Abstract
Objectives
Endoscopic sinus surgery (ESS) is performed for patients with chronic rhinosinusitis (CRS) that have failed maximal medical therapy. This study seeks to determine the prevalence of revision surgery and factors predicting the need for revision after ESS using a large statewide surgery database.
Study Design
Large retrospective cohort study using the State Ambulatory Surgery Database for the state of CA between 2005–2011.
Methods
We identified over 61,000 patients with CRS who underwent ESS, determined by CPT code. We identified which patients underwent a repeat surgery, and performed multivariable modeling to determine which factors (nasal polyps, age, gender, insurance, hospital setting, ethnicity) predicted the need for revision. Adjusted odds ratios (AOR) and 95% confidence intervals are presented.
Results
Of 61,339 patients who underwent ESS, 4,078 (6.65%) returned for revision ESS during the time period investigated. In a multivariable logistic regression model, positive predictors of revision were a diagnosis of nasal polyps (AOR 1.20, 95% CI 1.11–1.29, p<0.001) and female gender (AOR 1.20, 95% CI 1.11–1.29, p<0.001); public insurance was marginally predictive of increased re-operation (AOR 1.10, 95% CI 1.00–1.21, p=0.048). Patients of Hispanic ethnicity were less likely to have revision surgery (AOR 0.86, 95% CI 0.77–0.97, p=0.011). Age, income, and hospital setting were not significant predictors.
Conclusions
A minority of patients with CRS who undergo ESS will have a revision surgery. This likelihood is increased in female patients and those with nasal polyps, and decreased in patients of Hispanic ethnicity, even when controlling for income, insurance, and hospital setting.
Keywords: endoscopic sinus surgery, chronic rhinosinusitis, nasal polyps, revision
Introduction
Chronic rhinosinusitis (CRS) affects an estimated 4.5–12% of the North American and European population [1] and negatively impacts quality of life in both disease-specific and general health-related domains [2]. Endoscopic sinus surgery (ESS) is an effective intervention for patients with medically recalcitrant CRS with over 250,000 ambulatory surgeries performed in the U.S. each year [3]. Although surgery has been shown to improve quality of life [4], symptom persistence and recurrence can occur necessitating revision surgery [5]. Although short-term failure rates have been well characterized with patient reported outcome measures, little is known about revision sinus surgery rates on both short and long timelines.
Limited, small sample size data on the overall revision rates following ESS provide some insight into this potential outcome. One Canadian study performed a survival analysis in 549 patients with CRS with nasal polyps (CRSwNP) and found that surgery-free survival ranged from 63–90% at 5 years and 11–83% at 10 years, and patients with asthma and aspirin sensitivity had higher recurrence rates [6]. A retrospective analysis of 490 patients with CRSwNP who underwent revision surgery over a 25 year period by a single surgeon found that the mean time to revision surgery was 4.87 ± 3.61 years, and that time to revision was shorter in patients who smoke and longer in patients who underwent middle turbinate resection [5]. Factors that did not reduce time to revision included gender, prior surgery, asthma, nasal polyps, CT stage, allergic mucin, or eosinophilia. Data from the UK Chronic Rhinosinusitis Epidemiological Study gives perhaps the most comprehensive view of revision surgery, demonstrating that among 1459 patients who underwent any surgery for CRS, there was an overall revision rate of 19.1% at 5 years, and that this rate was higher in patients with nasal polyps and allergic fungal rhinosinusitis than those with CRS alone [7]. However, the generalizability of these results to the U.S. healthcare environment may not be valid.
The goal of this study is to clarify the rate of revision surgery after ESS. Specifically, to determine what percentage of patients who undergo ESS require at least one revision surgery, and how much time elapses between the original and revision surgery. To date there has been no large database analysis in the U.S. that has looked precisely at the overall revision rate following ESS for CRS with or without NP, and compared revision rates and time to revision among patients with different comorbidities. We hypothesize that patients with CRSwNP will have increased revision rates and shorter time to revision.
