Abstract
Objective:
The aim of this study was to test the efficacy of technology-based weight loss interventions for endometrial cancer (EC) survivors with obesity.
Methods:
EC survivors with obesity (n = 196) from three medical centers completed assessments for knowledge of obesity as a risk for EC and interest in weight management. Forty-one women were randomized to a 6-month intervention: telemedicine with Wi-Fi scales, text messaging (texting), or enhanced usual care (EUC). Changes in anthropometrics and psychosocial measures were analyzed.
Results:
One-third of survey participants lacked awareness that obesity increased the risk of EC, and 40% misclassified their body mass. There were no significant differences in weight loss across interventions (mean = −4.4 kg, SD = 6.5 kg). Telemedicine showed improvements in physical health and cancer-related body image (Ps = 0.04) compared to texting and in sexual functioning compared to EUC (P = 0.03). Total physical activity was increased in EUC compared with telemedicine (P = 0.01), and vigorous physical activity was increased in EUC compared with both interventions (P = 0.01–0.03); walking significantly increased in texting compared with telemedicine (P = 0.02).
Conclusions:
Technology-based lifestyle interventions in EC survivors with obesity were accessible and resulted in weight loss and improved quality of life. EUC also produced weight loss, demonstrating a potential for beginning weight management with information on specific diet and exercise goals.
Introduction
With an estimated 61,380 new diagnoses in 2017 (1), endometrial cancer (EC) is the fourth most common women’s cancer in the United States and the cancer most frequently linked to obesity (2). Obesity worsens cancer outcomes; women with a BMI > 40 kg/m2 have a 60% higher risk of dying from any cancer than women with normal weight (3). Obesity-related comorbidities (diabetes, hypertension, and cardiovascular disease) threaten long-term health and quality of life once cancer treatment is completed (4). Women with EC and obesity have a nine-fold higher mortality from all causes than women with EC but not obesity (5). Gynecologic oncologists caring for women through their cancer treatment and survivorship are uniquely positioned to counsel women on obesity’s associated risks and influence their long-term health outcomes. However, most EC survivors do not implement weight management efforts that could decrease these risks (6).
The American Society for Clinical Oncology recently released a call to action for obesity counseling and management by oncology providers (7). Despite promotion of such weight-loss strategies, weight management is not commonly integrated into gynecologic cancer care. Clinical trials have shown that sustained weight loss through exercise and a healthy diet is possible in EC patients (8), but scaling up such efforts is rarely feasible, as in-person requirements can be burdensome for patients and difficult for clinicians to implement practically.
Technology-based communication offered through the internet, text messaging, and telephone offers a potential solution. Previous studies of adults seeking weight loss have suggested that such programs generated losses between 1 and 6 kg with 4 to 6 months of treatment (9–11). One systematic review of eHealth interventions based on the Diabetes Prevention Program (DPP) (12) showed that stand-alone eHealth interventions produced a 3.3% weight loss, while eHealth plus a remote counselor produced a 4.3% loss (13). In a behavioral weight-loss intervention pilot, 20 women with endometrial hyperplasia/cancer and obesity were randomized to either a 6-month telemedicine program using a DPP-based intervention plus Wi-Fi weighing or a texting intervention; women in the telemedicine arm had a median decrease in percent body weight of 7.6%, compared to 4.1% in the texting arm (P = 0.041) (14).
While these findings were promising, the ability to implement such interventions on a larger scale is necessary for greater impact. Through the Transdisciplinary Research in Energetics and Cancer (TREC) initiative at Washington University, University of Pennsylvania, Harvard Medical Center, and the Fred Hutchinson Cancer Research Center (Coordinating Center) (U54 CA155496), we undertook a multicenter, randomized pilot study. The primary objective was to compare weight loss in each of two intervention arms to a minimal, nontechnology intervention. We hypothesized that, as in our pilot study (14), the more intense telemedicine intervention with telephone counseling sessions and Wi-Fi weight monitoring or the text messaging (texting) intervention, including three to five messages per day and reporting of weekly weights, would result in significantly more weight loss compared with enhanced usual care (EUC). Secondary objectives included assessment of knowledge regarding obesity and EC, psychosocial assessments, and demographic characteristics associated with weight in this population.
