Abstract
Objective
Germline alterations in the breast cancer susceptibility genes type 1 and 2, BRCA1 and BRCA2, predispose individuals to hereditary cancers, including breast, ovarian, prostate, pancreatic, and stomach cancers. Accumulating evidence suggests inherited genetic susceptibility to lung cancer. The present study aimed to survey the prevalence of pathogenic germline BRCA mutations (gBRCAm) and explore the potential association between gBRCAm and disease onset in Chinese advanced non-small cell lung cancer (NSCLC) patients.
Methods
A total of 6,220 NSCLC patients were screened using capture-based ultra-deep targeted sequencing to identify patients harboring germline BRCA1/2 mutations.
Results
Out of the 6,220 patients screened, 1.03% (64/6,220) of the patients harbored the pathogenic gBRCAm, with BRCA2 mutations being the most predominant mutations (49/64, 76.5%). Patients who developed NSCLC before 50 years of age were more likely to carry gBRCAm (P = 0.036). Among the patients harboring classic lung cancer driver mutations, those with concurrent gBRCAm were significantly younger than those harboring the wild-type gBRCA (P = 0.029). By contrast, the age of patients with or without concurrent gBRCAm was comparable to those of patients without the driver mutations (P = 0.972). In addition, we identified EGFR-mutant patients with concurrent gBRCAm who showed comparable progression-free survival but significantly longer overall survival (P = 0.002) compared to EGFR-mutant patients with wild-type germline BRCA.
Conclusions
Overall, our study is the largest survey of the prevalence of pathogenic gBRCAm in advanced Chinese NSCLC patients. Results suggested a lack of association between germline BRCA status and treatment outcome of EGFR-TKI. In addition, results showed a positive correlation between pathogenic gBRCAm and an early onset of NSCLC.
Keywords: Germline BRCA mutations, non-small cell lung cancer, prevalence, BRCA1, BRCA2
Introduction
Certain germline alterations predispose individuals to hereditary cancers. Approximately, 5% of breast cancer cases and 20%–30% of ovarian cancers are caused by germline alterations in the breast cancer susceptibility type 1 and 2 (BRCA1 and BRCA2) genes1,2. Women with germline BRCA mutation (gBRCAm) have significantly higher lifetime risk of developing breast, ovarian, tubal, and peritoneal cancers. The overall prevalence of gBRCAm in women ranged from 1 in 400 to 1 in 800 depending on the ethnicity3. In addition to breast and ovarian cancers, results showed that gBRCAm additionally conferred increased susceptibility to a spectrum of other cancers, including but not limited to, colorectal4,5, lung6, prostate7, pancreatic8, and stomach cancers7,9.
Abnormalities in the DNA repair system are central to the emergence of mutations, which ultimately result in the development of cancer10. BRCA1 and BRCA2 are tumor suppressor genes that participate in the DNA repair processes through homologous recombination repair (HRR) and thus contribute to the maintenance of genomic integrity. BRCA1 and BRCA2 act in concert to repair DNA lesions, which normally stall the DNA replication fork and/or cause DNA double-stranded breaks. Previous studies on BRCA1/2 have driven the development of therapeutic strategies targeting defects in HRR, such as poly (ADP-ribose) polymerase (PARP) inhibitors, whose function is based on the inability of BRCA-deficient cells to repair DNA lesions. Studies showed that cells with complete BRCA1/2 inactivation exhibit high sensitivity to DNA damaging agents11,12. Furthermore, numerous clinical trials have demonstrated an excellent response rate of gBRCAm carriers to PARP inhibitors13,14. The recent approval of olaparib for ovarian cancer combined with numerous promising results from trials involving other PARP inhibitors either as a single agent or in combination with other regimens for various cancer types has demonstrated the potential use of PARP inhibitors15. In addition, along with sensitivity to PARP inhibitors, BRCA mutations have been associated with sensitivity to platinum-based chemotherapy in vitro16 and in phase III clinical studies in breast17 and ovarian cancers18.