Materials and Methods
We utilized data from the Healthcare Cost and Utilization Project (HCUP), which is a family of health care databases and related software tools and products developed through a Federal-State-Industry partnerships and sponsored by the Agency for Healthcare Research and Quality (AHRQ) [8]. HCUP databases contain data from federal, state, hospital, and private organizations, and includes the largest collection of longitudinal hospital care data in the United States. Within HCUP is the State Ambulatory Surgery and Services Database (SASD), which includes encounter-level data for ambulatory surgery and other outpatient services from hospital-owned facilities. The SASD contains more than 100 clinical and nonclinical variables such as all-listed diagnoses and procedures (coded by ICD-9 and CPT), patient demographic characteristics (e.g., sex, age, race), expected payment source, and hospital characteristics (e.g., urban v. rural). The SASD is commonly used for research on a variety of topics including analyses of ambulatory surgeries and trends.
Inclusion & Exclusion Criteria
We acquired data from SASD for California for all the years for which the data were available: 2005–2011. We searched the data set for patients who had endoscopic sinus surgery, as determined by CPT code (31254, 31255, 31256, 31267, 31276, 31287, and 31288). Patients who were less than 18 years old at the time of surgery and any patients who did not have an ICD-9 coded diagnosis for chronic rhinosinusitis were excluded. Patients with a diagnosis of allergic fungal rhinosinusitis and cystic fibrosis were excluded.
Data collection
Each patient had been previously assigned a unique de-identified “person-number” which allowed us to determine the number of surgeries the patient underwent in the defined time period. If the patient had more than one surgery they were classified as a “revision” patient. Data from the first surgery they underwent was used for analysis. From the data set, we also extracted demographic data (age, gender, ethnicity), insurance status (public v. private), income quartile, and hospital setting (urban v. rural).
Statistical analysis
Statistical analysis was performed using the program R version 2.13.1 (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org; R Development Core Team, 2011). Univariate analysis was performed comparing variables between patients who underwent revision surgery and those who did not. Odds ratios and 95% confidence intervals were calculated for all categorical variables, and students t-test was performed to compare age. A multivariate logistic regression was performed with the need for revision surgery as the binomial outcome variable. Predictors included the presence of nasal polyps, age, gender, income quartile (as a factor), insurance status, hospital setting, and ethnicity (as a factor). The time until revision surgery was analyzed by the Kaplan-Meier method and compared with the log-rank test. Cox proportional hazards model for disease-free survival (revision-free survival) was performed with the same predicting variables used in the logistic regression model. Statistical significance was set at a p-value of <0.05.
Results
61,339 patients were identified who carried a diagnosis of CRS and underwent at least one ESS between 2005–2011. Of these patients, 4,078 patients were identified who had at least one revision ESS during the time period analyzed, yielding a revision rate of 6.65%. The mean time until revision surgery was 20.93 ± 17.29 months. 1,753 (43%) of all revision surgeries were performed within one year of the initial surgery. Demographic data are presented in Table 1. A concurrent diagnosis of nasal polyposis was present in 31.13% of all patients, and this proportion was statistically greater in those who underwent a revision surgery (34.09% v. 30.92%, P < 0.001). In the revision group there were also more women (50.29% v. 45.86%, P < 0.001), and fewer patients of Hispanic ethnicity (9.12% v. 10.33%, P = 0.014). An urban hospital setting was marginally more common in patients who underwent revision surgery (92.15% v. 91.20%, P = 0.037).
Table 1.
Baseline characteristics. Patient data split by those who underwent a revision surgery vs. those who did not. Data presented as the number of patients, and percentage of total (i.e. 30.92% of patients who did not have revision surgery had nasal polyps). T-test was performed for age. All other analyses are logistic regression yielding odds ratios with 95% confidence intervals.
