Abstract
This study sought to identify brain regions that underlie symptom changes in severely affected IBS patients undergoing cognitive therapy (CT). Five healthy controls and 6 Rome II diagnosed IBS patients underwent psychological testing followed by rectal balloon distention while brain neural activity was measured with O-15 water positron emission tomography (PET) before and after a brief regimen of CT. Pre-treatment resting state scans, without distention, were compared to post-treatment scans using statistical parametric mapping (SPM). Neural activity in the parahippocampal gyrus and inferior portion of the right cortex cingulate were reduced in the post-treatment scan, compared to pre-treatment (x, y, z coordinates in MNI standard space were −30, −12, −30, P = 0.017; 6, 34, –8, P = 0.023, respectively). Blood flow values at these two sites in the controls were intermediate between those in the pre- and post-treatment IBS patients. Limbic activity changes were accompanied by significant improvements in GI symptoms (e.g., pain, bowel dysfunction) and psychological functioning (e.g., anxiety, worry). The left pons (−2, −26, −28, P = 0:04) showed decreased neural activity which was correlated with post-treatment anxiety scores. Changes in neural activity of cortical-limbic regions that subserve hypervigilance and emotion regulation may represent biologically oriented change mechanisms that mediate symptom improvement of CT for IBS.
Keywords: Irritable bowel syndrome, Cognitive therapy, Pain, Brain imaging, Anxiety, Worry
Introduction
Irritable bowel syndrome (IBS) is a common, costly, and potentially disabling gastrointestinal (GI) disorder characterized by abdominal pain/discomfort with altered bowel habits (e.g., diarrhea, constipation). Lacking a well-defined biomarker, IBS is conceptualized as a manifestation of disturbance in the brain–gut axis (Mayer, Naliboff, Chang, & Coutinho, 2001). The brain–gut axis is a neural network comprising the central nervous system (CNS) and the enteric nervous system (ENS). Neural transmission lines of the brain–gut axis are bidirectional and reciprocal, with the CNS (brain-spinal cord) receiving information from the digestive tract and modulating the ENS (gut). Normal GI function involves a high degree of coordination between the brain and gut. In IBS patients, however, dysregulation in the brain–gut axis is expressed in enhanced sensitivity of the GI tract to common stimuli such as food, stress, stool passage; disturbances in contractions of the GI tract (i.e., altered motility); and psychological dysfunction.
Although alterations at any level of the brain–gut axis may result in dysmotilty and visceral sensitivity, multiple lines of evidence underscore the role of CNS activity in modulating IBS symptoms, particularly in more severely affected patients. These include: (a) high rates of psychiatric comorbidity (Sykes, Blanchard, Lackner, Keefer, & Krasner, 2003); (b) therapeutic value of centrally acting agents (e.g., antidepressants) for specific symptoms (e.g., pain, Greenbaum et al., 1987); (c) temporal pattern of symptoms during the sleep–wake cycle (e.g. disappearances of symptoms during sleep and the increase of propagating contraction velocity upon awakening (Kumar, Thompson, Wingate, Vesselinova-Jenkins, & Libby, 1992)); (d) high comorbidity of functional somatic disorders whose pathophysiology involves central processing abnormalities (e.g., benign headache, fibromyalgia (Whitehead, Palsson, & Jones, 2002)); (e) the lack of correspondence between pain intensity and measured gut motility (Kellow & Phillips, 1987); (f) neuroimaging studies showing differences in activity of brain regions (anterior cingulate cortex, prefrontal cortex) among IBS patients vs. healthy controls in response to actual or anticipated rectal stimuli (Silverman et al., 1997); and (g) the effect of psychosocial factors on IBS via CNS pathway (Lackner, 2003; Lackner, Morley, Dowzer, Mesmer, & Hamilton, 2004).
Among psychological factors contributing to IBS, cognitive factors such as the beliefs, expectations, and thoughts patients hold about their symptoms appear particularly important (Almy, Kern, & Tulin, 1949; Drossman, Li et al., 2000; Lackner, Quigley, & Blanchard, 2004). Cognitive factors can modulate pain experience (Accarino, Azpiroz, & Malagelada, 1997; Naliboff et al., 2001), induce IBS-like colonic motor activity (Almy et al., 1949), heighten stress reactivity (Mayer et al., 2001) and alter brain function (Naliboff et al., 2001). One measure of the importance of cognitive processes comes from outcome research featuring cognitive therapy (CT, Lackner, Morley et al., 2004). A major goal of CT is to modify habitual, negatively skewed thinking patterns that underlie emotional or physiological reactivity. Notwithstanding the apparent efficacy of CT for IBS, little is known about the mechanisms that mediate therapeutic change. Knowledge of the active change mechanisms is important because it may clarify our understanding of IBS and enhance the development of more efficient treatments. If a treatment achieves its effects by targeting a particular process, this finding establishes the importance of this process in the maintenance of the disorder (Kraemer, Wilson, Fairburn, & Agras, 2002). Attempts at specifying change mechanisms of behaviorally oriented treatments have focused on psychological variables (e.g., negative cognitions) that correspond weakly, if at all, with IBS symptoms at post-treatment (e.g., Payne & Blanchard, 1995). These data invite exploration of biologically oriented change mechanisms that explain treatment effects.
One hypothesis draws from Mayer et al. who contend that an information processing style preferentially oriented toward threat detection contributes to a breakdown of the neuroenteric axis (Mayer et al., 2001) by modulating activity of brain regions within the emotional motor system (EMS, Holstege, Bandler, & Saper, 1996). The EMS regulates all functions (pain, stress, anxiety) necessary for basic survival behavior. The EMS receives descending input from cortical areas during psychological stressors, and ascending input from the viscera during noxious visceral stimulation. Dysregulation in the EMS circuitry is manifested in: (1) abnormal patterns of GI motility; (2) perceptual abnormalities for visceral sensations (i.e., visceral sensitivity) such that IBS patients report more intense pain in response to balloon distension in the rectum and detect this sensation at lower pressure and lower volume than controls; and (3) negative emotional states (e.g., anxiety, stress) that aggravate IBS symptoms. On the basis of these data and research showing that voluntary cognitive control of emotion corresponds with changes in neural activity in brain structures comprising the EMS (Ochsner, Bunge, Gross, & Gabrieli, 2002), we were interested in whether a brief regimen of CT that teaches IBS patients to identify and challenge faulty threat appraisals would yield broad therapeutic benefit (e.g., pain, bowel disturbance, distress) by modulating activity in the brain regions comprising the EMS. If so, we predicted significant changes in brain neural activity would be observed in brain regions (e.g., anterior cingulate cortex, amygdala) subserving psychological processes of hypervigilance and emotional self-control in IBS patients at post-treatment.