Although environmental factors, such as tobacco smoking, are regarded as the primary causes of lung cancer, increasing evidence has suggested inherited genetic susceptibility to lung cancer6. Genome-wide association studies (GWAS) have revealed multiple polymorphic variants as determinants of lung cancer risk19-21. However, to date, the prevalence of gBRCAm in Chinese lung cancer patients remains elusive. In the present study, we screened 6,220 Chinese advanced NSCLC patients to survey the prevalence of gBRCAm by sequencing the entire coding region of BRCA1/2. We also investigated the clinical significance of gBRCAm in advanced NSCLC patients.
Patients and methods
Patients
Sequencing results of germline BRCA were retrieved and retrospectively screened from 6,220 advanced (stage IIIB to IV) Chinese NSCLC patients with various histology subtypes and who underwent somatic mutation profiling for the purpose of treatment selection and genetic testing. The study was approved by the Ethics Committee of Xiangya Hospital (Approval No. 201705818). Patients provided written informed consent for the use of their tissue and/or blood samples for analyzing their germline mutations, as well as their clinical data. The screened cohort was recruited from ten hospitals.
Preparation of plasma cell-free DNA
Peripheral blood (10 mL) was collected, stored in ethylenediaminetetraacetic acid (EDTA) acid tubes, and allowed to stand in 25°C for 2 h. The supernatant was transferred to a 15-mL centrifuge tube and then centrifuged for 10 min at 16,000 g at 4°C. Circulating cell-free DNA (cfDNA) was recovered from 4 to 5 mL of plasma using the QIAamp Circulating Nucleic Acid kit (Qiagen).
DNA preparation and capture-based targeted DNA sequencing
DNA quantification was performed using the Qubit 2.0 Fluorimeter with the dsDNA HS assay kits (Life Technologies, Carlsbad, CA). A minimum of 50 ng of DNA is required for NGS library construction. DNA shearing was performed using Covaris M220, followed by end repair, phosphorylation, and adaptor ligation. Fragments with sizes ranging from 200-400 bp were selected using AMPure beads (Agencourt AMPure XP Kit), followed by hybridization with capture probes baits, hybrid selection with magnetic beads, and PCR amplification. The quality and size range of amplified fragments were then assessed by performing bioanalyzer high-sensitivity DNA assay. Paired-end sequencing of the indexed samples was performed on a NextSeq 500 sequencer (Illumina, Inc., USA).
Germline variant calling and filtering
Germline SNVs were identified using Varscan with default parameters. Germline indels were identified using Varscan and GATK. Pathogenic BRCA mutations were determined by a clinical molecular geneticist according to the guidelines of American College of Medical Genetics (ACMG). ClinVar and Enigma were used during manual curation for final confirmation of the results.
Somatic sequencing data analysis
Sequence data were mapped to the reference human genome (hg19) using BWA aligner 0.7.10. Local alignment optimization, variant calling, and annotation were performed using GATK 3.2, and VarScan. Plasma samples were compared to their corresponding white blood cell controls to identify somatic variants. Variants were filtered using the VarScan fpfilter pipeline. Loci with depths of less than 100 filtered out. At least 2 and 5 supporting reads were required for INDELs in plasma and tissue samples, respectively, while 8 supporting reads were required for SNV calling in both plasma and tissue samples. According to the ExAC, 1000 Genomes, dbSNP, ESP6500SI-V2 database, variants with population frequencies higher than 0.1% were classified as SNPs and excluded from further analysis. After filtering, the remaining variants were annotated with ANNOVAR and SnpEff v3.6. DNA translocation analysis was performed using Factera 1.4.3.
Statistical analysis
All data were analyzed using R software. Survival data were analyzed by Kaplan-Meier and log-rank test to compare the differences between the survival groups. Differences in groups were calculated and presented using paired, two-tailed Student’s t-test. P < 0.05 was considered as statistically significant. The p-values were adjusted for variables, such as age, gender, and pathological stage, when applicable.