| Parameter | Total (N=61,339) | No revision (N=57,261) | Revision (N=4,078) | OR (95% CI) | P-value |
|---|---|---|---|---|---|
| Nasal Polyps - no. (%) | 19093 (31.1) | 17703 (30.9) | 1390 (34.1) | 1.16 (1.08–1.24) | <0.001 |
| Age - yr (mean ± SD) | 49.21 ± 14.77 | 49.20 ± 14.81 | 49.35 ± 14.20 | 0.512 | |
| Female sex - no. (%) | 28310 (46.2) | 26259 (45.9) | 2051 (50.3) | 1.19 (1.12–1.27) | <0.001 |
| Ethnicity - no. (%) | |||||
| White | 38863 (63.4) | 36260 (63.3) | 2603 (63.8) | 1.02 (0.96–1.09) | 0.517 |
| Black | 1310 (2.1) | 1213 (2.1) | 97 (2.4) | 1.13 (0.91=1.39) | 0.267 |
| Hispanic | 6289 (10.3) | 5917 (10.3) | 372 (9.1) | 0.87 (0.78–0.97) | 0.014 |
| Asian | 3002 (4.9) | 2820 (4.9) | 182 (4.5) | 0.90 (0.77–1.05) | 0.187 |
| Native | 26 (.04) | 26 (.05) | 0 | NA | 0.174 |
| Other | 11849 (19.3) | 11025 (19.3) | 824 (20.2) | 1.06 (0.98–1.15) | 0.137 |
| Insurance - no. (%) | |||||
| Public | 12696 (20.7) | 11841 (20.7) | 855 (21.0) | 1.02 (0.94–1.10) | 0.662 |
| Private | 46609 (76.0) | 43545 (76.0) | 3064 (75.1) | 0.95 (0.88–1.02) | 0.188 |
| Income - no. (%) | |||||
| First quartile | 9431 (15.4) | 8828 (15.4) | 603 (14.8) | 0.95 (0.87–1.04) | 0.281 |
| Second quartile | 13665 (22.3) | 12797 (22.4) | 868 (21.3) | 0.94 (0.87–1.02) | 0.115 |
| Third quartile | 16235 (26.5) | 15115 (26.4) | 1120 (27.5) | 1.06 (0.98–1.13) | 0.135 |
| Fourth quartile | 20791 (33.9) | 19385 (33.9) | 1406 (34.5) | 1.03 (0.96–1.10) | 0.416 |
| Urban setting | 55979 (91.3) | 52221 (91.2) | 3758 (92.1) | 1.13 (1.01–1.28) | 0.037 |
Significant differences (P<0.05) in bold type
In the subgroup of patients who had revision surgery performed within 1 year of the initial surgery (2.86% of all patients) compared to those who had revision greater than 1 year after initial surgery, there was a lower prevalence of nasal polyposis (27.32% v. 39.18%, P < 0.001), but no difference in female gender (51.3% v. 49.51%, P=0.1836), Hispanic ethnicity (9.01% v. 9.20%, P=0.8336), or urban hospital setting (92.76% v. 91.70%, P=0.2104).
In a multivariable logistic regression model (Table 2), positive predictors of revision were a diagnosis of nasal polyps (AOR 1.20, 95% CI 1.11–1.29, P <0.001) and female gender (AOR 1.20, 95% CI 1.11–1.29, P <0.001). Public insurance was marginally predictive of increased re-operation (AOR 1.10, 95% CI 1.00–1.21, P = 0.048). Patients of Hispanic ethnicity were less likely to have revision surgery (AOR 0.86, 95% CI 0.77–0.97, P=0.011). An urban hospital setting, which was significant in the univariate analysis, was not a significant predictor of revision in the multivariable analysis. Age and income were also not significant predictors.
Table 2.