Subjects
Subjects included five healthy controls (age: mean = 33 years, range SD = 9.8) and 8 (age: mean = 33 years, SD = 9.4) IBS patients recruited as part of an NIH clinical trial of two psychological treatments. Two IBS patients dropped out after the pre treatment assessment due to transportation problems. All subjects were female. All but one of the participants was Caucasian. To qualify, IBS subjects must have not been taking IBS, narcotic, or psychotropic agents, must have experienced IBS symptoms at least twice weekly for 6 months with some activity limitation (i.e., at least moderately severe), and must have met Rome II IBS diagnosis for IBS (Drossman, Corazziari, Talley, Thompson, & Whitehead, 2000) established by the study gastroenterologist (L. K.). Rome II criteria for IBS require abdominal pain/discomfort that is relieved with defecation or associated with a change in stool frequency or consistency for at least 12 weeks. The study gastroenterologist (LK) rated the severity of patients’ symptoms using a four-point rating scale (Drossman, Toner et al., 2003) where 1 = mild and 4 = very severe. The average severity score for the IBS group was 3.2, indicating a severely affected sample. Patients were excluded from participation if they had inflammatory bowel disease or lactose malabsorption syndrome; had a history of current or past psychotic disorders; or current depression with suicidal ideation. The average duration of IBS symptoms was 16 years. None of the participants were taking psychotropic medications. None of the IBS patients had undergone psychotherapy featuring cognitive-behavioral techniques. Because IBS patients often report significant exacerbation of their IBS symptoms with menses (Houghton, Lea, Jackson, & Whorwell, 2002), visceral distention/PET testing was not scheduled during the menstruation phase of their menstrual cycles. The comparison group of healthy controls was recruited from the community and were deemed eligible if they were 18 years or older and reported no current or previous history of GI or psychiatric disorder. None of the control subjects were taking either prescription or over the counter medications. At the time of enrollment, all subjects gave written informed consent to participate in protocols that had been approved by the Human Subjects, Research and Development, Radiation Safety and Radioactive Drug Research Committees in accord with the Declaration of Helsinki.
Study design
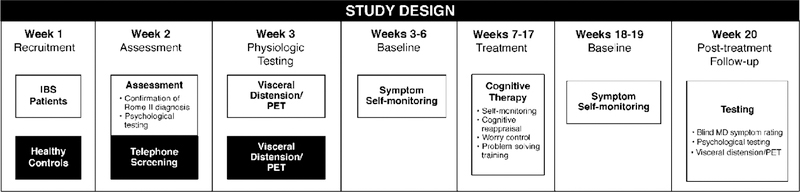
The design of the study is diagrammatically represented in Fig. 1. IBS patients underwent psychological testing followed by visceral distension PET scan protocol on two occasions: (1) approximately 4 weeks prior to the initiation of CT and (2) 2 weeks after the end of CT. With respect to healthy controls, we adopted the practice of Lieberman et al. (2004) who scanned their controls once. Their rationale for not rescanning healthy controls was based on the results of a number of previous studies (Amanzio & Benedetti, 1999; Benedetti et al., 2003; Vase, Robinson, Verne, & Price, 2003) that failed to find sufficient variation over time in the natural history control group to justify a second scan. The within-subject stability of repeated resting state scans has been previously demonstrated (Carroll et al., 2002; Matthew et al., 1993). Each component of the assessment is described in more detail below.
Fig. 1.
Study design.
Psychological assessment measures
Psychological distress
The Brief Symptom Inventory (BSI, Derogatis, 1993) is a 53-item measure designed to measure nine specific types of problems (e.g., anxiety, somatization, depression). Items are rated on a five-point scale (0 = not at all, 1 = at little bit, 2 = moderately, 3 = quite a bit, and 4 = extremely). The average intensity for all items (Global Severity Index) was used as an index of overall psychological distress.
Anxiety
In addition to the Anxiety subscale of the BSI, participants completed the Penn State Worry Questionnaire (PSWQ, Meyer, Miller, Metzger, & Borkovec, 1990) and the Anxiety Sensitivity Index (Peterson & Reiss, 1993). The PSWQ assesses the extent to which worry is pervasive, uncontrollable, and excessive (e.g., ‘‘I know I should not worry about things, but I just can’t help it’’). The PSWQ focuses on the process of worry rather than on particular domain of worry (e.g., health). The PSWQ presents 16 statements followed by a five-point Likert-type response scale (from 1 = not at all typical to 5 = very typical) representing how typical the individual feels the statements are of him or her. The Anxiety Sensitivity Index (Peterson & Reiss, 1993) is a self-report measure that measures fear of anxiety (e.g., ‘‘it scares me when I am anxious’’), arousal related bodily sensations (‘‘It scares me when my heart beats rapidly’’), and their consequences (e.g., ‘‘When I notice my heart is beating rapidly, I worry that I might have a heart attack’’). Each of the 16 items of the ASI is rated on a five-point Likert scale (from 0 = very little, 5 = very much). The test–retest reliability, internal consistency, and predictive validity of the ASI are well established (Zinbarg, Mohlman, & Hong, 1999).
Pain severity
The Bodily Pain (BP, Ware, Snow, & Kosinski, 2000) subscale of the SF-36 Health Survey (Ware & Sherbourne, 1992) is a weighted combination of two items measuring (1) the intensity of pain using a six-point verbal rating scale (1 = None, 6 = Very severe) and (2) the effect of pain on normal activities (0 = not at all, 5 = extremely). Items yield an empirically validated (Ware et al., 2000) composite index of the severity of pain and its effects. Higher scores indicate no pain/ limitations due to pain and lower scores indicate very intense/extremely limiting pain. Subjects’ baseline BP scores (M = 54) indicate that their pain was relatively severe and a cause of significant activity limitation. This scale has been used in previous research with IBS patients (Lackner, Quigley et al., 2004).
GI symptoms
Global ratings of IBS symptom severity were made by the study gastroenterologist who evaluated the global severity of each patient at baseline and 2 weeks after the last scheduled therapy session visit using a four-point scale (1 = mild, 4 = very severe) in a manner consistent with previous research (Drossman, Toner et al., 2003). Subjects also completed a GI symptom diary once daily during the 4 weeks before therapy and for 2 weeks before after the last scheduled therapy session. In the diary, patients rated the severity of GI symptoms (pain, diarrhea, bloating) on a five-point scale (0 = absent, 1 = mild, 2 = moderate, 3 = severe, 4 = debilitating scale). Values derived from this diary have been shown to be sensitive to treatment effects described on patients’ global ratings (Blanchard, 2001).
Visceral distension protocol
Before participants were positioned in the scanner, a Fleet phosphate enema was administered. After the desired effect, participants were placed in a lateral decubitus position, and a latex rubber balloon (external diameter, 5 cm; length of each balloon, 9 cm) was inserted into the rectal cavity by a board certified gastroenterologist (TM). The balloon was attached to the a computer-driven barostat (G & J Electronics, Toronto, Ontario) that controlled inflations at a maximal inflation rate 38 ml/s at pressures of 4, 25 and 50 mm Hg pressure using a protocol adapted from Mertz et al. (2000). The 4 mm Hg served as a ‘‘sham’’ condition in that pressure was expected but not delivered. Subjects were informed that each distension would last 60 s and be followed by a rest period of approximately 15 min with the balloon deflated. During the first six scans, participants were informed that the barostat would inflate the balloon to a variety of pressures. The order of the conditions was randomized except for the resting state scan which was always done last. Immediately following each scan/distension phase, subjects were asked to rate the intensity and unpleasantness of abdominal pain, the urge to defecate and associated distress, and anxiety experienced during the distension phases using a series of visual analogue scales. The last and seventh scan was done in a resting state (post-balloon insertion, no inflation) after the subject was informed that all of the distensions had been administered and she would experience no further distensions. The resting state condition at the end of each study was designed to assess basal neurophysiologic activity that was minimally influenced by the psychological state (anxiety, apprehension) associated with the distensions. The balloon was removed after the final scan.