Methods
Patient population
Three clinical sites participated in the trial: the Perelman School of Medicine at the University of Pennsylvania, Washington University School of Medicine, and the Dana Farber Cancer Institute at Harvard University. Women with a history of EC scheduled for follow-up visits in the gynecologic oncology clinic at each site were identified via electronic medical records. English-speaking women 18 years of age or older with biopsy-proven EC and a BMI ≥ 30 kg/m2 were recruited to participate first in a survey study focusing on this patient population’s knowledge of the link between obesity and cancer, their technology access, and their desire for weight management. Further inclusion criteria for patients interested in the randomized intervention included no concurrent cytotoxic chemotherapy, radiation therapy, or further planned treatment; no evidence of active EC as determined by physician evaluation prior to randomization; Eastern Cooperative Oncology Group performance status 0–1; life expectancy of at least 1 year; and access to either wireless internet or a smartphone.
Exclusion criteria for the intervention included current or recent participation in a weight loss program or use of weight loss medications (history of bariatric surgery was not specifically excluded); uncontrolled serious medical or psychiatric condition(s) that would affect the patient’s ability to participate in the interventional study; invasive malignancy other than EC or nonmelanoma skin cancer that required active treatment currently or within the last 5 years; or current pregnancy.
The Institutional Review Boards of each participating site approved the study, and written informed consent was obtained. Participants were able to keep their scales and, at the University of Pennsylvania, were compensated for travel to the intervention assessments.
Part I: Endometrial Cancer Questionnaire survey study
The Endometrial Cancer Questionnaire (ECQ) was developed by the authors and used to query women regarding their knowledge of the relationship between EC and weight (14). Questions were selected from a bank of previously validated questions provided by the Centers for Disease Control and Prevention, World Health Organization (WHO), Behavioral Risk Factor Surveillance System, and the Harvard Forums of Health Survey regarding health behaviors (14). The ECQ assessed the participants’ perception of their weight status, physical activity, and whether they had access to internet and/or a smartphone with texting capabilities. It queried whether they were interested in participating in a weight management intervention and, if so, what mode and frequency of intervention delivery would they prefer (e.g., in person weekly, telephone weekly, internet-based, or text-based).
Part II: Technology-based intervention
Survey participants who met eligibility for and desired to participate in the intervention trial were randomized 1:1:1 in clinic by random envelope selection by a trained research assistant into the following three arms.
1) Telemedicine weight management plus Wi-Fi scale (telemedicine group).
Participants received a Withings Wi-Fi Scale (www.Withings.com), which transmits information to a website through a smartphone and was accessible by password to the participant and study staff. Participants also received 15- to 20-minute telephone counseling sessions following a behavioral weight loss manual developed and previously piloted (14), which was based on the DPP (12) and Look AHEAD weight loss manuals (15). Phone sessions occurred weekly for 16 weeks, then biweekly during weeks 18 through 24. Doctoral students in clinical psychology and medical students at the University of Pennsylvania served as counselors for this study; they were trained and supervised by KCA. Participants recorded their dietary and physical activity using www.MyFitnessPal.com, sharing their records with study staff, with calorie goals of 1,200 to 1,500 kcal/d if they weighed <250 pounds and 1,500 to 1,800 kcal/d if they weighed >250 pounds at baseline. They also had an exercise goal starting at 50 minutes per week, increasing to 175 minutes per week of moderate physical activity, e.g., brisk walking.
2) Text messaging (texting) group.
Participants received three to five personalized and interactive text messages daily. The content was developed by SanTech, Inc. (Text4Diet) (11), and messages were delivered in the same way to all participants through the Sense Health platform. They received a conventional scale (Eat Smart Precision Digital Scale) and provided their weight as prompted once weekly via text message. Messages were sent that provided feedback, support, prompting, quiz items, and strategies to adhere to behaviors associated with long-term weight management. For example, in a given day, they may receive a physical activity tip, an eating pace multiple-choice question, and a fun fact about nutrition. Participants were encouraged to meet the same calorie and exercise goal as that of the telemedicine cohort and to record all food and beverage intake on paper or through www.MyFitnessPal.com.
3) Enhanced usual care group.
Participants were provided with 1- to 3-page handouts on 14 topics, including healthy eating, exercise, and behavioral eating strategies from materials provided on the American Cancer Society’s website. These materials encouraged weight loss through calorie counting, recording dietary intake, engaging in a walking program, and using portion control strategies. No specific calorie or physical activity goals were prescribed, and these recommendations were not reinforced or monitored by study staff.