Multivariable logistic regression showing predictors of revision surgery
| Predictor | B (standard error) | OR (95% CI) | P-value |
|---|---|---|---|
| Nasal Polyps | 0.18 (0.04) | 1.2 (1.11–1.29) | <0.001 |
| Age - yr | 0.00004 (0.001) | 1.0 (0.99–1.00) | 0.977 |
| Female sex | 0.18 (0.04) | 1.20 (1.11–1.29) | <0.001 |
| Income | |||
| 1st quartile | Reference | Reference | |
| 2nd quartile income | −0.05 (0.06) | 0.95 (0.84–1.07) | 0.390 |
| 3rd quartile income | 0.05 (0.06) | 1.05 (0.94–1.18) | 0.369 |
| 4th quartile income | 0.03 (0.06) | 1.03 (0.92–1.15) | 0.641 |
| Public insurance | 0.09 (0.05) | 1.10 (1.00–1.21) | 0.048 |
| Urban setting | 0.11 (0.08) | 1.12 (0.96–1.30) | 0.136 |
| Race | |||
| white | Reference | Reference | |
| black | 0.06 (0.11) | 1.07 (0.86–1.32) | 0.558 |
| hispanic | −0.15 (0.06) | 0.86 (0.77–0.97) | 0.011 |
| asian/pacific | −0.15 (0.08) | 0.86 (0.74–1.01) | 0.070 |
| native | −0.11 (0.01) | NA | 0.917 |
| other ethnicity | −0.14 (0.12) | 0.87 (0.70–1.09) | −0.236 |
Significant differences (P < 0.05) in bold type
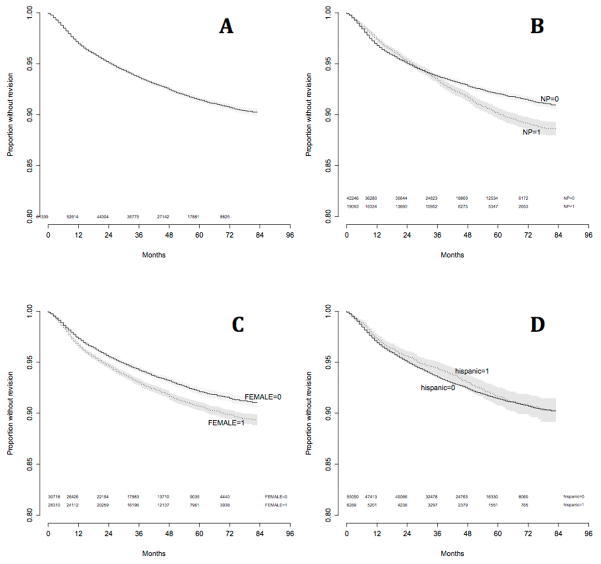
Kaplan-Meier survival curves were created for overall survival, and survival stratified by the presence of nasal polyps, female gender, and Hispanic ethnicity (Figure 1A,B,C,D respectively). Overall actuarial survival rate at 5 years was 91.4%. For patients without nasal polyps 5 year survival was 92.1%, versus 90.1% for those with polyps (logrank P < 0.001). Overall actuarial survival rate at 5 years for males was 93.0% versus 90.7% for females (logrank P < 0.001). For all non-Hispanic patients the 5 year survival was 91.4% versus 91.6% for Hispanics (logrank P = 0.245).
Figure 1.
Kaplan-Meier survival curves for A) entire cohort, and stratified by B) nasal polyposis, C) female gender, D) Hispanic ethnicity
A cox-proportional hazards model (Table 3) was performed to determine predictive variables. Variables included were the same for the multivariable described above model except age was excluded as it had a varying hazard ratio over time. The presence of nasal polyps was predictive of revision surgery (hazards ratio 1.19, 95% CI 1.11–1.27, P<0.001), as was female gender (hazards ratio 1.24, 95% CI 1.16–1.32, P<0.001), and public insurance (hazards ratio1.10, 95% CI 1.03–1.19, P=0.008). Hispanic ethnicity was not predictive, and no other covariates were significantly predictive.
Table 3.
Cox proportional hazards model showing which variables effected survival/need for revision surgery.