Results
Patient characteristics
We conducted a retrospective nationwide multicenter study across ten hospitals to survey the prevalence rate of gBRCAm in advanced (stage IIIB to IV) Chinese NSCLC patients with various histological subtypes. Patients who initially underwent NGS-based molecular testing for treatment guidance were included in the study. The WBCs were additionally sequenced for the purpose of filtering out germline mutations. Sequencing results of WBCs were retrieved and screened for gBRCAm status. The screened cohort comprised 3,412 males and 2,809 females with a median age of 63.2 years. The majority of the patients (4,752; 76.4%) were diagnosed with adenocarcinoma, followed by 765 patients (12.3%) with squamous cell carcinoma, 51 patients (0.82%) with large cell carcinoma (LCC), 31 patients (0.5%) with adenosquamous carcinoma, and the remaining 621 patients with other subtypes. A total of 1,089 patients were treatment-naïve, while the remaining 5,131 patients previously received treatment. We identified 64 patients with pathogenic gBRCAm and had a median age of 59 years; of these, 26 were females and 38 were males. Fifty-five patients were diagnosed with adenocarcinoma, two with LCC, two with squamous cell carcinoma, and the remaining five patients with NOS; of these, 43 patients were non-smokers, 13 were smokers, and the remaining eight patients had no information regarding smoking history. Thirty-five of them were treatment-naïve. Patient demographics of the cohort with pathogenic gBRCAm are summarized in Table 1.
1.
Demographics of 64 patients with pathogenic germline BRCA1/2 mutations
| Chrematistics | No. of patients (%) |
| Gender | |
| Male | 38 (59.4%) |
| Female | 26 (40.6%) |
| Age, years (median and range) | 59 (33-77) |
| Smoking status | |
| Yes | 13 |
| Non/ever | 43 |
| No information | 8 |
| Histology | |
| Adenocarcinoma | 55 (86%) |
| Squamous cell | 2 (3%) |
| Large cell | 2 (3%) |
| Not specified | 5 (8%) |
| Pathogenic/likely pathogenic germline mutations detected | |
| BRCA1 | 15 (23.4%) |
| BRCA2 | 49 (76.6%) |
| Treatment history | |
| Treatment-naive | 35 (54.7%) |
| Previously treated | 29 (45.3%) |
Prevalence of gBRCA1/2 mutations
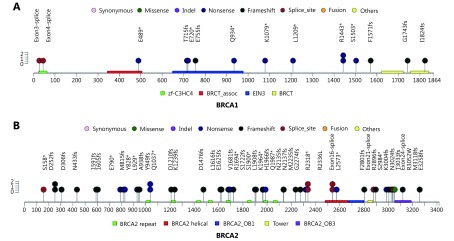
Of the 6,220 NSCLC patients screened, we detected 64 pathogenic and 699 variants of unknown significance (VUS) germline BRCA variants. Overall, we identified 64 patients harboring pathogenic gBRCAm, corresponding a prevalence of 1.03% (64/6,220). Results revealed a gBRCA2 predominance with 15 (23.4%) gBRCA1 loss-of-function (LOF) mutation carriers and 49 (76.6%) gBRCA2 LOF mutation carriers. Consistent with gBRCAm findings reported in other cancer types, the mutations were found to be evenly distributed between the two genes, and no particular hotspots were identified. The distributions of gBRCA1/2 mutations are presented in Supplementary Figure S1A-S1B. Among the pathogenic gBRCA2 variants, we observed four types of mutations, with frameshift mutations being the most predominant mutation type accounting for 57.2% (28/49), followed by nonsense/stop gain point mutations with 24.5% (12/49), splice-site variants with 14.3% (7/49), and the remaining missense variants with 4%. Meanwhile, the majority of the pathogenic BRCA1 variants were nonsense mutations (8/15), followed by frameshift mutations (5/15) and splice-site mutations. No gBRCA1 pathogenic missense mutations were identified in our cohort. Furthermore, we detected several mutations, including R1443* in gBRCA1 and D252fs, Q1037*, R2336L, and exon 16 splice site variant in gBRCA2, in at least two patients from our cohort.
S1.
Pathogenic germline BRCA mutations found in this cohort.
