Abstract
Objective.
To determine whether phenotypic differences exist among individuals with Prader-Willi syndrome with either type I or type II deletions of chromosome 15 or maternal disomy 15 leading to a better understanding of cause and pathophysiology of this classical genetic syndrome.
Methods.
We analyzed clinical, anthropometric, and behavioral data in 12 individuals (5 men, 7 women; mean age: 25.9 ± 8.8 years) with PWS and a type I (TI) deletion, 14 individuals (6 men, 8 women; mean age: 19.6 ± 6.5 years) with PWS and a type II (TII) deletion, and 21 individuals (10 men, 11 women; mean age: 23.6 ± 9.2 years) with PWS and maternal disomy 15 (UPD). The deletion type was determined by genotyping of DNA markers between proximal chromosome 15 breakpoints BP1 and BP2. TI deletions are ~500 kb larger than TII deletions. Several validated psychological and behavioral tests were used to assess phenotypic characteristics of individuals with PWS representing the 3 genetic subtypes.
Results.
Significant differences were found between the 2 deletion groups and those with UPD in multiple psychological and behavorial tests, but no differences were observed in other clinical or anthropometric data studied. Adaptive behavior scores were generally worse in individuals with PWS and the TI deletion, and specific obsessive-compulsive behaviors were more evident in the TI individuals compared with those with UPD. Individuals with PWS with TI deletions also had poorer reading and math skills as well as visual-motor integration.
Conclusions.
Our study indicates that individuals with TI deletion generally have more behavioral and psychological problems than individuals with the TII deletion or UPD. Four recently identified genes have been identified in the chromosome region between BP1 and BP2 with 1 of the genes (NIPA-1) expressed in mouse brain tissue but not thought to be imprinted. It may be important for brain development or function. These genes are deleted in individuals with TI deletion and are implicated in compulsive behavior and lower intellectual ability in individuals with TI versus TII.
Prader-Willi syndrome (PWS) is a genetic disorder that results from the absence of normally active paternally expressed genes from the 15q11-q13 chromosome region.1–3 A number of genes located in the 15q11-q13 region have been shown to be imprinted (active on only 1 member of the chromosome pair), and expression is dependent on the parent of origin of the chromosome 15. The major characteristics of this syndrome include infantile hypotonia with feeding problems, global developmental delay and mental deficiency, behavior problems, small hands and feet, hypogonadism and hyperphagia leading to marked obesity in early childhood, and a characteristic face.1,2,4 The majority of individuals, ~70%, have a paternally derived interstitial deletion of 15q11-q13, ~25% have maternal disomy 15 (UPD), and the remaining 2% to 5% of individuals have imprinting defects.2,3
PWS and Angelman syndrome (AS), an entirely different clinical disorder, were the first examples in humans of genomic imprinting or the differential expression of genetic material depending on the parent of origin. The majority of AS patients have a maternally derived interstitial deletion of 15q11-q13. At least 1 dozen imprinted genes have been identified in the 15q11-q13 region, and the majority are paternally expressed (maternally imprinted or inactivated). The paternally expressed genes are candidates for PWS, whereas a single maternally expressed gene (UBE3A) is thought to cause AS.
The typical deletion responsible for PWS encompasses most of the 15q11-q13 region; however, recent studies have shown that the proximal deletion breakpoint may occur at 1 of 2 sites within either of 2 large duplicons centromeric to locus ZNF127.3 The precise location of the breakpoints within the duplicons may vary,5 but the breakpoints seem to be confined to a relatively small region of chromosome 15, which allows for the identification of 2 classes of deletion subjects. Breakpoint 1 (BP1) is proximal to D15S541/S1035 loci, and BP2 lies between loci D15S541/S1035 and D15S543.6 The type I (TI) deletion involves BP1, which is close to the centromere, while the type II (TII) deletion involves breakpoint BP2 and is located ~500 kb distal to BP1. Therefore, the TI deletion results in the loss of ~500 kb of genetic material in addition to what is missing in the TII deletion. Recently, Chai et al7 reported 4 newly identified genes in the region between BP1 and BP2. BP3, located between loci D15S156 and D15S165, is the distal breakpoint in the 15q11-q13 region and is observed in both deletion subgroups.
Analyses of the genetic subtypes of PWS to date have compared deletion individuals and UPD individuals without grouping the deletion individuals into TI or TII. For example, hypopigmentation and homogeneous clinical presentations including dermatoglyphic patterns were more often seen in individuals with PWS and a deletion compared with those with normal chromosomes now recognized as having UPD.1,8 In addition, we reported significantly higher verbal intelligence quotient (IQ) scores in PWS individuals with UPD compared with individuals with deletion.9 PWS individuals from the UPD subgroup scored significantly higher than the deletion subgroup in 4 subcategories of verbal testing: information, arithmetic, vocabulary, and comprehension. Similarly, Dykens and others4,10–13 also reported behavioral and cognitive differences in PWS individuals with the UPD individuals having fewer maladaptive behaviors measured by the Child Behavior Checklist’s (CBC’s) internalizing, externalizing, and total domain scores and more symptom-related distress noted using the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS).
We further reported more self-injurious behavior in deletion individuals with PWS compared with those with UPD.14 Conversely, visual processing of complex stimuli was significantly poorer in individuals with UPD compared with those with the deletion.15 Gunay-Aygun et al16 also reported a decrease in severity of some of the minor behavioral characteristics associated with PWS, particularly in individuals with UPD compared with individuals with deletions. Thus, analyses of individuals with PWS to date have included cognitive, psychological, behavioral, physiologic, and biochemical data and grouped into deletion or UPD subgroup categories. Herein, we report the first clinical study of individuals with PWS grouped by deletion size (TI vs TII) and compare their findings with individuals with UPD.
