Abstract
Objective
The purpose of this study was to explore the optimal cutoffs of the three parameters of Ki67 during NAC for predicting patient prognosis and investigate whether the optimal cutoffs of the Ki67 values were associated with relapse-free survival (RFS) or breast cancer-specific survival (BCSS).
Methods
A total of 92 patients with locally advanced breast cancer (LABC), who had residual disease after NAC were retrospectively investigated. The optimal cutoff values of the Ki67 parameters were assessed by the online algorithm Cutoff Finder. Kaplan-Meier analysis, the log-rank test and Cox regression analysis were carried out to analyze survival.
Results
The optimal cutoff values for the postsurgical Ki67 level and the decrease in the Ki67 level during NAC were defined as 25% and 12.5%, respectively. According to the univariate survival analysis, a higher Ki67 level in residual disease was associated with poor RFS (P = 0.004) and BCSS (P = 0.014). In addition, a Ki67 expression decrease > 12.5% during NAC was related to favorable RFS ( P = 0.007), but was not related to BCSS (P = 0.452). Cox regression analysis showed that the Ki67 expression decrease (> 12.5%vs. ≤ 12.5%) and histological grade (grade 3 vs. grade 1-2) were the independent factors associated with RFS (P = 0.020 and P = 0.023, respectively), with HR values of 0.353 (95% CI: 0.147-0.850) and 3.422 (95% CI: 1.188-9.858), respectively.
Conclusions
The Ki67 decrease was one of the independent factors associated with RFS in LABC patients with residual disease after receiving NAC.
Keywords: Ki67, locally advanced breast cancer, neoadjuvant chemotherapy, prognosis, residual disease
Introduction
Neoadjuvant chemotherapy (NAC) is a standard management therapy for patients with locally advanced breast cancer (LABC). NAC routinely decreases tumor size to increase the success rate of resection and breast-conserving surgery1,2. NAC also lowers lymph node staging, allowing sentinel lymph node biopsy instead of axillary lymph node dissection3,4. Moreover, the surgical specimens after NAC even provide an opportunity for assessing biomarkers as therapeutic and prognostic predictors5. A meta-analysis comprising nine randomized trials showed that NAC was equivalent to adjuvant chemotherapy in terms of disease progression and overall survival (OS)6. Several studies also indicated that pathologically complete response (pCR) after NAC in either primary breast lesions or positive axillary nodes, but especially when both sites achieved pCR had a favorable long-term outcome compared with non-pCR7-9. However, the prognosis is quite different among the non-pCR patients10. The identification of prognostic factors for these patients is under investigation. It is very important to find a new prognostic marker for stratifying these patients and distinguishing which patients might benefit from subsequent therapy.
The proliferative index using Ki67 as a prognostic and predictive indictor has been extensively investigated in breast cancer. High levels of Ki67 were associated with an unfavorable prognosis. A meta-analysis of 46 studies including 12155 patients indicated that high Ki67 expression was associated with unfavorable relapse-free survival (RFS) and OS in early-stage breast cancer patients11. In the NAC setting, three parameters, the pretherapeutic Ki67 level, postsurgical Ki67 level and change in the Ki67 level, could be obtained and assessed. Pretherapeutic Ki67 cutoffs in the range between 4% and 58% have been reported to be associated with prognosis12. A recent study also showed that high Ki67 levels in residual disease after NAC were associated with early metastasis13. Von Minckwitz and colleagues reported that patients whose Ki67 levels decreased during NAC had an excellent outcome, which was even superior to the outcomes of patients who had lower baseline Ki67 levels in biopsy samples14. It is unclear which of the three variates has the most significance regarding the prognosis of patients with residual disease.
In this retrospective study, we explored the optimal cutoff values for the three Ki67 parameters during NAC to predict prognosis. Next, we investigated whether the optimal Ki67 cutoff value was associated with RFS or breast cancer-specific survival (BCSS).
Materials and methods
Patients
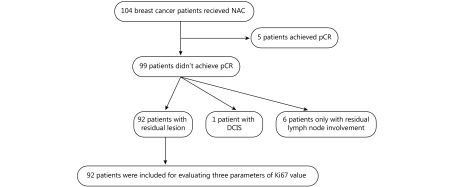
The Institutional Review Board of Shantou University Medical College Cancer Hospital approved this study. From December 2009 to September 2013, 1,329 patients with primary breast cancer who were hospitalized in the Breast Center at the Hospital were identified. Patients with bilateral breast cancer and distant metastasis were excluded. A total of 104 patients receiving NAC were preliminarily included in this study. An absence of pathologic evidence of residual disease in the breast and axillary lymph nodes was defined as a pCR. As shown in Figure 1, 99 (95.2%) patients did not achieve a pCR. Among the 99 patients, one patient had ductal carcinoma in situ, and 6 patients had only residual lymph node involvement. In total, 92 patients with residual disease in the breast, which can obtain paired Ki67 value before and after NAC in the primary breast cancer, were enrolled in this study. Their demographic and clinicopathologic characteristics were obtained from the hospital medical records.
1.
Flowchart of patient selection in present study. DCIS, ductal carcinoma in situ; NAC, neoadjuvant chemotherapy; pCR, pathologically complete response.