Somatic mutation spectrum of patients with gBRCAm
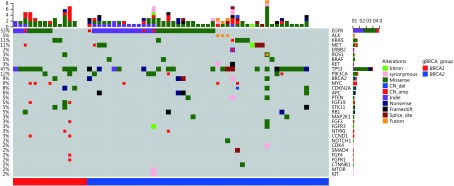
In addition to analyzing the gBRCAm status of the lung cancer patients in our cohort, we investigated somatic mutations associated with patients harboring gBRCAm. Somatic mutation profiling was conducted using a panel consisting of 56 lung cancer-related genes spanning 330 kb of the human genome. Collectively, we identified 164 somatic mutations spanning 31 genes. A total of 47 patients harbored the following classic lung cancer driver mutations: 33 with EGFR, 3 with ALK, 6 with KRAS, 5 with MET, 1 with ERBB2, 1 with ROS1, and 2 with BRAF mutations (Figure 1). Four patients had multiple driver mutations, and six patients had no mutations detected based on this panel. EGFR was most frequently mutated gene, occurring in 53% of patients, followed by TP53 and PIK3CA, occurring in 47% and 12% of patients, respectively. Six patients (9%) harbored somatic BRCA2 mutations corresponding to two nonsense, one synonymous, one splice site, and two missense mutations. Other frequently occurring somatic mutations included MYC (9%), CDKN2A (8%), and APC (6%). Next, we examined whether there is a difference in the somatic mutation spectra in patients with gBRCA1 and gBRCA2 mutations. Our findings revealed that patients harboring gBRCA1 or gBRCA2 mutations had comparable mutation profiles (Figure 1). Taken together, the somatic mutation profiles of patients with gBRCAm were comparable to those of patients harboring wild-type germline BRCA1/2. We observed no significant differences in the somatic mutation spectra of patients with the gBRCA1 and gBRCA2 mutations.
1.
Somatic mutational profiles of 64 patients carrying pathogenic gBRCAm. Columns represent patients. Rows represent genes. Top bars represent the number of mutations per patient. Side bars represent the percentage of patients with the mutation. Colored boxes indicate different mutation types.
gBRCAm is more prevalent in patients with early disease onset
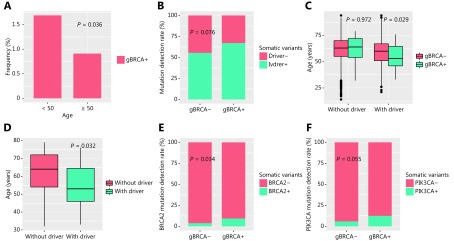
Numerous studies have suggested correlations between gBRCAm and the early onset of disease in several cancer types, including lung cancer. First, we investigated whether gBRCAm is associated with early onset of NSCLC in Chinese patients. The screened cohort comprised 947 patients diagnosed before age of 50; among them, 16 harbored gBRCAm, corresponding to a prevalence rate of 1.69%. A total of 4,945 patients were diagnosed at or after the age of 50; of these, 45 patients were gBRCAm carriers, corresponding to a prevalence of 0.91%. Our results revealed that patients with an onset of disease before 50 were more likely to have gBRCAm (P = 0.036) (Figure 2A). Importantly, it is well-established that ALK fusions tend to occur in younger patients; therefore, patients with ALK fusions were excluded from the analysis. In addition, results indicated that patients with gBRCAm tend to carry classic driver mutations for lung cancer (P = 0.076, Figure 2B). Further analysis revealed that among patients harboring driver mutations, those with concurrent gBRCAm were significantly younger compared to patients with wild-type (WT) gBRCA (P = 0.029, Figure 2C). We observed no significant differences in age between patients with gBRCAm and WT gBRCA among patients without the driver mutations (P = 0.972, Figure 2C). Furthermore, among the patients with gBRCAm, those with concurrent driver mutations were younger than patients without the driver mutations (P = 0.032, Figure 2D). In addition to driver mutations, we identified a positive correlation between gBRCAm with somatic BRCA2 mutations (P = 0.034, Figure 2E) and a marginal positive correlation between gBRCAm and PIK3CA mutations (P = 0.055, Figure 2F). Somatic BRCA2 mutations are likely to act as the second hit for tumorigenesis. In our cohort, we identified six patients with germline BRCA2 mutations who carried concurrent somatic BRCA mutations; five of these patients harbored a BRCA2 mutation and one had BRCA1 mutations. Taken together, our findings suggested that gBRCAm predisposes patients for the development of lung cancer.