METHODS
Individuals with PWS and their parents were recruited as part of a larger study on genotype/phenotype relationships. All individuals agreed to informed consent approved by the institutional review board before entry into the study. All individuals with PWS were diagnosed by a clinical geneticist (M.G.B.) and chromosome studies performed with fluorescent in situ hybridization showing a deletion of the 15q11-q13 region. In addition, DNA methylation and microsatellite analysis with 15q11-q13 probes were used following established protocols to confirm the deletion or UPD status.17–20 The UPD status was confirmed by informative chromosome 15 microsatellite studies using DNA isolated from the parents and the patient. No individuals with imprinting defects were included in this study.
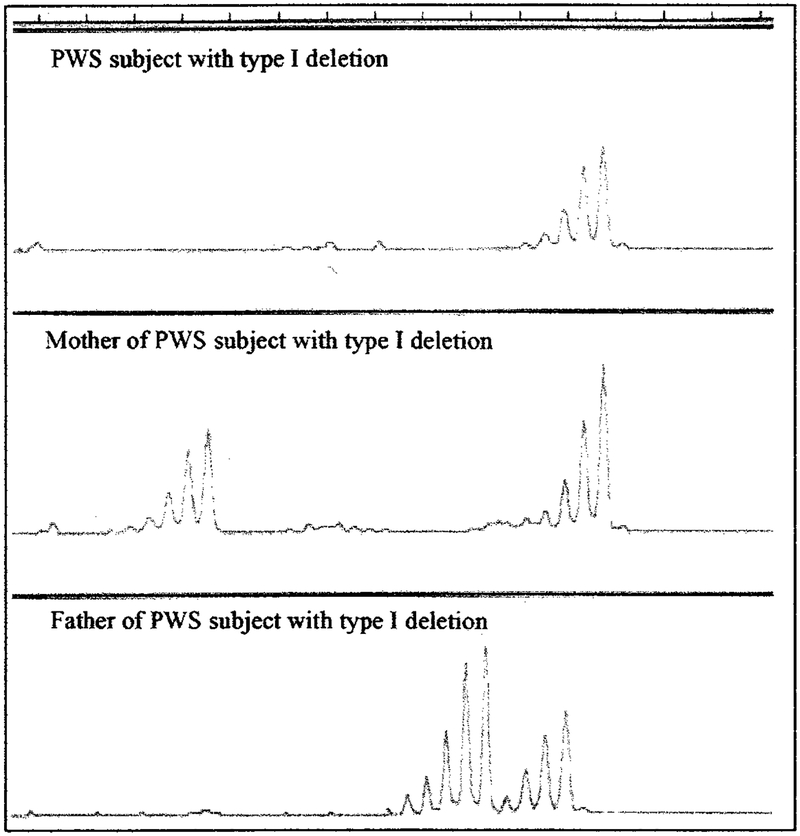
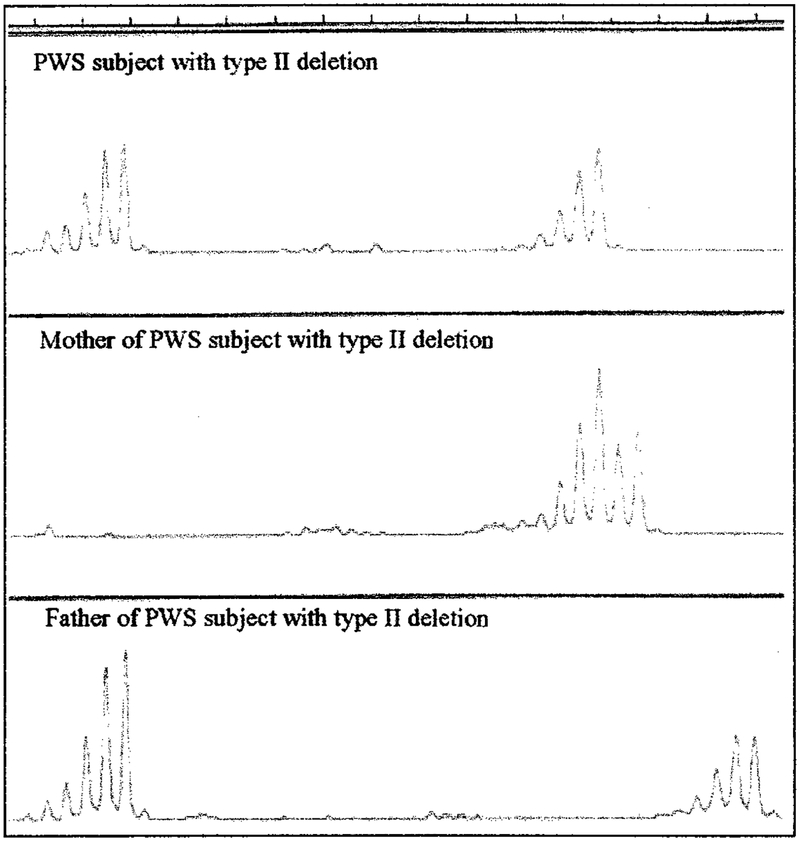
Individuals with deletions were further subdivided into TI or TII using microsatellite markers around and between BP1 and BP2. Additional molecular genetic testing confirmed the location of the distal breakpoint at BP3. The absence of the paternal D15S541/S1035 loci was classified as having the TI deletion and identified by routine microsatellite analysis using an ABI 310 automated capillary sequencer with PWS subject and parental DNA isolated from peripheral blood (Fig 1). The TII deletion was classified as having the presence of these loci, which are located between BP1 and BP2 (Fig 2). The deletion subtype status was confirmed with quantitative polymerase chain reaction using established protocols6,20 in individuals uninformative at these loci (data not shown).