Treatment regimens
The basic chemotherapy regimens were as follows: TEC (docetaxel 75 mg/m2, epirubicin 80 mg/m2, and cyclophosphamide 500 mg/m2 every 3 weeks for 18 weeks); FEC→T (5-fluorouracil 500 mg/m2, epirubicin 100 mg/m2, and cyclophosphamide 500 mg/m2 every 3 weeks for 9 weeks, followed by docetaxel 100 mg/m2 every 3 weeks for another 9 weeks); and CEF (cyclophosphamide 500 mg/m2, epirubicin 100 mg/m2, and 5-fluorouracil 500 mg/m2 on days 1 and 8 every 3 weeks for 18 weeks). Although thoroughly informed of the benefits of targeted therapy for patient outcome, only two patients with human epidermal growth receptor-2 (Her-2) positive breast cancer received neoadjuvant targeted therapy, with the others opting against the treatment for financial reasons. Surgery was performed after 4 or 6 NAC cycles depending on the patient’s response and their choice. All the patients underwent breast-conserving surgery or mastectomy and underwent axillary lymph node dissection while striving to completely remove residual disease. Patients received the remaining cycles of chemotherapy, radiotherapy, and endocrine therapy after surgery if eligible. A total of 64.5% of the Her-2 positive patients, generally divided according to economic status, received targeted therapy for 1 year.
Immunohistochemical examination
Pathological examinations of the tumor samples were performed by two experienced pathologists. Estrogen receptor (ER), progesterone receptor (PR), Her-2, and Ki67 expression levels were analyzed by immunohistochemical staining (EnVision). The antibodies used for immunohistochemistry were as follows: anti-ER (ab137738, 1 : 200 dilution; Abcam, USA), anti-PR (ab16661, 1 : 200 dilution; Abcam, USA), anti-Her-2 (ab2428, 1 : 200 dilution; Abcam, USA), and anti-Ki67 (MIB-1, 1 : 400 dilution; Dako, Denmark). We adopted the threshold of 10% positive tumor cell staining to interpret ER and PR status, with less than 10% positive staining regarded as negative, and ≥ 10% positive staining considered positive15. Her-2 expression was determined according to the American Society of Clinical Oncology/College of American Pathologists guideline recommendations16. Ki67 scoring was assessed according to the International Ki67 in Breast Cancer Working Group guidelines17. The Ki67 index depended on the percentage of positive cells among a total number of 1,000 tumor cells (at least 500 tumor cells were counted).
Statistical analysis
All patients were followed until July 2016 through outpatient clinic or telephone interview. RFS was defined as the time from the date of surgery to local recurrence or distant metastasis. BCSS was defined as the time from the date of diagnosis of breast cancer to breast cancer-related death. Patient death due to other diseases or survival at the last follow-up date was considered censored data. The Ki67 values before and after NAC were analyzed by the Wilcoxon test using MedCalc Statistical Software (version 15.8, MedCalc Software bvba, Ostend, Belgium). The optimal cutoff values of pretherapeutic Ki67 level as well as postsurgical Ki67 level and Ki67 level change during NAC was analyzed using an online Cutoff Finder algorithm ( http://molpath.charite.de/cutoff/)18. Kaplan-Meier survival analysis was used to analyze RFS and BCSS by MedCalc Statistical Software, and the log-rank test was used to evaluate the survival differences. Cox regression analysis was performed to determine the independent factors for survival using SPSS statistical software (version 18.0, SPSS Inc., Chicago, IL, USA). Significant variables from the univariate analysis were selected for the multivariable analysis by an enter procedure. All tests were two-tailed, and P < 0.05 was considered statistically significant.
Results
Clinicopathological characteristics of the patients
The patients’ clinicopathological characteristics used in this study are summarized in Supplementary Table S1. All the patients were female. Their median age was 51 years old (range from 28 to 80). Of these patients, 48 (52.2%) were premenopausal. The mean primary tumor size was 47.5 ± 20.0 mm. Additionally, 95.7% of patients had invasive ductal carcinomas, and 85.9% of them received anthracycline- and taxane-based chemotherapy regimens. The TNM staging was performed according to the American Joint Committee on Cancer 7th edition guidelines19. Before chemotherapy, sixty (65.2%) patients were ER-positive, 52 (56.5%) patients were PR-positive and 31 (33.7%) patients were Her-2-positive. When classified by breast cancer subtype, HR(+)/Her-2(–) accounted for 51(55.4%) cases, HR(+)/Her-2(+) accounted for 12 (13.0%) cases, HR(–)/Her-2(+) accounted for 19 (20.7%) cases, TN accounted for 10 (10.9%) cases. One (1.1%) patient was classified as stage IIB, 44 (47.8%) patients were stage IIIA, 19 (20.7%) patients were stage IIIB, and 28 (30.4%) patients were stage IIIC. After receiving NAC, 56.5% patients were downstaged. Post-NAC staging was as follows: 9 (9.8%) patients were stage I, 20 (21.6%) patients were stage II, 34 (37.0%) patients were stage IIIA, 11 (12.0%) patients were stage IIIB, and 18 (19.6%) patients were stage IIIC. Immunohistochemically, the median Ki67 value before NAC was 30% (range 1% to 80%). After NAC, the median Ki67 value was 20% (range 0% to 80%).
S1.