Positron emission tomography (PET) scanning protocol
Subjects were placed in a comfortable supine position in a Siemens ECAT 951/31R tomo-graph (CTI, Knoxville, TN) operated in the 3-D mode. The head was immobilized with a thermoplastic mask and the subject was positioned so that the inferior image plane coincided with the cantho-meatal line. A 20 min 68-Germanium transmission scan was obtained to correct for radiation attenuation due to brain structures and skull. At the beginning of each emission scan, the rectal balloon was inflated to the desired pressure and an intravenous bolus injection of approximately 7 mCi of 15O-water was administered. Coincidence counts were monitored visually and a 1-min emission scan was started when the bolus of activity arrived in the brain. The resulting images were reconstructed (31 planes, 128 × 128 matrix, a Hann filter with a cut-off filter set at 0.4 cycles per pixel, a zoom factor of 2.5 and the measured attenuation data).
Image data analysis
The raw images were converted to the Analyze format (Biodynamics Research Unit, Rochester, MN) and analyzed using the statistical parametric mapping (SPM2, http://www.fil.ion.ucl.ac.uk/spm/spm2.html) (Friston et al., 1995). SPM2 preprocessing included the following steps: (1) with-in subject realignment of all images to eliminate the effects of small inter-scan movements; (2) normalization into Montreal Neurological Institute (MNI) stereotatic standard space (Montreal Neurological Institute, Montreal, Canada); and (3) smoothing with a 15 mm Gaussian kernel. Statistical analyses were performed after accounting for differences in cerebral blood flow and the injected dose of the tracer by an analysis of covariance. The results of the analyses are displayed in the ‘‘glass brain’’ format in which suprathreshold voxels are projected onto coronal, sagittal, and transaxial planes. In addition, the MNI coordinates of sites of maximum effects and their size were listed along with Z-scores and P-values. MNI co-ordinates were converted to Talairach co-ordinates (http://www.mrc-cbu.cam.ac.uk/Imaging/Common/mnispace.shtml). The coordinate convention is x (right positive, left negative), y (anterior positive, posterior negative) and z (superior positive, inferior negative) in mm in relation to a perpendicular axis with the origin set at the anterior commissure. It should be noted that the extent of clusters reaches beyond the site of the voxel with the maximum Z-score and that several maxima may be found in any given cluster. The analytical threshold for displaying the Z-score images was set at z = 3.09 (P = 0:001, uncorrected for multiple comparisons). This is the threshold most commonly employed in the literature.
A multiple subject with conditions and covariates model was used for the image analysis. For the IBS patients, the pre- and post-treatment scans were treated as different conditions within a subject for a total of eight conditions (pre-treatment: rest1, sham1, 25 mm Hg distension1, 50 mm Hg distension1; post-treatment: rest2, sham2, 25 mm Hg distension, 50 mm Hg distension)
CT treatment protocol
IBS participants underwent 10 weekly sessions of CT whose structure and content was implemented in accord with an empirically validated treatment manual originally developed by Blanchard (2001) and refined by Lackner (2003). Treatment aims to teach patients to reduce GI symptoms and related distress by identifying and correcting maladaptive beliefs and information processing errors. To this end, cognitive interventions consist of four overlapping phases (a) educating the patient about IBS and the processes that maintain the disorder, with a focus on its situational, cognitive, and emotional triggers; (b) training in the identification and modification of cognitive appraisals, excessive worries (overestimating the probability of a negative event, catastrophizing), and interpretations of situations, thoughts, and behaviors; (c) changing underlying or ‘‘core’’ beliefs (e.g., perfectionism, overresponsbility, expecting approval) supporting negative cognitions; and (d) formal problem solving training to strengthen the ability to manage realistic stressors by developing a flexible repertoire of decision making skills (e.g., matching perceptions of stressor controllability to appropriate [emotion-focused vs. problem solving] coping response). Treatment was conducted in a small group (3–6 patients) setting. Homework assignments were assigned weekly to facilitate acquisition and generalization of cognitive coping skills. Treatment was administered by a clinical psychologist (J. M. L.) with over 15 years experience in CT.
Results
Between group psychological symptom severity (baseline)
The T-scores on the Global Symptom Index for IBS patients and healthy controls on the Global Severity Index were 53 and 49, respectively, using norms for adult, female non-patients (Derogatis, 1993). A T-score greater or equal to 63 is regarded as clinically significant (Derogatis, 1993). The only psychological variable that rose to a clinically remarkable level for IBS patients was worry (PSWQ). Patients’ average PSWQ score was 54 which corresponds to a percentile rank of 80 for 18–44 year olds (Gillis, Haaga, & Ford, 1995). By comparison, the average PSWQ score for healthy controls was 34, which differed significantly from IBS patients’ pretreatment worry score (p < 0.05).
Between group in vivo ratings
In vivo ratings for pain intensity, pain unpleasantness, and anxiety were averaged across each scan condition for both IBS patients and healthy controls. A series of ANOVAs indicated that IBS patients reported significantly higher pain intensity, pain unpleasantness, and anxiety ratings than healthy controls following 50 mm distension after applying the Bonferroni correction method (P < 0.05). No significant differences between groups were observed for the 4 (sham) or 25 mm conditions.
Within group changes
Psychological measures
The significance of change from pre- to post-treatment on measures of somatic symptoms and distress were analyzed in two ways. Statistical significance was analyzed by conducting a series of repeated measure ANOVAs. Because the small sample size may have lacked sufficient statistical power to detect statistical pre- and post-treatment differences (Type 2 error), we also analyzed data in light of the degree to which patients showed clinically meaningful gain (i.e., clinical significance). To this end, we adopted the practice (Borkovec & Costello, 1993) of using a 20% change from pre-therapy level as an index of clinically significant change. Table 1 shows the pre-and post-treatment values for primary outcomes measures. CT generated clinically meaningful changes on multiple measures of somatic complains (pain severity, bowel dysfunction, defecation urge) and psychological distress (anxiety, defecation urge distress, pain unpleasantness, worry, anxiety sensitivity). In vivo ratings of non-pain related distress (anxiety, defecation distress) decreased significantly (i.e., both statistically, clinically significant change) from pre- to post-treatment. These data are also presented in Table 1.
Table 1.
Pre- and post-values for outcome measures
| Measure | Pre-treatment |
Post-treatment |
% Change | Significance?a | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Daily GI symptom diaries | ||||||
| Pain | 1.59 | 0.41 | 0.91 | 0.73 | −42.8** | Yes |
| Diarrhea | 0.67 | 0.26 | 0.47 | 0.38 | −29.9* | Yes |
| Bloating | 0.65 | 0.41 | 0.36 | 0.32 | −44.6 | Yes |
| Composite | 0.97 | 0.33 | 0.58 | 0.43 | −40.2** | Yes |
| Anxiety | ||||||
| Anxiety sensitivity | 23.83 | 16.69 | 15.00 | 10.16 | −37.1 | Yes |
| BSI-Anxiety | 54.33 | 5.85 | 43.67 | 7.81 | −19.6* | Yes |
| PSWQ | 54.75 | 10.14 | 40.16 | 13.70 | −26.6 | Yes |
| Somatic symptoms | ||||||
| Pain severity (SF-36) | 54.50 | 15.25 | 77.00 | 11.91 | +41.3* | Yes |
| ‘‘Blind’’ MD ratings | 2.80 | 0.45 | 1.80 | 1.10 | −35.7 | Yes |
| Psychopathology | ||||||
| BSI-Global Severity Index | 51.67 | 8.48 | 43.00 | 10.33 | −16.8** | No |
| In vivo ratings (50 mm Hg) | ||||||
| Pain intensity | 52.50 | 19.65 | 47.58 | 21.28 | −9.4 | No |
| Pain unpleasantness | 66.17 | 11.59 | 50.75 | 28.69 | −23.3 | Yes |
| Defecation distress | 4.21 | 2.52 | 2.13 | 2.86 | −49.4** | Yes |
| Defecation urge | 5.48 | 1.32 | 4.38 | 1.99 | −20.0 | Yes |
| Anxiety | 59.33 | 14.80 | 36.91 | 26.63 | −37.8* | Yes |
Statistically significant = P < 0.05
Marginally significant = P < 0.07
Clinical significance = ⩾20% change from pre-treatment levels.