Clinical assessment of weight and body composition
For those randomized to the intervention, anthropomorphic measures were taken at baseline assessment and treatment end (6 months), and participants’ medical and reproductive histories were collected. Body weight was measured using a calibrated digital scale. Height was measured using a stadiometer, and waist circumference was measured according to the WHO Physical Measurements Guidelines (16).
Psychosocial assessment
Participants who were randomized to the intervention also completed several validated psychosocial assessment instruments at baseline and 6-month follow-up to measure quality of life metrics. These were administered on paper in a private area within the gynecologic oncology clinics. The assessments evaluated mood with the Patient Health Questionnaire 9-Item (PHQ-9) (17), health-related quality of life with the Quality of Life 12-Item Short Form Health Survey (SF-12) (18), physical activity with the International Physical Activity Questionnaire Short Form (IPAQ) (19), sexual and relationship functioning with the Female Sexual Function Index (FSFI) (20) and the Dyadic Adjustment Scale (DAS) (21), and general and cancer-specific body image utilizing the Multidimensional Body Self Relations Questionnaire–Appearance Subscales (MBSRQ-AS) (22) and the Cancer-Related Body Image Scale (23).
Statistical analysis
Descriptive statistics were used to characterize the sample and describe participants’ responses to the ECQ and other questionnaires and anthropometrics. The primary analysis for the intervention study was assessment of weight loss in each of the two intervention groups as compared to that of the EUC group. Examination of weight loss between the two intervention groups was also conducted. Secondary analyses included examination of changes of other body composition measures and psychosocial scales among the three study arms. For these analyses, variables indicating change between time points were calculated for each measure by subtracting the baseline measurement from the 6-month assessment. Analyses were restricted to participants with measurements at both time points, and due to the small sample size, nonparametric tests were used. Exact Wilcoxon rank sum tests were conducted to assess pair-wise differences between groups. Spearman rank correlation coefficients were computed to quantify associations of weight loss with change in psychosocial measures, in an analysis combining participants from the three arms. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina).
Results
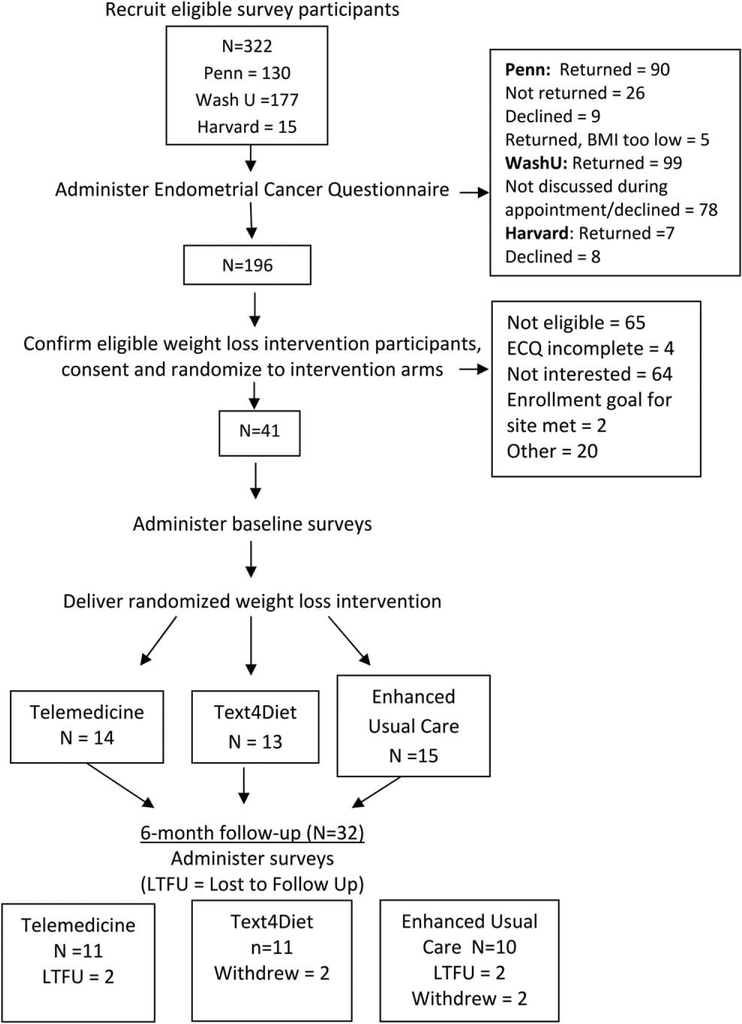
A total of 196 women (Wash U = 99, Penn = 90, Harvard = 7) completed the ECQ. Of those, 41 were eligible (Wash U = 31, Penn = 10), agreed to participate in the intervention, and were randomized 1:1:1 to one of the three arms for the 6-month weight loss intervention; 32 women completed the 6-month final assessment (Figure 1).