Patients characteristics
| Characteristics | Mean ± SD/median | n (%) |
| Age (years) | 51 (28–80) | |
| Female | 92 (100.0) | |
| Menstrual status | ||
| Premenopausal | 48 (52.2) | |
| Postmenopausal | 44 (47.8) | |
| Primary tumor size (mm) | 47.5 ± 20.0 | |
| Clinical tumor stage | ||
| cT1–2 | 54 (58.7) | |
| cT3–4 | 38 (41.3) | |
| Clinical lymph node stage | ||
| cN0–1 | 24 (26.1) | |
| cN2–3 | 68 (73.9) | |
| Clinical stage | ||
| II | 1 (1.1) | |
| IIIA | 44 (47.8) | |
| IIIB | 19 (20.7) | |
| IIIC | 28 (30.4) | |
| Pathological tumor stage | ||
| ypT1–2 | 70 (76.1) | |
| ypT3–4 | 22 (23.9) | |
| Pathological lymph stage | ||
| ypN0 | 17 (18.5) | |
| ypN1–3 | 75 (81.5) | |
| Pathological stage | ||
| I | 9 (9.8) | |
| II | 20 (21.6) | |
| IIIA | 34 (37.0) | |
| IIIB | 11 (12.0) | |
| IIIC | 18 (19.6) | |
| Histological grade | ||
| 1–2 | 30 (32.6) | |
| 3 | 58 (63.1) | |
| Unclearly | 4 (4.3) | |
| Continued | ||
Changes in Ki67 levels before and after NAC
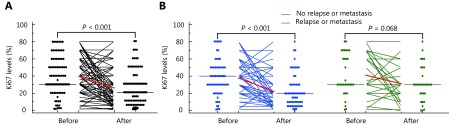
To determine the changes in Ki67 levels during NAC, Ki67 levels in the preoperative biopsy and surgical specimens after NAC from the same patient were assessed. As shown in Table 1, the median Ki67 value before NAC was 30%, and the interquartile range (IQR) was 30% to 57%. In the samples after receiving NAC, the median Ki67 value was 20% (IQR: 10%–30%). As shown in Supplementary Figure S1A, the Ki67 level was obviously decreased after NAC (P < 0.001). Next, we stratified the patients as with or without relapse/metastasis. In the group without relapse or metastasis (65.2%), the Ki67 value was significantly decreased after NAC ( P < 0.001, Supplementary Figure S1B), and the median pretherapeutic Ki67 value was 40% (IQR: 22%–50%), and the postsurgical median was 20% (IQR: 10%–30%) (Table 1). A reduction in the postsurgical Ki67 value was observed in the patients with relapse or metastasis (34.8%), but it was not a significant difference compared to the pretherapeutic Ki67 value (P = 0.068, Supplementary Figure S1B).
1.
Value of Ki67 before and after neoadjuvant chemotherapy
| Characteristics | n (%) | Median (IQR) | Mean ± SD | P |
| All patients | 92 (100.0) | 0.000* | ||
| Before | 30 (30–57) | 39 ± 22 | ||
| After | 20 (10–30) | 25 ± 20 | ||
| No relapse or metastasis | 60 (65.2) | 0.000* | ||
| Before | 40 (22–50) | 38 ± 21 | ||
| After | 20 (10–30) | 22 ± 19 | ||
| Relapse or metastasis | 32 (34.8) | 0.068 | ||
| Before | 30 (30–67) | 41 ± 24 | ||
| After | 30 (20–47) | 31 ± 20 |
S1.
Changes in Ki67 levels between core needle biopsy and surgical specimens. (A) Changes in Ki67 levels before and after neoadjuvant chemotherapy in all patients. (B) Changes in Ki67 levels before and after neoadjuvant chemotherapy stratifying by with or without relapse or metastasis.
Optimal cutoffs of the three parameters relevant to the Ki67 levels during NAC
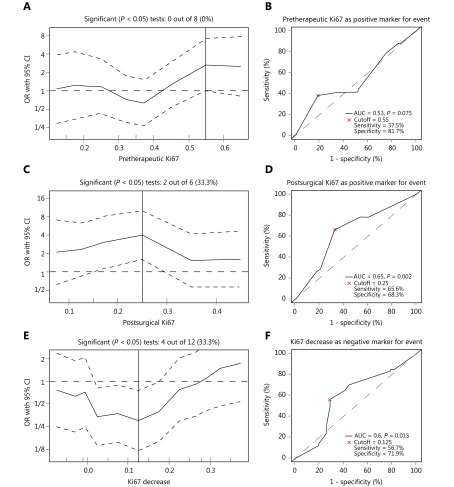
As shown in Figure 2A, 0 out of 8 cut points for the pretherapeutic Ki67 level were significant, and the optimal cutoff value was 55%. Receiver operating characteristic (ROC) analysis showed that when pretherapeutic Ki67 levels greater than 55% were used for prediction of RFS, the area under the curve (AUC) was 0.53 (P = 0.075), the sensitivity was 37.5% and the specificity was 81.7% (Figure 2B). For the postsurgical Ki67 level after NAC, 2 out of 6 cut points were significant, and the optimal cutoff value was 25% (Figure 2C). When a postsurgical Ki67 level larger than 25% was used for predicting RFS, the AUC was 0.65 (P = 0.002), the sensitivity was 65.6%, and the specificity was 68.3% (Figure 2D). Regarding the Ki67 change during NAC, 4 out of 12 cut points were significant, and the optimal cutoff value was 12.5% (Figure 2E). When a Ki67 decrease > 12.5% was used as a predictor for negative RFS, the AUC was 0.6 ( P = 0.015), the sensitivity was 56.7% and the specificity was 71.9% (Figure 2F).
2.
Analysis of different optimal cutoff for three parameters of Ki67 levels as predictors for relapse-free survival (RFS). (A) Odds ratio (OR) for the probability of RFS in dependence of cutoff point of pretherapeutic Ki67 value. Vertical line marked the optimal cutoff value of 55%. (B) Receiver operating curve (ROC) for determining the optimal cutoff value of pretherapeutic Ki67 in predicting RFS. (C) OR for the probability of RFS in dependence of cutoff point of postsurgical Ki67 value. Vertical line marked the optimal cutoff value of 25%. (D) ROC for determining the optimal cutoff value of postsurgical Ki67 in predicting RFS. (E) OR for the probability of RFS in dependence of cutoff point of Ki67 change levels. Vertical line marked the optimal cutoff value of 12.5%. (F) ROC for determining the optimal cutoff value of Ki67 change in predicting RFS. The plots were calculated by the online biostatistical tool Cutoff Finder18.