2.
Relationship between gBRCAm and clinical and molecular features. (A) Germline BRCA mutations are more likely to occur in patients < 50 years of age. (B) Association between germline BRCA status and the presence of driver mutations. Blue bars denote the presence of driver mutations; red bars denote the lack of driver mutations. X-axis denotes the status of germline BRCA. (C) In the absence of driver mutations, no age difference was observed between patients with (+) or without (–) germline BRCA mutations. (D) In the presence of driver mutations, patients with germline BRCA mutations are younger. X-axis denotes the presence or absence of classic lung cancer driver mutations. (E) Association between germline BRCA status and somatic BRCA2 mutation. (F) Association between germline BRCA status and somatic PIK3CA mutation. X-axis indicates the gBRCAm status, (–) denotes wild-type status, while (+) represents mutation carriers. Y-axis indicates the percentage of patients with the somatic molecular features being observed. P < 0.05 was considered statistically significant.
Survival analysis of patients with gBRCAm
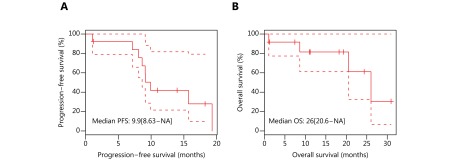
Previous studies reported that patients with gBRCAm are more sensitive to platinum-containing chemotherapy18,22. Next, we investigated the efficacy of chemotherapy in 13 gBRCAm treatment-naïve patients who were ineligible for targeted therapy. These patients had a median progression-free survival (PFS) of 9.9 months and median overall survival (OS) of 26 months (Figure 3A-3B).
3.
Treatment outcomes and overall survival of patients with pathogenic germline BRCA mutations treated with platinum-containing chemotherapy. (A) Progression-free survival (PFS) and (B) overall survival (OS) from the day of treatment of 13 patients who received platinum-containing chemotherapy as first-line treatment.
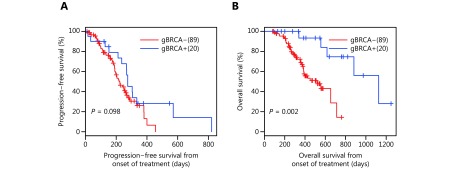
Next, we additionally evaluated the efficacy of EGFR tyrosine kinase inhibitor (TKI) in 20 patients harboring EGFR activating mutations and treated with first-generation EGFR-TKI. The study included a cohort of 89 patients harboring EGFR mutations but had WT gBRCA and treated with EGFR-TKI at the Xiangya Hospital. Patients with gBRCAm had a comparable median PFS with patients harboring WT gBRCA (9.2 vs. 7.3 months), after adjusting for age, gender, and disease stage (P = 0.098) (Figure 4A). Interestingly, the overall survival (OS) of patients with concurrent gBRCAm was significantly longer than that of EGFR-TKI-treated patients harboring WT gBRCA (37.5 months vs. 17.4 months, P = 0.002, Figure 4B).
4.
Treatment outcomes and overall survival of EGFR-mutant patients with pathogenic germline BRCA mutations treated with EGFR inhibitor. (A) Progression-free survival (PFS) and (B) overall survival (OS) from the day of treatment of 20 patients who received first-generation EGFR inhibitor as first-line treatment.
Discussion
In the present study, we analyzed the incidence of gBRCAm in advanced Chinese NSCLC patients (stage IIIB to IV). To the best of our knowledge, our study is the first and largest cohort to investigate the prevalence of gBRCAm in Chinese NSCLC patients, which was found to be 1.03% of gBRCAm with a predominance of BRCA2 (49/64, 76.5%). This BRCA2 predominance was previously observed in prostate cancer patients23. However, the majority of gBRCAm in ovarian cancer are predominantly composed of BRCA1 mutations (172/235, 73.2%)2. In prostate cancer, patients with germline BRCA2 mutations showed elevated global genomic instability and harbored unique mutations that were rarely or not yet been reported in sporadic localized prostate cancer24,25. However, our findings revealed that NSCLC patients harboring gBRCA2 mutations had comparable mutation spectra to those of patients harboring gBRCA1, as well as patients with WT gBRCA.