Figure 1.
CONSORT diagram and study procedures.
Demographics
For the 196 women completing the ECQ, mean age was 62.2 (SD = 8.7) years old. They were 78% white, 20% black, 2% Latina, and 2% other/declined to answer. From electronic medical record review, they had a mean BMI of 39.1 kg/m2 (range: 30–67 kg/m2). Income strata were spread for those reporting it: 17% at <$25,000, 24% between $25,000 and $50,000, 18% between $50,000 and $75,000, and 29% at ≥$75,000. For the 41 women randomized to the intervention, mean age was 59.7 (SD = 8.7) years, weight at baseline was 109.5 (SD = 21.9) kg, mean height was 164.3 (SD = 7.1) cm, and mean BMI was 40.6 (SD = 7.5) kg/m2. The racial makeup of the sample was 78% white and 22% black. There were no baseline differences among groups.
ECQ outcomes
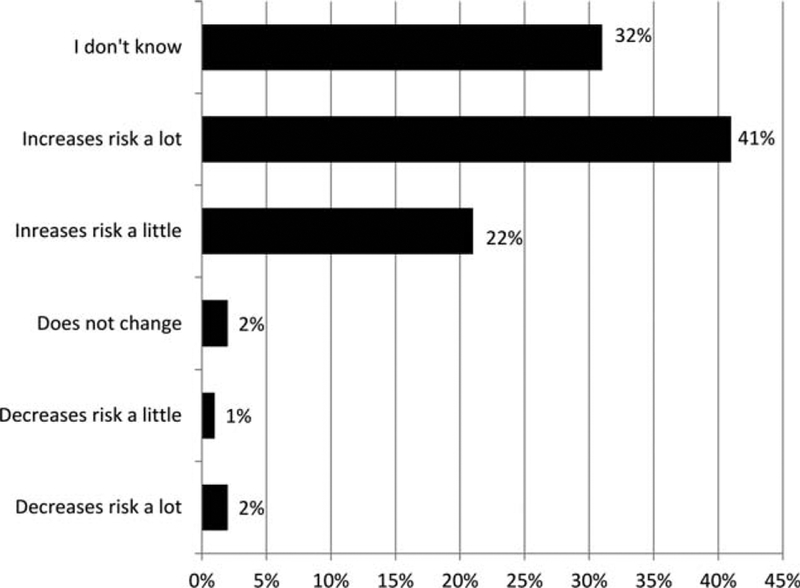
Of the 196 survey participants, 60.8% identified as having obesity, 36.6% identified as having overweight, 1.0% identified as having average weight, and 1.6% identified as having underweight. When asked whether obesity impacted risk of EC, 63% reported that obesity increased risk, 32% did not know, and the remainder believed it did not impact or reduced risk (Figure 2). Access to technology was also assessed, with 66% owning a smartphone; 88% of those with a cell phone had texting capability. Additionally, 80% reported having private wireless internet access. Sixty-eight percent were interested in participating in a weight loss program. Participants ranked their preferred modality for a weight loss intervention in the following order: text messaging, internet-based, telephone sessions, and, lastly, in-person sessions.
Figure 2.
Reported relationship between obesity and endometrial cancer.