Univariate survival analysis
All patients were followed until July 2016, and the median follow-up time was 44 (range 5 to 80) months. At the end of the follow-up time, 32 (34.8%) patients had presented local recurrence or distant metastasis, and 22 (23.9%) patients had died of breast cancer (Supplementary Table S1).
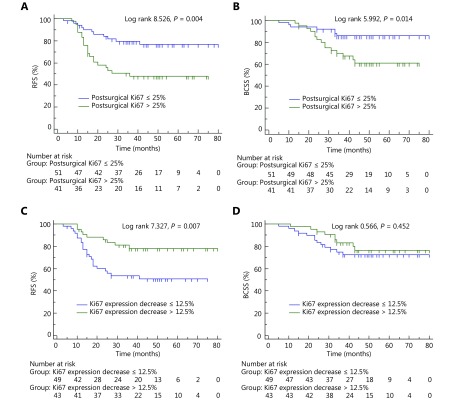
According to the optimal cutoffs for the Ki67 levels, the postsurgical Ki67 level and the decrease in the Ki67 level were applied to the survival analysis. As shown in Figure 3A, patients with postsurgical Ki67 levels ≤ 25% had a significantly longer RFS than those with postsurgical Ki67 levels > 25% ( P = 0.004). The average RFS times of patients were 65 (95% CI: 58–73) months and 44 (95% CI: 35–53) months, respectively (Table 2). The 5-year RFS rates were 76.5% and 47.9%, respectively. Patients with a Ki67 expression decrease > 12.5% had significantly longer RFS compared to those with a Ki67 expression decrease ≤ 12.5% (as shown in Figure 3C, P = 0.007). The average RFS times of patients were 66 (95% CI: 59–74) months and 46 (95% CI: 37–54) months, respectively (Table 2). The 5-year RFS rates were 78.1% and 51.0%, respectively. Other variables that correlated with RFS in the univariate analysis included histological grade (P = 0.008), ER status (P = 0.005), and PR status (P = 0.029) (Table 2). There were no statistically significant differences related to RFS in terms of age, menstrual status, clinical tumor stage, clinical lymph node stage, pathologic tumor stage, pathologic lymph node stage, histologic type, Her-2 status or molecular subtype (P > 0.05).
3.
Survival analysis of postsurgical Ki67 level and change in Ki67 level during NAC. (A) Postsurgical Ki67 level was associated with relapse-free survival (RFS). (B) Postsurgical Ki67 level was associated with breast cancer-specific survival (BCSS). (C) Ki67 expression decrease > 12.5% was associated with RFS. (D) Ki67 expression decrease > 12.5% was not associated with BCSS.
2.
Associated characteristics with relapse-free survival by uni- and multi-variate survival analysis
| Characteristics | Univariate analysis | Multivariate analysis | ||||
| Mean survival (months) (95% CI) | Log rank | P | HR (95% CI) | P | ||
| Age (years) | 2.034 | 0.154 | ||||
| ≤ 50 | 51 (41–60) | |||||
| > 50 | 61 (53–69) | |||||
| Menstrual status | 1.927 | 0.165 | ||||
| Premenopausal | 51 (42–60) | |||||
| Postmenopausal | 61 (53–70) | |||||
| Clinical tumor stage | 0.001 | 0.985 | ||||
| cT1–2 | 57 (48–65) | |||||
| cT3–4 | 53 (44–63) | |||||
| Clinical lymph node stage | 2.919 | 0.088 | ||||
| cN0–1 | 66 (55–77) | 1 | ||||
| cN2–3 | 52 (45–60) | 1.554 (0.563–4.291) | 0.395 | |||
| Pathologic tumor stage | 0.287 | 0.592 | ||||
| yT1–2 | 58 (50–65) | |||||
| yT3–4 | 50 (38–63) | |||||
| Pathologic lymph node stage | 0.195 | 0.658 | ||||
| yN0 | 60 (45–74) | |||||
| yN1–3 | 55 (48–62) | |||||
| Histological grade | 9.630 | 0.008* | ||||
| 1–2 | 67 (59–75) | 1 | ||||
| 3 | 49 (40–57) | 3.422 (1.188–9.858) | 0.023* | |||
| Unclearly | 57 (57–57) | |||||
| Histologic type | 1.921 | 0.166 | ||||
| Invasive ductal carcinoma | 56 (49–62) | |||||
| Other | 57 (57–57) | |||||
| ER | 8.005 | 0.005* | ||||
| Negative | 42 (32–53) | 1 | ||||
| Positive | 63 (56–70) | 0.477 (0.127–1.792) | 0.273 | |||
| PR | 4.765 | 0.029* | ||||
| Negative | 46 (37–55) | 1 | ||||
| Positive | 63 (55–71) | 0.795 (0.208–3.042) | 0.738 | |||
| Her-2 | 1.591 | 0.207 | ||||
| Negative | 60 (52–67) | |||||
| Positive | 50 (39–61) | |||||
| Postsurgical Ki67 | 8.526 | 0.004* | ||||
| ≤ 25% | 65 (58–73) | 1 | ||||
| Continued | ||||||
2.