Fig 1.
Microsatellite pattern for D15S1035 locus from an individual with PWS and parental DNA isolated from peripheral blood using an ABI 310 automated capillary sequencer. Only 1 DNA signal pattern is seen in the individual with PWS and inherited from the mother, whereas no DNA signal was observed from the father, indicating a paternal TI deletion in the individual with PWS.
Fig 2.
Microsatellite pattern for D15S1035 locus from an individual with PWS and parental DNA isolated from peripheral blood using an ABI 310 automated capillary sequencer. Two DNA signal patterns are seen from the individual with PWS, indicating inheritance of a D15S1035 allele from each parent. Genetic testing showed a deletion of the 15q11-q13 region in the individual with PWS but not for the D15S1035 locus, indicating a TII deletion.
We extensively analyzed clinical, anthropometric, physiologic, metabolic, cognitive, and behavioral data from a large clinical data set produced during a 5-year program project on PWS and obese comparison subjects. Twelve individuals with PWS and a TI deletion (5 men, 7 women; mean age: 25.9 ± 8.8 years), 14 individuals with PWS and a TII deletion (6 men, 8 women; mean age: 19.6 ± 6.9 years), and 21 individuals with PWS and UPD (10 men, 11 women; mean age: 23.6 ± 9.2 years) were analyzed for our study. No differences were found in the clinical or anthropometric data among the subjects with the deletion type or UPD, although differences were identified in behavior, academic, and intelligence parameters discussed below.
Several validated psychological and behavioral scales were used to assess phenotypic characteristics of individuals with PWS. The Y-BOCS21 was used in our study and is the most widely used standardized scale for measuring obsessions and compulsions in psychiatric patients. A caregiver report form of the scale (used here) has been used with individuals with PWS and found to be sensitive to compulsivity.22,23 Because it is difficult to assess obsessions among people with limited verbal capacity, we also used the Compulsive Behavior Checklist24 designed for people with intellectual disabilities, focusing on compulsive behavior rather than obsessions.
The Reiss Screen for Maladaptive Behavior25 is a caregiver report instrument for people older than 12 years that assesses psychiatric symptoms of people with developmental disabilities. The Scales of Independent Behavior26 is designed to assess both adaptive and maladaptive behavior of individuals with cognitive disabilities and is used most widely with individuals with moderate to severe intellectual disabilities. The Wechsler Intelligence Scale was used to evaluate intellectual ability.27,28 The Visual Motor Integrations Scale29 is a measure of visual-motor integration (VMI) and has been shown to detect the ability to coordinate motor responses with specific visual demands. Academic skills were assessed using the Woodcock Johnson Psycho-Educational Battery–Revised.30 Statistical analyses used throughout the study included mean and standard deviation, t test, and analysis of variance.
RESULTS
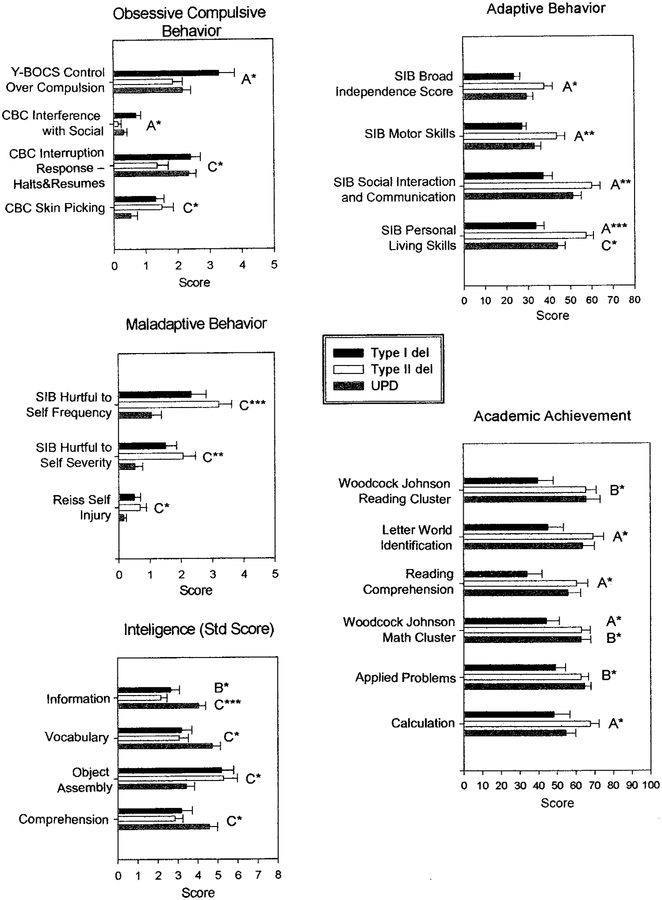
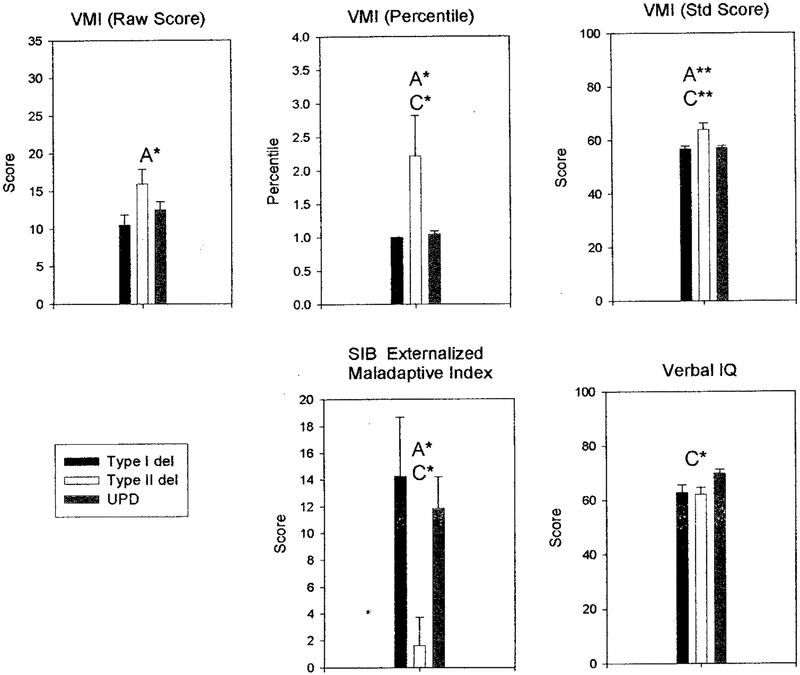