Previous studies have established the association between gBRCAm and the development of various cancers, including but not limited to breast, ovarian, prostate, pancreatic and stomach cancers. Germline BRCA mutation associated cancers present with distinct clinical behaviors often characterized by an earlier onset, improved efficacy of certain treatments, and more favorable survival26,27 However, the role of BRCA mutations in NSCLC remain elusive. Our results revealed a positive association between gBRCAm and the early onset of NCSLC. Patients with gBRCAm were significantly more likely to develop NSCLC before the age of 50 (P = 0.036). Interestingly, patients with concurrent gBRCAm and mutations in classic lung cancer driver genes were also found to develop NSCLC at a younger age (P = 0.029). However, this phenomenon was not observed in patients without mutations in driver genes.
While the clinical relevance of gBRCAm has been well-established in multiple types of cancer, the significance of somatic BRCA mutations is beginning to be understood. Our cohort has six gBRCAm carriers with concurrent somatic BRCA mutations, suggesting that the somatic BRCA mutation could act as a second hit for the development of tumorigenesis (Table 2). Interestingly, two of these patients, namely, patients 3 and 6, harbored the germline and somatic BRCA2 mutations on the same amino acid residue (i.e. Arg1694 and Ser2984) but with different mutation types.
2.
Mutational profile of the patients with germline and somatic mutations in BRCA1/2
| Patient number | Gender | Age (years) | Somatic mutations | Germline mutations | |||||||
| Gene | Mutation type | Mutation | AF | Gene | Mutation type | Mutation | AF | ||||
| 1 | Female | 48 | BRCA2 | Stop gained | c.1688G >
A (p.Trp563*) |
14.93% | BRCA2 | Stop gained | c.6952C >
T (p.Arg2318*) |
46.92% | |
| 2 | Male | 64 | BRCA2 | Missense | c.9065G >
C (p.Arg3022Thr) |
21.20% | BRCA2 | Stop gained | c.7718T >
G (p.Leu2573*) |
50.50% | |
| 3 | Male | 70 | BRCA2 | Synonymous | c.5082A >
G (p.Arg1694=) |
4.73% | BRCA2 | Stop gained | c.5080A >
T (p.Arg1694*) |
54.19% | |
| 4 | Male | 65 | BRCA2 | Stop gained | c.9299T >
A (p.Leu3100*) |
25.90% | BRCA2 | Splice donor | c.7805+1G >
A |
59.10% | |
| 5 | Male | 68 | BRCA2 | Missense | c.8026A >
T (p.Met2676Leu) |
1.11% | BRCA1 | Frameshift | c.2143_2155delinsTCTTT (p.Thr715fs) | 40.53% | |
| 6 | Male | 60 | BRCA2 | Splice region | c.8951C >
T (p.Ser2984Leu) |
0.70% | BRCA2 | Stop gained | c.8951C >
G (p.Ser2984*) |
47.83% | |
Despite very poor survival outcomes, platinum-based chemotherapy remains the standard therapy for the majority of heavily-treated advanced NSCLC patients, who have a median progression-free survival (PFS) and overall survival (OS) of 3.6 months and 7.9 months, respectively28. Enhanced sensitivity to platinum-containing chemotherapy has been associated with germline BRCA mutations based on pre-clinical and clinical studies on ovarian and breast cancers17,18. In the present study, we consistently observed the good efficacy of chemotherapy in gBRCAm treatment-naïve patients with PFS and OS of 9.9 months and 26 months, respectively. Interestingly, patients with concurrent gBRCAm and somatic EGFR mutations also showed a tendency to respond well to EGFR-TKI compared to patients without gBRCAm. In addition to investigating somatic mutations, studying the germline genetic status of lung cancer patients is also important for understanding their genetic landscape and for guiding clinical decisions.
