMed] [Google Scholar]
- Harwood NE, Batista FD. (2008). New insights into the early molecular events underlying B cell activation. Immunity , 609–619. [DOI] [PubMed] [Google Scholar]
- Hatada MH, Lu X, Laird ER, Green J, Morgenstern JP, Lou M, Marr CS, Phillips TB, Ram MK, Theriault K, et al. (1995). Molecular basis for interaction of the protein tyrosine kinase ZAP-70 with the T-cell receptor. Nature , 32–38. [DOI] [PubMed] [Google Scholar]
- Hess B. (2008). P-LINCS: a parallel linear constraint solver for molecular simulation. J Chem Theory Comput , 116–122. [DOI] [PubMed] [Google Scholar]
- Hughes CE, Pollitt AY, Mori J, Eble JA, Tomlinson MG, Hartwig JH, Callaghan CA, Fütterer K, Watson SP. (2010). CLEC-2 activates Syk through dimerization. Blood , 2947–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. (1995). Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell , 225–236. [DOI] [PubMed] [Google Scholar]
- Iwashima M, Irving BA, van Oers NS, Chan AC, Weiss A. (1994). Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science , 1136–1139. [DOI] [PubMed] [Google Scholar]
- Ji QS, Chattopadhyay A, Vecchi M, Carpenter G. (1999). Physiological requirement for both SH2 domains for phospholipase C-γ1 function and interaction with platelet-derived growth factor receptors. Mol Cell Biol , 4961–4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K, Zhong B, Ritchey C, Gilvary DL, Hong-Geller E, Wei S, Djeu JY. (2003). Regulation of Akt-dependent cell survival by Syk and Rac. Blood , 236–244. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Pleiman CM, Pao L, Schneringer J, Hippen K, Cambier JC. (1995). Phosphorylated immunoreceptor signaling motifs (ITAMs) exhibit unique abilities to bind and activate Lyn and Syk tyrosine kinases. J Immunol , 4596–4603. [PubMed] [Google Scholar]
- Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. (1983). Comparison of simple potential functions for simulating liquid water. J Chem Phys , 926–935. [Google Scholar]
- Kawakami Y, Kitaura J, Hartman SE, Lowell CA, Siraganian RP, Kawakami T. (2000). Regulation of protein kinase CβI by two protein-tyrosine kinases, Btk and Syk. Proc Natl Acad Sci USA , 7423–7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshvara LM, Isaacson C, Harrison ML, Geahlen RL. (1997). Syk activation and dissociation from the B-cell antigen receptor is mediated by phosphorylation of tyrosine 130. J Biol Chem , 10377–10381. [DOI] [PubMed] [Google Scholar]
- Keshvara LM, Isaacson CC, Yankee TM, Sarac R, Harrison ML, Geahlen RL. (1998). Syk- and Lyn-dependent phosphorylation of Syk on multiple tyrosines following B cell activation includes a site that negatively regulates signaling. J Immunol , 5276–5283. [PubMed] [Google Scholar]
- Kihara H, Siraganian RP. (1994). Src homology 2 domains of Syk and Lyn bind to tyrosine-phosphorylated subunits of the high affinity IgE receptor. J Biol Chem , 22427–22432. [PubMed] [Google Scholar]
- Klauda JB, Venable RM, Freites JA, O’Connor JW, Tobias DJ, Mondragon-Ramirez C, Vorobyov I, MacKerell AD, Jr, Pastor RW. (2010). Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J Phys Chem B , 7830–7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong GH, Bu JY, Kurosaki T, Shaw AS, Chan AC. (1995). Reconstitution of Syk function by the ZAP-70 protein tyrosine kinase. Immunity , 485–492. [DOI] [PubMed] [Google Scholar]
- Kuriyan J, Cowburn D. (1993). Structures of SH2 and SH3 domains. Curr Opin Struct Biol , 828–837. [Google Scholar]
- Ladbury JE, Lemmon MA, Zhou M, Green J, Botfield MC, Schlessinger J. (1995). Measurement of the binding of tyrosyl phosphopeptides to SH2 domains: a reappraisal. Proc Natl Acad Sci USA , 3199–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur F, Klausner RD. (1991). T-cell and basophil activation through the cytoplasmic tail of T-cell-receptor zeta family proteins. Proc Natl Acad Sci USA , 8905–8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu FT, Bohn JW, Ferry EL, Yamamoto H, Molinaro CA, Sherman LA, Klinman NR, Katz DH. (1980). Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J Immunol , 2728–2737. [PubMed] [Google Scholar]
- Lopez CA, Sethi A, Goldstein B, Wilson BS, Gnanakaran S. (2015). Membrane-mediated regulation of the intrinsically disordered CD3 cytoplasmic tail of the TCR. Biophys J , 2481–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGlashan D., Jr (2008). IgE receptor and signal transduction in mast cells and basophils. Curr Opin Immunol , 717–723. [DOI] [PubMed] [Google Scholar]