Parameters classified into specific groups (mal-adaptive behavior, adaptive behavior, obsessive compulsive behavior, visual processing, academic achievement, and intelligence) were found to show significant differences after analyzing a large data set collected over a 5-year study on the genotype/phenotype relationships in individuals with PWS. Three PWS subject groups (TI, TII, and UPD) were analyzed in our study (see Tables 1–3). Significant differences in maladaptive behavior assessment parameters were found in the 3 PWS subject groups for self-injurious behavior (SIB) externalized and internalized maladaptive index; SIB hurtful to self frequency and severity, and Reiss self-injury. However, no significant correlations with age were found with these variables in the 3 PWS subject groups. Significant differences in adaptive behavior assessment parameters were found among the 3 groups for SIB broad independence score: SIB motor skills, SIB social interaction and communication, and SIB personal living skills. Significant differences were also observed in measurements of behavioral difficulties related to functional living skills. These differences seemed to manifest in reduced independence scores for TI deletions compared with either TII or UPD. Generally, psychological, behavior, and academic achievement scores in individuals with PWS and TI deletions were significantly worse than in individuals with TII or UPD. Maladaptive difficulties were coupled with a reduction in independent behaviors, suggesting a requirement for closer supervision. Figures 3 and 4 show histograms of the behavior, visual processing, academic achievements, and cognitive data showing significant differences among our TI, TII, and UPD individuals.
TABLE 1.
Analysis of Behavior Data in Individuals With PWS and TI or TII Deletions or UPD
| Parameters | Test | Group | Mean | SD | N | P Value | |
|---|---|---|---|---|---|---|---|
| vs TII Deletion | vs UPD | ||||||
| Maladaptive behavior | SIB externalized maladaptive index | TI deletion | −14.25 | 15.28 | 12 | .019 | .830 |
| TII deletion | −1.64 | 7.86 | 14 | - | .033 | ||
| UPD | −11.88 | 10.73 | 21 | - | |||
| SIB hurtful to self frequency* | TI deletion | 2.33 | 1.67 | 12 | .328 | .068 | |
| TII deletion | 3.21 | 1.53 | 14 | - | .001 | ||
| UPD | 1.05 | 1.50 | 21 | - | |||
| SIB hurtful to self severity* | TI deletion | 1.50 | 1.24 | 12 | .494 | .097 | |
| TII deletion | 2.07 | 1.49 | 14 | - | .003 | ||
| UPD | 0.52 | 1.12 | 21 | - | |||
| Reiss self-injury* | TI deletion | 0.50 | 0.67 | 12 | .734 | .285 | |
| TII deletion | 0.68 | 0.77 | 14 | - | .048 | ||
| UPD | 0.16 | 0.37 | 19 | - | |||
| Adaptive behavior | SIB broad independence score | TI deletion | 23.75 | 10.06 | 12 | .027 | .452 |
| TII deletion | 37.86 | 15.07 | 14 | - | .186 | ||
| UPD | 29.62 | 13.78 | 21 | - | |||
| SIB motor skills | TI deletion | 27.42 | 6.88 | 12 | .007 | .445 | |
| TII deletion | 43.71 | 14.24 | 14 | - | .056 | ||
| UPD | 33.14 | 14.45 | 21 | - | |||
| SIB social interaction and communication | TI deletion | 37.25 | 15.01 | 12 | .002 | .057 | |
| TII deletion | 60.14 | 14.40 | 14 | - | .264 | ||
| UPD | 51.24 | 18.06 | 21 | - | |||
| SIB personal living skills | TI deletion | 33.67 | 13.29 | 12 | .001 | .154 | |
| TII deletion | 57.36 | 12.77 | 14 | - | .031 | ||
| UPD | 43.86 | 16.90 | 21 | - | |||
| Obsessive-compulsive behavior | Y-BOCS control over compulsion* | TI deletion | 3.30 | 1.57 | 10 | .021 | .061 |
| TII deletion | 1.86 | 1.17 | 14 | - | .774 | ||
| UPD | 2.16 | 1.12 | 19 | - | |||
| CBC significant interference with social* | TI deletion | 0.70 | 0.48 | 10 | .012 | .082 | |
| TII deletion | 0.14 | 0.36 | 14 | - | .518 | ||
| UPD | 0.32 | 0.48 | 19 | - | |||
| CBC interruption response-halts and resumes* | TI deletion | 2.40 | 0.97 | 10 | .071 | .987 | |
| TII deletion | 1.36 | 1.28 | 14 | - | .045 | ||
| UPD | 2.33 | 1.03 | 18 | - | |||
| CBC skin picking* | TI deletion | 1.30 | 0.82 | 10 | .891 | .157 | |
| TII deletion | 1.50 | 1.34 | 14 | - | .032 | ||
| UPD | 0.53 | 0.90 | 19 | - | |||
SD indicates standard deviation.
Categorical variables analyzed by analysis of variance for descriptive purposes.
TABLE 3.