Regional cerebral blood flow
Whole brain analyses
The comparison between the pre- and post-treatments resting state in the patients revealed five sites containing 40 or more voxels where neural activity was reduced after treatment as shown in Fig. 2 and detailed in Table 2. The threshold for image display was P < 0.001, uncorrected for multiple comparisons. This is the threshold most commonly employed in the literature. P-values for voxels reported in this paper are corrected for multiple comparisons, which were made using the False Discovery Rate (FDR: Nichols & Hayasaka, 2003) strategy. P-values for cluster size are corrected. Three of these clusters were in the limbic system. The largest cluster contained 1814 voxels (P < 0.001 for cluster size). It contained four maxima, three of which reached the P < 0.02 criterion at the voxel-level. The maximum with the highest Z-score was located in limbic gray matter of the medial surface of the left temporal lobe, predominately in the parahippocampal gyrus. The cluster extended through the temporal lobe to the superior and middle temporal gyri. A 42-voxel cluster was present in the contralateral parahippocampal gyrus of the right medial temporal lobe (22, −6, −28 (MNI) P = 0.047). An additional 200-voxel site of limbic system activation was present in the right anterior cingulate (ACC) gyrus, inferior to the genu of the corpus callosum (subgenual part of perigenual ACC). The other sites were located in the left superior frontal gyrus and in the left cingulate gyrus near the splenium of the corpus callosum. We observed no significant changes in neural activity during distension from pre to post treatment when the effects of the rest scan were subtracted.
Fig. 2.
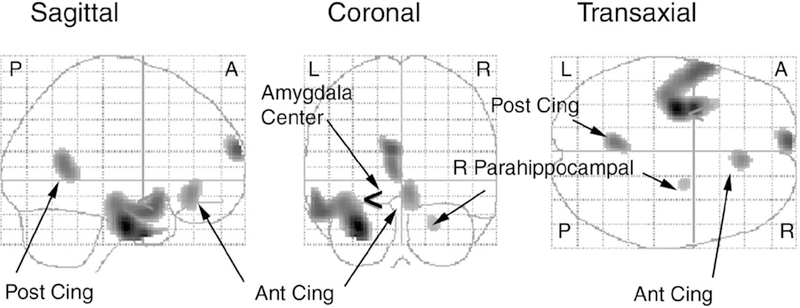
EMS regions showing reduced neural activity after CT (P < 0:05). Reductions in neural activity after CT. Sagittal, coronal, and transaxial projections of P-values for the SPM contrast (pre-therapy rest)—(post-therapy rest) are shown with a threshold P = 0.001, uncorrected for multiple comparisons. The < symbol, shown best on the coronal projection, marks the center of the a priori defined sphere delineating the left amygdala. Abbreviations Ant Cing and Post Cing are anterior and posterior cingulate, respectively, R = right, L = left, A = anterior, P = posterior. See Table 2 for cluster sizes and loci of maxima and associated P-values.
Table 2.
Reductions in neural activity after cognitive therapy
| Anatomical site | Cluster size (number of voxels) | P-value, corrected for multiple comparisons | Talairach coordinates x, y, z (mm)a |
|---|---|---|---|
| Left temporal lobe | 1814 | ||
| Parahippocampal gyrus | 0.017 | −30, −13, −25 | |
| Uncus | 0.017 | −32, −2, −30 | |
| Middle temporal gyrus | 0.018 | −57, 3, −17 | |
| Superior temporal gyrus | 0.055 | −34, 12, −28 | |
| Inferior portion, superior frontal gyrus | 181 | 0.018 | −6, 67, 15 |
| Left posterior cingulate | 245 | 0.020 | −6, −54, 12 |
| Right anterior cingulate | 200 | 0.023 | 6, 33, −8 |
| Right parahippocampal gyrus | 42 | 0.047 | 22, −7, −23 |
Loci of maxima generated by SPM software were converted to Talairach coordinates using formulae of Brett (http://www.mrc-cbu.cam.ac.uk/Imaging/Common/mnispace.shtml, accessed June 28, 2004).
Region of interest analysis: The Amygdala
Because SPM methodology identifies between-condition maxima, but provides little information concerning the extent of the significant activation, we sought to determine whether the temporal lobe cluster included the amygdala. Thus, we conducted an analysis restricted to the amygdala, which we defined as a sphere with an 8 mm radius centered at coordinates −26, −4, −14, based on the Talairach atlas (Talairach & Tournoux, 1988). There was a maximum within that sphere where P = 0.031. Thus, we conclude that at least portions of the amygdala (left) were within the site whose neural activity decreased following treatment.
Correlations with anxiety
A covariance analysis using a multi-subject conditions and covariance model was performed to identify areas of brain activity corresponding to pre- and post-treatment state anxiety scores There was a significant negative correlation between scores and neural activity in the pons, which contained a cluster of 860 voxels (P = 0.008 corrected), with a maximum effect seen on the left at coordinates, −2, −26, −28 P = 0.40 . There is considerable pre-clinical evidence for a relationship between this area and anxiety (Bremner, Krystal, Southwick, & Charney, 1996). Activation in the pons has been previously related to rectal distension (Hamaguchi et al., 2004; Naliboff et al., 2003; Berman et al., 2002) as well co-regulation of viscera and forebrain activity (Valentino, Miselis, & Pavcovich, 1999).
Conclusion
In this sample of severely affected, Rome II diagnosed IBS patients, we have shown that improvement in symptoms after CT corresponds with changes in baseline brain neural activity. Specifically, improvement was associated with reduced neural activity in portions of the limbic system, including the mesial surface of the left temporal lobe including the amygdala, and subgenual part of the perigenual anterior cingulate cortex. These brain regions have been identified as important parts of the central circuitry underlying both pain perception and self-regulation.
To our knowledge, this is the first study that has sought to identify neural changes in patients following completion of behavioral treatment for IBS. Based on previous research showing that enhanced responsiveness of brain regions that underlie altered brain–gut interactions characteristic of IBS, we sought to identify changes in CNS activity in patients whose symptoms were alleviated following a brief regimen of CT. We observed statistically significant reductions for pain severity, anxiety, and GI symptom severity. Scores of pathological worry met the criterion for clinically significant change in that they fell from clinical range at pre-treatment (percentile score = 80) to normal range (percentile = 40) at post-treatment (Gillis et al., 1995). Changes in pre- to post-treatment values of psychological data parallel outcomes of larger randomized controlled trials featuring CT either as a sole intervention (Payne & Blanchard, 1995) or as part of a multidimensional CBT protocol (Lackner, Morley et al., 2004).