Weight and adiposity intervention outcomes
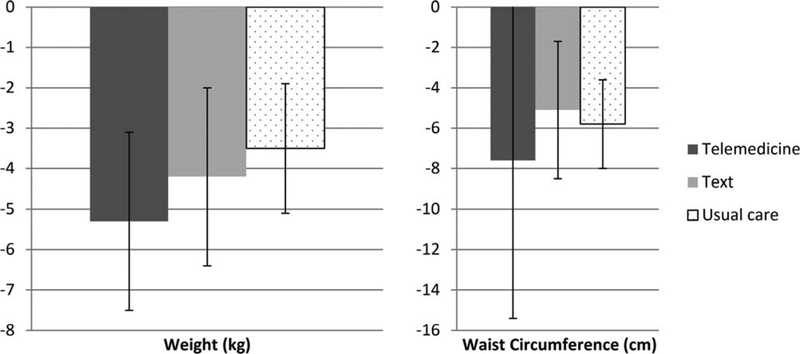
Participants in all three arms lost weight (−4.4 [SD = 6.5] kg; −4% weight change) and reduced their waist circumference (−6.1 [SD = 15.5] cm), with no significant differences between the intervention and the EUC groups (see Table 1 and Figure 3). Weight loss and waist circumference means, respectively, by group were as follows: telemedicine: −5.3 (SD = 7.2) kg, −7.6 (SD = 24.7) cm; texting: −4.2 (SD = 7.3) kg, −5.1 (SD = 11.2) cm; EUC −3.5 (SD = 5.1) kg, −5.8 (SD = 7.1) cm. No serious adverse events were reported.
TABLE 1.
Change (median, interquartile range) across intervention arms from baseline to 6 months
| Telemedicine, n = 11 | Text4Diet, n = 11 | Enhanced usual care, n = 10 | P value | |
|---|---|---|---|---|
| Weight change (kg); % total weight loss | −3.0 (−11.5 to −0.1); −4.6% | −4.4 (−7.9 to 1.1); −3.9% | −1.8 (−5.2 to −0.5); −3.3% | ns |
| Waist circumference change (cm) | −3.7 (−7.6 to 3.0) | −5.9 (−10.5 to 2.6) | −4.0 (−13.2 to 0.5) | ns |
| Physical health component (SF-12) | 5.4 (3.8 to 15.0)a | 0.9 (−0.7 to 4.8)b | 7.4 (1.8 to 11.0) | 0.044 |
| Cancer-related body image | −3.5 (−5.0 to −1.0)a | 0.0 (−1.0 to 0.0)b | −0.5 (1.5 to 0) | 0.035 |
| Female sexual functioning—satisfaction | 0.4 (0.4 to 0.8)a | 0.0 (0.0 to 1.2) | 0.0 (0.0 to 0.0)b | 0.028 |
| Walking activity (METs/wk; IPAQ) | 41.3 (−280.5 to 148.5)a | 430.7 (132.0 to 594.0)b | 24.8 (−198.0 to 429.0) | 0.022 |
| Total PA (METs/wk; IPAQ) | 175.5 (−343.5 to 348.5)a | 588.0 (88.0 to 931.2) | 1,454.5 (619.9 to 2,655.4)b | 0.046 |
| Vigorous PA (METs/wk; IPAQ) | 0 (0.0 to 0.0)a | 0 (0.0 to 480.0)a | 1,120.0 (0.0 to 1,840.0)b | 0.008; 0.034 |
Superscripts a and b denote that these groups differed statistically from each other at the P < 0.05 level using Wilcoxon rank sum tests.
Figure 3.
Weight and waist circumference changes from baseline to 6 months.
Psychosocial assessments
Psychosocial functioning generally improved in all three groups. Participants in the telemedicine arm improved their Physical Health Component Score of the SF-12 and Cancer-Related Body Image score as compared to the texting group (both P = 0.04). The telemedicine arm also showed significant improvement on the FSFI-Satisfaction subscale as compared to the EUC arm (P = 0.03). Walking activity (IPAQ) significantly increased in the texting arm as compared to the telemedicine condition (P = 0.02). The EUC arm significantly increased total physical activity as compared to the telemedicine condition (P = 0.01) and vigorous physical activity as compared to both technology-based conditions (Ps = 0.01–0.03). Of note, IPAQ results varied widely, particularly in the EUC group, and should be interpreted with caution. Relationship functioning (DAS) and mood (PHQ-9) did not differ by group. Psychosocial measures were largely not significantly correlated with weight change, with the exception of a negative relationship between weight change and the MBSRQ Overweight Preoccupation scale (Spearman’s ρ = −0.54, P = 0.002) and moderate physical activity (Spearman’s ρ = 0.44, P = 0.02).
Discussion
Our findings indicated that a technology-based weight loss intervention for EC survivors with obesity was accessible and resulted in significant weight loss and improved quality of life. Overall, the sample lost a similar amount of weight, as previously shown in telemedicine and texting interventions (13), which was encouraging, given the older age of this study’s population compared to the typical age of mid-40s for participants in weight loss interventions. The majority had access to smartphones, and even more to texting. Thus, a technology-based intervention may have appeal to EC survivors who are unable to attend in-person visits.