- Mahajan A, Barua D, Cutler P, Lidke DS, Espinoza FA, Pehlke C, Grattan R, Kawakami Y, Tung C-S, Bradbury ARM, et al. (2014). Optimal aggregation of FcεRI with a structurally defined trivalent ligand overrides negative regulation driven by phosphatases. ACS Chem Biol , 1508–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger H, Alcaraz G, Hohman R, Kinet JP, Pribluda V, Quarto R. (1986). The receptor with high affinity for immunoglobulin E. Annu Rev Immunol , 419–470. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Kollman PA. (1992). Settle: an analytical version of the SHAKE and RATTLE algorithm for rigid water models. J Comput Chem , 952–962. [Google Scholar]
- Mocsai A, Ruland J, Tybulewicz VL. (2010). The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol , 387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfetta R, Peruzzi G, Santoni A, Paolini R. (2007). Negative signals from FcεRI engagement attenuate mast cell functions. Arch Immunol Ther Exp , 219–229. [DOI] [PubMed] [Google Scholar]
- Mukai K, Tsai M, Saito H, Galli SJ. (2018). Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev , 121–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. (2008). Fcγ receptors as regulators of immune responses. Nat Rev Immunol , 34–47. [DOI] [PubMed] [Google Scholar]
- Ohashi T, Galiacy SD, Briscoe G, Erickson HP. (2007). An experimental study of GFP-based FRET, with application to intrinsically unstructured proteins. Protein Sci , 1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill SK, Getahun A, Gauld SB, Merrell KT, Tamir I, Smith MJ, Dal Porto JM, Li QZ, Cambier JC. (2011). Monophosphorylation of CD79a and CD79b ITAM motifs initiates a SHIP-1 phosphatase-mediated inhibitory signaling cascade required for B cell anergy. Immunity , 746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottinger EA, Botfield MC, Shoelson SE. (1998). Tandem SH2 domains confer high specificity in tyrosine kinase signaling. J Biol Chem , 729–735. [DOI] [PubMed] [Google Scholar]
- Panayotou G, Gish G, End P, Truong O, Gout I, Dhand R, Fry MJ, Hiles I, Pawson T, Waterfield MD. (1993). Interactions between SH2 domains and tyrosine-phosphorylated platelet-derived growth factor β-receptor sequences: analysis of kinetic parameters by a novel biosensor-based approach. Mol Cell Biol , 3567–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrinello M, Rahman A. (1981). Polymorphic transitions in single crystals: a new molecular dynamics method. J Appl Phys , 7182–7190. [Google Scholar]
- Pastor RW, Brooks BR, Szabo A. (1988). An analysis of the accuracy of Langevin and molecular dynamics algorithms. Mol Phys , 1409–1419. [Google Scholar]
- Pawson T. (1995). Protein modules and signalling networks. Nature , 573–580. [DOI] [PubMed] [Google Scholar]
- Pawson T, Scott JD. (2005). Protein phosphorylation in signaling—50 years and counting. Trends Biochem Sci , 286–290. [DOI] [PubMed] [Google Scholar]
- Pei D, Wang J, Walsh CT. (1996). Differential functions of the two Src homology 2 domains in protein tyrosine phosphatase SH-PTP1. Proc Natl Acad Sci USA , 1141–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluskey S, Wandless TJ, Walsh CT, Shoelson SE. (1995). Potent stimulation of SH-PTP2 phosphatase activity by simultaneous occupancy of both SH2 domains. J Biol Chem , 2897–2900. [DOI] [PubMed] [Google Scholar]
- Ra C, Jouvin MH, Kinet JP. (1989). Complete structure of the mouse mast cell receptor for IgE (FcεRI) and surface expression of chimeric receptors (rat–mouse–human) on transfected cells. J Biol Chem , 15323–15327. [PubMed] [Google Scholar]
- Rawat N, Biswas P. (2009). Size, shape, and flexibility of proteins and DNA. J Chem Phys , 165104. [DOI] [PubMed] [Google Scholar]
- Reth M. (1989). Antigen receptor tail clue. Nature , 383–384. [PubMed] [Google Scholar]
- Reth M. (2001). Oligomeric antigen receptors: a new view on signaling for the selection of lymphocytes. Trends Immunol , 356–360. [DOI] [PubMed] [Google Scholar]
- Richardson CD, Ray GJ, DeWitt MA, Curie GL, Corn JE. (2016). Enhancing homology-directed genome editing by catalytically active and inactive CRISPR-Cas9 using asymmetric donor DNA. Nat Biotechnol , 339–344. [DOI] [PubMed] [Google Scholar]
- Rivera J, Fierro NA, Olivera A, Suzuki R. (2008). New insights on mast cell activation via the high affinity receptor for IgE. Adv Immunol , 85–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Hua DP, Post CB. (2016). Analysis of multidomain protein dynamics. J Chem Theory Comput , 274–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryckaert J-P, Ciccotti G, Berendsen HJC. (1977). Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J Comput Phys , 327–341. [Google Scholar]
- Salomon-Ferrer R, Case DA, Walker RC. (2012). An overview of the Amber biomolecular simulation package. WIREs Comput Mol Sci , 198–210. [Google Scholar]