Our imaging data support the prediction that CT is associated with changes in the network of brain circuits (e.g., anterior cingulate cortex, amygdala) that subserve danger orientation, attention to fear-related stimuli, and vigilance (Pissiota et al., 2003). In non-clinical neuroimaging studies, the amygdala is activated in response to subtle (low arousal) affective stimuli cues that connote threat such as pictures depicting facial expressions of fear (Morris et al., 1998; Whalen et al., 1998). Whalen (Davis & Whalen, 2001; Whalen, 1998) interprets these data as evidence that the amygdala is responsible for modulating moment to moment levels of vigilance to environmental stimuli with threat potential . Amygdala activation appears particularly responsive to uncertain (i.e., ambiguous) environmental stimuli because they confer less information regarding the nature of potentially threatening stimuli. The amygdala responds to ambiguity by facilitating the acquisition of additional information regarding the threat value of environmental stimuli through a future oriented, negatively skewed style of thinking (Whalen, 1998) that is descriptively similar to worry (Borkovec, 1994). This thinking pattern characterizes more severely affected patients with IBS (Lackner & Quigley, in press) and GAD (Borkovec & Newman, 1999), an anxiety disorder highly comorbid with IBS (Sykes et al., 2003). While a ‘‘what if’’ thinking style may effectively reduce ambiguity (Whalen, 1998), its benefits come at the cost of increased scanning and hypervigilance. Hypervigilance is a key cognitive process underlying IBS (Naliboff, 1999) and other anxiety related disorders (Barlow, 2002). Because of negative reinforcement cycles, worry and subsequent vigilance may elevate anxiety which, in IBS patients, can aggravate GI symptoms and lead to further worry and illness behaviors (e.g., treatment seeking behaviors). The importance of the amygdala to worry is also suggested by neuroimaging research implicating the left amygdala in the cognitive representation of anxiety (Phelps et al., 2001; Tillfors et al., 2001). Worry has been conceptualized as the cognitive aspect of anxiety (Borkovec & Newman, 1999). If a left lateralized pattern of activation within the brain region involving the amygdala subserves cognitive aspects of anxiety (worry and anticipatory anxiety, Heller, Nitschke, Etienne, & Miller, 1997), then the therapeutic value of worry control techniques (‘‘decatastrophzing’’, evidence based logic) may lie in part in their ability to modulate vigilance by raising the threshold for amygdala response to emotionally noxious stimuli.
We also found that IBS patients showed decreased activity in the medial frontal cortex (medial frontal gyrus) and perigenual anterior cingulate cortex at the end of CT. The medial prefrontal cortex mediates the processing of affective meaning or interpretations of stimuli. Processing of higher-order emotion related meanings of stimuli plays a central role in the ‘‘cognitive’’ elicitation of affect (Andreasen, 1997; LeDoux, 1995; Teasdale et al., 1999). Because this route links cortical structures such as the medial prefrontal cortex to affect generation, it is regarded as of ‘‘central importance’’ (Teasdale et al., 1999, p. 210) to appraisal theories of emotion underlying cognitively oriented therapies (Beck, 1971; Lazarus, 1984). In the context of prior imaging research, our data suggest that not only does the medial prefrontal cortex play an important role in the generation of affect (Teasdale et al., 1999), it may also be involved in affect suppression via worry control strategies featured in the CT of IBS. This conclusion dovetails with the findings of other non-clinical studies that have implicated prefrontal cortex regions in the cognitive regulation of thoughts and feelings (Ochsner et al., 2002). Additional prospective, clinical studies are needed to determine whether the prefrontal cortex is a biologically oriented change mechanism that mediates response to CT for IBS.
At post-treatment, IBS patient showed decreased neural activity in the perigenual (pACC), a brain region implicated in the processing of the affective dimension of pain (i.e., the emotional unpleasantness or suffering component of pain experience, Hsieh, Belfrage, Stone-Elander, Hansson, & Ingvar, 1995; Lackner, Jaccard, & Blanchard, in press). The pACC has high opioid receptor density (Jones et al., 1991) with projections to two brain regions that subserve pain modulation (periaqueductal grey, pontine nuclei) (Room, Russchen, Groenewegen, & Lohman, 1985). For these reasons, activity in the pACC patients has been largely interpreted in light of its pain inhibition functions (Naliboff et al., 2001; Ringel et al., 2003; Silverman et al., 1997). The behavioral science literature, however, suggests that the ACC in general (Lane et al., 1998; Posner & Rothbart, 1998) and its perigenual region (pACC) in particular have broader emotion regulatory functions that extend beyond modulating pain affect (Devinsky, Morrell, & Vogt, 1995). For example, cognitively induced states of negative emotions elevate neural activity in pACC (George et al., 1995), while patients without pain who respond to pharmacotherapy for depression (Mayberg, 2003) or behavior therapy for social phobia (Furmark et al., 2002) show decreased neural activity in the pACC at post-treatment. Clinical neuroimaging studies with PTSD (i.e., non-pain) patients further highlight the contributing role of the pACC in stress modulation (Bremner, Southwick, Johnson, Yehuda, & Charney, 1993; Shin et al., 1997). Taken together, these data suggest that deactivations in the pACC might be more parsimoniously understood as a neural index of emotion regulatory capabilities (Furmark et al., 2002) of which the processing of pain affect is only one task. The notion that activity in the pACC reflects more generalized (vs. pain specific) emotional self-control capabilities would explain why IBS patients who at post-treatment showed reduced neural activity in the pACC also showed prospective changes in pain- and non-pain-related emotional intensity measures (e.g., in vivo ratings of pain unpleasantness, anxiety, urge to defecate distress, anxiety sensitivity). These data raise the interesting question of whether the cognitive (e.g., uncontrollable worry) and emotional (e.g., anxiety, depression) dysfunction that aggravates IBS symptoms are due in part to executive control and self-regulation deficits mediated by the pACC. If so, cognitive coping skills taught in CT may achieve their therapeutic objectives (self-regulation) reflected by dampening pACC activity.
Our imaging data dramatically converge with the results of two previous placebo controlled drug studies (Berman et al., 2002; Mayer et al., 2002) that found patterns of deactivations involving the left temporal cortex including the amygdala and parahippocampal gyrus at resting state in patients receiving the IBS agent alosetron (Lotronex). In light of these data, our results raise the intriguing notion that CBT and pharmacotherapy despite different primary mechanisms of action may achieve therapeutic objectives through a common limbic-cortical pathway. This similarity echoes the work of Baxter et al. (1992) who found that improvements following pharmacotherapy and behavior therapy for obsessive compulsive disorder were associated with similar changes in neural activity.