| Predictor | B (standard error) | HR (95% CI) | P-value |
|---|---|---|---|
| Nasal Polyps | 0.17 (0.03) | 1.19 (1.11–1.27) | <0.001 |
| Female sex | 0.22 (0.03) | 1.24 (1.16–1.32) | <0.001 |
| Income | |||
| 1st quartile | −0.18 (0.13) | 0.83 (0.64–1.08) | 0.173 |
| 2nd quartile | −0.18 (0.13) | 0.84 (0.64–1.09) | 0.179 |
| 3rd quartile | −0.13 (0.14) | 0.89 (0.69–1.17) | 0.406 |
| 4th quartile | −0.14 (0.14) | 0.87 (0.66–1.13 | 0.289 |
| Public insurance | 0.09 (0.04) | 1.1 (1.03–1.19) | 0.008 |
| Urban setting | 0.13 (0.07) | 1.14 (0.99–1.31) | 0.063 |
| Race | |||
| white | 0.02 (0.04) | 1.02 (0.94–1.11) | 0.649 |
| black | 0.14 (0.11) | 1.15 (0.92–1.42) | 0.218 |
| hispanic | −0.06 (0.07) | 0.94(0.83–1.07) | 0.366 |
| asian/pacific | −0.06 (0.08) | 0.94 (0.80–1.12) | 0.505 |
| native | NA | NA | 0.967 |
| other ethnicity | −0.02 (0.12) | 0.98 (0.7801.22) | 0.831 |
Significant differences (P < 0.05) in bold type
Women accounted for 48.0% of the total cohort but accounted for only 38.4% of patients with CRSwNP versus 52.4% of those with CRSsNP(P < 0.001). Among patients with CRSwNP, women accounted for 44.9% of patients who had a revision surgery versus 37.9% of those who did not require revision (P <0.001). Among patients with CRSsNP, women accounted for 56.4% of patients who had a revision surgery versus 52.1% of those who did not require revision (P <0.001).
Discussion
The present study investigated the rate of revision surgery after endoscopic sinus surgery for CRS in the largest data set published to date. This data reveals an overall revision rate of 6.65% and builds on smaller cohort data [6,7,9,10,11] reinforcing the association of revision surgery with CRSwNP. Furthermore, the present analysis identified female gender and Hispanic ethnicity associating with increased and decreased rates of revision, respectively. Kaplan-Meier survival curve analysis, which takes into account the varying length of follow-up for each patient, shows revision rates at 5 years are 7.9% for CRSsNP and 9.9% for CRSwNP.
Our data supports prior findings that the presence of nasal polyps increases the rate of revision surgery [7,12–16]. In addition to causing sinonasal obstruction, the presence of polyps inhibits normal mucociliary clearance [17]. It is still unclear whether worse outcomes and higher rates of revision in the presence of polyps are due to the more chronic and severe spectrum of the disease, or if there are other variables within the surgeon’s control that may allow for improved outcomes. Of note, patients who had a revision surgery within 1 year of the initial surgery actually tended to have lower rates of nasal polyposis than those who had revision more than 1 year out. This suggests that these early revisions may be due largely to what could be considered a technical or surgical failure as oppose to later revisions, which might be more likely to be related to progression of the disease. A recent cohort of patients with nasal polyposis has shown a 40% polyp recurrence rate at 18 months despite appropriate medical therapy [18]. It is possible that either patients with or without CRSwNP would benefit from more extensive initial surgery including nasalization of the ethmoids [19], frontal sinusotomy [20] or middle turbinate resection [5] to improve ventilation and access of ongoing topical medical therapy.
Furthermore, it is possible that medication regimens may need to be further optimized, and unfortunately the present study is limited by a lack of data frequency of postoperative exacerbations and need for systemic steroids.
Interestingly, this data demonstrates that women made up only 46% of the surgical population, but were more likely to undergo repeat surgery. This held true in univariate, multivariate, and cox proportional hazards modeling. A retrospective cohort analysis of 1393 patients with CRS showed that women accounted for only 38% of patients with CRSwNP. However, these women with CRSwNP had more severe disease and were more likely to require revision surgeries [21]. These authors suggested that estrogen may play a role by activating eosinophils and allowing autoreactive antibody-producing B cells to escape tolerance. In our cohort, women accounted for a similar minority of 38.4% of patients with CRSwNP, and women in the CRSwNP cohort were more likely to have revision surgery. However, we found that female gender was a risk factor for revision surgery in the CRSsNP cohort as well, suggesting there may be additional factors beyond disease severity that is driving increased revision rates in women.