| Continued | ||||||
| Characteristics | Univariate analysis | Multivariate analysis | ||||
| Mean survival (month) (95% CI) | Log rank | P | HR (95% CI) | P | ||
| *P < 0.05 was considered statistically significant. Abbreviations: ER, estrogen receptor; Her-2, human epidermal growth factor receptor 2; HR(+), hormonal receptor (+); PR, progestrone receptor; TN, triple negative. | ||||||
| > 25% | 44 (35–53) | 1.093 (0.449–2.664) | 0.844 | |||
| Ki67 decrease | 7.327 | 0.007* | ||||
| ≤ 12.5% | 46 (37–54) | 1 | ||||
| > 12.5% | 66 (59–74) | 0.353 (0.147–0.850) | 0.020* | |||
| Molecular subtype | 5.875 | 0.118 | ||||
| HR(+)/Her-2(–) | 62 (54–70) | |||||
| HR(+)/Her-2(+) | 62 (46–77) | |||||
| HR(–)/Her-2(+) | 43 (29–56) | |||||
| TN | 41 (23–58) | |||||
Moreover, patients with postsurgical Ki67 levels ≤ 25% had longer BCSS compared to those with postsurgical Ki67 levels > 25% ( P = 0.014) (Figure 3B). The average BCSS times of patients were 71 (95% CI: 65–77) months and 56 (95% CI: 49-64) months, respectively (Table 3). The 5-year BCSS rates were 85.8% and 61.2%, respectively. As shown in Table 3, other variables that correlated with BCSS in the univariate analysis were histological grade (P = 0.023) and ER status (P = 0.006). The molecular subtype had marginal significance in relation to BCSS (P = 0.051). There were no statistically significant differences related to BCSS in terms of age, menstrual status, clinical tumor stage, clinical lymph node stage, pathologic tumor stage, pathologic lymph node stage, histologic type, PR status, Her-2 status or Ki67 change (P > 0.05).
3.
Associated characteristics with breast cancer-specific survival by uni- and multi-variate survival analysis
| Characteristics | Univariate analysis | Multivariate analysis | ||||
| Mean survival (month) (95% CI) | Log rank | P | HR (95% CI) | P | ||
| Age (years) | 0.114 | 0.736 | ||||
| ≤ 50 | 65 (57–72) | |||||
| > 50 | 67 (60–73) | |||||
| Menstrual status | 1.488 | 0.223 | ||||
| Premenopausal | 63 (55–70) | |||||
| Postmenopausal | 69 (62–76) | |||||
| Clinical tumor stage | 0.733 | 0.392 | ||||
| cT1–2 | 68 (61–74) | |||||
| cT3–4 | 63 (55–71) | |||||
| Clinical lymph node stage | 2.221 | 0.136 | ||||
| cN0–1 | 72 (65–80) | |||||
| cN2–3 | 63 (57–70) | |||||
| Pathologic tumor stage | 2.215 | 0.137 | ||||
| yT1–2 | 68 (62–73) | |||||
| yT3–4 | 59 (48–71) | |||||
| Pathologic lymph node stage | 1.610 | 0.205 | ||||
| yN0 | 73 (64–82) | |||||
| yN1–3 | 64 (58–70) | |||||
| Histological grade | 5.139 | 0.023* | ||||
| 1–2 | 73 (67–80) | 1 | ||||
| 3 | 61 (54–68) | 3.579 (1.046–12.238) | 0.042* | |||
| Unclearly | 57 (57–57) | |||||
| Continued | ||||||
3.
| Continued | ||||||
| Characteristics | Univariate analysis | Multivariate analysis | ||||
| Mean survival (month) (95% CI) | Log rank | P | HR (95% CI) | P | ||
| *P < 0.05 was considered statistically significant.Abbreviations: ER, estrogen receptor; Her-2, human epidermal growth factor receptor 2; HR (+), hormonal receptor (+); PR, progestrone receptor; TN, triple negative. | ||||||
| Histologic type | 1.243 | 0.265 | ||||
| Invasive ductal carcinoma | 65 (60–70) | |||||
| Other | 57 (57–57) | |||||
| ER | 7.609 | 0.006* | ||||
| Negative | 54 (45–63) | 1 | ||||
| Positive | 71 (65–76) | 0.467 (0.042–5.207) | 0.536 | |||
| PR | 2.832 | 0.092 | ||||
| Negative | 58 (50–65) | 1 | ||||
| Positive | 69 (63–75) | 2.170 (0.220–21.410) | 0.507 | |||
| Her-2 | 1.032 | 0.310 | ||||
| Negative | 67 (61–74) | |||||
| Positive | 61 (53–70) | |||||
| Postsurgical Ki67 | 5.992 | 0.014* | ||||
| ≤ 25% | 71 (65–77) | 1 | ||||
| > 25% | 56 (49–64) | 1.771 (0.649–4.832) | 0.265 | |||
| Ki67 decrease | 0.566 | 0.452 | ||||
| ≤ 12.5% | 64 (56–71) | 1 | ||||
| > 12.5% | 68 (61–75) | 0.898 (0.349–2.311) | 0.824 | |||
| Molecular subtype | 7.766 | 0.051 | ||||
| HR(+)/Her-2(–) | 70 (64–76) | 1 | ||||
| HR(+)/Her-2(+) | 70 (60–80) | 0.662 (0.122–3.603) | 0.633 | |||
| HR(–)/Her-2(+) | 54 (42–65) | 2.591 (0.200–33.574) | 0.466 | |||
| TN | 49 (33–65) | 2.500 (0.172–36.273) | 0.502 | |||
Multivariate survival analysis
In a multivariate analysis, the Ki67 expression change (> 12.5%vs. ≤ 12.5%) and histological grade (grade 3 vs. grade 1–2) were the independent factors for RFS (P = 0.020 and P = 0.023, respectively), with HR values of 0.353 (95% CI: 0.147–0.850) and 3.422 (95% CI: 1.188–9.858), respectively (Table 2). However, only histological grade (grade 3 vs. grade 1–2) was an independent factor related to BCSS (P = 0.042), with an HR of 3.579 (95% CI: 1.046–12.238) (Table 3).