- Schwartz SL, Cleyrat C, Olah MJ, Relich PK, Phillips GK, Hlavacek WS, Lidke KA, Wilson BS, Lidke DS. (2017). Differential mast cell outcomes are sensitive to FcεRI–Syk binding kinetics. Mol Biol Cell , 3397–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SL, Yan Q, Telmer CA, Lidke KA, Bruchez MP, Lidke DS. (2015). Fluorogen-activating proteins provide tunable labeling densities for tracking FcεRI independent of IgE. ACS Chem Biol , 539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi A, Goldstein B, Gnanakaran S. (2011). Quantifying intramolecular binding in multivalent interactions: a structure-based synergistic study on Grb2–Sos1 complex. PLoS Comput Biol , e1002192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiue L, Zoller MJ, Brugge JS. (1995). Syk is activated by phosphotyrosine-containing peptides representing the tyrosine-based activation motifs of the high affinity receptor for IgE. J Biol Chem , 10498–10502. [DOI] [PubMed] [Google Scholar]
- Sigalov A. (2005). Multi-chain immune recognition receptors: spatial organization and signal transduction. Semin Immunol , 51–64. [DOI] [PubMed] [Google Scholar]
- Siraganian RP. (2003). Mast cell signal transduction from the high-affinity IgE receptor. Curr Opin Immunol , 639–646. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Surviladze Z, Gaudet EA, Backer JM, Mitchell CA, Wilson BS. (2001). p110β and p110δ phosphatidylinositol 3-kinases up-regulate FcεRI-activated Ca2+ influx by enhancing inositol 1,4,5-trisphosphate production. J Biol Chem , 17213–17220. [DOI] [PubMed] [Google Scholar]
- Smith-Garvin JE, Koretzky GA, Jordan MS. (2009). T cell activation. Annu Rev Immunol , 591–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z, Shoelson SE, Chaudhuri M, Gish G, Pawson T, Haser WG, King F, Roberts T, Ratnofsky S, Lechleider RJ, et al. (1993). SH2 domains recognize specific phosphopeptide sequences. Cell , 767–778. [DOI] [PubMed] [Google Scholar]
- Sugita Y, Okamoto Y. (1999). Replica-exchange molecular dynamics method for protein folding. Chem Phys Lett , 141–151. [Google Scholar]
- Tamir I, Cambier JC. (1998). Antigen receptor signaling: integration of protein tyrosine kinase functions. Oncogene , 1353–1364. [DOI] [PubMed] [Google Scholar]
- Turner H, Kinet JP. (1999). Signalling through the high-affinity IgE receptor FcεRI. Nature , 24–30. [DOI] [PubMed] [Google Scholar]
- Van den Herik-Oudijk IE, Capel PJ, van der Bruggen T, Van de Winkel JG. (1995). Identification of signaling motifs within human Fc gamma RIIa and Fc gamma RIIb isoforms. Blood , 2202–2211. [PubMed] [Google Scholar]
- Van Valen D, Haataja M, Phillips R. (2009). Biochemistry on a leash: the roles of tether length and geometry in signal integration proteins. Biophys J , 1275–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, Kurosaki T, Huang XY. (1996). Tyrosine kinases in activation of the MAP kinase cascade by G-protein-coupled receptors. Nature , 541–544. [DOI] [PubMed] [Google Scholar]
- Wang H, Kadlecek TA, Au-Yeung BB, Goodfellow HE, Hsu LY, Freedman TS, Weiss A. (2010). ZAP-70: an essential kinase in T-cell signaling. Cold Spring Harb Perspect Biol , a002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wester MJ, Lin J, Neumann AK. (2017). A computational model for regulation of nanoscale glucan exposure in Candida albicans. PLoS One , e0188599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BS, Kapp N, Lee RJ, Pfeiffer JR, Martinez AM, Platt Y, Letourneur F, Oliver JM. (1995). Distinct functions of the FcεR1 γ and β subunits in the control of FcεR1-mediated tyrosine kinase activation and signaling responses in RBL-2H3 mast cells. J Biol Chem , 4013–4022. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Pfeiffer JR, Oliver JM. (2000). Observing FcεRI signaling from the inside of the mast cell membrane. J Cell Biol , 1131–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Suzuki R, Backlund PS, Yamashita Y, Yergey AL, Rivera J. (2008). Differential dephosphorylation of the FcRγ immunoreceptor tyrosine-based activation motif tyrosines with dissimilar potential for activating Syk. J Biol Chem , 28584–28594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Barros T, Visperas PR, Deindl S, Kadlecek TA, Weiss A, Kuriyan J. (2013). Structural basis for activation of ZAP-70 by phosphorylation of the SH2-kinase linker. Mol Cell Biol , 2188–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Oh H, Burton RA, Burgner JW, Geahlen RL, Post CB. (2008). Tyr130 phosphorylation triggers Syk release from antigen receptor by long-distance conformational uncoupling. Proc Natl Acad Sci USA , 11760–11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H-X. (2001). Loops in proteins can be modeled as worm-like chains. J Phys Chem B , 6763–6766. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.