Behavior, Visual Processing, Academic Achievement, and Intelligence Parameters Found to be Significantly Different Among the Three PWS Subject Groups (TI deletion, TII deletion, or UPD) With the Two Subject Groups Listed Having Significant Differences
| Parameter | Group |
|---|---|
| Maladaptive behavior | |
| SIB externalized maladaptive index | TI deletion vs TII deletion* |
| TII deletion vs UPD* | |
| SIB hurtful to self frequency | TII deletion vs UPD‡ |
| SIB hurtful to self severity | TII deletion vs UPD† |
| Reiss self-injury | TII deletion vs UPD* |
| Adaptive behavior | |
| SIB broad independence score | TI deletion vs TII deletion* |
| SIB motor skills | TI deletion vs TII deletion† |
| SIB social interaction and communication | TI deletion vs TII deletion† |
| SIB personal living skills | TI deletion vs TII deletion‡ |
| TII deletion vs UPD* | |
| Obsessive-compulsive disorder | |
| Y-BOCS control over compulsion | TI deletion vs TII deletion* |
| CBC interference with social | TI deletion vs TII deletion* |
| CBC interruption response-halts and resumes | TII deletion vs UPD* |
| CBC skin picking | TII deletion vs UPD* |
| Visual processing | |
| Vineland Motor Inventory (raw score) | TI deletion vs TII deletion* |
| Vineland Motor Inventory (percentile) | TI deletion vs TII deletion* |
| TII deletion vs UPD* | |
| Vineland Motor Inventory (standard score) | TI deletion vs TII deletion† |
| TII deletion vs UPD† | |
| Academic achievement | |
| Woodcock-Johnson reading cluster | TI deletion vs UPD* |
| Letter-word identification | TI deletion vs TII deletion* |
| Reading comprehension | TI deletion vs TII deletion* |
| Woodcock-Johnson math cluster | TI deletion vs TII deletion* |
| TI deletion vs UPD* | |
| Applied problems | TI deletion vs UPD* |
| Calculation | TI deletion vs TII deletion* |
| Intelligence | |
| Verbal IQ | TII deletion vs UPD* |
| Information (standard score) | TI deletion vs UPD* |
| TII deletion vs UPD‡ | |
| Vocabulary (standard score) | TII deletion vs UPD* |
| Object assembly (standard score) | TII deletion vs UPD* |
| Comprehension (standard score) | TII deletion vs UPD* |
P < .05.
P < .01.
P ≤ .001.
Fig 3.
Histograms of maladaptive behavior, adaptive behavior, obsessive-compulsive behavior, academic achievement, and intelligence (standard score) data showing significant differences among the 3 PWS genetic subtypes (TI deletion, TII deletion, and UPD). A, TI deletion versus TII deletion; B, TI deletion versus UPD; C, TII deletion versus UPD; *P < .05; **P < .01; ***P ≤ .001.
Fig 4.
Histograms of visual processing (Vineland Motor Inventory), SIB externalized maladaptive index, and verbal IQ data showing significant differences among the 3 PWS genetic subtypes (TI deletion, TII deletion, and UPD). A, TI deletion versus TII deletion; B, TI deletion versus UPD; C, TII deletion versus UPD; *P < .05; **P < .01; ***P ≤ .001.
Significant obsessive-compulsive behavior measures were found for Y-BOCS control over compulsion, Y-BOCS resistance to compulsion, CBC significant interference with social, Y-BOCS repeating compulsion (rereading and erasing), Y-BOCS washing compulsion (bathing/toilet), and CBC clean/tidy compulsion (data not shown). Although there were a variety of compulsive measurements, only a subset was significant (see Table 1). Generally, the TI deletion group had greater difficulty controlling compulsions. This seemed to be confirmed by several measurements related to the control or resistance to compulsive behavior. The difficulties associated with compulsions seem to present with greater problems for routine daily activities found in individuals with TI versus TII and UPD. The measurements of repetitive behaviors indicate that TI individuals scored more poorly.
Significant differences were also found in visual processing scores among the 3 subject groups based on VMI assessments for VMI (raw score), VMI (percentile), and VMI (standing score). Significant differences were found in academic achievement scores among the 3 groups using the Woodcock-Johnson Psycho-Educational Battery, which included Woodcock-Johnson reading cluster, reading comprehension, letter-word identification, Woodcock-Johnson math cluster, applied problems, and calculation (see Table 2). Significant academic achievement measurements were the most strikingly different for TI deletions with poorer performances compared with individuals with TII or UPD. For example, in Woodcock-Johnson math cluster, individuals with TI performed more poorly than in individuals with either TII or UPD. These scores represented a convergent set of intellectual assessments that all suggested a reduction in scholastic aptitude by individuals with TI deletions compared with the other genetic subtypes. However, significant differences were observed in intelligence scores generated using the Wechsler Intelligence Scale, particularly with lower scores generated for both deletion types (TI and TII) compared with individuals with UPD. For these scores, observed significant differences were found between individuals with TII and UPD. Unlike the other intelligence scores, the object assembly scores were higher in individuals with TI and TII compared with individuals with UPD, in agreement with previous reports on deletion and UPD comparisons.
TABLE 2.