Discussion
NAC is increasingly used in the management of breast cancer. One of the topics of interest in NAC addresses the issue of exploring reliable prognostic and predictive markers. At present, most of the studies have focused on pCR associated with Ki67 expression. Only a few studies have investigated Ki67 expression in residual disease after NAC. Although the outcomes in patients with minimal residual cancer burden after NAC were not inferior to pCR patients, the prognosis of other patients with residual lesions remains unclear. NAC can provide an opportunity for evaluating the three parameters of Ki67 expression, the pretherapeutic Ki67 level, postsurgical Ki67 level and change in the Ki67 level during NAC. There have been very few studies about the role of the three parameters of the Ki67 level in prognosis during NAC. It is certainly necessary to investigate the relationship between these three Ki67 values and prognosis more thoroughly.
The optimal cutoffs for the use of three Ki67 values as prognostic factors in patients with residual disease currently remains uncertain. In this study, the optimal cutoff values for the pretherapeutic Ki67 level, postsurgical Ki67 level and Ki67 level change during NAC were 55%, 25% and 12.5%, respectively. In the ROC analysis, only the postsurgical Ki67 level and the decrease in the Ki67 level had significance for predicting RFS. Most of the Ki67 cutoff values in previous studies were determined through a dichotomy of direct calculations20-22, whereas the optimal cutoffs of the Ki67 level parameters in this study were acquired from an online biostatistical calculator, which is more appropriate and reliable from a methodological and statistical point of view18.
Which of the three Ki67 variables is the most significant for prognosis is undetermined. Numerous studies have reported that the prechemotherapy Ki67 level was the most significant predictor of therapeutic response and prognosis23-26. Our unpublished data demonstrated that higher Ki67 levels in core needle biopsy samples were associated with good therapeutic responses. Consistent with previous reports, high pretherapeutic Ki67 levels were significantly associated with pCR, but these levels were not significantly related to RFS or OS24. In line with this finding, Denkert and colleagues found that the core needle biopsy Ki67 level was not associated with either RFS or OS over a wide range of cutoff values in non-pCR patients12. Our data showed that the prechemotherapeutic Ki67 level had no significant association with RFS when using the optimal cutoff value. Therefore, we concluded that high Ki67 expression in the core needle biopsy was more likely a predictor of good responsiveness to chemotherapy, but not a prognostic predictor.
Whether the post-chemotherapy Ki67 level was a marker for predicting outcome in patients with residual disease was still undefined. A recent study showed that the Ki67 expression level in residual tumor tissue was correlated with patient prognosis, and those with lower Ki67 expression after chemotherapy had a better prognosis than those with higher Ki67 expression27. Several studies also demonstrated that higher Ki67 expression in the postsurgical specimen was independently associated with poorer RFS and OS compared to low Ki67 expression8,28. In these studies, post-treatment Ki67 expression level was found to be more useful for predicting outcomes than either the pretherapy Ki67 value or the change in the Ki67 value during NAC. These findings were also supported by other studies14,29. Although similar to the above outcomes, our study showed that high postsurgical Ki67 levels were significantly related to RFS and BCSS in univariate survival analysis, but were not significant in multivariate survival analysis. Consistent with our result, another investigator found that the postsurgical Ki67 level was not an independent factor for survival30-32. Our results found that only the histological grade of the tumor was independently associated with RFS and BCSS by Cox regression analysis.
In the present study, we observed that the Ki67 level was obviously decreased after NAC, which was consistent with several published studies29,31,33,34. Moreover, the Ki67 level was significantly decreased in the no relapse or metastasis group compared to that in the relapse or metastasis group. A recent study supported our result and confirmed that patients with higher Ki67 values in the postsurgical specimen were more prone to metastasis, particularly in the short term after surgery13. This phenomenon might be explained by several previous studies in which good NAC responders often exhibited reduced Ki67 expression14,31. Thus, we speculated that the cancer cells sensitive to chemotherapy were mostly killed during NAC, and consequently, those patients showed low or no Ki67 expression in their residual cancer cells.
It has been reported that high pretherapy Ki67 levels are stronger predictors for good therapeutic response and poor markers for prognosis23-26. This conflicting result may be partly due to the highly proliferative cancer cells being sensitive to drugs and killed by chemotherapy. Consequently, patients with a reduction in Ki67 expression can obtain a better treatment benefit. In this study, we demonstrated that the Ki67 expression change during NAC was an independent predictor for RFS. In agreement with our findings, Matsubara et al. found that the Ki67 expression decrease during NAC was an independent prognostic factor for RFS, but pre- and post-treatment Ki67 levels were not independently correlated with prognosis in a multivariate analysis33. Their further investigation indicated that the Ki67 expression change was an independent prognostic marker for RFS in the Luminal B (HR positive, Her-2 negative and Ki67 >14%), TN, and Her-2(+) subtypes. Taken together, these data may suggest that if a tumor belongs to the group with no apparent response to NAC, no apparent change or an increased in the Ki67 level may be an indicator of unfavorable prognosis. The selection of patients with residual disease at high risk after NAC is crucial to tailoring a more accurate individualized therapy, such as the administration of an LHRH analogue for premenopausal HR(+) patients, extension of targeted therapy for Her-2(+) patients, and administration of Capecitabine, which significantly increased OS in Her-2(–) patients with residual disease 35.
The present study also has certain limitations. First, this was a retrospective study with a small sample size. A Cox regression of the log hazard ratio based on a sample of 92 cases with a standard deviation of 0.502 achieves 72.2% power at a 0.05 significance level to detect a regression coefficient equal to –1.0410, which might compromise the study results. Second, although most patients (85.9%) received anthracycline- and taxane-based chemotherapy regimens, only 2 patients received trastuzumab as a neoadjuvant targeted therapy. This might influence the postsurgical Ki67 level and then affect the change in the Ki67 level. Finally, not all the Her-2(+) patients received trastuzumab as adjuvant targeted treatment because of economic status. This aspect might compromise the survival results.