There is a robust body of research detailing weight loss interventions, both in the general population as well in oncology patients, focusing on diet and/or physical activity in combination with behavioral weight management programs. A recent meta-analysis evaluated eight studies representing 1,022 subjects and determined more successful long-term weight loss was achieved with diet and physical activity combined, including programs that combined behavior modifications (24). This literature in cancer patients has often focused on breast cancer populations (25,26). Previous internet-based interventions have reported weight losses of 3 to 7 kg over 6 to 12 months (27), consistent with our results. A recent systematic review of 16 randomized trials evaluating weight loss in breast cancer patients showed significant weight reductions in most trials (28). This review identified only two previously published randomized trials in endometrial cancer patients (8,14); thus, our data add support for the accessibility and efficacy in achieving weight loss in this particular subset of oncology patients.
Women in all arms of the trial lost weight, but the weight loss in the EUC group was unexpected, as most control groups maintain or gain weight in similarly designed trials (29). However, a recent review (30) identified an ~1 kg weight loss (with several studies reporting 2–3 kg) achieved by subjects enrolled in randomized trials who were assigned to a minimal intervention arm, including the provision of self-help material or a single weight-management session. We feel this finding highlights the importance of the physician-patient relationship and the value of openly discussing (even just briefly) specific behavioral goals related to weight management with our patients, particularly in the context of using a diagnosis of cancer as an impetus for change.
Our survey results indicated that a third of the sample was not knowledgeable about the link between EC and obesity, which is consistent with our previous study (14), raising the question of how to increase awareness of this relationship earlier. While the diagnosis of cancer provides an important teachable moment for obesity management, it should ideally occur earlier for prevention in general women’s health and gynecology clinics before the onset of this largely preventable cancer (31). Further studies should address the barriers and facilitators of weight management discussions in women’s health care prior to a diagnosis of EC, especially as weight is the most important modifiable risk factor for EC and other gynecological cancers.
Previous surveys of gynecologic oncology providers have indicated willingness to engage in weight management facilitation (32). Practically, a busy clinic may not be the ideal setting unless resources are specifically provided for nutrition and weight management support. Presently, these skills are beyond the scope of standard gynecologic oncology training. Digital technology offers a promising opportunity to overcome this, but still requires monitoring by a staff member to provide at least periodic feedback and to monitor for technical difficulties. Thus, even for more minimal interventions, such as texting or telephone-based counseling, funding in the form of out-of-pocket charges for patients or reimbursable billing codes for insurance companies would be required.
Reproductive cancers can have a devastating effect on a woman’s self-concept, appraisal of her body image, and feelings of sexual desire and attractiveness (33), which may be aided in part by weight loss. Some psychosocial domains improved for the sample, including satisfaction with sexual functioning, physical quality of life, and body image, but likely the magnitude of weight change observed was not large enough to produce a stronger effect across more measures.
This pilot study had several limitations, including a small sample, self-reported physical activity, a short intervention period, and follow-up and technical issues. This study was designed to generate estimates for an intervention effect size achievable by the experimental intervention for a full-scale trial of a weight loss program in women with EC. Because hypothesis testing was not a primary goal and multiple tests were conducted on a small sample size, P values and findings should be interpreted with caution.
Recruitment differences among sites likely resulted from different methods of approaching patients. The IRB at Washington University required that physicians introduce the study to potential participants, whereas patients at University of Pennsylvania and Harvard University were approached first by research assistants. At Harvard, the IRB process was longer, and patients there were required to complete general paperwork regarding research, in addition to the usual consenting process, which likely acted as barriers, contributing to their unanticipated low enrollment. Examination of the roles of provider-and practice-specific facilitators (such as physician champions and carefully integrated research efforts in the clinic) and barriers to implementation (e.g., brief funding period of the trial) were not planned outcomes of this study. Our experience indicates that stakeholder buy-in is critical for implementation.
Given the scope and the budget of this study, the intervention period was relatively brief at 6 months, without a longer-term follow-up. Furthermore, we did not collect information on eligible patients who chose not to enroll. We also were not able to provide patients with actigraphy or other means of monitoring their physical activity objectively.