While the design of the present study improves upon aspects (e.g., sample size, pre- and post-treatment baseline, manualized treatment protocol, treatment specification, exclusion criteria, IBS diagnosis based on Rome criteria) of the only similar non-drug neuroimaging study (Drossman, Ringel et al., 2003), there are limitations that should be considered in interpreting our data. A larger sample size may have provided sufficient statistical power to detect robust group differences between patients and healthy controls. The value of a larger sample size may have been particularly useful for relating prospective changes in neural activity to symptom improvement. Without a post-treatment control condition, we are unable to discern whether changes in pre- and post-treatment values are due to active features of CT or non-specific treatment factors (e.g., group support, therapist–client relationship, suggestion, positive expectancy of patient). Also unknown is whether changes in clinical symptoms or neural activity among patients with comorbid distress is necessarily specific to IBS or a product of generalized improvement in psychological well-being. We note, however, that while this study included patients with comorbid psychological distress (e.g., pathological worry) at baseline, our imaging findings converge with data those from two prospective imaging studies whose IBS subjects’ were free of psychological distress (Berman et al., 2002; Mayer et al., 2002). One might contend that post-treatments reductions in neural activity are simply because patients habituated neurally to a second scanner/ rectal distension procedure. This argument would be more plausible had subjects demonstrated reduced neural activity in theoretically relevant brain areas across conditions (baseline, in vivo) without corresponding improvement in symptoms. The finding of symptom reduction and resting state (but not in vivo) changes in neural activity argues against the notion that habituation effects account for our pattern of results (Lieberman et al., 2004). In other words, a habitation effect explanation does not explain improvement in symptoms. Last, some may criticize the rigor of using Borkovec’s 20% criteria for clinical significance. We note that 8 of the 13 of pre–post changes deemed clinically significant using a 20% rule retained their clinical significance when a 33% criteria (Drossman, Patrick et al., 2000, p. 1003) was applied.
In our study, we have used 15O-water to measure cerebral blood flow, using positron emission tomography (PET). This widely used technique provides a quantitative index of neural activity at various brain regions under various experimental conditions (Posner, Petersen, Fox, & Raichle, 1988). By comparing scans obtained under different conditions, or by seeking correlations between variables such as test scores and blood flow, it is possible to attribute changes in neural activity to the difference between the experimental conditions (Frackowiack et al., 2004). The images in the figures are displayed at the usual threshold P = 0.001, uncorrected for multiple corrections. This is the usual threshold for visualization. However, in making statistical inferences, we have used more rigorous methods. In describing clusters, groups of contiguous voxels that exceed the display threshold, the threshold for significance is corrected for the fact that multiple comparisons have been made. Significance at the cluster level means that the number of voxels in a cluster exceeds the number expected due to chance. Significant clusters also contain information concerning the general location of the cluster. However an exact location, in terms of x, y, and z coordinates cannot be inferred. Within a cluster, one or more maxima are found. These maxima may reach the voxel level of significance after correction for multiple comparisons. This allows the investigator to conclude that at that exact location, the difference between conditions is statistically significant. Because the image set contains a very large number of voxels whose value is not completely independent from the value of adjacent voxels, a correction for this fact is applied using the false discovery rate method (FDR, Nichols & Hayasaka, 2003). This relatively new strategy controls for the expected proportion of false positives among the voxels that are identified, yielding the P-value included in the Table 1. In addition, we have conducted one analysis, made on an a priori basis, to test the hypothesis that a significant effect was found in the amygdala. By considering just this region, the need to correct P-values for multiple comparisons is eliminated. Although our sample size is limited, the very conservative statistical methods we employed lend a high level of confidence in the validity of our results.
In spite of our conservative approach, we obtained data—the first of its kind, to our knowledge—showing that an efficacious regimen of CT is associated with changes in neural activity deemed aberrant in severely affected IBS patients. Studies that build upon this preliminary study are needed to investigate how formal instruction in reappraisal strategies taught in CT can functionally ‘‘rewire’’ brain circuitry in a manner that decreases symptoms of IBS patients.
Acknowledgements
This research was supported in part by National Institutes of Health Grants DK-5421and DK-67878 and an Interdisciplinary Research and Creative Activities Fund Grant through the University at Buffalo, SUNY.
The authors thank Doug Drossman, Brent Vogt, and Paul Whalen for helpful comments regarding an earlier version of this manuscript. We would also like to thank Gregory Gudleski, Paul Galantowicz, and David Wack for their technical contributions.
References
- Accarino AM, Azpiroz F, & Malagelada JR (1997). Attention and distraction: Effects on gut perception. Gastroenterology, 113(2), 415–422. [DOI] [PubMed] [Google Scholar]
- Almy TP, Kern FJ, & Tulin M (1949). Alternation in colonic function in man under stress: Experimental production of sigmoid spasm in healthy persons’. Gastroenterology, 12, 425–436. [PubMed] [Google Scholar]
- Amanzio M, & Benedetti F (1999). Neuropharmacological dissection of placebo analgesia: Expectation-activated opioid systems versus conditioning-activated specific subsystems. Journal of Neuroscience, 19(1), 484–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC (1997). Linking mind and brain in the study of mental illnesses: A project for a scientific psychopathology. Science, 275(5306), 1586–1593. [DOI] [PubMed] [Google Scholar]
- Barlow DH (2002). Anxiety and its disorders New York: Guilford. [Google Scholar]
- Baxter LR Jr., Schwartz JM, Bergman KS, Szuba MP, Guze BH, Mazziotta JC, et al. (1992). Caudate glucose metabolic rate changes with both drug and behavior therapy for obsessive-compulsive disorder. Archives of General Psychiatry, 49(9), 681–689. [DOI] [PubMed] [Google Scholar]
- Beck AT (1971). Cognition, affect, and psychopathology. Archives of General Psychiatry, 24(6), 495–500. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Pollo A, Lopiano L, Lanotte M, Vighetti S, & Rainero I (2003). Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. Journal of Neuroscience, 23(10), 4315–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman SM, Chang L, Suyenobu B, Derbyshire SW, Stains J, Fitzgerald L, et al. (2002). Condition-specific deactivation of brain regions by 5-HT3 receptor antagonist Alosetron. Gastroenterology, 123(4), 969–977. [DOI] [PubMed] [Google Scholar]
- Blanchard EB (2001). Irritable bowel syndrome: Psychosocial assessment and treatment Washington: APA. [Google Scholar]