Analysis of Visual Processing, Academic Achievement, and Intelligence Data in Individuals With PWS and TI or TII Deletions or UPD
| Parameters | Test | Group | Mean | SD | N | P Value | |
|---|---|---|---|---|---|---|---|
| Vs TII Deletion | Vs UPD | ||||||
| Visual processing | Vineland Motor Inventory (raw score) | TI deletion | 10.50 | 4.66 | 12 | .045 | .579 |
| TII deletion | 15.93 | 7.25 | 14 | - | .191 | ||
| UPD | 12.52 | 4.72 | 21 | - | |||
| Vineland Motor Inventory (percentile) | TI deletion | 1.00 | 0.00 | 12 | .043 | .994 | |
| TII deletion | 2.21 | 2.26 | 14 | - | .024 | ||
| UPD | 1.05 | 0.22 | 21 | - | |||
| Vineland Motor Inventory (standard score) | TI deletion | 58.92 | 3.45 | 12 | .007 | .993 | |
| TII deletion | 64.08 | 8.69 | 13 | - | .003 | ||
| UPD | 57.14 | 3.88 | 21 | - | |||
| Academic achievement | Woodcock-Johnson reading cluster* | TI deletion | 43.27 | 27.43 | 11 | .113 | .040 |
| TII deletion | 65.57 | 20.99 | 14 | - | .927 | ||
| UPD | 69.11 | 30.42 | 19 | - | |||
| Letter-word identification* | TI deletion | 45.00 | 29.41 | 12 | .023 | .085 | |
| TII deletion | 69.29 | 21.37 | 14 | .543 | |||
| UPD | 63.75 | 28.50 | 20 | ||||
| Reading comprehension | TI deletion | 33.75 | 28.11 | 12 | .048 | .087 | |
| TII deletion | 60.50 | 22.85 | 14 | - | .881 | ||
| UPD | 55.85 | 30.64 | 20 | - | |||
| Woodcock-Johnson math cluster* | TI deletion | 44.08 | 24.51 | 12 | .031 | .038 | |
| TII deletion | 63.07 | 17.52 | 14 | - | .982 | ||
| UPD | 62.90 | 23.31 | 20 | - | |||
| Applied problems | TI deletion | 49.08 | 18.59 | 12 | .087 | .031 | |
| TII deletion | 62.93 | 14.65 | 14 | - | .950 | ||
| UPD | 64.65 | 15.69 | 20 | - | |||
| Calculation* | TI deletion | 48.17 | 29.60 | 12 | .047 | .508 | |
| TII deletion | 67.71 | 17.37 | 14 | - | .087 | ||
| UPD | 54.58 | 23.47 | 19 | - | |||
| Intelligence | Verbal IQ | TI deletion | 62.91 | 9.34 | 11 | .975 | .058 |
| TII deletion | 62.21 | 9.39 | 14 | - | .020 | ||
| UPD | 70.00 | 6.20 | 21 | - | |||
| Information (standard score) | TI deletion | 2.64 | 1.50 | 11 | .674 | .031 | |
| TII deletion | 2.14 | 1.17 | 14 | - | .001 | ||
| UPD | 4.05 | 1.56 | 21 | - | |||
| Vocabulary (standard score) | TI deletion | 3.18 | 1.72 | 11 | .987 | .068 | |
| TII deletion | 3.07 | 1.69 | 14 | - | .030 | ||
| UPD | 4.71 | 1.90 | 21 | - | |||
| Object assembly (standard score) | TI deletion | 5.18 | 1.99 | 11 | .992 | .077 | |
| TII deletion | 5.29 | 2.55 | 14 | - | .038 | ||
| UPD | 3.43 | 1.83 | 21 | - | |||
| Comprehension (standard score) | TI deletion | 3.18 | 1.78 | 11 | .887 | .090 | |
| TII deletion | 2.86 | 1.46 | 14 | - | .017 | ||
| UPD | 4.57 | 1.86 | 21 | - | |||
SD indicates standard deviation.
Variables analyzed by unpaired t tests only.
The following are assessment sets in which individuals with TII seemed to do better than individuals with TI or UPD: assessment of maladaptive and adaptive behavior (SIB externalized maladaptive index and SIB personal living skills) and for obsessive compulsive behavior (CBC interruption response-halts and resumes). For example, individuals with TII deletions had significantly better scores for 2 of the SIB measures, whereas 4 other measures were found to do more poorly. For visual processing, individuals with TI and UPD were similar, but individuals with TII were more different (performed better) than the other 2 genetic subtypes. These measurements suggest that individuals with TII deletions had better daily living skills than individuals with TI deletions or UPD. Intelligence as assessed by a number of subtests indicate that individuals with TI and TII did not differ from each other and were each lower than UPD for verbal IQ, which is in general agreement with our previous reports comparing UPD with deletion PWS individuals.
DISCUSSION
The percentage of individuals with PWS and TI or TII deletions in our study was similar to that reported by others.5 In addition, differences in the behavioral, psychological, intellectual, and physical characteristics of individuals with PWS and uncharacterized deletions compared with individuals with UPD have been reported previously. However, we present the first assessment of clinical differences in individuals with PWS categorized as having TI or TII deletion. We examined a large existing data set of measures (eg, biochemical, morphologic, metabolic, behavioral, psychological) and most were not significantly different between the 2 deletion types, but significant differences were found for several behavioral and intelligence measures. The average age for our individuals with PWS would fall within the young adulthood range; therefore, the behavioral differences found may not apply to children with PWS.
Psychobehavioral phenotypic characteristics of individuals with PWS and TI or longer deletions (ie, BP1) were similar in several respects to individuals with uncharacterized deletions previously reported31–34 but do differ from those with TII or shorter deletions (ie, BP2), the latter group resembling several features of individuals with UPD. Those with longer deletions had more compulsive behavior and more impairment of visual perception. Conversely, individuals with longer deletions did not display more self-injury than individuals with shorter deletions or UPD (eg, Scales of Independent Living, Reiss Self Injury Scale, Compulsive Behavior Checklist Skin Picking). Although both individuals with TI and TII deletion exhibited more SIB than individuals with UPD for several assessments, the differences were significant in only individuals with TII when compared with UPD. These differences may reflect the sample size with a larger number of individuals with TII and UPD studied compared with the number of individuals with TI. With larger sample sizes found in both the TII and UPD subject groups and thus higher degrees of freedom, higher t test values would meet the significance level for these parameters.