We encountered several technical issues during the study, including difficulty with accessing Wi-Fi for some of the participants, particularly those in rural areas (about 20% of the sample). In these instances, the women reported the weight on their scales to the interventionist, or took a picture and texted it to the interventionist. We also had issues with delivery of text messages through our platform twice, resulting in no message delivery for a week for some participants. Study staff was in contact with participants during this time. However, these missed messages were a very low proportion (2 weeks out of 24 weeks) of the total messages that participants received.
While this pilot study was successful in achieving weight loss utilizing novel technology in EC survivors with obesity, further research is needed. Tailored efforts to increase awareness of the link between obesity and EC, enrollment in future trials—including for women of minority populations and with lower socioeconomic status—and acceptable weight management interventions that can be utilized in a busy clinical practice will all be necessary to address the impact of obesity on survival and quality of life in EC survivors.
Conclusion
Weight loss was feasible in an older population of EC survivors using technology-based weight loss interventions. Unexpectedly, modest weight loss also occurred in the control group with only the attention of the study coupled with written information, perhaps due to increased motivation for lifestyle change during a teachable moment, such as recovery from cancer. However, knowledge of the link between excess weight and EC was still low for a population of survivors. Future larger-scale studies should focus on this knowledge gap and develop implementation strategies to ensure that all eligible patients are reached, with tailored approaches realistic for a busy gynecologic oncology clinic. This also speaks to a larger systematic need for funding, reimbursement of counseling time, and policies that stress the importance of weight management in cancer care. As EC incidence continues rising, mostly related to increasing rates of obesity, we should find more ways to remove barriers to effective behavioral interventions.
Acknowledgments
We would like to thank our participants for their time and effort in this trial. We would also like to thank the enormous efforts of our study coordinators and staff. At Penn, we would like to thank Dr. Kathryn Schmitz (TREC site PI), Christina Hopkins, and Sarah Huepenbecker. We would also like to thank our interventionists, Sarah Huepenbecker, Emily Feig, Stephanie Manasse, Shannon Patterson, Brandon Tong, and Daisy Vélez. At WashU, we would like to thank Lynne Lippman, Chrisann Winslow, Krystal Martin, and Lakesha Bowen. At Harvard, we would like to thank Dr. Frank Hu (Harvard TREC site PI), Dr. Colleen Feltmate, Taylor Ahlborn, and Christina Whalen. At the coordinating center (Fred Hutchinson Cancer Research Center), we would like to thank Diana Lowry, Suzanna Reid, Jeff Pittman, Kiarra Witcher, and Lori Schumacher.
Funding agencies: Funding for this study was provided by the Transdisciplinary Research on Energetics and Cancer (TREC) initiative through the following grants: NCI U54-CA155850, University of Pennsylvania; U54 CA155626, Harvard University; U54 CA155496CC, Washington University; U01 CA116850, Fred Hutchinson Cancer Research Center.
Footnotes
Disclosure: The authors declared no conflict of interest.
Clinical trial registration: ClinicalTrials.gov identifier .
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Jiang L, Stefanick ML, et al. Duration of adulthood overweight, obesity, and cancer risk in the Women’s Health Initiative: a longitudinal study from the United States. PLoS Med 2016;13:e1002081. doi: 10.1371/journal.pmed.1002081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flegal KM, Graubard BI. Estimates of excess deaths associated with body mass index and other anthropometric variables. Am J Clin Nutr 2009;89:1213–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fader AN, Frasure HE, Gil KM, Berger NA, von Gruenigen VE. Quality of life in endometrial cancer survivors: what does obesity have to do with it? Obstet Gynecol Int 2011;2011:308609. doi: 10.1155/2011/308609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. NEJM 2003; 348:1625–1638. [DOI] [PubMed] [Google Scholar]
- 6.Laskey RA, McCarroll ML, von Gruenigen VE. Obesity-related endometrial cancer: an update on survivorship approaches to reducing cardiovascular death. BJOG 2016;123:293–298. [DOI] [PubMed] [Google Scholar]
- 7.Ligibel JA, Alfano CM, Courneya KS, et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clinl Onco 2014;32:3568–3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Gruenigen V, Frasure H, Kavanagh MB, et al. Survivors of uterine cancer empowered by exercise and healthy diet (SUCCEED): a randomized controlled trial. Gynecol Oncol 2012;125:699–704. [DOI] [PubMed] [Google Scholar]