The present study has certain limitations. Numerous cancer predisposing genes, mostly tumor suppressors, have been identified29. This study was only limited to the identification of pathogenic mutations. Further studies are required to elucidate the clinical significance of other pathogenic cancer predisposing genes in NSCLC. Numerous studies revealed that not all BRCA germline mutations resulted in the locus-specific loss of heterozygosity (LOH) and further reported the differential clinical significance between BRCA LOH mutations and non-LOH mutations. Unfortunately, panels used in this study cannot detect LOH. Only patients with advanced disease were screened (stage IIIb to IV) and analyzed. Future studies that include patients in the early stages of the disease are needed to provide a more comprehensive understanding of gBRCA status. The lack of family history also limits further investigation.
In conclusion, to the best of our knowledge, we have conducted the largest and most comprehensive survey of the prevalence of pathogenic gBRCAm in Chinese NSCLC patients. Results revealed a positive correlation between pathogenic gBRCAm and early onset of NSCLC.
Acknowledgements
This work was supported by grant from the National Natural Science Foundation of China (Grant No. 81502699).
Conflict of interest statement
JY, HH-Z, AL, HL, XM and HH are employees of Burning Rock Biotech. The other authors declare no conflict of interest.
Contributor Information
Qian Chu, Email: qianchu@tjh.tjmu.edu.cn.
Chengping Hu, Email: huchengp28@csu.edu.cn.
References
- 1.Daly MB, Axilbund JE, Buys S, Crawford B, Farrell CD, Friedman S, et al Genetic/familial high-risk assessment: breast and ovarian. J Natl Compr Canc Netw. 2010;8:562–94. doi: 10.6004/jnccn.2010.0043. [DOI] [PubMed] [Google Scholar]
- 2.Wu XH, Wu LY, Kong BH, Liu JH, Yin RT, Wen H, et al The first nationwide multicenter prevalence study of germline BRCA1 and BRCA2 mutations in Chinese ovarian cancer patients . Int J Gynecol Cancer. 2017;27:1650–7. doi: 10.1097/IGC.0000000000001065. [DOI] [PubMed] [Google Scholar]
- 3.Petrucelli N, Daly MB, Feldman GL Hereditary breast and ovarian cancer due to mutations in BRCA1 and BRCA2 . Genet Med. 2010;12:245–59. doi: 10.1097/GIM.0b013e3181d38f2f. [DOI] [PubMed] [Google Scholar]
- 4.Phelan CM, Iqbal J, Lynch HT, Lubinski J, Gronwald J, Moller P, et al Incidence of colorectal cancer in BRCA1 and BRCA2 mutation carriers: results from a follow-up study . Br J Cancer. 2014;110:530–4. doi: 10.1038/bjc.2013.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sopik V, Phelan C, Cybulski C, Narod SA BRCA1 and BRCA2 mutations and the risk for colorectal cancer . Clin Genet. 2015;87:411–8. doi: 10.1111/cge.2015.87.issue-5. [DOI] [PubMed] [Google Scholar]
- 6.Wang YF, McKay JD, Rafnar T, Wang ZM, Timofeeva MN, Broderick P, et al Rare variants of large effect in BRCA2 and CHEK2 affect risk of lung cancer . Nat Genet. 2014;46:736–41. doi: 10.1038/ng.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breast Cancer Linkage Consortium Cancer risks in BRCA2 mutation carriers. J Nat Cancer Inst. 1999;91:1310–6. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- 8.Stadler ZK, Salo-Mullen E, Patil SM, Pietanza MC, Vijai J, Saloustros E, et al Prevalence of BRCA1 and BRCA2 mutations in ashkenazi jewish families with breast and pancreatic cancer . Cancer. 2012;118:493–9. doi: 10.1002/cncr.26191. [DOI] [PubMed] [Google Scholar]
- 9.Cavanagh H, Rogers KMA The role of BRCA1 and BRCA2 mutations in prostate, pancreatic and stomach cancers . Hered Cancer Clin Pract. 2015;13:16. doi: 10.1186/s13053-015-0038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lord CJ, Ashworth A Parp inhibitors: synthetic lethality in the clinic. Science. 2017;355:1152–8. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–7. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 12.Farmer H, McCabe N, Lord CJ, Tutt ANJ, Johnson DA, Richardson TB, et al Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy . Nature. 2005;434:917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 13.Tutt A, Robson M, Garber JE, Domchek S, Audeh MW, Weitzel JN, et al Phase II trial of the oral parp inhibitor olaparib in brca-deficient advanced breast cancer. J Clin Oncol. 2009;27:CRA501. doi: 10.1200/jco.2009.27.18_suppl.cra501. [DOI] [Google Scholar]