- Borkovec TD (1994). The nature, function, and origins of worry. In Davey GCL, & Tallis F (Eds.), Worrying: Perspective on theory, assessment, and treatment Chichester, England: Wiley. [Google Scholar]
- Borkovec TD, & Costello E (1993). Efficacy of applied relaxation and cognitive-behavioral therapy in the treatment of generalized anxiety disorder. Journal of Consulting and Clinical Psychology, 61(4), 611–619. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, & Newman MG (1999). Worry and generalized anxiety disorder. In Bellack AS, & Hersen M (Eds.), Comprehensive clinical psychology, Vol. 6 (pp. 439–459). Elsevier Science: Oxford. [Google Scholar]
- Bremner JD, Southwick SM, Johnson DR, Yehuda R, & Charney DS (1993). Childhood physical abuse and combat-related posttraumatic stress disorder in Vietnam veterans. American Journal of Psychiatry, 150(2), 235–239. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, & Charney DS (1996). Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse, 23(1), 28–38. [DOI] [PubMed] [Google Scholar]
- Carroll TJ, Teneggi V, Jobin M, Squassante L, Treyer V, Hany TF, et al. (2002). Absolute quantification of cerebral blood flow with magnetic resonance, reproducibility of the method, and comparison with H2(15)O positron emission tomography. Journal of Cerebral Blood Flow and Metabolism, 22(9), 1149–1156. [DOI] [PubMed] [Google Scholar]
- Davis M, & Whalen PJ (2001). The amygdala: Vigilance and emotion. Molecular Psychiatry, 6(1), 13–34. [DOI] [PubMed] [Google Scholar]
- Derogatis LR (1993). Brief symptom inventory Minneapolis, MN: National Computer Systems. [Google Scholar]
- Devinsky O, Morrell MJ, & Vogt BA (1995). Contributions of anterior cingulate cortex to behaviour. Brain, 118(Part 1), 279–306. [DOI] [PubMed] [Google Scholar]
- Drossman DA, Corazziari E, Talley NJ, Thompson GW, & Whitehead WE (2000). Rome II. The functional gastrointestinal disorders. Diagnosis, pathophysiology and treatment: A multinational consensus (2nd ed). McLean, VA: Degnon Associates. [Google Scholar]
- Drossman DA, Li Z, Leserman J, Keefe FJ, Hu YJ, & Toomey TC (2000). Effects of coping on health outcome among female patients with gastrointestinal disorders. Psychosomatic Medicine, 62, 309–317. [DOI] [PubMed] [Google Scholar]
- Drossman DA, Patrick DL, Whitehead WE, Toner BB, Diamant NE, Hu Y, et al. (2000). Further validation of the IBS-QOL: A disease-specific quality-of-life questionnaire. American Journal of Gastroenterology, 95(4), 999–1007. [DOI] [PubMed] [Google Scholar]
- Drossman DA, Ringel Y, Vogt BA, Leserman J, Lin W, Smith JK, et al. (2003). Alterations of brain activity associated with resolution of emotional distress and pain in a case of severe irritable bowel syndrome. Gastroenterology, 124(3), 754–761. [DOI] [PubMed] [Google Scholar]
- Drossman DA, Toner BB, Whitehead WE, Diamant NE, Dalton CB, Duncan S, et al. (2003). Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology, 125(1), 19–31. [DOI] [PubMed] [Google Scholar]
- Frackowiack RS, Friston KJ, Frith CD, Dolan RJ, Price CJ, Zeki S, Ashburner J, & Penny W (2004). Human brain function (2nd ed.). New York: Elsevier. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, & Frackowiak RSJ (1995). Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping, 2, 189–210. [Google Scholar]
- Furmark T, Tillfors M, Marteinsdottir I, Fischer H, Pissiota A, Langstrom B, et al. (2002). Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitive-behavioral therapy. Archives of General Psychiatry, 59(5), 425–433. [DOI] [PubMed] [Google Scholar]
- George MS, Ketter TA, Parekh PI, Horwitz B, Herscovitch P, & Post RM (1995). Brain activity during transient sadness and happiness in healthy women. American Journal of Psychiatry, 152(3), 341–351. [DOI] [PubMed] [Google Scholar]
- Gillis MM, Haaga DAF, & Ford GT (1995). Normative values for the Beck Anxiety Inventory, Fear Questionnaire, Penn State Worry Questionnaire, and Social Phobia and Anxiety Inventory. Psychological Assessment, 7(4), 450–455. [Google Scholar]
- Greenbaum DS, Mayle JE, Vanegeren LE, Jerome JA, Mayor JW, Greenbaum RB, et al. (1987). Effects of desipramine on irritable bowel syndrome compared with atropine and placebo. Digestive Diseases and Sciences, 32(3), 257–266. [DOI] [PubMed] [Google Scholar]
- Hamaguchi T, Kano M, Rikimaru H, Kanazawa M, Itoh M, Yanai K, et al. (2004). Brain activity during distention of the descending colon in humans. Neurogastroenterology and Motility, 16(3), 299–309. [DOI] [PubMed] [Google Scholar]
- Heller W, Nitschke JB, Etienne MA, & Miller GA (1997). Patterns of regional brain activity differentiate types of anxiety. Journal of Abnormal Psychology, 106(3), 376–385. [DOI] [PubMed] [Google Scholar]
- Holstege G, Bandler R, & Saper CS (1996). The emotional motor system New York: Elsevier. [DOI] [PubMed] [Google Scholar]
- Houghton LA, Lea R, Jackson N, & Whorwell PJ (2002). The menstrual cycle affects rectal sensitivity in patients with irritable bowel syndrome but not healthy volunteers. Gut, 50(4), 471–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh JC, Belfrage M, Stone-Elander S, Hansson P, & Ingvar M (1995). Central representation of chronic ongoing neuropathic pain studied by positron emission tomography. Pain, 63(2), 225–236. [DOI] [PubMed] [Google Scholar]
- Jones AK, Qi LY, Fujirawa T, Luthra SK, Ashburner J, Bloomfield P, et al. (1991). In vivo distribution of opioid receptors in man in relation to the cortical projections of the medial and lateral pain systems measured with positron emission tomography. Neuroscience Letters, 126(1), 25–28. [DOI] [PubMed] [Google Scholar]
- Kellow JE, & Phillips SF (1987). Altered small bowel motility in irritable bowel syndrome is correlated with symptoms. Gastroenterology, 92, 1885–1893. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Wilson GT, Fairburn CG, & Agras WS (2002). Mediators and moderators of treatment effects in randomized clinical trials. Archives of General Psychiatry, 59(10), 877–883. [DOI] [PubMed] [Google Scholar]
- Kumar D, Thompson PD, Wingate DL, Vesselinova-Jenkins CK, & Libby G (1992). Abnormal REM sleep in the irritable bowel syndrome. Gastroenterology, 103(1), 12–17. [DOI] [PubMed] [Google Scholar]
- Lackner JM (2003). Irritable bowel syndrome. In Collins F, & Cohen L (Eds.), Handbook of health psychology Thousand Oaks: Sage. [Google Scholar]
- Lackner JM, Jaccard J, & Blanchard EB (in press). Testing the sequential model of pain proccessing in irritable bowel syndrome: A structural equation modeling analysis. European Journal of Pain [DOI] [PubMed] [Google Scholar]
- Lackner JM, Morley S, Dowzer C, Mesmer C, & Hamilton S (2004). Psychological treatments for irritable bowel syndrome: A systematic review and meta-analysis. Journal of Consulting and Clinical Psychology, 72(6), 1100–1113. [DOI] [PubMed] [Google Scholar]
- Lackner JM, & Quigley BM (in press). Pain catastrophizing mediates the relationship between worry and pain suffering in patients with chronic abdominal pain. Behaviour Research and Therapy [DOI] [PubMed] [Google Scholar]
- Lackner JM, Quigley BM, & Blanchard EB (2004). Depression and abdominal pain in IBS patients: The mediating role of catastrophizing. Psychosomatic Medicine, 66(3), 435–441. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Axelrod B, Yun LS, Holmes A, & Schwartz GE (1998). Neural correlates of levels of emotional awareness. Evidence of an interaction between emotion and attention in the anterior cingulate cortex. Journal of Cognitive Neuroscience, 10(4), 525–535. [DOI] [PubMed] [Google Scholar]
- Lazarus RS (1984). On the primacy of cognition. American Psychologist, 39, 124–129. [Google Scholar]