It has been shown that high levels of anxiety and depression are common in patients with CRS, and that psychiatric comorbidity is associated with increased symptoms in CRS [22]. Increased rates of psychiatric comorbidities such as depression in women [23] may be one factor influencing the higher rates of revision surgery. Previous research has supported the notion that women have a higher prevalence of musculoskeletal pain, likely due to a combination of biological and psychosocial factors [24], and are more likely to report pain [25]. It is therefore possible that women are more likely to experience and report sinus symptoms and therefore require reoperation when compared to their male counterparts. Historically, there have also been gender disparities in patients being offered orthopedic and vascular surgery [26,27] which may exist in sinus surgery and lead to women not being offered intervention until their disease has progressed and is less likely to respond optimally to intervention.
The present study also found patients of Hispanic ethnicity were less likely to undergo repeat surgery. Data from the 2010 Census [28] indicates that 37.6% of the CA population is Hispanic, though this group represented only 10% of our study population. Non-Hispanic whites were disproportionately represented among those undergoing surgery, making up 40.3% of the CA population, but 63.4% of our patient population. At least some of this discrepancy may be due to a lower prevalence of CRS in Hispanics. A study which analyzed data from the National Health Interview Survey of 27,731 U.S. residents, which included 610 Hispanics, showed a sinusitis prevalence of 8.8% in Hispanics compared to 13% in Caucasians [29]. An additional explanation for the discrepancy in both initial surgery and likelihood of revision in Hispanics may be access to healthcare. Indeed, in the study by Soler et. al [29], Hispanics were more likely to be uninsured, delay medical care due to cost-related concerns, and were less likely to have seen a medical specialist or undergone a surgical procedure in the previous 12 months. Our multivariate model controlled for known variables associated with diminished access to healthcare including income, insurance status, and urban setting, and yet the diminished revision rates of Hispanics remained significant. It is still quite plausible however that reduced access to care resulted in lower revision rates in Hispanics, as our data only included insurance status etc. at a single time point and these variables can easily change over time.
It should be noted that the revision rates presented in this study are overall are on the lower end of reported revision rates [5–7,9–11,30–32] and may be in part a reflection of the limitations of the database as there was no guaranteed follow-up for specific patients, and we therefore may not be capturing a portion of revision patients. Alternatively, the lower revision rate may be due to an overall lower disease burden in our population, which unlike many of the prior cohort studies, included patients from both university tertiary practices as well as community practices. Our period of observation (mean 42 months) may also not be long enough to capture patients that will ultimately undergo repeat surgery. However, we did find that 43% of patients who get revision will have it done within the first year. Furthermore, this data did not capture the number of patients that had already failed prior surgery, which is a known risk for factor for failing a second surgery [30,33]. Further limitations of the present study are inherent in administrative data in a lack of granularity of likely clinically important factors such as patient reported and physician-reported measures of disease severity as well as concurrent therapies and variable coding practices among surgeons. The findings of the present study warrant further investigation in a prospective manner to clarify why patients of female gender and Hispanic ethnicity experience variable revision rates after ESS.
Conclusion
Over 61,000 patients were identified who underwent outpatient ESS in CA between 2005–2011. In this large data set we found that overall revision-free survival at 5 years post-ESS was 91.4%. Factors that increased the rate of revision surgery included the presence of nasal polyps and female gender. Patients of Hispanic ethnicity were less likely to undergo revision surgery. These findings illustrate the need for further investigation into proper patient selection and operative intervention to minimize need for reoperation.
Acknowledgments
Funding for this project was received from National Institutes of Health T32 Resident Training Grant 5T32DC000028-25.
Footnotes
Potential Conflicts of Interest: None
Financial Discloures: Adam S. DeConde is a consultant for IntersectENT, (Menlo Park, CA) and Stryker Endoscopy (San Jose, CA).
The results of this study were presented at the Triological Society Annual Meeting at COSM in San Diego on Saturday, April 29, 2017.