- 14.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–34. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 15.O'Cearbhaill RE Using parp inhibitors in advanced ovarian cancer. Oncology. 2018;32:339–43. [PMC free article] [PubMed] [Google Scholar]
- 16.Tassone P, Tagliaferri P, Perricelli A, Blotta S, Quaresima B, Martelli ML, et al BRCA1 expression modulates chemosensitivity of BRCA1-defective HCC1937 human breast cancer cells. Br J Cancer. 2003;88:1285–91. doi: 10.1038/sj.bjc.6600859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu XC, Zhang J, Xu BH, Cai L, Ragaz J, Wang ZH, et al Cisplatin plus gemcitabine versus paclitaxel plus gemcitabine as first-line therapy for metastatic triple-negative breast cancer (CBCSG006): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2015;16:436–46. doi: 10.1016/S1470-2045(15)70064-1. [DOI] [PubMed] [Google Scholar]
- 18.Tan DSP, Yap TA, Hutka M, Roxburgh P, Ang J, Banerjee S, et al Implications of BRCA1 and BRCA2 mutations for the efficacy of paclitaxel monotherapy in advanced ovarian cancer . Eur J Cancer. 2013;49:1246–53. doi: 10.1016/j.ejca.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 19.Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, et al A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452:633–7. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- 20.McKay JD, Hung RJ, Gaborieau V, Boffetta P, Chabrier A, Byrnes G, et al Lung cancer susceptibility locus at 5p15.33. Nat Genet. 2008;40:1404–6. doi: 10.1038/ng.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang YF, Broderick P, Webb E, Wu XF, Vijayakrishnan J, Matakidou A, et al Common 5p15.33 and 6p21.33 variants influence lung cancer risk. Nat Genet. 2008;40:1407–9. doi: 10.1038/ng.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egawa C, Miyoshi Y, Takamura Y, Taguchi T, Tamaki Y, Noguchi S Decreased expression of BRCA2 mrna predicts favorable response to docetaxel in breast cancer . Int J Cancer. 2001;95:255–9. doi: 10.1002/(ISSN)1097-0215. [DOI] [PubMed] [Google Scholar]
- 23.Ostrander EA, Udler MS The role of the BRCA2 gene in susceptibility to prostate cancer revisited . Cancer Epidemiol Biomarkers Prev. 2008;17:1843–8. doi: 10.1158/1055-9965.EPI-08-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Risbridger GP, Taylor RA, Clouston D, Sliwinski A, Thorne H, Hunter S, et al Patient-derived xenografts reveal that intraductal carcinoma of the prostate is a prominent pathology in BRCA2 mutation carriers with prostate cancer and correlates with poor prognosis . Eur Urol. 2015;67:496–503. doi: 10.1016/j.eururo.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Taylor RA, Fraser M, Livingstone J, Espiritu SMG, Thorne H, Huang V, et al Germline BRCA2 mutations drive prostate cancers with distinct evolutionary trajectories . Nat Commun. 2017;8:13671. doi: 10.1038/ncomms13671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alsop K, Fereday S, Meldrum C, deFazio A, Emmanuel C, George J, et al BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the australian ovarian cancer study group . J Clin Oncol. 2012;30:2654–63. doi: 10.1200/JCO.2011.39.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moschetta M, George A, Kaye SB, Banerjee S BRCA somatic mutations and epigenetic brca modifications in serous ovarian cancer. Ann Oncol. 2016;27:1449–55. doi: 10.1093/annonc/mdw142. [DOI] [PubMed] [Google Scholar]
- 28.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 29.Donner I, Katainen R, Sipila LJ, Aavikko M, Pukkala E, Aaltonen LA Germline mutations in young non-smoking women with lung adenocarcinoma. Lung Cancer. 2018;122:76–82. doi: 10.1016/j.lungcan.2018.05.027. [DOI] [PubMed] [Google Scholar]