- LeDoux JE (1995). Emotion: Clues from the brain. Annual Reviews in Psychology, 46, 209–235. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Jarcho JM, Berman S, Naliboff BD, Suyenobu BY, Mandelkern M, et al. (2004). The neural correlates of placebo effects: A disruption account. Neuroimage, 22(1), 447–455. [DOI] [PubMed] [Google Scholar]
- Matthew E, Andreason P, Carson RE, Herscovitch P, Pettigrew K, Cohen R, et al. (1993). Reproducibility of resting cerebral blood flow measurements with H2(15)O positron emission tomography in humans. Journal of Cerebral Blood Flow and Metabolism, 13(5), 748–754. [DOI] [PubMed] [Google Scholar]
- Mayberg HS (2003). Modulating dysfunctional limbic-cortical circuits in depression: Towards development of brain-based algorithms for diagnosis and optimised treatment. British Medical Bulletin, 65, 193–207. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Berman S, Derbyshire SW, Suyenobu B, Chang L, Fitzgerald L, et al. (2002). The effect of the 5-HT3 receptor antagonist, alosetron, on brain responses to visceral stimulation in irritable bowel syndrome patients. Alimentary Pharmacology and Therapeutics, 16(7), 1357–1366. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Naliboff BD, Chang L, & Coutinho SV (2001). V. Stress and irritable bowel syndrome. American Journal of Physiology-Gastrointestinal and Liver Physiology, 280(4), G519–524. [DOI] [PubMed] [Google Scholar]
- Mertz H, Morgan V, Tanner G, Pickens D, Price R, Shyr Y, et al. (2000). Regional cerebral activation in irritable bowel syndrome and control subjects with painful and non-painful rectal distention. Gastroenterology, 118, 842–848. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, & Borkovec TD (1990). Development and validation of the Penn State Worry Questionnaire. Behaviour Research and Therapy, 28, 487–495. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, et al. (1998). A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain, 121(Part 1), 47–57. [DOI] [PubMed] [Google Scholar]
- Naliboff BD (1999). Hypervigilence. In Evolving pathophysiological models of functional GI disorders New York: Health Education Alliance. [Google Scholar]
- Naliboff BD, Derbyshire SW, Munakata J, Berman S, Mandelkern M, Chang L, et al. (2001). Cerebral activation in patients with irritable bowel syndrome and control subjects during rectosigmoid stimulation. Psychosomatic Medicine, 63(3), 365–375. [DOI] [PubMed] [Google Scholar]
- Naliboff BD, Berman S, Chang L, Derbyshire SW, Suyenobu B, Vogt BA, et al. (2003). Sex-related differences in IBS patients: central processing of visceral stimuli. Gastroenterology, 124(7), 1738–1747. [DOI] [PubMed] [Google Scholar]
- Nichols T, & Hayasaka S (2003). Controlling the familywise error rate in functional neuroimaging: A comparative review. Statistical Methods in Medical Research, 12(5), 419–446. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, & Gabrieli JD (2002). Rethinking feelings: An FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience, 14(8), 1215–1229. [DOI] [PubMed] [Google Scholar]
- Payne A, & Blanchard EB (1995). A controlled comparison of cognitive therapy and self-help support groups in the treatment of irritable bowel syndrome. Journal of Consulting and Clinical Psychology, 63, 779–786. [DOI] [PubMed] [Google Scholar]
- Peterson RA, & Reiss S (1993). Anxiety Sensitivity Index: Revised test manual Worthington, OH: IDS Publishing Corporation. [Google Scholar]
- Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, & Davis M (2001). Activation of the left amygdala to a cognitive representation of fear. Nature Neuroscience, 4(4), 437–441. [DOI] [PubMed] [Google Scholar]
- Pissiota A, Frans O, Michelgard A, Appel L, Langstrom B, Flaten MA, et al. (2003). Amygdala and anterior cingulate cortex activation during affective startle modulation: A PET study of fear. European Journal of Neuroscience, 18(5), 1325–1331. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE, Fox PT, & Raichle ME (1988). Localization of cognitive operations in the human brain. Science, 240(4859), 1627–1631. [DOI] [PubMed] [Google Scholar]
- Posner MI, & Rothbart MK (1998). Attention, self-regulation and consciousness. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences, 353(1377), 1915–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringel Y, Drossman DA, Turkington TG, Bradshaw B, Hawk TC, Bangdiwala S, et al. (2003). Regional brain activation in response to rectal distension in patients with irritable bowel syndrome and the effect of a history of abuse. Digestive Diseases and Sciences, 48(9), 1774–1781. [DOI] [PubMed] [Google Scholar]
- Room P, Russchen FT, Groenewegen HJ, & Lohman AH (1985). Efferent connections of the prelimbic (area 32) and the infralimbic (area 25) cortices: An anterograde tracing study in the cat. Journal of Comparative Neurology, 242(1), 40–55. [DOI] [PubMed] [Google Scholar]
- Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, et al. (1997). A positron emission tomographic study of symptom provocation in PTSD. Annals of the New York Academy of Sciences, 821, 521–523. [DOI] [PubMed] [Google Scholar]
- Silverman D, Munakata J, Ennes H, Mandelkern M, Hoh C, & Mayer E (1997). Regional cerebral activity in normal and pathological perception of visceral pain. Gastroenterology, 112, 64–72. [DOI] [PubMed] [Google Scholar]
- Sykes MA, Blanchard EB, Lackner J, Keefer L, & Krasner S (2003). Psychopathology in irritable bowel syndrome: Support for a psychophysiological model. Journal of Behavioral Medicine, 26(4), 361–372. [DOI] [PubMed] [Google Scholar]
- Talairach J, & Tournoux P (1988). Co-planar stereotactic atlas of the human brain New York: Thieme Medical Publishers. [Google Scholar]
- Teasdale JD, Howard RJ, Cox SG, Ha Y, Brammer MJ, Williams SC, et al. (1999). Functional MRI study of the cognitive generation of affect. American Journal of Psychiatry, 156(2), 209–215. [DOI] [PubMed] [Google Scholar]
- Tillfors M, Furmark T, Marteinsdottir I, Fischer H, Pissiota A, Langstrom B, et al. (2001). Cerebral blood flow in subjects with social phobia during stressful speaking tasks: A PET study. American Journal of Psychiatry, 158(8), 1220–1226. [DOI] [PubMed] [Google Scholar]
- Vase L, Robinson ME, Verne GN, & Price DD (2003). The contributions of suggestion, desire, and expectation to placebo effects in irritable bowel syndrome patients. An empirical investigation. Pain, 105(1–2), 17–25. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Miselis RR, & Pavcovich LA (1999). Pontine regulation of pelvic viscera: pharmacological target for pelvic visceral dysfunctions. Trends in Pharmacological Sciences, 20(6), 253–260. [DOI] [PubMed] [Google Scholar]
- Ware JE, & Sherbourne CD (1992). The MOS 36-item short form health survey (SF-36). Medical Care, 30, 473–483. [PubMed] [Google Scholar]
- Ware JE, Snow KK, & Kosinski M (2000). SF-36 health survey and interpretation guide Lincoln, RI: QualityMetric Incoporated. [Google Scholar]
- Whalen PJ (1998). Fear, vigilance and ambiguity: Initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science, 7(6), 177–188. [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, & Jenike MA (1998). Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience, 18(1), 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead WE, Palsson O, & Jones KR (2002). Systematic review of the comorbidity of irritable bowel syndrome with other disorders: What are the causes and implications? Gastroenterology, 122, 1140–1156. [DOI] [PubMed] [Google Scholar]
- Zinbarg R, Mohlman J, & Hong N (1999). Dimensions of anxiety sensitivity. In Taylor S (Ed.), Anxiety sensitivity: Theory, research, and treatment of the fear of anxiety New York: Erlbaum. [Google Scholar]