- 9.Steinberg DM, Levine EL, Askew S, Foley P, Bennett GG. Daily text messaging for weight control among racial and ethnic minority women: randomized controlled pilot study. J Med Internet Res 2013;15:e244. doi: 10.2196/jmir.2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harvey-Berino J, West D, Krukowski R et al. Internet delivered behavioral obesity treatment. Prev Med 2010;51:123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patrick K, Raab F, Adams MA, Dillon L, Zabinski M, Rock CL, Griswold WG, Norman GJ. A text message-based intervention for weight loss: randomized controlled trial. J Med Internet Res 2009;11:e1. doi: 10.2196/jmir.1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diabetes Prevention Program Research Group. The Diabetes Prevention Program: baseline characteristics of the randomized cohort. Diabetes Care 2000;23:1619–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joiner KL, Nam S, Whittemore R. Lifestyle interventions based on the Diabetes Prevention Program delivered via eHealth: a systematic review and meta-analysis. Prev Med 2017;100:194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haggerty AF, Huepenbecker S, Sarwer DB, et al. The use of novel technology-based weight loss interventions for obese women with endometrial hyperplasia and cancer. Gynecol Oncol 2016;140:239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan DH, Espeland MA, Foster GD, et al. ; Look AHEAD Research Group. The Look AHEAD Research Group (2003) Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 2003:24;610–628 [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Waist Circumference and Waist–Hip Ratio: Report of a WHO Expert Consultation, Geneva, 8–11 December 2008. Geneva: World Health Organization; 2011. http://apps.who.int/iris/bitstream/10665/44583/1/9789241501491_eng.pdf [Google Scholar]
- 17.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ware J Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 19.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exer 2003;35: 1381–1395. [DOI] [PubMed] [Google Scholar]
- 20.Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther 2000;26:191–208. [DOI] [PubMed] [Google Scholar]
- 21.Spanier G, Thompson LA. Confirmatory analysis of the dyadic adjustment scale. J Marriage Fam 1982;44:731–738. [Google Scholar]
- 22.Cash TF. User’s Manual for the Multidimensional Body-Self Relations Questionnaire. Norfolk, VA: Old Dominion University; 2000. www.body-images.com. [Google Scholar]
- 23.Hopwood P, Fletcher I, Lee A, Al Ghazal S. A body image scale for use with cancer patients. Eur J Cancer 2001;37:189–197. [DOI] [PubMed] [Google Scholar]
- 24.Johns DJ, Hartmann-Boyce J, Jebb SA, et al. Diet or exercise interventions vs combined behavioral weight management programs: A systematic review and meta-analysis of direct comparisons. J Acad Nutr Diet 2014;114:1557–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stolley M, Sheean P, Gerber B et al. Efficacy of a weight loss intervention for African American breast cancer survivors. J Clin Oncol 2017;35:2820–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lynch SM, Stricker CT, Brown JC, et al. Evaluation of a web-based weight loss intervention in overweight cancer survivors aged 50 years and younger. Obes Sci Pract 2017;3:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcus B, Lewis BA, Williams DM et al. A comparison of Internet and print-based physical activity interventions. Arch Intern Med 2007;167:944–949. [DOI] [PubMed] [Google Scholar]
- 28.Chlebowski RT and Reeves MM. Weight loss randomized intervention trials in female cancer survivors. J Clin Oncol 2016:34:4238–4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wadden TA, Volger S, Tsai AG, et al. Managing obesity in primary care practice: an overview with perspective from the POWER-UP study. Int J Obes 2013;37 (suppl 1):S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johns DJ, Hartmann-Boyce J, Jebb SA et al. Weight change among people randomized to minimal intervention control groups in weight loss trials. Obesity 2016;24:772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarwer DB, Allison KC, Gibbons LM, Markowitz JT, Nelson DB. Pregnancy and obesity: a review and agenda for future research. J Womens Health 2006;15:720–733. [DOI] [PubMed] [Google Scholar]
- 32.Neff R, McCann GA, Carpenter KM, et al. Is bariatric surgery an option for women with gynecologic cancer? Examining weight loss counseling practices and training among gynecologic oncology providers. Gynecol Oncol 2013;134:540–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mc-Cuen-Wurst C, Culnan E, Stewart N, Allison KC. Weight and eating concerns in women’s repoductive health. Curr Psychiatry Rep 2017;19:68. doi: 10.1007/s11920-017-0828-0 [DOI] [PubMed] [Google Scholar]