The disassociation of compulsivity and skin picking is consistent with our previous factor analytic study revealing that skin picking does not factor with compulsivity using the Compulsive Behavior Checklist.35 Moreover, we recently reported that plasma γ-aminobutyric acid levels are inversely correlated with skin picking in PWS but unrelated to compulsive behavior scale scores (T. Thompson, PhD, I. Feurer, PhD, W. MacLean, PhD, D. Schmidt, PhD, and M.G. Butler, MD, PhD, unpublished data, 2002). Several academic achievement scores differed between shorter and longer deletions, which may reflect a difference in intellectual functioning as well as differences in visual perception that may affect reading ability.
The longer deletion results in the loss of an additional 500 kb of DNA compared with the shorter deletion. DNA sequences contained in this region may contribute to the differences observed between individuals with PWS and TI and TII deletions, which is supported by the identification of 4 genes between BP1 and BP2.7 Thus, individuals with the longer deletion are presumably missing the 4 genes compared with individuals with PWS and shorter deletions. One of these genes is NIPA-1, which is expressed in mouse brain tissue and is not thought to be imprinted but may be important for brain development or function.7 These or other unidentified genes in the BP1 and BP2 region may be implicated in compulsive behavior and lower intellectual ability that were seen in our patients.
Our previous studies indicate that 2 maternal copies of the 15q11-q13 region may predispose to less skin picking, more visual perceptual abnormalities,15 but a superior visual memory,36 which may ameliorate the IQ deficit in individuals with UPD. In addition, the UBE3A gene, which is maternally expressed in Purkinje cells, hippocampal neurons, and mitral cells of the olfactory bulb in mouse models,37 should also be considered as playing a role in 1 or more of these phenotypic features in PWS with UPD. Individuals with maternal disomy will have 2 expressed alleles of the UBE3A gene, and this overproduction of gene product may have an impact on the behavior or clinical phenotype compared with the individual with TI or TII deletion with only 1 active allele of this gene.
Our results indicate that having a paternal copy of genes between BP1 and BP2 is beneficial to having 2 maternal copies as seen in individuals with UPD. One would anticipate no distinction between maternal and paternal alleles in this region if they are biallelically expressed; however, the 15q11-q13 region contains imprinted genes. This imprinting process may have an impact on the function of other genes in the region. In addition, paternally expressed genes outside the PWS critical region would not be expressed in individuals with PWS and UPD but would be expressed in individuals with a deletion. Similarly, incorrect methylation may also play a role in this region. Hence, the above observations and speculations will require additional genetic testing and confirmation.
ACKNOWLEDGMENTS
Partial support was provided by grant PO1HD30329 and RO1HD41672 from the National Institute of Child Health and Human Development, Kansas City Area Life Sciences Institute, Children’s Mercy Hospital Physician Scientist Award (GL01.4871), and the Hall Family Foundation (GL01.3905).
We acknowledge Linda Heim and Deborah Moore for expert preparation of this manuscript.
ABBREVIATIONS.
- PWS
Prader-Willi syndrome
- UPD
maternal disomy 15
- AS
Angelman syndrome
- BP
breakpoint
- TI
type I
- TII
type II
- IQ
intelligence quotient
- CBC
Child Behavior Checklist
- Y-BOCS
Yale-Brown Obsessive-Compulsive Scale
- VMI
visual-motor integration
- SIB
self-injurious behavior
REFERENCES
- 1.Butler MG. Prader-Willi syndrome: current understanding of cause and diagnosis. Am J Med Genet. 1990;35:319–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butler MG, Thompson T. Prader-Willi syndrome: clinical and genetic findings. Endocrinologist 2000;10:3S–16S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicholls RD, Knepper JL. Genome organization, function, and imprinting in Prader-Willi and Angelman syndromes. Annu Rev Genom Hum Genet. 2001;2:153–175 [DOI] [PubMed] [Google Scholar]
- 4.Cassidy SB. Prader-Willi syndrome. J Med Genet. 1997;34:917–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mewborn SK, Milley NL, Fantes JA, et al. Break point junction fragments in Prader-Willi and Angelman syndrome (PWS/AS) deletion patients reveal variable breakpoints within large duplicons. Am J Hum Genet. 2002;71:A298 [Google Scholar]
- 6.Ungaro P, Christian SL, Fantes JA, et al. Molecular characterization of four cases of intrachromosomal triplication of chromosome 15q11–q14. Am J Med Genet. 2001;38:26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chai JH, Locke DP, Eichler EE, Nicholls RD. Evolutionary transposition of 4 unique genes mediated by flanking duplicons in the Prader-Willi/Angelman syndromes deletion region. Am J Hum Genet. 2002;71:A395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butler MG. Hypopigmentation: a common feature of Prader-Labhart-Willi syndrome. Am J Hum Genet. 1989;45:140–146 [PMC free article] [PubMed] [Google Scholar]