Level of Evidence: Level 4
References
- 1.DeConde AS, Soler ZM. Chronic rhinosinusitis: Epidemiology and burden of disease. Am J Rhinol Allergy. 2016;30(2):134–9. doi: 10.2500/ajra.2016.30.4297. [DOI] [PubMed] [Google Scholar]
- 2.Van Oene CM, van Reij EJ, Sprangers MA. Quality-assessment of disease-specific quality of life questionnaires for rhinitis and rhinosinusitis: a systematic review. Allergy. 2007;62(12):1359–71. doi: 10.1111/j.1398-9995.2007.01482.x. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharyya N. Ambulatory sinus and nasal surgery in the United States: demographics and perioperative outcomes. Laryngoscope. 2010;120:635–638. doi: 10.1002/lary.20777. [DOI] [PubMed] [Google Scholar]
- 4.Gliklich RE, Metson R. Effects of sinus surgery on quality of life. Otolaryngol Head Neck Surg. 1997;117:12–17. doi: 10.1016/S0194-59989770199-2. [DOI] [PubMed] [Google Scholar]
- 5.Wu AW, Ting JY, Platt MP, Tierney HT, Metson R. Factors affecting time to revision sinus surgery for nasal polyps: a 25-year experience. Laryngoscope. 2014;124:29–33. doi: 10.1002/lary.24213. [DOI] [PubMed] [Google Scholar]
- 6.Mendelsohn D, Jeremic G, Wright ED, Rotenberg BW. Revision rates after endoscopic sinus surgery: a recurrence analysis. Annals Otol Rhinol Laryngol. 2011;120:162–166. doi: 10.1177/000348941112000304. [DOI] [PubMed] [Google Scholar]
- 7.Philpott C, Hopkins C, Erskine S, Kumar N, Robertson A, Farboud A. The burden of revision sinonasal surgery in the UK-data from the Chronic Rhinosinusitis Epidemiology Study (CRES): a cross-sectional study. BMJ Open. 2015;5(4):1–8. doi: 10.1136/bmjopen-2014-006680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality. Overview of the State Ambulatory Surgery Database. Aug 15, 2011. 8-15-2012. [Google Scholar]
- 9.Ramadan HH. Surgical causes of failure in endoscopic sinus surgery. Laryngoscope. 1999;109:27–9. doi: 10.1097/00005537-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Wynn R, Har-EI G. Recurrence rates after endoscopic sinus surgery for massive sinus polyposis. Laryngoscope. 2004;114:811–3. doi: 10.1097/00005537-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Albu S, Tomescu E, Mexca Z, et al. Recurrence rates in endonasal surgery for polyposis. Acta Otorhinolaryngol Belg. 2004;58:79–86. [PubMed] [Google Scholar]
- 12.Naseri I, DelGaudio JM. Predictors of failure in primary surgery. In: Kountakis SE, Jacobs JB, Gosepath J, editors. Revision Sinus Surgery. Berlin: Springer; 2008. pp. 19–21. [Google Scholar]
- 13.Jiang RS, Hsu CY. Revision functional endoscopic sinus surgery. Ann Otol Rhinol Laryngol. 2010;111:155–159. doi: 10.1177/000348940211100208. [DOI] [PubMed] [Google Scholar]
- 14.King JM, Caldarelli DD, Pigato JB. A review of revision functional endoscopic sinus surgery. Laryngoscope. 1994;104:404–408. doi: 10.1288/00005537-199404000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Lazar RH, Younis RT, Long TE, Gross CW. Revision functional endonasal sinus surgery. Ear Nose Throat J. 1992;71:131–133. [PubMed] [Google Scholar]
- 16.Deal RT, Kountakis SE. Significance of nasal polyps in chronic rhinosinusitis: symptoms and surgical outcomes. Laryngoscope. 2004;114:1932–1935. doi: 10.1097/01.mlg.0000147922.12228.1f. [DOI] [PubMed] [Google Scholar]
- 17.Waguespack R. Mucociliary clearance patterns following endoscopic sinus surgery. Laryngoscope. 1995;105:1–40. [PubMed] [Google Scholar]
- 18.DeConde AS, Mace JC, Levy JM, Rudmik L, Alt JA, Smith TL. Prevalence of polyp recurrence after endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis. The Laryngoscope. 2016 doi: 10.1002/lary.26391. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jankowski R, Pigret D, Decroocq F, Blum A, Gillet P. Comparison of radical (nasalisation) and functional ethmoidectomy in patients with severe sinonasal polyposis. A retrospective study. Rev Laryngol Otol Rhinol (Bord) 2006;127:131–140. [PubMed] [Google Scholar]