Conclusions
A larger Ki67 expression decrease was one of the independent factors associated with favorable RFS in patients with residual disease after NAC. Our results suggested that both biopsy Ki67 and postsurgical Ki67 levels were important and provided different information for clinical practice. The postsurgical Ki67 level may be important to identify patients with highly proliferative residual disease, while pretherapeutic Ki67 level can be a good predictor for therapeutic responsiveness. We rationalized that the pivotal role of the change in the Ki67 level can act to integrate the intrinsic prognostic information and the benefit-related information from treatment. Our findings need to be further validated in a large cohort.
Acknowledgments
This work was partly supported by grants from the Department of Education of Guangdong Province (Grant No. 2016KQNCX051), Key International Collaborative Project of National Natural Science Fund Committee (Grant No. 81320108015), National Natural Science Foundation of China (Grant No. 31271068), and Natural Science Foundation of Guangdong Province (Grant No. 2015A030313429).
Conflict of interest statement
No potential conflicts of interest are disclosed.
Contributor Information
Wenhe Huang, Email: huangwenhe2009@163.com.
Guojun Zhang, Email: guoj_zhang@yahoo.com.
References
- 1.Wolmark N, Wang JP, Mamounas E, Bryant J, Fisher B Preoperative chemotherapy in patients with operable breast cancer: Nine-year results from national surgical adjuvant breast and bowel project b-18. J Natl Cancer Inst Monogr. 2001;2001:96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists' Collaborative Group Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: Meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19:27–39. doi: 10.1016/S1470-2045(17)30777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martelli G, Miceli R, Folli S, Guzzetti E, Chifu C, Maugeri I, et al Sentinel node biopsy after primary chemotherapy in cT2 N0/1 breast cancer patients: Long-term results of a retrospective study. Eur J Surg Oncol. 2017;43:2012–20. doi: 10.1016/j.ejso.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 4.Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: The acosog z1071 (alliance) clinical trial. JAMA. 2013;310:1455–61. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaufmann M, Hortobagyi GN, Goldhirsch A, Scholl S, Makris A, Valagussa P, et al Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: An update. J Clin Oncol. 2006;24:1940–9. doi: 10.1200/JCO.2005.02.6187. [DOI] [PubMed] [Google Scholar]
- 6.Mauri D, Pavlidis N, Ioannidis JPA Neoadjuvant versus adjuvant systemic treatment in breast cancer: A meta-analysis. J Natl Cancer Inst. 2005;97:188–94. doi: 10.1093/jnci/dji021. [DOI] [PubMed] [Google Scholar]
- 7.Bonnefoi H, Litière S, Piccart M, MacGrogan G, Fumoleau P, Brain E, et al Pathological complete response after neoadjuvant chemotherapy is an independent predictive factor irrespective of simplified breast cancer intrinsic subtypes: A landmark and two-step approach analyses from the eortc 10994/big 1-00 phase III trial. Ann Oncol. 2014;25:1128–36. doi: 10.1093/annonc/mdu118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones RL, Salter J, A'Hern R, Nerurkar A, Parton M, Reis-Filho JS, et al The prognostic significance of ki67 before and after neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat. 2009;116:53–68. doi: 10.1007/s10549-008-0081-7. [DOI] [PubMed] [Google Scholar]
- 9.Mougalian SS, Hernandez M, Lei XD, Lynch S, Kuerer HM, Symmans WF, et al Ten-year outcomes of patients with breast cancer with cytologically confirmed axillary lymph node metastases and pathologic complete response after primary systemic chemotherapy. JAMA Oncol. 2016;2:508–16. doi: 10.1001/jamaoncol.2015.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Symmans WF, Wei CM, Gould R, Yu X, Zhang Y, Liu M, et al Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol. 2017;35:1049–60. doi: 10.1200/JCO.2015.63.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Azambuja E, Cardoso F, de Castro G Jr, Colozza M, Mano MS, Durbecq V, et al Ki-67 as prognostic marker in early breast cancer: A meta-analysis of published studies involving 12 155 patients. Br J Cancer. 2007;96:1504–13. doi: 10.1038/sj.bjc.6603756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denkert C, Loibl S, Müller BM, Eidtmann H, Schmitt WD, Eiermann W, et al Ki67 levels as predictive and prognostic parameters in pretherapeutic breast cancer core biopsies: A translational investigation in the neoadjuvant gepartrio trial. Ann Oncol. 2013;24:2786–93. doi: 10.1093/annonc/mdt350. [DOI] [PubMed] [Google Scholar]
- 13.Tokuda E, Horimoto Y, Arakawa A, Himuro T, Senuma K, Nakai K, et al Differences in ki67 expressions between pre- and post-neoadjuvant chemotherapy specimens might predict early recurrence of breast cancer. Hum Pathol. 2017;63:40–5. doi: 10.1016/j.humpath.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 14.von Minckwitz G, Schmitt WD, Loibl S, Müller BM, Blohmer JU, Sinn BV, et al Ki67 measured after neoadjuvant chemotherapy for primary breast cancer. Clin Cancer Res. 2013;19:4521–31. doi: 10.1158/1078-0432.CCR-12-3628. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa Y, Moriya T, Kato Y, Oguma M, Ikeda K, Takashima T, et al Immunohistochemical assessment for estrogen receptor and progesterone receptor status in breast cancer: Analysis for a cut-off point as the predictor for endocrine therapy. Breast Cancer. 2004;11:267–75. doi: 10.1007/BF02984548. [DOI] [PubMed] [Google Scholar]