- 9.Roof E, Stone W, MacLean W, Feurer ID, Thompson T, Butler MG. Intellectual characteristics of Prader-Willi syndrome: comparison of genetic subtypes. J Intellect Disabil Res. 2000;44:25–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dykens EM, Cassidy SB. Correlates of maladaptive behavior in children and adults with Prader-Willi syndrome. Am J Med Genet. 1995;60:546–549 [DOI] [PubMed] [Google Scholar]
- 11.Mitchell J, Schinzel A, Langlois S, et al. Comparison of phenotype in uniparental disomy and deletion Prader-Willi syndrome: sex differences. Am J Med Genet. 1996;65:133–136 [DOI] [PubMed] [Google Scholar]
- 12.Dykens EM, Cassidy SB, King BH. Maladaptive behavior differences in Prader-Willi syndrome due to paternal deletion versus maternal uni-parental disomy. Am J Ment Retard. 1999;104:67–77 [DOI] [PubMed] [Google Scholar]
- 13.Dykens EM. Are jigsaw puzzle skills “spared’ in persons with Prader-Willi syndrome? J Child Psychol Psychiatry. 2002;43:343–352 [DOI] [PubMed] [Google Scholar]
- 14.Symons FJ, Butler MG, Sanders MD, Feurer ID, Thompson T. Self-injurious behavior and Prader-Willi syndrome: behavioral forms and body locations. Am J Med Genet. 1999;104:260–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox R, Yang GS, Feurer ID, Butler MG, Thompson T. Kinetic form discrimination in Prader-Willi syndrome. J Intellect Disabil Res. 2001;45:317–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gunay-Aygun M, Heeger S, Schwartz S, Cassidy SB. Delayed diagnosis in patients with Prader-Willi syndrome due to maternal uniparental disomy 15. Am J Med Genet. 1997;71:106–110 [PubMed] [Google Scholar]
- 17.Mutirangura A, Greenberg F, Butler MG, et al. Multiple PCR of three dinucleotide repeats in the Prader-Willi/Angelman critical region (15q11–q13): molecular diagnosis and mechanism of uniparental disomy. Hum Mol Genet. 1993;2:143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butler MG. Molecular diagnosis of Prader-Willi syndrome: comparison of cytogenetics and molecular genetic data including parent of origin dependent methylation DNA patterns. Am J Med Genet. 1996;61:188–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muralidhar B, Butler MG. Methylation PCR analysis of Prader-Willi syndrome, Angelman syndrome and control subjects. Am J Med Genet. 1998;80:263–265 [PMC free article] [PubMed] [Google Scholar]
- 20.Butler MG, Bittel D, Talebizadeh Z. Prader-Willi syndrome and a deletion/duplication within the 15q11–q13 region. J Med Genet. 2002;107:69–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. Arch Gen Psychiatry. 1989;46:1006–1011 [DOI] [PubMed] [Google Scholar]
- 22.Stein DJ, Keating J, Zar HJ, Hollander E. A survey of the phenomenology and pharmacotherapy of compulsive and impulsive-aggressive symptoms in Prader-Willi syndrome. J Neuropsychiatr Clin Neurosci. 1994;6:23–29 [DOI] [PubMed] [Google Scholar]
- 23.Dykens EM, Leckman JF, Cassidy SB. Obsessions and compulsions in Prader-Willi syndrome. J Child Psychol Psychiatry. 1996;37:995–1002 [DOI] [PubMed] [Google Scholar]
- 24.Gedye A Recognizing obsessive-compulsive disorder in clients with developmental disabilities. Habil Ment Healthcare Newslett. 1992;11:73–77 [Google Scholar]
- 25.Reiss S Test Manual for the Reiss Screen for Maladaptive Behavior. Orland Park, IL: International Diagnostic Systems; 1988 [Google Scholar]
- 26.Bruininks RH, Woodcock RW, Weatherman RF, Hill BK. Scales of Independent Behavior (SIB). Allen, TX: DCM Teaching Resources; 1984 [Google Scholar]
- 27.Wechsler D Wechsler Adult Intelligence Scale-Revised. San Antonio, TX: The Psychological Corporation; 1981 [Google Scholar]
- 28.Wechsler D Wechsler Intelligence Scale for Children-III. San Antonio, TX: The Psychological Corporation; 1991 [Google Scholar]
- 29.Beery KE, Buktenica NA. Developmental Test of Visual-Motor Integration. 3rd rev Cleveland, OH: Modern Curriculum Press; 1997 [Google Scholar]
- 30.Woodcock RW, Johnson MB. Woodcock-Johnson Tests of Achievement-Revised. Allen, TX: DLM Teaching Resources; 1990 [Google Scholar]
- 31.Butler MG, Meaney FJ, Palmer CG. Clinical and cytogenetic survey of 39 individuals with Prader-Labhart-Willi syndrome. Am J Med Genet. 1986;23:793–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magenis RE, Toth-Fejel S, Allen LJ, et al. Comparison of the 15q deletions in Prader-Willi and Angelman syndromes: specific regions, extent of deletions, parental origin, and clinical consequences. Am J Med Genet. 1990;35:333–349 [DOI] [PubMed] [Google Scholar]
- 33.Zori R, Williams C, Mattei JF, Moncla A. Parental origin of del (15) (q11–q13) in Angelman and Prader-Willi syndromes. Am J Med Genet. 1990;37:294–295 [DOI] [PubMed] [Google Scholar]
- 34.Robinson WP, Bottani A, Yagang X, et al. Molecular, cytogenetic, and clinical investigations of Prader-Willi syndrome patients. Am J Hum Genet. 1991;49:1219–1234 [PMC free article] [PubMed] [Google Scholar]
- 35.Feurer ID, Dimitropoulos A, Stone WL, Roof E, Butler MG, Thompson T. The latent variable structure of the Compulsive Behavior Checklist in people with Prader-Willi syndrome. J Intellect Disabil Res. 1998;42(suppl):472–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joseph B, Egli M, Sutcliffe JS, Thompson T. Possible dosage effect of maternally expressed genes on visual recognition memory in Prader-Willi syndrome. Am J Med Genet. 2001;105:71–75 [PubMed] [Google Scholar]
- 37.Albrecht U, Sutcliffe JS, Cattanach BM, et al. Imprinted expression of the murine Angelman syndrome gene, Ube3a, in hippocampal and Purkinje neurons. Nat Genet. 1997;17:75–78 [DOI] [PubMed] [Google Scholar]