- 20.Bassiouni A, Wormald P-J. Role of frontal sinus surgery in nasal polyp recurrence. Laryngoscope. 2013;123:36–41. doi: 10.1002/lary.23610. [DOI] [PubMed] [Google Scholar]
- 21.Stevens WW, Peters AT, Suh L, Norton JE, Kern RC, Conley DB, Chandra RK, Tan BK, Grammer LC, Harris KE, Carter RG, Kato A, Urbanek M, Schleimer RP, Hulse KE. A retrospective, cross-sectional study reveals that women with CRSwNP have more severe disease than men. Immunity, Inflammation and Disease. 2015;3(1):14–22. doi: 10.1002/iid3.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wasan A, Fernandez E, Jamison RN, Bhattacharyya N. Association of anxiety and depression with reported disease severity in patients undergoing evaluation for chornic rhinosinusitis. Annals of Otology, Rhinology, & Laryngology. 2007;116(7):491–497. doi: 10.1177/000348940711600703. [DOI] [PubMed] [Google Scholar]
- 23.Kessler RC. Epidemiology of women and depression. J Affective Disorders. 2003;71(1):5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- 24.LeResche L. Defining gender disapirities in pain management. Clin Orthop Relat Res. 2011;469:1871–1877. doi: 10.1007/s11999-010-1759-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson ME, Riley JL, 3rd, Myers CD, Papas RK, Wise EA, Waxenberg LB, Fillingim RB. Gender role expectations of pain: relationship to sex differences in pain. J Pain. 2001;2:251–257. doi: 10.1054/jpai.2001.24551. [DOI] [PubMed] [Google Scholar]
- 26.Mota RE, Tarricone R, Ciani O, Bridges JF, Drummond M. Determinants of demand for total hip and knee arthroplasty: a systematic literature review. BMC Health Serv Res. 2012;12:225. doi: 10.1186/1472-6963-12-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feinglass J, McDermott MM, Foroohar M, Pearce WH. Gender differences in interventional management of peripheral vascular disease: evidence from a blood flow laboratory population. Ann Vasc Surg. 1994;8:343–9. doi: 10.1007/BF02132995. [DOI] [PubMed] [Google Scholar]
- 28.U.S. Census Bureau. Race and Hispanic or Latino Origin: 2010 Census Summary File 1.; generated by Nathan Stein; using American FactFinder. 2016 Nov 15; < http://factfinder2.census.gov>;
- 29.Soler ZM, Mace JC, Litvack JR, Smith TL. Chronic rhinosinusitis, race, and ethnicity. Am J Rhinol Allergy. 2012;26(2):110–116. doi: 10.2500/ajra.2012.26.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JY, Lee SW, Lee JD. Comparison of the surgical outcome between primary and revision endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis. Am J Otolaryngology. 2008;29:379–384. doi: 10.1016/j.amjoto.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Koskinen A, Salo R, Huhtala H, Myller J, Rautiainen M, Kääriäinen J, Penttilä M, Renkonen R, Raitiola H, Mäkelä M, Toppila-Salmi S. Factors affecting revision rate of chronic rhinosinusitis Laryngoscope Investigative. Otolaryngology. 2016;1:96–105. doi: 10.1002/lio2.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hopkins C, Browne JP, Slack R, Lund V, Topham J, Reeves B, et al. The national comparative audit of surgery for nasal polyposis and chronic rhinosinusitis. Clin Otolaryngol. 2006;31(5):390–8. doi: 10.1111/j.1749-4486.2006.01275.x. [DOI] [PubMed] [Google Scholar]
- 33.Smith TL, Litvack JR, Hwang PH, Loehrl TA, Mace JC, Fong KJ, James KE. Determinants of outcome of sinus surgery: a multi-institutional prospective cohort study. Otolaryngol Head Neck Surg. 2010;142(1):55–63. doi: 10.1016/j.otohns.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]