- 16.Wolff AC, Hammond MEH, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al American society of clinical oncology/college of american pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–45. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 17.Dowsett M, Nielsen TO, A'Hern R, Bartlett J, Coombes RC, Cuzick J, et al Assessment of ki67 in breast cancer: Recommendations from the international Ki67 in breast cancer working group. J Natl Cancer Inst. 2011;103:1656–64. doi: 10.1093/jnci/djr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Budczies J, Klauschen F, Sinn BV, Győrffy B, Schmitt WD, Darb-Esfahani S, et al Cutoff finder: A comprehensive and straightforward web application enabling rapid biomarker cutoff optimization. PLoS One. 2012;7:e51862. doi: 10.1371/journal.pone.0051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edge SB, Compton CC The american joint committee on cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM . Ann Surg Oncol. 2010;17:1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 20.Ács B, Zámbó V, Vízkeleti L, Szász AM, Madaras L, Szentmártoni G, et al Ki-67 as a controversial predictive and prognostic marker in breast cancer patients treated with neoadjuvant chemotherapy. Diagn Pathol. 2017;12:20. doi: 10.1186/s13000-017-0608-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bustreo S, Osella-Abate S, Cassoni P, Donadio M, Airoldi M, Pedani F, et al Optimal ki67 cut-off for luminal breast cancer prognostic evaluation: A large case series study with a long-term follow-up. Breast Cancer Res Treat. 2016;157:363–71. doi: 10.1007/s10549-016-3817-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munzone E, Botteri E, Sciandivasci A, Curigliano G, Nolè F, Mastropasqua M, et al Prognostic value of Ki-67 labeling index in patients with node-negative, triple-negative breast cancer. Breast Cancer Res Treat. 2012;134:277–82. doi: 10.1007/s10549-012-2040-6. [DOI] [PubMed] [Google Scholar]
- 23.Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA Ki67 in breast cancer: Prognostic and predictive potential. Lancet Oncol. 2010;11:174–83. doi: 10.1016/S1470-2045(09)70262-1. [DOI] [PubMed] [Google Scholar]
- 24.Jones RL, Salter J, A'Hern R, Nerurkar A, Parton M, Reis-Filho JS, et al Relationship between oestrogen receptor status and proliferation in predicting response and long-term outcome to neoadjuvant chemotherapy for breast cancer. Breast Cancer Res Treat. 2010;119:315–23. doi: 10.1007/s10549-009-0329-x. [DOI] [PubMed] [Google Scholar]
- 25.Urruticoechea A, Smith IE, Dowsett M Proliferation marker ki-67 in early breast cancer. J Clin Oncol. 2005;23:7212–20. doi: 10.1200/JCO.2005.07.501. [DOI] [PubMed] [Google Scholar]
- 26.Fasching PA, Heusinger K, Haeberle L, Niklos M, Hein A, Bayer CM, et al Ki67, chemotherapy response, and prognosis in breast cancer patients receiving neoadjuvant treatment. BMC Cancer. 2011;11:486. doi: 10.1186/1471-2407-11-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshioka T, Hosoda M, Yamamoto M, Taguchi K, Hatanaka KC, Takakuwa E, et al Prognostic significance of pathologic complete response and ki67 expression after neoadjuvant chemotherapy in breast cancer. Breast Cancer. 2015;22:185–91. doi: 10.1007/s12282-013-0474-2. [DOI] [PubMed] [Google Scholar]
- 28.Sheri A, Smith IE, Johnston SR, A'Hern R, Nerurkar A, Jones RL, et al Residual proliferative cancer burden to predict long-term outcome following neoadjuvant chemotherapy. Ann Oncol. 2015;26:75–80. doi: 10.1093/annonc/mdu508. [DOI] [PubMed] [Google Scholar]
- 29.Tanei T, Shimomura A, Shimazu K, Nakayama T, Kim SJ, Iwamoto T, et al Prognostic significance of ki67 index after neoadjuvant chemotherapy in breast cancer. Eur J Surg Oncol. 2011;37:155–61. doi: 10.1016/j.ejso.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Colleoni M, Viale G, Zahrieh D, Pruneri G, Gentilini O, Veronesi P, et al Chemotherapy is more effective in patients with breast cancer not expressing steroid hormone receptors: A study of preoperative treatment. Clin Cancer Res. 2004;10:6622–8. doi: 10.1158/1078-0432.CCR-04-0380. [DOI] [PubMed] [Google Scholar]
- 31.Bottini A, Berruti A, Bersiga A, Brizzi MP, Bruzzi P, Aguggini S, et al Relationship between tumour shrinkage and reduction in ki67 expression after primary chemotherapy in human breast cancer. Br J Cancer. 2001;85:1106–12. doi: 10.1054/bjoc.2001.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneeweiss A, Katretchko J, Sinn HP, Unnebrink K, Rudlowski C, Geberth M, et al Only grading has independent impact on breast cancer survival after adjustment for pathological response to preoperative chemotherapy. Anticancer Drugs. 2004;15:127–35. doi: 10.1097/00001813-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Matsubara N, Mukai H, Fujii S, Wada N Different prognostic significance of Ki-67 change between pre- and post-neoadjuvant chemotherapy in various subtypes of breast cancer. Breast Cancer Res Treat. 2013;137:203–12. doi: 10.1007/s10549-012-2344-6. [DOI] [PubMed] [Google Scholar]
- 34.Cabrera-Galeana P, Muñoz-Montaño W, Lara-Medina F, Alvarado-Miranda A, Pérez-Sánchez V, Villarreal-Garza C, et al Ki67 changes identify worse outcomes in residual breast cancer tumors after neoadjuvant chemotherapy. Oncologist. 2018;23:670–8. doi: 10.1634/theoncologist.2017-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I, et al Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147–59. doi: 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]